Introduction
Alcohol-related liver disease (ALD) is a pressing
global health issue that leads to a spectrum of liver conditions,
from simple steatosis to severe outcomes such as steatohepatitis,
cirrhosis and hepatocellular carcinoma (1). The oxidative stress and lipid
peroxidation induced by chronic alcohol abuse played a critical
role in the pathogenesis of ALD, contributing to hepatocyte damage
(2,3). In this context, the body's defense
mechanisms against oxidative stress, particularly involving the
nuclear factor erythroid 2-related factor 2 (NRF2) signaling
pathways and key antioxidant enzymes such as catalase (CAT),
glutathione (GSH) and superoxide dismutase (SOD), become essential
for cellular protection (4,5).
The disruption of liver lipid metabolism due to
long-term alcohol consumption further exacerbates the development
of steatosis, highlighting the importance of maintaining cellular
energy balance through proteins such as AMP-activated protein
kinase (AMPK) (6). Ethanol
exposure alters lipid regulators and enzymes, such as sterol
regulatory element binding transcription factor 1 (SREBP-1c) and
stearyl-coA desaturase-1 (SCD1), leading to triglyceride (TG)
accumulation in the liver (7,8).
Despite current treatment options for ALD, including alcohol
cessation, nutritional support and pharmacological interventions,
their efficacy can be limited by side effects and costs (9). Therefore, it is urgent to find
natural products without toxic side effects for regulating and
preventing ALD.
Rosa roxburghii Tratt is a Rosaceae plant
species that is widely cultivated in Guizhou, China (10). This plant is widely recognized for
its multitude of health benefits, such as its ability to act as an
antioxidant, reduce inflammation, lower lipid levels, regulate the
immune system and decrease the body's burden of heavy metals
(11). The lotus is a plant in the
Nymphaeaceae family. Its lotus leaf is a dried leaf that is
considered both a medicinal herb and a food source. Research has
revealed that lotus leaf has antioxidant, anti-inflammatory and
antitumor effects (12). Grape
seed proanthocyanidins have been reported to have strong
antioxidant properties and the ability to eliminate oxygen free
radicals (13). Lactic acid
fermentation is an ancient and cost-effective method used for food
preservation, which is facilitated by lactic acid bacteria (LAB)
(14). This method not only
enhances the nutritional value and flavor of food but also
increases the content of functional components, such as phenolic
substances, organic acids and volatile compounds in fruits and
vegetables (15). Probiotic
fermentation has attracted considerable attention as a convenient
and easily prepared functional food (16). Taking into consideration the
antioxidant properties of the active substances in the
aforementioned plant materials, as well as the safety and
functionality of probiotics, the use of Lactobacillus
plantarum HH-LP56 to ferment these medicinal plants enhances
their sensory attributes and antioxidant properties. The aim of the
present study was to explore how the fermented Rosa roxburghii
Tratt (juice (FRRT) impacts ethanol-induced oxidative stress
and lipid metabolism in Alpha Mouse Liver 12 (AML-12) cells,
focusing on its potential hepatoprotective effects.
By investigating the pharmacological properties of
FRRT and its mechanisms of action in ethanol-induced hepatocyte
injury, the aim of the present study was to shed light on the
therapeutic potential of this fermented juice for liver disease
treatment. Emphasizing the application of FRRT in liver health, the
present study provided valuable insights into its hepatoprotective
effects and potential implications for clinical use.
Materials and methods
Strain, materials and chemicals
Rosa roxburghii tratt juice (freshly
squeezed, 100% purity) was obtained from East China Institute of
Medicinal Plants (Lishui, China), and stored in −4°C refrigerator
for use. Lactobacillus plantarum HH-LP56 was purchased from
Xian Miser Biotechnology Co., Ltd. Proanthocyanidins from grape
seeds were purchased from Shandong Saint Jia De Biotechnology Co.,
Ltd. Dimethyl sulfoxide (DMSO),
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT)
and 95% ethanol were purchased from MilliporeSigma. RIPA Lysis
Buffer (cat. no. P0013C), Increased Bicinchoninic Acid (BCA)
Protein Concentration Kit (cat. no. P0009), Bodipy 500/510 C1, C12
(Fatty Acid Green Fluorescence Probe) (cat. no. C2055), Hoechst
33342 (cat. no. C1022), SOD (cat. no. S0101S), malondialdehyde
(MDA) (cat. no. S0131S) and reactive oxygen species (ROS) assay kit
(cat. no. S0033S) were obtained from Beyotime Institute of
Biotechnology. 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging
capacity assay kit (cat. no. BL897A) and Hydroxyl free radical
(·OH) scavenging capacity assay kit (cat. no. BL1065A) were
purchased from Biosharp, sourced from Langjiako Technology Co.,
Ltd. Vitamin C (VC; cat. no. A009-1-1), TG (cat. no. A110-2-1) and
CAT assay kit (cat. no. A007-1-1) were purchased from Nanjing
Jiancheng Bioengineering Institute. Horseradish peroxidase
(HRP)-conjugated goat anti-mouse (cat. no. sc-2354) and goat
anti-rabbit (cat. no. sc-2357) antibodies were purchased from Santa
Cruz Biotechnology, Inc. Primary antibodies against NAD(P)H:
quinone oxidoreductase 1 (NQO1) (cat. no. 11451-1-AP), kelch like
ECH associated protein 1 (KEAP1) (cat. no. 80744-1-RR) and GAPDH
(cat. no. 60004-1-lg) (all 1:1,000 ratio, respectively) were
purchased from Proteintech Group, Inc. Primary antibodies against
AMPK (cat. no. PAB44300), NRF2 (cat. no. PAB37815), SCD1 (cat. no.
RMAB49932), and SREBP-1c (cat. no. PAB39550) (1:1,000,
respectively) were purchased from Bioswamp; Wuhan Bienle
Biotechnology Co., Ltd.
Preparation of FRRT
A total of 50 ml of Rosa roxburghii Tratt
juice were enzymatically treated with tannase and pectinase at 45°C
for 100 min, while stirring and filtering to remove precipitates.
Prior to preparing the additives, 0.012% (v/v) trichloro-sucrose
was dissolved in water at 70°C. A total of 1 g each of lotus leaf
extract and grape seed proanthocyanidin powder were separately
dissolved in water at ~45°C to form colloidal solutions. When
mixing, the procedure started by adding the dissolved
trichloro-sucrose, followed by the lotus leaf extract and grape
seed proanthocyanidin colloids, followed by stirring for 20 min to
ensure uniformity. For particle microencapsulation, the mixture was
heated to 65°C, homogenized under a pressure of 200 MPa, then
cooled to ~37°C before inoculating with the plant lactobacillus
HH-LP56 for fermentation. After 24 h, the fermentation juice
production process was completed with ultrasonic treatment (20–22
kHz).
Determination of active substance
concentration and antioxidant activity in FRRT and unfermented Rosa
roxburghii Tratt (RRT)
The fermented product was chemically characterized
by determining the active substance concentration and antioxidant
activity in FRRT. The biological activity of the fermented product
was assessed by measuring the VC and SOD content. The SOD content
in the fruit juice samples was determined using the WST-1 method in
an enzyme-linked immunosorbent assay reader, while the VC content
was assessed using UV–Visible spectrophotometry (UV–Vis) at a
wavelength of 536 nm. The process involved extracting the VC
content, leveraging its specific absorption characteristics at a
defined wavelength, and measuring the absorbance intensity to
quantify its concentration in the fruit juice. While the
antioxidant properties were determined by evaluating OH and DPPH
radical scavenging ability in FRRT and RRT juice using specific
assay kits according to the manufacturer's protocol.
Culture of AML-12 cells
AML-12 cells were obtained from the Cell Bank of the
Chinese Academy of Sciences in Shanghai, China. The cells were
cultured in Dulbecco's Modified Eagle's Medium (DMEM; HyClone;
Cytiva) supplemented with 10% fetal bovine serum (FBS; Hangzhou
Dacheng Biotechnology Co., Ltd.), penicillin (100 U/ml),
streptomycin (100 µg/ml), dexamethasone (40 ng/ml), insulin (5
mg/ml), transferrin (5 µg/ml) and sodium selenite (5 ng/ml) at 37°C
under a 95% air and 5% CO2 atmosphere. Passaging was
performed when the cells covered approximately the entire surface
of the culture flask, at a ratio of 1:3 to 1:5. Cells from the 3rd
to 8th passages in the logarithmic growth phase were selected for
subsequent experiments. Before cell treatment, both the FRRT and
RRT were filtered using a 0.22-µm membrane.
Cell viability assay
Cell viability was assessed using the MTT assay.
Hepatocytes were seeded in a 96-well culture plate at a density of
2×104 cells per well and cultured until reaching 80%
confluency. Subsequently, the cells were divided into different
groups. The negative control (NC) group received culture medium
without FRRT or ethanol, while the ethanol (EtOH) group was exposed
to various concentrations of ethanol (25, 50, 100, 200, 400 and 800
mM) for 24, 36 and 48 h. The experimental group was treated with
different concentrations of FRRT (2.5, 5, 10, 20, 40, 80 and 160
mg/ml) for 24 h in a 37°C incubator. The positive control group was
treated with different concentrations of RRT (2.5, 5, 10, 20, 40
mg/ml) for 24 h. After the respective incubation periods, MTT
solution was added to each well, and the cells were further
incubated in darkness at 37°C for 4 h. Following the dissolution of
formazan crystals with DMSO (150 µl/well), the absorbance was
measured at 490 nm using a microplate reader. Cell viability was
calculated using the following formula:
Determination of intracellular TG
AML-12 cells were inoculated on 24-well plates at a
density of 5×105 cells per well. After incubation for 24
h in a 37°C incubator, the cells were divided into different
groups: NC group, EtOH group (200 mM ethanol), lower dose FRRT
group (2.5 mg/ml), higher-dose FRRT group (5 mg/ml) and RRT
positive control group (10 mg/ml). AML-12 cells were pretreated
with FRRT (2.5, 5 mg/ml) and RRT (10 mg/ml) for 2 h, respectively,
and then stimulated with or without EtOH (200 mM) for 24 h. After
discarding the supernatant of the prepared cells, they were washed
with PBS and the cell pellet were retained. A total of 0.2 to 0.3
ml of homogenization medium was added for homogenization. Following
the manufacturer's protocol, TG assay kit was used to measure the
TG content in the homogenate. A BCA protein assay kit was used to
determine the protein concentration in the homogenate.
Determination of MDA, CAT, GSH and
SOD
After inoculating AML-12 cells on 6-well plates at a
density of 2×105 cells per well, they were grouped as
per section according to the aforementioned method. Following the
cultivation period, the supernatant was discarded and the activity
levels of MDA, CAT, GSH and SOD were assessed in the liver cell
homogenate using specific assay kits. Additionally, a BCA protein
assay kit was used to determine the protein concentration in the
liver cell homogenate.
Hoechst 33342 nuclear staining
The following culture methods were performed as
aforementioned. After the intervention, cells were stained with
Hoechst 33342 staining at 37°C for 10 min. Following staining, the
cells were washed twice with PBS, ensuring that enough PBS was
added to evenly cover them. Finally, the results were examined
under an inverted fluorescence microscope.
Bodipy dying
Following the intervention, cells were stained with
Bodipy dye at 37°C for 20 min and then examined under an inverted
fluorescence microscope.
ROS production
After being cultured, the cell culture medium was
treated with the fluorescent dye DCFH-DA at a final concentration
of 10 µmol/l. The cells were then cultured in a cell incubator at
37°C for 20 min. Subsequently, the cells were washed three times
with a serum-free cell medium. Finally, images of three different
areas of each well were captured using the Olympus IX84 inverted
fluorescence microscope.
Reverse transcription-quantitative
(RT-qPCR) analysis
After isolating total RNA from AML-12 cells using
the TRIzol® method (Invitrogen; Thermo Fisher
Scientific, Inc.), a reverse transcription reaction [Accurate
Biotechnology (Hunan) Co., Ltd.] was performed following the cDNA
synthesis kit protocol. qPCR was performed using the SYBR Green
premix Pro Taq HS qPCR Kit (Accurate Biotechnology (Hunan) Co.,
Ltd.) under the following conditions: Initial denaturation at 95°C
for 30 min, denaturation at 95°C for 5 sec, annealing/extension at
60°C for 30 sec and 40 cycles. A periodic threshold (Cq) was
determined and the relative expression of the target mRNA was
quantified by the 2−ΔΔCq method (17) with GAPDH as a normalized
reference gene. The qPCR primers used in the present study were
synthesized by Hunan Accurate Biotechnology Co., Ltd. and their
sequences are listed in Table
I.
 | Table I.Primer sequence for reverse
transcription-quantitative PCR. |
Table I.
Primer sequence for reverse
transcription-quantitative PCR.
| Target genes | Primer sequences
(5′-3′) |
|---|
| NQO1 | Forward:
CAGCCAATCAGCGTTCGGTA |
|
| Reverse:
CTTCATGGCGTAGTTGAATGATGTC |
| NRF2 | Forward:
TCTTGGAGTAAGTCGAGAAGTGT |
|
| Reverse:
GTTGAAACTGAGCGAAAAAGGC |
| GAPDH | Forward:
TGTGTCCGTCGTGGATCTGA |
|
| Reverse:
TTGCTGTTGAAGTCGCAGGAG |
| SCD1 | Forward:
ACCCGGCTGTCAAAGAGAAG |
|
| Reverse:
CGCAAGAAGGTGCTAACGAAC |
|
SREBP-1c | Forward:
TCAGAGCCGTGGTGAGAAG |
|
| Reverse:
GCAAGAAGCGGATGTAGTCG |
| KEAP1 | Forward:
GTCGCCCTGTGCCTCTATG |
|
| Reverse:
CGCCAATCCTCCGTGTCAA |
| HO-1 | Forward:
AAGCCGAGAATGCTGAGTTCA |
|
| Reverse:
GCCGTGTAGATATGGTACAAGGA |
| AMPK | Forward:
CTACTTGTCTGGGTCCTTCAACA |
|
| Reverse:
GCTGGTTACTATTGGCTCAGAAG |
Western blot analysis
To quantify protein expression in cells, RIPA lysis
buffer was used to lyse cells (contains 1% phosphatase inhibitor
and 1% protease inhibitor), and a BCA assay kit was used to
determine protein concentration. Each experimental group was
adjusted for protein concentration. A total of 20 µg/lane cell
lysates were subjected to electrophoresis on a 10%
SDS-polyacrylamide gel. Subsequently, 10% SDS-PAGE gels were
electrophoretically separated: 80 V for 30 min and 120 V for 60–90
min. Isolated proteins were transferred to a PVDF membrane at low
temperature using a transfer device (200 mA, 120 min). After
blocking with 5% skim milk at room temperature for 1 h, the
membrane was incubated overnight at 4°C with the primary antibody
against the target protein. TBST (0.1% Tween-20 solution was used
to prepare) was used for washing membranes to remove unbound
antibodies, and secondary antibodies (goat anti-rabbit or
anti-mouse IgG-horseradish peroxidase; cat. nos. sc-2357 and
sc-2354 respectively; Santa Cruz Biotechnology, Inc.) were applied
(1:50,000 dilution;.) at room temperature for 1 h. TBST was used to
remove unbound antibodies after incubation with the secondary
antibody. SuperPico ECL Chemiluminescence Kit (Vazyme Biotech Co.,
Ltd.) was used to visualize protein bands, and digital gel imaging
systems to capture images. Gray-level analysis of the images was
performed using ImageJ software (version 1.53e; National Institutes
of Health), and the relative expression of the target protein was
normalized to the expression of the valet protein GAPDH.
Statistical analysis
All experiments were performed in triplicate. The
experimental data were analyzed using GraphPad Prism 9.5.1 software
(Dotmatics), and the results are presented as the mean ± standard
deviation (SD) of ≥3 independent experiments performed under
identical conditions. For comparisons involving three or more
groups, one-way analysis of variance (ANOVA) followed by Tukey's
post hoc test was used to determine differences. In cases where
there were two groups being compared, unpaired Student's t-test was
conducted. Furthermore, ImageJ software was utilized to quantify
the grayscale values of protein bands. P<0.05 was considered to
indicate a statistically significant difference.
Results
Changes in active substance content
and antioxidant activity during fermentation
Changes in pH, VC content, SOD activity, ·OH and
DPPH radical scavenging rate during the fermentation process of
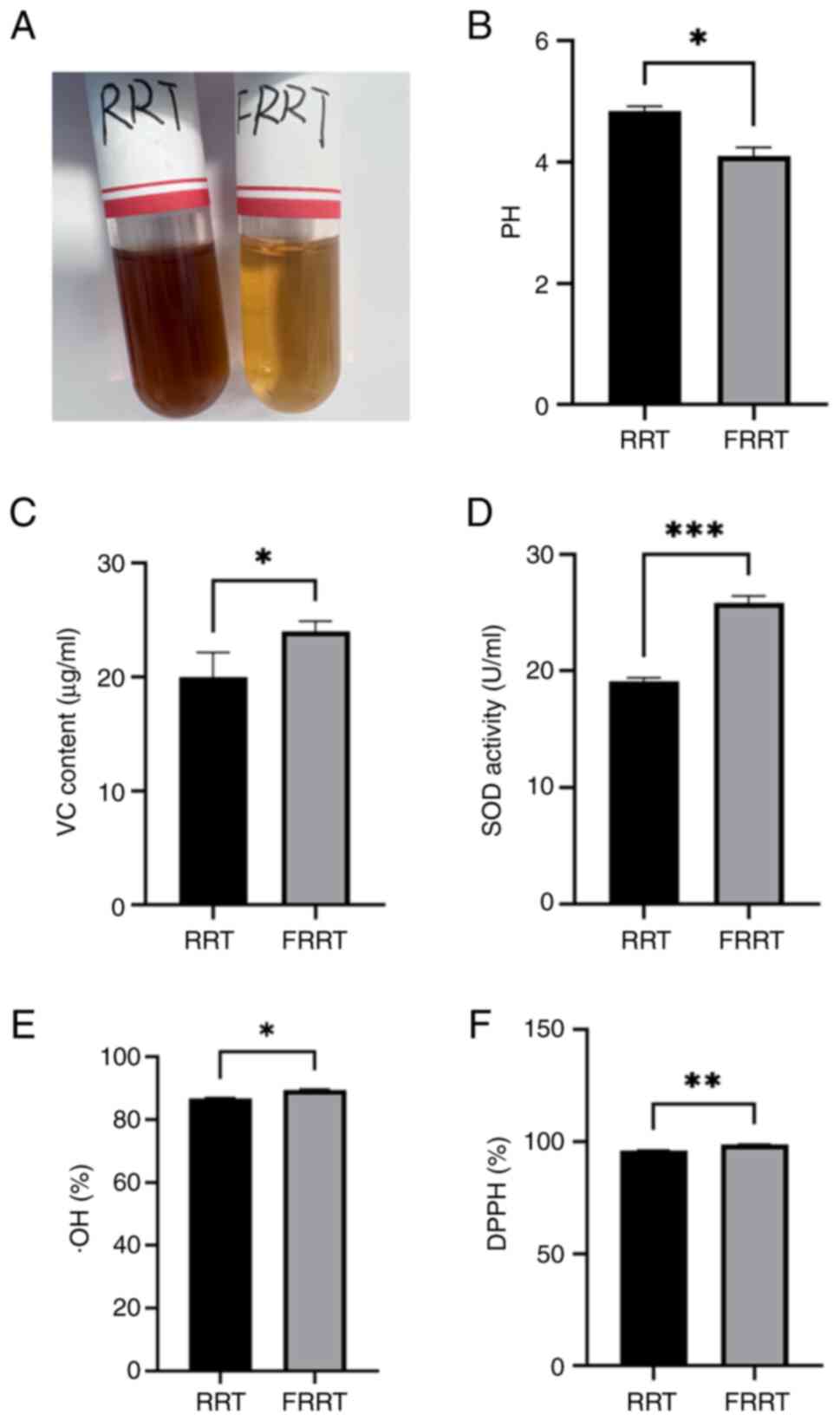
FRRT are demonstrated in Fig. 1.
The results demonstrated that the color of the fermented juice
significantly lightens (Fig. 1A),
and the production of LAB during fermentation led to a decrease in
pH of the FRRT compared with RRT (Fig.
1B). Furthermore, there was a significant increase in VC
content and SOD activity (Fig. 1C and
D). The ·OH and DPPH free radical scavenging abilities of FRRT
were enhanced compared with RRT (Fig.
1E and F), which indicated an improved antioxidant capacity
post-fermentation (P<0.05).
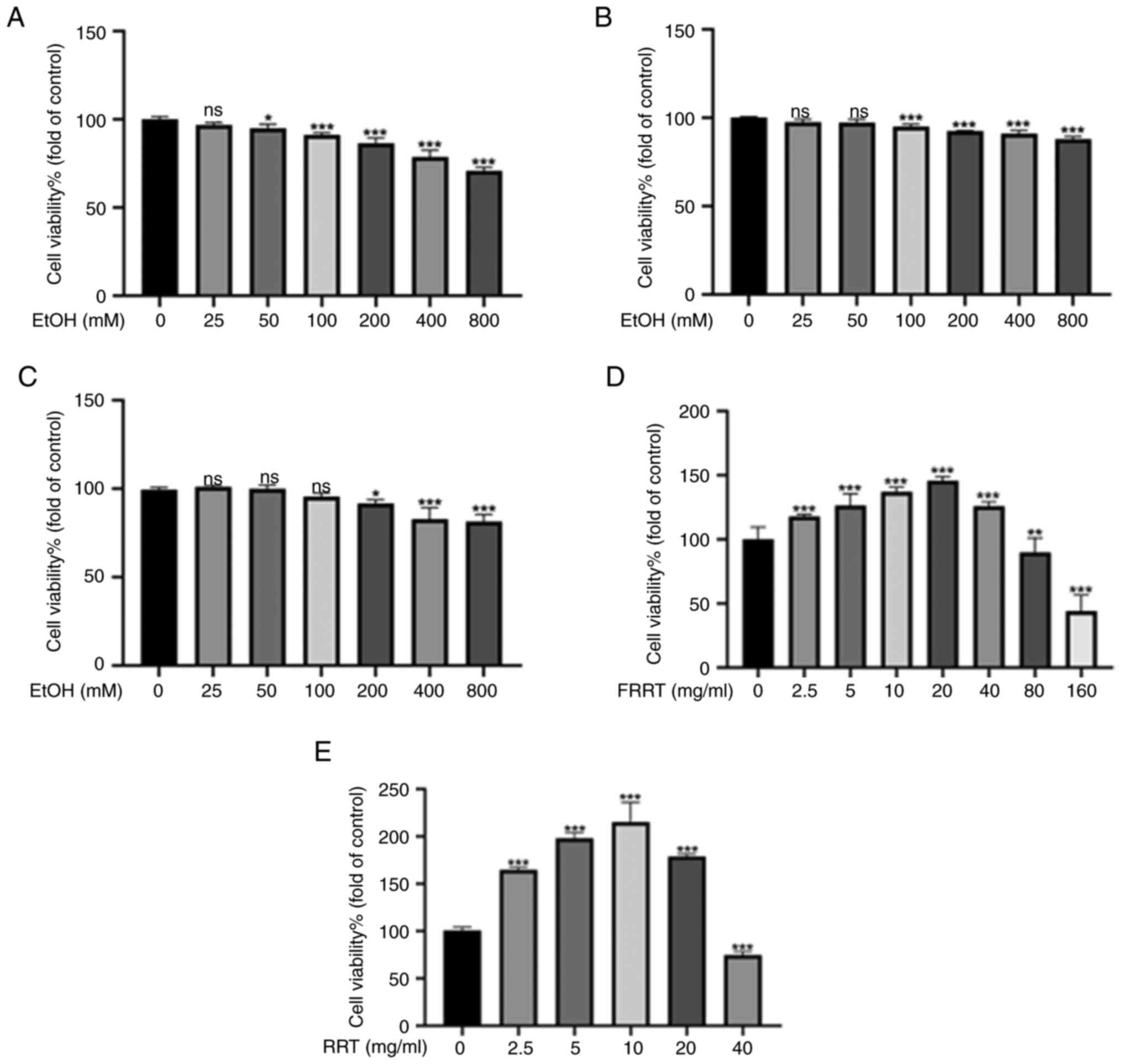
Effect of ethanol and FRRT on AML-12
cell viability
The results revealed that, as compared with the
control group (cells not exposed to ethanol), cell viability
decreased with the increase of ethanol concentration. This trend
was consistent across all three exposure periods (24, 36 and 48 h;
Fig. 2A-C). Specifically, after a
24 h exposure period, cell viability significantly decreased from
100±1.5% in the control group to 86.4±2.9% in cells exposed to 200
mM ethanol (P<0.001). These findings suggested that ethanol
hampers cell viability and inhibits the proliferation of AML-12
cells in a concentration-dependent manner. No significant changes
were observed in the data at 36 and 48 h when compared with the 24
h data. This could potentially be attributed to the volatility of
ethanol. Therefore, the 24 h exposure period and a concentration of
200 mM ethanol were selected to establish an ethanol-induced
hepatocyte injury model. Treatment with lower concentrations of
FRRT (FRRT-L) (2.5–40 mg/ml) significantly increased cell
viability, while higher concentrations (80–160 mg/ml) led to a
decrease in cell viability (P<0.001; Fig. 2D). The results of the preliminary
research revealed that the overall antioxidant effects of 2.5 and 5
mg/ml were greater than those of ≥10 mg/ml (Fig. S1). Therefore, in subsequent
studies, a total of 2.5 and 5 mg/ml as the intervention
concentrations were chosen. Additionally, the results demonstrated
that treatment with low concentrations of RRT (~2.5–20 mg/ml)
significantly increased cell viability, while high concentration of
RRT treatment (40 mg/ml) resulted in a decrease in cell viability
(P<0.001; Fig. 2E). Therefore,
the concentration of 10 mg/ml was selected, which exhibited the
most significant enhancement in cell viability, as the positive
control group.
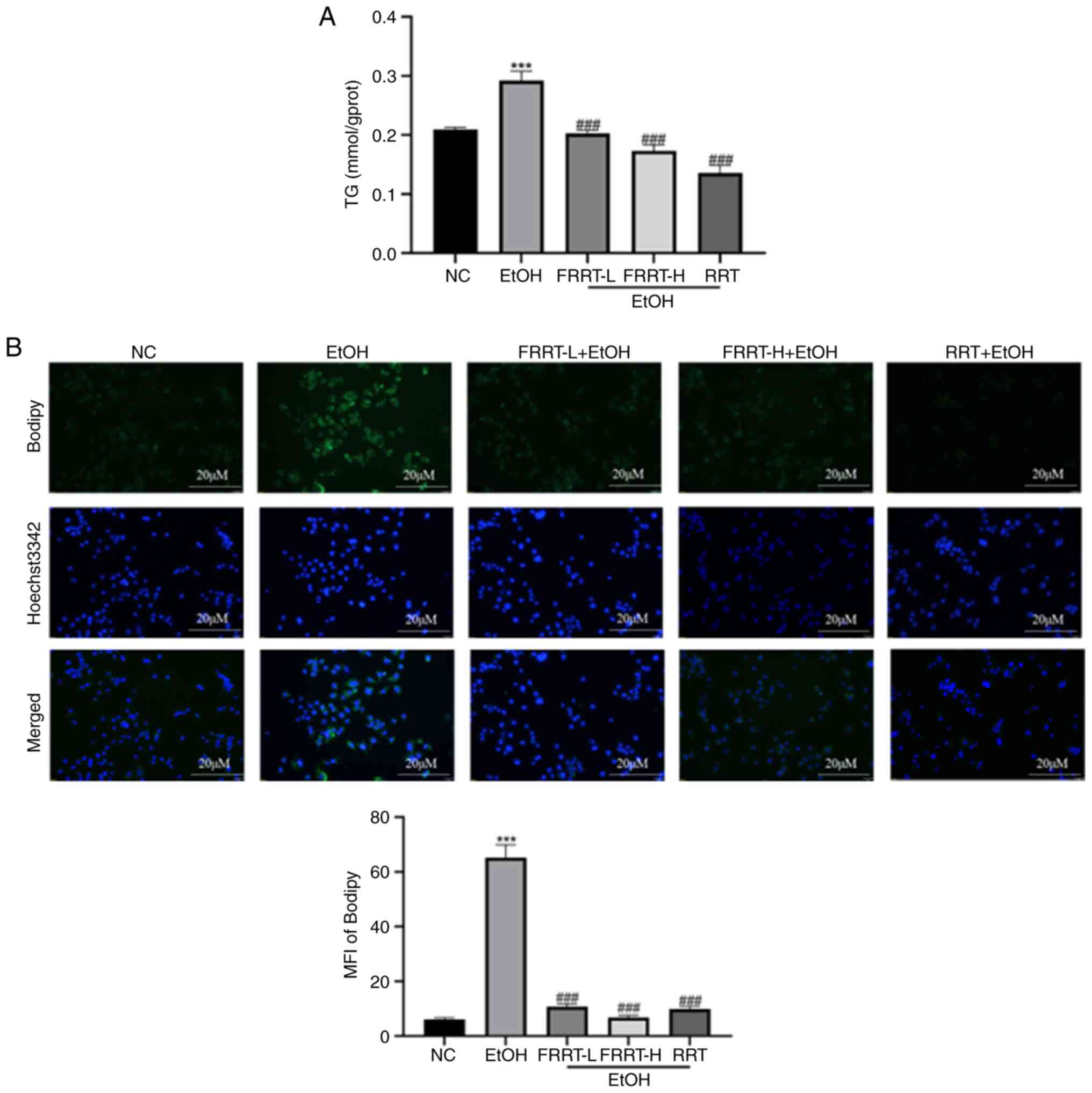
FRRT improves ethanol-induced hepatic
lipid metabolism in AML-12 cells
The results revealed that the EtOH group exhibited a
significant increase in TG content compared with the NC group
(P<0.001; Fig. 3A). However,
pretreatment with FRRT at concentrations of 2.5 and 5 mg/ml
significantly reduced the ethanol-induced increase in TG content in
the AML-12 cells when compared with the EtOH group. This reduction
occurred in a dose-dependent manner (P<0.001). To further
investigate the impact of FRRT on lipid accumulation, BODIPY™
staining was performed. The images obtained from BODIPY staining
revealed a substantial accumulation of lipid droplets in the EtOH
group (Fig. 3B). However, in the
FRRT pretreatment group, both the density and staining intensity of
lipid droplets in the cells were significantly reduced compared
with the ethanol-treated group. These reductions also exhibited a
dose-dependent relationship. These findings suggested that
pretreatment with FRRT at concentrations of 2.5 and 5 mg/ml can
effectively attenuate ethanol-induced TG accumulation and lipid
droplet formation in AML-12 cells. The dose-dependent response
indicated that a higher concentration of FRRT (FRRT-H) may provide
even stronger protection against lipid accumulation.
FRRT enhances hepatic lipid metabolism
by activating the AMPK-SREBP-1c-SCD1 pathway
To elucidate the molecular mechanism underlying the
regulatory effects of FRRT on intracellular lipid metabolism, the
expression levels of pivotal lipogenic genes, namely AMPK,
SREBP-1c and SCD1 were quantified using qPCR. It was
observed that, compared with the NC group, the gene expression of
the SREBP-1c and SCD1 gene was significantly
increased in the EtOH group, while that of the AMPK gene was
decreased (P<0.001). Of note, these alterations in gene
expression were effectively reversed by treatment with FRRT, as
depicted in Fig. 4B. Furthermore,
the protein levels of AMPK, SREBP-1c and SCD1 were examined using
western blotting. Compared with the NC group, the group exposed to
ethanol exhibited a statistically significant decrease in liver
AMPK protein levels (P<0.05). In addition, there was a
significant trend towards increased SREBP-1c and SCD1 protein
levels in the liver; however, this increase did not reach
statistical significance. Markedly, pretreatment with FRRT resulted
in a significant reversal of these alterations, particularly with
FRRT-H exerting pronounced effects on ethanol-induced changes in
AMPK, SREBP-1c and SCD1 protein levels (Fig. 4A).
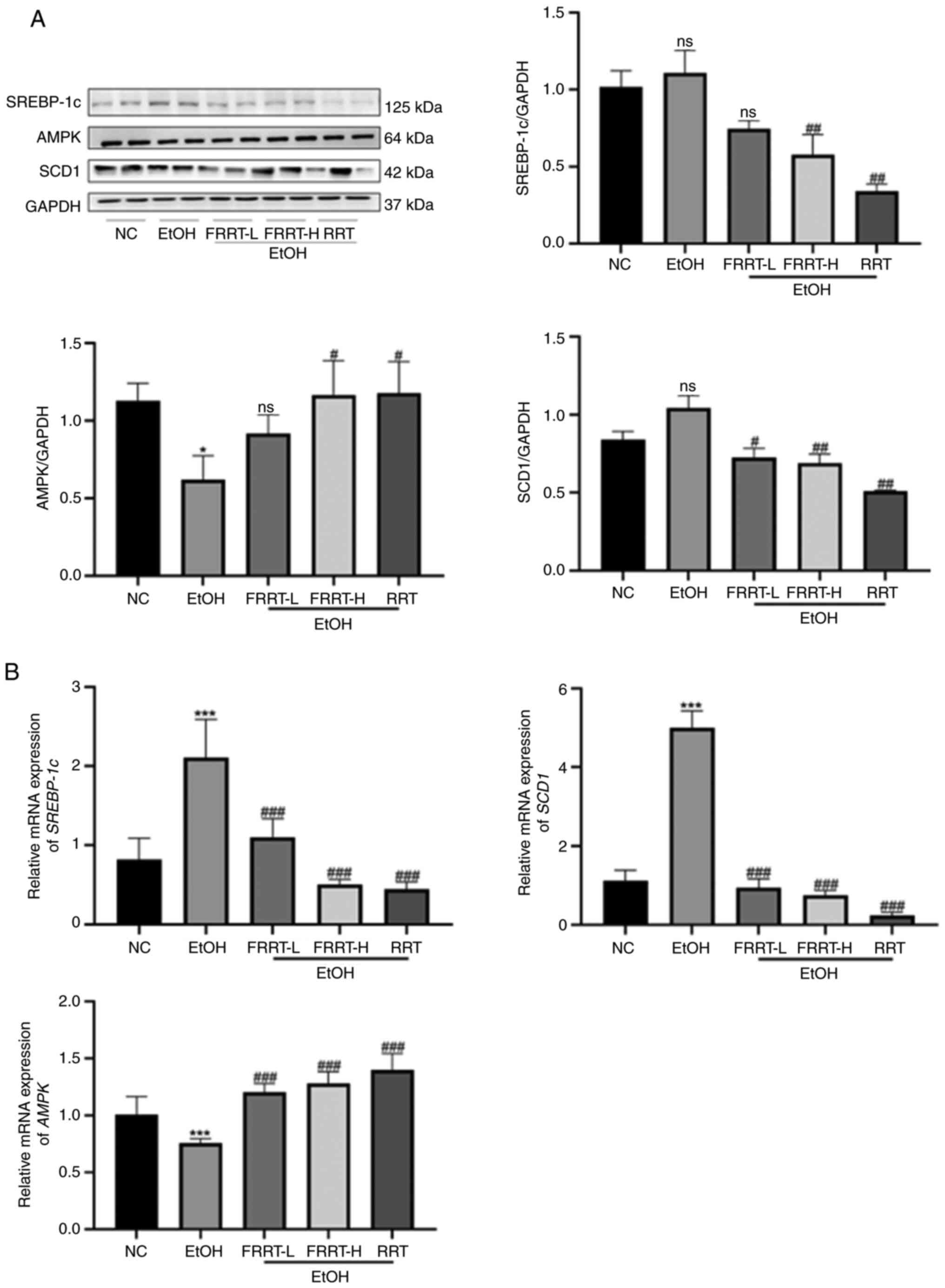 | Figure 4.FRRT enhances hepatic lipid
metabolism by activating the AMPK-SREBP-1c-SCD1 pathway. (A and B)
Western blotting and quantitative PCR were performed to verify the
effects of FRRT on the expression of AMPK, SREBP-1c and SCD1 with
ethanol treatment. *P<0.05 and ***P<0.001 compared with the
NC group; #P<0.05, ##P<0.01 and
###P<0.001 compared with the EtOH group. AMPK,
AMP-activated protein kinase; SREBP-1c, sterol regulatory element
binding transcription factor 1; SCD1, stearyl-coA desaturase-1;
FRRT, fermented Rosa roxburghii Tratt; NC, negative control;
L, low; H, high; RRT, unfermented Rosa roxburghii Tratt; ns,
no significance. |
FRRT alleviates ethanol-induced
hepatic oxidative stress in AML-12 cells
Superoxide anion is recognized as one of the primary
forms of ROS within mitochondria (18). Increased superoxide anion release
leads to oxidative stress and apoptosis (19). To visualize hepatocyte damage,
DCFH-DA staining was used to detect the distribution and quantity
of superoxide free radicals. Antioxidant levels in the cells were
assessed by measuring MDA, SOD, CAT and GSH. The results
demonstrated that ROS levels in AML-12 cells were significantly
increased following ethanol exposure but were reversed following
treatment with FRRT, as indicated by fluorescence intensity results
(Fig. 5A). Compared with the NC
group, the EtOH group exhibited a significant reduction in the
intracellular activities of SOD, CAT and GSH, along with a
significant increase in MDA levels (Fig. 5B-E; P<0.01). These findings
suggested an imbalance in cellular oxidative status, indicating the
presence of oxidative injury. However, the pretreatment of cells
with FRRT at concentrations of 2.5 and 5 mg/ml resulted in
significantly lower MDA levels compared with the EtOH group
(Fig. 5B; P<0.001), suggesting
that FRRT can suppress the ethanol-induced elevation of MDA in a
dose-dependent manner within this concentration range. Furthermore,
the addition of different concentrations of FRRT led to increased
SOD, GSH and CAT levels compared with the EtOH group (Fig. 5C-E; P<0.01). Of note, the lower
dose of FRRT intervention demonstrated a more pronounced reduction
in MDA levels and greater enhancement of GSH, CAT and SOD
activities compared with the RRT group (Fig. 5B-E). Collectively, these
experimental findings confirmed the protective effect of FRRT
against ethanol-induced liver cell damage through the modulation of
intracellular oxidative stress response within a specific
concentration range.
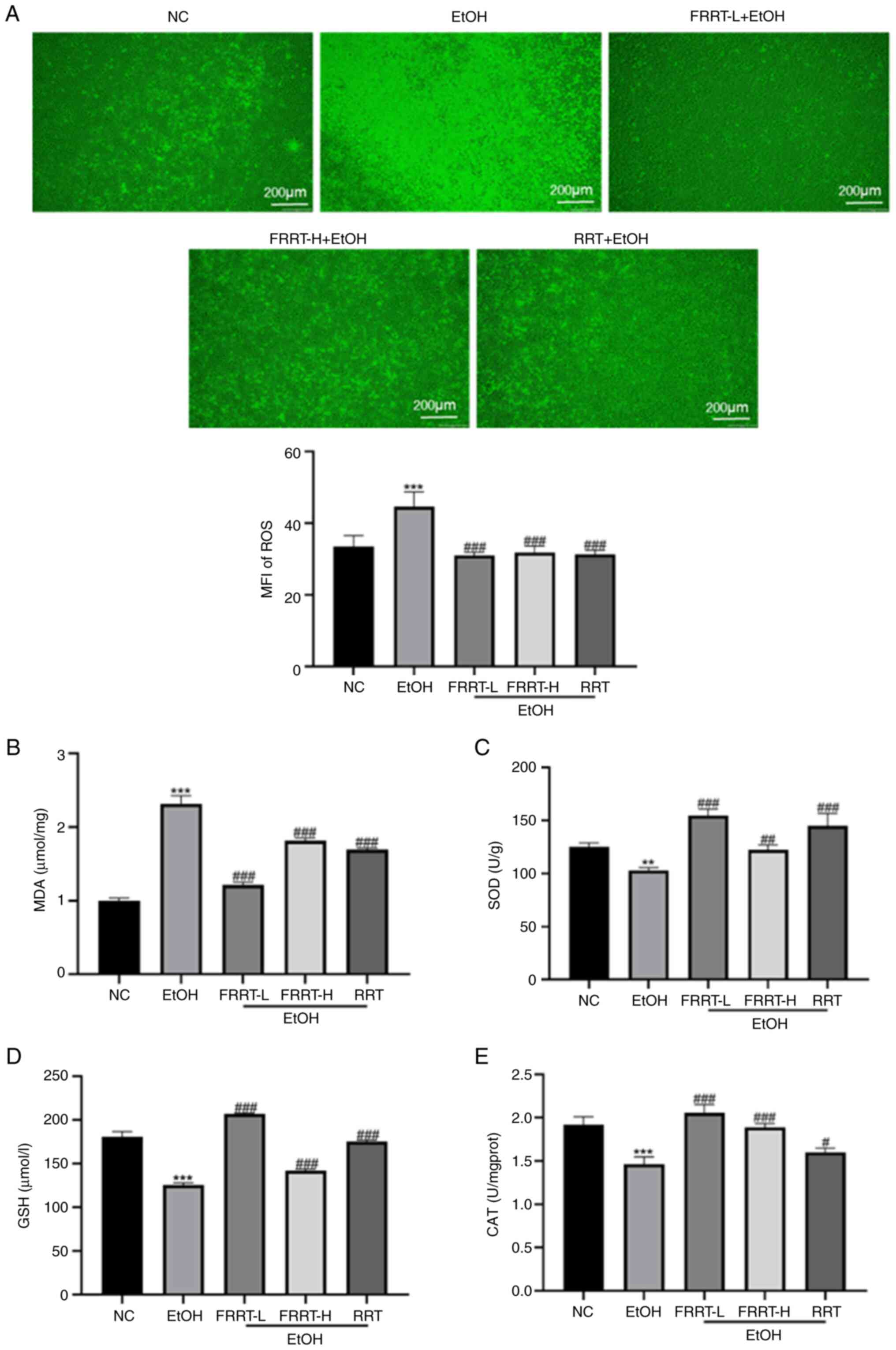 | Figure 5.FRRT alleviates ethanol-induced
hepatic oxidative stress in AML-12 cells. (A) The ROS level in
AML-12 cells. (B) The MDA level in AML-12 cells. (C-E) The
activities of SOD, GSH and CAT in AML-12 cells. **P<0.01 and
***P<0.001 compared with the NC group; #P<0.05,
##P<0.01 and ###P<0.001 compared with
the EtOH group. FRRT, fermented Rosa roxburghii Tratt; ROS,
reactive oxygen species; MFI, mean fluorescence intensity; MDA,
malondialdehyde; SOD, superoxide dismutase; CAT, catalase; GSH,
glutathione; NC, negative control; L, low; H, high; RRT,
unfermented Rosa roxburghii Tratt; ns, no significance. |
FRRT inhibits hepatic oxidative stress
by activating the NRF2-KEAP1-NQO1-heme oxygenase 1 (HO-1)
pathway
To study the molecular mechanism of the FRRT-induced
inhibition of oxidative stress, the total protein levels of NRF2,
NQO1, HO-1 and KEAP1 were detected by western blotting. As
demonstrated in Fig. 6A, compared
with the NC group, the hepatic protein levels of HO-1, NRF2 and
NQO1 were decreased, while the protein level of KEAP1 was increased
in the EtOH group (P<0.05). However, FRRT pretreatment
effectively reversed these changes, leading to the restoration of
NRF2, NQO1 and KEAP1 protein levels. In addition, the gene
expression levels of NRF2, KEAP1, HO-1 and NQO1 were
assessed in AML-12 cells using qPCR. The results included in
Fig. 6B revealed that the
FRRT-treated groups exhibited a significantly increased mRNA
expression of NRF2, HO-1 and NQO1, accompanied by a
decreased expression of KEAP1 compared with that in the EtOH
group (P<0.05). The dose-dependent response suggested that
FRRT-L may potentially exhibit a stronger protective effect against
oxidative stress.
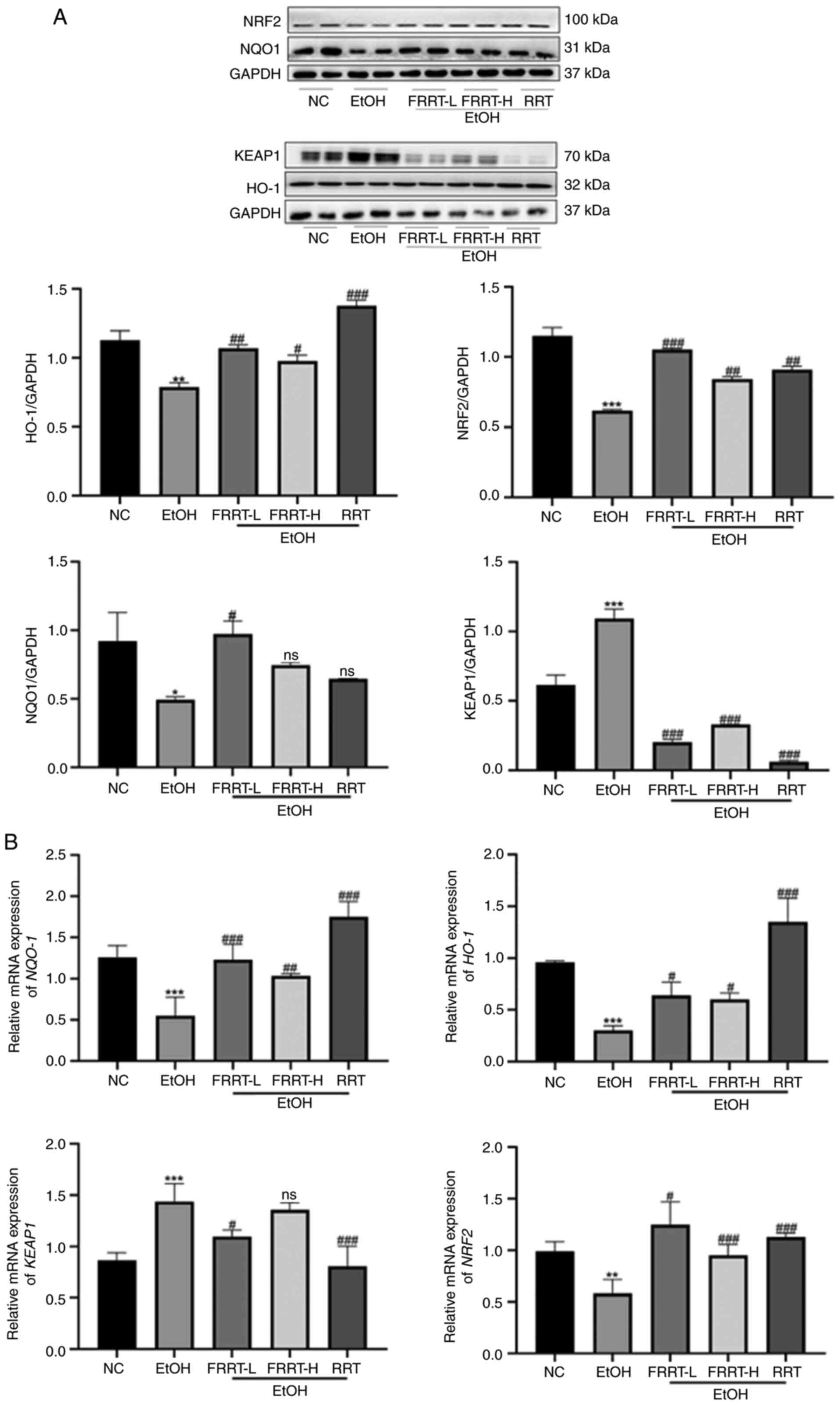 | Figure 6.FRRT inhibits hepatic oxidative
stress via activating NRF2-KEAP1-NQO1-HO-1 pathway. (A) Western
blot analysis verifying the total protein level of NRF2, NQO1, HO-1
and KEAP1. GAPDH was used as an internal control for protein
normalization. (B) Reverse transcription-quantitative PCR verifying
the expression levels of NRF2-KEAP1-related genes, including
NRF2, KEAP1, HO-1 and NQO1. *P<0.05, **P<0.01
and ***P<0.001 compared with the NC group;
#P<0.05, ##P<0.01 and
###P<0.001 compared with the EtOH group. FRRT,
fermented Rosa roxburghii Tratt; NRF2, nuclear factor
erythroid 2-related factor 2; KEAP1, kelch like ECH associated
protein 1; NQO1, quinone oxidoreductase 1; HO-1, heme oxygenase 1;
NC, negative control; L, low; H, high; RRT, unfermented Rosa
roxburghii Tratt; ns, no significance. |
Discussion
ALD remains a prominent global health risk. While
currently there is no ideal drug for the treatment of ALD,
accumulating evidence reveal that natural products can inhibit the
progression of ALD by regulating lipid metabolism, and by
inhibiting oxidative stress, apoptosis and programmed cell death,
among other ways (20–22). The present study successfully
developed a flavorful and refreshing FRRT juice with antioxidant
properties. During fermentation, the pH of the juice was
significantly decreased, indicating an increase in LAB production,
as well as increased SOD and VC activities, leading to a
significant improvement in the free radical scavenging ability.
Further in vitro cell experiments confirmed the protective
effect of FRRT against ethanol-induced liver cell damage. Although
research has revealed that Rosa roxburghii Tratt juice can
effectively prevent chronic alcohol liver injury by reducing
oxidative stress and improving lipid metabolism through modulating
the pathways mediated by nuclear receptor Chimeric Antigen
Receptor, peroxisome proliferator-activated receptors and NRF2,
there is currently lack of studies investigating the effects of
FRRT juice on ALD (23). The
present study demonstrated, for the first time to the best of our
knowledge, the relevance of the findings to human cell lines by
extrapolating from studies such as by Sefried et al
(24), which investigated the
suitability of hepatocyte cell lines HepG2, AML12 and THLE-2 for
insulin signaling and hepatokine gene expression (25). The authors acknowledge the
limitations of the present study, which only involved preliminary
validation of the efficacy of FRRT using AML-12 cells. In order to
ensure the scientific validity and applicability of the research
findings, future studies will be extended to human cell lines to
further validate the biological effects of FRRT.
Oxidative stress in ALD is caused by an imbalance
between free radicals and antioxidants, resulting in increased ROS
and MDA production and decreased antioxidant activity. This damages
cellular components and worsens liver injury, while endogenous
antioxidants such as SOD, CAT and GSH protect against oxidative
damage (25–27). In the present study, the cellular
oxidative stress levels were assessed by measuring the levels of
MDA, SOD, GSH, CAT and ROS. The findings confirmed that hepatocytes
exposed to ethanol exhibited elevated levels of ROS and MDA, a
marker of lipid peroxidation, along with decreased activities of
antioxidant enzymes, such as SOD, CAT and GSH. These findings
indicated increased oxidative stress, impaired antioxidant defense
and increased lipid peroxidation, suggesting cellular toxicity.
However, treatment with FRRT reversed these effects, restoring
antioxidant enzyme activities and reducing lipid peroxidation. This
suggested that FRRT can mitigate oxidative stress and restore
cellular antioxidant capacity in ethanol-induced hepatocyte injury.
In addition, excessive hepatic lipid production disrupts the
cellular redox state, leading to oxidative damage (28). To further investigate the molecular
mechanism of FRRT, the effects of FRRT on the NRF2-KEAP1-NQO1-HO-1
pathway were studied. The NRF2-KEAP1-antioxidant response element
(ARE) system is a defense mechanism that helps maintain cellular
homeostasis and counteract oxidative stress. KEAP1 acts as a
negative regulator of NRF2, but under oxidative stress conditions,
KEAP1 undergoes a conformational change and dissociates from NRF2,
leading to the activation and nuclear translocation of NRF2
(29,30). Subsequently, NRF2 binds to ARE in
the nucleus, initiating the transcriptional activation of several
downstream genes. This activation results in the upregulation of
protective proteins, including Heme-oxygenase 1 (HO-1), NQO1 and
SOD, among others, which play crucial roles in regulating
antioxidant responses (31,32).
HO-1 is an important antioxidant enzyme regulated by NRF2, playing
a crucial role in cellular redox homeostasis and serving as a
significant cellular protective enzyme (33). Upon stimulation, NRF2 is activated
and translocated to the nucleus, where it binds with ARE elements
to upregulate HO-1, thereby reducing oxidative stress (34). The study found that the targeted
overexpression of NQO1, specifically in AML-12 cells of mice,
effectively mitigated the excessive production of ROS and lipid
peroxidation caused by chronic alcohol exposure (35). The present study observed that FRRT
reversed the decreased expression of NRF2 and NQO1, as well as the
increased ethanol-induced KEAP1 expression in AML-12 cells. This
suggested that FRRT may activate the NRF2 pathway and enhance
antioxidant defense against alcohol-induced oxidative stress. Due
to its antioxidant effects, NRF2 has been widely studied as a
potential anti-inflammatory target, as it can reduce ROS levels and
restore redox homeostasis, thus protecting cells (36).
Furthermore, ROS produced during ethanol metabolism
act as critical regulatory factors that can influence lipid
metabolism. One important regulator of cellular metabolism is AMPK.
When activated, AMPK can restore impaired fatty acid β-oxidation
and influence lipid metabolism. AMPK activation inhibits hepatic
fatty acid synthesis by suppressing SREBP-1c, the primary regulator
of gene expression related to hepatic lipogenesis (37,38).
Another vital protein involved in lipid metabolism is SCD1. It acts
as a central lipogenic enzyme that catalyzes the conversion of
saturated fatty acids to unsaturated fatty acids. Its preferred
substrates include various structural lipids such as TG,
cholesterol esters and membrane phospholipids (39). In the present study, it was
observed that FRRT reduced the increased levels of ethanol-induced
TG in AML-12 cells. It also decreased the expression of SCD1 and
SREBP-1c, while increasing the expression of AMPK in AML-12 cells.
These findings indicated that FRRT has beneficial effects in
mitigating alcohol-induced liver injury by regulating lipid
metabolism and reducing lipid accumulation.
There is cross-talk between the AMPK and NRF2
pathways; when AMPK is activated, it phosphorylates NRF2, promoting
its translocation into the nucleus. This enhances the cell's
antioxidant capacity by activating genes involved in antioxidant
defense mechanisms (40–43). The study demonstrated that
empagliflozin may mitigate the onset of ferroptosis by facilitating
the AMPK-mediated NRF2 activation pathway (44). In xanthine-treated mouse embryonic
fibroblasts, NRFR/HO-1 expression was found to be reduced in the
context of AMPKα1 deletion (45).
These examples appear to show that there is an interdependent
signaling relationship between energy and redox homeostasis through
AMPK and NRF2. Activated AMPK appears to enhance NRF2 signaling. By
contrast, NRF2 responds negatively to this enhancement by restoring
the redox and metabolic balance, with some delay, thus limiting the
signal to activate AMPK (46).
In conclusion, the present study demonstrated that
FRRT significantly rescued ethanol-induced hepatocyte injury by
alleviating hepatic oxidative stress and improving lipid
metabolism. The antioxidant effects are mainly achieved through the
NRF2-KEAP1-NQO1-HO-1 axis, while the lipid-lowering effects are
regulated through the AMPK-SREBP-1c-SCD1 pathway (Fig. 7). These discoveries offer new
perspectives on the application of natural products for preventing
and managing ALD.
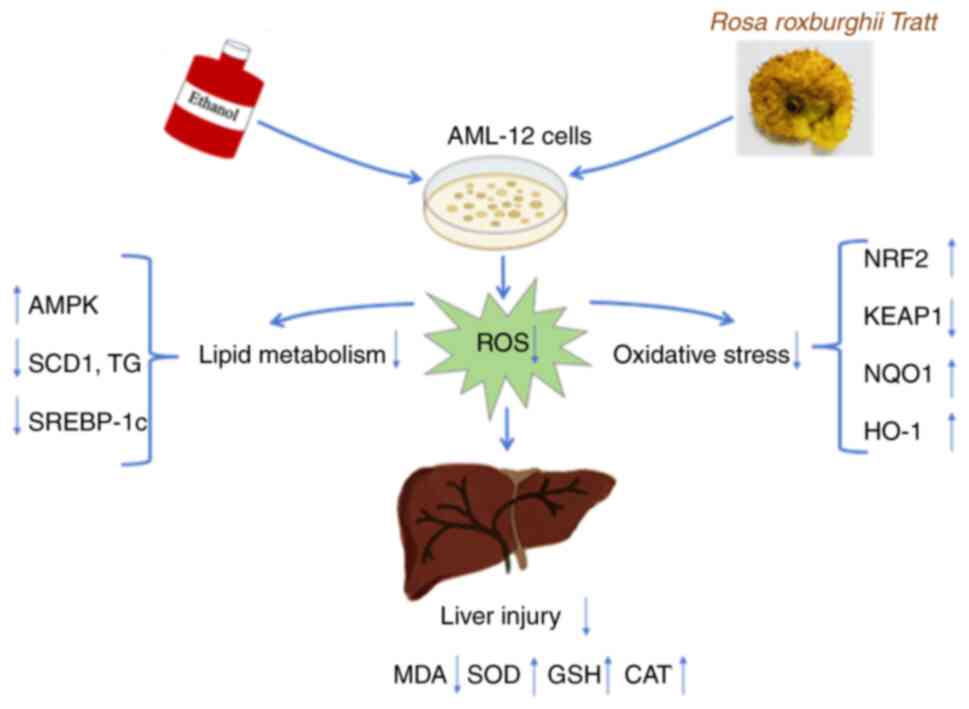 | Figure 7.Graphical abstract. The present study
evaluated the therapeutic potential of fermented FRRT in the
treatment of alcohol-related liver disease. The research utilized
an in vitro model established with AML-12 cells exposed to
ethanol to assess cell damage, lipid accumulation and oxidative
stress markers. Pre-treated FRRT significantly reduced the
formation of lipid droplets and triglyceride levels in liver cells
by regulating genes and proteins involved in lipid metabolism,
demonstrating antioxidant properties and reversing the changes in
genes and proteins associated with oxidative stress caused by
ethanol exposure. These findings provide a new strategy for the
management of ALD. FRRT, fermented Rosa roxburghii Tratt;
ALD, alcohol-related liver disease; AMPK, AMP-activated protein
kinase; SCD1, stearyl-coA desaturase-1; TG, triglyceride; SREBP-1c,
sterol regulatory element binding transcription factor 1; ROS,
reactive oxygen species; MDA, malondialdehyde; SOD, superoxide
dismutase; GSH, glutathione; CAT, catalase; NRF2, nuclear factor
erythroid 2-related factor 2; KEAP1, kelch like ECH associated
protein 1; NQO1, quinone oxidoreductase 1; HO-1, heme oxygenase
1. |
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key R&D Projects in
Zhejiang (grant no. 2021C02018) and the Research Project of
Zhejiang Chinese Medical University (grant nos. 2020ZR07, 2020ZG08
and 2023FSYYZY48).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LZ, ZY and JH collected the data, performed the data
analysis and drafted the manuscript. HZ and DW conceived and
designed the study, drafted the manuscript and supervised the
study. XW acquired the data and supervised the study. QH and SL
made substantial contributions to conception and design, and
revised the manuscript. QH, SL and LZ confirm the authenticity of
all the raw data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lamas-Paz A, Hao F, Nelson LJ, Vázquez MT,
Canals S, Del Moral MG, Martínez-Naves E, Nevzorova YA and Cubero
FJ: Alcoholic liver disease: Utility of animal models. World J
Gastroenterol. 24:5063–5075. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sommerfeld-Klatta K, Łukasik-Głębocka M
and Zielińska-Psuja B: Oxidative stress and biochemical indicators
in blood of patients addicted to alcohol treated for acute ethylene
glycol poisoning. Hum Exp Toxicol. 41:96032712110615022022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Louvet A and Mathurin P: Alcoholic liver
disease: Mechanisms of injury and targeted treatment. Nat Rev
Gastroenterol Hepatol. 12:231–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishida K, Kaji K, Sato S, Ogawa H, Takagi
H, Takaya H, Kawaratani H, Moriya K, Namisaki T, Akahane T and
Yoshiji H: Sulforaphane ameliorates ethanol plus carbon
tetrachloride-induced liver fibrosis in mice through the
Nrf2-mediated antioxidant response and acetaldehyde metabolization
with inhibition of the LPS/TLR4 signaling pathway. J Nutr Biochem.
89:1085732021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaur K, Narang RK and Singh S: Role of
Nrf2 in oxidative stress, neuroinflammation and autophagy in
Alzheimer's disease: Regulation of Nrf2 by different signaling
pathways. Curr Mol Med. 26:10.2174/156652402366623072614544.
2023.PubMed/NCBI
|
|
6
|
Herzig S and Shaw RJ: AMPK: Guardian of
metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol.
19:121–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lan T, Geng XJ, Zhang SJ, Zeng XX, Ying
JJ, Xu Y, Liu SY, Li P, Tong YH, Wang W, et al: Si-Ni-San inhibits
hepatic Fasn expression and lipid accumulation in MAFLD mice
through AMPK/p300/SREBP-1c axis. Phytomedicine. 123:1552092024.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Attal N, Marrero E, Thompson KJ and
McKillop IH: Role of AMPK-SREBP signaling in regulating fatty acid
binding-4 (FABP4) expression following ethanol metabolism. Biology
(Basel). 11:16132022.PubMed/NCBI
|
|
9
|
Kong LZ, Chandimali N, Han YH, Lee DH, Kim
JS, Kim SU, Kim TD, Jeong DK, Sun HN, Lee DS and Kwon T:
Pathogenesis, early diagnosis, and therapeutic management of
alcoholic liver disease. Int J Mol Sci. 20:27122019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Liu ZJ, Liu J, Liu LK, Zhang ES
and Li WL: Inhibition of metastasis and invasion of ovarian cancer
cells by crude polysaccharides from rosa roxburghii tratt in vitro.
Asian Pac J Cancer Prev. 15:10351–10354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang LT, Lv MJ, An JY, Fan XH, Dong MZ,
Zhang SD, WangJ D, Wang YQ, Cai ZH and Fu YJ: Botanical
characteristics, phytochemistry and related biological activities
of Rosa roxburghii Tratt fruit, and its potential use in functional
foods: A review. Food Funct. 12:1432–1451. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan P, Liu J, Huang Y, Li Y, Yu J, Xia J,
Liu M, Bai R, Wang N, Guo L, et al: Lotus leaf extract can
attenuate salpingitis in laying hens by inhibiting apoptosis. Poult
Sci. 102:1028652023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen L, Hao L, Yanshuo C, FangFang W,
Daqin C, Weidong X, Jian X, Shaodong C, Hongyu Z and Ke X: Grape
seed proanthocyanidins regulate mitophagy of endothelial cells and
promote wound healing in mice through p-JNK/FOXO3a/ROS signal
pathway. Arch Biochem Biophys. 749:1097902023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zapaśnik A, Sokołowska B and Bryła M: Role
of lactic acid bacteria in food preservation and safety. Foods
(Basel). 11:12832022.
|
|
15
|
Chen C, Lu Y, Yu H, Chen Z and Tian H:
Influence of 4 lactic acid bacteria on the flavor profile of
fermented apple juice. Food Biosci. 27:30–36. 2019. View Article : Google Scholar
|
|
16
|
Wei M, Feng D, Zhang Y, Zuo Y, Li J, Wang
L and Hu P: Effect and correlation of rosa roxburghii tratt juice
fermented by lactobacillus paracasei SR10-1 on oxidative stress and
gut microflora dysbiosis in streptozotocin (STZ)-induced type 2
diabetes mellitus mice. Foods (Basel). 12:32332023.
|
|
17
|
Wang X, Guo R, Yu Z, Zikela L, Li J, Li S
and Han Q: Torreya grandis Kernel oil alleviates loperamide-induced
slow transit constipation via up-regulating the colonic expressions
of Occludin/Claudin-1/ZO-1 and 5-HT3R/5-HT4R in BALB/c mice. Mol
Nutr Food Res. 68:e23006152024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Z, Tian R, She Z, Cai J and Li H:
Role of oxidative stress in the pathogenesis of nonalcoholic fatty
liver disease. Free Radic Biol Med. 152:116–141. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren Z, Wang X, Xu M, Yang F, Frank JA, Ke
ZJ and Luo J: Binge ethanol exposure causes endoplasmic reticulum
stress, oxidative stress and tissue injury in the pancreas.
Oncotarget. 7:54303–54316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Damjanovska S, Karb DB and Cohen SM:
Delivering health care education and information about excessive
alcohol consumption and risks of alcohol-associated liver disease.
Clin Liver Dis (Hoboken). 22:184–187. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang F, Li Y, Zhang YJ, Zhou Y, Li S and
Li HB: Natural products for the prevention and treatment of
hangover and alcohol use disorder. Molecules (Basel). 21:642016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan J, Nie Y, Luo M, Chen Z and He B:
Natural compounds: A potential treatment for alcoholic liver
disease? Front Pharmacol. 12:6944752021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang S, Huang XY, Zhou N, Wu Q, Liu J and
Shi JS: RNA-Seq analysis of protection against chronic alcohol
liver injury by rosa roxburghii fruit juice (Cili) in mice.
Nutrients. 14:19742022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sefried S, Häring HU, Weigert C and
Eckstein SS: Suitability of hepatocyte cell lines HepG2, AML12 and
THLE-2 for investigation of insulin signalling and hepatokine gene
expression. Open Biol. 8:1801472018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reyes RC, Brennan AM, Shen Y, Baldwin Y
and Swanson RA: Activation of neuronal NMDA receptors induces
superoxide-mediated oxidative stress in neighboring neurons and
astrocytes. J Neurosci. 32:12973–12978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsikas D: Assessment of lipid peroxidation
by measuring malondialdehyde (MDA) and relatives in biological
samples: Analytical and biological challenges. Anal Biochem.
524:13–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang B, Wang Y, Zhang J, Hu C, Jiang J, Li
Y and Peng Z: ROS-induced lipid peroxidation modulates cell death
outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis.
Arch Toxicol. 97:1439–1451. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Svegliati-Baroni G, Pierantonelli I,
Torquato P, Marinelli R, Ferreri C, Chatgilialoglu C, Bartolini D
and Galli F: Lipidomic biomarkers and mechanisms of lipotoxicity in
non-alcoholic fatty liver disease. Free Radic Biol Med.
144:293–309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Minelli A, Conte C, Grottelli S, Bellezza
I, Cacciatore I and Bolaños JP: Cyclo (His-Pro) promotes
cytoprotection by activating Nrf2-mediated up-regulation of
antioxidant defence. J Cell Mol Med. 13:1149–1161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bellezza I, Giambanco I, Minelli A and
Donato R: Nrf2-KEAP1 signaling in oxidative and reductive stress.
Biochim Biophys Acta Mol Cell Res. 1865:721–733. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rahim I, Sayed RK, Fernández-Ortiz M,
Aranda-Martínez P, Guerra-Librero A, Fernández-Martínez J, Rusanova
I, Escames G, Djerdjouri B and Acuña-Castroviejo D: Melatonin
alleviates sepsis-induced heart injury through activating the Nrf2
pathway and inhibiting the NLRP3 inflammasome. Naunyn Schmiedebergs
Arch Pharmacol. 394:261–277. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Higgins LG, Kelleher MO, Eggleston IM,
Itoh K, Yamamoto M and Hayes JD: Transcription factor Nrf2 mediates
an adaptive response to sulforaphane that protects fibroblasts in
vitro against the cytotoxic effects of electrophiles, peroxides and
redox-cycling agents. Toxicol Appl Pharmacol. 237:267–280. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ryter SW: Heme oxgenase-1, a cardinal
modulator of regulated cell death and inflammation. Cells.
10:5152021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guan D, Zhou W, Wei H, Wang T, Zheng K,
Yang C, Feng R, Xu R, Fu Y, Li C, et al: Ferritinophagy-mediated
ferroptosis and activation of KEAP1/Nrf2/HO-1 pathway were
conducive to EMT inhibition of gastric cancer cells in action of
2,2′-Di-pyridineketone hydrazone dithiocarbamate butyric acid
ester. Oxid Med Cell Longev. 21:39206642022.PubMed/NCBI
|
|
35
|
Dong H, Hao L, Zhang W, Zhong W, Guo W,
Yue R, Sun X and Zhou Z: Activation of AhR-NQO1 signaling pathway
protects against alcohol-induced liver injury by improving redox
balance. Cell Mol Gastroenterol Hepatol. 12:793–811. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu W, Wang B, Wang T, Liu X, He X, Liu Y,
Li Z and Zeng H: Ursodeoxycholic acid attenuates acute aortic
dissection formation in angiotensin II-infused apolipoprotein
E-deficient mice associated with reduced ROS and increased Nrf2
levels. Cell Physiol Biochem. 38:1391–1405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yap F, Craddock L and Yang J: Mechanism of
AMPK suppression of LXR-dependent Srebp-1c transcription. Int J
Biol Sci. 7:645–650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ha JH, Jang J, Chung SI and Yoon Y: AMPK
and SREBP-1c mediate the anti-adipogenic effect of
β-hydroxyisovalerylshikonin. Int J Mol Med. 37:816–824. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tracz-Gaszewska Z and Dobrzyn P:
Stearoyl-CoA Desaturase 1 as a therapeutic target for the treatment
of cancer. Cancers (Basel). 11:9482019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Day EA, Ford RJ and Steinberg GR: AMPK as
a therapeutic target for treating metabolic diseases. Trends
Endocrinol Metab. 28:545–560. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Robinson AJ, Darley RL and Tonks A:
Reactive oxygen species in leukemias: Maintaining cancer cell
proliferation via redox signaling and changing metabolic
homeostasis. Oncotarget. 12:952–954. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang X, Chen X, Zhou W, Men H, Bao T, Sun
Y, Wang Q, Tan Y, Keller BB, Tong Q, et al: Ferroptosis is
essential for diabetic cardiomyopathy and is prevented by
sulforaphane via AMPK/NRF2 pathways. Acta Pharm Sin B. 12:708–722.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park Y, Sung J, Yang J, Ham H, Kim Y,
Jeong HS and Lee J: Inhibitory effect of esculetin on
free-fatty-acid-induced lipid accumulation in human HepG2 cells
through activation of AMP-activated protein kinase. Food Sci
Biotechnol. 26:263–269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu Q, Yang L, Xiao JJ, Liu Q, Ni L, Hu JW,
Yu H, Wu X and Zhang BF: Empagliflozin attenuates the renal tubular
ferroptosis in diabetic kidney disease through AMPK/NRF2 pathway.
Free Radic Biol Med. 195:89–102. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zimmermann K, Baldinger J, Mayerhofer B,
Atanasov AG, Dirsch VM and Heiss EH: Activated AMPK boosts the
Nrf2/HO-1 signaling axis-A role for the unfolded protein response.
Free Radic Biol Med. 88:417–426. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Petsouki E, Cabrera SNS and Heiss EH: AMPK
and NRF2: Interactive players in the same team for cellular
homeostasis? Free Radic Biol Med. 190:75–93. 2022. View Article : Google Scholar : PubMed/NCBI
|