Introduction
With the increasing number of cancer patients every
year, the side effects caused by chemotherapy drugs have become the
focus of medical workers. Chemotherapy-induced neuropathic pain
seriously affects the quality of life of millions of individuals,
while bringing a great economic burden worldwide (1,2). At
present, opioids are the most widely used painkillers in clinical
practice, but their side effects cannot be ignored (3). Remifentanil has some intraoperatively
and postoperatively clinical value, but its unique pharmacokinetics
are associated with the development of opioid-induced hyperalgesia
(OIH). Hyperalgesia could hinder patient's recovery process and
reduce their quality of life after surgery (4). Therefore, to relieve pain, it is
urgent to find new targets for the treatment of neuropathic pain
and explore potential therapeutic drugs.
According to previous studies (5,6), the
members of the Transient Receptor Potential family are key
regulatory factors in the induction of hyperalgesia. As a
non-selective cationic channel, transient receptor potential cation
channel subfamily V Member 1 (TRPV1) could be activated by protons,
cold stimulation, and capsaicin to transmit neuropathic pain
signals, and it is highly expressed in dorsal root ganglion neurons
(7). TRPV1 antagonists could
effectively inhibit neuropathic pain mediated by various
neurotransmitters and activates the CaMKII-PKC signaling pathway in
DRG neurons, thus exacerbating the persistence of postoperative
remifentanil-induced hyperalgesia (RIH) (8). Meanwhile, TRPV1 is a potentially
meaningful target molecule for the treatment of opiate-induced
hyperalgesia.
Chinese herbal extracts are known to be effective in
treating various diseases including cancer, diabetes, kidney
disease and chronic pain. As a kind of traditional Chinese medicine
monomer extracted from plants of genus Pueraria, puerarin
has been widely reported for its anti-inflammatory and antioxidant
effects. Liu et al (9)
found that puerarin could regulate the NF-κB signaling pathway,
downregulate inflammatory factors, and relieve diabetic neuropathic
pain. In addition, puerarin could reduce the stress transmission
mediated by the P2X3 receptor and relieve pain hypersensitivity
after burn (10). It was
hypothesized that puerarin could alleviate postoperative RIH, but
the mechanism remains unclear. In the present study, it was
intended to explore the effects of puerarin on OIH and the
molecular mechanism of TRPV1 regulation in rat pain models.
Materials and methods
Animals and groups
The adult male Sprague-Dawley rats (80 rats; age,
8-week-old) used in the study, weighing 220–250 g, were purchased
from a company Beijing Vital River Laboratory Animal Technology Co.
Ltd. Animals were raised in an environment of 23±2°C in a 12/12-h
day-night cycle and they had access to a standard diet and water.
The surgical treatment of experimental animals was performed in
accordance with the guidelines of the General Hospital of the
Western Theater Command of the PLA on the use of experimental
animals. The animal experiments in the present study were approved
(approval no. 20210329015) by the Experimental Animal Ethics
Committee of the General Hospital of the Western Theater Command of
the PLA (Chengdu, China).
Drugs and groups
Remifentanil hydrochloride (Renfu Pharmaceutical
Co., Yichang, China) was administered 1.0 µg/kg/min for 60 min via
the tail vein. Moreover, intravenous saline (0.1 ml/min) for 60 min
was used as the control group. Puerarin was dissolved in 10% DMSO
and diluted with normal saline.
Puerarin was injected intraperitoneally every 24 h
for 21 days before remifentanil-induced pain. In the animal
experiment I, the rats were divided into five groups: Control, RI
(remifentanil-induced group), RI + 10 mg/kg puerarin (RI + 10 mg/kg
puerarin), RI + 20 mg/kg puerarin group (RI + 20 mg/kg puerarin)
and RI + 40 mg/kg puerarin group (RI + 40 mg/kg puerarin). In
experiment II, the rats were divided into five groups: Control, RI,
RI + puerarin (40 mg/kg), RI + MK-801 (0.3 mg/kg), and RI +
puerarin + MK-801. There were eight rats in each group, for a total
of 80 rats. Feed and water were supplemented at 10 a.m. every day,
and the rats were monitored. After 21 days of experimentation, 80
rats were euthanized by intraperitoneal injection of 120 mg/kg
pentobarbital sodium anesthesia. To confirm death, rats were
monitored for several signs such as no response when the toes were
pressed with tweezers, no rising and falling of the chest and no
palpable heartbeat. The tissues were harvested within 24 h after
the end of the last behavioral experiment for subsequent
detection.
Behavioral test
As previously reported (11), to assess the sensitivity to
mechanical and thermal pain, the mechanical paw withdrawal
threshold (PWT) and the paw withdrawal latency (PWL) were recorded
using electronic Von Frey filaments and a hot plate (Thermo Fisher
Scientific, Inc.) at 24 h (baseline) before remifentanil
intervention and at 2, 6, 24 and 48 h after remifentanil
intervention. Behavioral tests were performed in different groups,
and the observers of the behavioral tests were blind to the animal
randomization and treatment conditions throughout the
experiment.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from spinal cord tissue
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
The cDNA was synthesized from total RNA using Prime Script Reagent
kit following the manufacturer's instructions. RT-qPCR analysis was
performed by ABI 7500 PCR system using SYBRSYBRTM Green Master Mix
(Applied Biosystems). The following thermocycling conditions were
used for qPCR: 95°C for 30 s, 40 cycles of 95°C for 10 s and 60°C
for 30 s, followed by 95°C for 10 s, 65°C for 60 s and 97°C for 1
s. The relative mRNA expression of TRPV1 was calculated and
analyzed by the 2−ΔΔCq method (12), and GAPDH was used as the internal
control. Primer sequences were as follows: TRPV1 forward,
5′-TGCACAATGGGCAGAATGAC-3′ and reverse, 5′-GTCATGTTCCGCCGTTCAAT-3′;
and GAPDH forward, 5′-ACCACAGTCCATGCCATCAC-3′ and reverse,
5′-TCCACCACCACCCTGTTGCTGTA-3′.
Bioinformatics analysis
PharmMapper database
(lilab.ecust.edu.cn/pharmmapper/) was used to search potential
targets of puerarin, and then JASPAR database
(jaspar2020.genereg.net/) was used to predict the binding sites of
TRPV1 and PAX6.
Dual luciferase reporter assay
HEK293 cells were transiently transfected with the
wild-type construct of pGL3-TRPV1-promoter (WT), mutant construct
of pGL3-TRPV1-promoter (MUT), empty plasmid (Vector), or PAX6
expression plasmid (PAX6) using lipofectamine 3000. After 48 h, A
dual luciferase reporting assay system (cat. no. E1910, Promega
Corporation) was used to detect luciferase activity of firefly and
Renilla.
Western blotting
RIPA lysate (Solarbio, Beijing) was used to extract
the protein from spinal cord tissue and protein concentration was
determined by BCA kit (cat. no. ab287853; Abcam). As previously
reported (13), western blot assay
was performed in accordance with standardized procedures. Western
blot was carried out by separating 40 µg of protein by 10% SDS-PAGE
and electro-transferred onto a PVDF) membrane. The membranes were
blocked with 5% non-fat milk and washed with TBST buffer and then
incubated with primary antibody overnight in 4°C. Next, the
membranes were washed 3 times with TBST and incubated with
secondary antibody (ZB2306, 1:10,000, ZSGB-Bio) at a 1:5,000
dilutions for 1 h at room temperature. Finally, the expression of
the proteins was evaluated using enhanced chemiluminescence kit
(cat. no. PE0010; Beijing Solarbio, Beijing). The following primary
antibodies were used: anti-PAX6 (cat. no. ab195045; Abcam,
1:1,000), anti-t-NR1 (cat. no. 5704s; Cell Signaling Technology,
Inc., 1:1,000), anti-m-NR1 (cat. no. ab14596; Abcam, 1:1,000),
anti-NR2B (cat. no. 4207s; Cell Signaling Technology, Inc.,
1:1,000), anti-p-NR2B (cat. no. 4208s; Cell Signaling Technology,
Inc., 1:1,000) and anti-GAPDH (cat. no. 97166s; Cell Signaling
Technology, Inc., 1:1,000). GAPDH was used as the internal
reference. Finally, Image J software (National Institutes of
Health) was used to measure the gray value of protein bands and
calculate the relative expression of target proteins.
Immunofluorescent staining
The expression levels of PAX6, p-NR2B and TRPV1 were
determined by immunofluorescence staining according to the
previously reported methods (14).
Finally, the stained images were viewed under a fluorescence
microscope and images were captured. Image J software (National
Institutes of Health, Version 7.2) was used to analyze the
fluorescence intensity and evaluate the positive signal.
Chromatin immunoprecipitation
(Chip)-PCR
Chip-PCR analysis was performed as previously
reported (15). Finally, PCR was
used to quantify immunoprecipitated DNA, and all values were
normalized to the input.
Statistical analysis
The data in the study were expressed as the mean ±
SD. SPSS 22.0 software (IBM Corp.) package was used for statistical
analysis. Comparison between the two groups was performed by
unpaired t-test. One-way analysis of variance and minimum
significant difference (LSD) tests were used for statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference. In animal experiments, there were six rats
in each group.
Results
Effects of puerarin on RIH
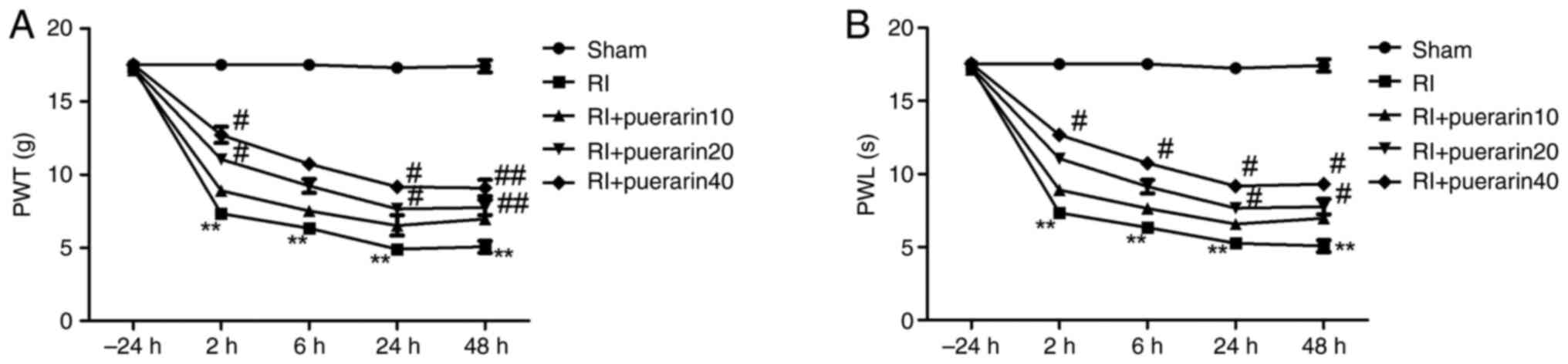
As shown in Fig. 1,
there were no significant differences between baseline PWLs to
thermal stimulation and baseline PWTs to von Frey filaments in the
treatment groups before the surgery (−24 h) (P<0.05, Fig. 1A and B). After surgery, PWT and PWL
in the incision and + remifentanil group decreased at 2, 6, 24 and
48 h, and postoperative RIH was noticeable and maintained from 2 to
48 h. Moreover, it reached a peak at 24 h and remifentanil appeared
to facilitate hyperalgesia induced by incision. On the contrary,
PWT and PWL did not demonstrate significant changes in saline rats
(P<0.05, Fig. 1A and B). The
results reflected that the postoperative RIH rat model was
successfully established.
Puerarin alleviates the expression of
PAX6 in remifentanil-induced spinal cord
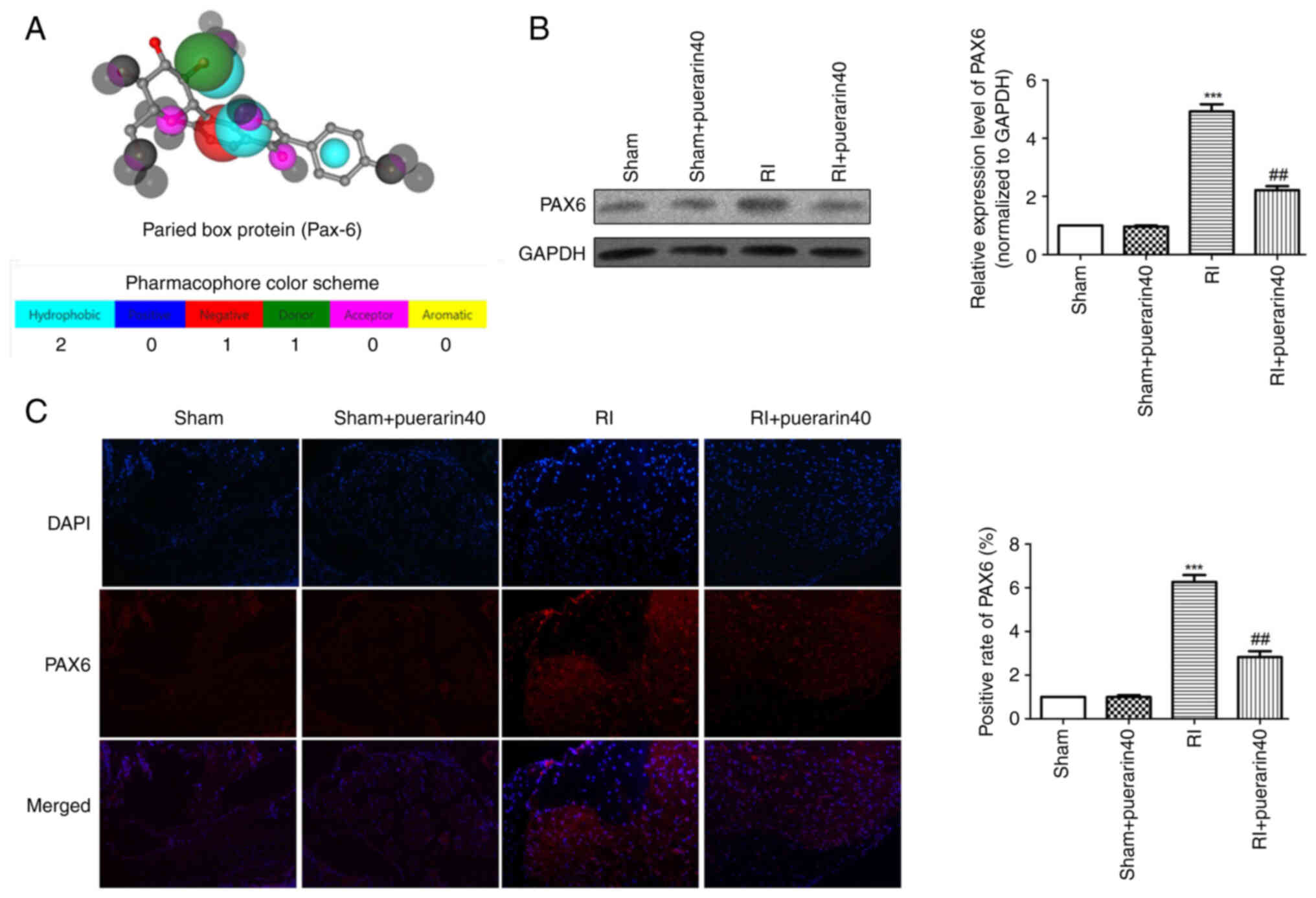
Combined with Pharmmapper prediction (Fig. 2A), the sites of targeted binding of
puerarin to PAX6 were analyzed and identified. Subsequently,
western blot results (Fig. 2B)
indicated that compared with the sham group, the expression levels
of PAX6 were increased significantly in the RI group. While the
expression of PAX6 in RI + puerarin40 group was reduced
significantly compared with the RI group. Consistent with western
blot results, immunofluorescence staining proved the same trend
(Fig. 2C). The present findings
suggested that Puerarin alleviates the expression of PAX6 in
remifentanil-induced spinal cord.
Puerarin regulates the transcription
of TRPV1 via targeting PAX6
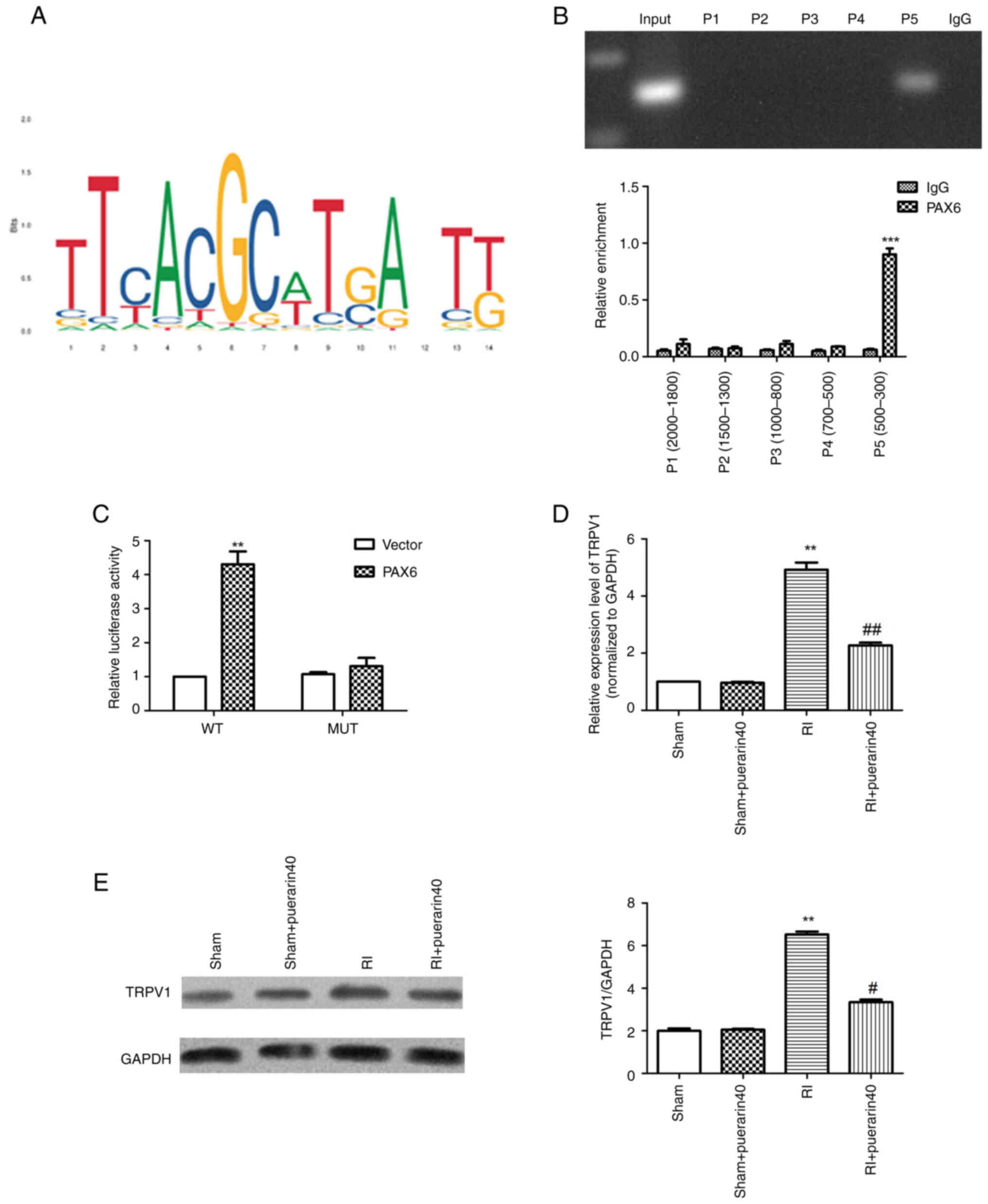
Next, JASPAR was used to predict the base binding
sequence between TRPV1 and PAX6 (Fig.
3A). Chip-PCR results (Fig.
3B) illustrated ~8-fold enrichment of PAX6-bound TRPV1 compared
with the IgG group. Furthermore, dual luciferase reporter assay
results (Fig. 3C) indicated that
compared with the vector group, the luciferase activity was
significantly increased. RT-qPCR results (Fig. 3D) demonstrated that the mRNA
expression levels of TRPV1 in the tissues were increased
significantly in the RI group compared with the sham group, while
the expression of TRPV1 was reduced significantly in RI + puerarin
group. Western blot results (Fig.
3E) revealed the same trend as RT-qPCR results. Overall, it was
found that puerarin could regulate the transcription of TRPV1 via
targeting PAX6.
The effects of puerarin on NR1
trafficking in postoperative RIH
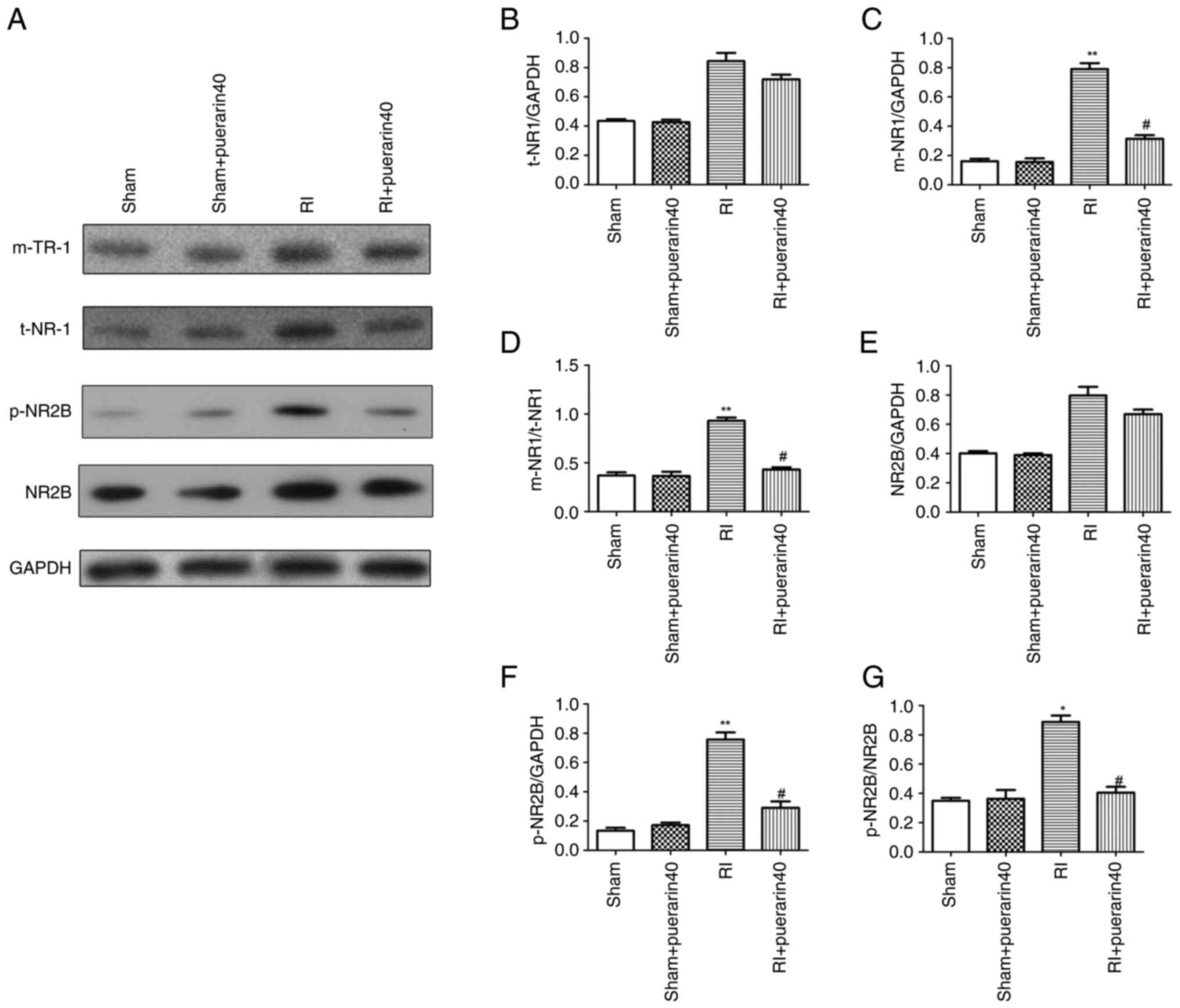
In order to confirm the effect of puerarin on NR1
transport after remifentanil hyperalgesia, the NR1 protein
expression level was detected by western blot assay. As
demonstrated in Fig. 4A-D,
compared with the sham group, the expression of NR1 protein and
membrane protein in the spinal cord were significantly upregulated
and downregulated after treatment. Moreover, western blot results
revealed that the expression of p-NR2B was reduced significantly
compared with the RI group (Fig.
4E-G). The aforementioned results suggested that puerarin may
play a role in influencing the NR1 trafficking and NR2B
phosphorylation of the spinal cord.
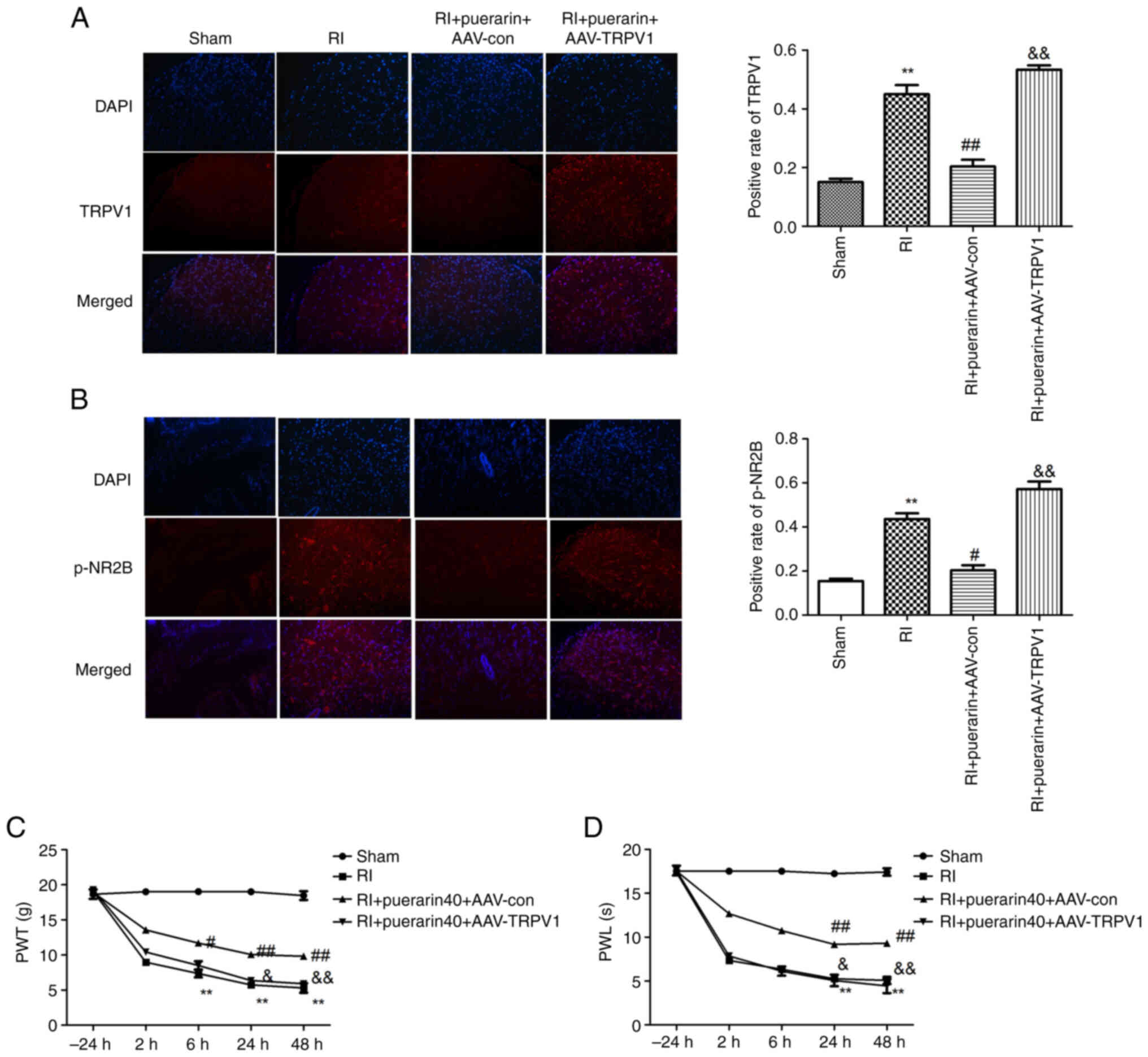
Overexpression of TRPV1 reverses the
effects of puerarin on postoperative RIH
Immunofluorescence staining results (Fig. 5A) identified that the positive rate
of TRPV1 in the tissue was significantly increased in the RI group
compared with sham group. Compared with RI group, the positive rate
of TRPV1 was reduced significantly in the RI + puerarin + AAV-con
group, while the positive rate of TRPV1 in the RI + puerarin +
AAV-TRPV1 group was reversed. Immunofluorescence staining results
(Fig. 5B) proved that the
expression level of p-NR2B had the same trend. After surgery, PWT
and PWL in the RI group decreased at 2, 6, 24 and 48 h, and
postoperative RIH was noticeable and maintained from 2 to 48 h
(Fig. 5C and D). In addition, both
PWT and PWL were significantly increased in the RI + puerarin +
AAV-con compared with the RI group. While the PWT and PWL showed a
downtrend compared with the RI + puerarin + AAV-TRPV1 group. These
results reflected that the overexpression of TRPV1 reversed the
effects of puerarin on postoperative RIH.
Discussion
Although opioids relieve the pain caused by cancer,
long-term exposure to opioids can lead to hyperalgesia (16,17).
The present study mainly investigated the effect of puerarin on
postoperative pain and allergy induced by remifentanil. It was
discovered that puerarin regulates NR1 transmembrane transport and
NR2B phosphorylation, and alleviates postoperative pain and
hypersensitivity induced by remifentanil. The protective mechanism
of puerarin is related to its targeted regulation of PAX6 and its
effects on TRPV1 transcription.
As a traditional Chinese medicine, the
pharmacological effects of puerarin have been widely reported,
including clinical applications (18–20).
It has been reported that puerarin can alleviate osteoporosis in
OVX-induced mice by inhibiting osteoclast generation by inhibiting
the TRAF6/ROS-dependent MAPK/NF-κB signaling pathway (21). In addition, puerarin could inhibit
apoptosis by regulating SIRT3/SOD2 signaling pathway and
alleviating nerve function defects in mice with subarachnoid
hemorrhage (22). Fu et al
(23) found that puerarin could
downregulate the PPARγ signaling pathway and has therapeutic effect
on atherosclerosis. Zhang et al (24) identified that puerarin may
preferentially block the β1 subunit of Nav1.8 in sensory neurons
and participate in the anti-paclitaxel-induced neuropathic pain.
Regarding opiate-induced postoperative hyperalgesia, it was found
that puerarin could inhibit NR1 transport and NR2B phosphorylation
and relieve OIH.
As a member of the paired box family, PAX6 plays a
key role in brain development (25). As a conserved transcription factor,
PAX6 has 2 distinct DNA-binding domains and also mediates embryonic
and adult neurogenesis (26).
DNMT3b-mediated hypomethylation of PAX6 gene may be involved in
mechanical allodynia after drug therapy (27). It was hypothesized that PAX6 may
also play an important role in opiate-induced postoperative
hyperalgesia. Most importantly, further molecular mechanism studies
(28,29) suggested that overexpression of
TRPV1 could reverse the inhibitory effect of puerarin on OIH. In
addition, it was demonstrated that puerarin could target and
regulate the expression of PAX6 and affect the transcription of
TRPV1.
Puerarin could relieve postoperative hyperalgesia
caused by remifentanil that may be associated with
N-methyl-D-aspartate (NMDA) activation through intracellular
pathways, thereby upregulating the function of NMDA activity
(30). The distribution of NMDA
receptor NR2B in the superficial dorsal horn is almost limited. The
expression of NR2B subunit in the superficial dorsal horn was
significantly increased due to the sensitivity signal transmission
of the spinal cord. The findings of the present study suggested
that puerarin could inhibit the transmembrane transport of NR1 and
the phosphorylation of NR2B.
The present results confirmed that puerarin could
alleviate remifentanil-induced postoperative pain by targeting PAX6
to regulate TRPV1 transcriptional expression. On the other hand,
the current findings explained the molecular mechanism through
which puerarin alleviates OIH, which may provide theoretical
support for the further expansion of its range of clinical
applications.
Acknowledgements
Not applicable.
Funding
The present study was supported by Scientific Research Project
of Military Logistic (grant no. CLB19J051) and the Scientific
Research Project of Sichuan Provincial Health Commission (grant no.
18PJ159).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY wrote the manuscript, conceptualized the study,
developed methodology and performed software analysis. YL collected
the data, wrote a draft of the manuscript, performed the data
presentation and produced the figures. YS visualized data and
conducted investigation. LR supervised the study and developed
methodology. XG performed software analysis, validated and curated
data. LC developed methodology and performed software analysis. GZ
visualized data and performed software analysis. XS validated data.
QH performed software analysis. XC developed methodology. GG
designed experiments and reviewed and edited the manuscript. YS and
GG confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The animal experiments in the present study were
approved (approval no. 20210329015) by the Experimental Animal
Ethics Committee of the General Hospital of the Western Theater
Command of the PLA (Chengdu, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fallon MT: Neuropathic pain in cancer. Br
J Anaesth. 111:105–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith EM, Pang H, Cirrincione C, Fleishman
S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C,
Le-Lindqwister N, et al: Effect of duloxetine on pain, function,
and quality of life among patients with chemotherapy-induced
painful peripheral neuropathy: A randomized clinical trial. JAMA.
309:1359–1367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Azzam A, McDonald J and Lambert D: Hot
topics in opioid pharmacology: Mixed and biased opioids. Br J
Anaesth. 122:e136–e145. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rivosecchi RM, Rice MJ, Smithburger PL,
Buckley MS, Coons JC and Kane-Gill SL: An evidence based systematic
review of remifentanil associated opioid-induced hyperalgesia.
Expert Opin Drug Saf. 13:587–603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han Q, Kim YH, Wang X, Liu D, Zhang ZJ,
Bey AL, Lay M, Chang W, Berta T, Zhang Y, et al: SHANK3 deficiency
impairs heat hyperalgesia and TRPV1 signaling in primary sensory
neurons. Neuron. 92:1279–1293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roberts LA and Connor M: TRPV1 antagonists
as a potential treatment for hyperalgesia. Recent Pat CNS Drug
Discov. 1:65–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katz B, Zaguri R, Edvardson S, Maayan C,
Elpeleg O, Lev S, Davidson E, Peters M, Kfir-Erenfeld S, Berger E,
et al: Nociception and pain in humans lacking a functional TRPV1
channel. J Clin Invest. 133:e1535582023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song C, Liu P, Zhao Q, Guo S and Wang G:
TRPV1 channel contributes to remifentanil-induced postoperative
hyperalgesia via regulation of NMDA receptor trafficking in dorsal
root ganglion. J Pain Res. 12:667–677. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu M, Liao K, Yu C, Li X, Liu S and Yang
S: Puerarin alleviates neuropathic pain by inhibiting
neuroinflammation in spinal cord. Mediators Inflamm.
2014:4859272014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu C, Li G, Gao Y, Liu S, Lin J, Zhang J,
Li X, Liu H and Liang S: Effect of puerarin on P2X3 receptor
involved in hyperalgesia after burn injury in the rat. Brain Res
Bull. 80:341–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Li Y, Wang H, Xie K, Shu R, Zhang
L, Hu N, Yu Y and Wang G: Inhibition of DOR prevents remifentanil
induced postoperative hyperalgesia through regulating the
trafficking and function of spinal NMDA receptors in vivo and in
vitro. Brain Res Bull. 110:30–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dalmau J, Furneaux HM, Gralla RJ, Kris MG
and Posner JB: Detection of the anti-Hu antibody in the serum of
patients with small cell lung cancer-a quantitative western blot
analysis. Ann Neurol. 27:544–552. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao L, Dai Z, Tang W, Liu C and Tang B:
Astragaloside IV Alleviates Cerebral Ischemia-Reperfusion Injury
through NLRP3 Inflammasome-Mediated Pyroptosis Inhibition via
Activating Nrf2. Oxid Med Cell Longev. 2021:99255612021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai W and Yu L: Insulin-like growth factor
1 ameliorates pre-eclampsia by inhibiting zinc finger E-box binding
homeobox 1 by up-regulation of microRNA-183. J Cell Mol Med.
27:1179–1191. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee M, Silverman SM, Hansen H, Patel VB
and Manchikanti L: A comprehensive review of opioid-induced
hyperalgesia. Pain Physician. 14:145–161. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santoni A, Mercadante S and Arcuri E:
Chronic cancer and non-cancer pain and opioid-induced hyperalgesia
share common mechanisms: Neuroinflammation and central
sensitization. Minerva Anestesiol. 87:210–222. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou B, Zhang J, Chen Y, Liu Y, Tang X,
Xia P, Yu P and Yu S: Puerarin protects against sepsis-induced
myocardial injury through AMPK-mediated ferroptosis signaling.
Aging (Albany NY). 14:3617–3632. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng F, Guo B, Ma YQ, Li KW and Niu FJ:
Puerarin: A review of its mechanisms of action and clinical studies
in ophthalmology. Phytomedicine. 107:1544652022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L: Pharmacokinetics and drug
delivery systems for puerarin, a bioactive flavone from traditional
Chinese medicine. Drug Deliv. 26:860–869. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao L, Zhong M, Huang Y, Zhu J, Tang W,
Li D, Shi J, Lu A, Yang H, Geng D, et al: Puerarin alleviates
osteoporosis in the ovariectomy-induced mice by suppressing
osteoclastogenesis via inhibition of TRAF6/ROS-dependent MAPK/NF-κB
signaling pathways. Aging (Albany NY). 12:21706–21729. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Yang X, Ge X and Zhang F:
Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved
caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid
hemorrhage mice. Biomed Pharmacother. 109:726–733. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu R, Zhang Y, Guo Y, Zhang Y, Xu Y and
Chen F: Digital gene expression analysis of the pathogenesis and
therapeutic mechanisms of ligustrazine and puerarin in rat
atherosclerosis. Gene. 552:75–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang XL, Cao XY, Lai RC, Xie MX and Zeng
WA: Puerarin Relieves Paclitaxel-Induced Neuropathic Pain: The Role
of Nav1.8 β1 subunit of sensory neurons. Front
Pharmacol. 9:15102019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wullimann ME and Rink E: Detailed
immunohistology of Pax6 protein and tyrosine hydroxylase in the
early zebrafish brain suggests role of Pax6 gene in development of
dopaminergic diencephalic neurons. Brain Res Dev Brain Res.
131:173–191. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Osumi N, Shinohara H, Numayama-Tsuruta K
and Maekawa M: Concise review: Pax6 transcription factor
contributes to both embryonic and adult neurogenesis as a
multifunctional regulator. Stem Cells. 26:1663–1672. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pires SF, de Barros JS, da Costa SS, de
Oliveira Scliar M, Van Helvoort Lengert A, Boldrini É, da Silva
SRM, Tasic L, Vidal DO, Krepischi ACV and Maschietto M: DNA
methylation patterns suggest the involvement of DNMT3B and TET1 in
osteosarcoma development. Mol Genet Genomics. 298:721–733. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rowan MP, Szteyn K, Doyle AP, Gomez R,
Henry MA and Jeske NA: β-arrestin-2-biased agonism of delta opioid
receptors sensitizes transient receptor potential vanilloid type 1
(TRPV1) in primary sensory neurons. Mol Pain. 10:502014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu Y, Chen J and Wang R: Puerarin
suppresses TRPV1, calcitonin gene-related peptide and substance P
to prevent paclitaxel-induced peripheral neuropathic pain in rats.
Neuroreport. 30:288–294. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao Y, Zhou S, Pan Y, Gu L, He Y and Sun
J: Wnt3a inhibitor attenuates remifentanil-Induced hyperalgesia
via downregulating spinal NMDA receptor in rats. J Pain Res.
13:1049–1058. 2020. View Article : Google Scholar : PubMed/NCBI
|