Globally, glaucoma is the primary cause of blindness
in individuals with eye diseases other than cataracts (1). An estimated 111.8 million people
worldwide will suffer from glaucoma by 2040, leading to unilateral
or bilateral vision loss if not diagnosed and treated promptly
(2). Glaucoma is now recognized as
a group of progressive optic neuropathies characterized by
excavation or cupping of the optic disc and corresponding visual
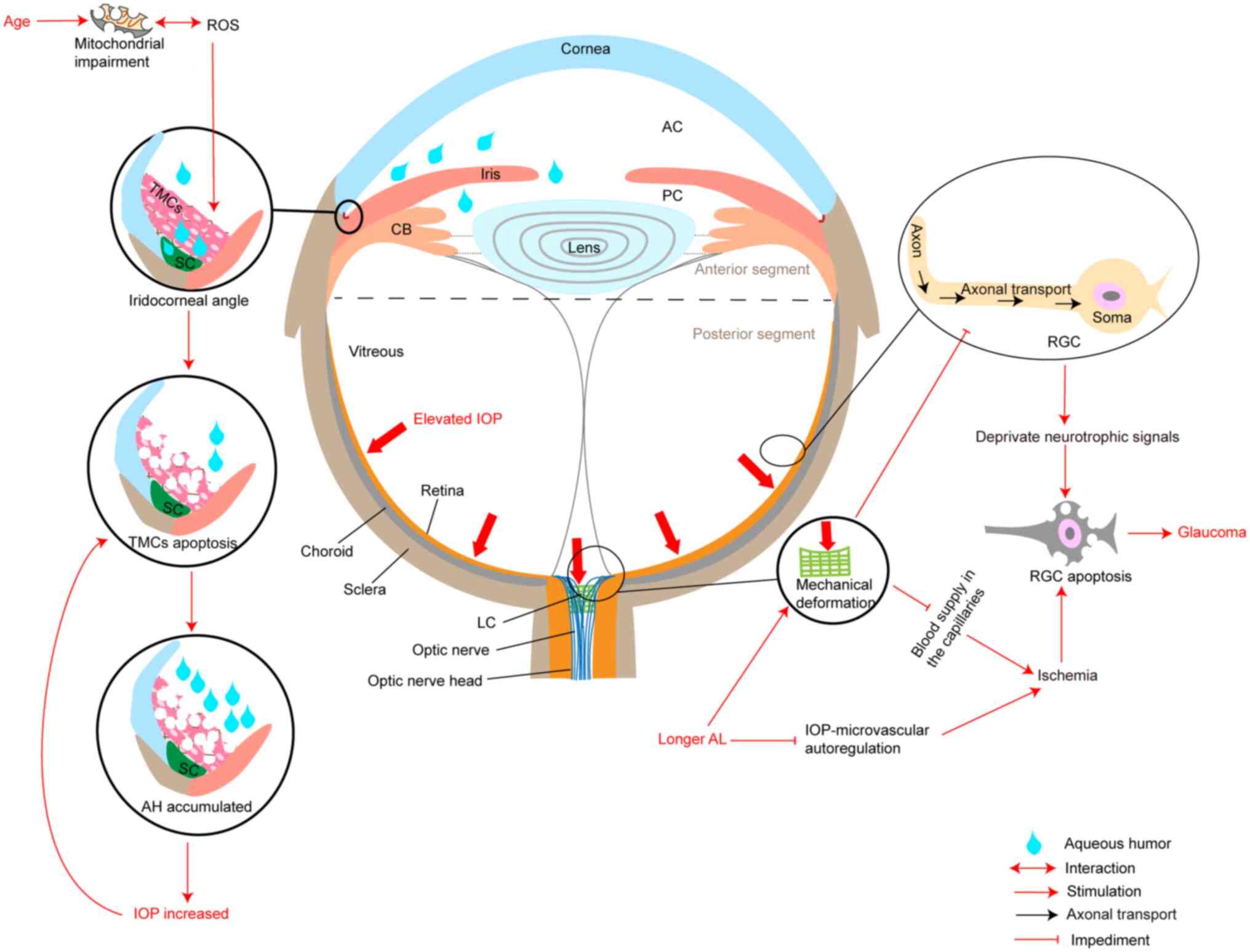
field loss (3,4). Elevated intraocular pressure (IOP), a
major risk factor for the development and progression of glaucoma,
is considered to impair the lamina cribrosa, resulting in a loss of
normal structural and metabolic support for retinal ganglion cell
(RGC) axon and impaired axoplasmic transport (5–7)
(Fig. 1). In addition,
meta-analyses have shown that individuals with myopia, especially
those with high myopia, have an increased risk of suffering from
primary open-angle glaucoma (POAG) (8,9). On
the one hand, the increased axial length of the myopic eye appears
to result in greater deformability of the lamina cribrosa, which
may contribute to a greater susceptibility to optic disc changes in
glaucoma (10,11). On the other hand, in patients with
glaucoma, a longer axial length disrupts the IOP-microvascular
autoregulation relationship and decreases the thickness of the
lamina cribrosa, increasing the susceptibility of eyes with longer
axial length to IOP-induced blood flow reduction (12,13).
However, for individuals with normal tension glaucoma (NTG), even
when the IOP is <21 mm Hg, there are also symptoms of optic
neuropathy, manifested as optic disc excavation and loss of vision
(14). Comprehensive reviews
indicated that the mechanisms of the pathophysiology of NTG are
related to the following factors: i) Lower tolerance of normal IOP
results in mechanical damage and generates stress on the axons in
the lamina cribrosa; ii) vascular dysregulation and perfusion
deficit; iii) greater than normal pressure gradient across the
lamina cribrosa; iv) impaired cerebrospinal fluid circulation in
the subarachnoid space of the optic nerve which results in a toxic
damage to the nerve; v) genetic predisposition (15,16)
and vi) abnormal function of neuroserpin (17). Upregulation of neural serine
proteases may protect the function of RGCs and restore the function
of biochemical networks related to autophagy, microglia and
synaptic function in glaucoma (17). Antioxidant defence weakens with
age, which is also associated with an increased risk of glaucoma
(18–20) (Fig.
1). Thus, a number of studies suggested that IOP-independent
factors in NTG, such as vasoconstriction, gliosis, glutamate
toxicity and oxidative stress, activate an apoptosis signalling
pathway similar to that involved in glaucoma with elevated IOP,
resulting in the loss of RGCs (21,22).
Although significant progress has been made in
understanding the pathogenic mechanism of glaucoma in recent years,
the pathogenesis of this disease has yet to be explored. Baleriola
et al (33) detected
apoptotic cells in the trabecular meshwork (TM) of patients with
glaucoma by TdT-mediated dUTP nick end labelling (TUNEL) staining.
Moreover, RGC apoptosis is also regarded as the earliest form of
cell loss in glaucoma (34). In
recent years, a number of studies have shown that apoptotic
signals, such as Fas signalling, contribute to the pathogenesis of
glaucoma by activating apoptosis signalling pathway and
inflammatory cytokines, suggesting that surgical injury and global
trauma also lead to rapid retinal inflammation and RGC apoptosis
(35,36). In glaucomatous mice after surgery
or corneal trauma, tumour necrosis factor-alpha (TNF-α) and other
inflammatory cytokines are produced in the anterior segment
(37). These inflammatory
cytokines rapidly spread to the retina, where they cause RGC
apoptosis, which is known to lead to glaucoma optic neuropathy
(37). The long-term
administration of antiglaucoma therapy may lead to TM cell
apoptosis, so the onset, development and prognosis of glaucoma
appear to be associated with apoptosis (33,37–39).
Lowering IOP with ocular hypotensive drops and surgery are
effective treatments for glaucoma, but these treatments simply
delay disease progression and preserve partial eye-sight. Besides,
some medications, such as anticholinergics and adrenergic agents,
are known to increase the risk of glaucoma, while
benzalkonium-containing beta-blockers and prostaglandin analogues
have been reported to trigger mild expression of apoptotic
molecules (40,41). Moreover, surgical treatments for
glaucoma depend on traditional filtering surgery, such as
trabeculectomy, which also has side effects. In more challenging
cases of glaucoma, tubes or antifibrotic agents are also needed to
improve the surgical success rate (42,43).
However, antifibrotic agents are associated with a higher incidence
of complications, some of which may be vision-threatening (44). Thus, identifying apoptotic signals
in glaucoma is essential for understanding the pathogenesis of this
disease and is also fundamental for developing new therapeutic
approaches. The present study reviewed the apoptosis and survival
pathways of patients with glaucoma and the progress of research on
apoptosis as a targeted therapy for glaucoma.
Clinical and experimental evidence has revealed a
rapidly initiated, inflammatory (TNF-α-mediated RGC apoptosis) and
IOP-independent glaucoma pathway induced by acute anterior segment
trauma or surgery, suggesting that cell apoptosis promotes the
development of glaucoma (36,37).
Administration of infliximab (a TNF-α antibody) or a TNF-α
inhibitor has been revealed to protect against cell apoptosis,
ameliorating neuroglial remodelling and inhibiting monocyte
infiltration (36,37,45,46).
Fas signalling reportedly contributes to the pathogenesis of
glaucoma by activating both apoptotic and inflammatory pathways and
the small peptide inhibitor of the Fas receptor, ONL1204, provides
potent neuroprotection (35,47).
In addition, several studies have shown that the apoptosis pathway
is an essential mediator of RGC death in glaucoma (48–52).
Moreover, in glaucoma, TM cells also undergo death through
apoptotic pathway, such as the Fas/Fas ligand (FasL) pathway and
ERS (51,53–55).
Notably, benzalkonium chloride, the most common preservative used
in glaucoma treatment also induces toxic changes in the TM and cell
apoptosis, which further leads to impaired function of the TM and
may worsen any glaucomatous process within the TM if used for a
long time (38,56,57).
Glaucoma-related cell death typically occurs through apoptosis,
triggered by oxidative stress through mitochondrial damage,
inflammation, endothelial dysregulation and dysfunction, hypoxia
and other factors (39,58).
TM cell apoptosis in the anterior chamber is the
most significant concern in glaucoma. It has been suggested that
mechanical stress, oxidative stress and intense phagocytic activity
of TM cells likely cause cell apoptosis (59). The extent of mechanical stress on
the TM depends on the IOP. Thus, glaucoma itself can also induce TM
cell apoptosis via mechanical stress or trabecular hypoperfusion
(59,60). TM cells die due to apoptosis, loss
of barrier function, alteration of aqueous humour outflow and
increased IOP (61,62) (Fig.
1).
Programmed cell death can be detected in the TM of
patients with glaucoma by using TUNEL (33). Moreover, the experimental results
regarding Fas monoclonal antibody-induced apoptosis in cultured
human TM cells demonstrated that human TM cells were stimulated to
undergo apoptosis through the Fas/FasL pathway (63). Upregulation of activating
transcription factor-4 (ATF4) and C/EBP homologous protein (CHOP)
and colocalization of ATF4 with endothelial leukocyte adhesion
molecule (ELAM-1) were found in the TM of patients with POAG and
inhibition of ATF4 reduced tunicamycin-induced caspase-3
activation, ROS production, ELAM-1 expression and human TM cell
phagocytosis impairment (53). An
in vivo study in mice revealed that overexpression of ATF4
in the TM induces CHOP expression and TM cell apoptosis, leading to
the production of inflammatory cytokines and possibly increased IOP
(53). The aforementioned study
demonstrated that the eukaryotic initiation factor 2 alpha
(eIF2α)/ATF4/CHOP branch of the unfolded protein response (UPR) was
activated in human TM cells from patients with glaucoma patients
following tert-butyl hydroperoxide exposure (53). In addition, alteration of the TM
cell extracellular matrix (ECM) is also considered to be one of the
mechanisms that induces IOP elevation by increasing TM resistance.
Furthermore, the effects of oxidative stress and latent
transforming growth factor beta-binding protein 2 knockdown on ECM
and TM cell apoptosis may be mediated by activation of the
transforming growth factor-beta (TGF-β)/bone morphogenetic protein
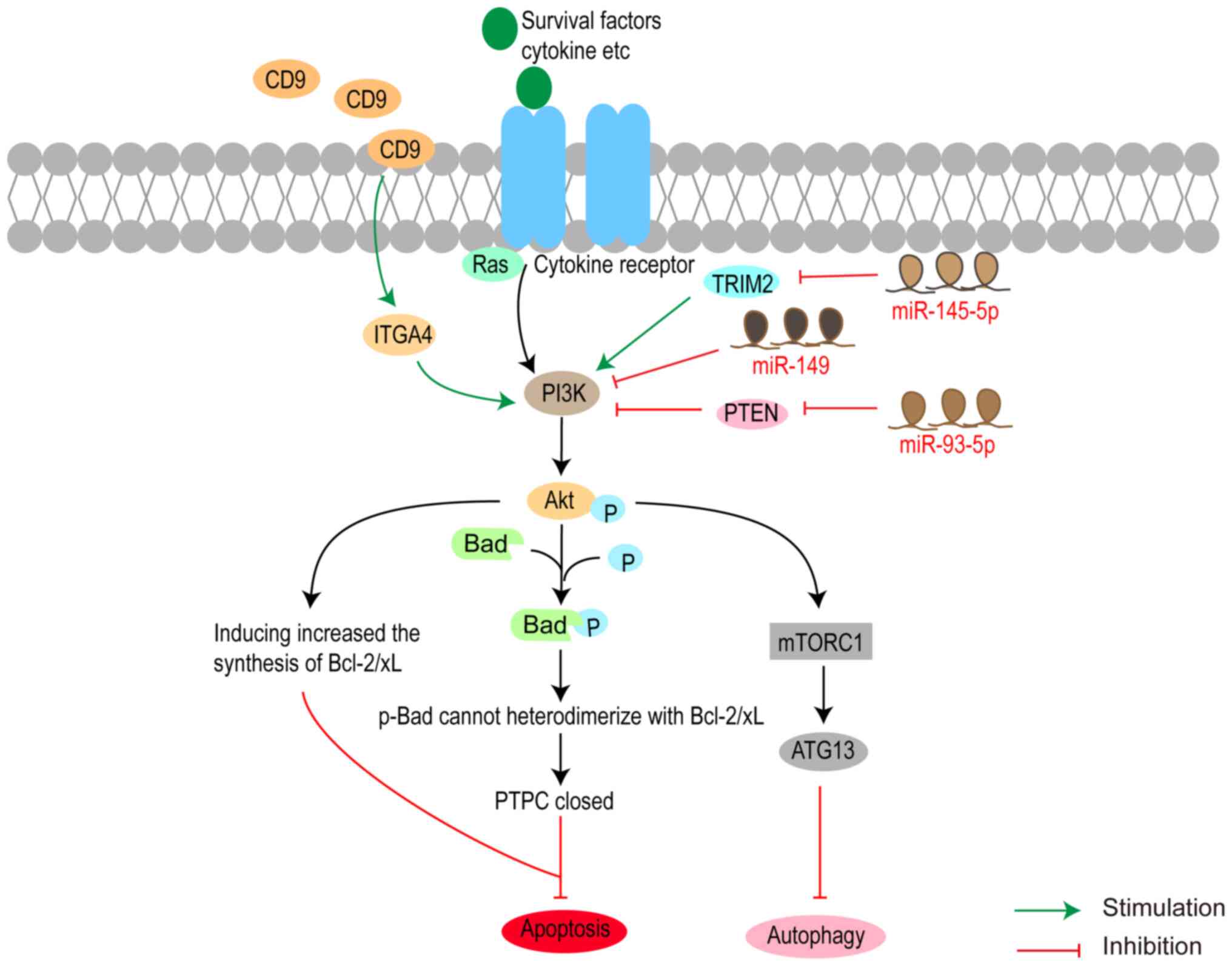
signalling pathway (64). CD9 was
found to be involved in a wide range of biological processes, such
as cell migration and differentiation and cell adhesion and
motility, by regulating the phosphatidylinositol 3-kinase
(PI3K)-protein kinase B (Akt) signalling pathway (65,66).
Yan et al (67) reported
that CD9 was downregulated in glaucoma and the overexpression of
CD9 could activate integrin α4 (ITGA4), PI3K and Akt, leading to
reduced TM cell apoptosis and alleviating glaucoma (Fig. 2). MicroRNAs (miRNAs) are
single-stranded noncoding RNAs that regulate cellular processes in
human TM cells and the effect of miRNAs on TM cell apoptosis in
glaucoma has been reported in previous studies. The expression of
miR-93 was significantly upregulated in human TM cells in glaucoma
and miR-93 induced human TM cells and inhibited their viability by
suppressing the expression of nuclear factor erythroid 2-like 2,
thus indicating that miR-93 is a vital regulator in glaucoma
(68). However, the effect of
miR-200c-3p on TM cell is opposite to that of miR-93. A different
study revealed that overexpression of miR-200c-3p negatively
regulates the expression of phosphatase and tensin homologue (PTEN)
to inhibit cleaved caspase-3, reduce Bax expression and activate
the PTEN/Akt/mammalian target of rapamycin (mTOR) signalling
pathway, thereby promoting cell proliferation and inhibiting TM
cell apoptosis (69). Furthermore,
the effects of miR-17-5p on the human TM cell apoptosis and
proliferation are similar to those of miR-200c-3p (70). Moreover, miR-181a enhances the
survival rate of TM cells by blocking the nuclear factor kappa-B
and c-Jun amino-terminal kinase (JNK) signalling pathway (71).
Therefore, elevated IOP resulting from TM
dysfunction caused by the apoptosis signalling pathway and changes
in stiffness, inflammation and oxidative stress, aggravates TM cell
damage and causes TM cell death. Moreover, TM-derived molecules
from damaged TM cells and harmful signals released from stressed TM
cells act as apoptotic stimulators, such as neuronal-like cell
apoptosis (55,75) (Fig.
1).
The exact mechanisms of RGC apoptosis are not fully
understood and increasing evidence suggests that RGC apoptosis may
involve blocking anterograde and retrograde axonal transport
resulting in the deprivation of neurotrophic signals (4). Moreover, this process, which is
accompanied by microglial activation, neuroinflammation and ECM
remodelling, was enhanced by high IOP (76–79).
Microglia are activated before RGC loss, and early
changes in the retina and optic nerve were detected in the DBA/2J
mouse model of glaucoma (80,81).
Moreover, the degree of microglial activation in the optic nerve
head (ONH) is proportional to the severity of optic nerve
degeneration (81). Numerous
studies have revealed that microglia may be essential modulators
involved in peripheral monocyte infiltration and retinal pigment
epithelial migration (36,45,82,83).
The depletion of these proteins leads to abnormal neuroglial
remodelling, exacerbating neuroretinal tissue damage (45). Furthermore, infiltrating monocytes
may amplify the inflammatory cascade response and contribute to the
activation of retinal microglia (36). Inflammatory cytokines, such as
TNF-α, lead to permanent changes in the immune function of the
retina, called ‘permanent neuroglial remodelling’ and the
anti-inflammatory agents have significant neuroprotective effects
on RGCs (36,37).
Furthermore, RGC apoptosis is associated with the
level of IOP. Elevated IOP causes axonal degeneration at the ONH in
the region of lamina cribriform, a process that occurs in parallel
to RGC apoptosis (84). Elevated
IOP is considered to damage the lamina cribrosa by mechanical
stress, resulting in loss of normal structural and metabolic
support of RGC axons and impaired axoplasmic transport. A reduction
in neurotrophic signals in RGCs may lead to the initiation of
apoptosis and ultimately to the RGC death (5,39).
Another novel mechanism of IOP-induced RGC apoptosis is that
IOP-induced changes in specific ECM components or cytokines in the
retina may increase the activity of matrix metalloproteinases
(MMPs), such as MMP-9 (79). One
of the reasons for the increase in MMP-9 may be that the mechanical
effects of elevated IOP may lead to the RGC axonal damage in the
ONH region, which further results in retrograde damage to the RGC
body and, in turn, may induce increased MMP-9 activity, causing
changes in the ECM (79). Another
explanation for the MMP-9 increase induced by elevated IOP is an
indirect effect mediated by glutamate (79). Glutamate is a major excitatory
neurotransmitter that is increased by stimuli, such as IOP,
ischaemia and injury (85).
Glutamate excitotoxicity has been reported to be one of the
critical pathophysiological causes of RGC injury in glaucoma
(86). Thus, glutamate-mediated
activation of MMP-9 may lead to RGC apoptosis.
An increase in MMP-9 activity during RGC apoptosis
parallels to a decrease in deposition of laminin in the RGC layer,
which may lead to disruption of the cell-ECM and cell-cell
interactions, increasing susceptibility to apoptosis (79). Tissue inhibitors of matrix
metalloproteinase-1 (TIMP-1) are generally considered to be
inhibitors of MMPs, particularly MMP-9, that maintain ECM
homeostasis (87). TIMP-1 activity
in the RGC layer increases with increasing MMP-9 activity and is
correlated with IOP exposure (79). An increase in retinal TIMP-1 may
contribute to its neuroprotective effects on RGCs by inhibiting
MMP-9 and antiapoptotic effects. This finding is consistent with
the theory that the ECM is continually remodelled after retinal
exposure to elevated IOP.
Molecules associated with ECM changes at the
glaucoma ONH site, such as TGF-β2 and collagen 1, are also involved
in glaucomatous RGC loss. The experimental results revealed that
TGF-β2 and collagen 1 deposition was significantly associated with
increased IOP at the ONH (73,79,88).
A possible mechanism for increased TGF-β expression in the ONH is
the stress response to elevated IOP, as TGF-β is a crucial molecule
stimulated by mechanical stress (89,90).
However, with increased IOP exposure, TGF-β2 deposition in the
retina significantly decreases, whereas MMP-9 increases. TGF-β1
regulates the mRNA and protein levels of MMP-9, similar to its
inhibitor TIMP-2 (91).
Furthermore, the effect of TGF-β2 depends on its concentration and
specificity in targeting cells (92). The biphasic behaviour of TGF-β
explains why both the low levels of TGF-β2 found in the RGC layer
and the high levels of TGF-β2 in the ONH may be involved in RGC
apoptosis (79).
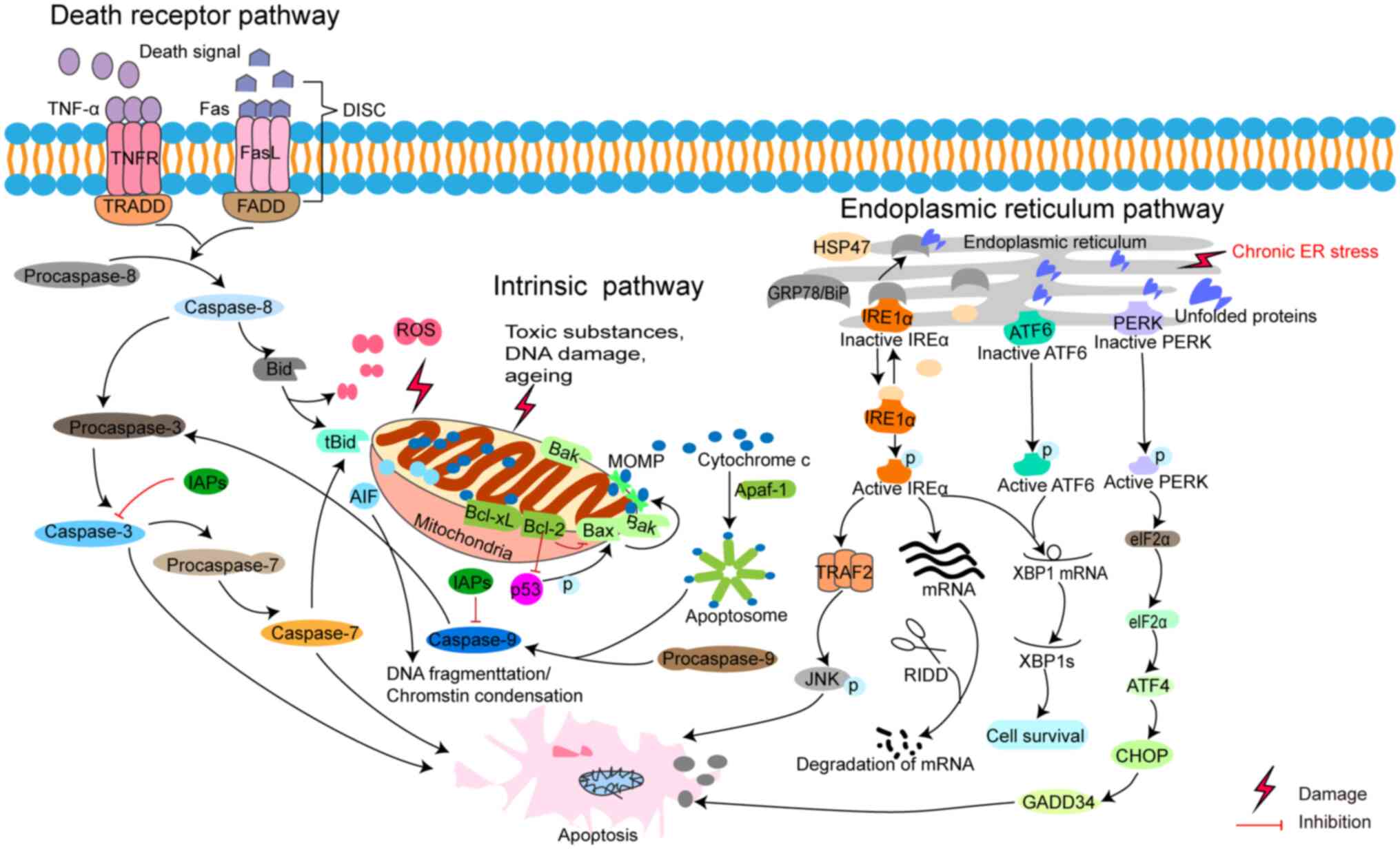
The intrinsic (or mitochondrial) and extrinsic (or
death receptor) pathways of apoptosis are two commonly described
pathways. Both pathways eventually lead to a common pathway or the
executive phase of apoptosis. The third pathway is an ERS-induced
pathway (28,93–95)
(Fig. 3).
Although several extrinsic DR pathways have been
described, the best-known pathway is triggered by death signals
including TNF-α and FasL (Fig. 3).
A previous study has shown that Fas signal contributes to glaucoma
pathogenesis by activating apoptotic and inflammatory pathways
(35). At the same time, a
marginal single nucleotide polymorphism association of TNF-α was
also found in human glaucoma and the assessment of the expression
levels of TNF-α may serve as a promising biomarker for POAG in
African Americans (96). In this
pathway, corresponding DRs of TNFα and FasL are TNF receptor1
(TNFR1) and Fas, respectively, which help transmit death signals
from the cell surface to intracellular pathways through their death
domain (DD). DD recruits adaptor proteins, such as Fas-associated
DD proteins (FADD), TNFR1-associated DD protein and cysteine
proteases such as caspase-8 (97).
Subsequently, the death-inducing signalling complex (DISC), a
ligand-receptor-adaptor protein complex, is formed by a sequential
process. The death ligand of DISC binds to DR to recruit an adaptor
protein in order (98,99). The DISC activates caspase-8, which
initiates apoptosis through cleavage of the downstream caspases
(97).
Previous studies have revealed that stimuli (toxic
substances, ROS and aging) and inherent DNA deficiencies can impair
mitochondrial structure and function, triggering the intrinsic
mitochondrial pathway in glaucoma (Fig. 3) (100,101). Meanwhile, these stimuli induce
the opening of the mitochondrial permeability transition pore and
hinder the mitochondrial transmembrane potential, thus accelerating
the release of proapoptotic proteins, such as cytochrome c (Cytc)
and apoptosis-inducing factors, such as apoptosis-inducing factor
(AIF) from mitochondria into the cytoplasm (102,103). Apoptotic protease activating
factor 1 (Apaf-1, homologous to cell death protein 4) with an
N-terminal caspase recruitment domain (CARD) consists of a
six-helix bundle, is an activator of procaspase-9 and is considered
to be a junction protein of the mitochondrial pathway (104). Apaf-1 oligomerizes in response to
the release of Cytc and forms a disc-shaped heptamer
(Cytc-Apaf-1)7 called apoptosome (104). Subsequently, the apoptosome
recruits and activates procaspase-9, an initiator of caspase in the
mitochondrial pathway, leading to downstream caspase-3 processing
(105).
Mitochondrial-initiated processes are mediated by
B-cell lymphoma 2 (Bcl-2) family proteins. Bcl-2 family proteins
are in the outer mitochondrial membrane that regulates
mitochondrial outer membrane permeabilization (MOMP) and the
release of the Cytc. The conformations of members of the Bcl-2
family all have one to four homology BH domains (BH1 to BH4), of
which the homology BH3 domain of the proapoptotic proteins (Bax,
Bak) is required for MOMP and the execution of intrinsic apoptosis
(106). Based on their function
in regulating MOMP and their domains, members of the Bcl-2 family
are classified into three categories. The first category is
antiapoptotic members, including Bcl-2, Bcl-extra-large, induced
myeloid leukemia cell differentiation protein Mcl-1, Bcl-w and
Bcl-2 related protein A1, characterized by all having four BH
domains (107). These proteins
inhibit Bax homo-oligomerization in MOMP by competing with Bax to
bind to the BH3 helix of Bax through the groove BH1-3 (108). The second category is
proapoptotic proteins comprising Bax, Bak and Bok, containing four
BH domains (107). Activated by
interaction with the BH3 domain of Bim or BH3 interacting-domain
death agonist (Bid), oligomerization of Bak and Bax results in the
formation of MOMP and induces Cytc release into the cytoplasm
(107,109). The BH3-only proteins are the
third subfamily, comprised of Bid, Bim, Bcl-2-interacting killer,
Bcl2 modifying factor, p53-upregulated modulator of apoptosis and
Noxa. Compared with the sequence homology of other subfamily
members, these proteins only have the BH3 domain (107,110). BH-3 only proteins act as
apoptotic signal receptors and Bax-like proteins contribute to
MOMP, which results in the release of proapoptotic proteins (Cytc,
AIF) from mitochondria (110). In
the late stages of apoptosis, AIF nuclear translocation induces
chromatin condensation or DNA fragmentation in a
caspase-independent manner (111). In the apoptosis signalling
pathway, Bcl-2 and Bax with negative or positive p53 response
elements are located downstream of p53 (112). Oxidative stress-induced DNA
damage upregulates Bax by phosphorylating p53 (113). Moreover, Bcl-2 inhibits
p53-mediated apoptosis and transcriptional activation (114,115).
The third apoptosis pathway is mediated by ERS,
which is critical for cell survival (Fig. 3). ERS is a condition in which some
physiological and pathological impairments impede the ability of
the cell to properly fold and post-translationally modify secretory
and transmembrane proteins in the ER, resulting in the accumulation
of misfolded proteins in the ER lumen (116). When protein misfolding persists
or is excessive, ERS triggers cell death, usually apoptosis. ER
proteostasis surveillance is sensed by the UPR, the highly
conserved signal transduction pathway in the ER lumen that senses
the fidelity of protein folding and determines cell fate in
response to ERS (117).
UPR is activated by three transmembrane ER proteins,
namely ATF6, inositol-requiring enzyme 1α (IRE1α) and protein
kinase RNA-like ER kinase (PERK) (118,119). These ERS sensors have a common
domain, namely the ER-luminal domain, which senses high
concentrations of misfolded proteins to alter the oligomerization
state of each senor and activates their downstream molecular signal
(116). The phosphorylated form
of IRE1α (p-IRE1α) activates chaperone genes and also activates JNK
by binding to TNF receptor associated factor-2 (TRAF2) (120). In a rat model of experimental
glaucoma, p-JNK is increased and may play a role in RGC death
(121). Thus, it is assumed that
IRE1α-JNK signalling pathway is involved in ERS of glaucoma.
Besides, p-IRE1α initiates mRNA splicing of the X-box binding
protein (XBP1) to produce a frameshift that code encodes a potent
transcription factor, XBP1s. XBP1s enters the nucleus and initiates
the transcription of a subset of UPR-related genes associated with
protein folding, secretion, ER-associated degradation and lipid
synthesis (122). In addition,
p-IRE1α is involved in the regulated IRE1-dependent decay process
to promote the degradation of mRNA and slow down the synthesis of
new polypeptide chains, thereby alleviating ERS (123). Thus, IRE1α is a crucial protein
that regulates cell survival or induces apoptosis in response to
surrounding ERS.
PERK is activated in a similar way to IRE1α.
Activation of PERK induces translational attenuation of p-eIF2α.
Under excessive or prolonged ERS, p-eIF2α translates the
transcription factor ATF4, which further induces the expression of
other transcription factors, CHOP and ATF3, thus participating in
the proapoptotic process (124,125). The expression of ATF4 and CHOP
significantly increased in human glaucomatous TM cells (126). However, in the DBA/2J mouse model
of glaucoma, an age-related, naturally occurring ocular
hypertensive mouse model of glaucoma, though CHOP plays a minor
role in contributing to RGC somal apoptosis, it does not lead to
axonal degeneration (127).
Additional studies have confirmed that the eIF2α/ATF4/CHOP pathway
induces TM cell apoptosis in experimental glaucoma (53,128,129).
Similarly, ATF6 activates the target transcription
factor, XBP1, to perform a pro-survival effect. XBP1 mRNA is
induced by ATF6 and spliced by IRE1α to form the spliced form of
XBP1 (130). TM cells treated
with DEX showed that IRE1, ATF6 and GRP78 were downregulated
(131). A recent study suggested
that loss of ATF6 exacerbates retinal degeneration (132). However, the specific mechanism of
ATF6-associated ERS in glaucoma has not been revealed.
The cysteine protease family is the core of the
caspase-dependent apoptosis pathway. A total of 13 caspases encoded
by the human genome belong to the peptidase C14A family and are
orthologous to CED-3 in C. elegans (133,134). Caspases are divided into
apoptotic and inflammatory caspases based on their biological
function; apoptotic caspase includes initiator caspase (caspase-2,
−8, −9 and −10) and effector caspase (caspase-3, −6 and −7). The
initial caspase is the first caspase to be activated by induced
self-cleavage. The primary structure of the initial caspase is
characterized by the presence of a long N-terminal propeptide and
two death effector domains (DED) or CARD and it mainly activates
the effector caspases (135). The
effector caspase, characterized by a short N-terminal propeptide
without DED and CARD, performs apoptosis by shearing various target
proteins (136). Cell pyroptosis
is a form of inflammatory programmed cell death pathway activated
by human and mouse caspase-1, human caspase-4 and caspase-5, or
mouse caspase-11 (137).
Inflammatory caspases (caspase-1, −4, −5, or caspase-11) with CARD
followed by the catalytic domain mediate cell pyroptosis by the
effector protein gasdermin D (137). A recent study reported that
melatonin reduced the expression of cleaved caspase-1, cleaved
gasdermin D and decreased the number of IL-1β-positive RGC cells
after acute ocular hypertension injury (138).
The initiator caspase of the intrinsic pathway is
caspase-9, while the extrinsic pathway is caspase-8 and both
converge to caspase-3. In the caspase-8/-9 cleavage process of Bid
in tBid to remodel mitochondria, favourable conditions are created
for ROS production, inhibited by caspase-3 and enhanced by
caspase-7 (105). Moreover,
caspase-3 is the primary executor of apoptosis.
In a rat model of chronic hypertensive glaucoma
induced by episcleral vein cauterization, activation of the
PI3K/Akt/mTOR pathway was shown to be neuroprotective against
glaucoma (139). Apoptosis
crosstalk with autophagy remains limited in glaucoma and the
balance between autophagy and apoptosis is crucial for the survival
of glaucoma cells (22). In recent
years, additional regulators have been found to be involved in the
development of glaucoma through the PI3K/Akt pathway. For example,
overexpression of CD9 decreases human TM cell apoptosis and
attenuates symptoms of human glaucoma by activating ITGA4, PI3K and
Akt (67). miR-145-5p induces RGC
apoptosis by suppressing tripartite motif-containing 2
(TRIM2)-mediated activation of the PI3K/Akt signalling pathway in a
rat model of glaucoma induced by intraocular injection of
N-methyl-D-aspartate (NMDA) (140). Using the same animal model of
glaucoma, miR-93-5p was revealed to negatively regulate phosphatase
and PTEN to promote the survival of RGCs by activating the Akt/mTOR
pathway (141). Moreover, p21/Ras
integrates survival signals and induces the activation of PI3K to
phosphorylate Akt (p-Akt) (142).
p-Akt increases Bcl-2/Bcl-xL synthesis and phosphorylates Bad,
preventing the heterodimerization of Bad with Bcl-2/Bcl-xL
(142). Subsequently, it
facilitates the closure of the permeability transition pore complex
and prevents the release of mitochondrial factors into the
cytoplasm (142). In addition,
Yan et al (62) reported
that accumulation of the Asn450Tyr mutant myocilin gene
(Myoc-N450Y) promotes apoptosis of primary human TM cell through
the ERS-induced apoptosis pathway, with the PI3K/Akt signalling
pathway playing a crucial role. This evidence suggested that
survival signals promote cell survival through the PI3K/Akt
signalling pathway in glaucoma (Fig.
2).
Microglial activation is an early change in the
retina and optic nerve in the DBA/2J mouse model of chronic
inherited glaucoma and may contribute to the onset or progression
of glaucoma (80,81). Detection of microglial activation
may be valuable for early glaucoma diagnosis, while modulation of
microglial responses may change disease progression (80). Increased Fas/FasL immunoreactivity
was found in microglia and FADD immunoreactivity was found in
Müller glial cells and RGCs (47).
FasL (CD95-L/APO-1L) is a signal membrane-bound type II
transmembrane protein that belongs to the TNF family, a central
pathway in the regulation of apoptosis by the immune system
(143,144). Membrane-bound FasL (mFasL)
induces apoptosis and promotes inflammation upon binding to Fas. By
contrast, the soluble Fas-ligand (sFasL), which is formed by
cleavage of the 103–137 amino acid region of mFasL by MMPs, blocks
the apoptosis and inhibits inflammation (145–148). Previous research has revealed
that the sFas concentration in the aqueous humour is lower in
patients with POAG than in control individuals, suggesting that a
low level of sFas may provide an appropriate microenvironment for
increased apoptosis of TM cells in glaucoma (149).
Currently, for the antagonistic effect of mFasL and
sFasL in glaucoma, Gregory-Ksande and Marshak-Rothstein (148) suggested that sFasL competes with
mFasL for binding to Fas, resulting in steric hindrance. However,
except for the Met12 small-molecule inhibitor, the steric hindrance
mechanism by which Met12 binds to Fas results in fewer receptors
available for FasL binding, directly interfering with FasL binding
to Fas (153). There is no better
answer to how the sFasL monomer effectively blocks the binding of
mFasL multimers to Fas. Nevertheless, other studies have reported
that the proapoptotic activity of trimeric sFasL in the ciliary
body is enhanced when sFasL binds to corneal ECM proteins (154). A specific cytokine in the ECM,
such as TGF-β, increases the local concentration of sFasL (154). Thus, the antagonistic effects of
mFasL/sFasL may be a treatment target in glaucoma in the
future.
Full-length tropomyosin receptor kinase C (TrkC) is
the primary receptor for neurotrophin-3. TrkCT1 is a truncated
receptor isoform of TrkC, that lacks the kinase domain and has a
unique short intracellular domain. TrkCT1 has been reported to
control the production of TNF-α in glial cells, leading to the
death of RGCs (155). TNFα is a
type II single-transmembrane protein (N-terminal at the cytoplasmic
face), that is expressed mainly by macrophages, natural killer
cells, T and B cells and plays a role in inflammation, cell
proliferation, apoptosis and morphology (155–157). TNFα binds to two types of TNF-α
receptors (TNFR1 and TNFR2) to perform its multiple biological
functions (158,159). Soluble TNF-α (sTNFα)
preferentially binds to TNFR1, which results in neuroinflammation
and cell death (160). In
addition, Cueva Vargas et al (161) reported that glia-derived sTNFα
modulates neuronal death in glaucoma via calcium-permeable AMPA
receptor activation. Conversely, transmembrane TNF-α (tTNFα) mainly
binds to TNFR2 by activating the prosurvival PI3K-Akt/PKB
signalling pathway which mediates neuroprotective effects (159,162,163). A reduction in the number of
activated microglia shifts the balance towards antiapoptotic
effects in glaucoma (164). A
mouse model of glaucoma generated by ocular surgery or trauma has
demonstrated that rapid inhibition of TNFα or IL-1β significantly
inhibits monocyte infiltration and RGC apoptosis caused by surgical
injury and global trauma (36).
Additionally, the expression levels of IL-1β and TNFα in the TM of
patients with POAG were significantly greater than those in the
control group and TNFα induced TM cell death (165,166). TNFα stimulates mitochondria to
form a stress response, which sequentially releases ROS, Cytc and
Bax to activate caspase-9 and a downstream caspase cascade to
initiate apoptosis (167).
Moreover, neutralizing the action of TNFα and IL-1β prevents the
loss of RGCs induced by elevated hydrostatic pressure or
lipopolysaccharide (168).
Previous studies demonstrated that patients with the TNFα-308 G/A
polymorphism may have increased susceptibility to glaucoma
(169,170). However, the relationship between
the TNFα-308 G/A polymorphism and glaucoma has not yet been
verified.
DR, which belongs to the TNFR superfamily, is a
type I signal transmembrane receptor (97). All members of the DR family are
characterized by the presence of a DD consisting of an ~80
amino-acid-long motif (171). DD
recruits various junction proteins to form a DR platform to mediate
cell death. In addition to TNFR1 (known as DR1) and Fas (known as
DR2, CD95, or APO-1), DR includes DR-3 (APO-3), DR4, known as
TNF-related apoptosis-inducing ligand receptor1 (TRAIL-R1), DR5
(TRAIL-R2), DR6 (CD358), nerve growth factor receptor and
ectodysplasin A receptor (172).
However, death ligands bind to decoy receptors without DDs, such as
TRAIL-R3, which cannot transform apoptotic signals, thus producing
antiapoptotic effect (172). The
level of DR plays a role in transmitting an apoptotic signal to
balance cell life or die. Regardless of the mechanism underlying
the upregulation of DR or enhancement of DR function, cell death
occurs.
For example, upregulation of the Fas receptor is a
marker of human glaucomatous neuropathy (173). In glaucoma, inhibiting Fas
expression via an inhibitor provides protection to the retinal
nerve (35,174). The glial production of TNFα is
increased in the glaucomatous retina and ONH which caused RGCs
death through its direct or indirect neurotoxicity (175). Increased immunostaining for TNFα
and TNFR1 is observed mainly in glial cells and in glial cells
processed around axons and blood vessels in the ONH (176). Under normal circumstances, only
TNFR1 is constitutively expressed in the vasculature of the ONH. In
human glaucoma, the expression of TNFα and TNFR1 in astrocytes and
microglia is increased (177). In
severe glaucomatous damage, RGC axons expresses TNFR1, which may be
a direct target for TNFα mediated optic nerve degeneration
(177). The mRNA and protein
expression levels of TNFα and TNFR1 are greater in the inner
retinal layers in glaucomatous eyes and TNFR1 is expressed at high
levels in RGCs, whereas TNFα, is expressed mainly in glial cells
(178). In rat model of
experimental glaucoma, TNFα strengthened the excitability of RGCs
by activating TNFR1 to upregulate the current density of Nav1.6,
namely, the voltage-gated Na+ channel, thereby promoting
RGC apoptosis, while choosing a suitable sodium channel blocker to
block Nav1.6 may be a useful strategy for treating glaucoma
(179). An explanation for the
cause of TNFR2-mediated RGC death, was that TNFα simulated ocular
hypertension, leading to the release of cytotoxic agents followed
by the activation of microglia and the loss of oligodendrocytes
that encapsulate RGCs, which in turn led to slow RGC death
(180).
The caspase family plays a significant role in the
execution of cell apoptosis. Hence, caspase activation is likely
related to cell apoptosis in glaucoma.
Activation of caspase-9, an initiator of the
intrinsic caspase cascade, was found to be involved in RGC death in
a rat model of experimental glaucoma (181). In addition, the downregulation of
caspase-8 significantly alleviated RGC death in a mouse model of
acute glaucoma by inhibiting the processing of IL-1β (182). Caspase-8 has various functions in
both RGCs and astroglia in glaucoma. In the C57BL/6J mouse model of
experimental glaucoma, caspase-8−/− astroglia protected
RGCs from glial-driven inflammatory impairment, while an inhibitor
of caspase-8 cleavage rescued RGCs against from apoptosis, as Yang
et al (183) reported that
caspase-8 plays a crucial role in RGC apoptosis and
astroglia-induced neuroinflammation in glaucoma. Additionally, in
caspase-7−/− mice, caspase-7−/− ameliorated
RGC death in optic nerve crush (ONC) injury and improved the
functional response of RGCs (184).
IAPs regulate the cell cycle, signal transduction,
cell apoptosis, cytokinesis and are composed of a group of proteins
similar in structure and function. IAPs are characterized by the
baculovirus IAP repeat (BIR) protein domain at the N-terminus; some
possess a new, interesting gene finger domain at the C-terminus
(202). To date, eight human IAPs
have been identified, including X-chromosome-linked IAP
(XIAP/BIRC4), cellular IAP1 (c-IAP1/BIRC2), cellular IAP2
(c-IAP2/BIRC3), IAP-like protein 2 (BIRC8), melanoma IAP
(Livin/BIRC7), neuronal apoptosis inhibitory protein (BIRC1),
survivin (BIRC5) and the BIR repeat-containing
ubiquitin-conjugating enzyme system (BIRC6, Apollo) (202). Although not all proteins with a
BIR domain antiapoptotic functions, the BIR domain is necessary for
the antiapoptotic effects of IAP family proteins.
As an endogenous inhibitor, IAP inhibits caspase
activity by binding its conserved BIR domain to the active sites of
caspases (202). By promoting the
degradation of caspases or separating caspases from their
substrates, IAPs inhibit caspases (202). Recently, dysregulated expression
of IAPs has attracted increased amounts of attention in eye disease
research. c-IAP1 is upregulated early in experimental glaucoma, as
part of the intrinsic neuroprotective mechanism (203). A reduction in the level of c-IAP1
and XIAP secreted by RGCs and the accumulation of TRAF2 result in
increased susceptibility to death in cells in the mature RGC layer
being more susceptible to death (204,205). Previous studies concluded that
ageing impaired the endogenous neuroprotective mechanism of RGCs
evoked by elevated IOP in glaucoma patients, namely, a reduction in
survival signals mediated by IAPs and TRAF (204,205).
Although lowering IOP with ocular hypotensive drops
and surgery are effective treatments for glaucoma, the disease
continues to progress. Thus, therapeutic strategies targeting the
activation of intrinsic and extrinsic apoptotic signalling pathways
for further investigation into the study of the pathogenesis of
glaucoma may provide a novel and practical neuroprotective approach
(230). Specific blockade or
interference with apoptosis is a potential new therapy for the
treatment of glaucoma (Table
II).
Blocking caspase activation is one of the targets
for the treatment of glaucoma. Tahzib et al (231) proposed that if caspase activation
results in prolonged apoptosis in RGCs, then the use of caspase
inhibitors (such as XIAP) to inhibit apoptosis extends the
therapeutic window. The pharmacological inhibitor Z-VDVAD-fmk
(Z-VDVAD) is a small molecular inhibitor and an available agent
that will enter clinical trials. Vigneswara et al (232) reported that Z-VDVAD protected
RGCs from apoptosis after ONC by specifically inhibiting caspase-2
activation but did not promote regeneration of RGC axons. Moreover,
these results resemble those reported by Monnier et al
(233), who used Z-VEID-FMK, a
selective inhibitor of caspase-6, to achieve similar effects on RGC
survival at 14 days after axotomy and promoted axonal regeneration
after ONC. In addition, the caspase-3 inhibitor, Z-DEVD-FMK has
neuroprotective effects and significantly promotes visual recovery
by inhibiting RGC apoptosis when injected 30 min after optic nerve
injury (234). Similarly, the
loss of RGCs was delayed by brain-derived neurotrophic factor, an
inhibitor of caspase-3 (235,236).
Minocycline, which has anti-inflammatory and
antiapoptotic properties is a second-generation tetracycline with
protease inhibitory properties (239). It is considered a candidate
neuroprotective drug for experimental glaucoma and other
neurodegenerative diseases (239). Minocycline has been reported to
reduce the number of activated microglia and improve RGC axon
transport in glaucoma (240).
Minocycline upregulates Bcl-2 expression and downregulates
Bax and Tp53bp2 expression, shifting the balance to
the antiapoptotic direction in experimental glaucoma, and is ready
for clinical trials of acute neurological injury (164,239). A similar drug, Asiatic acid,
ameliorates retinal dysfunction and protects RGCs from ocular
hypertension (241). The
antiapoptotic effect of a small molecule inhibitor
[2,6-diaminopyridine-3,5-bis(thiocyanate) (PR-619)] on RGCs is
mediated by modulation of parkin function and interaction with Bax
and Bcl-2 (242). PR-619 protects
RGCs from glutamate excitotoxicity-induced apoptosis by increasing
the levels of Bcl-2 in RGCs (242). PR-619 regulates
neurodegeneration-related stress by stabilizing the mitochondrial
membrane potential of RGCs, reducing cytotoxicity and apoptosis, as
well as Bax expression. Thus, PR-619 protects RGCs from
glutamate excitotoxicity by enhancing parkin-mediated mitochondrial
autophagy rather than the apoptotic pathway.
Injection of Szeto-Schiller peptide 31 (SS-31) into
a Sprague Dawley rat model of experimental glaucoma had
neuroprotective effects. SS-31 improved the a-wave and b-wave
amplitudes of the ERG and F-VEP amplitude in the eye, upregulated
the level of Bcl-2, downregulated the level of Bax, and inhibited
the release of Cytc (243).
Numerous experimental drugs have demonstrated that upregulating the
expression of Bcl-2 and downregulating the expression of Bax and
caspase-3 are potential methods for protecting RGCs (195,241,244).
IAPs, especially XIAP, are potent caspase
inhibitors and are attractive molecular targets. IAPs significantly
inhibit apoptosis by inhibiting the activity of caspase-9, −3 and
−7 (245). A number of novel
treatments include gene therapy, such as with XIAP, which
offers a new direction in glaucoma treatment. In a rat model of
glaucoma, recombinant adeno-associated viral (AAV) loaded with
XIAP promoted the survival of optic nerve axons (246). Moreover, the expression of the
XIAP prevents IOP elevation by regulating the production of
aqueous humour (246). Other
findings indicated that AAV-loaded XIAP protected both the
structure and function of the axons of RGCs and decreased glial
cell infiltration in a mouse model of glaucoma (247).
In addition, it has been revealed that tacrolimus
inhibits the expression of survivin to reduce cell proliferation
and induce cell apoptosis (248).
A small-molecule survivin inhibitor decreased TGF-β-induced cell
proliferation and migration during the epithelial mesenchymal
transition (249). Thus, survivin
depletion results in TGF-β1 induced cell cycle arrest and apoptosis
and reduces the phosphorylation of retinoblastoma proteins
(249). Therefore, a strategy to
improve survivin expression may be a useful for treating glaucoma.
Recently, ROCK inhibitors have been identified as a new class of
drugs that directly target TM cells to reduce IOP (250,251). In a transgenic mouse model, ROCKi
was reported to reduce IOP by promoting cell proliferation and
phagocytosis of TM cell, reducing actin cross-linking and cell
adhesion interactions (74,250,252).
Currently, two types of ROCKis, namely, ripasudil (K-115) in Japan
and netarsudil (known as AR-13324z), are approved for the clinical
treatment of glaucoma in the United States (253–256). These findings indicated that the
ROCKi may be a new first-line drug for glaucoma treatment.
The use of p53 as a therapeutic target has been
studied in glaucoma. The function the transcription factor
p53 depends on its phosphorylation and dephosphorylation.
Serine/threonine-protein phosphatase-1 has been reported to
directly dephosphorylate p53 to negatively regulate its
transcriptional and apoptotic activities, thus promoting cell
survival (257). Vitamin B1 has
been revealed to significantly reduce p53 expression
(258). Johnson et al
(259) demonstrated that the
adenoviral p53 gene replaced the role of mitomycin C and
5-fluorouracil in glaucoma surgery due to its antiproliferative
properties.
The PI3K/Akt pathway is a pivotal intracellular
signalling pathway that promotes cell proliferation, inhibits
apoptosis and induces angiogenesis by activating multiple
downstream regulatory elements. A sustained decrease in Akt
activation was observed in the ocular-hypertensive retina and optic
nerve of a rat model of glaucoma induced by injecting hypertonic
saline into the limbal veins (260). Factors that activate the PI3K/Akt
pathway constitute a novel molecular therapy for glaucoma (Fig. 2). Yan et al (67) demonstrated that CD9-knockdown
significantly reduced the expression of ITGA4, p-PI3K and p-Akt and
increased the apoptotic activity of TM cells, which was increased
dramatically in the CD9-overexpressing group. Knockdown of ITGA4
rescued p-PI3K and Akt expression, suggesting that overexpression
of CD9 activates the ITGA4/PI3K/Akt axis to attenuate TM cell
apoptosis in human glaucoma (67).
Moreover, several traditional Chinese medicines may
suppress glaucoma pathogenesis by activating PI3K/Akt signalling
both in vitro and in vivo. Husain et al
(260) reported that the δ-opioid
receptor agonist SNC-121 significantly increased the ERG amplitude
and RGC number in a rat model of chronic glaucoma by activating the
PI3K/Akt pathway. Moreover, baicalein inhibits NMDA-induced
apoptosis, autophagy and oxidative stress in RGCs by activating the
PI3K/Akt signalling pathway in vitro and in vivo
(261). A mouse model of glaucoma
induced by injection of NMDA confirmed that baicalin inhibited
autophagy and subsequently attenuated pathological changes in
retinal tissues by activating PI3K/Akt signalling (261). Furthermore, ligustrazine
increased protein levels of p-PI3K, p-Akt and p-mTOR in a rat model
of chronic hypertensive glaucoma, while rapamycin or Ly294002
attenuated these changes (139).
A recent study revealed that acteoside may be a potential
protective drug for maintaining RGC homeostasis and preventing
glaucoma-related blindness because of its therapeutic effects, such
as antioxidative stress, anti-inflammatory, anti-aging and
proliferative effects (262).
In addition to drugs, several microRNAs mediate
apoptosis in glaucoma through the PI3K/Akt pathway. miR-145-5p
suppresses TRIM2 expression by targeting the 3′-untranslated region
of TRIM2. Inhibition of miR-145-5p promotes cell survival and
suppresses RGCs apoptosis by activating the TRIM2/PI3K/Akt
signalling pathway (140).
miR-149 inhibition also promoted the viability of RGCs and
inhibited RGC apoptosis in a mouse model of glaucoma by increasing
the levels of BTC, p-PI3K and p-Akt (263).
Glaucoma is a complex group of eye diseases that
involve degeneration of the retinal nerve. The clinical symptoms of
glaucoma are aggravated with age, resulting in partial blindness or
lifelong blindness in patients with glaucoma and severely
influencing quality of life. In the early stages of glaucoma,
patients are usually treated with eye-drop drugs, such as
prostaglandin analogues or β-adrenergic antagonists. Although drugs
reduce IOP to some extent, they cannot maintain long-term
effectiveness or avoid producing side effects. Currently, the most
promising treatment for glaucoma is surgical intervention as the
primary treatment and medication as an adjunct. However, some
surgical treatments, including filtration surgery, still have
drawbacks, as visual function damage continues to progress.
There has been significant progress in
understanding the pathogenesis of glaucoma in the recent years and
several genes such as MYOC and CYP1B1, and epigenetic
regulators, have been found to be involved in this disease.
Additionally, an abundance of literature indicated that apoptosis
plays a vital role in glaucoma onset, development and prognosis and
that novel apoptosis-targeted strategies are promising approaches
for treating glaucoma. Furthermore, survival signals that activate
the PI3K/Akt pathway may lead to novel treatment options for
glaucoma. Among drugs and gene therapies, targeting apoptosis is
feasible in animal models and preclinical experiments. However,
further proof of the feasibility of treatment is needed.
Thus, there are several remaining problems to be
solved, for example: i) How can specific mechanisms and molecular
changes involved in glaucoma apoptosis be further explained? ii)
Identification of specific apoptotic genes or pathways that inhibit
apoptosis is essential. iii) How can the effect of existing drugs
or gene therapy on the inhibition of apoptosis last longer and be
more stable? iv) How can the balance between proapoptotic and
antiapoptotic signals be addressed? Future research will combine
mechanism-based assays with improved detection methods to further
explore changes in apoptotic proteins and their interrelationships
and identify targets for inhibiting cell apoptosis. Furthermore,
the necessary combination of interventions to maintain a subtly
inclined balance while preventing apoptosis may achieve long-term
and effective protection in the treatment of glaucoma.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 81470667), the Department of Science
and Technology of Sichuan (grant no. 2020YFS0435), the Health Care
of Sichuan Provincial cadres (grant no. 2020-227) and the Science
& Technology Bureau of Chengdu (grant nos. YF05-00198-SN and
YF05-00060-SN).
Not applicable.
QX designed and completed the editing of the
manuscript and created the figures and tables. QX and DZ revised
the manuscript. Both authors read and approved the final version of
the manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Qiongrong Xia, ORCID: 0000-0001-8642-9139.
|
1
|
GBD 2019 Blindness and Vision Impairment
Collaborators: Vision Loss Expert Group of the Global Burden of
Disease Study, . Causes of blindness and vision impairment in 2020
and trends over 30 years, and prevalence of avoidable blindness in
relation to VISION 2020: The right to sight: An analysis for the
global burden of disease study. Lancet Glob Health. 9:e144–e160.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tham YC, Li X, Wong TY, Quigley HA, Aung T
and Cheng CY: Global prevalence of glaucoma and projections of
glaucoma burden through 2040: A systematic review and
meta-analysis. Ophthalmology. 121:2081–2090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang JM and Tanna AP: Glaucoma. Med Clin
North Am. 105:493–510. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quigley HA: Glaucoma. Lancet.
377:1367–1377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burgoyne CF, Downs JC, Bellezza AJ, Suh JK
and Hart RT: The optic nerve head as a biomechanical structure: A
new paradigm for understanding the role of IOP-related stress and
strain in the pathophysiology of glaucomatous optic nerve head
damage. Prog Retin Eye Res. 24:39–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weinreb RN, Aung T and Medeiros FA: The
pathophysiology and treatment of glaucoma: A review. JAMA.
311:1901–1911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L and Song F: Biomechanical research
into lamina cribrosa in glaucoma. Natl Sci Rev. 7:1277–1279. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marcus MW, de Vries MM, Junoy Montolio FG
and Jansonius NM: Myopia as a risk factor for open-angle glaucoma:
A systematic review and meta-analysis. Ophthalmology.
118:1989–1994.e2. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ha A, Kim CY, Shim SR, Chang IB and Kim
YK: Degree of myopia and glaucoma risk: A dose-response
meta-analysis. Am J Ophthalmol. 236:107–119. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fong DS, Epstein DL and Allingham RR:
Glaucoma and myopia: Are they related? Int Ophthalmol Clin.
30:215–218. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saw SM, Gazzard G, Shih-Yen EC and Chua
WH: Myopia and associated pathological complications. Ophthalmic
Physiol Opt. 25:381–391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Juliano J, Burkemper B, Lee J, Nelson A,
LeTran V, Chu Z, Zhou G, Jiang X, Wang RK, Varma R and Richter GM:
Longer axial length potentiates relationship of intraocular
pressure and peripapillary vessel density in glaucoma patients.
Invest Ophthalmol Vis Sci. 62:372021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren R, Wang N, Li B, Li L, Gao F, Xu X and
Jonas JB: Lamina cribrosa and peripapillary sclera histomorphometry
in normal and advanced glaucomatous Chinese eyes with various axial
length. Invest Ophthalmol Vis Sci. 50:2175–2184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim KE and Park KH: Update on the
prevalence, etiology, diagnosis, and monitoring of normal-tension
glaucoma. Asia Pac J Ophthalmol (Phila). 5:23–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Killer HE and Pircher A: Normal tension
glaucoma: Review of current understanding and mechanisms of the
pathogenesis. Eye (Lond). 32:924–930. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mi XS, Yuan TF and So KF: The current
research status of normal tension glaucoma. Clin Interv Aging.
9:1563–1571. 2014.PubMed/NCBI
|
|
17
|
Chitranshi N, Rajput R, Godinez A,
Pushpitha K, Mirzaei M, Basavarajappa D, Gupta V, Sharma S, You Y,
Galliciotti G, et al: Neuroserpin gene therapy inhibits retinal
ganglion cell apoptosis and promotes functional preservation in
glaucoma. Mol Ther. 31:2056–2076. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miglior S, Torri V, Zeyen T, Pfeiffer N,
Vaz JC and Adamsons I; EGPS Group, : Intercurrent factors
associated with the development of open-angle glaucoma in the
European glaucoma prevention study. Am J Ophthalmol. 144:266–275.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzuki Y, Iwase A, Araie M, Yamamoto T,
Abe H, Shirato S, Kuwayama Y, Mishima HK, Shimizu H, Tomita G, et
al: Risk factors for open-angle glaucoma in a Japanese population:
The Tajimi study. Ophthalmology. 113:1613–1617. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saccà SC and Izzotti A: Oxidative stress
and glaucoma: Injury in the anterior segment of the eye. Prog Brain
Res. 173:385–407. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Flammer J and Mozaffarieh M: What is the
present pathogenetic concept of glaucomatous optic neuropathy? Surv
Ophthalmol. 52 (Suppl 2):S162–S173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen WC, Huang BQ and Yang J: Regulatory
mechanisms of retinal ganglion cell death in normal tension
glaucoma and potential therapies. Neural Regen Res. 18:87–93. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fleisher TA: Apoptosis. Ann Allergy Asthma
Immunol. 78:245–250. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li C, Liu W, Wang F, Hayashi T, Mizuno K,
Hattori S, Fujisaki H and Ikejima T: DNA damage-triggered
activation of cGAS-STING pathway induces apoptosis in human
keratinocyte HaCaT cells. Mol Immunol. 131:180–190. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu J, Sun W, Song Y, Liu J, Xue F, Gong K,
Yang X and Kang Q: SIRT6 protects retinal ganglion cells against
hydrogen peroxide-induced apoptosis and oxidative stress by
promoting Nrf2/ARE signaling via inhibition of Bach1. Chem Biol
Interact. 300:151–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Osakue D, Yang E, Zhou Y, Gong H,
Xia X and Du Y: Endoplasmic reticulum stress response of trabecular
meshwork stem cells and trabecular meshwork cells and protective
effects of activated PERK pathway. Invest Ophthalmol Vis Sci.
60:265–273. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang M, Tan J, Miao Y, Li M and Zhang Q:
Role of Ca2+ and ion channels in the regulation of
apoptosis under hypoxia. Histol Histopathol. 33:237–246.
2018.PubMed/NCBI
|
|
30
|
Liu Z, Fu G and Liu A: The relationship
between inflammatory mediator expression in the aqueous humor and
secondary glaucoma incidence after silicone oil tamponade. Exp Ther
Med. 14:5833–5836. 2017.PubMed/NCBI
|
|
31
|
Saraste A and Pulkki K: Morphologic and
biochemical hallmarks of apoptosis. Cardiovasc Res. 45:528–537.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Geske FJ and Gerschenson LE: The biology
of apoptosis. Hum Pathol. 32:1029–1038. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baleriola J, García-Feijoo J,
Martínez-de-la-Casa JM, Fernández-Cruz A, de la Rosa EJ and
Fernández-Durango R: Apoptosis in the trabecular meshwork of
glaucomatous patients. Mol Vis. 14:1513–1516. 2008.PubMed/NCBI
|
|
34
|
Galvao J, Davis BM and Cordeiro MF: In
vivo imaging of retinal ganglion cell apoptosis. Curr Opin
Pharmacol. 13:123–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Krishnan A, Kocab AJ, Zacks DN,
Marshak-Rothstein A and Gregory-Ksander M: A small peptide
antagonist of the Fas receptor inhibits neuroinflammation and
prevents axon degeneration and retinal ganglion cell death in an
inducible mouse model of glaucoma. J Neuroinflammation. 16:1842019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen X, Lei F, Zhou C, Chodosh J, Wang L,
Huang Y, Dohlman CH and Paschalis EI: Glaucoma after ocular surgery
or trauma: The role of infiltrating monocytes and their response to
cytokine inhibitors. Am J Pathol. 190:2056–2066. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dohlman CH, Zhou C, Lei F, Cade F,
Regatieri CV, Črnej A, Dohlman JG, Shen LQ and Paschalis EI:
Glaucoma after corneal trauma or surgery-A rapid, inflammatory,
IOP-independent pathway. Cornea. 38:1589–1594. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rasmussen CA, Kaufman PL and Kiland JA:
Benzalkonium chloride and glaucoma. J Ocul Pharmacol Ther.
30:163–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Almasieh M, Wilson AM, Morquette B, Cueva
Vargas JL and Di Polo A: The molecular basis of retinal ganglion
cell death in glaucoma. Prog Retin Eye Res. 31:152–181. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu A, Khawaja AP, Pasquale LR and Stein
JD: A review of systemic medications that may modulate the risk of
glaucoma. Eye (Lond). 34:12–28. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hamard P, Blondin C, Debbasch C, Warnet
JM, Baudouin C and Brignole F: In vitro effects of preserved and
unpreserved antiglaucoma drugs on apoptotic marker expression by
human trabecular cells. Graefes Arch Clin Exp Ophthalmol.
241:1037–1043. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gedde SJ, Schiffman JC, Feuer WJ, Herndon
LW, Brandt JD and Budenz DL; Tube versus Trabeculectomy Study
Group, : Treatment outcomes in the tube versus trabeculectomy (TVT)
study after five years of follow-up. Am J Ophthalmol.
153:789–803.e2. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Javaid U, Ali MH, Jamal S and Butt NH:
Pathophysiology, diagnosis, and management of glaucoma associated
with Sturge-Weber syndrome. Int Ophthalmol. 38:409–416.
2018.PubMed/NCBI
|
|
44
|
Yoon PS and Singh K: Update on
antifibrotic use in glaucoma surgery, including use in
trabeculectomy and glaucoma drainage implants and combined cataract
and glaucoma surgery. Curr Opin Ophthalmol. 15:141–146. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Paschalis EI, Lei F, Zhou C, Chen XN,
Kapoulea V, Hui PC, Dana R, Chodosh J, Vavvas DG and Dohlman CH:
Microglia regulate neuroglia remodeling in various ocular and
retinal injuries. J Immunol. 202:539–549. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cade F, Paschalis EI, Regatieri CV, Vavvas
DG, Dana R and Dohlman CH: Alkali burn to the eye: Protection using
TNF-α inhibition. Cornea. 33:382–389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ju KR, Kim HS, Kim JH, Lee NY and Park CK:
Retinal glial cell responses and Fas/FasL activation in rats with
chronic ocular hypertension. Brain Res. 1122:209–221. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Quigley HA, Nickells RW, Kerrigan LA,
Pease ME, Thibault DJ and Zack DJ: Retinal ganglion cell death in
experimental glaucoma and after axotomy occurs by apoptosis. Invest
Ophthalmol Vis Sci. 36:774–786. 1995.PubMed/NCBI
|
|
49
|
Libby RT, Li Y, Savinova OV, Barter J,
Smith RS, Nickells RW and John SW: Susceptibility to
neurodegeneration in a glaucoma is modified by Bax gene dosage.
PLoS Genet. 1:17–26. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kerrigan LA, Zack DJ, Quigley HA, Smith SD
and Pease ME: TUNEL-positive ganglion cells in human primary
open-angle glaucoma. Arch Ophthalmol. 115:1031–1035. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tök L, Nazıroğlu M, Uğuz AC and Tök O:
Elevated hydrostatic pressures induce apoptosis and oxidative
stress through mitochondrial membrane depolarization in PC12
neuronal cells: A cell culture model of glaucomaz: A cell culture
model of glaucoma. J Recept Signal Transduct Res. 34:410–416. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Erisgin Z, Ozer MA, Tosun M, Ozen S and
Takir S: The effects of intravitreal H2 S application on
apoptosis in the retina and cornea in experimental glaucoma model.
Int J Exp Pathol. 100:330–336. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ying Y, Xue R, Yang Y, Zhang SX, Xiao H,
Zhu H, Li J, Chen G, Ye Y, Yu M, et al: Activation of ATF4 triggers
trabecular meshwork cell dysfunction and apoptosis in POAG. Aging
(Albany NY). 13:8628–8642. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Saccà SC, Pulliero A and Izzotti A: The
dysfunction of the trabecular meshwork during glaucoma course. J
Cell Physiol. 230:510–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Saccà SC, Gandolfi S, Bagnis A, Manni G,
Damonte G, Traverso CE and Izzotti A: From DNA damage to functional
changes of the trabecular meshwork in aging and glaucoma. Ageing
Res Rev. 29:26–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ammar DA and Kahook MY: Effects of
benzalkonium chloride- or polyquad-preserved fixed combination
glaucoma medications on human trabecular meshwork cells. Mol Vis.
17:1806–1813. 2011.PubMed/NCBI
|
|
57
|
Goldstein MH, Silva FQ, Blender N, Tran T
and Vantipalli S: Ocular benzalkonium chloride exposure: Problems
and solutions. Eye (Lond). 36:361–368. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Baudouin C, Kolko M, Melik-Parsadaniantz S
and Messmer EM: Inflammation in glaucoma: From the back to the
front of the eye, and beyond. Prog Retin Eye Res. 83:1009162021.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liton PB and Gonzalez P: Stress response
of the trabecular meshwork. J Glaucoma. 17:378–385. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rohen JW, Lütjen-Drecoll E, Flügel C,
Meyer M and Grierson I: Ultrastructure of the trabecular meshwork
in untreated cases of primary open-angle glaucoma (POAG). Exp Eye
Res. 56:683–692. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Stamer WD and Acott TS: Current
understanding of conventional outflow dysfunction in glaucoma. Curr
Opin Ophthalmol. 23:135–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yan X, Wu S, Liu Q, Li Y, Zhu W and Zhang
J: Accumulation of Asn450Tyr mutant myocilin in ER promotes
apoptosis of human trabecular meshwork cells. Mol Vis. 26:563–573.
2020.PubMed/NCBI
|
|
63
|
Agarwal R, Talati M, Lambert W, Clark AF,
Wilson SE, Agarwal N and Wordinger RJ: Fas-activated apoptosis and
apoptosis mediators in human trabecular meshwork cells. Exp Eye
Res. 68:583–590. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Suri F, Yazdani S and Elahi E: LTBP2
knockdown and oxidative stress affect glaucoma features including
TGFβ pathways, ECM genes expression and apoptosis in trabecular
meshwork cells. Gene. 673:70–81. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Oritani K, Aoyama K, Tomiyama Y, Kincade
PW and Matsuzawa Y: Stromal cell CD9 and the differentiation of
hematopoietic stem/progenitor cells. Leuk Lymphoma. 38:147–152.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jiang X, Teng M, Ji R, Zhang D, Zhang Z,
Lv Y, Zhang Q, Zhang J and Huang Y: CD9 regulates keratinocyte
differentiation and motility by recruiting E-cadherin to the plasma
membrane and activating the PI3K/Akt pathway. Biochim Biophys Acta
Mol Cell Res. 1867:1185742020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yan J, Yang X, Jiao X, Yang X, Guo M, Chen
Y, Zhan L and Chen W: Integrative transcriptomic and proteomic
analysis reveals CD9/ITGA4/PI3K-Akt axis mediates trabecular
meshwork cell apoptosis in human glaucoma. J Cell Mol Med.
24:814–829. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang Y, Li F and Wang S: MicroRNA-93 is
overexpressed and induces apoptosis in glaucoma trabecular meshwork
cells. Mol Med Rep. 14:5746–5750. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shen Y, Zhu Y and Rong F: miR-200c-3p
regulates the proliferation and apoptosis of human trabecular
meshwork cells by targeting PTEN. Mol Med Rep. 22:1605–1612. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang X, Li Z, Bai J, Song W and Zhang F:
miR-17-5p regulates the proliferation and apoptosis of human
trabecular meshwork cells by targeting phosphatase and tensin
homolog. Mol Med Rep. 19:3132–3138. 2019.PubMed/NCBI
|
|
71
|
Wang Y, Zhou H, Liu X, Han Y, Pan S and
Wang Y: MiR-181a inhibits human trabecular meshwork cell apoptosis
induced by H2O2 through the suppression of
NF-κB and JNK pathways. Adv Clin Exp Med. 27:577–582. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang K, Read AT, Sulchek T and Ethier CR:
Trabecular meshwork stiffness in glaucoma. Exp Eye Res. 158:3–12.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Fuchshofer R and Tamm ER: The role of
TGF-β in the pathogenesis of primary open-angle glaucoma. Cell
Tissue Res. 347:279–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang J, Liu X and Zhong Y:
Rho/Rho-associated kinase pathway in glaucoma (review). Int J
Oncol. 43:1357–1367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Vernazza S, Tirendi S, Passalacqua M,
Piacente F, Scarfì S, Oddone F and Bassi AM: An innovative in vitro
open-angle glaucoma model (IVOM) shows changes induced by increased
ocular pressure and oxidative stress. Int J Mol Sci. 22:121292021.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Soto I and Howell GR: The complex role of
neuroinflammation in glaucoma. Cold Spring Harb Perspect Med.
4:a0172692014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ebneter A, Casson RJ, Wood JP and Chidlow
G: Microglial activation in the visual pathway in experimental
glaucoma: Spatiotemporal characterization and correlation with
axonal injury. Invest Ophthalmol Vis Sci. 51:6448–6460. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bordone MP, González Fleitas MF, Pasquini
LA, Bosco A, Sande PH, Rosenstein RE and Dorfman D: Involvement of
microglia in early axoglial alterations of the optic nerve induced
by experimental glaucoma. J Neurochem. 142:323–337. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Guo L, Moss SE, Alexander RA, Ali RR,
Fitzke FW and Cordeiro MF: Retinal ganglion cell apoptosis in
glaucoma is related to intraocular pressure and IOP-induced effects
on extracellular matrix. Invest Ophthalmol Vis Sci. 46:175–182.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bosco A, Steele MR and Vetter ML: Early
microglia activation in a mouse model of chronic glaucoma. J Comp
Neurol. 519:599–620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bosco A, Romero CO, Breen KT, Chagovetz
AA, Steele MR, Ambati BK and Vetter ML: Neurodegeneration severity
can be predicted from early microglia alterations monitored in vivo
in a mouse model of chronic glaucoma. Dis Model Mech. 8:443–455.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Williams PA, Marsh-Armstrong N and Howell
GR; Lasker/IRRF Initiative on Astrocytes and Glaucomatous
Neurodegeneration Participants, : Neuroinflammation in glaucoma: A
new opportunity. Exp Eye Res. 157:20–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tribble JR, Kokkali E, Otmani A, Plastino
F, Lardner E, Vohra R, Kolko M, André H, Morgan JE and Williams PA:
When is a control not a control? Reactive microglia occur
throughout the control contralateral pathway of retinal ganglion
cell projections in experimental glaucoma. Transl Vis Sci Technol.
10:222021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Unlu M, Aktas Z, Gocun PU, Ilhan SO,
Hasanreisoglu M and Hasanreisoglu B: Neuroprotective effect of
systemic and/or intravitreal rosuvastatin administration in rat
glaucoma model. Int J Ophthalmol. 9:340–347. 2016.PubMed/NCBI
|
|
85
|
Dyka FM, May CA and Enz R: Metabotropic
glutamate receptors are differentially regulated under elevated
intraocular pressure. J Neurochem. 90:190–202. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wu X, Dou YN, Fei Z and Fei F: Parkin
prevents glutamate excitotoxicity through inhibiting NLRP3
inflammasome in retinal ganglion cells. Neuroscience. 478:1–10.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Murphy G, Knäuper V, Lee MH, Amour A,
Worley JR, Hutton M, Atkinson S, Rapti M and Williamson R: Role of
TIMPs (tissue inhibitors of metalloproteinases) in pericellular
proteolysis: The specificity is in the detail. Biochem Soc Symp.
65–80. 2003.PubMed/NCBI
|
|
88
|
Mathew DJ, Livne-Bar I and Sivak JM: An
inducible rodent glaucoma model that exhibits gradual sustained
increase in intraocular pressure with distinct inner retina and
optic nerve inflammation. Sci Rep. 11:228802021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sakata R, Ueno T, Nakamura T, Ueno H and
Sata M: Mechanical stretch induces TGF-beta synthesis in hepatic
stellate cells. Eur J Clin Invest. 34:129–136. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kirwan RP, Crean JK, Fenerty CH, Clark AF
and O'Brien CJ: Effect of cyclical mechanical stretch and exogenous
transforming growth factor-beta1 on matrix metalloproteinase-2
activity in lamina cribrosa cells from the human optic nerve head.
J Glaucoma. 13:327–334. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gomes LR, Terra LF, Wailemann RA, Labriola
L and Sogayar MC: TGF-β1 modulates the homeostasis between MMPs and
MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive
breast cancer cells. BMC Cancer. 12:262012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cordeiro MF, Bhattacharya SS, Schultz GS
and Khaw PT: TGF-beta1, -beta2, and -beta3 in vitro: Biphasic
effects on Tenon's fibroblast contraction, proliferation, and
migration. Invest Ophthalmol Vis Sci. 41:756–763. 2000.PubMed/NCBI
|
|
93
|
Nguyen TTM, Gillet G and Popgeorgiev N:
Caspases in the developing central nervous system: Apoptosis and
beyond. Front Cell Dev Biol. 9:7024042021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem J. 326:1–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Abate M, Festa A, Falco M, Lombardi A,
Luce A, Grimaldi A, Zappavigna S, Sperlongano P, Irace C, Caraglia
M and Misso G: Mitochondria as playmakers of apoptosis, autophagy
and senescence. Semin Cell Dev Biol. 98:139–153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Alapati T, Sagal KM, Gudiseva HV, Pistilli
M, Pyfer M, Chavali VRM and O'Brien JM: Evaluating TNF-α and
interleukin-2 (IL-2) levels in African American primary open-angle
glaucoma patients. Genes (Basel). 13:542021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wilson NS, Dixit V and Ashkenazi A: Death
receptor signal transducers: Nodes of coordination in immune
signaling networks. Nat Immunol. 10:348–355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Mahdizadeh SJ, Thomas M and Eriksson LA:
Reconstruction of the Fas-based death-inducing signaling complex
(DISC) using a protein-protein docking meta-approach. J Chem Inf
Model. 61:3543–3558. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Hillert-Richter LK and Lavrik IN:
Measuring composition of CD95 death-inducing signaling complex and
processing of procaspase-8 in this complex. J Vis Exp. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yang XJ, Ge J and Zhuo YH: Role of
mitochondria in the pathogenesis and treatment of glaucoma. Chin
Med J (Engl). 126:4358–4365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zeng Z, You M, Fan C, Rong R, Li H and Xia
X: Pathologically high intraocular pressure induces mitochondrial
dysfunction through Drp1 and leads to retinal ganglion cell
PANoptosis in glaucoma. Redox Biol. 62:1026872023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bock FJ and Tait SWG: Mitochondria as
multifaceted regulators of cell death. Nat Rev Mol Cell Biol.
21:85–100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Saelens X, Festjens N, Vande Walle L, van
Gurp M, van Loo G and Vandenabeele P: Toxic proteins released from
mitochondria in cell death. Oncogene. 23:2861–2874. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Shakeri R, Kheirollahi A and Davoodi J:
Apaf-1: Regulation and function in cell death. Biochimie.
135:111–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kale J, Osterlund EJ and Andrews DW: BCL-2
family proteins: Changing partners in the dance towards death. Cell
Death Differ. 25:65–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Leibowitz B and Yu J: Mitochondrial
signaling in cell death via the Bcl-2 family. Cancer Biol Ther.
9:417–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ding J, Mooers BHM, Zhang Z, Kale J,
Falcone D, McNichol J, Huang B, Zhang XC, Xing C, Andrews DW and
Lin J: After embedding in membranes antiapoptotic Bcl-XL protein
binds both Bcl-2 homology region 3 and helix 1 of proapoptotic Bax
protein to inhibit apoptotic mitochondrial permeabilization. J Biol
Chem. 289:11873–11896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Luna-Vargas MPA and Chipuk JE: The deadly
landscape of pro-apoptotic BCL-2 proteins in the outer
mitochondrial membrane. FEBS J. 283:2676–2689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Huang DC and Strasser A: BH3-Only
proteins-essential initiators of apoptotic cell death. Cell.
103:839–842. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Bano D and Prehn JHM: Apoptosis-inducing
factor (AIF) in physiology and disease: The tale of a repented
natural born killer. EBioMedicine. 30:29–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Porat S and Simantov R: Bcl-2 and p53:
Role in dopamine-induced apoptosis and differentiation. Ann N Y
Acad Sci. 893:372–375. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Sawada O, Perusek L, Kohno H, Howell SJ,
Maeda A, Matsuyama S and Maeda T: All-trans-retinal induces Bax
activation via DNA damage to mediate retinal cell apoptosis. Exp
Eye Res. 123:27–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wang Y, Okan I, Szekely L, Klein G and
Wiman KG: bcl-2 inhibits wild-type p53-triggered apoptosis but not
G1 cell cycle arrest and transactivation of WAF1 and bax. Cell
Growth Differ. 6:1071–1075. 1995.PubMed/NCBI
|
|
115
|
Chylicki K, Ehinger M, Svedberg H and
Gullberg U: Characterization of the molecular mechanisms for
p53-mediated differentiation. Cell Growth Differ. 11:561–571.
2000.PubMed/NCBI
|
|
116
|
Oakes SA and Papa FR: The role of
endoplasmic reticulum stress in human pathology. Annu Rev Pathol.
10:173–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Hetz C, Zhang K and Kaufman RJ:
Mechanisms, regulation and functions of the unfolded protein
response. Nat Rev Mol Cell Biol. 21:421–438. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Lafleur MA, Stevens JL and Lawrence JW:
Xenobiotic perturbation of ER stress and the unfolded protein
response. Toxicol Pathol. 41:235–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Urano F, Wang X, Bertolotti A, Zhang Y,
Chung P, Harding HP and Ron D: Coupling of stress in the ER to
activation of JNK protein kinases by transmembrane protein kinase
IRE1. Science. 287:664–666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kwong JMK and Caprioli J: Expression of
phosphorylated c-Jun N-terminal protein kinase (JNK) in
experimental glaucoma in rats. Exp Eye Res. 82:576–582. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Hwang J and Qi L: Quality control in the
endoplasmic reticulum: Crosstalk between ERAD and UPR pathways.
Trends Biochem Sci. 43:593–605. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Maurel M, Chevet E, Tavernier J and Gerlo
S: Getting RIDD of RNA: IRE1 in cell fate regulation. Trends
Biochem Sci. 39:245–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Hayashi T, Saito A, Okuno S, Ferrand-Drake
M, Dodd RL and Chan PH: Oxidative injury to the endoplasmic
reticulum in mouse brains after transient focal ischemia. Neurobiol
Dis. 15:229–239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Wek RC, Jiang HY and Anthony TG: Coping
with stress: eIF2 kinases and translational control. Biochem Soc
Trans. 34:7–11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Peters JC, Bhattacharya S, Clark AF and
Zode GS: Increased endoplasmic reticulum stress in human
glaucomatous trabecular meshwork cells and tissues. Invest
Ophthalmol Vis Sci. 56:3860–3868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Marola OJ, Syc-Mazurek SB and Libby RT:
DDIT3 (CHOP) contributes to retinal ganglion cell somal loss but
not axonal degeneration in DBA/2J mice. Cell Death Discov.
5:1402019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kasetti RB, Patel PD, Maddineni P, Patil
S, Kiehlbauch C, Millar JC, Searby CC, Raghunathan V, Sheffield VC
and Zode GS: ATF4 leads to glaucoma by promoting protein synthesis
and ER client protein load. Nat Commun. 11:55942020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Doh SH, Kim JH, Lee KM, Park HY and Park
CK: Retinal ganglion cell death induced by endoplasmic reticulum
stress in a chronic glaucoma model. Brain Res. 1308:158–166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Yoshida H, Matsui T, Yamamoto A, Okada T
and Mori K: XBP1 mRNA is induced by ATF6 and spliced by IRE1 in
response to ER stress to produce a highly active transcription
factor. Cell. 107:881–891. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Watanabe M, Ida Y, Furuhashi M, Tsugeno Y,
Ohguro H and Hikage F: Screening of the drug-induced effects of
prostaglandin EP2 and FP agonists on 3D cultures of
dexamethasone-treated human trabecular meshwork cells.
Biomedicines. 9:9302021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Lee EJ, Chan P, Chea L, Kim K, Kaufman RJ
and Lin JH: ATF6 is required for efficient rhodopsin clearance and
retinal homeostasis in the P23H rho retinitis pigmentosa mouse
model. Sci Rep. 11:163562021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Julien O and Wells JA: Caspases and their
substrates. Cell Death Differ. 24:1380–1389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Yuan J, Shaham S, Ledoux S, Ellis HM and
Horvitz HR: The C. elegans cell death gene ced-3 encodes a protein
similar to mammalian interleukin-1 beta-converting enzyme. Cell.
75:641–652. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Van Opdenbosch N and Lamkanfi M: Caspases
in cell death, inflammation, and disease. Immunity. 50:1352–1364.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Ramirez MLG and Salvesen GS: A primer on
caspase mechanisms. Semin Cell Dev Biol. 82:79–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Man SM, Karki R and Kanneganti TD:
Molecular mechanisms and functions of pyroptosis, inflammatory
caspases and inflammasomes in infectious diseases. Immunol Rev.
277:61–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Ye D, Xu Y, Shi Y, Fan M, Lu P, Bai X,
Feng Y, Hu C, Cui K, Tang X, et al: Anti-PANoptosis is involved in
neuroprotective effects of melatonin in acute ocular hypertension
model. J Pineal Res. 73:e128282022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Du HY, Wang R, Li JL, Luo H, Xie XY, Yan
R, Jian YL and Cai JY: Ligustrazine protects against chronic
hypertensive glaucoma in rats by inhibiting autophagy via the
PI3K-Akt/mTOR pathway. Mol Vis. 27:725–733. 2021.PubMed/NCBI
|
|
140
|
Xu K, Li S, Yang Q, Zhou Z, Fu M, Yang X,
Hao K, Liu Y and Ji H: MicroRNA-145-5p targeting of TRIM2 mediates
the apoptosis of retinal ganglion cells via the PI3K/AKT signaling
pathway in glaucoma. J Gene Med. 23:e33782021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Li R, Jin Y, Li Q, Sun X, Zhu H and Cui H:
MiR-93-5p targeting PTEN regulates the NMDA-induced autophagy of
retinal ganglion cells via AKT/mTOR pathway in glaucoma. Biomed
Pharmacother. 100:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Tatton WG, Chalmers-Redman RM, Sud A,
Podos SM and Mittag TW: Maintaining mitochondrial membrane
impermeability. An opportunity for new therapy in glaucoma? Surv
Ophthalmol. 45 (Suppl 3):S277–S283. S295–S296. 2001.PubMed/NCBI
|
|
143
|
Miano M, Madeo A, Cappelli E, Lanza F,
Lanza T, Stroppiano M, Terranova P, Venè R, Bleesing JJH and Di
Rocco M: Defective FAS-mediated apoptosis and immune dysregulation
in gaucher disease. J Allergy Clin Immunol Pract. 8:3535–3542.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Li-Weber M and Krammer PH: Function and
regulation of the CD95 (APO-1/Fas) ligand in the immune system.
Semin Immunol. 15:145–157. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Levoin N, Jean M and Legembre P: CD95
structure, aggregation and cell signaling. Front Cell Dev Biol.
8:3142020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Guégan JP and Legembre P: Nonapoptotic
functions of Fas/CD95 in the immune response. FEBS J. 285:809–827.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Krishnan A, Fei F, Jones A, Busto P,
Marshak-Rothstein A, Ksander BR and Gregory-Ksander M:
Overexpression of soluble fas ligand following adeno-associated
virus gene therapy prevents retinal ganglion cell death in chronic
and acute murine models of glaucoma. J Immunol. 197:4626–4638.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Gregory-Ksander M and Marshak-Rothstein A:
The FasLane to ocular pathology-metalloproteinase cleavage of
membrane-bound FasL determines FasL function. J Leukoc Biol.
110:965–977. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Razeghinejad MR and Kamali-Sarvestani E:
Aqueous humor levels of soluble Fas and Fas-ligand in patients with
primary open angle and pseudoexfoliation glaucoma. Iran J Immunol.
4:215–219. 2007.PubMed/NCBI
|
|
150
|
Gregory MS, Hackett CG, Abernathy EF, Lee
KS, Saff RR, Hohlbaum AM, Moody KS, Hobson MW, Jones A, Kolovou P,
et al: Opposing roles for membrane bound and soluble Fas ligand in
glaucoma-associated retinal ganglion cell death. PLoS One.
6:e176592011. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
O' Reilly LA, Tai L, Lee L, Kruse EA,
Grabow S, Fairlie WD, Haynes NM, Tarlinton DM, Zhang JG, Belz GT,
et al: Membrane-bound Fas ligand only is essential for Fas-induced
apoptosis. Nature. 461:659–663. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Wax MB, Tezel G, Yang J, Peng G, Patil RV,
Agarwal N, Sappington RM and Calkins DJ: Induced autoimmunity to
heat shock proteins elicits glaucomatous loss of retinal ganglion
cell neurons via activated T-cell-derived fas-ligand. J Neurosci.
28:12085–12096. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Besirli CG, Chinskey ND, Zheng QD and
Zacks DN: Inhibition of retinal detachment-induced apoptosis in
photoreceptors by a small peptide inhibitor of the fas receptor.
Invest Ophthalmol Vis Sci. 51:2177–2184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Aoki K, Kurooka M, Chen JJ, Petryniak J,
Nabel EG and Nabel GJ: Extracellular matrix interacts with soluble
CD95L: Retention and enhancement of cytotoxicity. Nat Immunol.
2:333–337. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
155
|
Bai Y, Shi Z, Zhuo Y, Liu J, Malakhov A,
Ko E, Burgess K, Schaefer H, Esteban PF, Tessarollo L and Saragovi
HU: In glaucoma the upregulated truncated TrkC.T1 receptor isoform
in glia causes increased TNF-alpha production, leading to retinal
ganglion cell death. Invest Ophthalmol Vis Sci. 51:6639–6651. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Aggarwal BB, Gupta SC and Kim JH:
Historical perspectives on tumor necrosis factor and its
superfamily: 25 Years later, a golden journey. Blood. 119:651–665.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Kelker HC, Oppenheim JD, Stone-Wolff D,
Henriksen-DeStefano D, Aggarwal BB, Stevenson HC and Vilcek J:
Characterization of human tumor necrosis factor produced by
peripheral blood monocytes and its separation from lymphotoxin. Int
J Cancer. 36:69–73. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Subedi L, Lee SE, Madiha S, Gaire BP, Jin
M, Yumnam S and Kim SY: Phytochemicals against TNFα-mediated
neuroinflammatory diseases. Int J Mol Sci. 21:7642020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Pegoretti V, Baron W, Laman JD and Eisel
ULM: Selective modulation of TNF-TNFRs signaling: Insights for
multiple sclerosis treatment. Front Immunol. 9:9252018. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Sivakumar V, Foulds WS, Luu CD, Ling EA
and Kaur C: Retinal ganglion cell death is induced by microglia
derived pro-inflammatory cytokines in the hypoxic neonatal retina.
J Pathol. 224:245–260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Cueva Vargas JL, Osswald IK, Unsain N,
Aurousseau MR, Barker PA, Bowie D and Di Polo A: Soluble tumor
necrosis factor alpha promotes retinal ganglion cell death in
glaucoma via calcium-permeable AMPA receptor activation. J
Neurosci. 35:12088–12102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Lee JC, Park CW, Shin MC, Cho JH, Lee HA,
Kim YM, Park JH, Ahn JH, Cho JH, Tae HJ, et al: Tumor necrosis
factor receptor 2 is required for ischemic preconditioning-mediated
neuroprotection in the hippocampus following a subsequent longer
transient cerebral ischemia. Neurochem Int. 118:292–303. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Agarwal R and Agarwal P: Glaucomatous
neurodegeneration: An eye on tumor necrosis factor-alpha. Indian J
Ophthalmol. 60:255–261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Levkovitch-Verbin H, Waserzoog Y, Vander
S, Makarovsky D and Piven I: Minocycline upregulates pro-survival
genes and downregulates pro-apoptotic genes in experimental
glaucoma. Graefes Arch Clin Exp Ophthalmol. 252:761–772. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Taurone S, Ripandelli G, Pacella E,
Bianchi E, Plateroti AM, De Vito S, Plateroti P, Grippaudo FR,
Cavallotti C and Artico M: Potential regulatory molecules in the
human trabecular meshwork of patients with glaucoma:
Immunohistochemical profile of a number of inflammatory cytokines.
Mol Med Rep. 11:1384–1390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Choi JA, Maddala R, Karnam S, Skiba NP,
Vann R, Challa P and Rao PV: Role of vasorin, an anti-apoptotic,
anti-TGF-β and hypoxia-induced glycoprotein in the trabecular
meshwork cells and glaucoma. J Cell Mol Med. 26:2063–2075. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Morgan MJ and Liu ZG: Reactive oxygen
species in TNFalpha-induced signaling and cell death. Mol Cells.
30:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Madeira MH, Elvas F, Boia R, Gonçalves FQ,
Cunha RA, Ambrósio AF and Santiago AR: Adenosine A2AR blockade
prevents neuroinflammation-induced death of retinal ganglion cells
caused by elevated pressure. J Neuroinflammation. 12:1152015.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Bozkurt B, Mesci L, Irkec M, Ozdag BB,
Sanal O, Arslan U, Ersoy F and Tezcan I: Association of tumour
necrosis factor-alpha-308 G/A polymorphism with primary open-angle
glaucoma. Clin Exp Ophthalmol. 40:e156–e162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Lin HJ, Tsai FJ, Chen WC, Shi YR, Hsu Y
and Tsai SW: Association of tumour necrosis factor alpha-308 gene
polymorphism with primary open-angle glaucoma in Chinese. Eye
(Lond). 17:31–34. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Singh A, Ni J and Aggarwal BB: Death
domain receptors and their role in cell demise. J Interferon
Cytokine Res. 18:439–450. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Lavrik I, Golks A and Krammer PH: Death
receptor signaling. J Cell Sci. 118:265–267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Zalewska R, Zalewski B, Reszec J, Mariak
Z, Zimnoch L and Proniewska-Skretek E: The expressions of Fas and
caspase-3 in human glaucomatous optic nerve axons. Med Sci Monit.
14:BR274–BR278. 2008.PubMed/NCBI
|
|
174
|
Pawar M, Busov B, Chandrasekhar A, Yao J,
Zacks DN and Besirli CG: FAS apoptotic inhibitory molecule 2 is a
stress-induced intrinsic neuroprotective factor in the retina. Cell
Death Differ. 24:1799–1810. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Tezel G: TNF-alpha signaling in
glaucomatous neurodegeneration. Prog Brain Res. 173:409–421. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Yan X, Tezel G, Wax MB and Edward DP:
Matrix metalloproteinases and tumor necrosis factor alpha in
glaucomatous optic nerve head. Arch Ophthalmol. 118:666–673. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Yuan L and Neufeld AH: Tumor necrosis
factor-alpha: A potentially neurodestructive cytokine produced by
glia in the human glaucomatous optic nerve head. Glia. 32:42–50.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Tezel G, Li LY, Patil RV and Wax MB:
TNF-alpha and TNF-alpha receptor-1 in the retina of normal and
glaucomatous eyes. Invest Ophthalmol Vis Sci. 42:1787–1794.
2001.PubMed/NCBI
|
|
179
|
Cheng S, Wang HN, Xu LJ, Li F, Miao Y, Lei
B, Sun X and Wang Z: Soluble tumor necrosis factor-alpha-induced
hyperexcitability contributes to retinal ganglion cell apoptosis by
enhancing Nav1.6 in experimental glaucoma. J Neuroinflammation.
18:1822021. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Nakazawa T, Nakazawa C, Matsubara A, Noda
K, Hisatomi T, She H, Michaud N, Hafezi-Moghadam A, Miller JW and
Benowitz LI: Tumor necrosis factor-alpha mediates oligodendrocyte
death and delayed retinal ganglion cell loss in a mouse model of
glaucoma. J Neurosci. 26:12633–12641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Hänninen VA, Pantcheva MB, Freeman EE,
Poulin NR and Grosskreutz CL: Activation of caspase 9 in a rat
model of experimental glaucoma. Curr Eye Res. 25:389–395. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Chi W, Li F, Chen H, Wang Y, Zhu Y, Yang
X, Zhu J, Wu F, Ouyang H, Ge J, et al: Caspase-8 promotes
NLRP1/NLRP3 inflammasome activation and IL-1β production in acute
glaucoma. Proc Natl Acad Sci USA. 111:11181–11186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Yang X, Zeng Q and Tezel G: Regulation of
distinct caspase-8 functions in retinal ganglion cells and
astroglia in experimental glaucoma. Neurobiol Dis. 150:1052582021.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Choudhury S, Liu Y, Clark AF and Pang IH:
Caspase-7: A critical mediator of optic nerve injury-induced
retinal ganglion cell death. Mol Neurodegener. 10:402015.
View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Vigneswara V, Akpan N, Berry M, Logan A,
Troy CM and Ahmed Z: Combined suppression of CASP2 and CASP6
protects retinal ganglion cells from apoptosis and promotes axon
regeneration through CNTF-mediated JAK/STAT signalling. Brain.
137:1656–1675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Seong H, Ryu J, Yoo WS, Kim SJ, Han YS,
Park JM, Kang SS and Seo SW: Resveratrol ameliorates retinal
ischemia/reperfusion injury in C57BL/6J mice via downregulation of
caspase-3. Curr Eye Res. 42:1650–1658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Thomas CN, Berry M, Logan A, Blanch RJ and
Ahmed Z: Caspases in retinal ganglion cell death and axon
regeneration. Cell Death Discov. 3:170322017. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Ngan BY, Chen-Levy Z, Weiss LM, Warnke RA
and Cleary ML: Expression in non-Hodgkin's lymphoma of the bcl-2
protein associated with the t(14;18) chromosomal translocation. N
Engl J Med. 318:1638–1644. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Maes ME, Schlamp CL and Nickells RW: BAX
to basics: How the BCL2 gene family controls the death of retinal
ganglion cells. Prog Retin Eye Res. 57:1–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Singh R, Letai A and Sarosiek K:
Regulation of apoptosis in health and disease: The balancing act of
BCL-2 family proteins. Nat Rev Mol Cell Biol. 20:175–193. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Cottet S and Schorderet DF: Triggering of
Bcl-2-related pathway is associated with apoptosis of
photoreceptors in Rpe65-/- mouse model of Leber's congenital
amaurosis. Apoptosis. 13:329–342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Zalewska R, Reszeć J, Mariak Z and
Sulkowski S: The expression of Bcl-2, Bcl-xl, Bak and Bax proteins
in axons of the optic nerve in closed-angle glaucoma. Klin Oczna.
106 (1–2 Suppl):S155–S157. 2004.(In Polish). PubMed/NCBI
|
|
193
|
Takahashi A, Masuda A, Sun M, Centonze VE
and Herman B: Oxidative stress-induced apoptosis is associated with
alterations in mitochondrial caspase activity and Bcl-2-dependent
alterations in mitochondrial pH (pHm). Brain Res Bull. 62:497–504.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Tatton WG, Chalmers-Redman RM and Tatton
NA: Apoptosis and anti-apoptosis signalling in glaucomatous
retinopathy. Eur J Ophthalmol. 11 (Suppl 2):S12–S22.
2001.PubMed/NCBI
|
|
195
|
Ye D, Shi Y, Xu Y and Huang J: PACAP
attenuates optic nerve crush-induced retinal ganglion cell
apoptosis via activation of the CREB-Bcl-2 pathway. J Mol Neurosci.
68:475–484. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
González-García M, García I, Ding L,
O'Shea S, Boise LH, Thompson CB and Núñez G: bcl-x is expressed in
embryonic and postnatal neural tissues and functions to prevent
neuronal cell death. Proc Natl Acad Sci USA. 92:4304–4308. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Liu XH, Collier RJ and Youle RJ:
Inhibition of axotomy-induced neuronal apoptosis by extracellular
delivery of a Bcl-XL fusion protein. J Biol Chem. 276:46326–46332.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Malik JMI, Shevtsova Z, Bähr M and Kügler
S: Long-term in vivo inhibition of CNS neurodegeneration by Bcl-XL
gene transfer. Mol Ther. 11:373–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Donahue RJ, Fehrman RL, Gustafson JR and
Nickells RW: BCLX(L) gene therapy moderates neuropathology in the
DBA/2J mouse model of inherited glaucoma. Cell Death Dis.
12:7812021. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Näpänkangas U, Lindqvist N, Lindholm D and
Hallböök F: Rat retinal ganglion cells upregulate the pro-apoptotic
BH3-only protein Bim after optic nerve transection. Brain research.
Brain Res Mol Brain Res. 120:30–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Donahue RJ, Maes ME, Grosser JA and
Nickells RW: BAX-depleted retinal ganglion cells survive and become
quiescent following optic nerve damage. Mol Neurobiol.
57:1070–1084. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Wei Y, Fan T and Yu M: Inhibitor of
apoptosis proteins and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 40:278–288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Levkovitch-Verbin H, Dardik R, Vander S,
Nisgav Y, Kalev-Landoy M and Melamed S: Experimental glaucoma and
optic nerve transection induce simultaneous upregulation of
proapoptotic and prosurvival genes. Invest Ophthalmol Vis Sci.
47:2491–2497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Kisiswa L, Albon J, Morgan JE and Wride
MA: Cellular inhibitor of apoptosis (cIAP1) is down-regulated
during retinal ganglion cell (RGC) maturation. Exp Eye Res.
91:739–747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Levkovitch-Verbin H, Vander S, Makarovsky
D and Lavinsky F: Increase in retinal ganglion cells'
susceptibility to elevated intraocular pressure and impairment of
their endogenous neuroprotective mechanism by age. Mol Vis.
19:2011–2022. 2013.PubMed/NCBI
|
|
206
|
Ayub H, Micheal S, Akhtar F, Khan MI,
Bashir S, Waheed NK, Ali M, Schoenmaker-Koller FE, Shafique S,
Qamar R and Hollander AI: Association of a polymorphism in the
BIRC6 gene with pseudoexfoliative glaucoma. PLoS One.
9:e1050232014. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Carbone MA, Chen Y, Hughes GA, Weinreb RN,
Zabriskie NA, Zhang K and Anholt RR: Genes of the unfolded protein
response pathway harbor risk alleles for primary open angle
glaucoma. PLoS One. 6:e206492011. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Kernt M, Neubauer AS, Eibl KH, Wolf A,
Ulbig MW, Kampik A and Hirneiss C: Minocycline is cytoprotective in
human trabecular meshwork cells and optic nerve head astrocytes by
increasing expression of XIAP, survivin, and Bcl-2. Clin
Ophthalmol. 4:591–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Liu L and Yang X, Zhang J, Jiang W, Hou T,
Zong Y, Bai H, Yang K and Yang X: Long non-coding RNA SNHG11
regulates the Wnt/β-catenin signaling pathway through rho/ROCK in
trabecular meshwork cells. FASEB J. 37:e228732023. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Levkovitch-Verbin H, Makarovsky D and
Vander S: Comparison between axonal and retinal ganglion cell gene
expression in various optic nerve injuries including glaucoma. Mol
Vis. 19:2526–2541. 2013.PubMed/NCBI
|
|
211
|
Murray-Zmijewski F, Lane DP and Bourdon
JC: p53/p63/p73 isoforms: an orchestra of isoforms to harmonise
cell differentiation and response to stress. Cell Death Differ.
13:962–972. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Lane DP, Lu X, Hupp T and Hall PA: The
role of the p53 protein in the apoptotic response. Philos Trans R
Soc Lond B Biol Sci. 345:277–280. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Peng Q, Lao X, Chen Z, Lai H, Deng Y, Wang
J, Mo C, Sui J, Wu J, Zhai L, et al: TP53 and MDM2 gene
polymorphisms, gene-gene interaction, and hepatocellular carcinoma
risk: evidence from an updated meta-analysis. PLoS One.
8:e827732013. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Lin HJ, Chen WC, Tsai FJ and Tsai SW:
Distributions of p53 codon 72 polymorphism in primary open angle
glaucoma. Br J Ophthalmol. 86:767–770. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Chipuk JE, Kuwana T, Bouchier-Hayes L,
Droin NM, Newmeyer DD, Schuler M and Green DR: Direct activation of
Bax by p53 mediates mitochondrial membrane permeabilization and
apoptosis. Science. 303:1010–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Acharya M, Mitra S, Mukhopadhyay A, Khan
M, Roychoudhury S and Ray K: Distribution of p53 codon 72
polymorphism in Indian primary open angle glaucoma patients. Mol
Vis. 8:367–371. 2002.PubMed/NCBI
|
|
217
|
Ara S, Lee PS, Hansen MF and Saya H: Codon
72 polymorphism of the TP53 gene. Nucleic Acids Res. 18:49611990.
View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Fan BJ, Liu K, Wang DY, Tham CC, Tam PO,
Lam DS and Pang CP: Association of polymorphisms of tumor necrosis
factor and tumor protein p53 with primary open-angle glaucoma.
Invest Ophthalmol Vis Sci. 51:4110–4116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Gupta S, Chatterjee S, Chandra A, Maurya
OPS, Mishra RN, Mukherjee A and Mutsuddi M: TP53 codon 72
polymorphism and the risk of glaucoma in a north Indian cohort: A
genetic association study. Ophthalmic Genet. 39:228–235. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Guo Y, Zhang H, Chen X, Yang X, Cheng W
and Zhao K: Association of TP53 polymorphisms with primary
open-angle glaucoma: A meta-analysis. Invest Ophthalmol Vis Sci.
53:3756–3763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Gohari M, Neámatzadeh H, Jafari MA,
Mazaheri M, Zare-Shehneh M and Abbasi-Shavazi E: Association
between the p53 codon 72 polymorphism and primary open-angle
glaucoma risk: Meta-analysis based on 11 case-control studies.
Indian J Ophthalmol. 64:756–761. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Blanco-Marchite C, Sánchez-Sánchez F,
López-Garrido MP, Iñigez-de-Onzoño M, López-Martínez F,
López-Sánchez E, Alvarez L, Rodríguez-Calvo PP, Méndez-Hernández C,
Fernández-Vega L, et al: WDR36 and P53 gene variants and
susceptibility to primary open-angle glaucoma: analysis of
gene-gene interactions. Invest Ophthalmol Vis Sci. 52:8467–8478.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Neamatzadeh H, Soleimanizad R, Atefi A,
Zare-Shehneh M, Gharibi S, Shekari A and Rahimzadeh AB: Association
between p53 codon 72 (Arg72Pro) polymorphism and primary open-angle
glaucoma in Iranian patients. Iran Biomed J. 19:51–56.
2015.PubMed/NCBI
|
|
224
|
Wiggs JL, Hewitt AW, Fan BJ, Wang DY,
Figueiredo Sena DR, O'Brien C, Realini A, Craig JE, Dimasi DP,
Mackey DA, et al: The p53 codon 72 PRO/PRO genotype may be
associated with initial central visual field defects in caucasians
with primary open angle glaucoma. PLoS One. 7:e456132012.
View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Jeong BS, Hu W, Belyi V, Rabadan R and
Levine AJ: Differential levels of transcription of p53-regulated
genes by the arginine/proline polymorphism: p53 with arginine at
codon 72 favors apoptosis. FASEB J. 24:1347–1353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Dimasi DP, Hewitt AW, Green CM, Mackey DA
and Craig JE: Lack of association of p53 polymorphisms and
haplotypes in high and normal tension open angle glaucoma. J Med
Genet. 42:e552005. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Silva RE, Arruda JT, Rodrigues FW and
Moura KKVO: Primary open angle glaucoma was not found to be
associated with p53 codon 72 polymorphism in a Brazilian cohort.
Genet Mol Res. 8:268–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Mabuchi F, Sakurada Y, Kashiwagi K,
Yamagata Z, Iijima H and Tsukahara S: Lack of association between
p53 gene polymorphisms and primary open angle glaucoma in the
Japanese population. Mol Vis. 15:1045–1049. 2009.PubMed/NCBI
|
|
229
|
Saglar E, Yucel D, Bozkurt B, Ozgul RK,
Irkec M and Ogus A: Association of polymorphisms in APOE, p53, and
p21 with primary open-angle glaucoma in Turkish patients. Mol Vis.
15:1270–1276. 2009.PubMed/NCBI
|
|
230
|
Chen SD, Wang L and Zhang XL:
Neuroprotection in glaucoma: Present and future. Chin Med J (Engl).
126:1567–1577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Tahzib NG, Ransom NL, Reitsamer HA and
McKinnon SJ: Alpha-fodrin is cleaved by caspase-3 in a chronic
ocular hypertensive (COH) rat model of glaucoma. Brain Res Bull.
62:491–495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Vigneswara V, Berry M, Logan A and Ahmed
Z: Pharmacological inhibition of caspase-2 protects axotomised
retinal ganglion cells from apoptosis in adult rats. PLoS One.
7:e534732012. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Monnier PP, D'Onofrio PM, Magharious M,
Hollander AC, Tassew N, Szydlowska K, Tymianski M and Koeberle PD:
Involvement of caspase-6 and caspase-8 in neuronal apoptosis and
the regenerative failure of injured retinal ganglion cells. J
Neurosci. 31:10494–10505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Liu Y, Yan H, Chen S and Sabel BA:
Caspase-3 inhibitor Z-DEVD-FMK enhances retinal ganglion cell
survival and vision restoration after rabbit traumatic optic nerve
injury. Restor Neurol Neurosci. 33:205–220. 2015.PubMed/NCBI
|
|
235
|
Sánchez-Migallón MC, Valiente-Soriano FJ,
Nadal-Nicolás FM, Vidal-Sanz M and Agudo-Barriuso M: Apoptotic
retinal ganglion cell death after optic nerve transection or crush
in mice: Delayed RGC loss with BDNF or a caspase 3 inhibitor.
Invest Ophthalmol Vis Sci. 57:81–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Nakazawa T, Tamai M and Mori N:
Brain-derived neurotrophic factor prevents axotomized retinal
ganglion cell death through MAPK and PI3K signaling pathways.
Invest Ophthalmol Vis Sci. 43:3319–3326. 2002.PubMed/NCBI
|
|
237
|
Tawfik M, Zhang X, Grigartzik L,
Heiduschka P, Hintz W, Henrich-Noack P, van Wachem B and Sabel BA:
Gene therapy with caspase-3 small interfering RNA-nanoparticles is
neuroprotective after optic nerve damage. Neural Regen Res.
16:2534–2541. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Ahmed Z, Kalinski H, Berry M, Almasieh M,
Ashush H, Slager N, Brafman A, Spivak I, Prasad N, Mett I, et al:
Ocular neuroprotection by siRNA targeting caspase-2. Cell Death
Dis. 2:e1732011. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Elewa HF, Hilali H, Hess DC, Machado LS
and Fagan SC: Minocycline for short-term neuroprotection.
Pharmacotherapy. 26:515–521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Bosco A, Inman DM, Steele MR, Wu G, Soto
I, Marsh-Armstrong N, Hubbard WC, Calkins DJ, Horner PJ and Vetter
ML: Reduced retina microglial activation and improved optic nerve
integrity with minocycline treatment in the DBA/2J mouse model of
glaucoma. Invest Ophthalmol Vis Sci. 49:1437–1446. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Huang W, Gao F, Hu F, Huang J, Wang M, Xu
P, Zhang R, Chen J, Sun X, Zhang S and Wu J: Asiatic acid prevents
retinal ganglion cell apoptosis in a rat model of glaucoma. Front
Neurosci. 12:4892018. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Hu X, Zhuang D, Zhang R, Sun X, Lu Q and
Dai Y: The small molecule inhibitor PR-619 protects retinal
ganglion cells against glutamate excitotoxicity. Neuroreport.
31:1134–1141. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Wu X, Pang Y, Zhang Z, Li X, Wang C, Lei
Y, Li A, Yu L and Ye J: Mitochondria-targeted antioxidant peptide
SS-31 mediates neuroprotection in a rat experimental glaucoma
model. Acta Biochim Biophys Sin (Shanghai). 51:411–421. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Shi Y, Ye D, Huang R, Xu Y, Lu P, Chen H
and Huang J: Down syndrome critical region 1 reduces oxidative
stress-induced retinal ganglion cells apoptosis via CREB-Bcl-2
pathway. Invest Ophthalmol Vis Sci. 61:232020. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Dai Y, Lawrence TS and Xu L: Overcoming
cancer therapy resistance by targeting inhibitors of apoptosis
proteins and nuclear factor-kappa B. Am J Transl Res. 1:1–15.
2009.PubMed/NCBI
|
|
246
|
McKinnon SJ, Lehman DM, Tahzib NG, Ransom
NL, Reitsamer HA, Liston P, LaCasse E, Li Q, Korneluk RG and
Hauswirth WW: Baculoviral IAP repeat-containing-4 protects optic
nerve axons in a rat glaucoma model. Mol Ther. 5:780–787. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Visuvanathan S, Baker AN, Lagali PS,
Coupland SG, Miller G, Hauswirth WW and Tsilfidis C: XIAP gene
therapy effects on retinal ganglion cell structure and function in
a mouse model of glaucoma. Gene Ther. 29:147–156. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Wang XC, Wang T, Zhang Y, Wang LL, Zhao RY
and Tan W: Tacrolimus inhibits proliferation and induces apoptosis
by decreasing survivin in scar fibroblasts after glaucoma surgery.
Eur Rev Med Pharmacol Sci. 22:2934–2940. 2018.PubMed/NCBI
|
|
249
|
Lee J, Choi JH and Joo CK: TGF-β1
regulates cell fate during epithelial-mesenchymal transition by
upregulating survivin. Cell Death Dis. 4:e7142013. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Honjo M and Tanihara H: Impact of the
clinical use of ROCK inhibitor on the pathogenesis and treatment of
glaucoma. Jpn J Ophthalmol. 62:109–126. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Wang J, Wang H and Dang Y: Rho-kinase
inhibitors as emerging targets for glaucoma therapy. Ophthalmol
Ther. 12:2943–2957. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Chen W and Yang X, Fang J, Zhang Y, Zhu W
and Yang X: Rho-associated protein kinase inhibitor treatment
promotes proliferation and phagocytosis in trabecular meshwork
cells. Front Pharmacol. 11:3022020. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Garnock-Jones KP: Ripasudil: First global
approval. Drugs. 74:2211–2215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Sturdivant JM, Royalty SM, Lin CW, Moore
LA, Yingling JD, Laethem CL, Sherman B, Heintzelman GR, Kopczynski
CC and de Long MA: Discovery of the ROCK inhibitor netarsudil for
the treatment of open-angle glaucoma. Bioorg Med Chem Lett.
26:2475–2480. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Tanna AP and Johnson M: Rho kinase
inhibitors as a novel treatment for glaucoma and ocular
hypertension. Ophthalmology. 125:1741–1756. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Testa V, Ferro Desideri L, Della Giustina
P, Traverso CE and Iester M: An update on ripasudil for the
treatment of glaucoma and ocular hypertension. Drugs Today (Barc).
56:599–608. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Li DW, Liu JP, Schmid PC, Schlosser R,
Feng H, Liu WB, Yan Q, Gong L, Sun SM, Deng M and Liu Y: Protein
serine/threonine phosphatase-1 dephosphorylates p53 at Ser-15 and
Ser-37 to modulate its transcriptional and apoptotic activities.
Oncogene. 25:3006–3022. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Yang Z, Ge J, Yin W, Shen H, Liu H and Guo
Y: The expression of p53, MDM2 and Ref1 gene in cultured retina
neurons of SD rats treated with vitamin B1 and/or elevated
pressure. Yan Ke Xue Bao. 20:259–263. 2004.(In Chinese). PubMed/NCBI
|
|
259
|
Johnson KT, Rödicker F, Heise K, Heinz C,
Steuhl KP, Pützer BM and Hudde T: Adenoviral p53 gene transfer
inhibits human Tenon's capsule fibroblast proliferation. Br J
Ophthalmol. 89:508–512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Husain S, Ahmad A, Singh S, Peterseim C,
Abdul Y and Nutaitis MJ: PI3K/Akt pathway: A role in δ-opioid
receptor-mediated RGC neuroprotection. Invest Ophthalmol Vis Sci.
58:6489–6499. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Zhao N, Shi J, Xu H, Luo Q, Li Q and Liu
M: Baicalin suppresses glaucoma pathogenesis by regulating the
PI3K/AKT signaling in vitro and in vivo. Bioengineered.
12:10187–10198. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Xi X, Chen Q, Ma J, Wang X, Xia Y, Wen X,
Cai B and Li Y: Acteoside protects retinal ganglion cells from
experimental glaucoma by activating the PI3K/AKT signaling pathway
via caveolin 1 upregulation. Ann Transl Med. 10:3122022. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Nie XG, Fan DS, Huang YX, He YY, Dong BL
and Gao F: Downregulation of microRNA-149 in retinal ganglion cells
suppresses apoptosis through activation of the PI3K/Akt signaling
pathway in mice with glaucoma. Am J Physiol Cell Physiol.
315:C839–C849. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Perkins TW, Faha B, Ni M, Kiland JA,
Poulsen GL, Antelman D, Atencio I, Shinoda J, Sinha D, Brumback L,
et al: Adenovirus-mediated gene therapy using human p21WAF-1/Cip-1
to prevent wound healing in a rabbit model of glaucoma filtration
surgery. Arch Ophthalmol. 120:941–949. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Heatley G, Kiland J, Faha B, Seeman J,
Schlamp CL, Dawson DG, Gleiser J, Maneval D, Kaufman PL and
Nickells RW: Gene therapy using p21WAF-1/Cip-1 to modulate wound
healing after glaucoma trabeculectomy surgery in a primate model of
ocular hypertension. Gene Ther. 11:949–955. 2004. View Article : Google Scholar : PubMed/NCBI
|