Introduction
High-mobility group box 1 protein (HMGB1) is a
pro-inflammatory cytokine that can either be passively secreted by
necrotic cells or actively secreted in response to inflammatory
signals. In our previous study, we showed that HMGB1 is
overexpressed in thyroiditis and in papillary thyroid cancer
(1). HMGB1 can be actively secreted
by a variety of cells including macrophages, activated monocytes,
dendritic cells, endothelial cells and certain cancer cells
(2). It functions as an alarmin and
its extracellular release signals damage to the immune system.
HMGB1 signals through the receptor for advanced glycation
end-products (RAGE) and via Toll-like receptor (TLR)2 and TLR4. The
activation of these receptors results in the activation of NFκB
which induces the secretion of cytokines, growth factors (3) and angiogenic factors (4). Apart from its connection to NFκB, RAGE
activation is linked to many signaling pathways that upregulate
protein synthesis, cell survival, proliferation and movement. In
certain cases NFκB may enhance the expression of oncogenic factors,
such as microRNAs (miRNAs) (5). The
intracellular pathways starting from different kinases (MAPKs)
(6), PI3K/AKT (7), JAK/STAT (8) that converge on AKT are positively or
negatively controlled by oncogenes or tumor suppressor genes
(9) which are in turn enhanced or
inhibited by various factors, such as miRNAs. miRNAs are small
non-coding RNAs that control gene expression at the
post-transcriptional level by targeting mRNAs. They have been shown
to play an important role in various processes, such as cellular
development, proliferation and apoptosis (10). Their aberrant expression has been
linked to several human cancers and metastasis (11). Some of them function as oncogenes or
tumor suppressors (12). MiR-221
and -222 have been found to be deregulated in human papillary
thyroid carcinomas (13) and are
involved in thyroid cell transformation as well as cell
proliferation through the inhibition of a cell cycle regulator
(p27kip1) (14). A
single miRNA can regulate different target genes as perfect
complementarity is not required. Many miRNAs such as miR-221 and
-222 form genetic clusters and therefore exert co-ordinated
expression and function. In gastric cancer cells for example, the
miR-221/222 cluster has been shown to target the tumor suppressor
gene, phosphatase and tensin homolog (PTEN), resulting in an
increase in cancer cell proliferation and radio resistance
(15).
We have previously demonstrated (1,3) that
inflammatory infiltrates are present in papillary cancer and may
contribute to tumor transformation and escape from immune
surveillance. We showed that HMGB1 is overexpressed in the
papillary cancer microenvironment. In this study, we analyzed the
influence of HMGB1 on the expression of the miR-221/222 cluster in
a papillary cancer cell line and in primary cultures of thyreocytes
obtained from patients who underwent thyroidectomy for papillary
cancer.
Materials and methods
Cell lines
The long-term thyroid carcinoma cell line, BC PAP
(16), was kindly provided by
Professor M. Russo (University of Sapienza, Rome, Italy) and
maintained in RPMI-1640 (Gibco) supplemented with heat inactivated
10% FCS containing 2 mM L-glutamine.
Patients and collection of samples
Patients included in this study were selected from a
group of 30 patients with papillary cancer who had undergone total
thyroidectomy for a pre-operative diagnosis of thyroid cancer. All
patients provided written informed consent, agreeing to participate
in this study, in accordance with the ethical standards of our
institution. Standard histology confirmed the diagnosis and allowed
us to select 11 patients (7 females and 4 males, aged from 28 to 73
years) of which 9 were used for the preparation of primary cultures
as previously described (1) and 2
female patients with papillary cancer (pT3NxMx) were selected for
chemotaxis experiments.
Primary cultures
Normal and neoplastic thyroid specimens were
obtained from patients who had undergone surgical treatment at the
Department of Surgery at our institution. Histological
classification was carried out in accordance with the WHO
recommendations. Upon removal, specimens were divided into 2
groups: in the first group, the samples were processed for routine
histopathological examination and in the other group, the samples
were used for establishing primary short-term cultures as
previously described (1). Cells
were cultured for 24 h in RPMI at 37°C, 5% CO2. Where
required, 10 nM HMGB1 (Sigma) were added to the cultures. Cell
viability, determined by the Trypan blue exclusion test, was always
95–98%.
Monocytes were obtained from healthy donors by the
monocyte isolation method. Human peripheral blood mononuclear cells
(PBMCs) from buffy coats, obtained from healthy donors, were
isolated by Lymphoprep (Nycomed AS Pharma Diagnostic Division,
Oslo, Norway) density-gradient centrifugation, washed 3 times in
PBS, pH 7.4, and isolated by density gradient separation
(Lympholite; Cedarlane, Ontario, Canada). CD14+
monocytes were purified by incubation with anti-CD14-coated
microbeads (Miltenyi Biotec, Bergish Gladbach, Germany), followed
by sorting with a magnetic device (MiniMacs Separation Unit;
Miltenyi Biotec), according to the manufacturer’s instructions.
The purity of the isolated monocytes was evaluated
by staining with a fluorescein isothiocyanate-conjugated antibody
against monocytes (FITC-conjugated anti-CD14) and analysis by flow
cytometry. Viable monocytic cells (86.7+3%) (mean ± SEM) were
obtained. Cells were cultured for 4 h in RPMI-1640, containing 2 mM
L-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, and 250
pg/ml Fungizone (Gibco), in the absence of antioxidant agents, at
37°C in a humified 5% CO2 atmosphere before the assay.
U937, a well-established cell line derived from human monocytic
leukemia was used as the positive control for western blot
analysis.
Chemotaxis
Cell migration was assessed in the BC PAP cells, in
primary cultures from 2 patients with papillary cancer. Specimens
were obtained from nodules and healthy contralateral lobes.
Monocytes from healthy donors were used as the positive controls.
The cells were seeded on a 24-well migration plate (ECM 509;
Chemicon International). The assay is based on the Boyden chamber
principle. It provides a system for quantitative determination of
cells migrating through 8-μm porous membrane towards a chemotactic
agent. In our case the lower chamber was loaded with RPMI medium
with or without 10 μM HMGB1 (Sigma). Incubation was carried out at
37°C, 5% CO2 for 12 h. At the end of the incubation
time, migration inserts were removed from the migration chambers,
placed into wells containing cell detachment solution and lysed.
Samples were dyed and read with a photometric plate reader using a
480-nm filter set. Samples without cells but containing cell
detachment buffer, lysis buffer and the dye were used as the blank
controls for interpretation of the data.
Western blot analysis
Whole cell lysates were separated as previously
described (1) on 15%
SDS-polyacrylamide electroforesis gel for HMGB1 and 10% for RAGE.
Samples were heat-denatured for 5 min, loaded on standard Tris-HCl
polyacrylamide gel and run on ice at 40 V for the stacking gel and
80 V for the running gel. Proteins were transferred onto a PVDF
membrane (Bio-Rad, Hercules), previously activated in 100% methanol
for 15 sec. The membrane was blocked in TBS-T and 5% albumin for 1
h, probed overnight at 4°C by the specific antibody (monoclonal
anti-HMGB1 by Sigma and monoclonal anti-RAGE (Millipore). At the
end of the incubation time, the membrane was washed and incubated
with anti-mouse IgG peroxidase conjugated secondary antibodies
(1:10000 Sigma) for 1 h at room temperature. The signal was
detected by autoradiography (Kodak Biomax light film;
Sigma-Aldrich) using the chemiluminescent peroxidase substrate kit
(Sigma-Aldrich) then quantified by densitometric analysis using
software (Quantity-One, Bio-Rad).
MiRNA relative quantification by
real-time RT-PCR
Total RNA was extracted from the cells using TRIzol
reagent (Life Technologies), according to the manufacturer’s
instructions.
TaqMan® microRNA assays (Applied
Biosystems, Foster City, CA, USA) that included RT primers and
TaqMan probes were used for reverse transcription and to quantify
the expression of mature miR-221 and -222 in the tissue samples and
cell lines. The ubiquitously expressed U6b small nuclear RNA
(snRNA) was used as the internal control. Relative quantification
was calculated using the ΔCt method.
Results
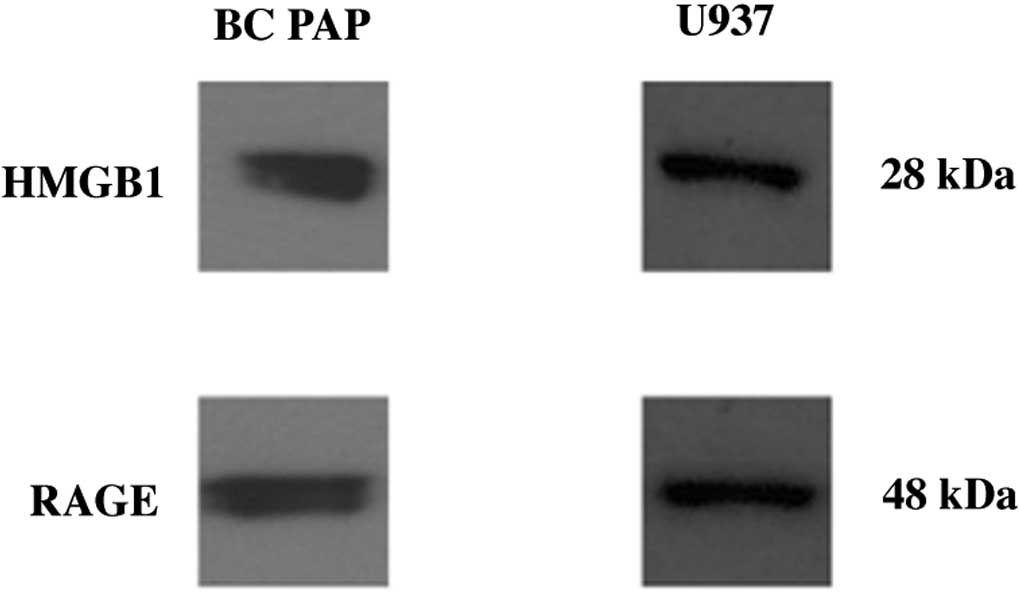
BC PAP cells express HMGB1 and RAGE
receptors
We previously (1)
provided evidence that thyreocytes from papillary cancer and
thyroiditis express HMGB1. We also demonstrated that the
inflammatory microenvironment in thyroiditis contributes to
neoplastic transformation. Therefore, we aimed to investigate
whether HMGB1 can be produced by a papillary cancer cell line.
Fig. 1 shows that BC PAP cells
express both HMGB1 and RAGE. This suggests that HMGB1 can bind to
RAGE receptors. The activation of RAGE activates the intracellular
signal transduction pathways, leading to various stimulatory
functions.
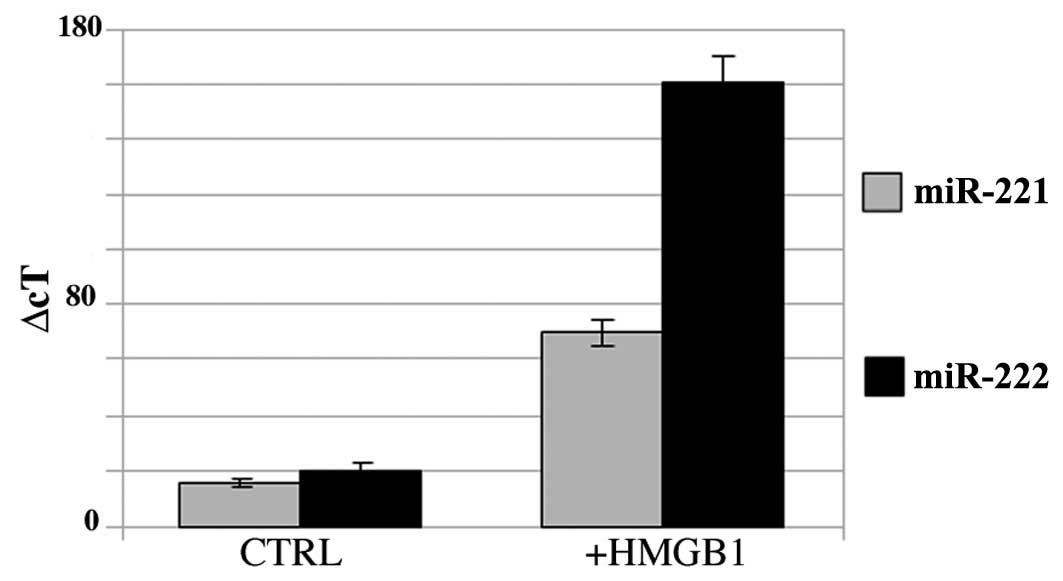
HMGB1 modulates the expression of miR-221
and miR-222 in BC PAP cells and in primary cultures of papillary
cancer cells
BC PAP cells express miR-221 and -222 as shown in
Fig. 2. The addition of HMGB1 to
the culture medium increased the expression of miR-221 (3-fold
increase) and miR-222 (7-fold increase).
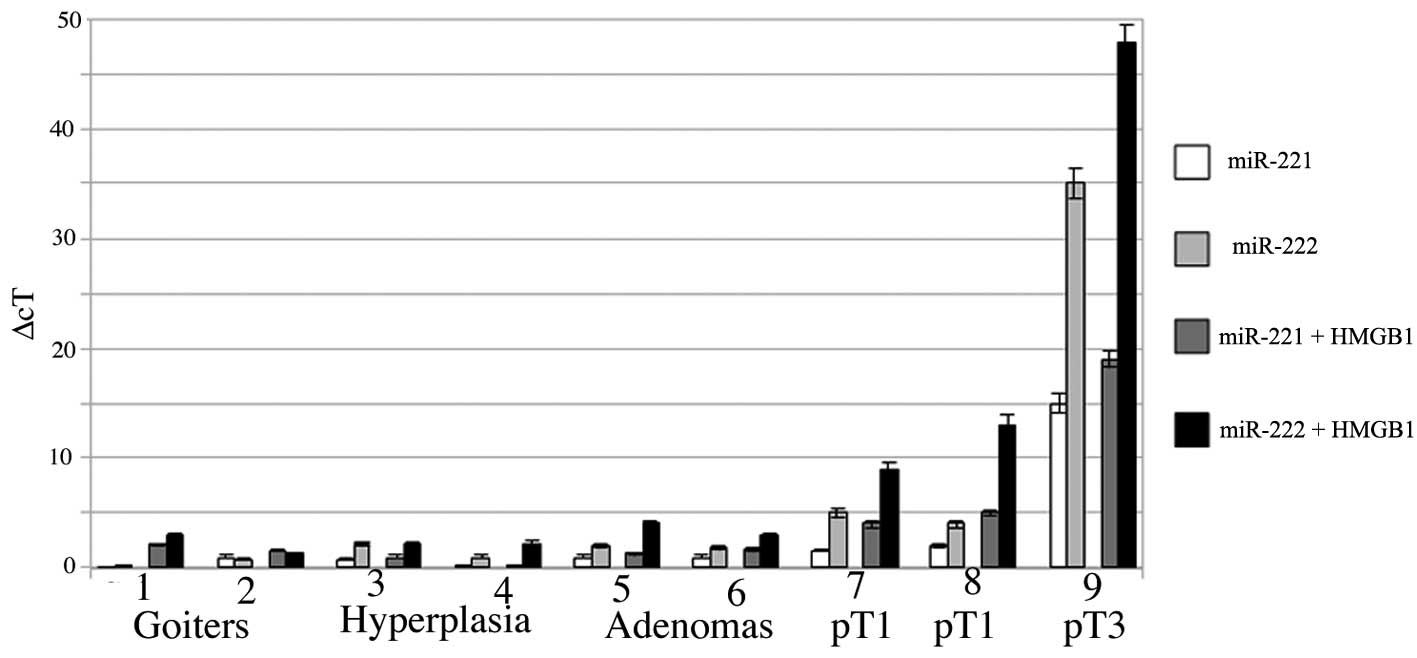
A particularly interesting result was obtained in
the thyreocytes from patients with papillary tumors, with adenomas
and micro-macrofollicular goiters. Fig.
3 shows a comparative analysis of the expression of miR-221 and
miR-222 in primary short-term cultures treated with 10 μM HMGB1.
The expression of miR-221 and -222 was low in the goiters, as well
as in the samples of hyperplasia and adenoma, even though the
addition of HMGB1 led to an increase in the expression of miR-222.
This positive trend was higher in the primary cultures of papillary
cancers and it reached its peak in the papillary cancer (pT3)
sample.
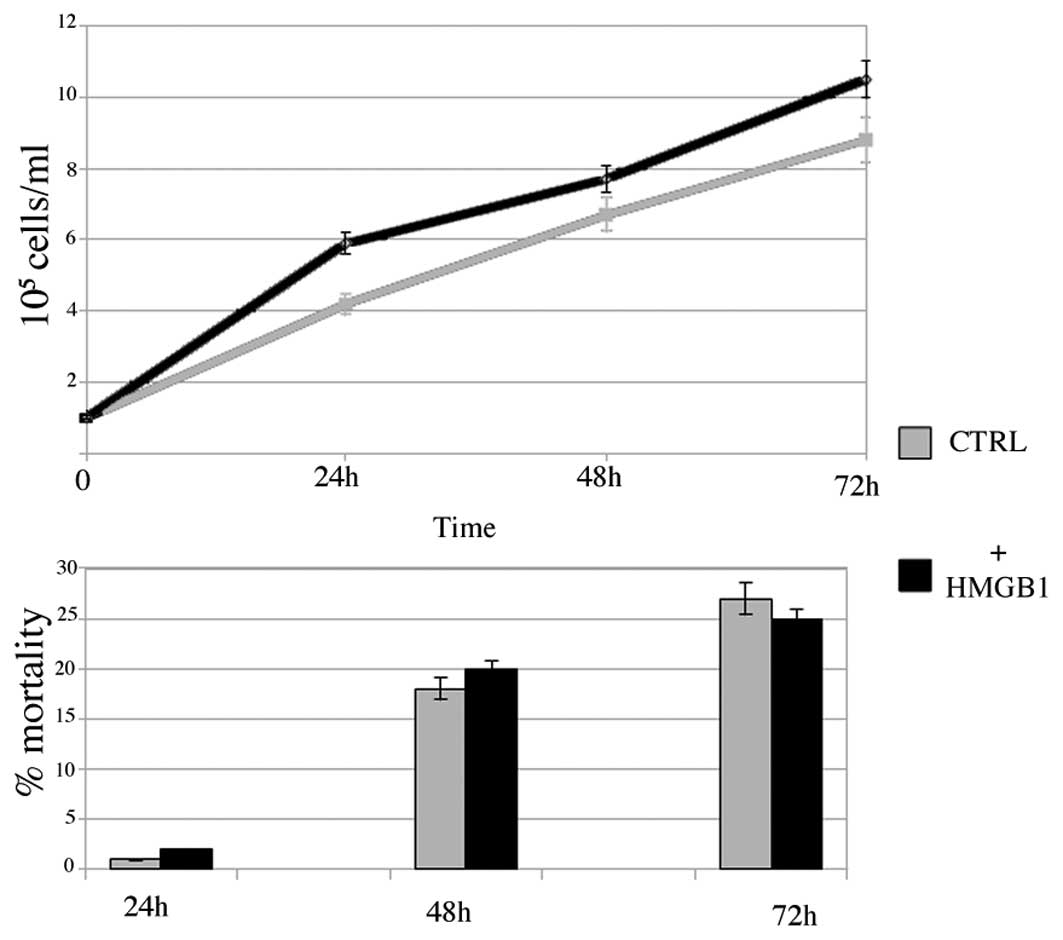
HMGB1 increases cell growth in BC PAP
cells
In order to investigate whether HMGB1 promotes
growth in BC PAP cells, we performed cell growth studies by seeding
105 cells in 24-microwell plates in the presence of 10
μM HMGB1. As shown in Fig. 4, HMGB1
increased cell growth after 24 h (2-fold) and at 48 and 72 h
(1.5-fold). Cell mortality increased in the controls and stimulated
cells with a small but not significant difference in the 2
populations, possibly due to overcrowding in the plates resulting
from the high growth rate.
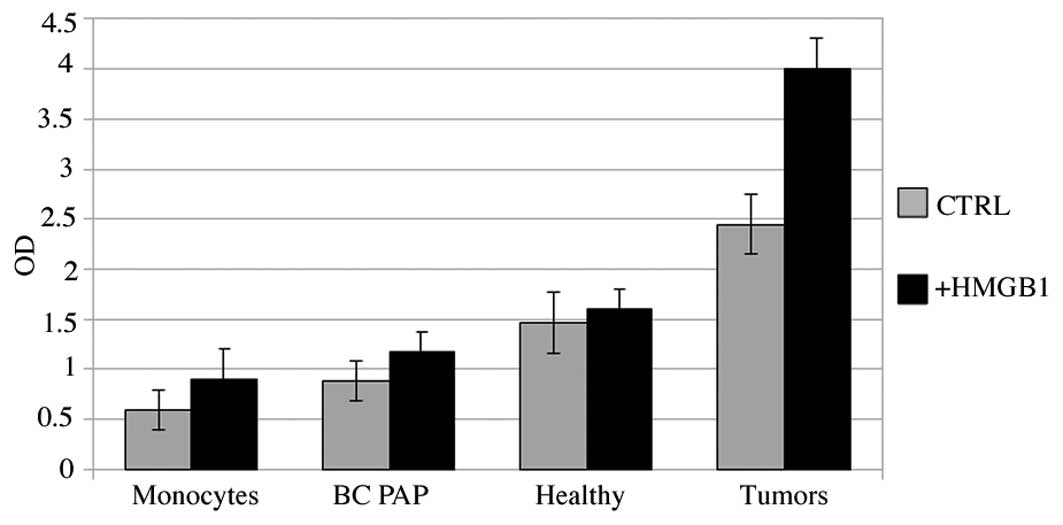
HMGB1 induces chemotaxis in papillary
cancer cells and in BC PAP cells
Cell migration was assessed in the BC PAP cells in
primary cultures from 2 patients with papillary cancer (pT3) and in
cells from healthy lobes from the same patients. Monocytes from
healthy donors were used as the positive controls. Fig. 5 shows that both the cell line and
cells from primary cultures were more active than the monocytes in
terms of movement through the membranes. This phenomenon was more
pronounced in the cells obtained from the patients than in the BC
PAP cells and was more evident with the addition of HMGB1,
suggesting that HMGB1 attracts tumor cells.
Discussion
This study reports on a way of signaling between the
inflammatory microenvironment (1)
of papillary thyroid cancer and the P13K/AKT/PTEN oncogenic
pathway. Due to difficulty in obtaining primary cultures, most
studies available on cell migration in thyroid cancer have used
cell lines; however, our results obtained from primary cultures
were similar to those obtained in the cell line.
We demonstrate that the binding of HMGB1 to RAGE
increases the expression of miR-221 and -222 in the tumor samples
and the cell line. Papillary cancer is highly differentiated and it
very rarely metastasizes. However, there are some cases in which
the clinical management of patients is difficult, as the tumors
remain very small in size but still lead to metastases. Cell growth
and motility are often evaluated as malignancy scores in many
oncology studies. In this study, we show that the expression of
miR-222/-221 is increased in pT3 samples, suggesting that there is
a direct correlation between the degree of malignancy and oncogenic
cluster expression.
The same correlation was observed between cell
motility and HMGB1. This means that a higher number of cells of
more malignant phenotype migrated as opposed to cytokines.
HMGB1 in cancer is associated with invasion and
metastasis through the high variety of RAGE transduction pathways.
These include the activation of NFκB, MAPKs, PI3K/Akt, Rho GTPases,
JAK/STAT and Src family kinases. Many tumor suppressor genes such
as p53 and PTEN (17) are involved
in the RAGE transduction network as essential regulators of the
cell cycle. PI3K generates phosphatidylinositol-3,4, 5-triphosphate
(PIP3) from phosphatidylinositol 4,5-bisphosphate (PIP2), which
then activates Akt. PTEN dephosphorylates PIP3 to inhibit Akt
activation (18). PTEN mutations
enhance Akt activity in certain cancer cells (19).
Akt activation and decreased PTEN function have been
observed in liver cancer (20). A
recent study on miRs showed that TGFβ activates AKT kinase through
PTEN downregulation by miR-216 and -217 (21). Since PTEN is a potential target of
miR-221 (22) and miR-222, it may
constitute a a link between cytokine external signaling and Akt
activation. Of note, miR-221 and miR-222 respond to cellular
stresses, such as radiation by activating NFκB and AP1 promoters
(23).
The results from the present study suggest that the
abnormal expression of miR-221 and -222 promoted by HMGB1 may
interfere with the PTEN regulation of the cell cycle. Further
studies on PTEN inhibition by miR-221 and -222 are required to
confirm this hypothesis.
Acknowledgements
The authors would like to thank Professor Hugo
Bowles from Tor Vergata University of Rome for language
proofreading. This study was supported by grant Ateneo 60% (2009)
to Professor Alfredo Antonaci and Ateneo 60% (2011) to Dr
Alessandra Zicari.
References
|
1
|
Mardente S, Zicari A, Consorti F, Mari E,
Di Vito M, Leopizzi M, Della Rocca C and Antonaci A: Cross-talk
between NO and HMGB1 in limphocytic thyroiditis and papillary
thyroid cancer. Oncol Rep. 24:1455–1461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-κB funtions as a tumor promoter in
inflammation-associated cancer. Nature. 431:261–266. 2004.
|
|
3
|
Mardente S, Lenti L, Lococo E, Consorti F,
Della Rocca C, Romeo S, Misasi R and Antonaci A: Phenotypic and
functional characterization of lymphocytes in autoimmune
thyroiditis and in papillary carcinoma. Anticancer Res.
25:2483–2488. 2005.PubMed/NCBI
|
|
4
|
Van Beijnum JR, Buurman WA and Griffioen
AW: Convergence and amplification of toll-like receptor (TLR) and
receptor for advanced glycation end products (RAGE) signaling
pathways via high mobility group B1 (HMGB1). Angiogenesis.
11:91–99. 2008.PubMed/NCBI
|
|
5
|
Galardi S, Mercatelli N, Farace MG and
Ciafrè SA: NF-κB and c-Jun induce the expression of the oncogenic
miR-221 and miR-222 in prostate carcinoma and glioblastoma cells.
Nucleic Acids Res. 39:3892–3902. 2011.
|
|
6
|
Palumbo R, De Marchis F, Pusterla T, Conti
A, Alessio M and Bianchi ME: Src family kinases are necessary for
cell migration induced by extracelllular HMGB1. J Neurooncol.
86:617–623. 2009.PubMed/NCBI
|
|
7
|
Toure F, Zahm JM, Garnotel R, Lambert E,
Bonnet N, Schmidt AM, Vitry F, Chanard J, Gillery P and Rieu P:
Receptor for advanced glycation end-products (RAGE) modulates
neutrophil adhesion and migration on glycoxidated extracellular
matrix. Biochem J. 416:255–261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JY, Park HK, Yoon JS, Kim SJ, Kim ES,
Ahn KS, Kim DS, Yoon SS, Kim BK and Lee YY: Advanced glycation end
product (AGE)-induced proliferation of HEL cells via receptor for
AGE-related signal pathways. Int J Oncol. 33:493–501.
2008.PubMed/NCBI
|
|
9
|
Maehama T and Dixon JE: PTEN: a tumour
suppressor that functions asa phospholipid phosphatase. Trends Cell
Biol. 9:125–128. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Si ML, Zhu S, Wu H, Wu F and Mo YY:
miR-21-mediated tumor growth. Oncogene. 26:2799–2803. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He H, Jazdzewski K, Li W, Liyanarachchi S,
Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, Kloos
RT, Croce CM and de la Chapelle A: The role of microRNA genes in
papillary thyroid carcinoma. Proc Natl Acad Sci USA. 102:1975–1980.
2005.
|
|
14
|
Visone R, Russo L, Pallante P, De Martino
I, Ferraro A, Leone V, Borbone E, Petrocca F, Alder H, Croce CM and
Fusco A: MicroRNAs (miR)-221 and miR-222, both overexpressed in
human thyroid papillary carcinomas, regulate p27Kip1
protein levels and cell cycle. Endocr Relat Cancer. 14:791–798.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chun-Zhi Z, Lei H, An-Ling Z, Yan-Chao F,
Xiao Y, Guang-Xiu W, Zhi-Fan J, Pei-Yu P, Qing-Yu Z and Chun-Sheng
K: MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell
proliferation and radioresistance by targeting PTEN. BMC Cancer.
10:3672010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fabien N, Fusco A, Santoro M, Barbier Y,
Dubois PM and Paulin C: Description of a human papillary thyroid
carcinoma cell line. Morphologic study and expression of tumoral
markers. Cancer. 15:2206–2212. 1994. View Article : Google Scholar
|
|
17
|
Hummel R, Hussey DJ and Haier J:
MicroRNAs: predictors and modifiers of chemo- and radiotherapy in
different tumour types. Eur J Cancer. 46:298–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cully M, You H, Levine AJ and Mak TW:
Beyond PTEN mutations: the PI3K pathway as an integrator of
multiple inputs during tumorigenesis. Nat Rev Cancer. 6:184–192.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Q, Li M, Wang YL, Fauzee NJ, Yang Y,
Pan J, Yang L and Lazar A: RNA interference of PARG could inhibit
the metastatic potency of colon carcinoma cells via PI3-kinase/Akt
pathway. Cell Physiol Biochem. 29:361–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong QW, Ching AK, Chan AW, Choy KW, To
KF, Lai PB and Wong N: MiR-222 overexpression confers cell
migratory advantages in hepatocellular carcinoma through enhancing
AKT signaling. Clin Cancer Res. 16:867–875. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kato M, Putta S, Wang M, Yuan H, Lanting
L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ and
Natarajan R: TGF-beta activates Akt kinase through a
microRNA-dependent amplifying circuit targeting PTEN. Nat Cell
Biol. 11:881–889. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng F, Henson R, Lang M, Wehbe H,
Maheshwari S, Mendell JT, Jiang J, Schmittgen TD and Patel T:
Involvement of human micro-RNA in growth and response to
chemotherapy in human cholangiocarcinoma cell lines.
Gastroenterology. 130:2113–2129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vincenti S, Brillante N, Lanza V, Bozzoni
I, Presutti C, Chiani F, Etna MP and Negri R: HUVEC respond to
radiation by inducing the expression of pro-angiogenic microRNAs.
Radiat Res. 175:535–546. 2011. View
Article : Google Scholar : PubMed/NCBI
|