Introduction
Tumor cells express MHC class I-related chain
molecules (MICs) A and B (MICA and MICB), which are ligands of the
NKG2D receptor that is expressed on the surface of cytotoxic immune
cells, such as natural killer (NK), γδ+ T and
CD8+ αβ+ T cells (1,2). The binding of the
NKG2D receptor to its ligands activates NK and γδ+ T
cells, and co-stimulates tumor-antigen-specific CD8+
αβ+ T cells (1,2). Therefore, the NKG2D-MIC system plays
an important role in the cytotoxicity of immune cells. However,
tumor cells produce soluble MICs and thus are able to avoid being
attacked by cytotoxic immune cells. Soluble MICs, which are
produced by the proteolytic cleavage of their extracellular domain
by proteases (1,3–6),
interfere with the binding of MICs on the surface of tumor cells to
NKG2D receptors on the surface of cytotoxic immune cells, and the
binding of soluble MICs to NKG2D receptors downregulates the NKG2D
receptors on the surface of cytotoxic immune cells (1,6–9).
The gene expression in tumor cells is altered by
both genetic and epigenetic events, and epigenetic modifiers, such
as histone deacetylase (HDAC) and DNA methylation inhibitors, alter
their gene expression profiles (10,11). A
number of studies have demonstrated that HDAC inhibitors stimulate
the expression of cell surface MICA and MICB in a variety of tumors
(12–15). Valproic acid (VPA), a HDAC
inhibitor, increases the expression of MICA and MICB on the surface
of human osteosarcoma cells (16).
Furthermore, VPA decreases the production of soluble MICA and MICB
in these cells (16). However, the
mechanisms by which VPA decreases the production of soluble MICA
and MICB in osteosarcoma cells remains to be elucidated.
Soluble MICA and MICB are produced by the
proteolytic cleavage of cell surface MICA and MICB as
broad-spectrum metalloproteinase inhibitors suppress the shedding
of MICs (3–6). Human osteosarcoma cells produce high
levels of matrix metalloproteinase (MMP)-2 and -9 (17,18),
and HDAC inhibitors, including VPA have been shown to decrease the
expression of MMP-2 and -9 in thyroid, gastric and lung cancer
cells (19–21). Therefore, in this study, we
investigated the effect of VPA on the mRNA expression of these MMPs
in osteosarcoma cells and the roles of these MMPs in the cleavage
of MICA and MICB on the cell surface. The present study shows that
the downregulation of MMP-9 mRNA by VPA is involved in the
inhibitory action of VPA on the shedding of MICA and MICB on the
surface of human osteosarcoma cells.
Materials and methods
Reagents and antibodies
Sodium valproate was purchased from Wako (Osaka,
Japan), the PE-conjugated anti-human MICA/B mouse monoclonal
antibody (IgG2b) from R&D Systems (Minneapolis, MN,
USA) and the control mouse IgG2b from BioLegend (San
Diego, CA, USA). The MMP-2/MMP-9 inhibitor (an inhibitor of MMP-2
and -9), GM 6001 (a broad-spectrum inhibitor of MMPs), and the GM
6001 negative control were purchased from Calbiochem (Merck, Tokyo,
Japan).
Cells
U-2 OS and SaOS-2 human osteosarcoma cells were
purchased from the American Type Culture Collection (ATCC, Manasas,
VA, USA), and Riken BRC Cell Bank (Tsukuba, Ibaragi, Japan),
respectively. The U-2 OS and SaOS-2 cells were cultured in McCoy’s
5A modified medium (Invitrogen, Carlsbad, CA, USA). All these media
contained 10% fetal bovine serum (FBS) (MP Biomedical, Inc., Morgan
Irvine, CA, USA), penicillin (100 U/ml) and streptomycin (100
μg/ml). All cells were cultured in a humidified atmosphere of 5%
CO2 in air at 37°C.
Flow cytometric analysis
U-2 OS and SaOS-2 cells were seeded at 2 and
4×103 cells/dish, respectively, in 6 cm-tissue culture
dishes containing 3 ml of medium/dish. After 24 h (day 0), VPA was
added to the medium at 1.0 mM, and the cells were cultured for
another 7 days, with a medium change on day 3. The cells were
detached from the dishes, and the expression of membrane-bound
MICA/B was analyzed by a flow cytometric analysis, as described
previously (16). The percentages
of membrane-bound MICA/B-positive cells were determined by flow
cytometric analysis, and the effects of VPA were evaluated by
determining the ratio of the percentage of positive cells in the
treated cultures to the average percentage of positive cells in the
untreated control cultures.
Enzyme-linked immunosorbent assays
(ELISAs)
U-2 OS and SaOS-2 cells were seeded at 1 and
2×105 cells/dish, respectively, in 10 cm-tissue culture
dishes containing 5 ml of medium/dish. After 24 h (day 0), VPA was
added to the medium at 1.0 mM, and the cells were cultured for
another 7 days, with a medium change on day 3. The medium was
collected for an assay of the soluble MICA and soluble MICB levels
using ELISA systems for human soluble MICA and MICB (R&D
Systems) and a microplate reader (Bio-Rad Laboratories, Tokyo,
Japan). The amount of soluble MICA or MICB/104 viable
cells in the treated cultures was expressed as a ratio of the
average value in the untreated control cultures.
Treatment with MMP inhibitors
U-2 OS and SaOS-2 cells were seeded at 2 and
4×105 cells/dish, respectively, in 10 cm-tissue culture
dishes containing 5 ml of medium/dish. After 24 h, VPA (1.0 mM),
MMP-2/MMP-9 inhibitor (10 μM), GM6001 (a broad-spectrum inhibitor
of MMPs) (2 μM) or the GM 6001 negative control (2 μM) was added to
the medium and the cells were cultured for another 48 h. The
soluble MICA and MICB levels in the medium were assayed using the
ELISA systems as described above. The amount of soluble MICA or
MICB/104 viable cells in the treated cultures was
expressed as a ratio of the average value in the untreated control
cultures.
Quantitative real-time PCR
U-2 OS and SaOS-2 cells were cultured in medium with
or without 1.0 mM VPA, for 7 days, with a medium change on day 3,
and on days 3 and 7, the total RNA was extracted from the cells in
each culture dish with TRIzol reagent (Invitrogen). An aliquot of
RNA was reverse-transcribed using Superscript II reverse
transcriptase (Invitrogen) according to the manufacturer’s
instructions. Real-time PCR for MMP-2, -9 or -14, or a disintegrin
and metalloproteinase (ADAM)-17 mRNA was performed using TaqMan
Gene Expression assays (Applied Biosystems, Foster City, CA, USA).
The primer sets used were Hs01548733_ml for MMP-2 mRNA,
Hs00957562_ml for MMP-9 mRNA, Hs00237119_ml for MMP-14 mRNA and
Hs01041915_ml for ADAM-17 mRNA (Applied Biosystems). The amount of
GAPDH mRNA as an internal reference was estimated using human GAPDH
as the endogenous control (Applied Biosystems), and the amount of
MMP-2, -9 or -14, or ADAM-17 mRNA in each sample was corrected by
the amount of GAPDH mRNA in the corresponding sample. The amount of
MMP-2, -9 or -14, or ADAM-17 mRNA in the treated cultures was
expressed as a ratio of the average value in the untreated control
cultures.
Effects of small interfering RNA (siRNA)
for MMP-2 or -9 on the secretion of soluble MICA and MICB
The siRNAs designed for MMP-2 and -9 mRNAs were
5′-GGAAAGAUUGAUGCGGUAtt-3′ (sense strand) and
5′-CAUCACCUAUUGGAUCCAAtt-3′ (sense strand), respectively, and were
synthesized by Applied Biosystems. U-2 OS and SaOS-2 cells were
seeded at 1 and 2×105 cells/well, respectively, in 6
well-tissue culture plates and cultured in 2 ml of medium for 24 h.
The culture medium was changed to Opti-MEM medium (Invitrogen), and
the cells were transfected with 10 nM of MMP-2 or MMP-9 siRNA and
negative control siRNA using an RNAiMAX reagent (Invitrogen), and
were cultured for another 48 h. The medium was collected, and the
levels of soluble MICA and MICB in the medium were determined as
described above, and the expression of MMP-2 and -9 mRNA in the
cells was examined by reverse transcription polymerase chain
reaction (RT-PCR). The amount of soluble MICA or
MICB/104 viable cells in the treated cultures was
expressed as a ratio of the average value in the untreated control
cultures.
RT-PCR
Total cell RNA was extracted from the U-2 OS and
SaOS-2 cells using TRIzol reagent (Invitrogen) according to the
manufacturer’s instructions. The reverse transcription of 2 μg of
total RNA was performed at 42°C for 1 h using random primers (Roche
Applied Science, Indianapolis, IN, USA) and Transcriptor Reverse
Transcriptase (Roche Applied Science), and cDNAs produced were
sequentially amplified by PCR with Takara Ex Taq™ DNA polymerase
(Takara Bio, Inc., Ohtsu, Shiga, Japan) using specific primer sets
as follows: sense, 5′-ACGATGATGACCGCAAGTGG-3′ and antisense, 5′-GGA
GCTCAGGCCAGAATGTG-3′ for MMP-2; sense, 5′-AGGAC GGCAATGCTGATGGG-3′
and antisense, 5′-GAGGTGCCG GATGCCATTCA-3′ for MMP-9; and sense,
5′-GTCATCAAT GGAAATCCCATCACC-3′ and antisense, 5′-GCTCAGGGAT
GACCTTGCCC-3′ for GAPDH. The amplification conditions of the PCR
for MMP-2 and -9 were 25 cycles at 95°C for 30 sec, 55°C for 30
sec, and 72°C for 1 min, followed by heating at 72°C for 7 min, and
that for GAPDH was 25 cycles at 95°C for 30 sec, 57°C for 30 sec,
and 72°C for 1 min, followed by heating at 72°C for 7 min. The
amplified fragments were resolved by electrophoresis on 1.5%
agarose gels, and were detected by ethidium bromide staining.
Statistical analysis
The data are presented as the means + SE. The data
of 2 groups were analyzed by the Student’s t-test, and the data of
3 groups or more by the two-tailed Dunnett’s t-test for multiple
comparisons. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Expression of MICA/B on the surface of
osteosarcoma cells and secretion of soluble MICA and MICB
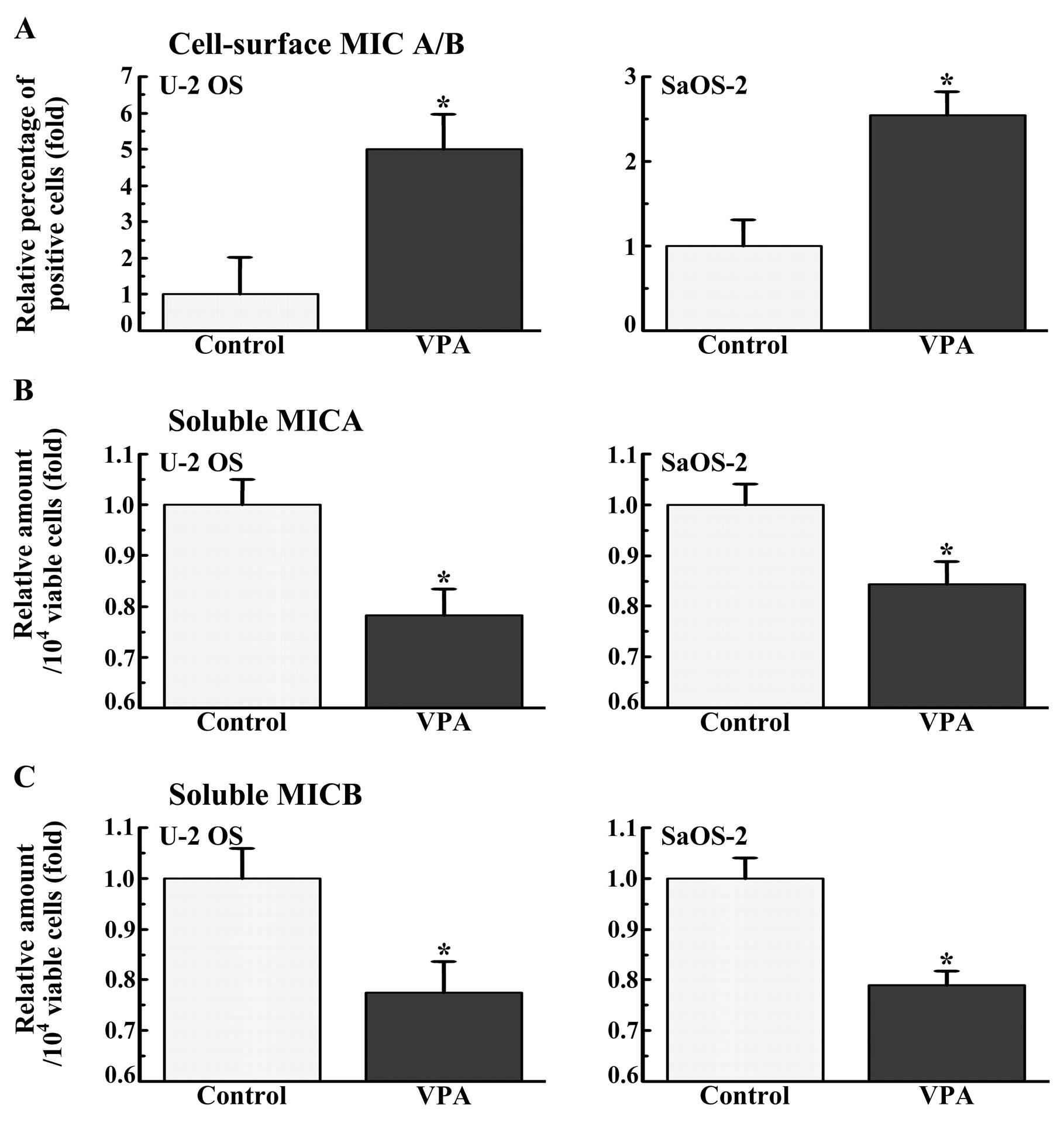
The osteosarcoma cells were cultured in the presence
or absence of VPA (1.0 mM) for 7 days, and the cell surface
expression of MICA/B was examined by flow cytometry (Fig. 1A). Cell surface MICA/B was expressed
in 7.2 and 5.1% of the U-2 OS and SaOS-2 cells, respectively. VPA
increased the expression of MICA/B on the surface of U-2 OS and
SaOS-2 cells by approximately 5.0- and 2.6-fold, respectively.
The amount of soluble MICA or MICB in the medium of
the osteosarcoma cells during the last 4 days of a 7-days culture
(from days 3 to 7) was estimated (Fig.
1B and C). VPA (1.0 mM) significantly decreased the amount of
both soluble MICA and MICB in the culture medium of the 2
osteosarcoma cell lines.
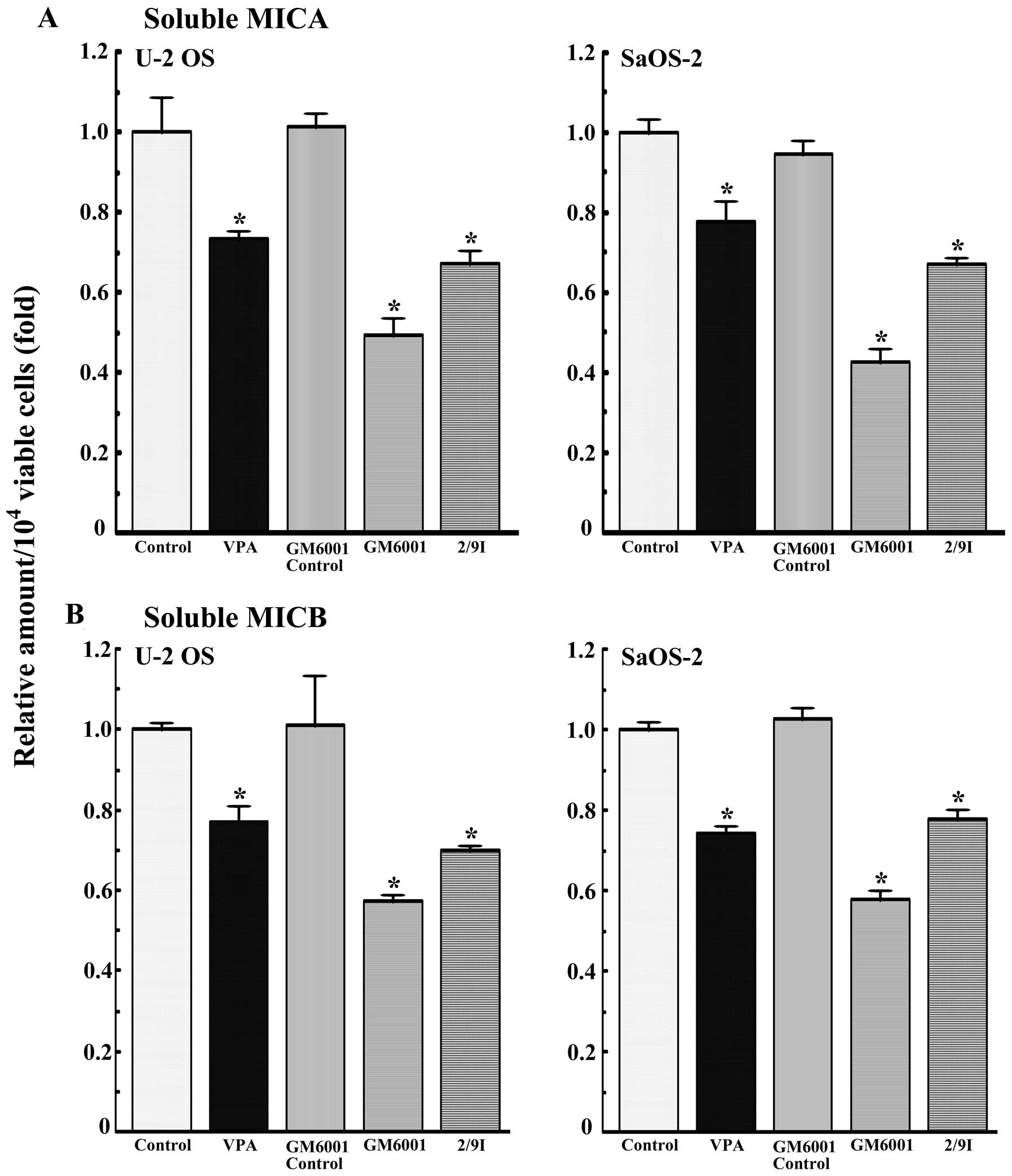
Role of MMP in the secretion of soluble
MICA and MICB
Soluble MICA and MICB are produced by the
proteolytic cleavage of cell surface MICA and MICB and human
osteosarcoma cells produce MMP-2 and -9. Therefore, the effects of
GM6001 (a broad-spectrum metalloproteinase inhibitor) and
MMP-2/MMP-9 inhibitor (an inhibitor of MMP-2 and -9) on the
shedding of MICA and MICB were examined (Fig. 2). The MMP-2/MMP-9 inhibitor (10 μM)
and GM 6001 (2 μM) as well as VPA (1 mM) decreased the amount of
soluble MICA and MICB in the U-2 OS and SaOS-2 cells.
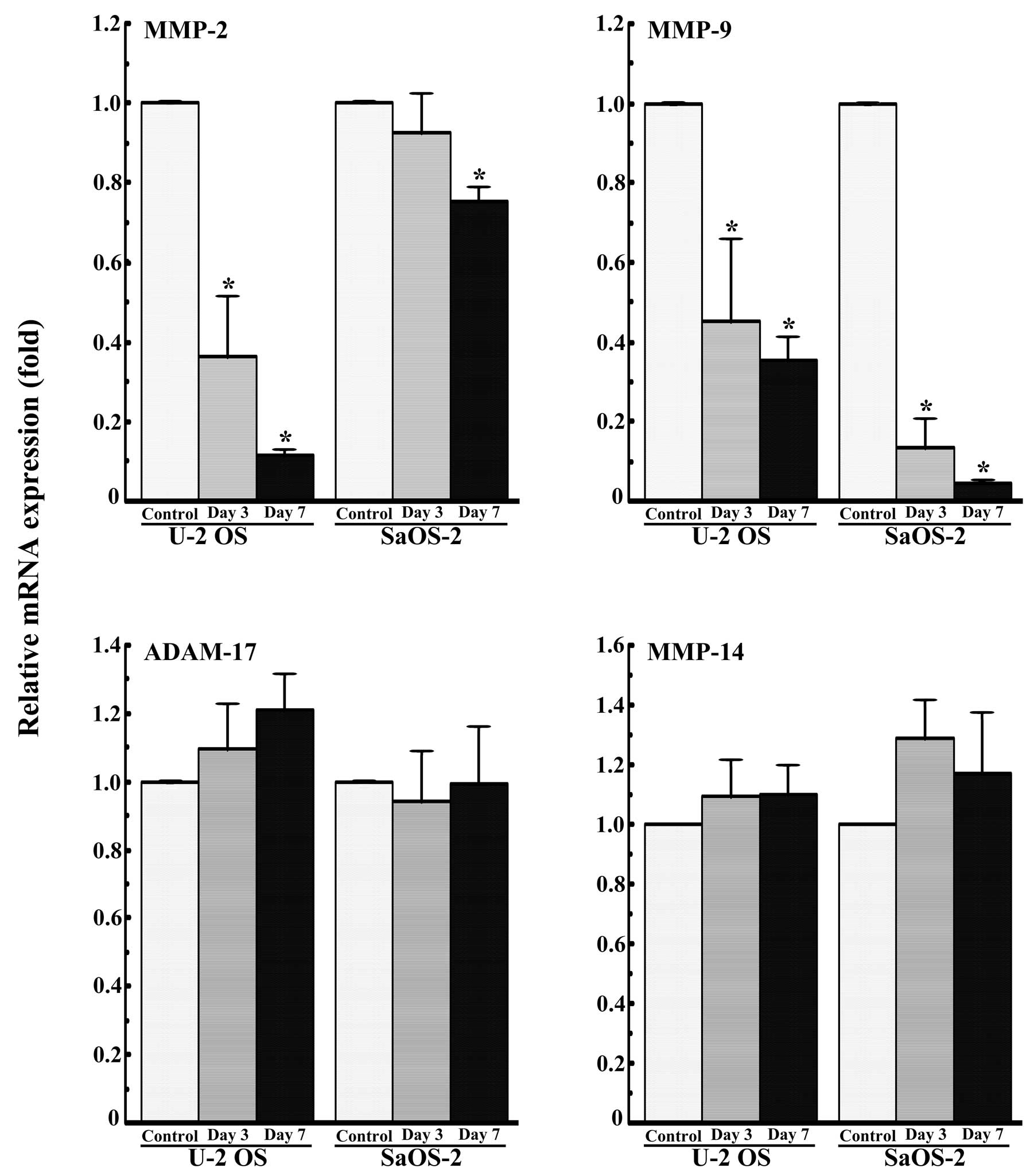
Subsequently, the effects of VPA on the expression
of MMP-2, -9 and -14, and ADAM-17 mRNA in U-2 OS and SaOS-2 cells
cultured for 3 or 7 days were examined by quantitative real-time
PCR. VPA (1.0 mM) markedly decreased the expression of MMP-9 mRNA
in the U-2 OS and SaOS-2 cells (Fig.
3). VPA also markedly decreased the expression of MMP-2 mRNA in
the U-2 OS cells, but showed little effect on the expression of
MMP-2 mRNA in the SaOS-2 cells (Fig.
3). VPA did not decrease the expression of MMP-14 and ADAM-17
mRNA (Fig. 3).
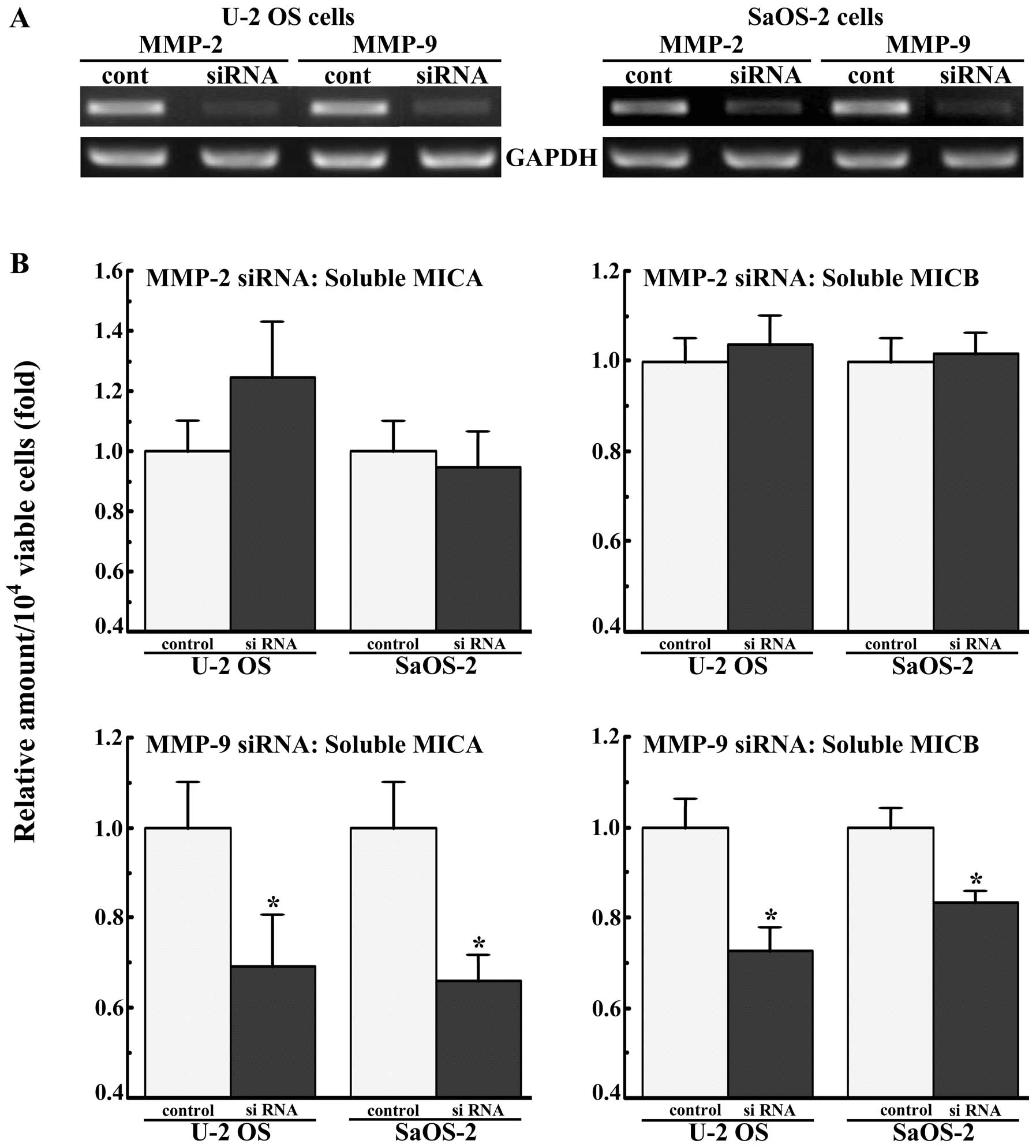
The U-2 OS and SaOS-2 cells were transfected with
siRNAs for MMP-2 and -9, and the amount of soluble MICA and MICB in
the medium was assayed after 2 days of culture. siRNAs for MMP-2
and -9 markedly decreased the expression levels of these mRNAs
(Fig. 4A). siRNA for MMP-9
decreased the amounts of soluble MICA and MICB secreted by the U-2
OS and SaOS-2 cells by approximately 20–30%, while siRNA for MMP-2
did not (Fig. 4B).
Discussion
Culture with 1.0 mM VPA increased the expression of
MICA/B on the surface of osteosarcoma cells and inhibited their
secretion of soluble MICA and MICB, confirming the results of our
previous report (16). Our previous
study showed that 1.0 mM VPA increased the acetylation of histones,
suggesting that at least a part of the action of VPA can be
ascribed to its action as a HDAC inhibitor (16).
GM6001 (a broad-spectrum inhibitor of MMPs) and the
inhibitor of MMP-2 and -9 decreased the secretion of MICA and MICB
by osteosarcoma cells and siRNA for MMP-9 decreased the secretion
of MICA and MICB, whereas that for MMP-2 did not. These results
indicate that MMP-9 is responsible for the shedding of MICA and
MICB on the surface of osteosarcoma cells. Several proteases have
been reported to be responsible for the shedding of cell surface
MICA and MICB; MMP-14 for MICA, ADAM-10 for MICA and ADAM-17 for
MICA and MICB (6,22,23).
Therefore, these proteases may also be responsible for the shedding
of MICA and MICB on the surface of osteosarcoma cells.
VPA markedly decreased the expression of MMP-9 mRNA
in the osteosarcoma cells, consistent with previous studies using
other types of cancer cells (19–21).
Therefore, the inhibitory action of VPA on the secretion of soluble
MICA and MICB is ascribed at least in part to the downregulation of
MMP-9 mRNA by VPA. VPA did not affect the expression of MMP-14 and
ADAM-17 mRNA. However, other proteases, including ADAM-10 may be
related to the inhibitory action of VPA on the shedding of MICA and
MICB on the surface of osteosarcoma cells.
In conclusion, the present study shows that the
downregulation of MMP-9 mRNA by VPA is involved in the inhibitory
action of VPA on the secretion of soluble MICA and MICB from the
surface of osteosarcoma cells. To our knowledge, this is the first
study to demonstrate that MMP-9 plays a role in the shedding of
MICA and MICB from the surface of tumor cells.
Acknowledgements
This study was supported in part by a Grant-in-Aid
for Young Scientists (B) (23792159) from the Ministry of Education,
Culture, Sports, Science and Technology of Japan, a Strategic
Program Grant for Research Infrastructure Development in Private
Institutes and a Grant-in-Aid for Promotion of Technical Seeds in
Advanced Medicine, the Hyogo College of Medicine.
References
|
1
|
Waldhauer I and Steinle A: NK cells and
cancer immunosurveillance. Oncogene. 27:5932–5943. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nausch N and Cerwenka A: NKG2D ligands in
tumor immunity. Oncogene. 27:5944–5958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salih HR, Rammensee HG and Steinle A:
Cutting edge: down-regulation of MICA on human tumors by
proteolytic shedding. J Immunol. 169:4098–4102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salih HR, Goehlsdorf D and Steinle A:
Release of MICB molecules by tumor cells: mechanism and soluble
MICB in sera of cancer patients. Hum Immunol. 67:188–195. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waldhauer I and Steinle A: Proteolytic
release of soluble UL16-binding protein 2 from tumor cells. Cancer
Res. 66:2520–2526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boutet P, Agüera-González S, Atkinson S,
Pennington CJ, Edwards DR, Murphy G, Reyburn HT and Valés-Gómez M:
Cutting edge: the metalloproteinase ADAM17/TNF-α-converting enzyme
regulates proteolytic shedding of the MHC class I-related chain B
protein. J Immunol. 182:49–53. 2009.PubMed/NCBI
|
|
7
|
Groh V, Wu J, Yee C and Spies T:
Tumour-derived soluble MIC ligands impair expression of NKG2D and
T-cell activation. Nature. 419:734–738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raffaghello L, Prigione I, Airoldi I,
Camoriano M, Levreri I, Gambini C, Pende D, Steinle A, Ferrone S
and Pistoia V: Downregulation and/or release of NKG2D ligands as
immune evasion strategy of human neuroblastoma. Neoplasia.
6:558–568. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Märten A, von Lilienfeld-Toal M, Büchler
MW and Schmidt J: Soluble MIC is elevated in the serum of patients
with pancreatic carcinoma diminishing γδT cell cytotoxicity. Int J
Cancer. 119:2359–2365. 2006.PubMed/NCBI
|
|
10
|
Yoo CB and Jones PA: Epigenetic therapy of
cancer: past, present and future. Nat Rev Drug Discov. 5:37–50.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kristensen LS, Nielsen HM and Hansen LL:
Epigenetics and cancer treatment. Eur J Pharmacol. 625:131–142.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Armeanu S, Bitzer M, Lauer UM, Venturelli
S, Pathil A, Krusch M, Kaiser S, Jobst J, Smirnow I, Wagner A, et
al: Natural killer cell-mediated lysis of hepatoma cells via
specific induction of NKG2D ligands by the histone deacetylase
inhibitor sodium valproate. Cancer Res. 65:6321–6329. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmudde M, Braun A, Pende D, Sonnenmann
J, Klier U, Beck JF, Moretta L and Bröker BM: Histone deacetylase
inhibitors sensitize tumour cells for cytotoxic effects of natural
killer cells. Cancer Lett. 272:110–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang C, Wang Y, Zhou Z, Zhang J and Tian
Z: Sodium butyrate upregulates expression of NKG2D ligand MICA/B in
HeLa and HepG2 cell lines and increases their susceptibility to NK
lysis. Cancer Immunol Immunother. 58:1275–1285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poggi A, Catellani S, Garuti A, Pierri I,
Gobbi M and Zocchi MR: Effective in vivo induction of NKG2D ligands
in acute myeloid leukaemias by all-trans-retinoic acid or sodium
valproate. Leukemia. 23:641–648. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamanegi K, Yamane J, Kobayashi K,
Kato-Kogoe N, Ohyama H, Nakasho K, Yamada N, Hata M, Nishioka T,
Fukunaga S, et al: Sodium valproate, a histone deacetylase
inhibitor, augments the expression of cell-surface NKG2D ligands,
MICA/B, without increasing their soluble forms to enhance
susceptibility of human osteosarcoma cells to NK cell-mediated
cytotoxicity. Oncol Rep. 24:1621–1627. 2010. View Article : Google Scholar
|
|
17
|
Cho HJ, Lee TS, Park JB, Park KK, Choe JY,
Sin DI, Park YY, Moon YS, Lee KG, Yeo JH, et al: Disulfiram
suppresses invasive ability of osteosarcoma cells via the
inhibition of MMP-2 and MMP-9 expression. J Biochem Mol Biol.
40:1069–1076. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xin ZF, Kim YK and Jung ST: Risedronate
inhibits human osteosarcoma cell invasion. J Exp Clin Cancer Res.
28:1052009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee KH, Choi EY, Kim MK, Kim KO, Jang BI,
Kim SW, Kim SW, Song SK and Kim JR: Inhibition of histone
deacetylase activity down-regulates urokinase plasminogen activator
and matrix metalloproteinase-9 expression in gastric cancer. Mol
Cell Biochem. 343:163–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mitmarker EJ, Griff NJ, Grogan RH, Sarkar
R, Kebebew E, Duh QY, Clark OH and Shen WT: Modulation of matrix
metalloproteinase activity in human thyroid cancer cell lines using
demethylating agents and histone deacetylase inhibitors. Surgery.
149:504–511. 2011. View Article : Google Scholar
|
|
21
|
Vinodhkumar R, Song YS, Ravikumar V,
Ramakrishnan G and Devaki T: Depsipeptide a histone deacetylase
inhibitor down regulates levels of matrix metalloproteinases 2 and
9 mRNA and protein expressions in lung cancer cells (A549). Chem
Biol Interact. 165:220–229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waldhauer I, Goehlsdorf D, Gieseke F,
Weinschenk T, Wittenbrink M, Ludwig A, Stevanovic S, Rammensee HG
and Steinle A: Tumor-associated MICA is shed by ADAM proteases.
Cancer Res. 68:6368–6376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu G, Atteridge CL, Wang X, Lundgren AD
and Wu JD: The membrane type matrix metalloproteinase MMP14
mediates constitutive shedding of MHC class I chain-related
molecule A independent of a disintegrin and metalloproteinases. J
Immunol. 184:3346–3350. 2010. View Article : Google Scholar : PubMed/NCBI
|