Introduction
Thymidine phosphorylase (TP), an enzyme involved in
pyrimidine catabolism, is identical with an angiogenic factor,
platelet-derived endothelial cell growth factor (PD-ECGF) (1). TP is overexpressed in various tumors
and plays an important role in angiogenesis, tumor growth, invasion
and metastasis (2). The enzymatic
activity of TP is required for the angiogenic effect of TP
(3). A novel, specific TP
inhibitor, TPI, inhibits angiogenesis induced by TP in KB/TP cells
(human KB epidermoid carcinoma cells transfected with TP
cDNA), as well as growth and metastasis of KB/TP cells in
vivo(4,5).
2-Deoxy-d-ribose (DR), one of the degradation
products of thymidine generated by TP activity, has both angiogenic
and chemotactic activity (6). Both
DR and TP inhibit a hypoxia-induced apoptotic pathway (7). These findings suggest that DR is a
downstream mediator of TP function. 2-Deoxy-l-ribose, a
stereoisomer of DR, inhibits the promotion of angiogenesis, tumor
growth and metastasis by TP (8,9).
Recent evidence suggests that DR affects endothelial cell migration
through activation of the integrin downstream signaling pathway
(10). Rapamycin completely
abrogates DR-induced endothelial cell migration and angiogenesis,
correlating with a blockade of DR-induced p70S6 kinase activation
in endothelial cells (11).
Thymidine-derived DR and deoxy-d-ribose 1-phosphate (DR1P) are
enzymatically converted to 2-deoxy-d-ribose 5-phosphate (DR5P)
(12). Bijnsdorp et al
observed that DR1P and DR5P accumulate at high levels in
TP-overexpressing cells and DR is extensively secreted by these
cells (13).
Brown and Bicknell inferred that DR may be an
important energy source under hypoxic conditions (14). Brown et al also reported that
TP overexpression in cells treated with thymidine induces hemo
oxygenase-1 (HO-1), a classical cellular oxidative stress marker
(15). Although our results
(4) and many reports from other
laboratories suggest that TP is pivotal for tumor progression, the
molecular basis for the induction of reactive oxygen species (ROS)
and angiogenic factors by TP is not completely understood.
In this study, we indicate that TP activity is
required for the enhanced ROS generation and IL-8 mRNA
expression in human cancer cells and agents which inhibit TP
activity are strong candidates for new anticancer drugs.
Materials and methods
Chemicals and cell culture
NAC was obtained from Sigma-Aldrich.
H2DCF-DA was obtained from Molecular Probes. KB (human
epidermoid carcinoma), EJ (human bladder cancer), Yumoto (human
cervical carcinoma), THP-1 (human monocyte) and MCF-7 (human breast
carcinoma) cells were grown in DMEM (Nissui Seiyaku Co.) containing
10% calf serum, 2 mM glutamine and 100 U/ml of penicillin at 37°C
in a 5% CO2 humidified atmosphere. The medium was
changed to fresh serum-free media before experiments.
Transfection of TP/PD-ECGF cDNA into KB
cell
TP/PD-ECGF full-length cDNA plasmid, TP/PD-ECGF
mutant plasmid (L148R, Leu-148→Arg) (3) or the empty vector was transfected into
KB cells by electroporation (16).
After selection with geneticin, expression of TP in each clone was
determined by immunoblot analysis using an anti-TP monoclonal
antibody as described (17). A
TP-positive clone (KB/TP cells) and a control vector-transfected
clone (KB/CV cells) were used for further analyses.
Immunoblot analysis
Samples were subjected to 6 or 12.5% sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) according to
the method of Laemmli (18). Gel
proteins were electrophoretically transferred onto polyvinylidene
difluoride membranes (Immobilon-P transfer membrane; Millipore)
using the Bio-Rad Transblot SD apparatus (19). The membrane was treated with
blocking buffer containing 3% skimmed milk, 350 mM NaCl, 10 mM
Tris-HCl (pH 8.0) and 0.05% Tween-20 for 1 h and incubated with the
indicated primary antibody overnight at 4°C. Following 4 washes,
the membrane was incubated with a secondary antibody in the buffer
for 1 h at room temperature. The membrane was then washed and
developed using enhanced chemiluminescence western blotting
detection system (Amersham Pharmacia). Primary antibodies against
HO-1 (Santa Cruz Biotechnology), α-tubulin (Calbiochem) and β-actin
(Santa Cruz Biotechnology) and HRP-conjugated secondary antibodies
(Amersham Pharmacia) were used.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total cellular RNA was extracted from cells using
the TRIzol reagent according to the manufacturer’s instructions
(Invitrogen). RT-PCR was performed using the SuperScript One-Step
RT-PCR system and gene-specific primers according to the
instructions of the manufacturer (Invitrogen). Reaction mixtures
containing total RNA (500 ng of each), 0.2 mM dNTPs, 0.2 μM of each
primer and an enzyme mixture composed of SuperScript II RT,
Platinum Taq DNA polymerase and 1X buffer with 1.2 mM
MgSO4 were maintained at 50°C for 20 min, then at 94°C
for 2 min and PCR was performed as follows: 30 cycles at 94°C for
15 sec, 55°C for 30 sec and 70°C for 30 sec. The primers for
RT-PCRs were designed based on human sequences in GenBank. The
forward and reverse primers used for the amplification of
IL-8 (1–422; 422 bp; GenBank accession no. NM_000584)
fragment were: 5′-ATGACTTCCAAGCTGGCCGTGG-3′ and
5′-TTATGAATTCTCAGCCCTCTTC-3′; and those for GAPDH (611–885; 275 bp;
GenBank accession no. NM_002046) were:
5′-AGAACATCATCCCTGCCTCTACTGG-3′ and
5′-AAAGGTGGAGGAGTGGGTGTCGCTG-3′.
Real-time PCR analysis
One microgram of RNA was reverse-transcribed using a
first-strand cDNA synthesis kit (ReverTra Aceα; Toyobo).
Quantitative real-time PCR was performed using SYBR premix Ex Taq
(Takara) on the CFX96™ Real-Time PCR Detection System (Bio-Rad)
according to the technical brochure of the company. Quantitative
measurements were determined using the ΔΔCt method and expression
of GAPDH was used as the internal control. Melt curve analyses of
all real-time PCR products were performed and shown to produce the
sole DNA duplex. A standard curve was prepared for each target gene
and PCR efficiency was determined to be in excess of 90% for all
primer sets.
TP activities
Enzyme activity of TP was assayed by the
spectrophotometric method. Cell lysates were incubated in a
potassium phosphate buffer (pH 6.5) and 10 mM thymidine at 37°C for
1 h. The thymine formed was quantitated by the absorbance at 300
nm.
Cellular ROS measurement
ROS production was measured using
H2DCF-DA, an uncharged cell-permeable fluorescent probe.
Cells were treated with H2DCF-DA (10 μM), then washed,
re-suspended in PBS and analyzed using a fluorescence microscope
(BZ-9000 Biorevo, Keyence) and FACScan (FACSCalibur, BD
Biosciences) as previously described (20).
Enzyme linked immunosorbent assays
(ELISA) of IL-8
IL-8 concentrations in the culture medium were
determined by ELISA (R&D Systems) according to the instructions
of the manufacturer.
RNA interference
TP and scramble siRNA duplexes were purchased
from Sigma. The siRNA transfection was carried out using
Lipofectamine 2000 (Invitrogen) according to the instructions of
the manufacturer.
Determination of cellular glutathione
levels
Total glutathione levels were measured using the
Total Glutathione Quantification kit (Dojindo Molecular
Technologies, Inc.) according to the manufacturer’s instructions.
Harvested cells were suspended in 80 μl of 10% HCl and lysed by
freezing and thawing. Twenty microliters of 5% 5-sulfosalicylic
acid was added to the lysates and centrifuged at 8000 × g for 10
min at 4°C. The supernatant was used to measure glutathione levels.
5,5′-Dithiobis-(2-nitrobenzoic acid) and GSH react to generate
2-nitro-5-thiobenzoic acid. Concentrations of GSH were determined
by measuring absorbance at 412 nm.
Statistical analysis
Results were statistically analyzed using the
GraphPad Prism v5.0 software. Statistical analyses for all
experiments including more than two groups were carried out using
one-way ANOVA. Student’s t-tests were used for experiments
including two groups. Data are presented as the means ± SD. The
differences were considered significant at P<0.05.
Results
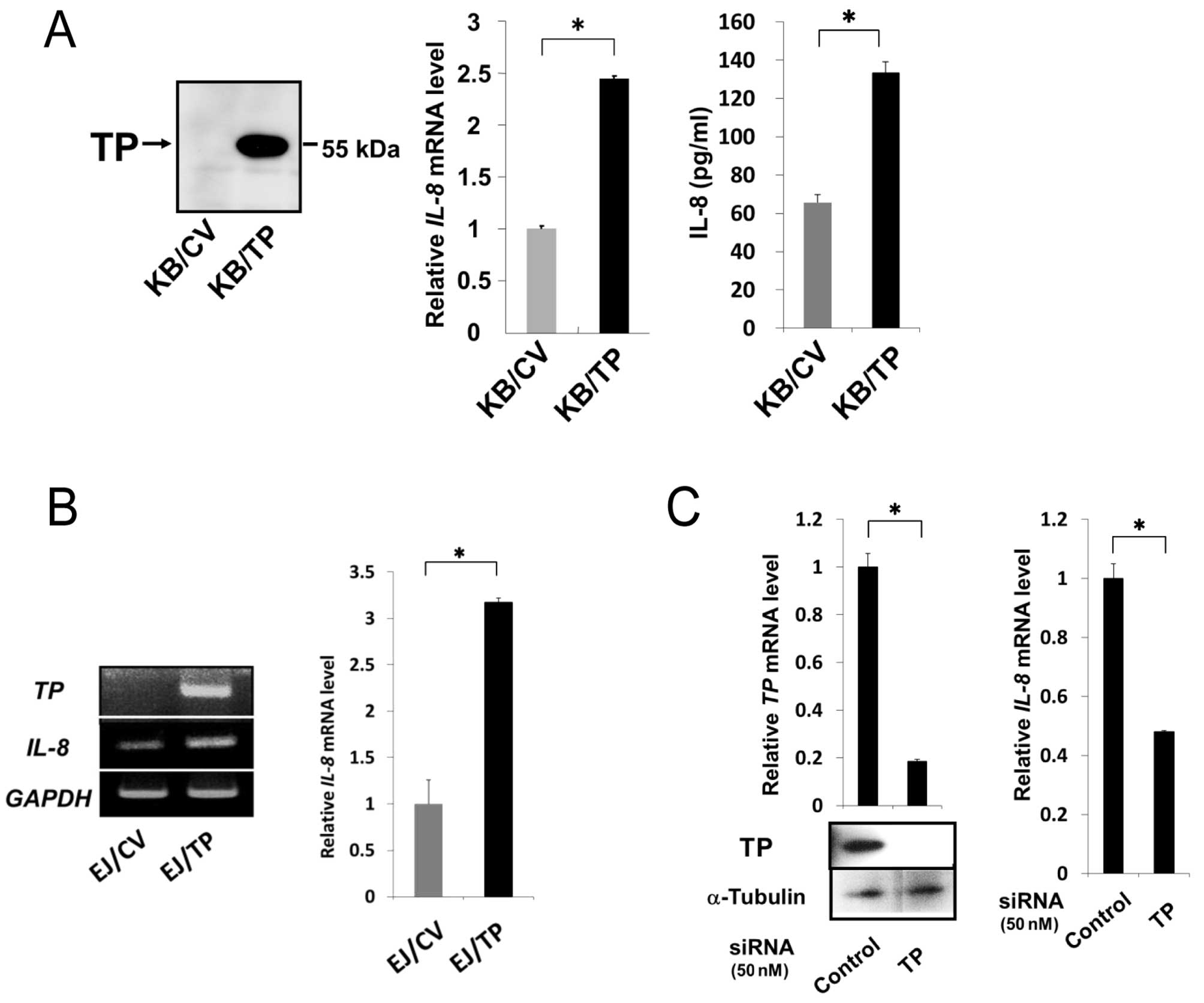
Role of TP in the induction of IL-8
We have previously reported that expression levels
of IL-8 mRNA and protein in KB/TP cells, which overexpress
TP, were higher than those in KB/CV cells that do not express TP
(9). In this study, we confirmed
that TP is implicated in the expression of IL-8 in KB/TP
cells (Fig. 1A) and examined
whether TP is also involved in IL-8 expression in human bladder
carcinoma EJ and human cervical cancer Yumoto cells. TP mRNA
was expressed at high levels in EJ/TP cells, but not detected in
EJ/CV cells. The expression level of IL-8 mRNA in EJ/TP
cells was 3.3-fold higher than in EJ/CV cells (Fig. 1B), suggesting that the induction of
IL-8 by TP is not restricted in KB cells. TP siRNA
efficiently downregulated expression of TP in Yumoto cells, and
suppressed expression of IL-8 mRNA in the cells, indicating
that the intrinsic TP is also implicated in the expression of
IL-8 in Yumoto cells (Fig.
1C).
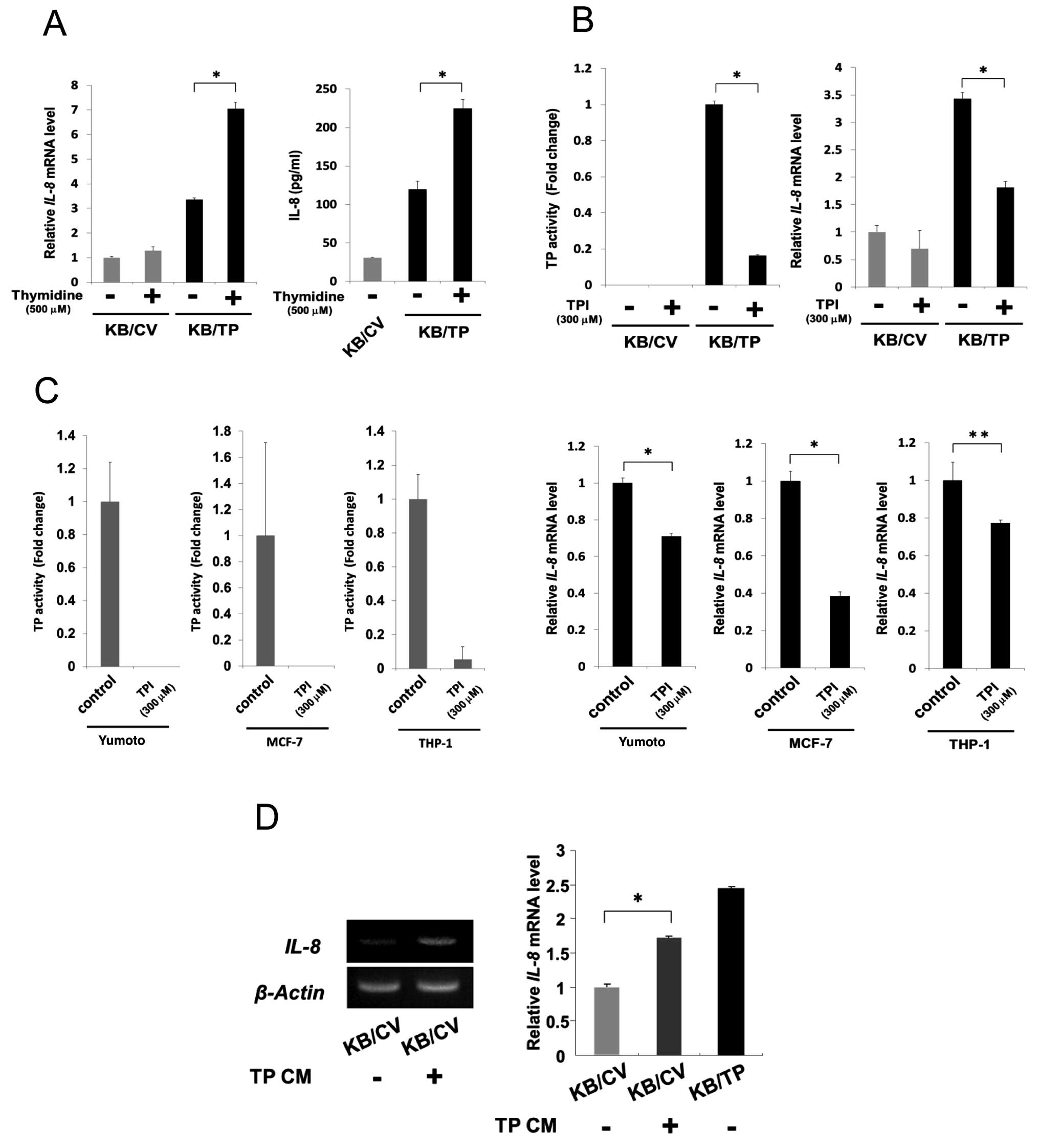
TP activity is needed for the enhanced
expression of IL-8
We then examined whether TP activity is required for
the enhanced expression of IL-8 in KB/TP cells (Fig. 2). KB/CV and KB/TP cells were
incubated in the absence or presence of thymidine for 48 h, then
levels of IL-8 mRNA in the cells were determined by
real-time PCR (Fig. 2A, left panel)
and the amount of IL-8 protein secreted from the cells was
determined by ELISA (Fig. 2A, right
panel). Expression of IL-8 mRNA in KB/TP cells, but not in
KB/CV cells, was considerably increased in the presence of
thymidine. The secreted IL-8 protein from KB/TP cells was also
significantly increased by thymidine.
KB cells were incubated in the medium without or
with 300 μM TPI for 48 h, then TP activities in the cytosol were
determined photometrically. IL-8 mRNA levels in the cells
were determined by real-time PCR. TPI at 300 μM, which is not
cytotoxic to KB/TP cells, decreased TP activity in KB/TP cells to
17% of that in untreated KB/TP cells (Fig. 2B, left panel). TPI at the same
concentration significantly decreased the expression of IL-8
mRNA in KB/TP cells, but not in KB/CV cells (Fig. 2B, right panel). Human cancer cell
lines, MCF-7 and THP-1 as well as Yumoto, express intrinsic TP. TPI
at 300 μM, which was not cytotoxic to those cells, completely
inhibited TP activity in Yumoto and MCF-7 cells and decreased it to
5% of the control levels in THP-1 cells (Fig. 2C left panel). IL-8 mRNA
expression in those cells was considerably decreased by the same
concentration of TPI (Fig. 2C right
panel). These results suggest that TP activity is required for the
enhanced expression of IL-8 mRNA and thymidine-derived
metabolites are involved in the enhanced expression of IL-8 in
those cells.
Bijnsdorp et al reported that DR, one of the
thymidine-derived sugars, is extensively secreted from
TP-overexpressing cells (13). When
KB/CV cells were incubated in conditioned medium of KB/TP cells
(TPCM), expression level of IL-8 mRNA in KB/CV cells was
significantly increased (Fig. 2D).
These results suggested that DR were secreted from KB/TP cells in
the medium and enhanced IL-8 mRNA expression in KB/CV
cells.
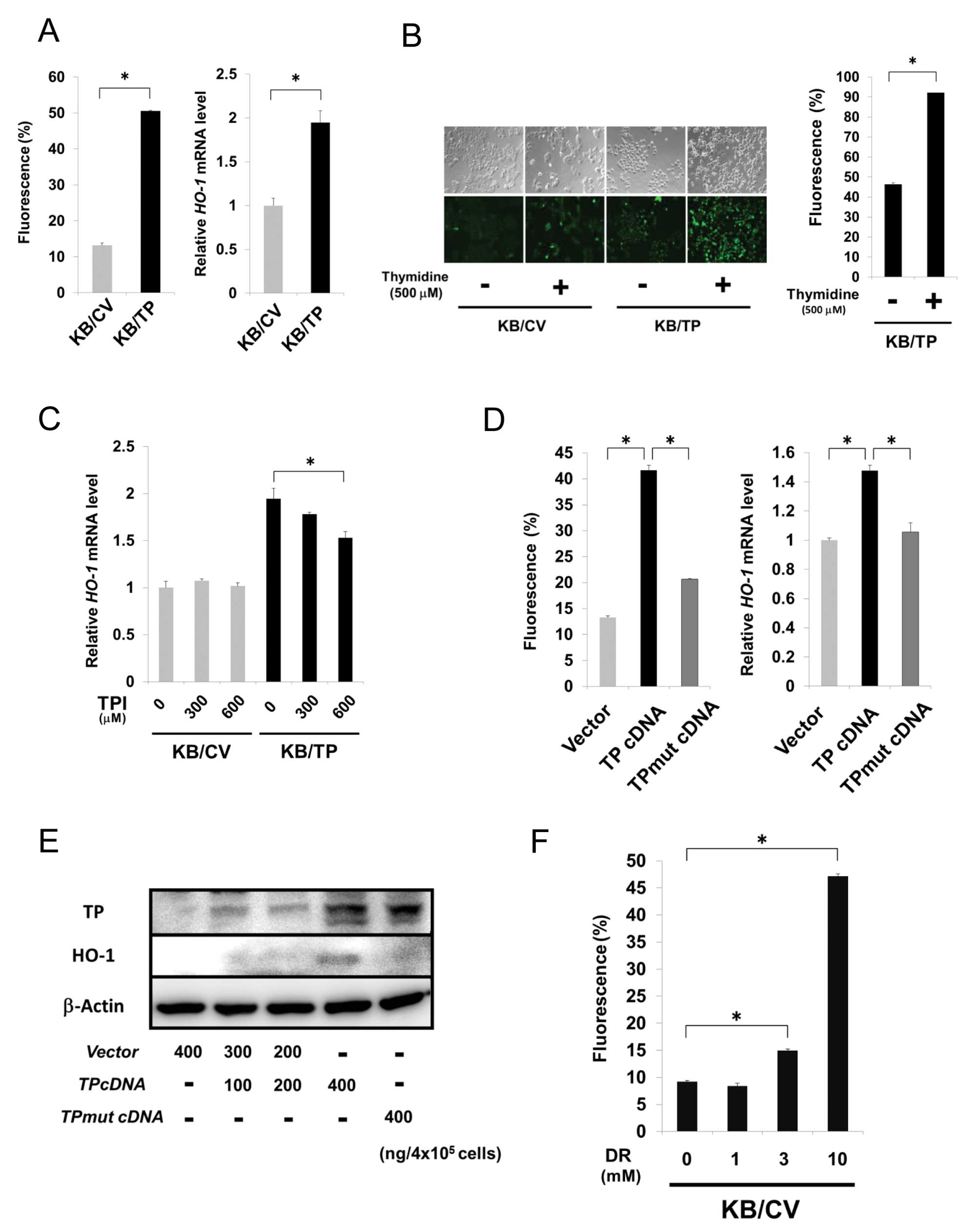
ROS generation by TP
TP-overexpressing cells treated with thymidine
induced expression of hemo oxygenase-1 (HO-1), a classical marker
of cellular oxidative stress (15).
We firstly investigated ROS and HO-1 mRNA levels in KB/CV
and KB/TP cells using the fluorescent probe H2DCF-DA and
real-time PCR, respectively. Expression levels of ROS and
HO-1 mRNA in KB/TP cells were about 5- and 2-fold higher
than those in KB/CV cells, respectively (Fig. 3A). ROS level in KB/TP cells was
higher than that in KB/CV cells and considerably increased when
thymidine was added in the medium (Fig.
3B). Expression level of HO-1 mRNA in KB/TP cells, but
not in KB/CV cells, was decreased by TPI (Fig. 3C). We then examined ROS levels in KB
cells transiently transfected with empty vector, TP cDNA or
TPmut cDNA (400 ng/4×105 cells) using
H2DCF-DA (Fig. 3D, left
panel). HO-1 mRNA and protein levels in the cells were also
determined by real-time PCR (Fig.
3D, right panel) and immunoblot analysis (Fig. 3E), respectively. ROS and HO-1 levels
in KB cells transiently transfected with TP cDNA were higher
than those in KB cells transfected with empty vector or
TPmut cDNA that codes mutant TP lacking the enzymatic
activity. ROS level in KB/CV cells was dose-dependently increased
by DR (Fig. 3F). These results
indicated that transiently expressed TP enhanced ROS generation and
the enzymatic activity of TP is needed for the enhanced generation
of ROS.
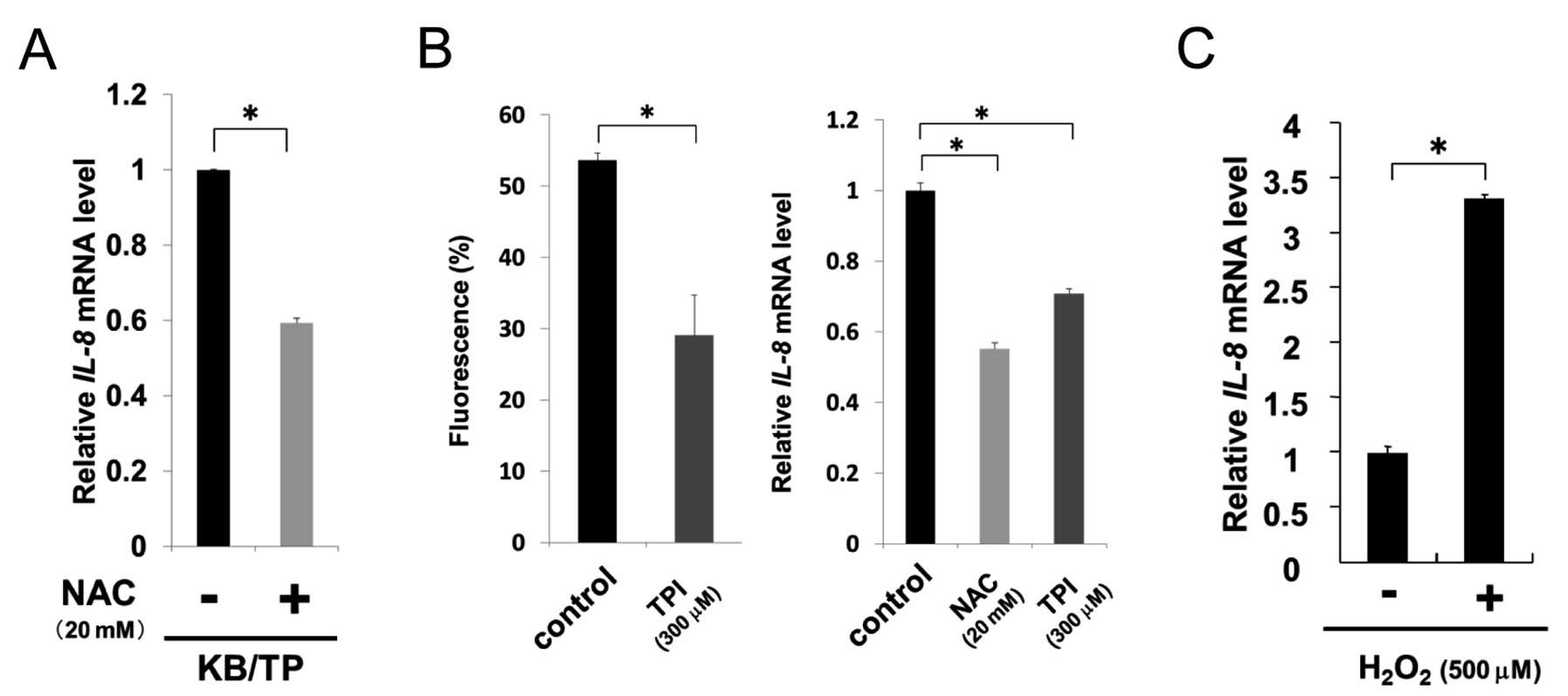
TP-induced ROS results in increased
expression of IL-8
To examine whether TP-induced ROS augmented IL-8
expression, we assessed the levels of IL-8 in KB/TP cells
treated with an antioxidant, NAC. IL-8 mRNA expression in
KB/TP cells was considerably decreased by NAC, suggesting that ROS
is involved in the enhanced expression of IL-8 mRNA in these
cells (Fig. 4A). TPI significantly
suppressed the levels of ROS and IL-8 mRNA in Yumoto cells
(Fig. 4B). NAC also decreased
IL-8 mRNA expression in Yumoto cells (Fig. 4B, right panel).
H2O2 at 500 μM increased IL-8 mRNA
levels up to 3.3-fold in Yumoto cells (Fig. 4C). These results suggest that TP is
involved in the generation of ROS in Yumoto cells which express
intrinsic TP and ROS cause the enhanced IL-8 mRNA expression
in those cells.
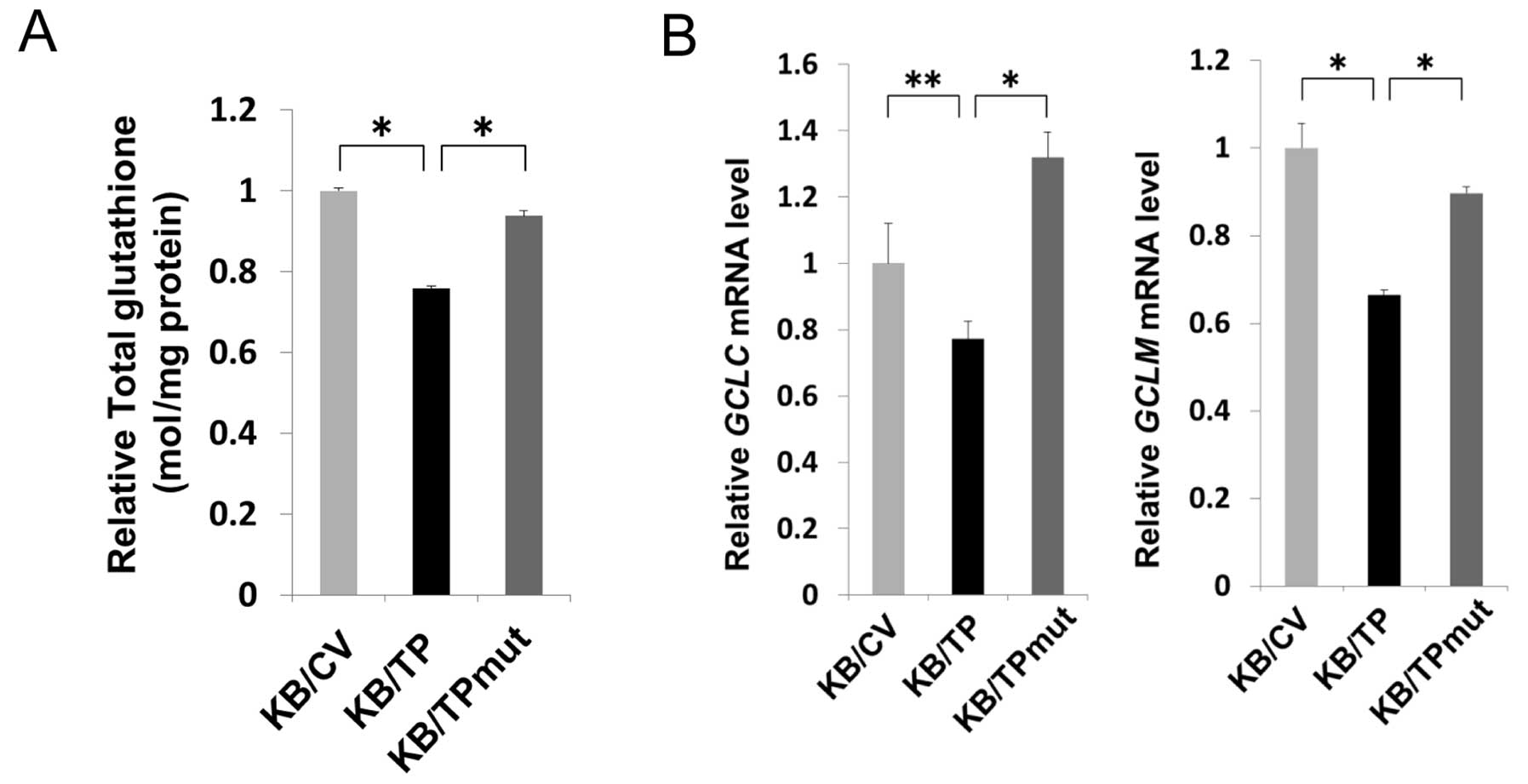
TP decreases the levels of cellular
glutathione
DR decreased cellular glutathione level in various
types of cells (16,17). We examined the glutathione levels in
KB/CV, KB/TP and KB/TPmut cells. Glutathione levels in KB/TP cells
were about 20–30% lower than those in KB/CV and KB/TPmut cells
(Fig. 5A). γ-glutamylcysteine
synthetase (γ-GCS) is the first rate-limiting enzyme of glutathione
synthesis. The enzyme consists of two subunits, a heavy catalytic
subunit (GCLC) and a light regulatory subunit (GCLM). Both
GCLC and GCLM mRNAs in KB/TP cells were significantly
decreased compared with those in KB/CV and KB/TPmut cells (Fig. 5B). These results suggested that TP
activity is involved in the decreased expression of γ-GCS and the
lowered level of cellular glutathione in KB/TP cells.
Discussion
TP is expressed in various malignant tumors and
plays a pivotal role in angiogenesis, tumor growth, invasion and
metastasis of TP-expressing tumors (2). DR, one of the thymidine-derived
sugars, has similar functions to TP. We have previously suggested
that DR is a downstream mediator of TP function (2,6).
Brown et al observed that thymidine
upregulated HO-1 in a dose-dependent manner in human bladder
carcinoma RT112-TP cells with high TP expression (15). Since cellular oxidative stress is
responsible for HO-1 induction, they suggested that TP induced
cellular oxidative stress in the cells. In this study, we directly
measured ROS levels in KB cells and indicated that TP enhanced ROS
generation. TP activity was required for the enhanced generation of
ROS and DR also enhanced ROS generation. High concentrations of DR
cause ROS generation and lowered intracellular glutathione levels
in various cells (21,22).
The level of cellular glutathione in KB/TP cells was
significantly lower than that in KB/CV and KB/TPmut cells (Fig. 5A). Transcription of γ-GCS in
KB/TP cells was also attenuated compared with those in KB/CV and
KB/TPmut cells (Fig. 5B).
Glutathione is considered as a main intracellular defense against
oxidative stress. Decreased glutathione levels in KB/TP cells may
be in part implicated in the augmented ROS in the cells.
Brown et al suggested that thymidine
catabolism by TP increased carcinoma cell secretion of angiogenic
factors induced by oxidative stress (15). We observed that TP augmented the
expression of IL-8 mRNA in human cancer KB, EJ and Yumoto
cells. TP activity was again needed to enhance IL-8 mRNA
expression and DR increased IL-8 mRNA expression in KB/CV
cells that do not express TP. Furthermore, NAC suppressed the
increased expression of IL-8 mRNA as well as the augmented
generation of ROS. These findings indicate that ROS induced by TP
enhanced the IL-8 mRNA expression. ROS was previously
suggested to stimulate cell growth by direct activation of certain
redox sensitive transcription factors such as NF-κB (23). The IL-8 promoter region contains
binding sites for the transcription factors, AP-1 (−126 to −120
bp), NF-κB (−80 to −71 bp) and NF-IL-6 (−94 to −81 bp) (24). ROS may activate NF-κB, which is
supposed to bind the IL-8 promoter and to enhance
IL-8 gene transcription.
In conclusion, our study demonstrated that the
enzymatic activity of TP is required for the enhanced ROS
generation and IL-8 expression by TP. DR, one of the
thymidine-derived sugars, enhanced the generation of ROS in
TP-negative KB/CV cells. NAC suppressed the enhanced IL-8
mRNA expression in KB/TP cells. The level of cellular glutathione
was decreased in TP-overexpressing cells. Decreased glutathione
levels in the TP-overexpressing cells may be in part implicated in
the augmented ROS generation in the cells, since glutathione is
considered as a main intracellular defense against oxidative
stress. These findings suggest that ROS generated by
thymidine-derived sugars enhance the expression of IL-8. TPI
inhibited the enzymatic activity of TP and attenuated the
TP-induced ROS generation and IL-8 mRNA expression in human
cancer cells. The results support the notion that compounds that
inhibit TP activity, such as TPI, are good candidates for new
progressive anticancer agents.
Further study of the molecular mechanisms for the
generation of ROS by thymidine-derived sugars and for the enhanced
IL-8 expression by ROS will contribute to our understanding
of the roles of TP in the malignant progression of tumors.
Acknowledgements
This study was supported by a Grant-in-Aid for
Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology of Japan and the Research Support
Foundation of The University of Tokushima and TAIHO Pharmaceutical
Co., Ltd.
Abbreviations:
|
TP
|
thymidine phosphorylase
|
|
IL-8
|
interleukin-8
|
|
ROS
|
reactive oxygen species
|
|
NAC
|
N-acetylcysteine
|
|
HO-1
|
hemo oxygenase-1
|
|
DR
|
2-deoxy-d-ribose
|
|
γ-GCS
|
γ-glutamylcysteine synthetase
|
References
|
1
|
Furukawa T, Yoshimura A, Sumizawa T, et
al: Angiogenic factor. Nature. 356:6681992.
|
|
2
|
Akiyama S, Furukawa T, Sumizawa T,
Takebayashi Y, Nakajima Y, Shimaoka S and Haraguchi M: The role of
thymidine phosphorylase, an angiogenic enzyme, in tumor
progression. Cancer Sci. 95:851–857. 2004.
|
|
3
|
Miyadera K, Sumizawa T, Haraguchi M,
Yoshida H, Konstanty W, Yamada Y and Akiyama S: Role of thymidine
phosphorylase activity in the angiogenic effect of platelet derived
endothelial cell growth factor/thymidine phosphorylase. Cancer Res.
55:1687–1690. 1995.
|
|
4
|
Matsushita S, Nitanda T, Furukawa T, et
al: The effect of a thymidine phosphorylase inhibitor on
angiogenesis and apoptosis in tumors. Cancer Res. 59:1911–1916.
1999.
|
|
5
|
Takao S, Akiyama S, Nakajo A, et al:
Suppression of metastasis by thymidine phosphorylase inhibitor.
Cancer Res. 60:5345–5348. 2000.
|
|
6
|
Haraguchi M, Miyadera K, Uemura K, et al:
Angiogenic activity of enzymes. Nature. 368:1981994.
|
|
7
|
Kitazono M, Takebayashi Y, Ishitsuka K, et
al: Prevention of hypoxia-induced apoptosis by the angiogenic
factor, thymidine phosphprylase. Biochem Biophys Res Commun.
253:797–803. 1998.
|
|
8
|
Uchimiya H, Furukawa T, Okamoto M, et al:
Suppression of thymidine phosphorylase-mediated angiogenesis and
tumor growth by 2-deoxy-l-ribose. Cancer Res. 62:2834–2839.
2002.
|
|
9
|
Nakajima Y, Gotanda T, Uchimiya H, et al:
Inhibition of metastasis of tumor cells overexpressing thymidine
phosphorylase by 2-deoxy-l-ribose. Cancer Res. 64:1749–1801.
2004.
|
|
10
|
Hotchkiss KA, Ashton AW and Schwartz EL:
Thymidine phosphorylase and 2-deoxyribose stimulate human
endothelial cell migration by specific activation of the integrins
α5β1 and αVβ3. J Biol
Chem. 278:19272–19279. 2003.
|
|
11
|
Seeliger H, Guba M, Koehl GE, et al:
Blockage of 2-deoxy-d-ribose induced angiogenesis with rapamycin
counteracts a thymidine phosphorylase-based escape mechanism
available for colon cancer under 5-fluorouracil therapy. Clin
Cancer Res. 10:1843–1852. 2004.
|
|
12
|
Hoffee PA: 2-Deoxyribose gene-enzyme
complex in Salmonella typhimurium I. Isolation and enzymatic
characterization of 2-deoxyribose-negative mutants. J Bacteriol.
95:449–457. 1968.
|
|
13
|
Bijnsdorp IV, Azijli K, Jansen EE, et al:
Accumulation of thymidine-derived sugars in thymidine phosphorylase
overexpressing cells. Biochem Pharmacol. 80:786–792. 2010.
|
|
14
|
Brown NS and Bicknell R: Thymidine
phosphorylase, 2-deoxy-d-ribose and angiogenesis (Review). Biochem
J. 334:1–8. 1988.
|
|
15
|
Brown NS, Jones A, Fujiyama C, Harris AL
and Bicknell R: Thymidine phosphorylase induces carcinoma cell
oxidative stress and promotes secretion of angiogenic factors.
Cancer Res. 60:6298–6302. 2000.
|
|
16
|
Potter H, Weir L and Leder P:
Enhancer-dependent expression of human kappa immunoglobulin genes
introduced into mouse pre-B lymphocytes by electroporation. Proc
Natl Acad Sci USA. 81:7161–7165. 1984.
|
|
17
|
Takebayashi Y, Yamada K, Miyadera K, et
al: The activity and expression of thymidine phosphorylase in human
solid tumours. Eur J Cancer. 32A:1227–1232. 1996.
|
|
18
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970.
|
|
19
|
Andersen JK: Electroblotting of multiple
gels: a simple apparatus without buffer tank for rapid transfer of
proteins from polyacrylamide to nitrocellulose. J Biochem Biophys
Methods. 10:203–209. 1984.
|
|
20
|
Tuvdendorj D, Oketani M, Ikeda R, et al:
Aspirin induces hepatoma-derived cell apoptosis via a hydrogen
peroxide-dependent pathway. Hepatol Res. 26:47–54. 2003.
|
|
21
|
Fico A, Manganelli G, Cigliano L, et al:
2-Deoxy-d-ribose induces apoptosis by inhibiting the synthesis and
increasing the efflux of glutathione. Free Radic Biol Med.
45:211–217. 2008.
|
|
22
|
Schmidt MM, Greb H, Koliwer-Brandl H, Kelm
S and Dringen R: 2-Deoxyribose deprives cultured astrocytes of
their glutathione. Neurochem Res. 35:1848–1856. 2010.
|
|
23
|
Brar SS, Kennedy TP, Sturrock AB, et al:
NADPH oxidase promotes NF-κB activation and proliferation in human
airway smooth muscle. Am J Physiol Lung Cell Mol Physiol.
282:L782–L795. 2002.
|
|
24
|
Murayama T, Ohara Y, Obuchi M, Khabar KSA,
Higashi H, Mukaida N and Matsushima K: Human cytomegalovirus
induces interleukin-8 production by a human monocytic cell line,
THP-1, through acting concurrently on AP-1- and NF-κB-binding sites
of the interleukin-8 gene. J Virol. 71:5692–5695. 1997.
|