Introduction
Malignant lymphomas observed in the breast are most
commonly non-Hodgkin lymphomas. They could be primary breast
lymphoma (PBL) or most frequently a sign of systemic disease
(1,2). The PBL represent 0.38–0.70% of all
non-Hodgkin lymphomas (NHL), 1.7–2.2% of all extra nodal NHL and
only 0.04–0.5% of all breast cancer (1–3). The
WHO criteria for the diagnosis of PBL are clinicopathological
demonstration of lymphomatous infiltration within breast tissue
with or without involvement of axillary lymph nodes, but in absence
of systemic disease (1,4). The most common histotypes of PBL are
diffuse large B-cell lymphoma and extra nodal marginal zone B-cell
lymphoma of mucosa-associated lymphoid tissue MALT-type (MALT
lymphomas) (1,2).
The molecular studies of MALT lymphoma in extra
nodal sites have shown the presence of different chromosomal
translocations that appear to be mutually exclusive with
substantial differences in their frequency in relation to extra
nodal anatomic sites. These translocations include the
t(11;18)(q21;q21), t(14;18)(q32;q21), t(1;14)(q22;q32), and the
most recently described, t(3;14)(p14.1;q32) (5–7). These
translocations result in the production of a chimeric protein
(API2-MALT1) or in BCL10, MALT1, FOXP1 genes over expression,
because of direct control of IgH promoter (8–10).
The purpose of this study is the evaluation of
chromosomal rearrangements t(11;18)(q21;q21), t(14;18)(q32;q21),
t(1;14)(q22;q32) and BCL10 expression in a series of nine cases of
primary breast MALT lymphomas.
Materials and methods
Clinical information
Cases were selected from the pathological files of
the Cardarelli Hospital and National Tumor Institute Pascale,
Naples, and University of Trieste, from January 1993 to December
2010. The WHO criteria to establish the diagnosis of PBL have been
strictly applied. Thus cases included in our study are
characterized by the following features: i) histologically the
lymphomatous infiltrate should demonstrate a close relationship
with the breast parenchyma with or without ipsilateral axillary
lymph node involvement; ii) no evidence of systemic disease after
staging; iii) imaging studies clearly identified the neoplasm to be
within the breast in absence of other localization. Clinical
information was recovered from clinical files. A total of 9 cases
of MALT-like lymphomas were identified.
Specimens had been routinely fixed and processed.
Haematoxylin and eosin (H&E) stained sections and appropriate
immunohistochemical staining were performed in order to confirm
diagnosis of MALT-like PBL (Table
I). As part of this study, additional immunostaining has been
performed using antibodies specific for BCL10.
 | Table IImmunohistochemical antibodies. |
Table I
Immunohistochemical antibodies.
| Antigen clone | Source | Dilution | Reactivity | Threshold | Internal control |
|---|
| CD20 (clone
L-26) | Dako | 1:100 |
Positive/negative | Any positive
neoplastic cells | Reactive
lymphocyte |
| CD10 (clone56C6) | Novocastra | 1:10 |
Positive/negative | Any tumoral cell
positive | GC* B cells |
| Bcl6
(clonePG-B6p) | Dako | 1:10 |
Positive/negative | >10% neoplastic
cells | GC* B cells |
| CK (clone
AE1/AE3) | Dako | 1:50 |
Positive/negative | Any positive
neoplastic cells | Breast epithelial
cell |
| Bcl10 | Dako | 1:200 |
Positive/negative | >10% neoplastic
cells | Reactive
lymphocyte |
| CD3 | Dako | 1:25 |
Positive/negative | Any tumoral cell | Reactive
lymphocyte |
| CD5 | Novocastra | 1:50 |
Positive/negative | >10% positive
cells | Reactive
lymphocyte |
Stained sections were evaluated by four different
pathologists (R.F., A.D., M.D.B., G.B.) using uniform criteria.
Discrepancies were resolved through simultaneous evaluation and
discussion of the results. Single-marker expression was recorded as
negative/positive and high/low level, after consideration of the
expression in reactive compared with tumoral cells and the specific
cut-off of each marker. As proposed, cytoplasmic BCL10 expression
was scored as strong when it was similar to tonsil centroblast
positivity, moderate when similar to centrocyte positivity, and
weak/absent when similar to tonsil mantle-zone positivity. Nuclear
positivity has been also recorded (11).
Fluorescent in situ hybridization
study
Tissue array sections from paraffin-embedded tissue
were heated for 4 h at 62°C and immediately deparaffinized in two
rinses of 100% xylene for 10 min each. The slides were then treated
with 0.3 M sodium chloride and 0.03 M sodium citrate for 20 min at
80°C, and with 0.05 mg/ml proteinase for 10 min at 37°C. For
t(11;18)(q21;q21) detection, we used LSI API2/MALT1
t(11;18)(q21;q21) dual-colour, dual-fusion translocation probe, and
for t(14;18) (q32;q21) we used LSI IGH/MALT1 t(14;18)(q32;q21)
dual-colour, dual-fusion translocation probe. For detection of
BCL10 translocation BCL10 FISH DNA Probe, Split Signal, and
Histology FISH kit (Dako) were used. The cut-off value for the
diagnosis of rearrangement involving IGH and MALT1 was 5.3%, which
is above the mean percentage of cells with a false positive signal
plus 3 SD, as assessed in tissue from reactive tonsils present in
TMA. Moreover, IGH dual-colour break-apart rearrangement probes
(Vysis Inc., Downers Grove, IL, USA) were applied to cells of all
t(14;18)(q32;q21)-positive lymphomas to confirm the
translocation.
The Spectrum Green-labelled LSI IGVH probe covers
the entire IGH variable region, while the Spectrum Orange-labelled
probe lies completely within the IGH locus. As a result of this
probe design, any translocation with a breakpoint at the J
segments, or within switch sequences, should produce separate
orange and green signals. Additionally, FISH with
centromere-specific probes for chromosome 18 (Vysis) was performed
in all cases. The appropriate probe mix (10 ml) was applied to the
tissue sections and covered with a coverslip. Both probe and target
DNA were simultaneously denatured at 75°C for 5 min and incubated
overnight at 37°C using the Hybrite System. Post-hybridization
washes were performed according to the ‘rapid wash protocol’
provided by Vysis. Slides were counterstained with
406-diamidino-2-phenylindole 2HCl (DAPI). FISH was performed
according to the manufacturer’s instructions (Vysis). FISH data
were collected using an Olympus BX 61 fluorescence microscope
equipped with a cooled black-and-white camera controlled by the
associated software (Olympus, Italy).
Statistical analysis
The Pearson’s χ2 test was used where
appropriate, to establish whether there were any relationships
between the frequencies of different markers included in this
study. Differences were considered to be significant for values of
P<0.05. All statistical analyses were performed using the SPSS
98 v.12 program.
Results
Clinical features
The main clinicopathological data are reported in
the Table II. All patients were
female, with mean age of 72 years (range: 39–93). In 6 cases right
breast was involved. Mean time of follow-up was 51 months (range:
33–65) and at the end of follow-up, 2 patients were alive, 6 alive
with disease and 1 dead from disease.
 | Table IIMain clinicopathological data. |
Table II
Main clinicopathological data.
| Case no. | Gender | Age | Site | BCL10 IHC | t(11;18) | t(14;18) | t(1;14) | Status |
|---|
| 1 | F | 71 | R | − | − | − | − | AWD |
| 2 | F | 82 | R | 1+ | − | − | − | AWD |
| 3 | F | 93 | R | − | + | − | − | AWD |
| 4 | F | 73 | R | − | − | − | − | Alive |
| 5 | F | 74 | L | − | − | − | − | Dead |
| 6 | F | 71 | L | 2+ | − | − | − | AWD |
| 7 | F | 72 | R | 2+ | + | − | − | AWD |
| 8 | F | 73 | L | 2+ | + | − | − | AWD |
| 9 | F | 39 | R | − | − | + | − | Alive |
Histological findings
All nine cases of MALT lymphoma had a diffuse or
vaguely nodular growth pattern. We have identified three types of
lymphoid cells: i) small to medium lymphoma cells with irregular
nuclear contours, inconspicuous nucleoli and pale-staining
cytoplasm (centrocyte-like cells), prevalent in all cases; ii)
small lymphoid cells with round nuclei, clumped chromatin and
sparse cytoplasm, some with prominent plasmacytic differentiation
admixed with mature plasma cells; iii) lymphoid cells with abundant
pale or clear cytoplasm resembling monocytoid cells. These elements
are present in a variable number in all cases. Lymphoepithelial
lesions were present but were not prominent. Transformed
lymphocytes resembling centroblasts or immunoblasts were present in
all cases, but rarely formed small clusters or confluent sheets.
Lymphoid follicle with reactive germinal centre was also present in
breast tissue in two cases. Mitotic activity was low and necrosis
areas were not observed (Fig.
1).
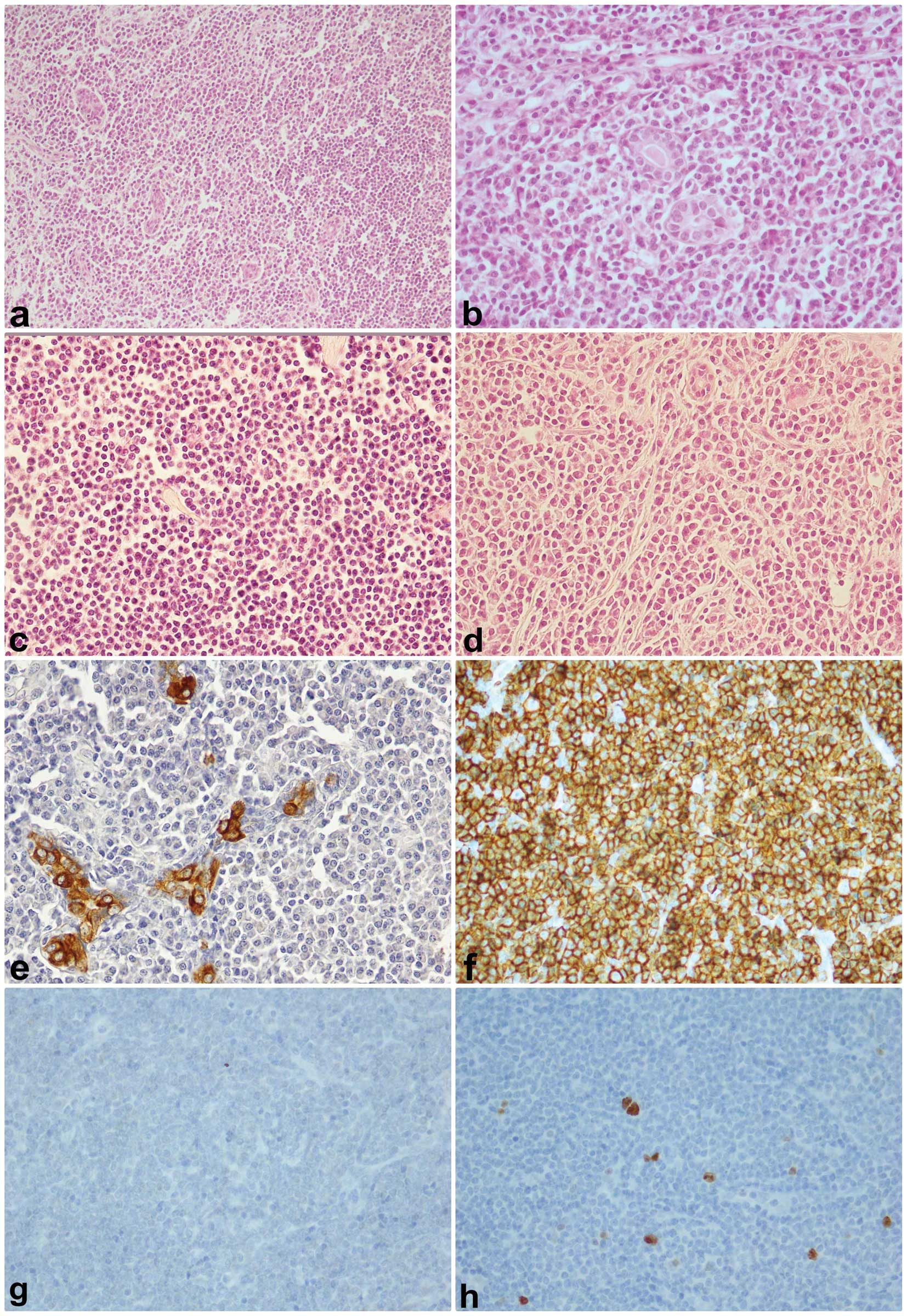 | Figure 1(a) Epithelial islands in a diffuse
pattern of lymphoma proliferation, ×10, hematoxylin and eosin
stain; (b) Lymphoma proliferation surrounds epithelial ducts, ×40;
(c) Monocytoid differentiation of MALT lymphoma, ×40;
(d)Plasmocytoid differentiation in MALT lymphoma, ×40; (e) CK
immunostaining, ×40, in trapped duct cells by lymphoma; (f) CD20
immunostaining, ×40, diffuse neoplastic cells positivity; (g) BCL6
immunostaining, ×40, BCL6 negativity in MALT lymphoma; (h) Ki67
immunostaining, ×40, low proliferation inde× of lymphoma. |
Immunohistochemical findings
Immunohistochemical results are summarized in
Table II. Lymphoma cells express
pan B-lymphocyte antigens CD20 and CD79a in all cases and 8 show κ
light chain restriction. CD43 was expressed in 8/9 cases, while
CD5, CD10, BCL6 and CD10 that were usually negative. BCL10 was
positive with cytoplasmic high intensity in 4 cases and negative in
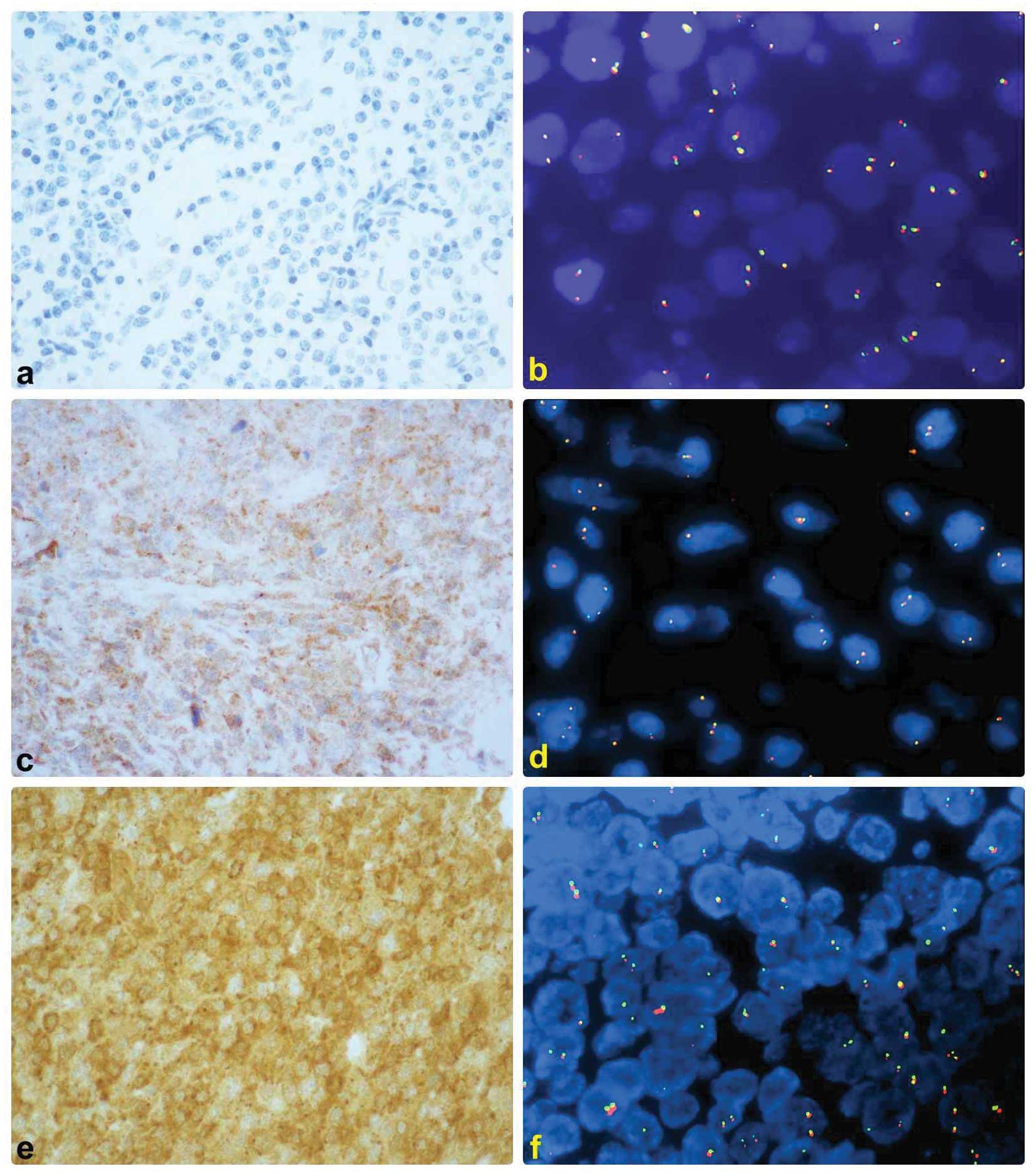
5. No nuclear positive case was observed (Fig. 2).
FISH analysis
t(11;18)(q21;q21) was present in 3 cases and
t(14;18)(q32;q21) in one case. There was no evidence of (1;14)
(q22;q32) in any of the lymphoma assessed (Fig. 2).
Statistical analysis
Only association between t(11;18) and high intensity
cytoplasmic expression of BCL10 (P=0.046) was observed.
Discussion
MALT type extra nodal marginal zone B-cell lymphoma
(MALT lymphoma) represents <10% of all B-cell lymphomas
(1). They are most frequent in the
gastrointestinal tract, salivary glands, ocular adnexa, lungs,
thyroid and breast (8). Due to the
lack of native lymphoid tissue in these organs, a chronic
inflammatory stimulus or an autoimmune disease can generate
mucosa-associated lymphoid tissue from which a MALT lymphoma could
arise (1,2,3,5–9).
Histologically MALT lymphomas show a case-to-case variability in
the neoplastic cytotype. Usually we have observed small cell
lymphomas constituted by centrocyte-like cells with irregular
nuclei, monocytoid B-cell with pale to clear cytoplasm with round
nuclei and more rarely plasmacytoid cells. The different
histological features of the tumor cells do not impact on clinical
prognosis (9). Generally MALToma
have an indolent behavior and recurrence can occur after many years
also in other extra nodal sites. Moreover, in some cases a
progression to diffuse large B-cell lymphoma may occur (1,9). In
particular in a recent work primary breast MALT lymphomas seem to
have a more indolent behavior in respect to other PBLs (12).
The molecular events underlying the origin of a
mucosa-associated lymphoid tissue (MALT) lymphoma and their
progression and prognosis are largely unknown. However, some
chromosomal translocations have been identified in a subset of MALT
lymphomas, including the t(11;18)(q21;q21), t(14;18)(q32;q21),
t(1;14)(p22;q32), and t(3;14)(p14.1;q32). These translocations are
responsible for deregulation of MALT1, BCL10 and FOXP1 genes that
cooperate in the NFκB anti-apoptotic pathway (13). Particularly, t(11;18)(q21;q21) and
t(14;18)(q32;q21) generate chimeric transcripts of MALT1 gene
respectively with the API2 and IgH gene, whereas t(1;14) (p22;q32)
causes aberrant BCL10 expression with nuclear localization
(5–9).
In addition, nuclear BCL10 expression also occurs in
MALT lymphomas without t(1;14)(p22;q32), suggesting an important
role for BCL10 in lymphoma development (14–17).
All these abnormalities also appear to be mutually exclusive and
their frequency correlates with the anatomic site. The
translocation (11;18) is frequently described in gastrointestinal
and pulmonary sites in contrast with presence of t(14;18) in ocular
adnexa, skin and salivary gland (1–7,10,11).
Different series regarding the most common extra nodal sites of
MALT lymphomas have been screened for the presence of the most
common translocations involving MALT1 gene and BCL10 expression
with relation to progression of disease or prognosis (11,14–18).
t(11;18)(q21;q21) is associated with advanced gastric MALT-lymphoma
that expresses nuclear BCL10. Moreover, nuclear expression of BCL10
or nuclear factor κB predicts Helicobacter
pylori-independent status of early-stage, high-grade gastric
mucosa-associated lymphoid tissue lymphomas (17). Moreover, nuclear BCL10 expression
characterizes a group of ocular adnexa MALT lymphomas with shorter
failure-free survival (11).
While the frequency of these translocations has been
studied in the most common extra nodal sites, their presence has
not been well characterized in the breast presumably because these
neoplasms are very infrequent. In a previous study, Talwakar et
al (18) studied eight cases of
primary MALT breast lymphoma and 14 cases of primary breast diffuse
large B-cell lymphoma for MALT1 gene rearrangements through FISH
and for BCL10, NF-κB p65, p50 using immunohistochemical methods.
None of the cases showed MALT1 gene rearrangements and NF-κB
activation was not demonstrated. Similar results were obtained by
Streubel et al (7) in five
additional cases of breast MALT lymphoma assessed for the t(11;18)
and t(14;18) and by Mulligan et al in a small series of PBL
MALT lymphomas assessed for MALT1 gene rearrangement (19). All these authors concluded that
MALT1 gene rearrangements are absent or rare in primary breast MALT
lymphoma compared to other extra nodal MALToma. In our series both
the t(11;18) and t(14;18) were observed. Our results showed
evidence of MALT1 gene rearrangements in four PBL: t(11;18)(q21;
q21) was observed in 3 cases and t(14;18)(q32; q21) in one case.
The expression of BCL10 was only cytoplasmic in 4 cases, with
low/moderate intensity, while nuclear expression was not observed.
No association with chromosomal aberration was observed.
In conclusion, our data indicated that MALT1 gene
rearrangements are not rare in primary breast MALT lymphoma, in
contrast with results of previous series, involving mainly
t(11;18)(q21;q21).
References
|
1
|
Tavassoli FA and Devilee P: Malignant
lymphoma and metastatic tumours. World Health Organization
Classification of Tumours: Pathology and Genetics. Tumors of the
Breast and Female Genital Organs. IARC (ed); Lyon: pp. 107–109.
2003
|
|
2
|
Brogi E and Harris NL: Lymphomas of the
breast: pathology and clinical behaviour. Semin Oncol. 26:357–364.
1999.PubMed/NCBI
|
|
3
|
Avenia N, Sanguinetti A, Cirocchi R,
Bistoni G, Trastulli S, D’Ajello F, Barberini F, Cavallaro G, Rulli
A, Sidoni A, et al: Primary breast lymphomas: a multicentric
experience. World J Surg Oncol. 8:532010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wiseman C and Liao KT: Primary lymphoma of
the breast. Cancer. 29:1705–1712. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ye H, Liu H and Attygalle A: Variable
frequencies of t(11;18)(q21;q21) in MALT lymphomas of different
sites: significant association with CagA strains of H.
pylori in gastric lymphoma. Blood. 102:1012–1018. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Streubel B, Simonitsch-Klupp I and
Mullauer L: Variable frequencies of MALT lymphoma-associated
genetic aberrations in MALT lymphomas of different sites. Leukemia.
18:1722–1726. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Streubel B, Vinatzer U and Lamprecht A:
t(3;14)(p14.1;q32) involving IGH and FOXP1 is a novel recurrent
chromosomal aberration in MALT lymphoma. Leukemia. 9:652–658.
2005.PubMed/NCBI
|
|
8
|
Lucas PC, Yonezumi M and Inohara N: Bcl10
and MALT1, independent targets of chromosomal translocation in Malt
lymphoma, cooperate in a novel NF-kappa B signalling pathway. J
Biol Chem. 276:19012–19019. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Isaacson PG and Du MQ: MALT lymphoma: from
morphology to molecules. Nat Rev Cancer. 4:644–653. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakagawa M, Hosokawa Y and Yonezumi M:
MALT1 contains nuclear export signals and regulates cytoplasmic
localization of BCL10. Blood. 106:4210–4216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Franco R, Camacho FI and Caleo A: Nuclear
bcl10 expression characterizes a group of ocular adnexa MALT
lymphomas with shorter failure-free survival. Mod Pathol.
19:1055–1067. 2006.PubMed/NCBI
|
|
12
|
Martinelli G, Ryan G, Seymour JF, Nassi L,
Steffanoni S, Alietti A, Calabrese L, Pruneri G, Santoro L,
Kuper-Hommel M, et al: Primary follicular and marginal-zone
lymphoma of the breast: clinical features, prognostic factors and
outcome: a study by the International Extranodal Lymphoma Study
Group. Ann Oncol. 20:1993–1999. 2009. View Article : Google Scholar
|
|
13
|
Liu H, Ye H, Dogan A, Ranaldi R, et al:
T(11;18)(q21;q21) is associated with advanced mucosa-associated
lymphoid tissue lymphoma that expresses nuclear BCL10. Blood.
98:1182–1187. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye H, Dogan A and Karran L: BCL10
expression in normal and neoplastic lymphoid tissue. Nuclear
localization in MALT lymphoma. Am J Pathol. 157:1147–1154. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yeh KH, Kuo SH and Chen LT: Nuclear
expression of BCL10 or nuclear factor kappaB helps predict
Helicobacter pylori-independent status of low-grade gastric
mucosa-associated lymphoid tissue lymphomas with or without
t(11;18)(9q21;q21). Blood. 106:1037–1041. 2005. View Article : Google Scholar
|
|
16
|
Sagaert X, Laurent M and Baens M: MALT1
and BCL10 aberrations in MALT lymphomas and their effect on the
expression of BCL10 in the tumor cells. Mod Pathol. 19:225–232.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuo SH, Chen LT, Yeh KH and Wu MS: Nuclear
expression of BCL10 or nuclear factor kappa B predicts Helicobacter
pylori-independent status of early-stage, high-grade gastric
mucosa-associated lymphoid tissue lymphomas. J Clin Oncol.
22:3491–3497. 2004. View Article : Google Scholar
|
|
18
|
Talwalkar SS, Valbuena JR, Abruzzo LV and
Admirand JH: MALT1 gene rearrangements and NF-kappaB activation
involving p65 and p50 are absent or rare in primary MALT lymphomas
of the breast. Mod Pathol. 19:1402–1408. 2006.PubMed/NCBI
|
|
19
|
Mulligan S, Hu P, Murphy A, Han J, Tam M,
Lin P, Konopley S and Lennon PA: Variations in MALT1 gene
disruptions detected by FISH in 109 MALT lymphomas occurring in
different primary sites. J Assoc Genet Technol. 37:76–79.
2011.PubMed/NCBI
|