Introduction
It has long been recognized that metabolism is
altered in human cancers. Warburg first observed enhanced anaerobic
glycolysis in cancer in 1926 (1).
Elevated glucose catabolism produces excessive levels of the
glycolytic end-product, pyruvate. Most of this pyruvate is
converted into lactate, but some of it is converted into
acetyl-CoA, which, in turn, is used in de novo lipid
synthesis. Rapidly proliferating cancer cells synthesize fatty
acids de novo to provide lipids for membrane production and
protein modification.
It is increasingly evident that enzymes involved in
the lipogenic pathways play direct roles in tumorigenesis and
cancer progression (2,3). ATP citrate lyase (ACL), a key enzyme
of de novo lipid synthesis, is involved in the generation of
cytosolic acetyl-CoA and oxaloacetate from citrate and thus
contributes to the translocation of acetyl-CoA from mitochondria to
cytosol. As a first step in lipid synthesis, pyruvate is converted
to acetyl-CoA in the mitochondria. Acetyl-CoA is then incorporated
into the TCA cycle which produces citrate in the presence of
sufficient amounts of ATP. Accumulated citrate is then exported to
the cytoplasm, where it is catalyzed by ACL to generate cytosolic
acetyl-CoA, the lipogenic building block (4). The role of ACL in tumor growth has
been highlighted by experiments showing that ACL RNAi or the
chemical inhibitor SB-204990 suppresses tumor cell proliferation
in vitro, reduces tumor growth, and induces differentiation
in vivo (5,6). Migita et al reported that ACL
was activated via Akt -mediated ACL phosphorylation in lung cancer,
while selective ACL inhibition suppressed tumor cell growth both
in vitro and in vivo (7). More recently, investigators have
discovered the importance of ACL in promoting tumor invasion via
its protection of the glycolytic pathway in glioblastomas (8). However, the role of ACL in human
epithelial ovarian cancer has yet to be determined.
Ovarian cancer is the third most common neoplasm of
the female reproductive tract and the leading cause of death
related to a gynecological malignancy. Epithelial ovarian cancer
(EOC) accounts for more than 90% of all ovarian cancers. Several
lines of evidence have demonstrated that the dysregulation of lipid
metabolism is associated with tumorigenesis and progression of
ovarian cancer (9,10). Thus, targeting dysregulated
metabolism may be an attractive strategy for ovarian cancer
treatment.
In this study, we examined the role of ACL in the
pathogenesis of ovarian cancer, and explored its prognostic and
therapeutic potential. Overexpression and increased phosphorylation
of ACL were found in patients with epithelial ovarian cancer.
Phosphorylated ACL was a significant prognostic factor. ACL
inhibition by RNAi altered the proliferative behavior and cell
cycle distribution of A2780 cells. Our findings suggest that ACL
may contribute to ovarian cancer pathogenesis and could serve as a
novel therapeutic target.
Materials and methods
Cell culture and clinical samples
The human ovarian carcinoma cell line A2780 was
obtained from the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China) and grown in RPMI-1640 supplemented with
10% fetal bovine serum and 1% penicillin/streptomycin in an
atmosphere of 5% CO2 at 37°C.
Tumor samples were obtained from patients who
underwent surgical resection at the Qilu Hospital of Shandong
University. All tumors were pathologically diagnosed based on the
World Health Organization criteria and staged according to the
classification of the International Federation of Gynecology and
Obstetrics. Clinicopathological para-meters and patient prognosis
data were also obtained. Normal ovaries removed during surgery for
benign conditions were collected as controls. This study was
approved by the Ethics Committee of Qilu Hospital, and signed
informed consent was obtained from each patient.
Immunohistochemistry and image
analysis
Tumor samples were fixed in 10% neutral formalin and
embedded in paraffin, and serial 5-μm-thick sections were cut.
After deparaffinization, antigen retrieval was performed for 30 min
in citrate buffer (pH 6.0). The slides were incubated in blocking
serum and then probed overnight at 4°C with primary antibodies
against ACL (Abcam, Cambridge, MA), phosphorylated ACL (p-ACL)
(Abcam), and SREBP-1 (Santa Cruz Biotechnology, Santa Cruz, CA).
Subsequently, biotinylated second antibodies and
streptavidin-peroxidase conjugates were applied, and the
immunostaining was visualized with 3,3-diaminobenzidine. Finally,
the sections were counterstained with hematoxylin. To evaluate the
immunoreactivity of ACL and p-ACL, we applied a scoring method that
was validated in a previous study (7). The staining intensity of ACL or p-ACL
was scored on a scale of 0–4 (0, no staining; 1, weak; 2, moderate;
3, moderate to strong; 4, strong). The patients were classified
into two groups: low ACL/p-ACL (scores of 0, 1, or 2) and high
ACL/p-ACL (scores of 3 or 4). SREBP-1 expression was also divided
into two groups according to the ACL classification.
Quantitative real-time PCR
Total RNA was extracted from frozen tissue samples
using the TRIzol reagent (Invitrogen, Carlsbad, CA) according to
the supplier’s protocol. First-strand cDNA was synthesized from
total RNA using the ReverTra Ace qPCR RT Kit (Toyobo, Tokyo,
Japan). Quantitative RT-PCR was carried out using SYBR-Green
Real-Time PCR Master Mix (Toyobo) on a Light Cycler (Roche Applied
Science, Indianapolis, IN). The specific primer sequences for ACL
and β-actin were as follows: 5′-GAAGGGAGTGA CCATCATCG-3′ (ACL
forward); 5′-TTAAAGCACCCAG GCTTGAT-3′ (ACL reverse);
5′-TTGCCGACAGGATGCA GAA-3′ (β-actin forward); and 5′-GCCGATCCACACGG
AGTACT-3′ (β-actin reverse). All reactions were performed in at
least triplicate. The comparative CT method was used to
determine the relative amounts of mRNA (11).
Cell lysate preparation and western
blotting
To obtain total protein lysates, frozen tissue and
cells were homogenized in a lysis buffer (Beyotime, Shanghai,
China) containing proteinase inhibitors and phosphatase inhibitors.
The protein concentration of each lysate was determined using a
protein assay reagent kit (Beyotime). Equal amounts of total
cellular proteins were separated onto a 10% SDS-PAGE gel and
transferred to a PVDF membrane (Millipore, Billerica, MA). The
membranes were then incubated with an anti-β-actin (Sigma-Aldrich,
St. Louis, MO), anti-ACL (Cell Signaling Technology, Danvers, MA),
anti-p-ACL (Cell Signaling Technology), or anti-SREBP-1 (Santa Cruz
Biotechnology) primary antibody at 4°C overnight. After being
washed and probed with HRP-conjugated secondary antibody, the
proteins were detected using ECL Western Blotting Detection
Reagents (Millipore).
RNA interference
Two independent siRNA oligonucleotides for ACL as
well as a negative control were purchased from GenePharma
(Shanghai, China). The ACL siRNA sequences were designed to
correspond to a previous study (5).
Transfection was performed using Lipofectamine 2000 (Invitrogen)
according to the manufacturer’s protocol. Briefly, 10 μl of a 20 μM
siRNA solution and 5 μl of Lipofectamine 2000 were co-incubated in
500 μl of Opti-MEM medium (Invitrogen) for 20 min and overlaid onto
cells plated in a 6-well plate.
MTT assay
After transfection with ACL-siRNA or negative
control siRNA for the indicated time periods, cells were incubated
with 5 mg/ml MTT (Sigma-Aldrich) for 4 h and then mixed with DMSO
after the supernatant was removed. Cell viability was quantitated
according to the dye absorption (A) at 550 nm (A1) and 630 nm (A2)
with an automatic multiwall spectrophotometer (Bio-Rad
Laboratories, Richmond, CA). Each experiment was repeated in
triplicate.
Flow cytometry assay
For cell cycle analysis, cells were harvested and
fixed with 70% ice-cold ethanol at 4°C overnight. After washing
with PBS, the cells were suspended with 200 μg RNaseA (1 mg/ml) at
37°C for 30 min and later stained with 800 μl (100 μg/ml) propidium
iodide (Invitrogen) at 37°C for 30 min. For the apoptosis analysis,
cells were washed, resuspended in ice-cold binding buffer, and
subsequently incubated with 5 μl Annexin-V-FITC (Jingmei Biotech,
Shanghai, China) and 10 μl propidium iodide for 15 min. Cell cycle
and cell apoptosis analyses were performed with a flow cytometer
(FCM) (BD Biosciences, San Jose, CA). Each experiment was repeated
in triplicate.
Statistical analysis
The data from the cell culture experiments were
compared using Student’s t-tests. For the clinical samples, the
correlations between ACL/p-ACL expression and clinicopathological
parameters were assessed using Fisher’s exact test. Univariate
survival analysis was performed using the Kaplan-Meier method and
log-rank statistics for comparisons of survival curves.
Multivariate survival analysis was determined by the Cox regression
model. Comparisons of ACL expression between ovarian cancer tissues
and normal tissues were carried out using the Mann-Whitney U Test.
For all analyses, a p-value of <0.05 was considered
statistically significant. Statistical analyses were performed
using SPSS software.
Results
ACL is overexpressed in and serves as a
prognostic factor for human epithelial ovarian cancer
To evaluate the role of ACL in epithelial ovarian
cancer, we assessed ACL expression in 18 epithelial ovarian cancer
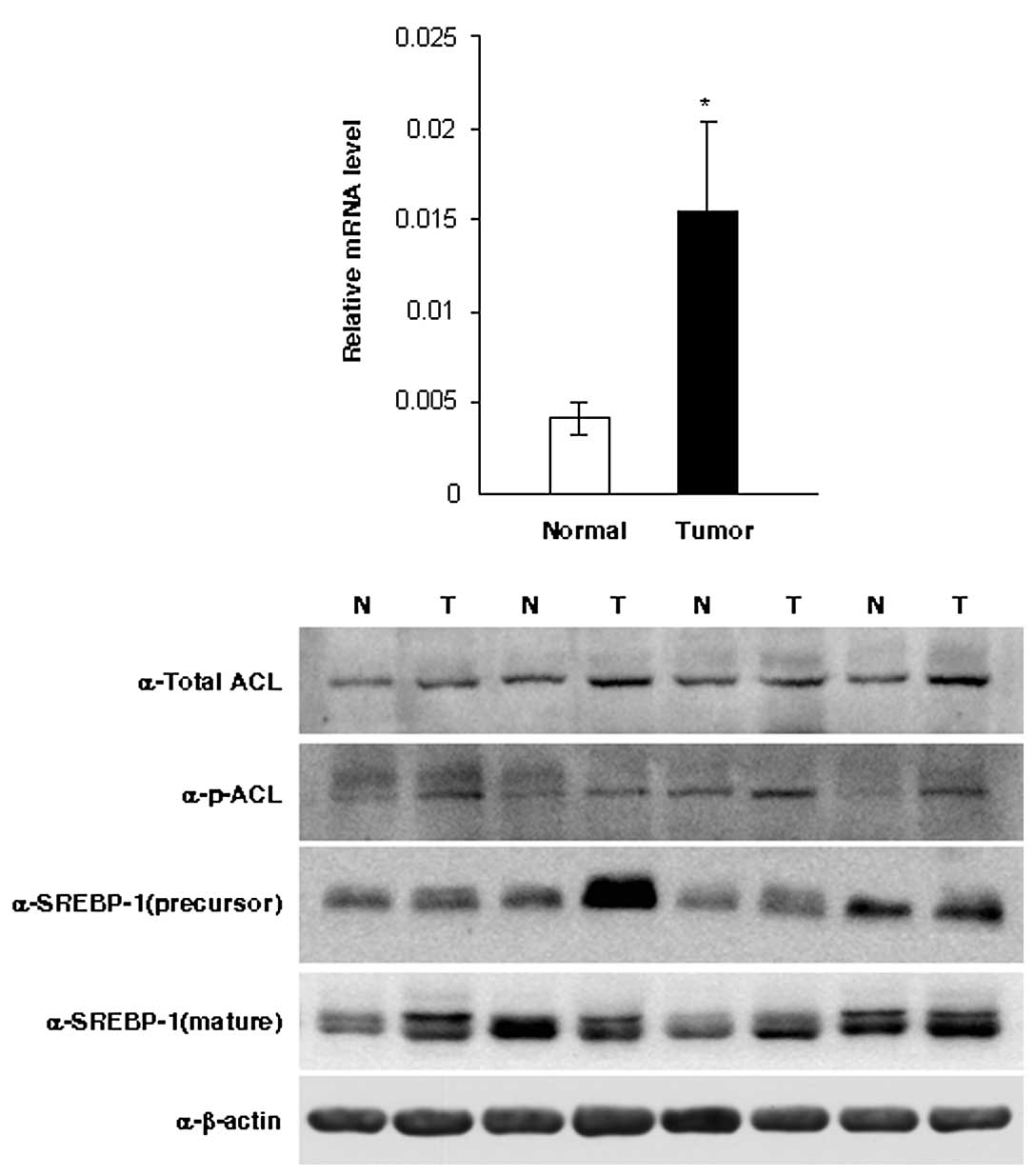
tissues and 12 normal ovarian tissues. ACL mRNA was upregulated
3.7-fold in ovarian cancer tissues compared to normal tissues
(p<0.05; Fig. 1A). Western blot
analysis confirmed the increased expression of ACL and p-ACL
protein in ovarian cancer tissues compared to normal tissues
(Fig. 1B).
SREBP-1 is a basic-helix-loop-helix-leucine zipper
protein that regulates the transcription of lipogenic enzymes. To
dissect the relationship of SREBP-1 with ACL in ovarian cancer, we
detected SREBP-1 expression by western blot analysis (Fig. 1B). However, our data showed that ACL
and p-ACL expression were independent of either the 125-kDa
precursor form of SREBP-1 or the 68-kDa mature and cleaved nuclear
form (Fig. 1B).
To determine whether ACL was associated with
clinicopathologic parameters, ACL and p-ACL expression in the 82
epithelial ovarian cancer samples were analyzed by
immunohistochemistry. The clinicopathological characteristics of
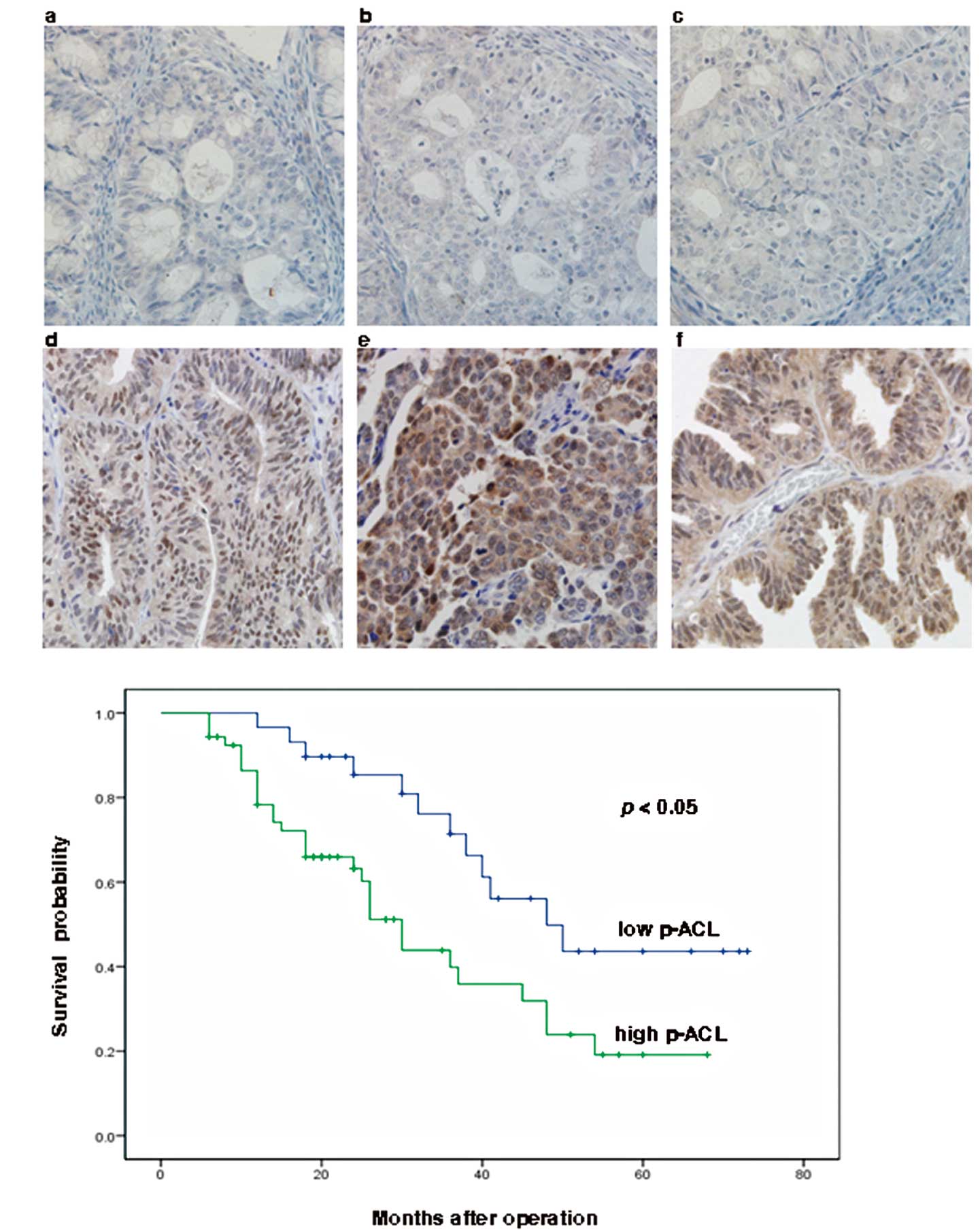
these 82 patients are summarized in Table I. Immunohistochemical results showed
that ACL and p-ACL were expressed at different levels in different
samples, which were classified as low expression (scores of 0, 1,
or 2) or high expression (scores of 3 or 4) groups (Fig. 2A). High ACL and high p-ACL scores
were recorded in 51 (62%) and 53 (65%) cases, respectively.
Comparisons of p-ACL levels and clinicopathological para-meters
revealed that high p-ACL expression was significantly correlated
with poor differentiation (p=0.044) and advanced FIGO stage
(p=0.020; Table I). Total ACL
expression was not correlated with any of the clinicopathological
parameters studied (Table I).
 | Table IRelationships between ACL/p-ACL
expression and clinicopathological parameters in epithelial ovarian
cancer. |
Table I
Relationships between ACL/p-ACL
expression and clinicopathological parameters in epithelial ovarian
cancer.
| N | ACL low, n | ACL high, n | p-value | p-ACL low, n | p-ACL high, n | p-value |
|---|
| Age (years) | 82 | 31 | 51 | 0.649 | 29 | 53 | 0.164 |
| <60 | 44 | 18 | 26 | | 19 | 25 | |
| ≥60 | 38 | 13 | 25 | | 10 | 28 | |
| Tumor grade | 82 | 31 | 51 | 0.623 | 29 | 53 | 0.044 |
| 1 | 10 | 5 | 5 | | 7 | 3 | |
| 2 | 19 | 6 | 13 | | 7 | 12 | |
| 3 | 53 | 20 | 33 | | 15 | 38 | |
| Preoperative maximal
diameter of tumor (cm) | 82 | 31 | 51 | 0.365 | 29 | 53 | 0.760 |
| <10 | 63 | 22 | 41 | | 22 | 41 | |
| ≥10 | 14 | 7 | 7 | | 6 | 8 | |
| Unknown | 5 | 2 | 3 | | 1 | 4 | |
| FIGO stage | 82 | 31 | 51 | 0.610 | 29 | 53 | 0.020 |
| I, II | 21 | 9 | 12 | | 12 | 9 | |
| III, IV | 61 | 22 | 39 | | 17 | 44 | |
| Histological
type | 82 | 31 | 51 | 1.000 | 29 | 53 | 0.788 |
| Serous | 62 | 24 | 38 | | 21 | 41 | |
| Others | 20 | 7 | 13 | | 8 | 12 | |
| SREBP1 | 82 | 31 | 51 | 0.113 | 29 | 53 | 0.248 |
| Low | 36 | 10 | 26 | | 10 | 26 | |
| High | 46 | 21 | 25 | | 19 | 27 | |
We also detected SREBP-1 expression using
immunohistochemistry (Fig. 2A).
Statistical analysis showed that neither ACL nor p-ACL
immunostaining patterns were correlated with SREBP-1 expression
(Table I). These findings were
consistent with our western blot analyses. In our study, ACL and
p-ACL expression was detected in both the cytoplasm and the nucleus
of tumor cells in some samples.
Next, we examined the relationship between p-ACL
expression and survival in patients with epithelial ovarian cancer
(n=82). The median survival for patients with high p-ACL ovarian
cancer was significantly shorter than the median survival for those
patients with low p-ACL ovarian cancer (30.0 months; 95% CI,
25.0–34.9 months vs. 48.0 months; 95% CI, 32.1–63.9 months;
p=0.010), and the 5-year survival rates in these two groups were
19.1% and 43.6% (p=0.010; Fig. 2B),
respectively. Univariate survival analysis showed that age, tumor
grade, FIGO stage and p-ACL expression were significantly
associated with survival (p<0.05). Furthermore, in a
multivariate Cox regression model with these significant
covariates, age and FIGO stage were significant factors for
prediction of a poor prognosis (p=0.001 and p=0.031, respectively).
Total ACL expression was not significantly associated with survival
(data not shown).
Knockdown of ACL by siRNA impairs the
proliferation of A2780 cells
To study whether ACL has potential as a therapeutic
target in ovarian cancer, we used ACL-specific siRNAs to
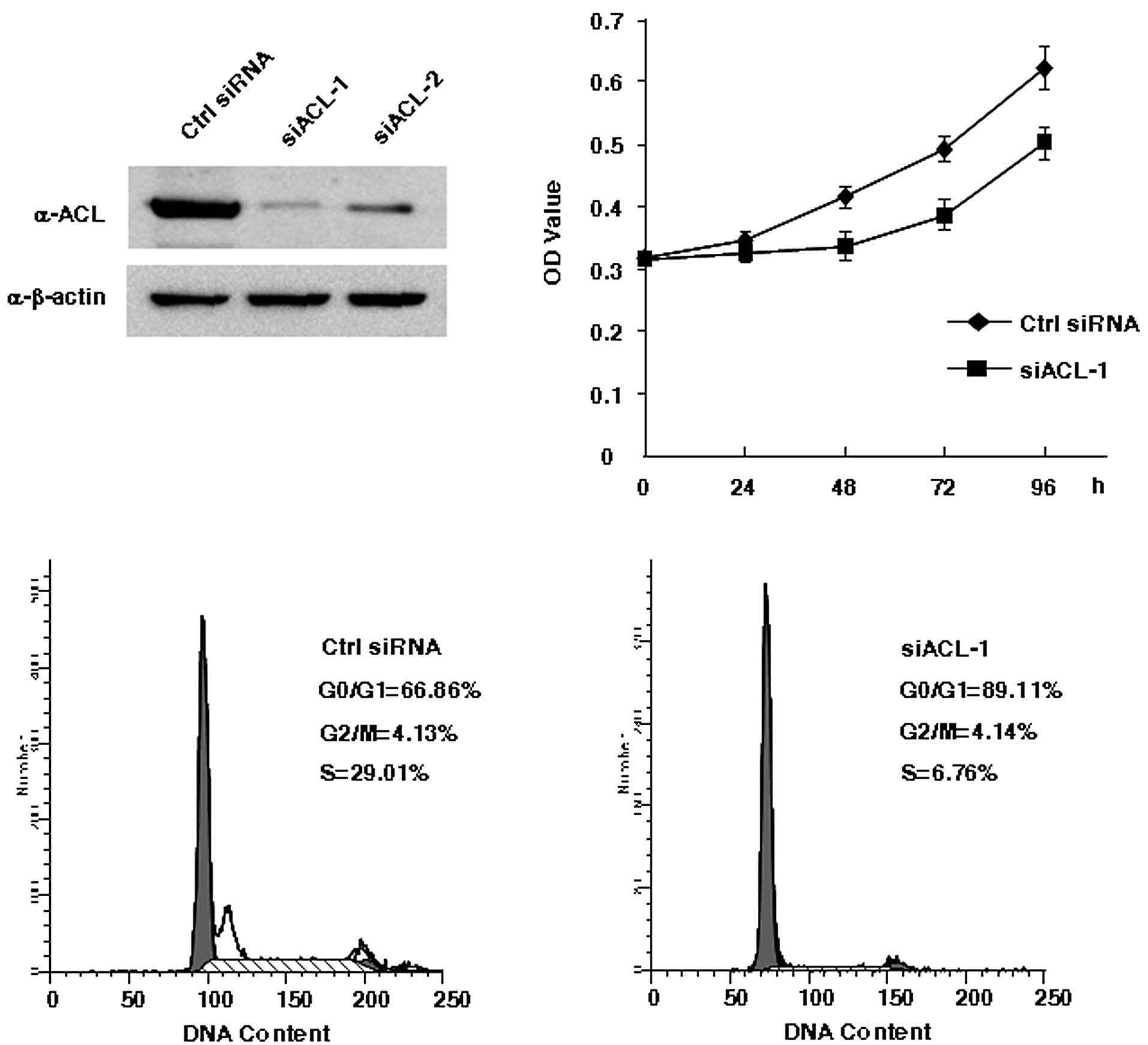
selectively reduce ACL gene expression in A2780 cells. Both
oligonucleotides (siACL-1 and siACL-2) efficiently reduced total
ACL protein levels. However, because western blot analyses showed
that siACL-1 was more effective (Fig.
3A), siACL-1 was used in all following experiments. MTT assays
were performed to examine the effect of ACL inhibition on cellular
proliferation. After transfection with siRNA, the ACL knockdown
cells proliferated more slowly than did the control cells, and
significant differences between the ACL knockdown cells and control
cells were observed at 48, 72 and 96 h (p<0.05, Fig. 3B).
Effect of ACL-siRNA on cell cycle
progression and apoptosis
After transfection with siRNAs for 72 h, cell cycle
distribution and apoptosis were analyzed with flow cytometry. The
cell cycle analysis indicated that ACL knockdown induced a
progressive reduction in the S-phase population that was associated
with a progressive parallel accumulation of G1-phase cells
(negative control siRNA, G1, 66.86%; S, 29.01%; and G2-M, 4.13%;
siACL-1, G1, 89.11%; S, 6.76%; and G2-M, 4.14%; Fig. 3C). We concluded that cell cycle
arrest may contribute to the cellular proliferation defect in the
ACL-siRNA-treated cells. We did not observe significant change in
apoptosis after ACL knockdown (data not shown).
Discussion
Altered metabolism is one of the most significant
features of cancer cells. High glycolysis and lipogenesis occur in
cancers to satisfy the increased demand for bioenergy and
macromolecules need for autonomous growth. It has been reported
that de novo fatty acid synthesis occurs at low rates in
normal tissues because normal cells acquire lipids via circulation;
in contrast, fatty acid synthesis occurs at very high rates in
tumor tissues (12). Ookhtens et
al confirmed that almost all fatty acids are derived from de
novo synthesis in tumor cells despite an abundant supply of
extracellular lipids (13). Thus,
glucose and lipid metabolism-targeting therapeutics have attracted
great attention (14,15).
Accumulating evidence indicates that various steps
of lipid synthesis contribute to cancer progression. Akt was
originally identified as a potent proto-oncogene, and constitutive
PI3K/Akt activation is commonly observed in a variety of cell types
(16,17). PI3K/Akt signaling is sufficient to
stimulate glucose uptake, as well as glycolysis and fatty acid
biosynthesis in cancer cells (18).
The blockade of HMG CoA reductase is widely considered to be a
useful strategy for inhibiting cancer cell growth and inducing
apoptosis both in vitro and in vivo (19,20).
Fatty acid synthase (FASN), the enzyme responsible for the terminal
steps in de novo fatty acid synthesis, is highly expressed
in a wide variety of human epithelial cancers (21,22).
Furthermore, lipogenic enzymes that function upstream of FASN such
as acetyl-CoA carboxylase-a (ACACA) and ATP citrate lyase (ACL) are
also elevated in various cancers. These enzymes have also been
considered to play critical roles in tumorigenesis and cancer
progression (23,24).
ACL regulates a key step that can convert high
glycolytic flux into increased lipid synthesis. ACL overexpression
or activation has been reported in bladder, breast, liver, stomach
and lung tumors (7,25–28).
As the first committed step in the glucose-dependent lipid
synthesis pathway, ACL inhibition can affect the cholesterol,
isoprenoid, and fatty acid synthesis pathways in combination. Thus,
ACL could be a particularly attractive target for ovarian cancer
therapy.
We detected ACL expression in human epithelial
ovarian cancer and normal ovarian tissues using quantitative RT-PCR
and western blot analysis. Significantly elevated levels of ACL and
p-ACL were observed in ovarian cancer tissues compared to normal
tissues, which suggests that ACL could promote ovarian cancer
tumorigenesis and progression.
The prognoses of women with epithelial ovarian
cancers are invariably poor. Recent attention has focused on the
identification of potential biological prognostic markers. We
assessed ACL expression in epithelial ovarian cancer tissues using
immunohistochemistry and analyzed the relationships between ACL
expression and the clinicopathological characteristics of the
patients. Our findings showed that p-ACL expression was
significantly correlated with tumor grade and FIGO stage,
suggesting that increased ACL phosphorylation could be generally
related to the aggressive behavior of ovarian cancer. p-ACL
expression was significantly correlated with overall survival.
Thus, ACL may be a useful marker for epithelial ovarian cancers
with especially poor prognoses.
As the rate-limiting enzyme that is responsible for
generating cytosolic acetyl-CoA from citrate, ACL is considered to
be localized in the cytoplasm. However, we observed both nuclear
and cytoplasmic localization of ACL and p-ACL proteins. This
finding is consistent with a previous study in lung tissue
(7). To date, the function of ACL
in the nucleus remains unclear, and further research is required to
clarify the role of differential ACL cellular localization.
SREBP-1 is a transcription factor that is involved
in cholesterol and lipid metabolism, and it has been reported that
ACL is transcriptionally regulated by SREBP-1 (29,30).
We assessed SREBP-1 protein levels in epithelial ovarian cancer
tissues using western blot analysis and immunohistochemistry. The
results showed that both ACL and p-ACL expression were independent
of SREBP-1.
Given the positive role of ACL in cancer
pathogenesis, we sought to determine whether the decreased
expression of ACL influenced the biological behavior of A2780
cells. We observed markedly impaired proliferation coupled with
delayed S-phase entry in cells transfected with ACL-siRNA.
In conclusion, our findings indicate that ACL is
overexpressed in and could serve as a prognostic factor for human
epithelial ovarian cancer. ACL knockdown inhibits cellular
proliferation and induces cell cycle arrest in vitro. The
present study suggests that ACL may be a novel therapeutic target
for epithelial ovarian cancer. However, more ovarian cancer cell
lines should be investigated, and further research will be required
to elucidate the detailed changes in lipid metabolism induced by
ACL inhibition in ovarian cancer cells.
References
|
1
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mashima T, Seimiya H and Tsuruo T: De novo
fatty-acid synthesis and related pathways as molecular targets for
cancer therapy. Br J Cancer. 100:1369–1372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Furuta E, Okuda H, Kobayashi A and Watabe
K: Metabolic genes in cancer: their roles in tumor progression and
clinical implications. Biochim Biophys Acta. 1805:141–152.
2010.PubMed/NCBI
|
|
4
|
Wellen KE, Hatzivassiliou G, Sachdeva UM,
Bui TV, Cross JR and Thompson CB: ATP-citrate lyase links cellular
metabolism to histone acetylation. Science. 324:1076–1080. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hatzivassiliou G, Zhao F, Bauer DE, et al:
ATP citrate lyase inhibition can suppress tumor cell growth. Cancer
Cell. 8:311–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bauer DE, Hatzivassiliou G, Zhao F,
Andreadis C and Thompson CB: ATP citrate lyase is an important
component of cell growth and transformation. Oncogene.
24:6314–6322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Migita T, Narita T, Nomura K, et al: ATP
citrate lyase: activation and therapeutic implications in non-small
cell lung cancer. Cancer Res. 68:8547–8554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beckner ME, Fellows-Mayle W, Zhang Z, et
al: Identification of ATP citrate lyase as a positive regulator of
glycolytic function in glioblastomas. Int J Cancer. 126:2282–2295.
2010.PubMed/NCBI
|
|
9
|
Zhou W, Han WF, Landree LE, et al: Fatty
acid synthase inhibition activates AMP-activated protein kinase in
SKOV3 human ovarian cancer cells. Cancer Res. 67:2964–2971. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iorio E, Mezzanzanica D, Alberti P, et al:
Alterations of choline phospholipid metabolism in ovarian tumor
progression. Cancer Res. 65:9369–9376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuhajda FP: Fatty-acid synthase and human
cancer: new perspectives on its role in tumor biology. Nutrition.
16:202–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ookhtens M, Kannan R, Lyon I and Baker N:
Liver and adipose tissue contributions to newly formed fatty acids
in an ascites tumor. Am J Physiol. 247:R146–R153. 1984.PubMed/NCBI
|
|
14
|
Swinnen JV, Brusselmans K and Verhoeven G:
Increassed lipogenesis in cancer cells: new players, novel target.
Curr Opin Clin Nutr Metab Care. 9:358–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pelicano H, Martin DS, Xu RH and Huang P:
Glycolysis inhibition for anticancer treatment. Oncogene.
25:4633–4646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo J, Manning BD and Cantley LC:
Targeting the PI3K-Akt pathway in human cancer: rationale and
promise. Cancer Cell. 4:257–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mazzoletti M and Broggini M: PI3K/AKT/mTOR
inhibitors in ovarian cancer. Curr Med Chem. 17:4433–4447. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and Gonzalez-Baron M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004.PubMed/NCBI
|
|
19
|
Bababeygy SR, Polevaya NV, Youssef S, et
al: HMG-CoA reductase inhibition causes increased necrosis and
apoptosis in an in vivo mouse glioblastoma multiforme model.
Anticancer Res. 29:4901–4908. 2009.PubMed/NCBI
|
|
20
|
Relja B, Meder F, Wilhelm K, Henrich D,
Marzi I and Lehnert M: Simvastatin inhibits cell growth and induces
apoptosis and G0/G1 cell cycle arrest in hepatic cancer cells. Int
J Mol Med. 26:735–741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Walter K, Hong SM, Nyhan S, et al: Serum
fatty acid synthase as a marker of pancreatic neoplasia. Cancer
Epidemiol Biomarkers Prev. 18:2380–2385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Migita T, Ruiz S, Fornari A, et al: Fatty
acid synthase: a metabolic enzyme and candidate oncogene in
prostate cancer. J Natl Cancer Inst. 101:519–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hilvo M, Denkert C, Lehtinen L, et al:
Novel theranostic opportunities offered by characterization of
altered membrane lipid metabolism in breast cancer progression.
Cancer Res. 71:3236–3245. 2011. View Article : Google Scholar
|
|
24
|
Wang C, Xu C, Sun M, Luo D, Liao DF and
Cao D: Acetyl-CoA carboxylase-alpha inhibitor TOFA induces human
cancer cell apoptosis. Biochem Biophys Res Commun. 385:302–306.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Turyn J, Schlichtholz B, Dettlaff-Pokora
A, et al: Increased activity of glycerol 3-phosphate dehydrogenase
and other lipogenic enzymes in human bladder cancer. Horm Metab
Res. 35:565–569. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Szutowicz A, Kwiatkowski J and Angielski
S: Lipogenetic and glycolytic enzyme activities in carcinoma and
nonmalignant diseases of the human breast. Br J Cancer. 39:681–687.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yahagi N, Shimano H, Hasegawa K, et al:
Co-ordinate activation of lipogenic enzymes in hepatocellular
carcinoma. Eur J Cancer. 41:1316–1322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Varis A, Wolf M, Monni O, et al: Targets
of gene amplification and overexpression at 17q in gastric cancer.
Cancer Res. 62:2625–2629. 2002.PubMed/NCBI
|
|
29
|
Moon YA, Lee JJ, Park SW, Ahn YH and Kim
KS: The roles of sterol regulatory element-binding proteins in the
transactivation of the rat ATP citrate-lyase promoter. J Biol Chem.
275:30280–30286. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sato R, Okamoto A, Inoue J, et al:
Transcriptional regulation of the ATP citrate-lyase gene by sterol
regulatory element-binding proteins. J Biol Chem. 275:12497–12502.
2000. View Article : Google Scholar : PubMed/NCBI
|