Introduction
Periodontitis is a chronic inflammatory and destructive disease of periodontal tissue caused by microorganisms, host immune factors and other promoting factors (1). Periodontitis, with high prevalence in all countries, is the main cause of tooth loss and edentulous jaws in adults, with ~12% of adults worldwide affected by severe periodontal disease (1,2). Tooth loss caused by periodontitis not only damages tooth function, but also damages the patient's speech and self-confidence. Furthermore, the cost of treating periodontitis also brings a significant burden on social medical resources (2). Bacteria and their products in dental plaque are essential initiating factors of periodontitis, which directly or indirectly participate in its pathogenesis (3). In previous years, with the development of periodontal medicine, periodontitis has been found to be associated with numerous systemic diseases, such as nervous system disease, diabetes, cardiovascular disease, rheumatoid arthritis, cancer, pregnancy complications and pulmonary diseases (4–6).
Previous research has shown that the oral bacterial community in patients with endocarditis is different from that of healthy individuals (7). Oral bacteria enter the bloodstream through ruptured periodontal pockets or epithelium, which is beneficial for the pathogenesis of infectious endocarditis (7). Haraszthy et al (8) found that ~50% of carotid endarterectomy samples of patients with atherosclerosis contain periodontal pathogens [such as Actinobacillus actinomycetemcomitans (A. actinomycetemcomitans), Bacteroides forsythus (now named Tannerella forsythia), Porphyromonas gingivalis (P. gingivalis) and Prevotella intermedia (P. intermedia)], which supports the view that periodontal pathogens enter the blood circulation (i.e. bacteremia) and cause infection of distant organs and systemic diseases (9). Similar mechanisms have also been discussed in the association between periodontitis and Alzheimer's disease, adverse pregnancy outcomes and rheumatoid arthritis (10,11). It has been reported that periodontal bacteria and their products (including lipopolysaccharide, gingipain and P. gingivalis peptidyl-arginine deiminase) directly enter the bloodstream and promote the development of existing systemic diseases by destroyed gingival epithelium (10,11). On the other hand, the systemic chronic inflammation caused by periodontitis also appears to play an important role in the aforementioned systemic diseases. A previous study has shown that periodontitis is associated with the risk factors of cardiovascular disease via proinflammatory markers such as interleukin (IL)-6, tumor necrosis factor-α (TNF-α) and C-reactive protein (CRP), and periodontal therapy can reduce the risk of cardiovascular disease by reducing these biomarkers (12). The European Federation of Periodontology and the International Diabetes Federation indicated that the associated mechanisms between periodontitis and diabetes included the expression of inflammation-related molecules such as IL-1β, TNF-α, IL-6, nuclear factor B-receptor activator ligand, and Toll-like receptor (TLR) 2 and 4, and suggested that professional periodontal therapy may help reduce glycated hemoglobin (HbA1C) in patients with diabetes (13). Similarly, the erythrocyte sedimentation rate, CRP, anti-citrullinated protein antibodies and joint disease activity score of patients with rheumatoid arthritis were improved after non-surgical periodontal treatment (14). Periodontal treatment was also beneficial for the quality of life and cognitive impairment of patients with Alzheimer's disease (10). The treatment principle of periodontitis is to control plaque. Related interventional studies have shown that professional periodontal therapy can reduce systemic inflammatory markers of periodontitis complications, thus reducing the risk of occurrence and aggravation of systemic diseases (9).
Previous studies have investigated the association between periodontitis and lung diseases, such as pneumonia (15), asthma (16), chronic obstructive pulmonary disease (COPD) (17), coronavirus disease 2019 (COVID-19) (18) and lung cancer (19). The periodontal status of patients with lung disease is relatively severe and the majority of patients have a high periodontal index for risk of infection (20). Bacteria and their products in plaque may play a crucial role in the association between pulmonary diseases and periodontitis. Periodontopathogens in periodontal pockets can be inhaled into the lungs and play a role in the initiation and development of pulmonary diseases by participating in the apoptosis of airway epithelial cells, enhancing the adhesion of respiratory pathogens, promoting the expression of mucin and regulating the immune system.
The present study aimed to review the published epidemiological evidence of the association between periodontitis and lung diseases, summarize the possible pathophysiological mechanisms of periodontal pathogens in lung diseases and highlight important research issues that require further attention.
Literature search
A bibliographical search was performed on PubMed using the following search terms: ‘(Periodontitis or periodontal disease) and (oral care or oral health or periodontal treatment or periodontal therapy) and (diabetes) and (cardiovascular diseases) and (rheumatoid arthritis) and (cancer) and (pregnancy complication or adverse pregnancy outcomes) and (Alzheimer's disease) and (lung diseases or respiratory diseases or pulmonary diseases) and (pneumonia or community-acquired pneumonia or ventilator-associated pneumonia) and (chronic obstructive pulmonary disease) and (asthma) and (lung cancer) and (Corona Virus Disease 2019 or severe acute respiratory syndrome coronavirus 2) and (Porphyromonas gingivalis) and (Prevotella intermedia) and (Tannerella forsythia or Bacteroides forsythia) and (Treponema denticola) and (Fusobacterium nucleatum) and (Aggregatibacter actinomycetemcomitans or Actinobacillus actinomycetemcomitans)’. The inclusion criteria involved articles published in English up to November 2023, including systematic reviews, literature reviews, and in vitro and in vivo studies related to periodontal bacteria and pulmonary diseases or other systemic diseases. Although publications were generally confined to the last 10 years, older significant publications were not excluded.
Periodontal disease and respiratory diseases
Pneumonia
Pneumonia is inflammation of the lung parenchyma, and can be caused by microbial, immune, physical and chemical factors, as well as allergies and drugs (21). It has been reported that clinical attachment loss (CAL) and bleeding on probing (BOP) in patients with community-acquired pneumonia (CAP) were more severe, and patients with moderate or severe periodontitis patients had an ~4-fold higher risk of suffering from CAP than individuals without periodontal infection (22). A 7-year cohort study also found that, compared with patients with periodontal disease, pneumonia mortality was significantly lower in patients with hemodialysis without periodontal disease, and periodontal disease could be a predictor of pneumonia-related mortality in these patients (23). Previous studies have shown that the mouth is a reservoir for pulmonary infection. When suffering from periodontal disease, dental plaque aggregates in the mouth, thus promoting the colonization of respiratory pathogens. Respiratory pathogens such as Pseudomonas aeruginosa (P. aeruginosa) and Klebsiella pneumoniae (K. pneumoniae), have been detected in dental plaque. In addition, periodontal pathogens have been found in the pulmonary aspiration of patients with aspiration pneumonia (4,24–26).
Previous research has focused on the association between periodontitis and hospital-acquired pneumonia, a type of ventilator-associated pneumonia (VAP). Critically ill and long-term bedridden patients, as well as patients with mobility difficulties tend to have poor oral status and increased bacterial load due to reduced oral saliva, decreased self-cleaning ability and the wearing of dentures. Thus, respiratory pathogens such as Staphylococcus aureus (S. aureus), P. aeruginosa and K. pneumoniae have more opportunities to colonize in the oral cavity (4). These patients have therefore a higher risk of inhalation, and when they inhale oral secretions containing respiratory pathogens, the risk of respiratory infection increases (4). For these patients, it is of great importance to maintain oral hygiene, and to prevent oral biofilm and dental plaque formation in order to reduce the potential risk of pneumonia (27,28). A previous systematic review showed that oral care provided by professionals could decrease the risk of pneumonia by reducing the colonization of pathogens in the oral cavity (29). Due to the anatomical continuity of the oral cavity and respiratory tract, bacteria in the oral cavity may cause lower respiratory tract infections due to aspiration to the lungs, and poor oral status appears to be associated with respiratory infection (30). The incidences of respiratory infections and VAP rates in patients within the intensive care unit (ICU) that have undergone professional dental treatment are both significantly lower than those of patients within the ICU receiving routine oral care, which suggests that professional dental treatment could prevent respiratory infections, and highlights the importance of professional dental treatment to prevent pulmonary infections in critically ill patients (30). The use of 0.12% chlorhexidine can reduce oropharyngeal microbial colonization (31), thus preventing saliva-containing bacteria from being inhaled into the respiratory tract and causing lung infections. Additionally, oral care for premature infants can effectively prevent the occurrence of early VAP after re-intubation by reducing the number of bacteria in the mouth (32). The aforementioned results indicate that oral interventions aimed at reducing dental plaque buildup can reduce the bacteria load in the mouth and the risk of pneumonia.
COPD
COPD is one of the most common airway diseases in humans, and is characterized by progressive obstruction of air flow caused by chronic airway inflammation (33). The predicted percentage of forced expiratory volume in 1 sec (FEV1%) is used to evaluate the severity of airflow limitation (33). The association between periodontitis and COPD has long attracted the attention of numerous researchers. In 1998, a study showed that the risk of COPD increased with the increase in alveolar bone loss (34). A previous study has shown that chronic marginal periodontitis is prevalent in patients with severe COPD, and there is a significant association between the two diseases (35). Similarly, compared with that of patients with COPD, the rate of periodontitis was significantly lower in patients without COPD (58.1% vs. 34.0%, P<0.001), which emphasized the importance of promoting oral health in patients with COPD (36). Additionally, a significant negative association between oral hygiene index-simplified CAL and FEV1% has been found in patients with COPD (37). It has also been reported that there is a significant association between CAL and decreased respiratory function in Northern Irish men (38). Compared with patients with COPD without periodontal treatment, the incidence of adverse outcomes (including risks of deterioration, pneumonia and respiratory failure) was significantly lower in patients with COPD receiving periodontal therapy. Moreover, the mortality rate in the periodontal treatment group was reduced by 37%. These results indicated that periodontal therapy could reduce the risk of adverse respiratory events in patients with COPD (39).
Various studies have confirmed the association between periodontitis and COPD, but it is not possible to establish a causal association between the two diseases because the majority of those studies are cross-sectional (40,41). Furthermore, those studies had several limitations including firstly, that there were differences in the diagnostic criteria of periodontitis and COPD used in the studies. Secondly, certain studies had certain shortcomings including small sample size and limited population selection, such as selecting only male patients; thus, the results are not representative. Thirdly, certain studies did not rule out common risk factors for both diseases such as smoking. Therefore, those findings are only preliminary, and large-scale prospective studies should be designed to investigate the association between periodontitis and COPD in the future. Furthermore, to the best of our knowledge, there are no reports to date on whether improving lung function improves oral health status.
COVID-19
COVID-19 is a life-threatening respiratory illness triggered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (42). Globally, the number of COVID-19 cases and mortalities is still increasing, greatly affecting society and medical care (42). Previous studies have shown that periodontitis, dental plaque and gingival bleeding are associated with COVID-19, and the presence of periodontal disease may elevate the risk of COVID-19 (18,43). The clinical manifestations of periodontal disease (including probing depth, gingival recession and CAL) are also significantly associated with COVID-19 complications (44). Similarly, poor oral hygiene has also been reported to be associated with complications of COVID-19 (45). Moreover, patients who have both periodontal disease and COVID-19 have a higher risk of mortality (43). Thus, periodontitis may be associated with COVID-19 and may promote the occurrence and development of COVID-19 to a certain extent. However, the current evidence is preliminary, and additional studies are needed in the future to confirm the association between the two diseases, which may contribute to the treatment of COVID-19.
Asthma
Asthma is a chronic inflammation of the airways that is characterized by variable airway obstruction and bronchial hypersensitivity (46), affecting >200 million individuals worldwide (47). Increasing evidence has shown that asthma is linked to oral health problems such as tooth decay, dental erosions, oral candidiasis and in particular, periodontal disease (48,49). Compared with those of patients without asthma, the mean and median of BOP, visible plaque index and incidence of periodontitis in the group with severe asthma were significantly higher (50). In addition, patients with periodontitis had a 2-4-fold higher risk of developing asthma than those without periodontitis (50,51). A meta-analysis in 2019 showed that the presence of papillary bleeding, salivary calculi and CAL were more common in asthmatic patients than in healthy individuals and concluded that the presence of asthma appeared to imply an increased risk of periodontal disease (52). These data suggest there may be a potential association between asthma and periodontal disease.
It has been reported that periodontitis is more common in Jordanian adults with bronchial asthma (BA) than in those without BA and BA was positively associated with periodontitis (53). A possible reason for this result is the use of corticosteroids in patients with asthma. Inhaled corticosteroids (ICs) are usually the first choice of treatment for asthmatic patients (54). The use of ICs in asthmatic patients results in a significant reduction in bone mineral density in the mandible, which is regarded as a risk factor for tooth loss (54). Notably, decreases in alveolar bone density and tooth loss are important signs of the occurrence and development of periodontitis (55). The use of ICs was also found to be associated with an improved risk of bacterial plaque (56). Additionally, the asthmatic patients who used ICs were more susceptible to develop periodontal disease (57). Thus, the use of ICs in asthmatic patients may promote the occurrence and development of periodontitis. Other reasons why patients with asthma are more likely to develop periodontitis include: i) The protective mechanism is impaired by reduced salivation and secretory IgA content; ii) a higher level of calcium and phosphorous in saliva contributing to an increase of calculi; iii) dehydration of oral mucosa caused by mouth breathing; iv) elevated level of IgE in gingival tissue leads to immune dysregulation; and v) since periodontitis is a chronic disease, it is not easy to detect in the early stage (49). In addition, more attention is paid to asthma as it can cause emergency events, which means periodontal health is often overlooked (49,50).
Lung cancer
As one of the most common tumors in the world, lung cancer has high mortality rates (58). Previous studies have indicated that periodontal disease was significantly associated with an increased risk of developing lung cancer (59) and patients with periodontitis were more likely to suffer from lung cancer than healthy individuals (19). A previous cohort study also reported that periodontitis was a hazard factor for women with lung cancer (60). Similarly, periodontal disease indices including BOP, probing pocket depth and CAL were significantly associated with the risk of developing lung carcinoma (61). Furthermore, history of tooth loss and periodontitis were regarded to be associated with the risk of developing lung carcinoma (62,63). These results suggest that periodontitis may promote the development of lung cancer; however, the role of periodontitis in the pathogenesis of lung cancer needs to be further investigated.
Periodontal pocket may be a potential source of respiratory pathogens
Accumulating evidence suggests that periodontal pathogens play a direct or indirect role in promoting systemic diseases, such as cardiovascular disease, colorectal cancer, diabetes, pregnancy complications, nervous system diseases and pulmonary diseases (6,64–66). Periodontal pathogens may directly cause systemic diseases by producing virulence factors, or by transfer to various parts of the body through aspiration and blood, thus causing diseases in distant organs (64,67).
Periodontal pathogens may be a mediator between periodontitis and lung disease, and periodontitis may have an influence on the development of lung disease through periodontal pathogens (9,68). Oral microbiota is one of the most complex groups of microorganisms in the human body, comprising of 700-1,000 prevalent taxa at the species level (64,69). These microbes can colonize in the teeth, gingival epithelium, tongue, gingival sulcus, hard and soft palates, and dentures, and concentrate in dental plaque (69). It has been reported that dental plaque accumulation and periodontitis are known risk factors for aspiration pneumonia (70) and ~50% of healthy individuals inhale saliva accidentally when they sleep (71). Thus, it is not unusual that adults with periodontal disease inhale pathogenic bacteria colonized in dental plaque into the lungs, which triggers inflammation.
The following sections will introduce relevant evidence of the potential link between periodontal pathogens and various lung diseases (Table I).
 |
Table I.
Association between various periodontal pathogens and lung diseases.
|
Table I.
Association between various periodontal pathogens and lung diseases.
| Periodontal bacteria |
Lung diseases |
(Refs.) |
| Porphyromonas gingivalis |
Pneumonia |
(25,70,77,78,82,117,124,138) |
| |
Bronchopneumonia and lung abscess |
(76) |
| |
COPD |
(37,107,124) |
| |
Lung cancer |
(100) |
| |
COVID-19 |
(94) |
| Treponema denticola |
Pneumonia |
(82) |
| |
Bronchopneumonia and lung abscess |
(76) |
| |
COPD |
(107) |
| Fusobacterium nucleatum |
Pneumonia |
(82) |
| |
COPD |
(71,80,99,107) |
| |
COVID-19 |
(93,119) |
| Prevotella intermedia |
Pneumonia |
(82) |
| |
Asthma |
(102) |
| |
Cystic fibrosis |
(74) |
| |
COPD |
(108) |
| |
COVID-19 |
(94) |
| Aggregatibacter actinomycetemcomitans |
Pneumonia |
(82) |
| |
COPD |
(107) |
| |
COVID-19 |
(94) |
| Tannerella forsythia |
Pneumonia |
(82) |
| |
COPD |
(107) |
Pneumonia
‘Red-complex’ bacteria including T. forsythia, P. gingivalis and Treponema denticola (T. denticola) are the key bacterial groups closely related to periodontitis (72). It has been reported that aspiration pneumonia is a disease resulting from inhaling saliva containing oral bacteria, and periodontitis-related bacteria such as P. gingivalis, T. forsythia, T. denticola and P. intermedia, which could be detected in lung samples in nearly 50% of patients with aspiration pneumonia (25).
P. intermedia
P. intermedia, which is a gram-negative bacterium related to moderate or severe gingivitis, acute necrotizing ulcerative gingivitis and chronic periodontitis, can induce severe bacterial pneumonia and pulmonary hemorrhage in mice (73). Similarly, immunohistochemistry revealed that neutrophils and macrophages were infiltrated in the lung tissue of C57BL/6 mice treated with the culture supernatant of P. intermedia, but the ability to trigger pulmonary inflammation was associated with the high proliferation rate of P. intermedia (74). It has been reported that a proliferation rate of P. intermedia reaching 108 colony-forming unit/lung may lead to pulmonary pathological changes (74).
P. gingivalis and T. denticola
P. gingivalis is the dominant pathogen of periodontal disease, known as the keystone pathogen in chronic periodontitis (75). A study has also shown that co-infection of P. gingivalis and T. denticola in mice can lead to excessive production of cytokines, resulting in severe bronchopneumonia and lung abscess. This lung lesion, caused by periodontal bacteria P. gingivalis and T. denticola, resulted in the mortality of ~50% of mice within 3 days (76). Notably, mice intratracheally inoculated only with P. gingivalis had abundant inflammatory cell infiltration in the lung and the pathological results show pulmonary edema, necrosis and bleeding (25). Similarly, severe bronchopneumonia, pulmonary parenchymal necrosis and lung abscesses were observed in mice infected with P. gingivalis 24–72 h after inoculation (70,77). In addition, ligature plus P. gingivalis-induced periodontitis can induce pulmonary inflammation in mice (78).
Fusobacterium nucleatum (F. nucleatum)
F. nucleatum is another dominant bacterium in the supragingival plaque, subgingival plaque and periodontal pocket, and is often detected in extra-oral infections such as in atherosclerotic plaques, amniotic fluid, the fetal membrane and respiratory tract (79–81), suggesting that infection may be caused by periodontal disease. In a study involving 87 pneumonia cases, the detection rate of Fusobacterium DNA in lower airway specimens was 36.8%, which suggested that F. nucleatum was a potential causative agent of pneumonia (82). Additionally, pulmonary F. nucleatum infection could also be presented as aspiration pneumonia and necrotic pneumonia (83). Previous research has indicated that F. nucleatum and Fusobacterium necrophorum were the major pathogens in Lemierre's Syndrome, a rare upper airway infection (79) that usually starts from oropharyngeal infection and presents with internal jugular vein septic thrombophlebitis and other metastatic diseases such as pulmonary emboli, pneumonia and pleural empyema (84–86). Lung tissue is affected in >80% of cases, and lung lesions can present as necrotic cavity lesions, empyema, lung abscess and necrotizing mediastinitis (86). Additionally, pulmonary F. nucleatum infection caused endobronchial lesions in a healthy 8-year-old male patient, and F. nucleatum was found in the surgical drainage of pulmonary abscess (83). Similarly, F. nucleatum was also detected in the lower respiratory tract specimen of patients with empyema and the pleural fluid of patients with lung abscess (81). Although the source of infection is unknown, F. nucleatum colonized in the oropharynx or periodontal pocket may be a source of lung infection in individuals who are prone to aspiration (87). These results indicate the potential role of F. nucleatum in respiratory diseases.
Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans)
In addition to the aforementioned periodontal pathogens, A. actinomycetemcomitans, as a periodontal pathogen highly associated with aggressive periodontitis, could also cause aspiration pneumonia or empyema associated with dental disease and oral mucosal injury (88). A literature search showed that 3 previously published cases of pneumonia caused by A. actinomycetemcomitans resembled malignant tumors (89–91). Patients with this type of pneumonia had similar symptoms and signs, including: i) Cough, hemoptysis, weight loss and fever; ii) chest radiography revealing consolidation in the lung; iii) pathology revealing a chronic inflammation process, with presence of malignant cells with or without sulfur granules; iv) effectiveness of antibiotic treatment; v) potential co-infection with Actinomyces spp.; and vi) poor oral health (such as periodontitis and missing teeth). Pulmonary infection can be caused by A. actinomycetemcomitans by either inhalation of bacteria into the lungs due to poor oral hygiene or by bacteria entering the bloodstream from the broken inner wall of the mouth (hematogenous dissemination) (89–91). A. actinomycetemcomitans is an important pathogenic bacterium in aggressive periodontitis. Therefore, the pathogenicity of A. actinomycetemcomitans in this particular type of pneumonia should be noted.
Overall, this suggests that periodontal pathogens may play a certain role in the pathogenesis of pneumonia. Thus, aspiration of pathogens in oral secretion and plaque in patients with periodontitis appears to be a potential mechanism by which patients with periodontitis are susceptible to lung disease (25). Based on the association between periodontitis and systemic diseases, it may be speculated that periodontal pathogens can enter the blood circulation through the ruptured periodontal pocket (8), colonize in the lungs and play a pathogenic role. However, the aforementioned studies only describe the fact that periodontal pathogens can cause lung inflammation; thus, the specific mechanism needs to be explored by conducting more rigorous in vivo experiments.
COVID-19
The initiating factor of periodontitis is the plaque attached to the surface of the teeth, especially the subgingival plaque in the periodontal pocket (92). It has been reported that periodontal bacteria can activate potential viruses such as human immunodeficiency virus and Epstein-Barr virus (93). The interaction between viruses and bacteria can cause an imbalance of microflora, promote the attachment and colonization of harmful pathogens, and lead to the development of diseases such as COPD, asthma and cystic fibrosis (94,95). Single cell RNA-sequencing analysis of oral tissues based on a public database showed that angiotensin converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) receptors, which are the binding receptors of SARS-CoV-2 in the human body, were highly expressed in periodontal pocket epithelial cells (95,96). These scenarios provided favorable conditions for SARS-CoV-2 infection.
When periodontitis occurs, oral bacteria and their metabolites can activate innate immune cells and release large levels of cytokines and chemokines to the periodontal tissue, which can also increase the cytokine levels in blood (97). Importantly, the cytokine storm caused by immune dysregulation (overexpression of proinflammatory cytokines and chemokines), is considered to be one of the major characteristics of patients with COVID-19 (95). In addition, periodontitis shares immune-inflammatory pathways such as the NF-κB, NLRP3/IL-1β and IL-6 pathway with COVID-19 (97): i) Periodontopathogens and SARS-CoV-2 activate the TLRs/MyD88/NF-κB signaling pathway to promote the expression of cytokines, chemokines and adhesion molecules; ii) the pathogenic components of periodontal bacteria (LPS and peptidoglycan) and SARS-CoV-2 first activate the NF-kB signaling pathway, and then activate the NLR family pyrin domain containing 3 inflammasome. In this process, caspase-1 is also activated, and continues to cleave pro-IL-1 β, pro-IL-18 and Gasdermin D (GSDMD), releasing IL-1β, IL-18 and GSDMD to induce pyroptosis and inflammation; iii) periodontal pathogens and SARS-CoV-2 induce pathological IL-6- and IL-23-dependent accumulation in T helper (Th) 17 cells, disrupt the balance of Th17/regulatory T cells (Tregs) by making the balance tend towards Th17 cells, and then release a large number of proinflammatory mediators, thus causing inflammation (97).
Whole-genome sequencing data on oral microbiome in patients with COVID-19 showed that the relative abundance of genera related to periodontitis (namely Prevotella, Lactobacillus, Capnocytophaga, Porphyromonas, Abiotrophia, Aggregatibacter and Atopobium) were higher in patients with COVID-19 than in SARS-CoV-2-negative subjects affected by non-respiratory diseases (94). In addition, numerous bacteria associated with periodontitis such as Prevotella melaninogenica, jejuni, denticola and oris; Eikenella corrodens; Capnocytophaga sputigena and gingivalis; and Aggregatibacter aphrophilus, were markedly increased in patients with COVID-19 (94). Previous research has shown that the use of P. gingivalis-derived lipopolysaccharide stimulates human gingival fibroblasts, leading to increased expression of pro-inflammatory molecules such as IL-1β, TNF-α and prostaglandin E2, which further promotes the expression of ACE2 and TMPRSS2 (96). On the other hand, pathogens and their products present in the periodontal pocket could enter the lower respiratory tract through the oropharyngeal pathway (inhalation or invasive mechanical ventilation), colonize the respiratory system and cause lung tissue damage (95). Huang et al (98) reported that, by detecting cells shed from oral mucosal, ACE-2 expressing SARS-CoV-2-positive ciliated cells, which are common in the respiratory tract, could be identified in saliva. In addition, periodontal bacteria exist in the bronchoalveolar lavage fluid of COVID-19 (94). Therefore, it is reasonable to suspect that poor periodontal condition of patients with COVID-19 may promote the development of COVID-19 disease.
Although several articles on the association between COVID-19 and periodontitis have been published in the past several years, these are epidemiological investigations, control studies, case reports, reviews and systematic analyses, and there are few reports on the mechanism of periodontal pathogens acting on the development of COVID-19 disease, and how to use the association between the two conditions for disease prediction, diagnosis and treatment management. Therefore, future research should be carried out focusing on those aspects.
COPD
The prevalence of P. gingivalis in patients with COPD was significantly higher than in patients without COPD (37). Moreover, the content of P. gingivalis in the subgingival plaques of patients with COPD was negatively associated with FEV1% (37), suggesting that P. gingivalis could be used as an index to assess pulmonary function in patients with COPD. An additional study showed that the rate of infection of F. nucleatum and mixed infection of F. nucleatum and P. aeruginosa in the airway samples of patients with acute exacerbation of COPD (AECOPD) was 60.8 and 45.3%, respectively (99). Furthermore, the lung function in patients with AECOPD co-infected with P. aeruginosa and F. nucleatum was weakened as the load of F. nucleatum increased (99). Accordingly, F. nucleatum may be used as an indicator of decreased lung function in patients with AECOPD (99).
Lung cancer, asthma and cystic fibrosis
Periodontopathogens are not only associated with the appearance and development of the aforementioned lung diseases, but also participate in the occurrence and development of lung cancer, asthma and cystic fibrosis. A previous study found P. gingivalis in cancer and para-cancerous tissues of patients with lung cancer, and the presence of P. gingivalis reduced the 5-year survival rate of patients with lung cancer (100). This indicated that the colonization of P. gingivalis could change the tumor microenvironment and promote tumor metastasis and deterioration. Moreover, it has been reported that P. intermedia is associated with severe asthma in adults as it is present in relatively high levels of the subgingival plaque in these patients (101). This may be attributed to the regulation of oral pathogens in allergic airway inflammation (102). In addition, P. intermedia and antibodies against P. intermedia antigens were detected in airway samples and serum, respectively, of patients with cystic fibrosis (74). Due to its cytotoxic effect on respiratory epithelial cells, it was speculated that this periodontal pathogen contributed to the pathophysiology of cystic fibrosis.
The aforementioned results suggest that there is an association between periodontitis-related pathogens and pulmonary diseases, and the existence of periodontal pathogens may be a potential diagnostic indicator to predict susceptibility to pulmonary disease. If this finding is fully confirmed, the removal of periodontal microorganisms and periodontitis intervention may provide novel ideas for the treatment of common pulmonary diseases.
It has been reported that the mouth, more specifically dental plaque, is the source of respiratory pathogens (4,103,104). Poor oral status and periodontal diseases are considered to be associated with pathogen colonization (103,105,106). Dental plaque samples are rich in various pulmonary pathogens such as S. aureus, P. aeruginosa, Streptococcus pneumoniae and Haemophilus influenzae (104). Potential respiratory pathogens colonized in the dental plaque biofilm could be inhaled into the lung, causing infection (106). On the other hand, periodontal bacteria (including P. gingivalis, F. nucleatum, P. intermedia, T. forsythia and T. denticola) could also be found in respiratory specimens of patients with pulmonary disease (82,107). Thus, periodontal anaerobic bacteria in dental plaque and oral secretions could also be transferred to the airway and participate in the development of respiratory diseases (106,108). Previous studies revealed that pathogenic microorganisms were indistinguishable in airway and dental plaque samples (104,107,109,110). In addition, bacteria in the dental plaque and respiratory specimens from the same patient were highly homologous (107), which reinforces the view that dental plaque is a crucial origin of airway pathogens. Therefore, maintenance of oral hygiene, especially periodontal health, may be a feasible way to preclude the occurrence and/or progression of respiratory diseases.
Potential mechanisms of periodontal pathogens involved in respiratory diseases
Periodontal pathogens may be involved in respiratory diseases by regulating apoptosis
It has been demonstrated that in the presence of periodontal bacteria (including P. gingivalis, F. nucleatum and A. actinomycetemcomitans), P. aeruginosa invasion to the HEp-2 cells was significantly enhanced, caspase-3 activity was stronger and release of proinflammatory cytokines was increased (111). Similarly, P. gingivalis could increase the levels of inflammatory cytokines and the expression of inducible nitric oxide synthase in BEAS-2B cells infected with H1N1 influenza virus, thereby increasing the production of nitric oxide, which in turn promoted the apoptosis of pulmonary epithelial cells through the Bcl-2/Bax/caspase-3 signaling pathway (112). These results suggested that periodontopathogens may increase the damage caused by respiratory pathogens to epithelial cells and promote the initiation and progression of respiratory diseases by promoting invasion and apoptosis, inducing the release of cytokines and changing the local microenvironment.
It has also been reported that P. gingivalis temporarily suppresses the apoptosis of airway epithelial cells induced by P. aeruginosa by activating STAT3 and increasing the expression of the anti-apoptotic genes, survivin and Bcl-2 (113). At the same time, the pro-apoptotic protein Bad was downregulated and the activity of caspase-3 was suppressed (113). Another study showed that P. gingivalis transiently inhibited influenza A virus (IAV)-induced respiratory epithelial cell apoptosis by promoting autophagy levels, which resulted in an improvement of the survival rate of pathogens, and the promotion of P. gingivalis and IAV proliferation in cells (114). Therefore, inhibition of respiratory pathogen-induced apoptosis may be a strategy for periodontal pathogens to survive and proliferate in cells. This mechanism enables pathogens to escape the immune defense of the host, which leads to the spread of infection and aggravates tissue damage (113). The aforementioned results showed that the co-regulation of apoptosis by respiratory pathogens by periodontal pathogens may function in the pathogenesis of lung diseases.
In addition, periodontal pathogens could also induce the apoptosis of pulmonary epithelial cells. A previous study has shown that P. intermedia has cytotoxic effects on respiratory epithelial cells, whereby the periodontal bacterium caused the injury and death of A549 lung epithelial cells (74). Furthermore, He et al (115) reported that after treatment with outer membrane vesicles (OMVs) of P. gingivalis, the survival rate of A549 cells was significantly decreased. Changes in cell morphology were characterized by cell contraction, membrane vesicles and cytoplasmic secretion. This phenomenon was confirmed to be caused by apoptosis induced by P. gingivalis OMVs (115). Furthermore, the cells separated from each other, suggesting the loss of adherence between epithelial cells and the disruption of the epithelial barrier (115). Therefore, the ability of periodontal pathogens to induce apoptosis of alveolar epithelial cells may play a role in the pathology of pulmonary infections independently from respiratory pathogens.
Periodontal pathogens regulate the attachment and invasion of airway pathogens to respiratory epithelial cells
It is well known that adhesion and invasion of host cells by bacteria are important conditions for bacteria to cause host infection (116). Grigg (116) has shown that periodontopathogens can participate in the pathogenesis of pulmonary diseases by promoting the attachment and invasion of airway pathogens to respiratory epithelial cells. Platelet activating factor receptor (PAFR) is an important bacterial adhesion receptor in respiratory epithelial cells (116). S. pneumoniae evades the defense mechanism of the host by the binding of phosphocholine on its cell wall to PAFR on respiratory epithelial cells, which leads to pneumonia (116). In addition, susceptibility to pneumococcal pneumonia increases with the increase in PAFR expression (116). Notably, certain periodontal pathogens can promote the adhesion of S. pneumoniae to airway epithelial cells by overexpressing PAFR. Compared with the group of S. pneumoniae-infected mice without the supernatant of P. intermedia (Pi Sup), the survival rates were significantly lower, the levels of S. pneumoniae in the lungs, spleen and blood were higher, and the levels of inflammatory cytokines in the early phases significantly increased in the group of S. pneumoniae-infected mice with Pi Sup (73). Pi Sup stimulated the expression of PAFR in respiratory cells in mice, thus enhancing the adhesion of pneumococcus to respiratory cells, which could therefore be inhibited using PAFR inhibitors (73). Similarly, another study showed that gingipains, a virulence factor secreted by P. gingivalis, also stimulated the expression of PAFR in respiratory epithelial cells, thereby promoting the adhesion of pneumococcus to pulmonary epithelial cells (117). Previous studies have shown that F. nucleatum and its virulence factor, butyric acid, could also upregulate the expression of ACE2, the main receptor that facilitates the entry of SARS-CoV-2 into cells, in respiratory epithelium, thus increasing the risk of COVID-19 (93,118). This periodontal pathogen also shows strong auto-aggregation because it expresses multiple surface adhesins (119). This property promotes the accumulation of F. nucleatum and P. aeruginosa on the surface of lung epithelial cells and promotes the invasion of P. aeruginosa on lung epithelial cells (119). In conclusion, periodontal pathogens and their secreted virulence factors can cause lung infection by changing the expression of receptors on the mucosal surface of airway epithelium or promoting pathogen aggregation, and by promoting pathogen adhesion and invasion, suggesting that periodontal pathogens may participate in the pathogenesis of pulmonary diseases through this mechanism. Therefore, when a patient suffers from periodontal disease, periodontal pathogens and their virulence factors may cause persistent lung infection, which increases the risk of lung diseases.
Periodontal pathogens upregulate mucin expression in respiratory cells
Airway obstruction due to mucus hypersecretion is one of the characteristics of OPD) (120). Mucin is an essential component of mucus, and mucin-5AC (MUC5AC) is the most important mucin secreted by the respiratory tract, which has critical influence on the pathogenesis of asthma (121), bronchiectasis (122) and COPD (123). Notably, the relatively low concentrations of culture supernatants of F. nucleatum upregulated MUC5AC expression in respiratory cells by phosphorylation of ERK1/2 (80). Similarly, P. gingivalis culture supernatant could increase the expression of the MUC5AC gene and the protein levels of MUC5AC in airway epithelial cells and in mouse lungs (124). It was also found that virulence factors (lipopolysaccharide and FimA fimbriae) of P. gingivalis did not affect MUC5AC expression in the airway. However, compared with PBS-treated mice (control) and P. gingivalis mutant (gingipain knockout)-treated mice, the airway epithelial cells of mice treated with P. gingivalis mutant accumulated more MUC5AC protein and mucins, which suggested that the gingipain contributed to the expression of MUC5AC in airway epithelial cells (124).
These results indicate that periodontal pathogens can induce excessive secretion of airway mucus in the lungs, resulting in airway obstruction, thus aggravating OPDs. This mechanism suggests that patients with periodontal disease can aspirate saliva with periodontal bacteria, which promotes the development of OPDs such as COPD and asthma, and further verifies the hypothesis that periodontitis may be a risk factor for COPD and asthma. Therefore, treating periodontitis in individuals with OPD may be a meaningful measure to alleviate the progression of pulmonary diseases.
Periodontal pathogens participate in lung diseases by regulating the host immune response
When periodontitis occurs, an imbalance in the oral microbial system directly or indirectly damages the immune system of the host, causing an inflammatory reaction in local periodontal tissue, which in turn causes damage to the body, including the cardiovascular system, nervous system, lungs, uterus and joints (125). With the increased understanding of periodontitis and its comorbidities, the systemic hypoinflammatory response caused by periodontitis is considered to be a possible mechanism associated with periodontitis and chronic systemic diseases (126), in which immune cells [including neutrophils, macrophages, dendritic cells (DCs) and T cells] play an important role (127–129).
It has been previously shown that periodontal bacteria can migrate into the systemic circulation via damaged periodontal pockets or enter the respiratory tract through accidental inhalation or invasive mechanical ventilation (24). When the body is challenged by microorganisms, neutrophils can release a series of chemokines and cytokines to recruit macrophages, DCs and T cells into the infected site, resulting in the imbalance between Th17 cells and Tregs, which is manifested in the higher expression of Th17 cells and lower Treg responses (128,129), thus promoting the progression of lung diseases (130). For instance, P. gingivalis could use its pathogenic factor fimbriae adhesins to invade DCs in tissue or blood, promote CD4+ T cells to differentiate into Th17 cells, and release IL-23, which can further induce the expression of IL-17, IL-22, granulocyte-macrophage colony-stimulating factor and TNF-α, thus recruiting monocytes and granulocytes to the infected site, causing inflammation and tissue damage (128,129,131). Among them, IL-17 can recruit inflammatory cells such as neutrophils to the site of infection (128). These neutrophils are considered to be highly reactive and promote the production of proinflammatory cytokines (IL-8, IL-4, TNF-α and IL-1β) in peripheral blood (126). Macrophages and DCs secrete IL-6 when stimulated by IL-23, which is beneficial for the differentiation of Th17 cells (132,133). This interaction between cytokines and immune cells can further exaggerate the inflammatory response in the infected site and cause tissue damage (126,128,129). It has been reported that a low Treg/Th17 ratio can lead to the uncontrolled release of cytokines and chemokine cascades, thus damaging the tissue (128,130).
In the peripheral blood of patients with gingivitis and healthy subjects the ratio of Treg/Th17 cells in patients with gingivitis was significantly downregulated using flow cytometry (134). Additionally, the results of an ELISA showed that the levels of Th17 cytokines (TGF-β, IL-17, IL-4, IL-6 and IL-10) in patients with gingivitis were significantly elevated (134). Notably, the imbalance between Treg/Th17 cells plays a similar role in tissue damage in lung disease. A recently published review suggested that Th17 cytokines (IL-17A, IL-6 and IL-23) could promote the progression of COPD, while decreased number of Treg cells could increase the levels of IL-1β and IL-6, which in turn aggravate lung injury (130). Th17/Treg imbalance can aggravate the severity of pulmonary infectious diseases such as asthma, COPD, acute respiratory distress syndrome, acute lung injury, mycoplasma pneumoniae infection and COVID-19 (130,135–137). Therefore, it is reasonable to suspect that high expression of cytokines and chemokines in blood caused by local periodontal inflammation can also indirectly promote the occurrence and development of lung diseases.
In addition, periodontal bacteria can directly induce the release of pro-inflammatory cytokines in airway epithelial cells and participate in the initiation and progression of lung diseases. A previous study showed that F. nucleatum could upregulate the expression of IL-8 and IL-6 in bronchial, pharyngeal and alveolar epithelial cells, and the expression levels of proinflammatory cytokine increased with the increase in F. nucleatum concentrations (71). The study also reported that the expression of IL-6 in the lung, trachea and serum of mice inoculated intratracheally with F. nucleatum was increased (71). This role of F. nucleatum in the respiratory cells and organs is partly attributed to butyric acid, a virulence factor of F. nucleatum (93), and may lead to the exacerbation of COPD and COVID-19 (71,93). A previous study showed that P. gingivalis upregulated the levels of IL-8 and IL-6 in respiratory epithelial cells via TLR2, thus playing a pro-inflammatory role in aspiration pneumonia (138). Furthermore, when respiratory epithelial cells were co-infected with P. aeruginosa and F. nucleatum, F. nucleatum had an additive effect on the inflammatory cytotoxicity of lung epithelial cells induced by respiratory pathogens, as characterized by excessive release of proinflammatory cytokines IL-6 and TNF-α, which may cause aggravation of lung damage and AECOPD (119).
Together, these studies indicate that the cytokines released by respiratory cells stimulated by periodontal pathogens can change the local inflammatory environment and epithelial state by participating in inflammatory responses or regulating the immune response, so as to maintain or aggravate respiratory tract infection (Fig. 1). Therefore, when patients with lung diseases also suffer from periodontal disease, periodontopathic bacteria can increase the production of cytokines in the lung and lead to the aggravation and deterioration of lung diseases. Thus, it would be beneficial for these patients to carry out periodontal disease management and maintain regular oral care.
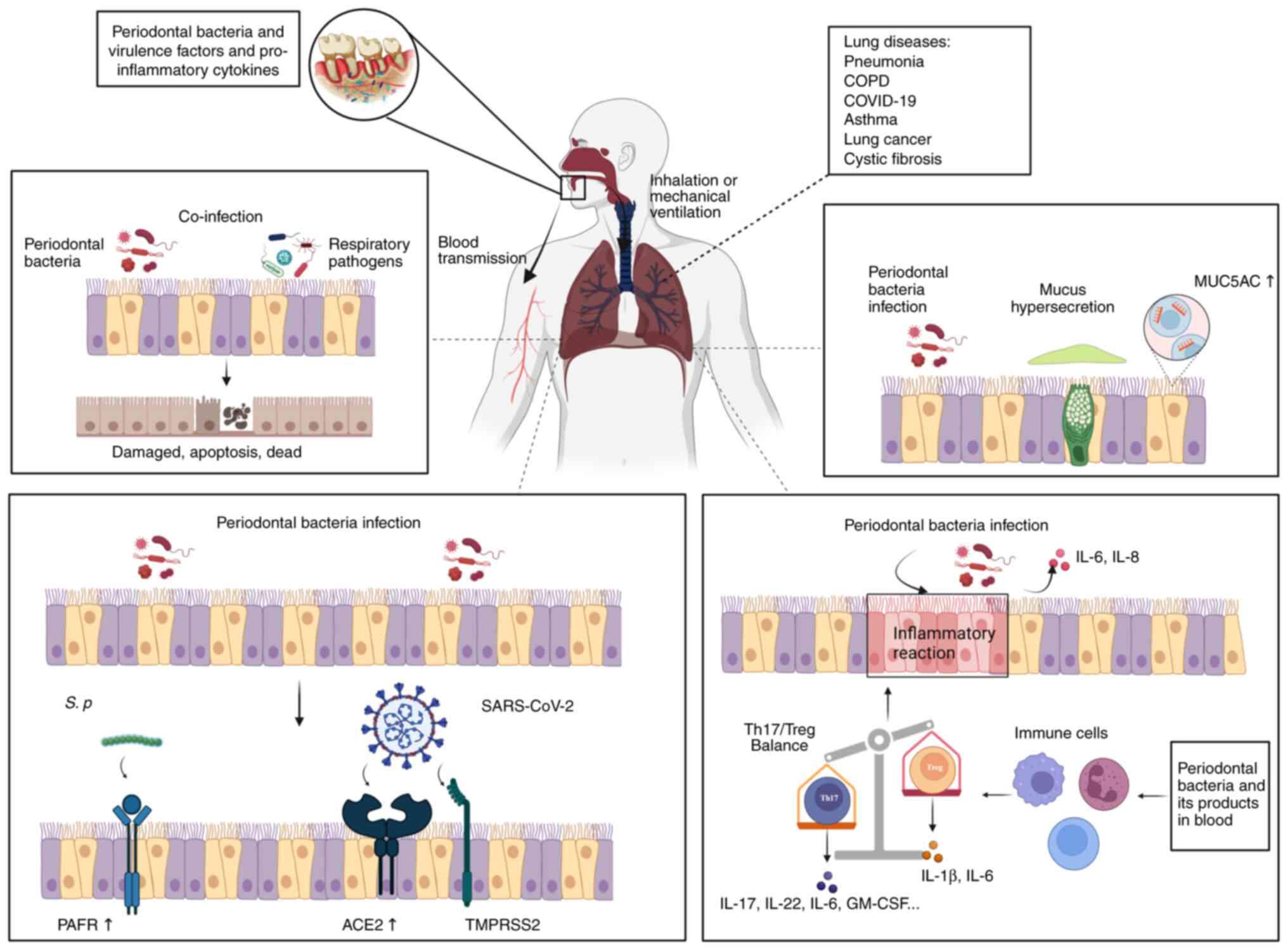 |
Figure 1.
Potential mechanisms of periodontal pathogens involved in respiratory diseases. Periodontitis is associated with a variety of respiratory diseases, such as pneumonia, asthma, COPD, COVID-19, lung cancer and cystic fibrosis. Periodontal bacteria, along with their virulence factors and pro-inflammatory cytokines, have the potential to enter the respiratory tract through aspiration or mechanical assisted ventilation, leading to pathogenic effects. Additionally, they can enter the bloodstream through ruptured periodontal pockets and reach the lungs, thereby playing a causative role in pulmonary disease: i) Periodontal bacteria and respiratory pathogens co-infect respiratory epithelial cells, leading to respiratory epithelial cell damage, apoptosis and potential fatality; ii) periodontal bacteria stimulate respiratory epithelial cells, resulting in increased expression of MUC5AC and excessive mucus production; iii) periodontal bacteria promote the expression of PAFR, ACE2 and TMPRSS2 in respiratory epithelial cells, facilitating the adhesion and binding of respiratory pathogens; and iv) periodontal bacteria can directly enter respiratory epithelial cells and release pro-inflammatory cytokines. Furthermore, systemic low inflammation caused by periodontal bacteria can modulate immune cells, disrupt the balance between Th17/Treg and induce Th17 cells to release pro-inflammatory cytokines. Created with BioRender.com. COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; PAFR, platelet activating factor receptor; ACE2, angiotensin converting enzyme 2; TMPRSS2, transmembrane serine protease 2; IL, interleukin; Th, T helper cell; Treg, regulatory T cell; GM-CSF, granulocyte-macrophage colony-stimulating factor.
|
Potential strategy for reducing adverse respiratory events
Pneumonia
Given the important role of oral microorganisms in the pathogenesis of VAP, removal of plaque biofilm is a simple and effective method to prevent VAP. A prospective study found that the combination of toothbrushing and chlorhexidine gel in ICU patients decreased the incidence of VAP and the length of ICU stay by reducing the microbial load (139). Labeau and Blot (140) proposed that brushing teeth could be essential for the prevention of VAP in mechanically ventilated patients, since it could reduce dental plaque in the mouth, help alleviate oral discomfort in patients and reduce the risk of oropharyngeal secretions being inhaled into the lower respiratory tract. Furthermore, the majority of these patients are elderly and more likely to possess dental defects or missing teeth; thus, their chances of using dentures are also increased (140,141). Dentures are more likely to harbor bacteria and are less efficient at chewing than healthy teeth, which leads to an increased risk of bacteria being inhaled into the respiratory tract; therefore, it is recommended that denture cleaner is used regularly to reduce the accumulation of plaque biofilm (24–26,140,141). In addition, dentures should be removed and soaked in the cold water while sleeping (142). Finally, it has been reported that preoperative professional periodontal treatment for patients with lung cancer (143) is associated with a decreased rate of postoperative pneumonia and similar results have been reported by Iwata et al (144).
COPD
It has been reported that patients with frequent exacerbations of COPD have worse oral health habits, which is characterized by lower frequency of teeth brushing and higher plaque index (145). Poor oral hygiene conditions are important risk factors for COPD exacerbation, suggesting that healthy periodontal status and oral hygiene may be beneficial to preventing COPD exacerbation (145). A randomized controlled clinical trial found that, compared with patients with COPD and chronic periodontitis who only received oral hygiene guidance without periodontal treatment, patients who received scaling and root planing therapy as well as supragingival scaling therapy had lower periodontal index, significantly improved pulmonary function and decreased COPD exacerbation frequency (146). In addition, the results of a previous meta-analysis showed that periodontal treatment was beneficial for reducing the rate of pulmonary function decline in patients with COPD and periodontitis, as well as the frequency of hospitalization in patients with COPD, thereby effectively saving the use of medical resources (147). Patients with COPD should use antibiotics to gargle before periodontal treatment, which should reduce the microbial load in the aerosol caused by dental ultrasound devices, thereby reducing the risk of bacterial infection (146).
Asthma
Long-term use of drugs (β2-agonists and inhaled corticosteroids) in patients with asthma could increase their susceptibility to oral diseases such as dental caries and periodontitis by changing the oral microenvironment (16). Thus, it could be recommended that patients with asthma use special equipment to deliver inhaled drugs directly into the airway instead of entering the respiratory tract through the oropharynx, and should be encouraged to drink more water to alleviate their reduced salivary flow (16,48). Previous research has shown that patients with asthma tend to suffer from gingivitis caused by plaque biofilm. The periodontal pathogen P. intermedia is considered to be a driver for the significant positive association between asthma and periodontitis. The possible mechanism lies in the anatomical proximity between the oral cavity and the respiratory tract. Periodontal anaerobic bacteria can easily enter the anaerobic environment of the respiratory tract, change the respiratory mucosa and reshape the airways by releasing cytokines, toxic products and hydrolases, thus facilitating the adhesion of pathogens to the respiratory epithelium, thereby causing local allergic and inflammatory reactions (101). Therefore, regular oral examinations, periodontal treatment aimed at mechanical plaque removal and preventive oral hygiene management with antibacterial mouthwash may also effectively prevent the occurrence of periodontal disease in patients with asthma (48). Compared with patients with asthma and periodontitis receiving periodontal therapy, the incidence of deterioration of asthma, pneumonia, and respiratory failure (referred to as adverse respiratory events), the rate of hospitalization and admission to ICU due to these detrimental events, and the mortality rate were all significantly lower than in patients with asthma without periodontal disease (148). However, due to the low heterogeneity and high bias in research, such evidence is limited (26). In the future, rigorous clinical controlled trials or prospective trials should be performed to clarify whether periodontal therapy is really beneficial to patients with asthma.
COVID-19
It has been reported that periodontal therapy can reduce systemic inflammatory markers in serum, which are harmful to systemic diseases, thus benefitting overall health (13). Considering the important role of a cytokine storm in the pathogenesis of periodontitis and COVID-19, periodontal therapy aimed at improving the systemic low inflammatory state caused by periodontitis may be also beneficial for patients with COVID-19 (149). This view is supported by a previous study whereby systemic inflammatory markers such as CRP, ferritin, urea, HbA1C and IL-6 decreased in patients with COVID-19 who received periodontal treatment (149). In addition, due to the interaction between SARS-CoV-2 and periodontal bacteria, and the oral cavity being a reservoir for SARS-CoV-2, removal of dental calculus and plaque biofilm could effectively prevent SARA-CoV-2 from colonizing the oral cavity and help reduce bacterial infection risk (18,45,97). A previous study found that the risk of death, probability of pulmonary infection, requirement of assisted ventilation and ICU occupancy rate decreased significantly in patients with COVID-19 and periodontitis who received periodontal therapy, and periodontal therapy could significantly reduce in serum the level of D-dimer, a biomarker of blood coagulation function, suggesting that periodontal treatment may be a potential way to reduce COVID-19-associated complications and mortality (149,150).
These results indicate that periodontal therapy can decrease the colonization of potential respiratory pathogens and the number of periodontal pathogens by removing dental plaque to improve the prognosis of patients with lung diseases, as well as reducing the incidence of postoperative pneumonia and the frequency of exacerbation of existing pulmonary diseases. Therefore, physicians and patients should be aware of the potential association between periodontitis and lung disease and since disease management requires contributions from both patients and physicians, the following are different recommendations: i) Patients with pulmonary diseases should develop good oral hygiene habits, including brushing their teeth twice a day for 2–3 min each time, using dental floss, dental drills and antibacterial mouthwash for self-control of plaque and having preventive oral health examinations once or twice a year, while avoiding visiting the dentist during an acute infection; ii) physicians should refer patients with lung disease to oral/periodontal health checks, and advise them to quit smoking, which is a common risk factor for periodontitis and lung disease; iii) dentists should conduct detailed oral health education for patients with lung diseases and make detailed oral health examination plans. In addition, the tilt angle of the dental chair should be conducive to the breathing of the patient, and emergency inhalers should be kept in the dental office in case of need (142,151).
To sum up, patients with respiratory diseases should pay attention to their periodontal health and have oral examinations regularly. Periodontal treatments aimed at eliminating and controlling plaque may be an effective adjuvant for patients with respiratory diseases. At the same time, dentists and physicians should be aware of the connection between periodontal disease and respiratory disease in clinical work. In addition, as dental plaque is relatively easy to obtain, the detection of specific periodontal pathogens is expected to be a biomarker for predicting susceptibility to pulmonary diseases.
Conclusions, limitations and prospects
Periodontitis is a potential risk factor for pulmonary disease, and relevant epidemiological studies have been extensively reported; however, such studies have certain shortcomings, including small sample size, inconsistent diagnostic criteria and differences in evaluation criteria (152). These deficiencies may lead to false negative results (152). By contrast, intervention studies on periodontal therapy and pulmonary health or respiratory disease prognosis and complications are rarely reported.
It is worth noting that the potential pathogenic role of periodontal pathogens in lung disease may be the mechanism behind the association between periodontitis and lung disease (153). On the one hand, plaque biofilm, as the initiating factor in periodontitis, may be the reservoir of respiratory pathogens (153). Microorganisms and their metabolites in the mouth can first enter the airway through aspiration or assisted mechanical ventilation, and directly play a pathogenic role in the respiratory system (153). Their potential role mainly includes regulating the apoptosis of respiratory epithelial cells (112–114), changing the characteristics of respiratory epithelium, and promoting the colonization and adhesion of respiratory pathogens to the epithelium (117,119). On the other hand, periodontal bacteria and their products can indirectly affect the development of lung diseases by regulating the immunity of the body and causing a low inflammatory response (71,93,119,126,138).
Previous studies have suggested that periodontal pathogens may play an important role in pulmonary diseases (153). However, such evidence is limited, and there are still numerous aspects worth exploring in greater depth, including: i) Whether periodontal microorganisms can be used as biomarkers to predict the development of lung diseases; ii) whether changes in oral microorganisms (such as adding beneficial microorganisms or removing harmful pathogens) are favorable to lung health; and iii) identifying the specific biological mechanisms of metabolites and pathogenic factors of periodontal microorganisms in the progression of lung diseases.
Acknowledgements
Not applicable.
Funding
This study was supported by National Natural Science Foundation of China (grant no. 82270966).
Availability of data and materials
Not applicable.
Authors' contributions
ZZ, SW, JL and YO wrote the manuscript; ZS, DC and ZL made substantial contributions to the reviewing and editing process; LG and YW were responsible for the manuscript design; and QJ and TL provided financial support for this paper and critically reviewed and revised the manuscript. All authors actively participated in the development of the article and have read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Slots J: Periodontitis: Facts, fallacies and the future. Periodontol 2000. 75:7–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Genco RJ and Sanz M: Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontol 2000. 83:7–13. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanz M, Beighton D, Curtis MA, Cury JA, Dige I, Dommisch H, Ellwood R, Giacaman RA, Herrera D, Herzberg MC, et al: Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J Clin Periodontol. 44 (Suppl 18):S5–S11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cardoso EM, Reis C and Manzanares-Céspedes MC: Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad Med. 130:98–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fiorillo L, Cervino G, Laino L, D'Amico C, Mauceri R, Tozum TF, Gaeta M and Cicciù M: Porphyromonas gingivalis, periodontal and systemic implications: A systematic review. Dent J (Basel). 7:1142019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mei F, Xie M, Huang X, Long Y, Lu X, Wang X and Chen L: Porphyromonas gingivalis and its systemic impact: Current status. Pathogens. 9:9442020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang C and Shi G: Smoking and microbiome in oral, airway, gut and some systemic diseases. J Transl Med. 17:2252019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haraszthy VI, Zambon JJ, Trevisan M, Zeid M and Genco RJ: Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 71:1554–1560. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hajishengallis G: Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontol 2000. 89:9–18. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liccardo D, Marzano F, Carraturo F, Guida M, Femminella GD, Bencivenga L, Agrimi J, Addonizio A, Melino I, Valletta A, et al: Potential bidirectional relationship between periodontitis and Alzheimer's disease. Front Physiol. 11:6832020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singhrao SK and Olsen I: Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer's disease. J Oral Microbiol. 11:15634052019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beck JD, Philips KH and Rao SS: Periodontal disease and systemic interactions (periodontal medicine): Current epidemiological evidence. Curr Oral Health Rep. 7:54–61. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, Herrera D, Jepsen S, Lione L, Madianos P, et al: Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the international diabetes federation and the European federation of periodontology. J Clin Periodontol. 45:138–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
González-Febles J and Sanz M: Periodontitis and rheumatoid arthritis: What have we learned about their connection and their treatment? Periodontol 2000. 87:181–203. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kelly N and El Karim I: Periodontitis may be associated with respiratory diseases such as asthma, COPD, and pneumonia. J Evid Based Dent Pract. 20:1014982020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moraschini V, Calasans-Maia JA and Calasans-Maia MD: Association between asthma and periodontal disease: A systematic review and meta-analysis. J Periodontol. 89:440–455. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takeuchi K, Matsumoto K, Furuta M, Fukuyama S, Takeshita T, Ogata H, Suma S, Shibata Y, Shimazaki Y, Hata J, et al: Periodontitis is associated with chronic obstructive pulmonary disease. J Dent Res. 98:534–540. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anand PS, Jadhav P, Kamath KP, Kumar SR, Vijayalaxmi S and Anil S: A case-control study on the association between periodontitis and coronavirus disease (COVID-19). J Periodontol. 93:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Yang X, Zou X, Zhang Y, Wang J and Wang Y: Relationship between periodontal disease and lung cancer: A systematic review and meta-analysis. J Periodontal Res. 55:581–593. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rastogi T, Chowdhary Z, Krishna MK, Mehrotra S and Mohan R: Prevalence of periodontitis in patients with pulmonary disease: A cross-sectional survey in the industrial district of India. J Indian Soc Periodontol. 23:269–274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ottosen J and Evans H: Pneumonia: Challenges in the definition, diagnosis, and management of disease. Surg Clin North Am. 94:1305–1317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Melo Neto JP, Melo MS, dos Santos-Pereira SA, Martinez EF, Okajima LS and Saba-Chujfi E: Periodontal infections and community-acquired pneumonia: A case-control study. Eur J Clin Microbiol Infect Dis. 32:27–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iwasaki M, Taylor GW, Awano S, Yoshida A, Kataoka S, Ansai T and Nakamura H: Periodontal disease and pneumonia mortality in haemodialysis patients: A 7-year cohort study. J Clin Periodontol. 45:38–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scannapieco FA and Shay K: Oral health disparities in older adults: Oral bacteria, inflammation, and aspiration pneumonia. Dent Clin North Am. 58:771–782. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Benedyk M, Mydel PM, Delaleu N, Płaza K, Gawron K, Milewska A, Maresz K, Koziel J, Pyrc K and Potempa J: Gingipains: Critical factors in the development of aspiration pneumonia caused by Porphyromonas gingivalis. J Innate Immun. 8:185–198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gomes-Filho IS, Cruz SSD, Trindade SC, Passos-Soares JS, Carvalho-Filho PC, Figueiredo ACMG, Lyrio AO, Hintz AM, Pereira MG and Scannapieco F: Periodontitis and respiratory diseases: A systematic review with meta-analysis. Oral Dis. 26:439–446. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hua F, Xie H, Worthington HV, Furness S, Zhang Q and Li C: Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst Rev. 10:CD0083672016.PubMed/NCBI
|
|
28
|
Liu C, Cao Y, Lin J, Ng L, Needleman I, Walsh T and Li C: Oral care measures for preventing nursing home-acquired pneumonia. Cochrane Database Syst Rev. 9:CD0124162018.PubMed/NCBI
|
|
29
|
Khadka S, Khan S, King A, Goldberg LR, Crocombe L and Bettiol S: Poor oral hygiene, oral microorganisms and aspiration pneumonia risk in older people in residential aged care: A systematic review. Age Ageing. 50:81–87. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bellissimo-Rodrigues WT, Menegueti MG, Gaspar GG, Nicolini EA, Auxiliadora-Martins M, Basile-Filho A, Martinez R and Bellissimo-Rodrigues F: Effectiveness of a dental care intervention in the prevention of lower respiratory tract nosocomial infections among intensive care patients: A randomized clinical trial. Infect Control Hosp Epidemiol. 35:1342–1348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kes D, Aydin Yildirim T, Kuru C, Pazarlıoglu F, Ciftci T and Ozdemir M: Effect of 0.12% chlorhexidine use for oral care on ventilator-associated respiratory infections: A randomized controlled trial. J Trauma Nurs. 28:228–234. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Katayama Y, Takanishi H, Sato Y, Fujita S and Enomoto M: Effect of oral care in reducing the incidence of early-onset ventilator-associated pneumonia in preterm infants. Pediatr Pulmonol. 56:2570–2575. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mirza S, Clay RD, Koslow MA and Scanlon PD: COPD guidelines: A review of the 2018 GOLD report. Mayo Clin Proc. 93:1488–1502. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hayes C, Sparrow D, Cohen M, Vokonas PS and Garcia RI: The association between alveolar bone loss and pulmonary function: The VA dental longitudinal study. Ann Periodontol. 3:257–261. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leuckfeld I, Obregon-Whittle MV, Lund MB, Geiran O, Bjørtuft Ø and Olsen I: Severe chronic obstructive pulmonary disease: Association with marginal bone loss in periodontitis. Respir Med. 102:488–494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chung JH, Hwang HJ, Kim SH and Kim TH: Associations between periodontitis and chronic obstructive pulmonary disease: The 2010 to 2012 Korean national health and nutrition examination survey. J Periodontol. 87:864–871. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tan L, Tang X, Pan C, Wang H and Pan Y: Relationship among clinical periodontal, microbiologic parameters and lung function in participants with chronic obstructive pulmonary disease. J Periodontol. 90:134–140. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Winning L, Patterson CC, Cullen KM, Kee F and Linden GJ: Chronic periodontitis and reduced respiratory function. J Clin Periodontol. 46:266–275. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shen TC, Chang PY, Lin CL, Chen CH, Tu CY, Hsia TC, Shih CM, Hsu WH, Sung FC and Kao CH: Periodontal treatment reduces risk of adverse respiratory events in patients with chronic obstructive pulmonary disease: A propensity-matched cohort study. Medicine (Baltimore). 95:e37352016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Prasanna SJ: Causal relationship between periodontitis and chronic obstructive pulmonary disease. J Indian Soc Periodontol. 15:359–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tamiya H, Mitani A, Abe M and Nagase T: Putative bidirectionality of chronic obstructive pulmonary disease and periodontal disease: A review of the literature. J Clin Med. 12:59352023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yüce M, Filiztekin E and Özkaya KG: COVID-19 diagnosis-A review of current methods. Biosens Bioelectron. 172:1127522021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Larvin H, Wilmott S, Wu J and Kang J: The impact of periodontal disease on hospital admission and mortality during COVID-19 pandemic. Front Med (Lausanne). 7:6049802020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gupta S, Mohindra R, Singla M, Khera S, Sahni V, Kanta P, Soni RK, Kumar A, Gauba K, Goyal K, et al: The clinical association between periodontitis and COVID-19. Clin Oral Investig. 26:1361–1374. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sampson V, Kamona N and Sampson A: Could there be a link between oral hygiene and the severity of SARS-CoV-2 infections? Br Dent J. 228:971–975. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mims JW: Asthma: Definitions and pathophysiology. Int Forum Allergy Rhinol. 5 (Suppl 1):S2–S6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stern J, Pier J and Litonjua AA: Asthma epidemiology and risk factors. Semin Immunopathol. 42:5–15. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Thomas MS, Parolia A, Kundabala M and Vikram M: Asthma and oral health: A review. Aust Dent J. 55:128–133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gani F, Caminati M, Bellavia F, Baroso A, Faccioni P, Pancera P, Batani V and Senna G: Oral health in asthmatic patients: A review: Asthma and its therapy may impact on oral health. Clin Mol Allergy. 18:222020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gomes-Filho IS, Soledade-Marques KR, Seixas da Cruz S, de Santana Passos-Soares J, Trindade SC, Souza-Machado A, Fischer Rubira-Bullen IR, de Moraes Marcílio Cerqueira E, Barreto ML and Costa de Santana T: Does periodontal infection have an effect on severe asthma in adults? J Periodontol. 85:e179–e187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Soledade-Marques KR, Gomes-Filho IS, da Cruz SS, Passos-Soares JS, Trindade SC, Cerqueira EMM, Coelho JMF, Barreto ML, Costa MDCN, Vianna MIP, et al: Association between periodontitis and severe asthma in adults: A case-control study. Oral Dis. 24:442–448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ferreira MKM, Ferreira RO, Castro MML, Magno MB, Almeida APCPSC, Fagundes NCF, Maia LC and Lima RR: Is there an association between asthma and periodontal disease among adults? Systematic review and meta-analysis. Life Sci. 223:74–87. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Khassawneh B, Alhabashneh R and Ibrahim F: The association between bronchial asthma and periodontitis: A case-control study in Jordan. J Asthma. 56:404–410. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Han ER, Choi IS, Kim HK, Kang YW, Park JG, Lim JR, Seo JH and Choi JH: Inhaled corticosteroid-related tooth problems in asthmatics. J Asthma. 46:160–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ramseier CA, Anerud A, Dulac M, Lulic M, Cullinan MP, Seymour GJ, Faddy MJ, Bürgin W, Schätzle M and Lang NP: Natural history of periodontitis: Disease progression and tooth loss over 40 years. J Clin Periodontol. 44:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Santos NC, Jamelli S, Costa L, Baracho Filho C, Medeiros D, Rizzo JA and Sarinho E: Assessing caries, dental plaque and salivary flow in asthmatic adolescents using inhaled corticosteroids. Allergol Immunopathol (Madr). 40:220–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shen TC, Chang PY, Lin CL, Wei CC, Tu CY, Hsia TC, Shih CM, Hsu WH, Sung FC and Kao CH: Risk of periodontal disease in patients with asthma: A nationwide population-based retrospective cohort study. J Periodontol. 88:723–730. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bade BC and Dela Cruz CS: Lung cancer 2020: Epidemiology, etiology, and prevention. Clin Chest Med. 41:1–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zeng XT, Xia LY, Zhang YG, Li S, Leng WD and Kwong JSW: Periodontal disease and incident lung cancer risk: A meta-analysis of cohort studies. J Periodontol. 87:1158–1164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tai SH, Chen JP, Chang HS and Kuo HC: Periodontitis as a risk factor for lung cancer among women: A nationwide matched cohort study. Kaohsiung J Med Sci. 35:123–124. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chrysanthakopoulos NA: Correlation between periodontal disease indices and lung cancer in Greek adults: A case-control study. Exp Oncol. 38:49–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yoon HS, Wen W, Long J, Zheng W, Blot WJ and Cai Q: Association of oral health with lung cancer risk in a low-income population of African Americans and European Americans in the Southeastern United States. Lung Cancer. 127:90–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen Y, Zhu BL, Wu CC, Lin RF and Zhang X: Periodontal disease and tooth loss are associated with lung cancer risk. Biomed Res Int. 2020:51076962020.PubMed/NCBI
|
|
64
|
Bui FQ, Almeida-da-Silva CLC, Huynh B, Trinh A, Liu J, Woodward J, Asadi H and Ojcius DM: Association between periodontal pathogens and systemic disease. Biomed J. 42:27–35. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, et al: Porphyromonas gingivalis in Alzheimer's disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 5:eaau33332019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Stockham S, Stamford JE, Roberts CT, Fitzsimmons TR, Marchant C, Bartold PM and Zilm PS: Abnormal pregnancy outcomes in mice using an induced periodontitis model and the haematogenous migration of Fusobacterium nucleatum sub-species to the murine placenta. PLoS One. 10:e01200502015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Scannapieco FA and Cantos A: Oral inflammation and infection, and chronic medical diseases: Implications for the elderly. Periodontol 2000. 72:153–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hajishengallis G and Chavakis T: Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 21:426–440. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mosaddad SA, Tahmasebi E, Yazdanian A, Rezvani MB, Seifalian A, Yazdanian M and Tebyanian H: Oral microbial biofilms: An update. Eur J Clin Microbiol Infect Dis. 38:2005–2019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Nemec A, Pavlica Z, Nemec-Svete A, Eržen D, Milutinović A and Petelin M: Aerosolized clindamycin is superior to aerosolized dexamethasone or clindamycin-dexamethasone combination in the treatment of severe Porphyromonas gingivalis aspiration pneumonia in an experimental murine model. Exp Lung Res. 38:9–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hayata M, Watanabe N, Tamura M, Kamio N, Tanaka H, Nodomi K, Miya C, Nakayama E, Ueda K, Ogata Y and Imai K: The periodontopathic bacterium Fusobacterium nucleatum induced proinflammatory cytokine production by human respiratory epithelial cell lines and in the lower respiratory organs in mice. Cell Physiol Biochem. 53:49–61. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Darveau RP: Periodontitis: A polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 8:481–490. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Nagaoka K, Yanagihara K, Morinaga Y, Nakamura S, Harada T, Hasegawa H, Izumikawa K, Ishimatsu Y, Kakeya H, Nishimura M and Kohno S: Prevotella intermedia induces severe bacteremic pneumococcal pneumonia in mice with upregulated platelet-activating factor receptor expression. Infect Immun. 82:587–593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ulrich M, Beer I, Braitmaier P, Dierkes M, Kummer F, Krismer B, Schumacher U, Gräpler-Mainka U, Riethmüller J, Jensen PØ, et al: Relative contribution of Prevotella intermedia and Pseudomonas aeruginosa to lung pathology in airways of patients with cystic fibrosis. Thorax. 65:978–984. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jia L, Han N, Du J, Guo L, Luo Z and Liu Y: Pathogenesis of important virulence factors of Porphyromonas gingivalis via toll-like receptors. Front Cell Infect Microbiol. 9:2622019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kimizuka R, Kato T, Ishihara K and Okuda K: Mixed infections with Porphyromonas gingivalis and Treponema denticola cause excessive inflammatory responses in a mouse pneumonia model compared with monoinfections. Microbes Infect. 5:1357–1362. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Nemec A, Pavlica Z, Svete AN, Erzen D, Crossley DA and Petelin M: Lack of soluble tumor necrosis factor alpha receptor 1 and 2 and interleukin-1beta compartmentalization in lungs of mice after a single intratracheal inoculation with live Porphyromonas gingivalis. Exp Lung Res. 35:605–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tian H, Zhang Z, Wang X, Liu W and Wang Z: Role of experimental periodontitis in inducing pulmonary inflammation in mice. Oral Dis. 28:2294–2303. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Han YW: Fusobacterium nucleatum: A commensal-turned pathogen. Curr Opin Microbiol. 23:141–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Nagaoka K, Yanagihara K, Harada Y, Yamada K, Migiyama Y, Morinaga Y, Hasegawa H, Izumikawa K, Kakeya H, Nishimura M and Kohno S: Macrolides inhibit Fusobacterium nucleatum-induced MUC5AC production in human airway epithelial cells. Antimicrob Agents Chemother. 57:1844–1849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Nagaoka K, Yanagihara K, Morinaga Y and Kohno S: Detection of Fusobacterium nucleatum in two cases of empyema and lung abscess using paromomycin-vancomycin supplemented Brucella HK agar. Anaerobe. 43:99–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Nagaoka K, Yanagihara K, Harada Y, Yamada K, Migiyama Y, Morinaga Y, Izumikawa K and Kohno S: Quantitative detection of periodontopathic bacteria in lower respiratory tract specimens by real-time PCR. J Infect Chemother. 23:69–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gedik AH, Cakir E, Soysal O and Umutoğlu T: Endobronchial lesion due to pulmonary Fusobacterium nucleatum infection in a child. Pediatr Pulmonol. 49:E63–E65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Laurencet ME, Rosset-Zufferey S and Schrenzel J: Atypical presentation of Lemierre's syndrome: Case report and literature review. BMC Infect Dis. 19:8682019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sattar Y, Susheela AT, Karki B, Liaqat A, Ullah W and Zafrullah F: Diagnosis and management of Lemierre's syndrome presented with multifocal pneumonia and cerebral venous sinus thrombosis. Case Rep Infect Dis. 2020:63962742020.PubMed/NCBI
|
|
86
|
Lee WS, Jean SS, Chen FL, Hsieh SM and Hsueh PR: Lemierre's syndrome: A forgotten and re-emerging infection. J Microbiol Immunol Infect. 53:513–517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Shi T, Wang J, Dong J, Hu P and Guo Q: Periodontopathogens Porphyromonas gingivalis and Fusobacterium nucleatum and their roles in the progression of respiratory diseases. Pathogens. 12:11102023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Homsi N and Kapila R: Aggregatibacter actinomycetemcomitans causing empyema necessitans and pyomyositis in an immunocompetent patient. Cureus. 12:e94542020.PubMed/NCBI
|
|
89
|
Shilo S, Kassis I, Hakim F and Shachor-Meyouhas Y: Aggregatibacter actinomycemcomitans pneumonia in children: Two case reports and a review of the literature. Pediatr Infect Dis J. 34:100–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Matzumura-Kuan M and Jennings J: Aggregatibacter actinomycetemcomitans infection mimicking lung cancer: A case report. Scand J Infect Dis. 46:669–672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Storms I, van den Brand M, Schneeberger P and van't Hullenaar N: Aggregatibacter actinomycetemcomitans pneumonia with chest and abdominal wall involvement. BMJ Case Rep. 2017:bcr20162173772017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kwon T, Lamster IB and Levin L: Current concepts in the management of periodontitis. Int Dent J. 71:462–476. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Takahashi Y, Watanabe N, Kamio N, Yokoe S, Suzuki R, Sato S, Iinuma T and Imai K: Expression of the SARS-CoV-2 receptor ACE2 and proinflammatory cytokines induced by the periodontopathic bacterium Fusobacterium nucleatum in human respiratory epithelial cells. Int J Mol Sci. 22:13522021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Soffritti I, D'Accolti M, Fabbri C, Passaro A, Manfredini R, Zuliani G, Libanore M, Franchi M, Contini C and Caselli E: Oral microbiome dysbiosis is associated with symptoms severity and local immune/inflammatory response in COVID-19 patients: A cross-sectional study. Front Microbiol. 12:6875132021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Brandini DA, Takamiya AS, Thakkar P, Schaller S, Rahat R and Naqvi AR: Covid-19 and oral diseases: Crosstalk, synergy or association? Rev Med Virol. 31:e22262021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Drozdzik A and Drozdzik M: Oral pathology in COVID-19 and SARS-CoV-2 infection-molecular aspects. Int J Mol Sci. 23:14312022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Qi M, Sun W, Wang K, Li W, Lin J, Gong J and Wang L: Periodontitis and COVID-19: immunological characteristics, related pathways, and association. Int J Mol Sci. 24:30122023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC, Conde CD, Gasmi B, Stein S, Beach M, et al: SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 27:892–903. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li Q, Tan L, Wang H, Kou Y, Shi X, Zhang S and Pan Y: Fusobacterium nucleatum interaction with Pseudomonas aeruginosa induces biofilm-associated antibiotic tolerance via Fusobacterium adhesin A. ACS Infect Dis. 6:1686–1696. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu Y, Yuan X, Chen K, Zhou F, Yang H, Yang H, Qi Y, Kong J, Sun W and Gao S: Clinical significance and prognostic value of Porphyromonas gingivalis infection in lung cancer. Transl Oncol. 14:1009722021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lopes MP, Cruz ÁA, Xavier MT, Stöcker A, Carvalho-Filho P, Miranda PM, Meyer RJ, Soledade KR, Gomes-Filho IS and Trindade SC: Prevotella intermedia and periodontitis are associated with severe asthma. J Periodontol. 91:46–54. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Card JW, Carey MA, Voltz JW, Bradbury JA, Ferguson CD, Cohen EA, Schwartz S, Flake GP, Morgan DL, Arbes SJ Jr, et al: Modulation of allergic airway inflammation by the oral pathogen Porphyromonas gingivalis. Infect Immun. 78:2488–2496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hong C, Aung MM, Kanagasabai K, Lim CA, Liang S and Tan KS: The association between oral health status and respiratory pathogen colonization with pneumonia risk in institutionalized adults. Int J Dent Hyg. 16:e96–e102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Marino PJ, Wise MP, Smith A, Marchesi JR, Riggio MP, Lewis MAO and Williams DW: Community analysis of dental plaque and endotracheal tube biofilms from mechanically ventilated patients. J Crit Care. 39:149–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Brennan MT, Bahrani-Mougeot F, Fox PC, Kennedy TP, Hopkins S, Boucher RC and Lockhart PB: The role of oral microbial colonization in ventilator-associated pneumonia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 98:665–672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Porto AN, Cortelli SC, Borges AH, Matos FZ, Aquino DR, Miranda TB, Oliveira Costa F, Aranha AF and Cortelli JR: Oral and endotracheal tubes colonization by periodontal bacteria: A case-control ICU study. Eur J Clin Microbiol Infect Dis. 35:343–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tan L, Wang H, Li C and Pan Y: 16S rDNA-based metagenomic analysis of dental plaque and lung bacteria in patients with severe acute exacerbations of chronic obstructive pulmonary disease. J Periodontal Res. 49:760–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yamasaki K, Kawanami T, Yatera K, Fukuda K, Noguchi S, Nagata S, Nishida C, Kido T, Ishimoto H, Taniguchi H and Mukae H: Significance of anaerobes and oral bacteria in community-acquired pneumonia. PLoS One. 8:e631032013. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Bahrani-Mougeot FK, Paster BJ, Coleman S, Barbuto S, Brennan MT, Noll J, Kennedy T, Fox PC and Lockhart PB: Molecular analysis of oral and respiratory bacterial species associated with ventilator-associated pneumonia. J Clin Microbiol. 45:1588–1593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Heo SM, Haase EM, Lesse AJ, Gill SR and Scannapieco FA: Genetic relationships between respiratory pathogens isolated from dental plaque and bronchoalveolar lavage fluid from patients in the intensive care unit undergoing mechanical ventilation. Clin Infect Dis. 47:1562–1570. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Pan Y, Teng D, Burke AC, Haase EM and Scannapieco FA: Oral bacteria modulate invasion and induction of apoptosis in HEp-2 cells by Pseudomonas aeruginosa. Microb Pathog. 46:73–79. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Chen Y, Zhou R, Yi Z, Li Y, Fu Y, Zhang Y, Li P, Li X and Pan Y: Porphyromonas gingivalis induced inflammatory responses and promoted apoptosis in lung epithelial cells infected with H1N1 via the Bcl-2/Bax/Caspase-3 signaling pathway. Mol Med Rep. 18:97–104. 2018.PubMed/NCBI
|
|
113
|
Li Q, Pan C, Teng D, Lin L, Kou Y, Haase EM, Scannapieco FA and Pan Y: Porphyromonas gingivalis modulates Pseudomonas aeruginosa-induced apoptosis of respiratory epithelial cells through the STAT3 signaling pathway. Microbes Infect. 16:17–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Li X, Li C, Liu JC, Pan YP and Li YG: In vitro effect of Porphyromonas gingivalis combined with influenza A virus on respiratory epithelial cells. Arch Oral Biol. 95:125–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
He Y, Shiotsu N, Uchida-Fukuhara Y, Guo J, Weng Y, Ikegame M, Wang Z, Ono K, Kamioka H, Torii Y, et al: Outer membrane vesicles derived from Porphyromonas gingivalis induced cell death with disruption of tight junctions in human lung epithelial cells. Arch Oral Biol. 118:1048412020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Grigg J: The platelet activating factor receptor: A new anti-infective target in respiratory disease? Thorax. 67:840–841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kamio N, Hayata M, Tamura M, Tanaka H and Imai K: Porphyromonas gingivalis enhances pneumococcal adhesion to human alveolar epithelial cells by increasing expression of host platelet-activating factor receptor. FEBS Lett. 595:1604–1612. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Scialo F, Daniele A, Amato F, Pastore L, Matera MG, Cazzola M, Castaldo G and Bianco A: ACE2: The major cell entry receptor for SARS-CoV-2. Lung. 198:867–877. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Li Q, Wang H, Tan L, Zhang S, Lin L, Tang X and Pan Y: Oral pathogen Fusobacterium nucleatum coaggregates with Pseudomonas aeruginosa to modulate the inflammatory cytotoxicity of pulmonary epithelial cells. Front Cell Infect Microbiol. 11:6439132021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Mall MA: Unplugging mucus in cystic fibrosis and chronic obstructive pulmonary disease. Ann Am Thorac Soc. 13 (Suppl 2):S177–S185. 2016.PubMed/NCBI
|
|
121
|
Bonser LR and Erle DJ: Airway mucus and asthma: The role of MUC5AC and MUC5B. J Clin Med. 6:1122017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Cho HY, Park S, Miller L, Lee HC, Langenbach R and Kleeberger SR: Role for mucin-5AC in upper and lower airway pathogenesis in mice. Toxicol Pathol. 49:1077–1099. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Li J and Ye Z: The potential role and regulatory mechanisms of MUC5AC in chronic obstructive pulmonary disease. Molecules. 25:44372020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Miya C, Cueno ME, Suzuki R, Maruoka S, Gon Y, Kaneko T, Yonehara Y and Imai K: Porphyromonas gingivalis gingipains potentially affect MUC5AC gene expression and protein levels in respiratory epithelial cells. FEBS Open Bio. 11:446–455. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kapila YL: Oral health's inextricable connection to systemic health: Special populations bring to bear multimodal relationships and factors connecting periodontal disease to systemic diseases and conditions. Periodontol 2000. 87:11–16. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Vitkov L, Muñoz LE, Knopf J, Schauer C, Oberthaler H, Minnich B, Hannig M and Herrmann M: Connection between periodontitis-induced low-grade endotoxemia and systemic diseases: Neutrophils as protagonists and targets. Int J Mol Sci. 22:46472021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hajishengallis G: Periodontitis: From microbial immune subversion to systemic inflammation. Nat Rev Immunol. 15:30–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bunte K and Beikler T: Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci. 20:33942019. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
El-Awady AR, Elashiry M, Morandini AC, Meghil MM and Cutler CW: Dendritic cells a critical link to alveolar bone loss and systemic disease risk in periodontitis: Immunotherapeutic implications. Periodontol 2000. 89:41–50. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Thomas R, Qiao S and Yang X: Th17/Treg imbalance: Implications in lung inflammatory diseases. Int J Mol Sci. 24:48652023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhu J, Yamane H and Paul WE: Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 28:445–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T and Cua DJ: TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 8:1390–1397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, et al: Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 13:991–999. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wang W, Wang X, Lu S, Lv H, Zhao T, Xie G, Du Y, Fan Y and Xu L: Metabolic disturbance and Th17/Treg imbalance are associated with progression of gingivitis. Front Immunol. 12:6701782021. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Lopes FDTQS, Tibério IDFLC, Leme A and Fairclough L: Editorial: The importance of Th17/Treg imbalance in asthma and COPD development and progression. Front Immunol. 13:10252152022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Muyayalo KP, Huang DH, Zhao SJ, Xie T, Mor G and Liao AH: COVID-19 and Treg/Th17 imbalance: Potential relationship to pregnancy outcomes. Am J Reprod Immunol. 84:e133042020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Lin S, Wu H, Wang C, Xiao Z and Xu F: Regulatory T cells and acute lung injury: Cytokines, uncontrolled inflammation, and therapeutic implications. Front Immunol. 9:15452018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Watanabe N, Yokoe S, Ogata Y, Sato S and Imai K: Exposure to Porphyromonas gingivalis induces production of proinflammatory cytokine via TLR2 from human respiratory epithelial cells. J Clin Med. 9:34332020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
de Lacerda Vidal CF, Vidal AKDL, Monteiro JGDM, Cavalcanti A, Henriques APC, Oliveira M, Godoy M, Coutinho M, Sobral PD, Vilela CÂ, et al: Impact of oral hygiene involving toothbrushing versus chlorhexidine in the prevention of ventilator-associated pneumonia: A randomized study. BMC Infect Dis. 17:1122017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Labeau SO and Blot SI: Oral care for mechanically ventilated patients involving toothbrushing. Crit Care Med. 41:e136–e137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Osmari D, Fraga S, Braun KO and Unfer B: Behaviour of the elderly with regard to hygiene procedures for and maintenance of removable dentures. Oral Health Prev Dent. 14:21–26. 2016.PubMed/NCBI
|
|
142
|
Coffee L and Sockrider M: Dental health and lung disease. Am J Respir Crit Care Med. 199:P9–P10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Jia C, Sun M, Wang W, Li C, Li X and Zhang X: Effect of oral plaque control on postoperative pneumonia following lung cancer surgery. Thorac Cancer. 11:1655–1660. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Iwata E, Hasegawa T, Yamada SI, Kawashita Y, Yoshimatsu M, Mizutani T, Nakahara H, Mori K, Shibuya Y, Kurita H and Komori T: Effects of perioperative oral care on prevention of postoperative pneumonia after lung resection: Multicenter retrospective study with propensity score matching analysis. Surgery. 165:1003–1007. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Liu Z, Zhang W, Zhang J, Zhou X, Zhang L, Song Y and Wang Z: Oral hygiene, periodontal health and chronic obstructive pulmonary disease exacerbations. J Clin Periodontol. 39:45–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhou X, Han J, Liu Z, Song Y, Wang Z and Sun Z: Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: A 2-year pilot randomized controlled trial. J Clin Periodontol. 41:564–572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Apessos I, Voulgaris A, Agrafiotis M, Andreadis D and Steiropoulos P: Effect of periodontal therapy on COPD outcomes: A systematic review. BMC Pulm Med. 21:922021. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Shen TC, Chang PY, Lin CL, Wei CC, Tu CY, Hsia TC, Shih CM, Hsu WH, Sung FC and Kao CH: Impact of periodontal treatment on hospitalization for adverse respiratory events in asthmatic adults: A propensity-matched cohort study. Eur J Intern Med. 46:56–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Said KN, Al-Momani AM, Almaseeh JA, Marouf N, Shatta A, Al-Abdulla J, Alaji S, Daas H, Tharupeedikayil SS, Chinta VR, et al: Association of periodontal therapy, with inflammatory biomarkers and complications in COVID-19 patients: A case control study. Clin Oral Investig. 26:6721–6732. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Herrera D, Serrano J, Roldán S, Alonso B and Sanz M: Oral and systemic health: Is there a ‘new’ link with COVID-19? Clin Oral Investig. 27:3–13. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Kouanda B, Sattar Z and Geraghty P: Periodontal diseases: Major exacerbators of pulmonary diseases? Pulm Med. 2021:47124062021. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Mammen MJ, Scannapieco FA and Sethi S: Oral-lung microbiome interactions in lung diseases. Periodontol 2000. 83:234–241. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Dong J, Li W, Wang Q, Chen J, Zu Y, Zhou X and Guo Q: Relationships between oral microecosystem and respiratory diseases. Front Mol Biosci. 8:7182222021. View Article : Google Scholar : PubMed/NCBI
|















