Introduction
Heat shock proteins (HSPs) are important
intracellular molecular chaperones that play essential roles in the
regulation of protein folding and translocation (1). They are specifically induced in
response to various stress conditions such as heat, anoxia, and
certain chemicals. HSPs can act as cytoprotective agents by binding
to misfolded proteins, thereby protecting them from denaturation
under cellular stress (2,3).
HSPs are overexpressed in many tumors and act at the
crossroads of key intracellular processes in their role as
molecular chaperones. HSPs associate with a vast array of tumor
antigenic peptides and are implicated as chaperones in the
formation of immunogenic complexes called HSP-peptide complexes
(HSP-PCs) (4–6). Immunization with HSP-PCs purified from
cancer cells provides protection against tumors derived from the
same cancer cells from which the complexes were purified. The
immunogenicity of HSP preparations is attributed to the antigenic
peptides that they chaperone (7–11).
Studies on the immunogenic mechanisms of HSP preparations have
shown that tumor-derived HSP-PCs exhibit antigens associated with
antigen-presenting cells such as DCs. Consequently,
antigen-specific cytotoxic CD8+ T cells are induced
(1,12). Hence, HSP-PCs purified from tumor
cells possess the qualities of tumor vaccines. Numerous reports
have confirmed HSP70 as one of the best choices for HSP-based tumor
vaccine preparations (13,14).
Recent research on HSP70-based tumor immunotherapy
is focused on how to obtain more effective and powerful peptides by
the purification of HSP70-PCs from tumor cells. Membrane proteins
containing many important tumor antigens, such as HER-2 protein,
cannot be obtained from cancer cells through HSP70 using previous
purification methods. Traditional methods cannot completely
separate the cellular membrane, resulting in the loss of
membrane-associated HSP70-PCs in the purified product. This
limitation attenuates the antitumor immunological activities of
purified HSP70-PCs.
A preliminary experiment by our group revealed that
HER-2 protein and HSP70 are both highly expressed in the human
breast cancer cell line SKBR-3. A co-immunoprecipitation experiment
also indicated that HER-2 protein and HSP70 are bound in the tumor
cells. The traditional method, combining hypotonic buffer with
column chromatography (using ConA-Sepharose and ADP-agarose), was
utilized to purify the HSP70-PCs from SKBR-3 cells. However, no
HER-2 protein was found by sodium dodecyl sulfate polyacrylamide
gel electrophoresis (SDS-PAGE) and immunodot analysis in the final
product (data not provided).
In the current study, an improved HSP70-associated
vaccine was produced based on a purification process involving the
detergent 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
(CHAPS). HER-2 protein was used to represent the membrane tumor
antigens. HER-2 proteins with HSP70-PCs were obtained from the
HER-2-overexpressed human breast cancer cell line SKBR-3. The new
product was temporarily named HSP70-HER-2-PCs. Their immunological
activities were determined by pulsing DCs and inducing specific
CD8+ T cells. Traditionally purified HSP70-PCs and the
recombinant human HSP70-HER-2 protein complex were used as
controls.
Materials and methods
Culture of the cell line
Human breast cancer cell line SKBR-3 was purchased
from Peking Union Medical College. Cells were cultured in RPMI-1640
medium (Gibco, Invitrogen Co., Carlsbad, CA, USA) supplemented with
10% heat-inactivated fetal calf serum (FCS) (HyClone, Logan, UT,
USA), 2 mol/l L-glutamine (Gibco, Invitrogen Co.), 100 U/ml
penicillin G and 100 μg/ml streptomycin at 37°C in a humidified
atmosphere of 5% CO2.
Purification of HSP70-HER-2-PCs
SKBR-3 cells were heated in a water bath at 42°C for
12 h followed by recovery for 2 h at 37°C in an atmosphere
containing 5% CO2. After the treatment of heat shock,
SKBR-3 cells were digested by 0.02% trypsin and then
1×108 cells were homogenized in hypotonic buffer with
CHAPS (Sigma Chemical Co., St. Louis, MO, USA) (50 mM Tris-Hcl, 150
mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium fluoride,
and 5 mM CHAPS, pH 7.2) for 15 min at 0°C. After ultrasonication at
0°C for 30 min, the homogenate was centrifuged at 14,000 rpm at 4°C
for 90 min. The supernatant was dialyzed against buffer A (20 mM
Tris-Hcl, 150 mM NaCl, 1 mM CaCl2, 1 mM
MnCl2, 0.5 mM phenylmethylsulfonyl fluoride, and 15 mM
β-mercaptoethanol, pH 7.4) overnight at 4°C. The sample was then
loaded onto an ConA-Sepharose column (Sigma Chemical Co.). Fluid
was collected at a flow rate of 12 ml/h, that consisted of the
ConA-Sepharose-unbound protein. The fraction was dialyzed against
buffer B (20 mM Tris-HCl, 20 mM NaCl, 3 mM MgCl2, 1 mM
MnCl2, 0.5 mM phenylmethylsulfonyl fluoride and 15 mM
β-mercaptoethanol, pH 7.4) overnight at 4°C. The sample was applied
to an ADP-agarose column (Sigma Chemical Co.) equilibrated
previously with buffer B at a flow rate of 12 ml/h. The column was
eluted by buffer B and buffer B containing 0.5 M NaCl until the
protein was not detected by the Bradford method. The target protein
was eluted by buffer B containing 3 mM ADP (Sigma Chemical Co.).
The endotoxin level in the preparations was determined by Limulus
amebocyte lysate (LAL) assay (Ocean Biologicals Co., China).
Identification of HSP70-HER-2-PCs
The target protein obtained by purification was run
on SDS-PAGE and detected by Coomassie Brilliant Blue staining. In
an immunodot analysis, several harvested parts were separately
dotted on the nitrocellulose membrane and dried at 37°C for 30 min.
The membrane was blocked by TBST (20 mM Tris-HCl, 150 mM NaCl and
0.05% Tween-20, pH 7.4) as well as 5% (w/v) skimmed milk powder at
room temperature for 1 h, and then incubated with mouse anti-human
monoclonal antibody against HSP70 (Santa Cruz Biotechnology, Inc.,
USA) at a 1:500 dilution in TBST/milk at 4°C for 1 h. The membrane
was thrice washed in TBST, incubated with horseradish peroxidase
(HRP)-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology,
Inc.) at a 1:10,000 dilution in TBST/milk at 37°C for 1 h, and
again thrice washed in TBST. Finally, the membrane was incubated
with a chemiluminescence reagent (Santa Cruz Biotechnology, Inc.)
for 1 min, and then exposed to Kodak autoradiography film for 20
sec.
Using the above described method, rabbit anti-human
monoclonal antibody against HER-2 and fluorescein isothiocyanate
(FITC)-conjugated goat anti-rabbit IgG (both from Santa Cruz
Biotechnology, Inc.) were used to detect the HER-2 protein of the
complex. The contents of HER-2 protein in the purified products
were quantified by HER-2 ELISA kits (R&D Systems) according to
the manufacturer’s instructions.
Recombinant human HSP70-HER-2 protein
complex prepared in vitro
Studies have shown that recombinant HSP protein
complexes in vitro are able to strengthen the cytotoxic
response of T cells against tumor cells (15). In this step, we produced the
recombinant human HSP70-HER-2 protein complex for the control for
the following research. The recombinant human HSP70 and the
recombinant human HER-2 protein were both purchased from R&D
Systems. The recombinant HSP70-HER-2 protein complex was generated
by incubation of the recombinant human HSP70 and the recombinant
human HER-2 protein in a 1:1 molar ratio at 43°C for 30 min and
then at 37°C for 1 h. The binding was evaluated by
co-immunoprecipitation and western bolt analysis. Briefly, the
HSP70-HER-2 protein complex was incubated with rabbit anti-human
monoclonal antibody against HER-2 (1:100) at room temperature for 2
h. The immune complex was then precipitated by incubation with
protein A-Sepharose CL-4B (20 μl/ml; Amersham Pharmacia Biotech AB,
Uppsala, Sweden) and rotating for 8 h on ice. Samples were then
washed eight times with washing buffer (1 M Tris-HCl, 5 M NaCl, 0.5
M EDTA and 0.1% Triton X-100, pH 7.4) at 4°C to remove any
nonspecific binding of the recombinant proteins to protein
A-Sepharose. The beads were then added with 2X SDS sample buffer,
boiled for 5 min, and subjected to SDS-PAGE, followed by probing
with the mouse anti-human monoclonal antibody against HSP70 (1:100)
at room temperature for 1 h. Western blot analysis was performed
using HRP-conjugated goat anti-mouse IgG (1:10,000) and
FITC-conjugated goat anti-rabbit IgG (1:10,000). During analysis,
the nitrocellulose membrane was incubated for 1 min with a
chemiluminescence reagent followed by exposure to Kodak
autoradiography film for 20 sec.
Preparation of DCs and CD8+ T
cells
DCs were generated as described with some
modifications (16). Briefly,
peripheral blood mononuclear cells (PBMCs) were isolated from
heparinized venous blood of healthy donors (from Inner Mongolia Red
Cross Blood Center, China) by Ficoll-Hypaque (1.077 g, Bei Jing
Zhong Shan Jin Qiao Co., China) density gradient centrifugation and
cultured in RPMI-1640 medium containing 10% FCS for 2 h. The
non-adherent cells were collected for generating CD8+ T
cells and the adherent cells were cultured for 7 days in RPMI-1640
medium containing 10% FCS, 800 U/ml recombinant human
granulocyte-macrophage colony stimulating factor (GM-CSF) and 500
U/ml recombinant human interleukin-4 (IL-4) (both from R&D
Systems, Inc., USA) for generating DCs. The media along with the
necessary cytokines were half-refreshed every other day and 50 U/ml
tumor necrosis factor-α (TNF-α) (R&D Systems, Inc.) was added
to the culture medium on the sixth day.
CD8+ T cells were harvested from the
non-adherent fraction. Briefly, non-adherent cells were resuspended
in RPMI-1640 medium containing 10% FCS, 100 U/ml penicillin G, and
100 μg/ml streptomycin. Recombinant human interferon (IFN)-γ (1,000
IU/ml) (PeproTech Inc., USA) was added on day 0. After 24 h of
incubation, 50 ng/ml of mouse anti-human monoclonal antibody
against CD3 (Becton, Dickinson and Co., BD, USA), 100 U/ml
recombinant human interleukin (IL)-1β (R&D Systems, Inc.) and
300 U/ml recombinant IL-2 (PeproTech Inc., USA) were added. Cells
were incubated at 37°C in a humidified atmosphere of 5%
CO2 and subcultured every third day in fresh complete
medium with 300 U/ml IL-2 at 2×106 cells/ml.
Fluorescence immunostaining
DCs (1×105) were pulsed with 10 μg of the
HSP70-HER-2-PCs purified from SKBR-3 at 37°C for 12 h. After
washing with phosphate-buffered saline (PBS), fixation in 4%
paraformaldehyde, and permeabilization with 0.5% Triton X-100, the
cells were blocked in 3% bovine serum albumin (BSA) at 4°C for 1 h.
The cells were then incubated with mouse anti-human monoclonal
antibody against HSP70 and rabbit anti-human monoclonal antibody
against HER-2 at a 1:100 dilution in PBS at 37°C for 1 h, and then
thrice washed in PBS. Rhodamine-conjugated goat anti-mouse IgG
(Santa Cruz Biotechnology, Inc.) and FITC-conjugated goat
anti-rabbit IgG were added at 1:50 dilution in PBS at 37°C for 30
min in the dark. The cells were again thrice washed in PBS and
smeared on the slides. Images were acquired using an Olympus DP71
microscope and analyzed using basic software (Olympus).
ELISPOT assay
An ELISPOT assay was performed to assess the IFN-γ
production of autologous T cells using an IFN-γ ELISPOT kit
(R&D Systems, Inc.). DCs were divided into four groups of
1×105 cells each, and pulsed by different ways for 12 h.
Group A received GM-CSF and IL-4 only, group B received 10 μg of
HSP70-HER-2-PCs purified from SKBR-3, group C received 10 μg of the
HSP70-PCs purified from SKBR-3 by a method without CHAPS, and group
D received 10 μg of recombinant human HSP70-HER-2 protein complex.
After washing with PBS, the four groups of DCs were cocultured with
autologous T cells isolated by a nylon wool column at a 1:10 ratio
in a 96-well culture plate (NUNC, Roskilde, Denmark) in the
presence of 20 U/ml IL-2 for 7 days, respectively. The stimulated T
cells (1×104/well) as effector cells and SKBR-3 cells
(5×103/well) as target cells were transferred to the
ELISPOT plate and incubated at 37°C for 18 h. The level of IFN-γ
was detected as described in the IFN-γ ELISPOT kit manual with an
automated ELISPOT reader system (Biosys Co., Germany).
Induction of specific CD8+ T
cells by DCs pulsed with HSP70-HER-2-PCs and in vitro cytotoxicity
test
Specific cytolytic activities of CD8+ T
cells were determined by an LDH release assay. Autologous
CD8+ T cells were obtained by using the method described
above. Cells were divided into four groups of 1×106
cells each. Then four groups of CD8+ T cells were
cocultured with four groups of autologous DCs which was pulsed by
different ways (mentioned above) at a 10:1 ratio respectively. All
the mixture was treated with 300 U/ml IL-2 in a 96-well plate for
one week and named group A, B, C and D, correspondingly. After one
week of coculture, four groups of CD8+ T cells were used
as effector cells in the assay using LDH cytotoxicity detection kit
(BioVision Inc., USA). SKBR-3 cells were used as target cells in
the assay. Briefly, target cells and effector cells were
resuspended in assay medium (RPMI-1640 with 1% BSA), and then
target cells (1×104 cells/well) were cocultured with
effector cells at different ratios (1:5, 1:10, and 1:20) in a
96-well round-bottomed culture plate at 37°C. After incubation for
4 h, cells were centrifuged at 250 rpm for 10 min and the cell-free
supernatant was collected and transferred to another ELISA plate
for LDH assay. LDH detection mixture (100 μl/well) was then added
and incubated in the dark for 30 min at room temperature. After
adding 50 μl stop solution per well, the absorbance of the samples
was measured by ELISA reader at 490 nm as reference wavelength. The
spontaneous release of LDH by target cells or effector cells was
assessed by incubation of target cells in the absence of effector
cells and vice versa. The maximum release of LDH was determined by
incubation of target cells in 1% Triton X-100 in assay medium. The
percentage of specific cell-mediated cytotoxicity was determined by
the following formula: Cytotoxicity (%) = [(effector and target
mixture - effector spontaneous - target spontaneous)/(maximum -
target spontaneous)] ×100.
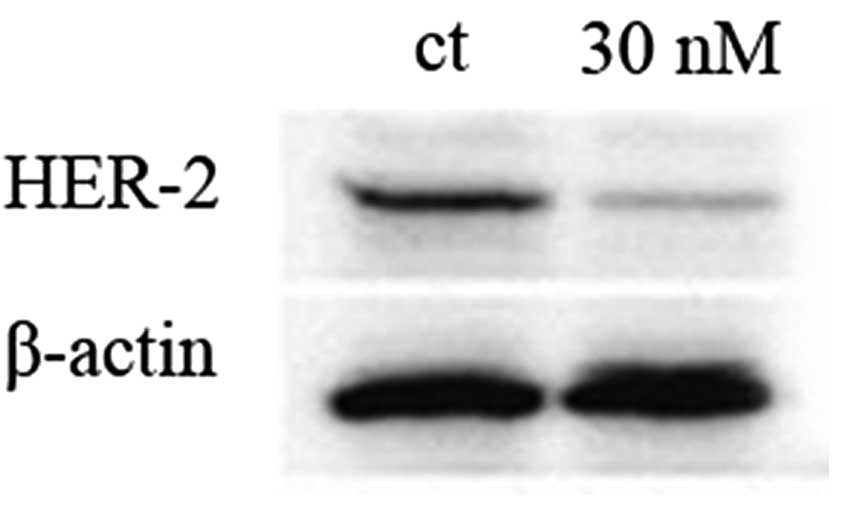
Generation of HER-2-knockdown cells
HER-2 small interfering (si)RNA (human HER-2 siRNA,
Santa Cruz Biotechnology, Inc.) was added at a final concentration
of 30 nM to knock down the expression of HER-2 in SKBR-3 cells.
Lipofectamine 2000 (Invitrogen Co., Carlsbad, CA, USA) was used to
transfect the siRNA into cells according to the instructions of the
manufacturer. Western blot analysis was performed, as described
previously, to investigate the ability of siRNA to suppress HER-2
expression. β-actin was used as the internal control.
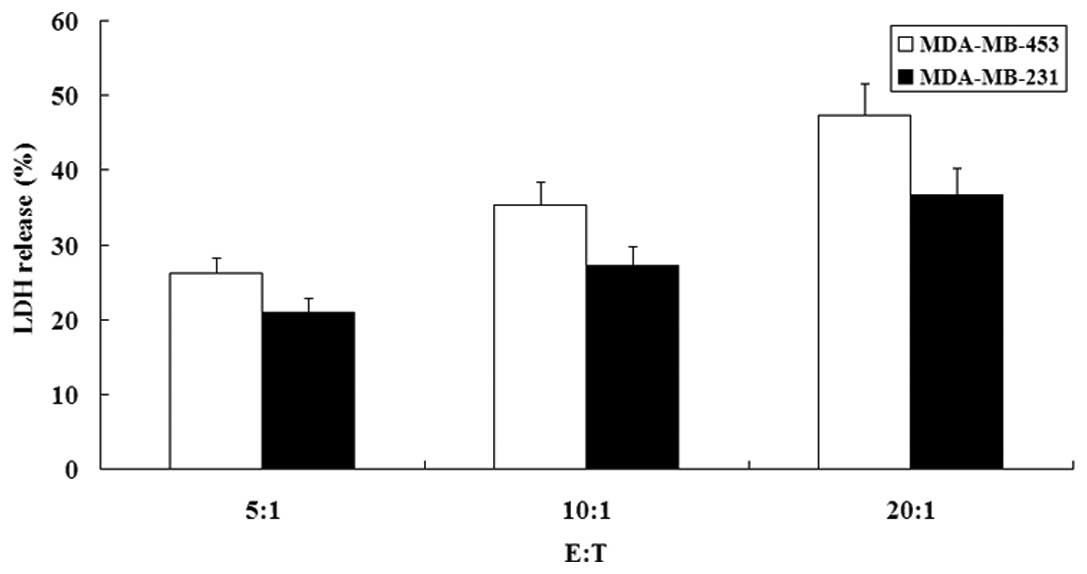
HER-2 specificity detection
The anti-tumor activities of the HSP70-HER-2-PCs
were further detected against MDA-MB-453 (HER-2 overexpression) and
HER-2-knockdown SKBR-3 cells to demonstrate the HER-2 specificity
of HSP70-HER-2-PCs. CD8+ T cells were induced by
autologous DCs pulsed with HSP70-HER-2-PCs purified from SKBR-3.
Cytolytic activities were determined by LDH release assay, as
described above, and MDA-MB-231 cells (HER-2 low-expression) were
used as controls. The expression of HER-2 in both MDA-MB-453 and
MDA-MB-231 cells was previously confirmed by flow cytometry.
Statistical analysis
Values were expressed as means ± SD or percent (%).
All analyses were conducted using the SPSS 13.0 software. The
results were considered statistically significant at P<0.05.
Results
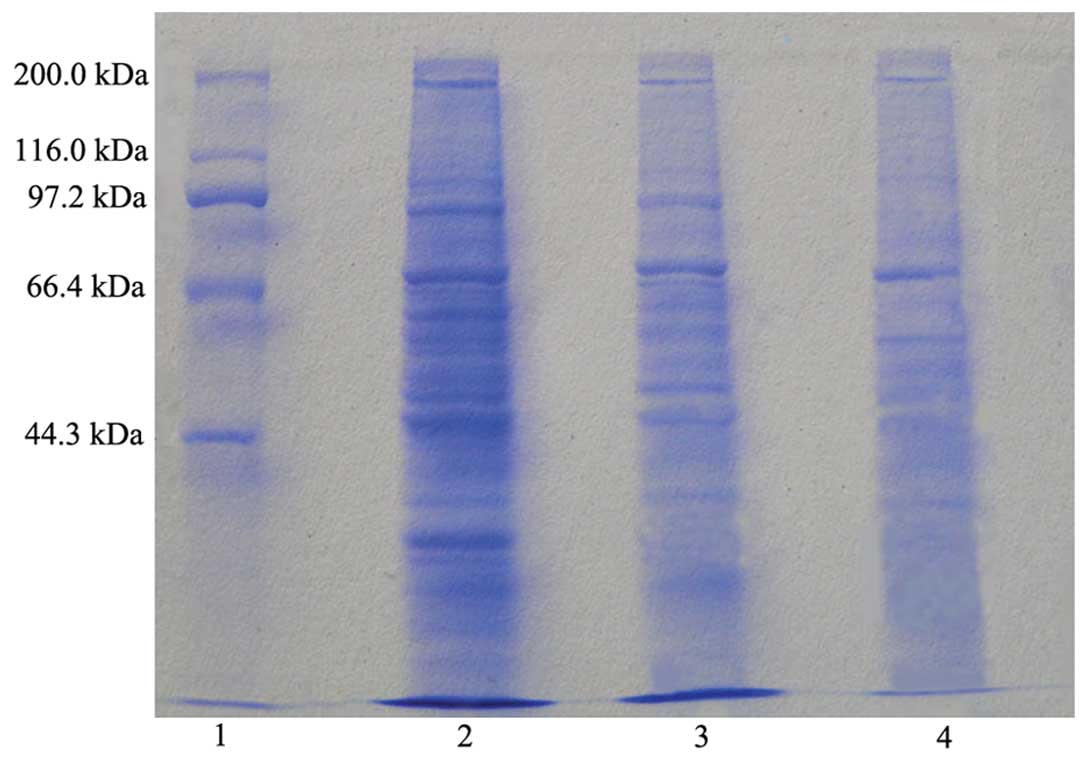
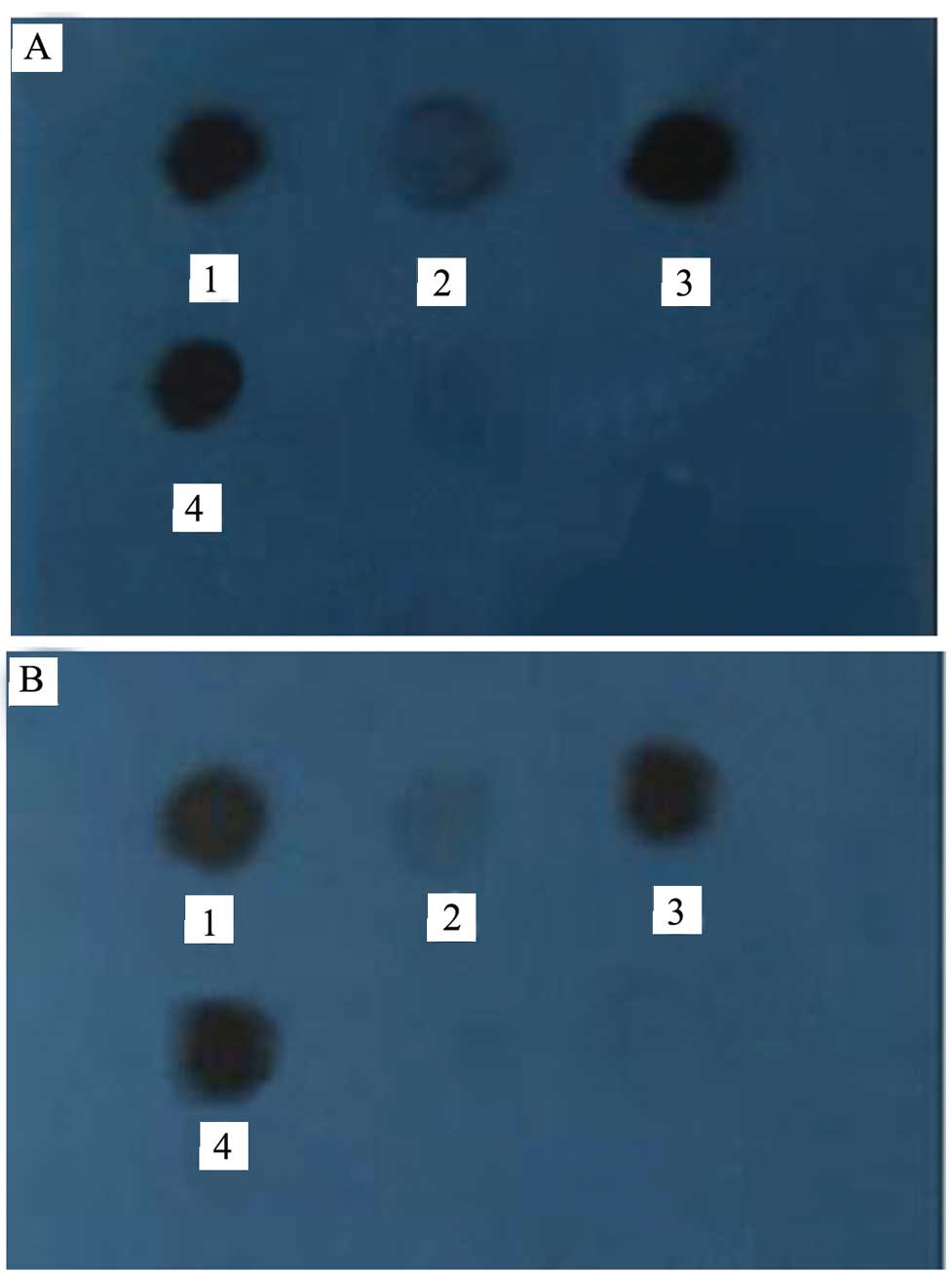
Identification of HSP70-HER-2-PCs
As described in Materials and methods,
HSP70-HER-2-PCs were purified from SKBR-3 cells as well as
identified by SDS-PAGE with Coomassie Brilliant Blue staining and
immunodotting using HSP70-specific and HER-2-specific antibodies.
The purified products were found to contain ~70- and 185-kDa
proteins (Fig. 1). The purified
protein hybridized with the HSP70-specific and HER-2-specific
antibodies (Fig. 2A and B),
demonstrated that the obtained complex contained HSP70 and HER-2
protein. The other protein straps apart from HSP70 and HER-2 in the
result may typify other tumor antigen peptides in the SKBR-3 cells
that were bound by HSP70.
Quantitative detection was performed using HER-2
ELISA kits. The content of HER-2 protein in the HSP70-HER-2-PCs was
~2.09 μg. The endotoxin levels in the preparations were lower than
0.03 EU/mg, as determined by an LAL assay.
Recombinant human HSP70-HER-2 protein
complex prepared in vitro
Recombinant human HSP70 was incubated with
recombinant human HER-2 protein at a 1:1 molar ratio at 43°C for 30
min, and then at 37°C for 1 h. The complex was then detected by
co-immunoprecipitation and western blot analysis. The protein
A-Sepharose-immune complex was found to bind with both mouse
anti-human monoclonal antibody against HSP70 and rabbit anti-human
monoclonal antibody against HER-2. Using a chemiluminescence
reagent, the successful in vitro preparation of the
recombinant human HSP70-HER-2 protein complex was confirmed
(Fig. 3).
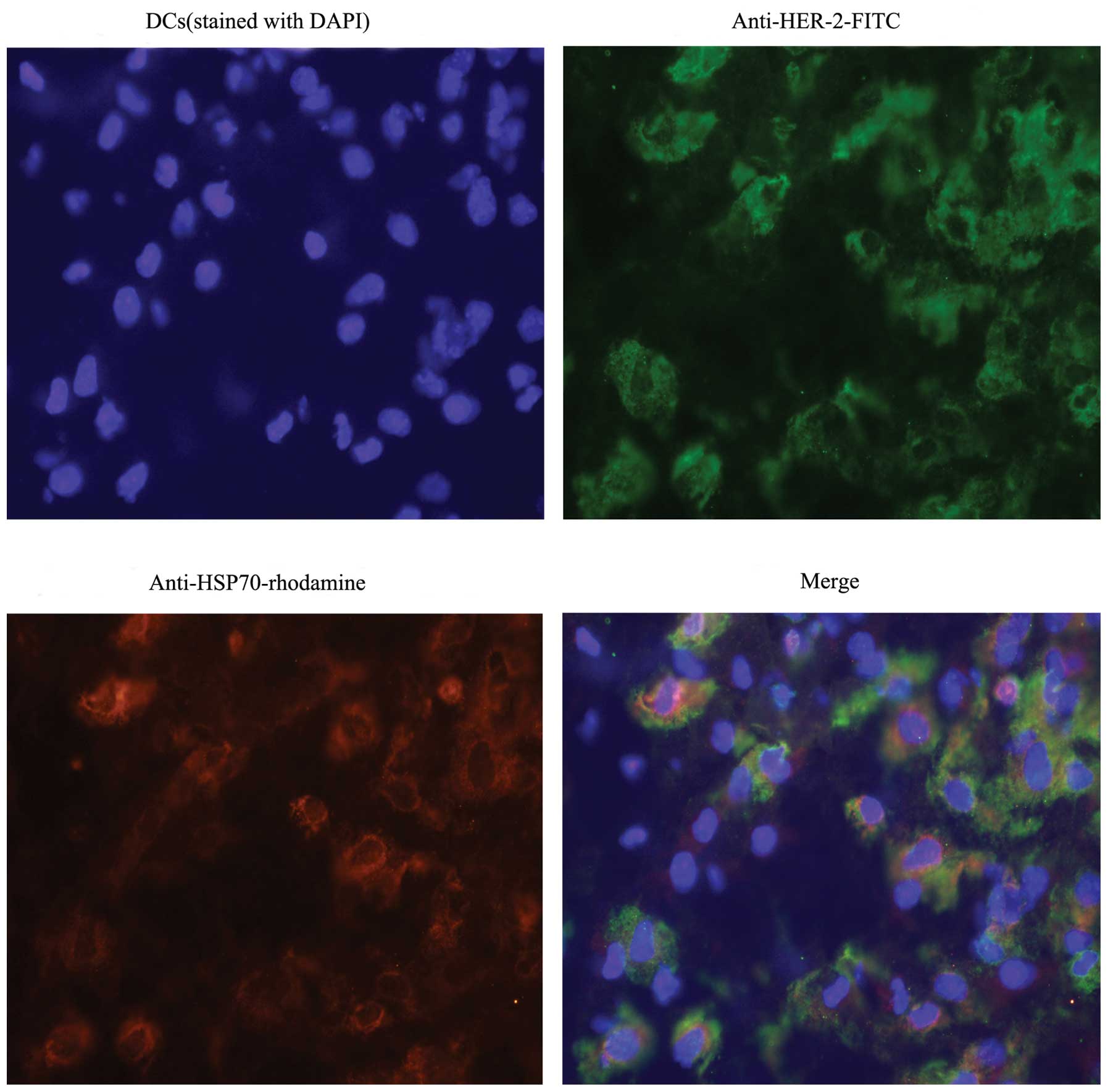
Fluorescence immunostaining
The ability of DCs to uptake HSP70-HER-2-PCs
purified from SKBR-3 was subsequently determined. DCs were pulsed
with HSP70-HER-2-PCs at 37°C for 12 h. After the numerous
aforementioned treatments and extensive washing to remove unbound
proteins, the DCs were characterized by an Olympus DP71 microscope.
Fig. 4 shows that DCs were
identified by the presence of HSP70 (red color) and HER-2 protein
(green color) expression. Evidently, the HSP70-HER-2-PCs were by
taken up by the DCs (Fig. 4).
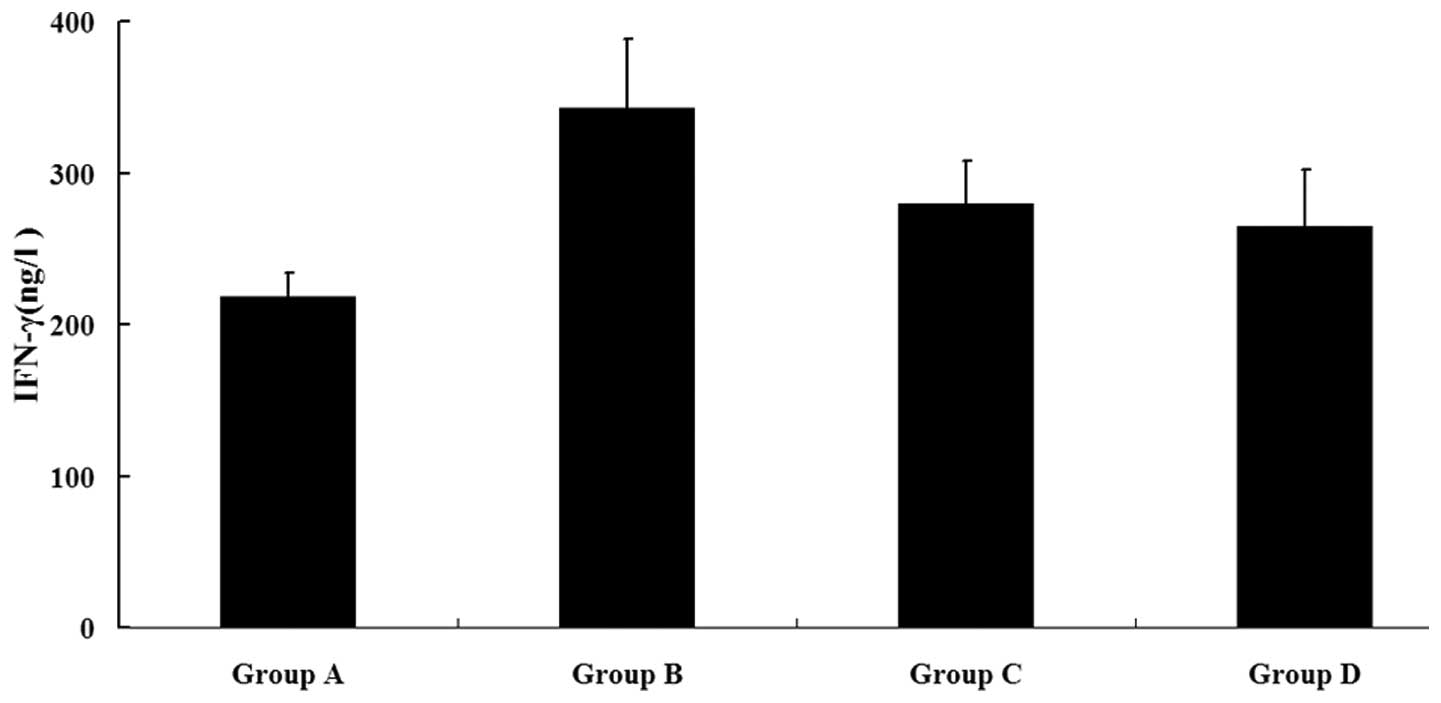
Antigen-specific IFN-γ production induced
by DCs pulsed with HSP70-HER-2-PCs
Cell-mediated immunity, which is particularly
important in tumor suppression, is characterized by the production
of type I cytokines. Consequently, the possible ability of DCs
pulsed with HSP70-HER-2-PCs to stimulate autologous T cells and
induce IFN-γ secretion was explored. Autologous T cells cocultured
with the four DC groups were used as effector cells, and SKBR-3
cells were used as target cells. These cells were then detected in
the four sample groups using an IFN-γ ELISPOT kit and an ELISA
reader system. Fig. 5 shows that
the IFN-γ level was significantly higher in group B than in the
three other groups (P<0.05). This phenomenon confirmed that the
DCs pulsed by HSP70-HER-2-PCs were capable of stimulating
autologous T cells and inducing type I cytokine secretion at high
levels.
Induction of specific CD8+ T
cells by DCs pulsed with HSP70-HER-2-PCs and in vitro cytotoxicity
test
The four groups of CD8+ T cells were
induced by coculturing with four groups of autologous DCs which
were pulsed by different ways (mentioned above). The specific
cytolytic activities of CD8+ T cells against SKBR-3
cells were next examined by detecting the level of LDH release.
This detection was performed after 4 h of coculturing effector
cells (CD8+ T cells) with target cells (SKBR-3 cells) at
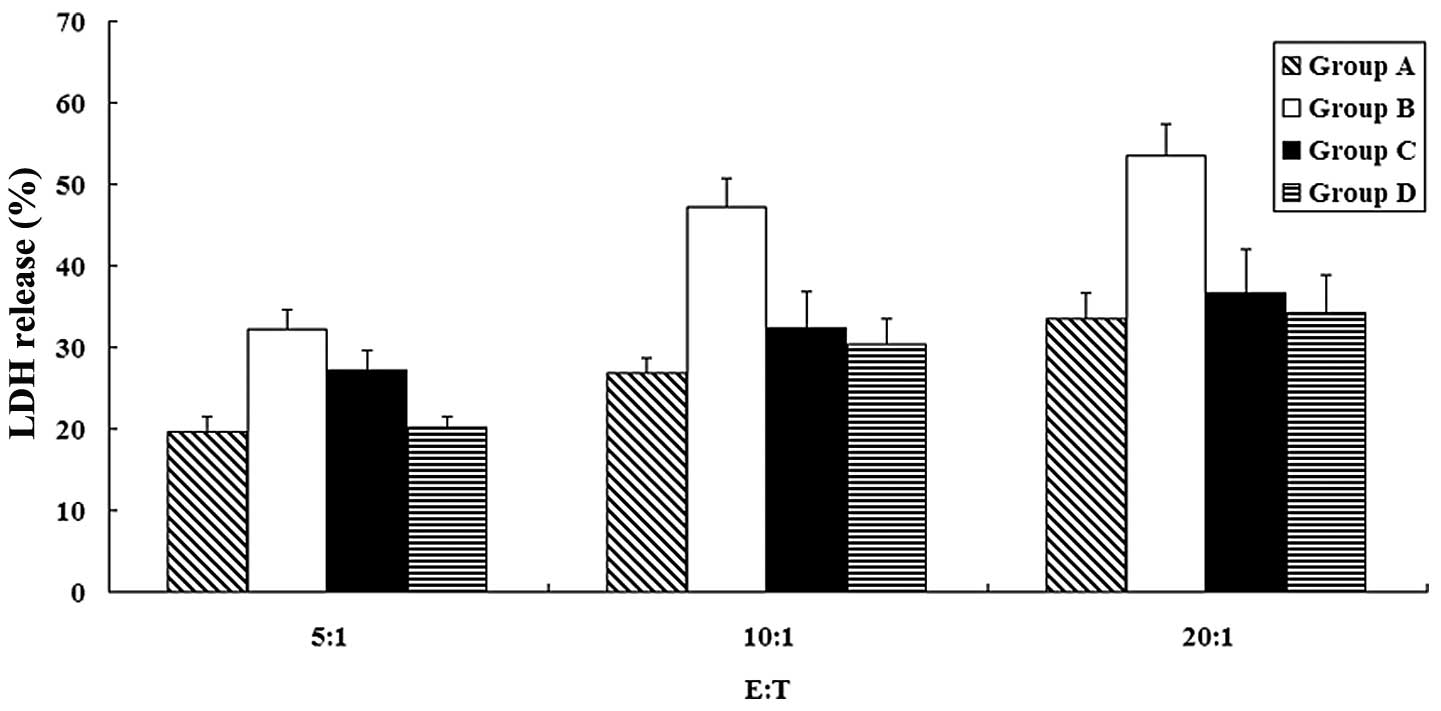
different ratios (5:1, 10:1 and 20:1). The LDH release level was
found to be significantly higher in group B than in the other
groups (P<0.05) (Fig. 6). This
finding indicated that the CD8+ T cells induced by DCs
which were pulsed with HSP70-HER-2-PCs had stronger cytotoxicities
against the SKBR-3 cells. Therefore, the HSP70-HER-2-PCs were an
improved individual tumor vaccine that had a more effective
antigenicity to SKBR-3 cells than other types of HSP70-PCs, and
induced more tumor-specific CD8+ T cells.
Inhibition of HER-2 expression by
siRNA
The knockdown of HER-2 was performed in SKBR-3 cells
using siRNA techniques. Expression was evaluated by western blot
analysis. The knockdown of HER-2 using the method described above
was confirmed to be efficient, as shown in Fig. 7.
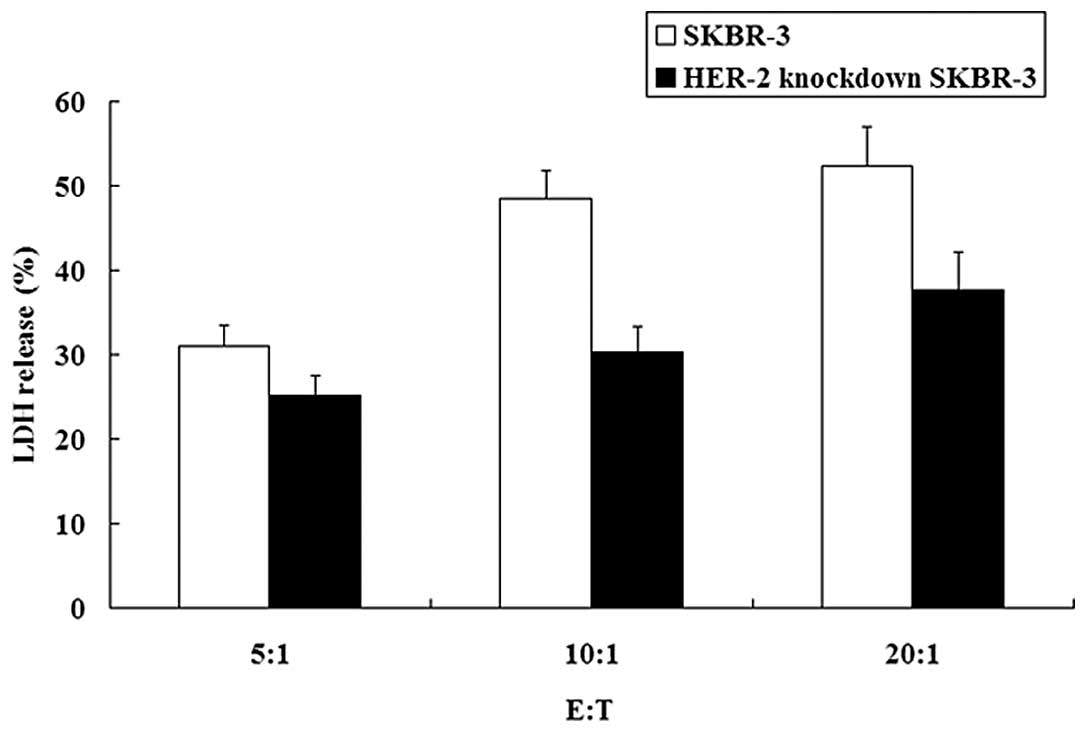
Detection of HER-2 specificity
HER-2 specificity with HSP70-HER-2-PCs will
determine the application scope of the immune complex. We confirmed
the characteristics of the complex using two trials. First, the
cytotoxicity of effector cells against MDA-MB-453 and MDA-MB-231
cells were compared. Effector cells showed significant cytotoxicity
against MDA-MB-453 cells, but exhibited minimal lysis against
MDA-MB-231 cells (P<0.05) (Fig.
8). Moreover, the cytotoxicities of effector cells against
SKBR-3 and HER-2-knockdown SKBR-3 cells were compared. Effector
cells showed stronger cytotoxicity against SKBR-3 cells than
HER-2-knockdown SKBR-3 cells (P<0.05) (Fig. 9).
Discussion
HSP70-PCs purified from tumor cells elicit
anticancer immunity when used as a tumor vaccine (17). The immune response stimulated by
HSP70-PCs is considered to consist of two parts. First is the
delivery of antigens for cross-presentation on the MHC class I
molecules of DCs. Second is the stimulation of DCs to excrete
cytokines and express costimulatory molecules, thereby creating the
immunogenic environment required for the induction of
CD8+ T cell responses (18). Previous studies have shown that
immunization of mice with HSP70-PCs provides protection against
tumor cells from which the HSP70-PCs were purified or slows the
progression of those tumors (19,20).
Based on these results, HSP70-based vaccines are purified from the
resected tumors of patients to whom the vaccine will be applied.
Therefore, HSP70 in itself has no antigenicity, and its
immunogenicity is attributed to the peptides it chaperones or binds
with (8,11,21,22).
The content and kind of antigenic peptides in HSP70-PCs that are
isolated from tumor cells determine the immune response against the
same tumor. Consequently, research on HSP70-based tumor
immunotherapy is focused on how to obtain more effective and
powerful peptides by the purification of HSP70-PCs from tumor
cells.
Previously purified HSP70-PC-based vaccines, while
eliciting immunological responses, have been proven to be
insufficient in providing protection against tumor growth. Thus,
the efficacy of HSP70-PCs as a therapeutic tumor vaccine requires
further improvement. To date, no evidence showing that membrane
tumor-associated peptides, such as HER-2 protein, are produced by
the purification of HSP70-PCs from tumor cells is available. Since
older HSP70-based purification methods cannot completely separate
the cellular membrane, losses in membrane antigenic peptides are
often observed.
In this study, HER-2 protein was used to represent
membrane antigens to verify the new product. HER-2 is an important
and ideal tumor antigen peptide that is mostly expressed on the
cell membrane. It is highly relevant to breast cancer and other
cancers (e.g. ovarian, prostate, lung, and colon). Some patients
with cancer, especially that of the breast, have a pre-existing
immune responses directed against HER-2 (23,24).
Therefore, an effective cancer vaccine targeting HER-2 protein will
be able to boost this immunity to therapeutic levels (25). In addition, HER-2 protein can be
easily identified.
Some researchers have used recombinant in
vitro heat shock-bound HSP-HER-2 protein complexes as cancer
vaccines for HER-2 target treatment. They confirmed that
recombinant HSP-HER-2 protein complexes elicit an antigen-specific
CD8+ T cell response. Nevertheless, this immune response
is insufficient since there are various types of important
antigenic peptides in tumor cells besides HER-2 protein. Although
the concrete identity of these peptides has yet to be identified,
they still contribute to the antitumor immunocompetence of
HSP70-based vaccines. The recombinant human HSP70-HER-2 protein
complex was successfully prepared in vitro and used as an
important control to support this theory (Fig. 3).
The present study extended previous findings and
established a new method using CHAPS to purify HSP70-PCs containing
membrane-associated tumor peptides from human cancer cells. CHAPS
was chosen because it is suitable for laminar analyses and easily
depleted by dialysis.
The product, HSP70-HER-2-PCs containing both
membrane-resident tumor peptides, HER-2 protein as a
representative, and other antigenic peptides bound by HSP70, were
able to induce tumor-specific CD8+ T cells with higher
specificity against SKBR-3 cells than traditional HSP70-PCs and
recombinant human HSP70-HER-2 protein complexes (Figs. 5 and 6). Moreover, HSP70-HER-2-PCs possessed
HER-2 specificity and could be used as a HER-2 target vaccine for
treating other HER-2 overexpressed tumor cells (Figs. 8 and 9).
In summary, an improved method for preparing an
advanced HSP70-based vaccine from cancer cells was designed. This
approach was based on HSP70 and a purification process involving
the detergent CHAPS to obtain membrane-resident tumor-associated
peptides from cancer cells. The product exhibited stronger
immunogenic activity and served as a better tumor antigen source
for pulsing DCs. The pulsed DCs were able to induce more effective
tumor-specific CD8+ T cells. Animal experiments are
ongoing by our group to determine the immunocompetence of the new
HSP70-based vaccine in vivo. The findings of this study
provide the basis for a new approach for enhancing HSP70-based
immunotherapies for HER-2-associated or other membrane antigenic
peptide-related tumors. We firmly believe that the new customized
vaccine will provide tumor patients with better individualized
treatments.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (30660206). The authors
gratefully acknowledge the Inner Mongolia Red Cross Blood Center
for supplying blood samples, and the laboratory staff of the Inner
Mongolia Agricultural University for performing the flow
cytometry.
References
|
1
|
Hightower LE: Heat shock, stress proteins,
chaperones, and proteotoxicity. Cell. 66:191–197. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pandey M, Mathew A and Nair MK: Cancer
vaccines: a step towards prevention and treatment of cancer. Eur J
Surg Oncol. 25:209–214. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hartl FU: Molecular chaperones in cellular
protein folding. Nature. 381:571–579. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Srivastava PK: Immunotherapy for human
cancer using heat shock protein-peptide complexes. Curr Oncol Rep.
7:104–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Srivastava PK: Peptide-binding heat shock
proteins in the endoplasmic reticulum: role in immune response to
cancer and in antigen presentation. Adv Cancer Res. 62:153–177.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sherman M and Multhoff G: Heat shock
proteins in cancer. Ann NY Acad Sci. 1113:192–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang C, Yu H, Wang Q, et al: Potent
antitumor effect elicited by superantigen-linked tumor cells
transduced with heat shock protein 70 gene. Cancer Sci. 95:160–167.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Srivastava PK, DeLeo AB and Old LJ: Tumor
rejection antigens of chemically induced sarcomas of inbred mice.
Proc Natl Acad Sci USA. 83:3407–3411. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Udono H, Levey DL and Srivastava PK:
Cellular requirements for tumor-specific immunity elicited by heat
shock proteins: tumor rejection antigen gp96 primes CD8+
T cells in vivo. Proc Natl Acad Sci USA. 91:3077–3081. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tamura Y, Peng P, Liu K, Daou M and
Srivastava PK: Immunotherapy of tumors with autologous
tumor-derived heat shock protein preparations. Science.
278:117–120. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vanaja DK, Grossmann ME, Celis E and Young
CY: Tumor prevention and antitumor immunity with heat shock protein
70 induced by 15-deoxy-delta12, 14-prostaglandin J2 in transgenic
adenocarcinoma of mouse prostate cells. Cancer Res. 60:4714–4718.
2000.PubMed/NCBI
|
|
12
|
Suto R and Srivastava PK: A mechanism for
the specific immunogenicity of heat shock protein-chaperoned
peptides. Science. 269:1585–1588. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Noessner E, Gastpar R, Milani V, et al:
Tumor-derived heat shock protein70 peptide complexes are
cross-presented by human dendritic cells. J Immunol. 169:5424–5432.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumar S, Deepak P and Acharya A:
Autologous HSP70 immunization induces anti-tumor immunity and
increases longevity and survival of tumor-bearing mice. Neoplasma.
56:259–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manjili MH, Henderson R, Wang XY, et al:
Development of a recombinant HSP110-HER-2/neu vaccine using the
chaperoning properties of HSP110. Cancer Res. 62:1737–1742.
2002.PubMed/NCBI
|
|
16
|
Thurner B, Roder C, Dieckmann D, et al:
Generation of large numbers of fully mature and stable dendritic
cells from leukapheresis products for clinical application. J
Immunol Methods. 223:1–15. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Udono H and Srivastava PK: Heat shock
protein 70-associated peptides elicit specific cancer immunity. J
Exp Med. 178:1391–1396. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bendz H, Ruhland SC, Pandya MJ, et al:
Human heat shock protein 70 enhances tumor antigen presentation
through complex formation and intracellular antigen delivery
without innate immune signaling. J Biol Chem. 282:31688–31702.
2007. View Article : Google Scholar
|
|
19
|
Ciupitu AM, Petersson M, Kono K, Charo J
and Kiessling R: Immunization with heat shock protein 70 from
methylcholanthrene-induced sarcomas induces tumor protection
correlating with in vitro T cell responses. Cancer Immunol
Immunother. 51:163–170. 2002. View Article : Google Scholar
|
|
20
|
Chen DX, Su YR, Shao GZ and Qian ZC:
Purification of heat shock protein 70-associated tumor peptides and
their antitumor immunity to hepatoma in mice. World J
Gastroenterol. 10:361–365. 2004.PubMed/NCBI
|
|
21
|
Palladino MA Jr, Srivastatva PK, Oettgen
HF and DeLeo AB: Expression of a shared tumor-specific antigen by
two chemically induced BALB/c sarcomas. Cancer Res. 47:5074–5079.
1987.PubMed/NCBI
|
|
22
|
Srivastava PK and Udono H: Heat shock
protein-peptide complexes in cancer immunotherapy. Curr Opin
Immunol. 6:728–732. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Disis ML and Cheever MA: HER-2/neu
protein: a target for antigen-specific immunotherapy of human
cancer. Adv Cancer Res. 71:343–371. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Disis ML, Grabstein KH, Sleath PR and
Cheever MA: Generation of immunity to the HER-2/neu oncogenic
protein in patients with breast and ovarian cancer using a
peptide-based vaccine. Clin Cancer Res. 5:1289–1297.
1999.PubMed/NCBI
|
|
25
|
Lin KY, Guarnieri FG, Staveley-O’Carroll
KF, Levitsky HI, August JT, Pardoll DM and Wu TC: Treatment of
established tumors with a novel vaccine that enhances major
histocompatibility class II presentation of tumor antigen. Cancer
Res. 56:21–26. 1999.PubMed/NCBI
|