Introduction
Reactive oxygen species (ROS), such as superoxide
anion (O2−), hydroxyl radical (·OH), and
hydrogen peroxide (H2O2), are natural
byproducts of oxygen metabolism that serve key roles in cell
proliferation and immune responses (1,2).
Excessive ROS can damage cellular molecules, leading to DNA damage,
lipid peroxidation and protein oxidation. These processes are key
in the development of various diseases, including cancer and lung
fibrosis (3–5). Cells contain a range of antioxidant
enzymes, including superoxide dismutase (SOD), catalase (CAT), and
glutathione peroxidase (GPx) and non-enzymatic antioxidants, such
as reduced glutathione (GSH), for defense against ROS (6,7).
SOD, a metalloenzyme, catalyzes the conversion of
O2− to molecular O2 and
H2O2 and functions as a key component of the
cellular antioxidant defense mechanism (8). CAT breaks H2O2
into O2 and H2O and GPx uses GSH as an
electron donor to convert H2O2 into its
corresponding alcohol or water (9,10).
In addition, heme oxygenase-1 (HO-1) serves as a catalyst for the
oxidative transformation of heme to carbon monoxide, iron and
biliverdin, which is then transformed into bilirubin by biliverdin
reductase (11). Furthermore, ROS
levels are notably boosted by exposure to environmental stressors,
such as ultraviolet light, pollutants and heavy metals (12).
Lung fibroblasts serve a major role in lung
development, such as alveolar unit development, aid production of
extracellular matrix, and facilitate wound healing and tissue
repair (13). Physiological
stresses (ROS and disrupted metabolism) may turn normal fibroblasts
into cancer-associated fibroblasts (CAFs) (14). CAFs are key factors in the tumor
microenvironment and serve a crucial role in non-small cell lung
cancer drug resistance (15).
Therefore, the present study aimed to identify a potent antioxidant
compound capable of ameliorating oxidative stress-mediated cellular
defects in fibroblasts.
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is a
flavonoid found abundantly in vegetables and fruits, including
green pepper and chamomile tea (16). It exhibits antitumor effects
against gastric, ovarian, and hepatocellular carcinomas (17–20).
Luteolin also possesses numerous biological benefits, such as
anti-inflammatory, anti-allergic, and antioxidant properties
(21). Given its wide range of
therapeutic potentials, the present study aimed to evaluate
cytoprotective effects of luteolin on lung fibroblasts via the
initiation of antioxidant enzyme activity.
Materials and methods
Reagents and antibodies
Luteolin, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO),
xanthine, xanthine oxidase, 2′,7′-dichlorodihydrofluorescein
diacetate (H2DCFDA), MTT, thiobarbituric acid (TBA),
Hoechst 33342, N-acetyl cysteine (NAC), and propidium iodide (PI)
were obtained from Sigma-Aldrich (Merck KGaA). Additionally,
7-amino-4-chloromethylcoumarin (CMAC) and
diphenyl-1-pyrenylphosphine (DPPP) were purchased from Molecular
Probes (Thermo Fisher Scientific, Inc.) The primary Bax, Bcl-2,
GPx, CAT and HO-1 antibodies were obtained from Santa Cruz
Biotechnology, Inc.; primary β-actin, phosphorylated (phospho)-H2A
histone family member X (H2A.X), H2A.X, caspase-3 and caspase-9
antibodies were obtained from Cell Signaling Technology, Inc.;
primary γ-glutamylcysteine ligase (γ-GCL) antibody was obtained
from Thermo Fisher Scientific, Inc.; primary Cu/Zn SOD was obtained
from Enzo Life Science.
Cell culture
Chinese hamster lung fibroblasts (V79-4) were
purchased from American Type Culture Collection and cultured in
Dulbecco's modified Eagle medium (Gibco; Thermo Fisher Scientific,
Inc.), supplemented with 10% heat-inactivated fetal calf serum, at
37°C in a humidified incubator with 5% CO2.
MTT assay
Cells (1.5×105 cells/ml) were treated
with 0.625, 1.250, 2.500, 5.000 or 10.000 µg/ml luteolin for 24 h
at 37°C. To investigate the cytoprotective effect of luteolin
against H2O2 exposure, cells were pretreated
with 2.5 µg/ml luteolin for 1 h before exposure to 1 mM
H2O2 for 24 h, all at 37°C. The MTT assay was
performed and formazan crystals were dissolved in dimethyl
sulfoxide, then absorbance was measured using a scanning multi-well
spectrophotometer at 540 nm, as previously described (22).
Evaluation of ROS levels
Cells were exposed to luteolin (0.625, 1.250, 2.500,
5.000 or 10.000 µg/ml, respectively) and 2 mM NAC for 30 min,
followed by 1 mM H2O2 treatment for 1 h, all
at 37°C. Following staining with 25 µM H2DCFDA at 37°C
for 10 min, the fluorescence was monitored and quantified using a
spectrofluorometer (PerkinElmer FL 6500 Fluorescence Spectrometer
with Spectrum FL Software 1.1 version, PerkinElmer Inc.) or a
confocal microscope (Zeiss LSM 510 confocal microscope with Zen 2.5
version, Carl Zeiss Inc.), as previously described (23) using 40× magnification.
Detection of superoxide anion
The xanthine/xanthine oxidase system was used to
produce O2−, which was captured by the
nitrone spin trap, DMPO. The resulting DMPO/·OOH adducts were
identified using electron spin resonance (ESR) spectrometer (JEOL,
Ltd.) as previously described (23).
Detection of hydroxyl radical
Hydroxyl radical was generated by the Fenton
reaction (H2O2 + FeSO4) and
detected by capturing with DMPO to form DMPO/·OH adducts, measured
using ESR spectrometer as previously described (23).
Assessment of lipid peroxidation
Following cell treatment with 5 µM DPPP at 37°C for
30 min, a fluorescence microscope (Zeiss LSM 510 confocal
microscope with Zen 2.5 version; 20× magnification) was used to
assess images of DPPP fluorescence. The cells were rinsed with cold
PBS, scraped and homogenized in ice-cold 1.15% KCl, resulted cell
lysate was subjected to further assessment. For the detection of
TBA reactive substances (TBARS), 100 µl cell lysates were mixed
with 0.2 ml sodium dodecyl sulfate (SDS, 8.1%), 1.5 ml 20% acetic
acid (pH 3.5) and 1.5 ml TBA (0.8%) and combined with 5 ml 15:1
(v/v) n-butanol and pyridine solutions. The resulting supernatant
absorbance was measured using a spectrophotometer at 532 nm.
Comet assay
Following treatment with luteolin and
H2O2, cells were collected and centrifuged at
15,000 × g for 5 min, following washing with PBS to obtain cell
pellets. Cell pellets on 1% agarose-coated slides were subjected to
gel electrophoresis at 300 mA and 25 V for 20 min in darkness at
20°C. Ethidium bromide (20 µg/ml) stained slides at 20°C for 5 min
were examined under a fluorescence microscope (Komet 7 with Zyla
5.5 USB 3.0 sCMOS camera, Andor Technology; 20× magnification).
Each slide contained 50 cells and data on tail length and total
fluorescence percentages were analyzed using Komet version 5.5
image analyzer software (Andor Technology).
Western blot analysis
Total proteins from the cells was extract by using
PRO-PREP™ protein extraction solution (iNtRON Biotechnology).
Thereafter total protein levels were estimated by protein assay
reagent kit (Bio-Rad). A total of 30 µg/lane cell lysates were
subjected to electrophoresis on a 10% SDS-polyacrylamide gel,
transferred to nitrocellulose membranes, subjected for blocking
with 3% bovine serum albumin (Bovogen Biologicals Pty Ltd.) for 1 h
at 20°C, and then incubated with the corresponding primary
antibodies whereas phospho-H2A.X (cat. #9718), H2A.X (cat. #2595),
β-actin (cat. #4967), Bax (cat. sc-7480), Bcl-2 (cat. sc-7382),
caspase-9 (cat. #9508), caspase-3 (cat. #9662), γ-GCL (cat.
#RB-1697-P0, -P1), Cu/Zn SOD (cat. ADI-SOD-100), CAT (cat.
sc-34285), GPx (cat. sc-22145), HO-1 (cat. sc-10789) (all 1:1,000
ratio, respectively) and for overnight at 4°C subsequently treated
with the relevant secondary (diluted 1:10,000, respectively) goat
anti-rabbit IgG (H+L) Secondary antibody, HRP (cat. #31460) and
goat anti-mouse IgG (H+L) secondary antibody, HRP (cat. #31430;
Thermo Fisher Scientific, Inc.) at 20°C for 1 h. Each corresponding
protein band was observed on an X-ray film following treat with
enhanced chemiluminescence western blotting detection kit
(Amersham) as previously described (23).
Hoechst 33342 nuclear staining
Cells were stained with Hoechst 33342, a
DNA-specific probe, at 37°C for 10 min. The degree of nuclear
condensation was evaluated using a fluorescence microscope
(BH2-RFL-T3; Olympus; 20× magnification) fitted with a CoolSNAP-Pro
color digital camera (Media Cybernetics) to observe stained
cells.
Detection of sub-G1
cells
Cells were fixed with 70% ethanol at 4°C for 30 min,
subjected for washing with PBS, mixed in 1 ml PBS containing 100 µg
PI solution and 100 µg RNase A and incubated in dark conditions at
37°C for 30 min. The percentage of apoptotic sub-G1
cells was determined using FACSCalibur flow cytometer with
CellQuest pro software 4.02 (Becton Dickinson).
GSH detection
Cells were incubated with 5 µM CMAC at 37°C, a
GSH-sensitive fluorescent dye, for 30 min. Images of CMAC
fluorescence in response to GSH were analyzed using a fluorescence
microscope (BH2-RFL-T3; Olympus; 10× magnification) (23).
Assessment of SOD activity
Collected cells were sonicated twice for 15 sec in
10 mM phosphate buffer (pH 7.5) on ice to lyse. 1% Triton X-100 was
added to the lysates and incubated on ice for 10 min. After
centrifugation at 5,000 × g for 10 min at 4°C, the lysates were
cleared of debris and the protein concentration of the supernatant
was measured utilizing using the Bradford method. Cell lysates were
mixed with 500 mM phosphate buffer (pH 10.2) and 1 mM epinephrine
at 20°C, which auto-oxidizes to form adrenochrome, and the
reactants were measured at 480 nm using a ultraviolet/visible
spectrophotometer in kinetic mode. SOD activity was calculated as
unit/mg protein as previously described (23).
Assessment of CAT activity
Harvested cells were suspended in 10 mM phosphate
buffer (pH 7.5) and sonicated twice for 15 sec on ice. After adding
1% TritonX-100 to the lysates, they were incubated on ice for 10
min. Protein content was measured after centrifuging lysates at
5,000 × g for 30 min at 4°C to eliminate cellular debris. Then cell
lysates were reacted with 50 mM phosphate buffer (pH 7) and 100 mM
H2O2, at 37°C for 2 min. Absorbance changes
at 240 nm over 5 min were measured by spectrophotometer (X-ma 1000;
Human Corporation) to determine the rate of
H2O2 decomposition.
Assessment of GPx activity
Cells were lysed by sonicating twice for 15 sec in
10 mM phosphate buffer (pH 7.5) on ice. 1% Triton X-100 was added
to lysates and incubated on ice for 10 min. After centrifugation at
5,000 × g for 10 min at 4°C, debris was removed and protein
concentration was assessed using the Bradford technique. Then cell
lysates were mixed with 25 mM phosphate buffer (pH 7.5), 1 mM EDTA,
NaN3, GSH, 0.25 units of glutathione reductase, and 0.1
mM NADPH. Following 10 min incubation at 37°C, 1 mM
H2O2 was added for 1 min at 37°C and
absorbance was measured using spectrophotometer (X-ma 1000, Human
Co.) at 340 nm for 5 min.
Assessment of HO-1 activity
Cells were washed with PBS, collected in PBS (pH
7.4), allowed to 15 min incubation on ice facilitating brief
sonication and added sucrose solution obtaining 0.25 M sucrose as
final concentration. Homogenates were centrifuged 10 min at 1,000 ×
g at 4°C. The supernatants were centrifuged at 12,000 × g for 15
min at 4°C and afterward at 105,000 × g for 60 min at 4°C. Resulted
pellet was resuspended in 50 mM PBS (pH 7.4) and protein
concentration was measured using the Bradford technique. Cell
lysates were suspended in a reaction mixture containing 0.2 mM
hemin, 0.5 mg/ml rat liver cytosol (supplying biliverdin reductase;
Creative Bioarray; cat. no. DDM-M063), 2 mM glucose-6-phosphate, 1
unit/ml glucose-6-phosphate dehydrogenase, 1 mM NADPH and 50 mM PBS
(pH 7.4) for 2 h at 37°C. The chloroform-extracted layer was
assessed using a spectrophotometer at 464 and 530 nm.
Statistical analysis
All experiments were performed in triplicate, with
results presented as the mean ± standard error. Data were analyzed
using one way analysis of variance, followed by Tukey's post hoc
test to determine the differences using SigmaStat 3.5 version
software (Systat Software Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of luteolin on ROS
scavenging
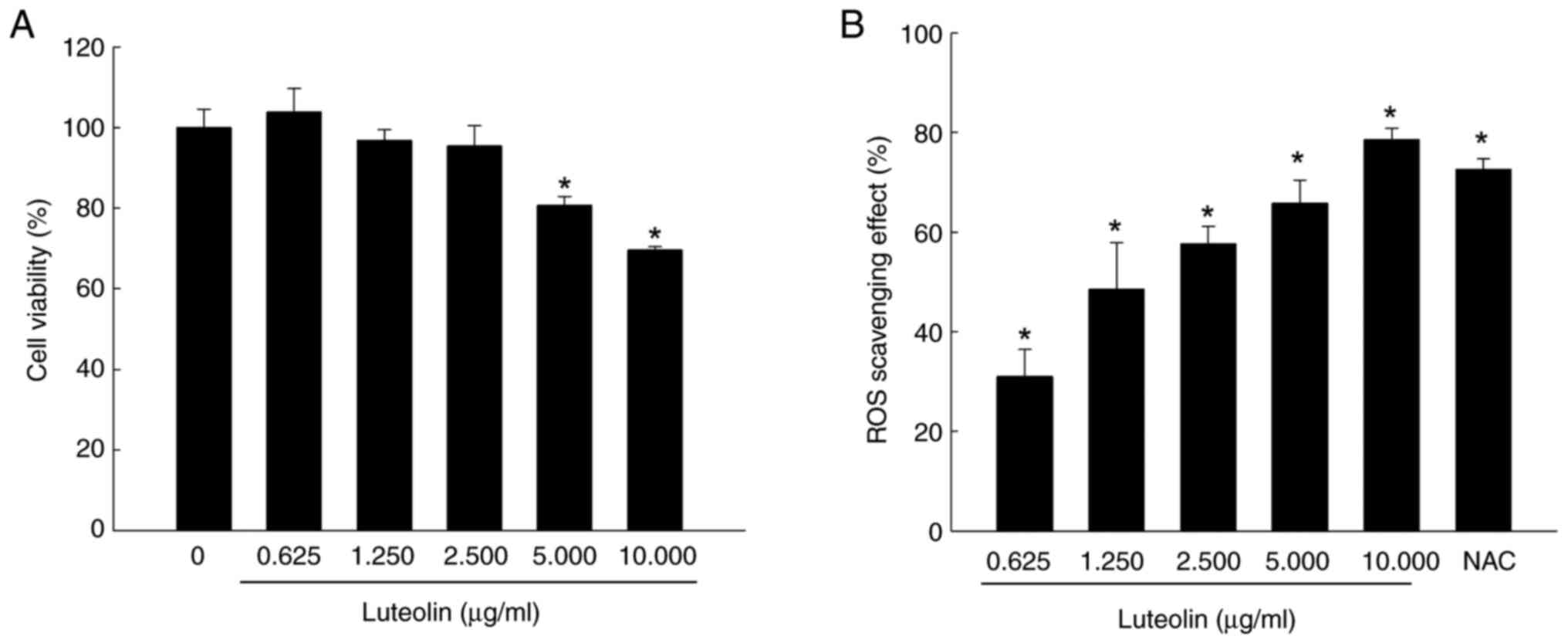
Luteolin exhibited no cytotoxicity toward V79-4
cells at concentrations of 0.625, 1.25 and 2.5 µg/ml. Cytotoxic
effects were observed at concentrations of 5 and 10 µg/ml (Fig. 1A). Fluorescence spectrometry
indicated that luteolin scavenged intracellular ROS in a
dose-dependent manner, decreasing ROS by 31% at 0.625, 51% at
1.250, 58% at 2.500, 68% at 5.000 and 75% at 10.000 µg/ml (Fig. 1B). The ROS scavenger NAC, serving
as a positive control, eliminated 72% of ROS. Given its cell
viability and ROS-scavenging ability, 2.5 µg/ml luteolin was
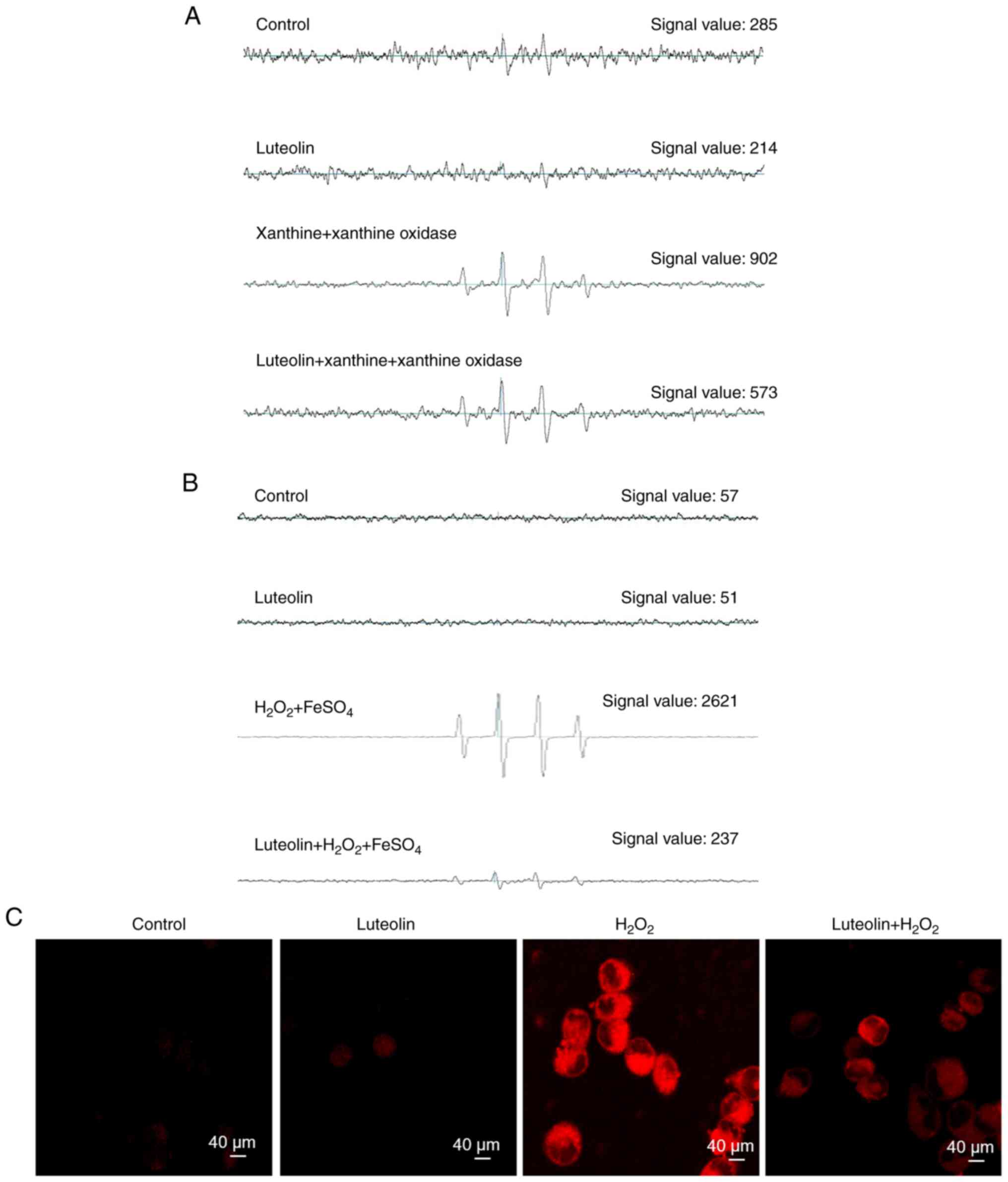
selected as the optimal dose for further evaluation. ESR
spectrometry was used to quantify the superoxide anion generated by
the xanthine/xanthine oxidase system. ESR measurement revealed an
elevation in the superoxide anion signal to 902 in this system.
However, when the superoxide anion was treated with luteolin, the
superoxide anion signal was reduced to 573, indicating the direct
scavenging effect of luteolin on the superoxide anion produced via
the xanthine/xanthine oxidase pathway (Fig. 2A). Furthermore, ESR spectrometry
was used to detect the hydroxyl radical produced via the Fenton
reaction. ESR measurement demonstrated that neither the control nor
luteolin at 2.5 µg/ml exhibited a signal, whereas the signal of the
hydroxyl radical increased to 2,621 in the Fenton reaction system.
Treatment with luteolin markedly decreased the hydroxyl radical
signal to 237, indicating the capacity of luteolin to directly
mitigate the hydroxyl radical generated through the Fenton reaction
system (Fig. 2B). Additionally,
confocal microscopy images from the H2DCFDA assay showed
that luteolin suppressed red fluorescence intensity, which
increased in response to H2O2 treatment in
association with elevated ROS levels (Fig. 2C).
Effect of luteolin on
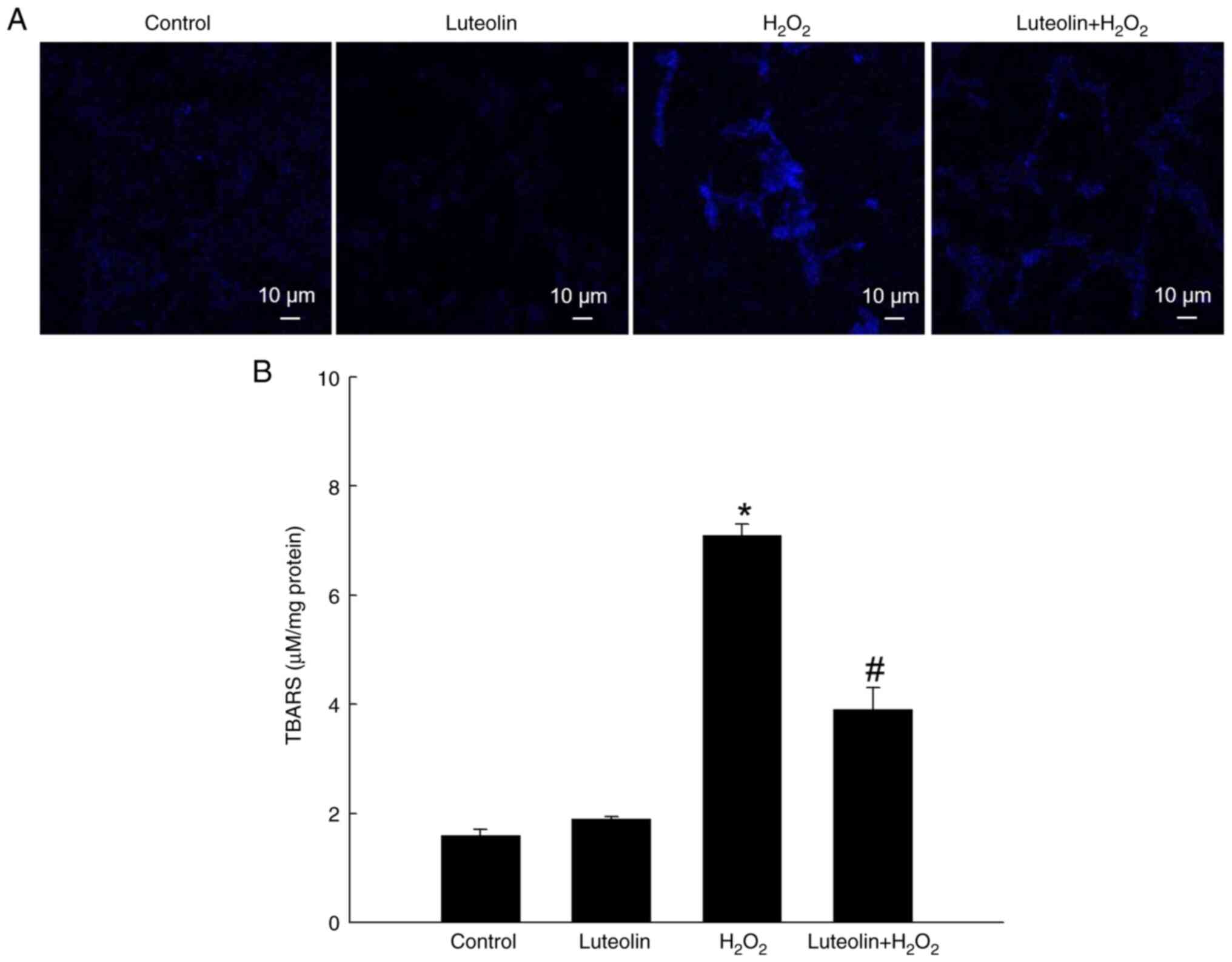
H2O2-induced lipid peroxidation
The capacity of luteolin to counteract membrane
lipid peroxidation in cells exposed to H2O2
was assessed. Lipid peroxidation, evidenced by formation of the
highly fluorescent compound DPPP oxide upon DPPP stoichiometric
reaction with lipid hydroperoxides, was assessed (24). H2O2 treatment
enhanced DPPP fluorescence intensity, indicating the elevation of
lipid peroxidation levels (Fig.
3A). However, this increase was notably attenuated by treatment
with 2.5 µg/ml luteolin. Additionally, protective effect of
luteolin against lipid peroxidation was demonstrated by TBARS
formation in H2O2-treated V79-4 cells whereas
luteolin significantly decreased H2O2-induced
TBARS formation (Fig. 3B).
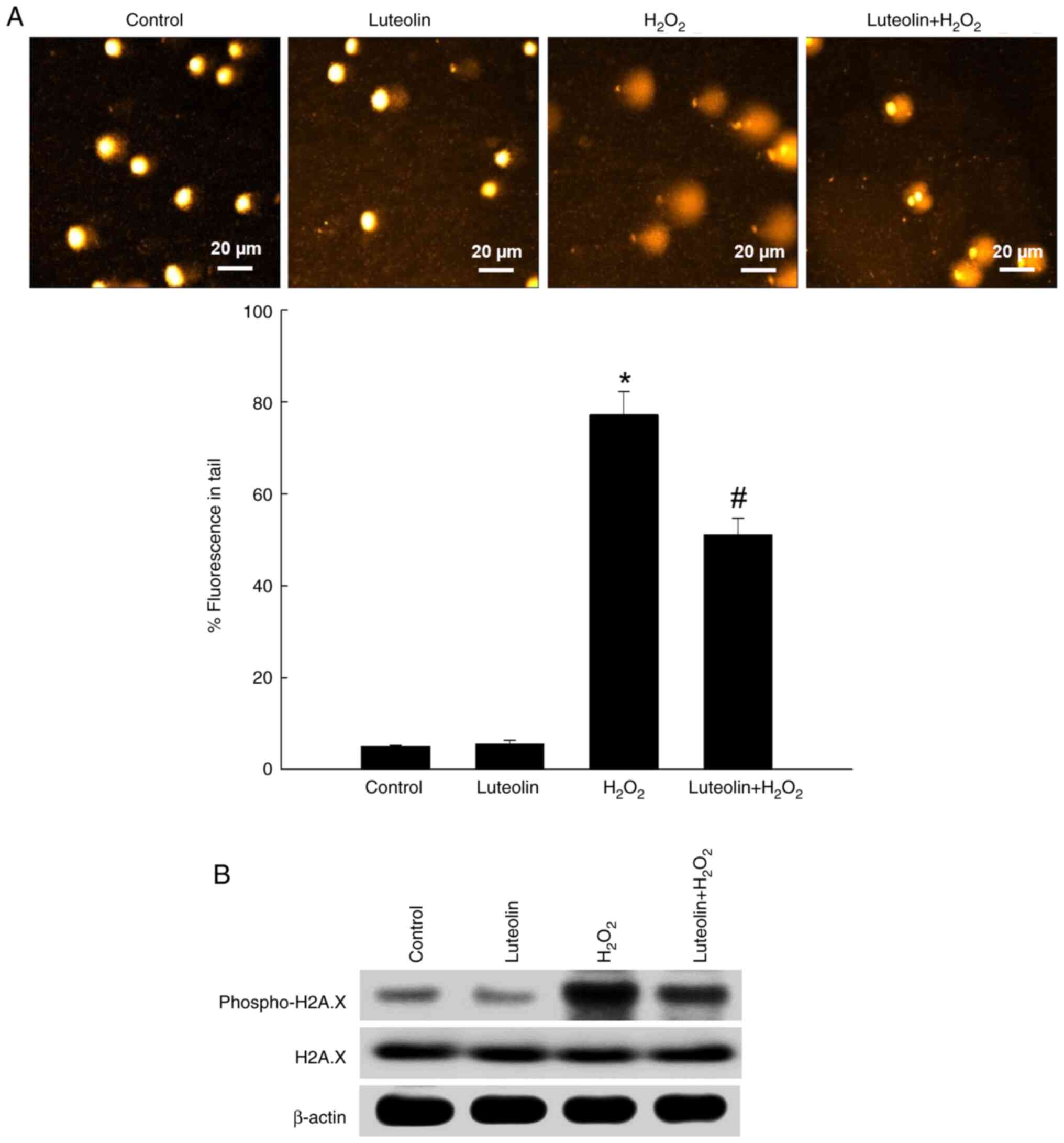
Effect of luteolin against DNA
damage
The comet assay, a sensitive technique for measuring
DNA damage (25), demonstrated
that H2O2 resulted in a 77% increase in the
length and quantity of DNA in the comet tail, signifying
considerable DNA damage; however, pretreatment with luteolin
reduced this increase to 51% (Fig.
4A). Furthermore, evaluation of phospho-H2A.X, a marker for DNA
double-strand breaks (25), showed
that luteolin pretreatment diminished expression levels of
phospho-H2A.X in H2O2-exposed cells (Fig. 4B). There was no change in total
H2A.X expression.
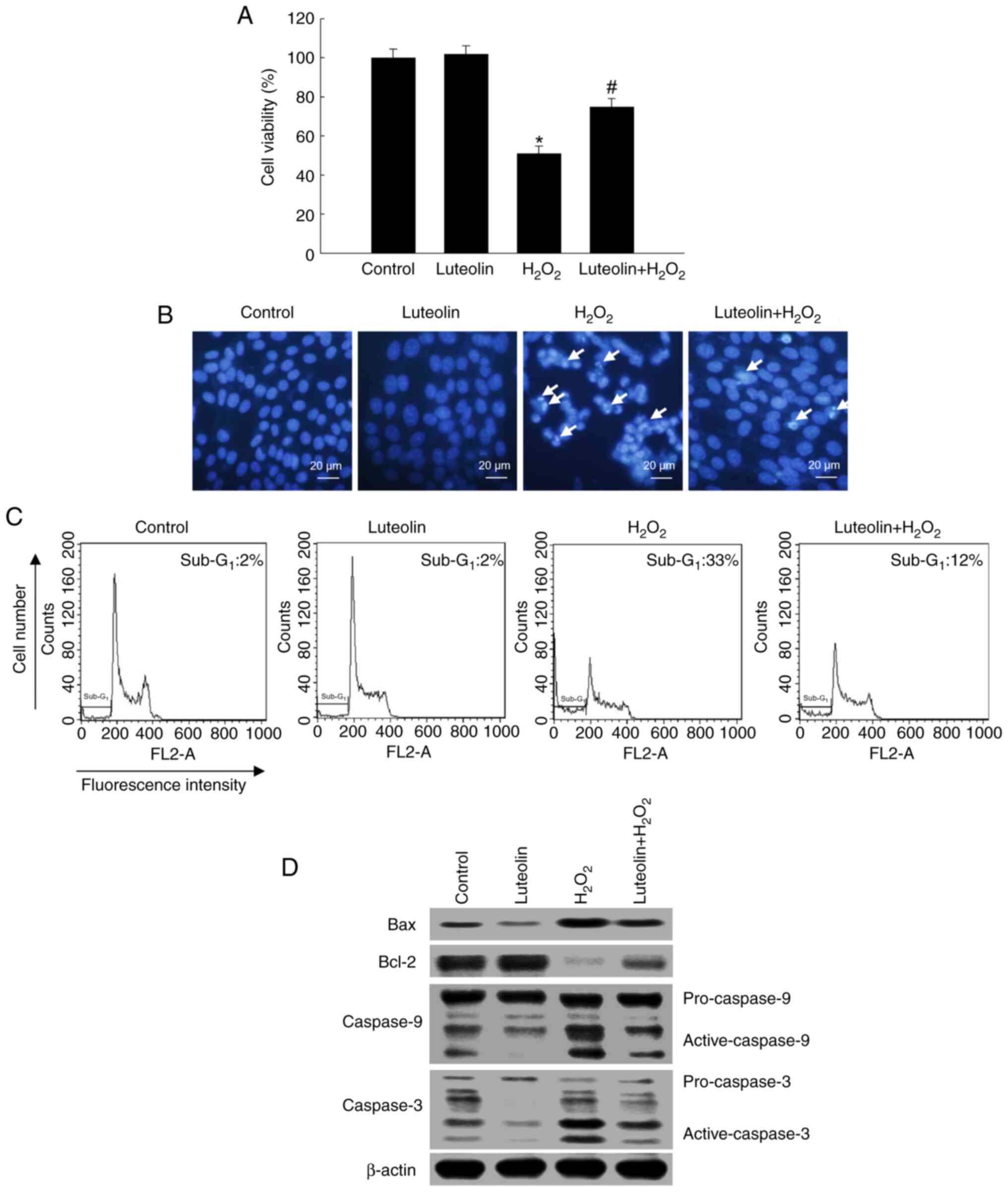
Effect of luteolin on cell survival
following H2O2 treatment
Pretreatment with 2.5 µg/ml luteolin in conjunction
with H2O2 significantly increased cell
viability to 77% compared with 54% in cells treated solely with
H2O2 (Fig.
5A). The cytoprotective effect of luteolin against
H2O2-induced apoptosis was determined by
staining nuclei of cells with Hoechst 33342 and observing them
under a microscope. H2O2-treated cells showed
considerable nuclear fragmentation and apoptotic morphology;
however, luteolin pretreatment alleviated
H2O2-induced cellular apoptosis (Fig. 5B). The proportion of
sub-G1 in H2O2-treated cells was
33% (a 31% increase relative to the control; Fig. 5C); however, pretreatment with
luteolin decreased the sub-G1 apoptotic cells to 12%.
Pretreatment with luteolin led to an alleviation of
H2O2-induced pro-apoptotic protein Bax
expression and increased the anti-apoptotic Bcl-2 protein
expression, which was decreased by H2O2
(Fig. 5D). Furthermore, luteolin
alleviated H2O2-mediated activation of
caspase-9 and caspase-3 (Fig.
5D).
Effect of luteolin on antioxidant
systems
Confocal microscopy showed a notable reduction in
GSH level in H2O2-treated cells, showing
lower fluorescence intensity than that of the control. However, an
increase in GSH levels was observed in the luteolin-pretreated
group (Fig. 6A). As GSH synthesis
involves γ-GCL and GSH synthase (26), γ-GCL expression was evaluated via
western blotting. The expression of γ-GCL was notably downregulated
by H2O2-treated cells but upregulated by
luteolin pretreatment (Fig. 6B).
Moreover, enzymatic assays demonstrated that luteolin pretreatment
enhanced activities of SOD, CAT, GPx, and HO-1 to 29, 17 and 17
unit/mg protein and 7,162 pmol bilirubin/mg protein, respectively,
compared with diminished activities in cells treated with
H2O2-alone (23, 9, and 9 unit/mg protein and
4,987 pmol bilirubin/mg protein, respectively; Fig. 6C and D). Also, the levels of SOD,
CAT, GPx and HO-1 were reduced in
H2O2-treated cells; however, luteolin
partially restored expression of these proteins (Fig. 6E).
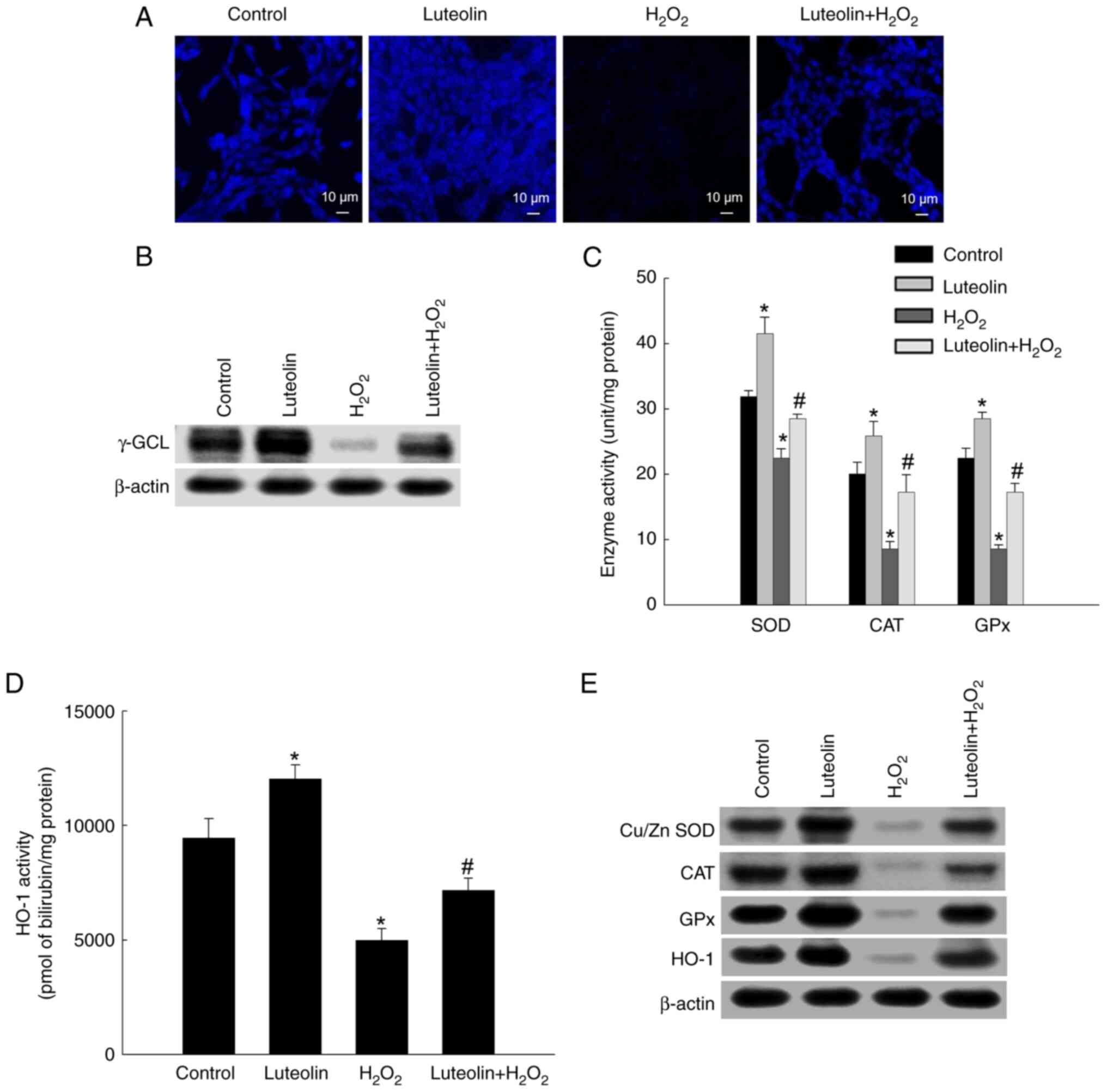 | Figure 6.Effect of luteolin on antioxidant
systems. (A) 7-Amino-4-chloromethylcoumarin dye was used to detect
intracellular GSH levels. (B) Western blot analysis with antibody
against γ-GCL was assessed. (C) The activities of SOD, CAT, and GPx
were presented as unit per mg of protein. (D) HO-1 enzyme activity
was shown as pmol bilirubin/mg protein. (E) Western blotting
analysis with antibodies for Cu/Zn SOD, CAT, GPx and HO-1. β-actin
was employed as a loading control. *P<0.05 vs. control;
#P<0.05 vs. H2O2. GSH,
glutathione; γ-GCL, γ-glutamylcysteine ligase; SOD, superoxide
dismutase; CAT, catalase; GPx, glutathione peroxidase; HO-1, heme
oxygenase-1. |
Discussion
Flavonoids are categorized based on their molecular
structures into flavan-3-ols, flavones, flavonols, flavanones,
isoflavones, and anthocyanins (27). These substances are abundantly
found in coffee, fruit, vegetables and cocoa-containing products
(28). Flavonoids are known for
broad therapeutic benefits, including anticancer, antioxidant,
anti-inflammatory, anti-microbial and antiangiogenic effects
(29). Extensive research,
including our prior studies, has demonstrated that luteolin induces
apoptosis in various types of cancer cells, such as human colon,
melanoma, lung, cervical, monocytic leukemia and breast cancer
cells (23,30–32).
Notably, Liu et al (33)
reported the protective effects of luteolin against angiotensin
II-induced renal damage in apolipoprotein E-deficient mice. The
present study evaluated the antioxidant potential of luteolin
against H2O2-induced oxidative stress in lung
fibroblasts and demonstrated that luteolin inhibited
H2O2-mediated cellular damage by upregulating
antioxidant enzymes.
ROS induce cellular damage and cause disease
progression, including chronic obstructive pulmonary disease (COPD)
and pulmonary fibrosis (34,35).
Here, luteolin showed potent scavenging capabilities for
O2− and ·OH in a cell-free system.
Additionally, pretreatment with luteolin resulted in a significant
decrease in intracellular levels of ROS. Given its efficacy in
neutralizing ROS, including O2− and ·OH,
luteolin may serve as a promising therapeutic agent for management
and treatment of conditions such as COPD and pulmonary
fibrosis.
ROS-induced lipid peroxidation compromises cellular
membranes (36). Polyunsaturated
fatty acids, particularly those vulnerable to ROS, undergo
peroxidation, leading to a cascade of free radical reactions
(34,37,38).
Here, H2O2 strongly promoted lipid
peroxidation and TBARS production; however, luteolin inhibited
lipid peroxidation in the cell membranes. GPx catalyzes conversion
of H2O2 into water and lipid peroxides into
their corresponding alcohols (6).
Moreover, bilirubin, a product of HO-1 activity, offers protection
against lipid peroxidation (39).
The present study indicates that luteolin directly scavenges ROS
and upregulates antioxidant enzymes such as GPx and HO-1, thereby
mitigating lipid peroxidation. Additionally, luteolin decreased DNA
damage and phospho-H2A.X protein levels that were elevated by
H2O2 treatment. The present study
investigated proteins associated with the mitochondrial cell death
pathway to gain insight into the mechanisms of apoptosis as
mitochondria-mediated apoptosis can be induced in response to
H2O2 exposure (40,41).
Luteolin suppressed active caspase-9 and caspase-3 while lowering
the levels of the pro-apoptotic protein Bax and increasing the
levels of anti-apoptotic protein Bcl-2. As luteolin attenuated
cellular lipid peroxidation and DNA damage and inhibits
H2O2-mediated cell apoptosis in the present
study, we focused on the antioxidant capacity of luteolin to
decrease the adverse effect of H2O2.
The cellular antioxidant system, comprising enzymes
such as SOD, CAT, GPx, and HO-1, serves a crucial role in
mitigating oxidative stress-induced cellular damage (42,43).
As evidenced by CMAC staining and western blotting, luteolin
pretreatment restored H2O2 mitigated GSH and
γ-GCL levels. H2O2 treatment reduced levels
and enzymatic activity of SOD, CAT, GPx, and HO-1 proteins, which
were subsequently restored by luteolin pretreatment. Previous
research has indicated that luteolin upregulates NRF2 and HO-1
expression, thereby decreasing H2O2-induced
oxidative damage in intestinal epithelial cells (44). Additionally, luteolin is known to
promote autophagy and antioxidant processes via activation of the
p62/KEAP1/NRF2 pathway, which has been identified for its
neuroprotective effect (45).
Luteolin scavenges ROS by enhancing antioxidant enzymes by
regulating the NRF2 signaling pathway (46,47).
The NRF2 signaling pathway is integral to cellular antioxidant
defenses and maintenance of redox homeostasis, regulating the
expression of antioxidant and drug-metabolizing enzymes such as
SOD, CAT, GPx, and HO-1 (48). The
present study demonstrates that luteolin activates these
antioxidant enzymes to counteract
H2O2-induced oxidative stress, suggesting
induction of these enzymes via the NRF2 signaling pathway. Taken
together, the present results suggested that luteolin can prevent
ROS generation by mitigating cellular damage and improving
antioxidant enzyme activity.
In conclusion, luteolin inhibited
H2O2-induced lipid peroxidation, DNA damage,
and cell apoptosis while augmenting the activity of cellular
antioxidant enzymes (CAT, SOD, GPx, and HO-1), thus safeguarding
V79-4 cells from oxidative harm. These properties position luteolin
as a potential agent for protecting lung fibroblasts against
oxidative damage, warranting further clinical investigation to
assess its efficacy in alleviating oxidative stress-associated
pulmonary conditions.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Research
Foundation of Korea, funded by the Ministry of Education (grant no.
RS-2023-00270936) and the Ministry of Science and ICT (grant no.
NRF-2023R1A2C1002770).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PDSMF, DOK, and JWH conceived and designed the study
and wrote the manuscript. DOK, HMULH, MJP, and KAK performed the
experiments and data analysis and interpretation. PDSMF and JWH
confirm the authenticity of all the raw data. PDSMF, DOK, HMULH,
and JWH revised the manuscript for important intellectual content.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shah S, Sun A and Chu XP: Modulation of
ASIC1a by reactive oxygen species through JFK signaling. Int J
Physiol Pathophysiol Pharmacol. 14:276–280. 2022.PubMed/NCBI
|
|
2
|
Banerjee S, Ghosh S, Mandal A, Ghosh N and
Sil PC: ROS-associated immune response and metabolism: A
mechanistic approach with implication of various diseases. Arch
Toxicol. 94:2293–2317. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meher PK and Mishra KP: Radiation
oxidative stress in cancer induction and prevention. J Radiat
Cancer Res. 8:44–52. 2017. View Article : Google Scholar
|
|
4
|
Martins SG, Zilhão R, Thorsteinsdóttir S
and Carlos AR: Linking oxidative stress and DNA damage to changes
in the expression of extracellular matrix components. Front Genet.
12:6730022021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakai T, Takagaki H, Yamagiwa N, Ui M,
Hatta S and Imai J: Effects of the cytoplasm and mitochondrial
specific hydroxyl radical scavengers TA293 and mitoTA293 in
bleomycin-induced pulmonary fibrosis model mice. Antioxidants
(Basel). 10:13982021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ighodaro OM and Akinloye OA: First line
defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and
glutathione peroxidase (GPX): Their fundamental role in the entire
antioxidant defence grid. Alexandria J Med. 54:287–293. 2018.
View Article : Google Scholar
|
|
7
|
Averill-Bates DA: The antioxidant
glutathione. Vitam Horm. 121:109–141. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saxena P, Selvaraj K, Khare SK and
Chaudhary N: Superoxide dismutase as multipotent therapeutic
antioxidant enzyme: Role in human diseases. Biotechnol Lett.
44:1–22. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaushal J, Mehandia S, Singh G, Raina A
and Arya SK: Catalase enzyme: Application in bioremediation and
food industry. Biocatal Agric Biotechnol. 16:192–199. 2018.
View Article : Google Scholar
|
|
10
|
Sharapov MG, Gudkov SV and Lankin VZ:
Hydroperoxide-reducing enzymes in the regulation of free-radical
processes. Biochemistry (Mosc). 86:1256–1274. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ryter SW: Therapeutic potential of heme
oxygenase-1 and carbon monoxide in acute organ injury, critical
illness, and inflammatory disorders. Antioxidants (Basel).
9:11532020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pizzino G, Irrera N, Cucinotta M, Pallio
G, Mannino F, Arcoraci V, Squadrito F, Altavilla D and Bitto A:
Oxidative stress: Harms and benefits for human health. Oxid Med
Cell Longev. 2017:84167632017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nova Z, Skovierova H and Calkovska A:
Alveolar-capillary membrane-related pulmonary cells as a target in
endotoxin-induced acute lung injury. Int J Mol. 20:8312019.
View Article : Google Scholar
|
|
14
|
Sahai E, Astsaturov I, Cukierman E,
DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR,
Hunter T, et al: A framework for advancing our understanding of
cancer-associated fibroblasts. Nat Rev Cancer. 20:174–186. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen C, Hou J, Yu S, Li W, Wang X, Sun H,
Qin T, Claret FX, Guo H and Liu Z: Role of cancer-associated
fibroblasts in the resistance to antitumor therapy, and their
potential therapeutic mechanisms in non-small cell lung cancer.
Oncol Lett. 21:4132021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan X, Liu B, Lu J, Li S, Baiyun R, Lv Y,
Lu Q and Zhang Z: Dietary luteolin protects against HgCl2-induced
renal injury via activation of Nrf2-mediated signaling in rat. J
Inorg Biochem. 179:24–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu J, Li G, He K, Jiang W, Xu C, Li Z,
Wang H, Wang W, Wang H, Teng X and Teng L: Luteolin exerts a marked
antitumor effect in cMet-overexpressing patient-derived tumor
xenograft models of gastric cancer. J Transl Med. 13:422015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Luo Y, Qiao T, Wu Z and Huang Z:
Luteolin sensitizes the antitumor effect of cisplatin in
drug-resistant ovarian cancer via induction of apoptosis and
inhibition of cell migration and invasion. J Ovarian Res.
11:932018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu Q, Zhang M, Ying Q, Xie X, Yue S, Tong
B, Wei Q, Bai Z and Ma L: Decrease of AIM2 mediated by luteolin
contributes to non-small cell lung cancer treatment. Cell Death
Dis. 10:2182019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu H, Yang T, Liu X, Tian Y, Chen X, Yuan
R, Su S, Lin X and Du G: Luteolin synergizes the antitumor effects
of 5-fluorouracil against human hepatocellular carcinoma cells
through apoptosis induction and metabolism. Life Sci. 144:138–147.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Imran M, Rauf A, Abu-Izneid T, Nadeem M,
Shariati MA, Khan IA, Imran A, Orhan IE, Rizwan M, Atif M, et al:
Luteolin, a flavonoid, as an anticancer agent: A review. Biomed
Pharmacother. 112:1086122019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fernando PDSM, Piao MJ, Zhen AX, Ahn MJ,
Yi JM, Choi YH and Hyun JW: Extract of cornus officinalis protects
keratinocytes from particulate matter-induced oxidative stress. Int
J Med Sci. 17:63–70. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang KA, Piao MJ, Ryu YS, Hyun YJ, Park
JE, Shilnikova K, Zhen AX, Kang HK, Koh YS, Jeong YJ and Hyun JW:
Luteolin induces apoptotic cell death via antioxidant activity in
human colon cancer cells. Int J Oncol. 51:1169–1178. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cinelli G, Sbrocchi G, Iacovino S,
Ambrosone L, Ceglie A, Lopez F and Cuomo F: Red wine-enriched olive
oil emulsions: Role of wine polyphenols in the oxidative stability.
Colloid Interfac. 3:592019. View Article : Google Scholar
|
|
25
|
Herath HMUL, Piao MJ, Kang KA, Zhen AX,
Fernando PDSM, Kang HK, Yi JM and Hyun JW: Hesperidin exhibits
protective effects against PM2.5-mediated mitochondrial damage,
cell cycle arrest, and cellular senescence in human HaCaT
keratinocytes. Molecules. 27:48002022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang KA, Zhang R, Chae S, Lee SJ, Kim J,
Kim J, Jeong J, Lee J, Shin T, Lee NH and Hyun JW: Phloroglucinol
(1,3,5-trihydroxybenzene) protects against ionizing
radiation-induced cell damage through inhibition of oxidative
stress in vitro and in vivo. Chem Biol Interact. 185:215–226. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dias MC, Pinto DCGA and Silva AMS: Plant
flavonoids: Chemical characteristics and biological activity.
Molecules. 26:53772021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rudrapal M and Chetia D: Plant flavonoids
as potential source of future antimalarial leads. Sys Rev Pharm.
8:13–18. 2017. View Article : Google Scholar
|
|
29
|
Ullah A, Munir S, Badshah SL, Khan N,
Ghani L, Poulson BG, Emwas AH and Jaremko M: Important flavonoids
and their role as a therapeutic agent. Molecules. 25:52432020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang KA, Zhang R, Piao MJ, Zhen AX, Herath
HMUL, Fernando PDSM and Hyun JW: Luteolin triggered apoptosis in
human colon cancer cells mediated by endoplasmic reticulum stress
signaling. Food Suppl Biomater Health. 2:e242022. View Article : Google Scholar
|
|
31
|
Kang KA, Piao MJ, Hyun YJ, Zhen AX, Cho
SJ, Ahn MJ, Yi JM and Hyun JW: Luteolin promotes apoptotic cell
death via upregulation of Nrf2 expression by DNA demethylase and
the interaction of Nrf2 with p53 in human colon cancer cells. Exp
Mol Med. 51:1–14. 2019. View Article : Google Scholar
|
|
32
|
Park J, Kang KA, Zhang R, Piao MJ, Park S,
Kim JS, Kang SS and Hyun JW: Antioxidant and cytotoxicity effects
of luteolin. Toxicol Res. 22:391–395. 2006.
|
|
33
|
Liu YS, Yang Q, Li S, Luo L, Liu HY, Li XY
and Gao ZN: Luteolin attenuates angiotensin II-induced renal damage
in apolipoprotein E-deficient mice. Mol Med Rep. 23:1572021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boukhenouna S, Wilson MA, Bahmed K and
Kosmider B: Reactive oxygen species in chronic obstructive
pulmonary disease. Oxid Med Cell Longev. 2018:57303952018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Son B, Kwon T, Lee S, Han I, Kim W, Youn H
and Youn B: CYP2E1 regulates the development of radiation-induced
pulmonary fibrosis via ER stress-and ROS-dependent mechanisms. Am J
Physiol Lung Cell Mol Physiol. 313:L916–L929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Su LJ, Zhang JH, Gomez H, Murugan R, Hong
X, Xu D, Jiang F and Peng ZY: Reactive oxygen species-induced lipid
peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med
Cell Longev. 2019:50808432019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee JS, Kim YR, Song IG, Ha SJ, Kim YE,
Baek NI and Hong EK: Cyanidin-3-glucoside isolated from mulberry
fruit protects pancreatic β-cells against oxidative stress-induced
apoptosis. Int J Mol Med. 35:405–412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Upadhyay S, Vaish S and Dhiman M: Hydrogen
peroxide-induced oxidative stress and its impact on innate immune
responses in lung carcinoma A549 cells. Mol Cell Biochem.
450:135–147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Campbell NK, Fitzgerald HK and Dunne A:
Regulation of inflammation by the antioxidant haem oxygenase 1. Nat
Rev Immunol. 21:411–425. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park C, Lee H, Noh JS, Jin CY, Kim GY,
Hyun JW, Leem SH and Choi YH: Hemistepsin a protects human
keratinocytes against hydrogen peroxide-induced oxidative stress
through activation of the Nrf2/HO-1 signaling pathway. Arch Biochem
Biophys. 691:1085122020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hua W, Li S, Luo R, Wu X, Zhang Y, Liao Z,
Song Y, Wang K, Zhao K, Yang S and Yang C: Icariin protects human
nucleus pulposus cells from hydrogen peroxide-induced
mitochondria-mediated apoptosis by activating nuclear factor
erythroid 2-related factor 2. Biochim Biophys Acta Mol Basis Dis.
1866:1655752020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oh Y, Ahn CB, Nam KH, Kim YK, Yoon NY and
Je JY: Amino acid composition, antioxidant, and cytoprotective
effect of blue mussel (Mytilus edulis) hydrolysate through the
inhibition of caspase-3 activation in oxidative stress-mediated
endothelial cell injury. Mar Drugs. 17:1352019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim EN, Lee HS and Jeong GS:
Cudratricusxanthone O inhibits H2O2-induced
cell damage by activating Nrf2/HO-1 pathway in human chondrocytes.
Antioxidants (Basel). 9:7882020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xia Y, Tan W, Yuan F, Lin M and Luo H:
Luteolin attenuates oxidative stress and colonic hypermobility in
water avoidance stress rats by activating the Nrf2 signaling
pathway. Mol Nutr Food Res. 68:e23001262024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tan X, Yang Y, Xu J, Zhang P, Deng R, Mao
Y, He J, Chen Y, Zhang Y, Ding J, et al: Luteolin exerts
neuroprotection via modulation of the p62/Keap1/Nrf2 pathway in
intracerebral hemorrhage. Front Pharmacol. 10:15512020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rajput SA, Shaukat A, Wu K, Rajput IR,
Baloch DM, Akhtar RW, Raza MA, Najda A, Rafał P, Albrakati A, et
al: Luteolin alleviates aflatoxinB1-induced apoptosis and oxidative
stress in the liver of mice through activation of Nrf2 signaling
pathway. Antioxidants (Basel). 10:12682021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li L, Luo W, Qian Y, Zhu W, Qian J, Li J,
Jin Y, Xu X and Liang G: Luteolin protects against diabetic
cardiomyopathy by inhibiting NF-κB-mediated inflammation and
activating the Nrf2-mediated antioxidant responses. Phytomedicine.
59:1527742019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mapuskar KA, Pulliam CF, Zepeda-Orozco D,
Griffin BR, Furqan M, Spitz DR and Allen BG: Redox regulation of
Nrf2 in cisplatin-induced kidney injury. Antioxidants (Basel).
12:17282023. View Article : Google Scholar : PubMed/NCBI
|