Introduction
The insulin superfamily comprises insulin,
insulin-like growth factors (IGF-1 and -2), relaxin and the
insulin-like (INSL) peptides 3, 4, 5, 6 and 7 (1). Similar to the other INSL members,
INSL5 is composed of a B- and A-chain connected by 2 disulfide
bonds. The Insl5 gene is expressed in various tissues,
including the gastrointestinal tract (2–4). INSL5
is the ligand for the orphan receptor RXFR4/GPCR142/GPR100 and this
receptor-ligand interaction results in the inhibition of
intracellular cAMP levels (3,5,6). INSL5
shares high sequence homology with INSL7/relaxin 3. The latter
engages in low affinity receptor interactions with RXFP4 but
preferentially binds to the orphan receptor RXFR3/GPCR135 (3,7). The
biological consequences of the INSL5-RXFP4 activated signaling
pathway remain largely elusive.
Enterochromaffin cells constitute the most abundant
enteroendocrine cells (EECs) of the GI tract and are found in the
highest number (>70%) in the small intestine, with a frequency
decreasing to around 40% in the large intestine. Despite
representing only a small minority (<1%) of dispersed cells
within the large epithelial cell population, overall EECs
constitute one of the largest endocrine systems in the body. EECs
alone and in bidirectional interaction with the enteric nervous
system have important roles in gut motility, intestinal transit,
absorption of nutrients and energy homeostasis. Their role as an
important component in the gut-brain, gut-pancreas and
immuno-endocrine axis has initiated a new and exciting area of
endocrine research (8,9). EECs have high turnover rates of 4–6
days and are scattered throughout the entire gastrointestinal
mucosa (10). Currently, at least
15 different EEC types can be distinguished based on the
ultrastructural characteristics of their secretory granules and the
diverse number of hormones produced (11). EECs are endoderm-derived (12) and develop from the same pluripotent
stem cells as the other 3 epithelial cell lineages which include
the absorptive enterocytes, Paneth cells and goblet cells (13). Studies in transgenic mice have
identified a cascade of basic helix-loop-helix transcription
factors involved in EEC differentiation, among them neurogenin-3,
the downstream target of Math-1, which is essential for the
commitment of progenitor cells to the EEC lineage. Notch signaling
initiates lateral inhibition, thus, preventing adjacent cells to
differentiate into EECs (14,15).
Serving as general EEC markers, chromogranin A and synaptophysin
are used in diagnostic pathology to identify an
entero-/neuroendocrine cell component in intestinal tumours
(16). Different sections of the
gastrointestinal tract contain distinct EEC populations, with the
richest diversity of EECs in the small intestine. The types of EECs
found in the large intestine are less complex and lacks EEC
secreting secretin (S-cells), gastric inhibitory polypeptide,
cholecystokinin, motilin (M-cells) and neurotensin (N-cells)
(11,17).
Clinical and animal data show an involvement of EEC
populations in gastrointestinal disorders stretching from
inflammatory bowel disease (IBD) to neuroendocrine tumours (NETs;
also named carcinoids). Patients suffering from IBD have a higher
incidence of developing NETs (10,18).
Carcinoids are rare and slow-growing tumours derived from EEC
populations representing approximately 0.5% of all malignancies
(19–22). Even small-sized NETs can display
unexpectedly high aggressiveness in the metastasis rate and these
patients have a significantly reduced 5-year survival rate
(20,23,24).
In the present study, we demonstrated the tissue
localization of human INSL5 and its cognate receptor RXFP4 in the
normal large intestinal tract, thus, providing first evidence for a
potential autocrine/paracrine signaling role of the INSL5-RXFP4
ligand receptor in the human large intestine and NET/carcinoid
tumours.
Materials and methods
Human tissues
Formalin-fixed human normal colorectal tissues
(n=10) embedded in paraffin blocks were cut into 5-μm sections for
histology and immunohistochemistry. Three cryopreserved invasive
carcinoid tumour tissues (n=3) derived from the stomach (male) and
rectum (female) and 1 lymph node metastasis of a carcinoid cancer
(male) composed of chromogranin A+ and cytokeratin
KL-1+ carcinoid cells of unknown primary origin were
obtained. Tissues were cryosectioned (8 μm) and processed for
immunofluorescence. All tissues were collected at the Department of
Surgery, University of Halle, Germany, by surgical resection for
clinical indications. This study was approved by the ethics
committee of the Martin Luther University, Faculty of Medicine,
Germany and all patients gave written consent.
Animal models
We used dextran sulfate sodium (DSS)-induced acute
colitis (25), a
lymphocyte-independent model, by oral administration of DSS and 2,4
dinitrobenzene-sulfonic acid (DNBS), a lymphocyte-dependent model
(26), by intracolonic
administration. Male C57BL/6 (7–9 weeks old) were purchased from
Charles River suppliers and maintained in the animal care facility
at the University of Manitoba under specific pathogen-free (SPF)
conditions. Mice were housed under standard conditions for a
minimum of 1 week before experimentation. DSS [molecular weight
(MW), 40 kDa; ICN Biomedicals, Inc., Soho, OH, USA] was added to
the drinking water at a final concentration of 5% (wt/vol) for 5
days (25,27). Controls were all time-matched and
consisted of mice that received normal drinking water only. Mean
DSS consumption was noted/cage each day. Five or 3 days after the
beginning of the DSS or DNBS treatments, the mice were sacrificed,
the abdominal cavity was opened and the colon was located.
Formalin-fixed colon segments were paraffin-embedded and 3-μm
sections were stained with hematoxylin and eosin (H&E). Colonic
damage was scored based on a published scoring system that
considers architectural derangements, goblet cell depletion,
oedema/ulceration and degree of inflammatory cell infiltrate
(28). All experiments were
approved by the University of Manitoba Animal Ethics Committee and
were conducted under the Canadian guidelines for animal research.
Insl5-deficient mice (29)
were housed under normal conditions at an ambient temperature in a
12-h light/12-h dark cycle, and mice had free access to standard
mouse chow and tap water. The collection of mouse tissues was
approved by the Institutional Animal Care and Use Committee of the
University of Göttingen.
Immunodetection in tissues
Paraffin-embedded tissue sections (5 μm) were
deparaffinized and washed in 0.1% Tween-20 in Tris-buffered saline
at pH 7.4 (TBS/T). Sections were incubated with 3% hydrogen
peroxide in methanol for 20 min in the dark to quench endogenous
peroxidase. Sections were pretreated with 20 μg/ml pepsin
(Sigma-Aldrich, St. Louis, MO, USA) in 0.01 M HCl for 10 min at
37°C before performing the antigen retrieval step by boiling tissue
sections in citrate buffer for 4 min, and incubating sections at
90°C for 35 min before cooling down to room temperature (RT) for 30
min. After washing with TBS/T, tissue sections were incubated for 1
h at RT in 10% goat normal serum (Sigma-Aldrich) in TBS/T to block
non-specific binding sites. The tissue sections were incubated with
primary antibody against INSL5 (1:100; Sigma-Aldrich),
synaptophysin (1:200; Thermo Scientific, Waltham, MA, USA) and
chromogranin A (1:100; Abcam, Cambridge, MA, USA) overnight at 4°C.
The pretreatment step was not necessary for synaptophysin and
chromogranin A. As a negative control, the primary antibody was
replaced with rabbit isotype IgG1 control at the same
concentration. After washing with TBS/T, the tissue sections were
incubated with 1:200 biotinylated anti-rabbit IgG (Vector
Laboratories, Burlingame, CA, USA) for 1 h at RT, followed by a
30-min incubation with streptavidin conjugated to horseradish
peroxidase (Vectastain Elite ABC kit; Vector Laboratories).
Specific immunostain was developed with DAB substrate (Thermo
Scientific). Tissue sections were counterstained with hematoxylin
and embedded prior to bright field imaging with a Zeiss A2
microscope (Zeiss, Jena, Germany).
Cryosections (8 μm) of human colon and carcinoid
tissues were fixed in 3.7% paraformaldehyde in phosphate-buffered
saline (PBS) for 20 min at RT, washed twice in PBS for 10 min and
treated for 1 min with permeabilization buffer (1.7 mM EGTA, 5 mM
PIPES, 1% Triton X-100 pH 6,7). Tissues were incubated with the
primary antibody against INSL5 (1:50) in Dako S2022 blocking buffer
overnight at 4°C. For the detection of INSL5, a goat anti-rabbit
secondary antiserum conjugated to TRITC (1:100; Dako, Hamburg,
Germany) was incubated for 1 h prior to 3×10 min washes in PBS. For
the co-localization of INSL5 and synaptophysin, we performed double
immunofluorescence and used a rhodamine-labeled synaptophysin
antiserum (1:50; Acris, San Diego, CA, USA). Primary antibodies
were replaced with rabbit isotype IgG1 control to serve as the
negative control. Nuclei were stained for 2 min with Hoechst stain
at 1:100 and washed twice in PBS (Sigma-Aldrich). Stained sections
were embedded in fluorescent mounting medium (Dako S3023) and
viewed with a Zeiss Axioplan 2 fluorescence microscope. Images were
captured with an AxioCam Zeiss digital camera at ×40 magnification.
The same buffer conditions were used for the fluorescence detection
of RXFP4. A goat anti-GPR100/RXFP4 antibody (ab79155; Abcam) was
used at 1:500, and specific binding was detected with a goat
anti-rabbit secondary antiserum conjugated to TRITC (1:100;
Dako).
For co-localization of mouse Insl5 and
synaptophysin, mouse tissue sections of the descending colon and
rectum (5 μm) were incubated overnight at 4°C with a primary rabbit
antiserum against mouse Insl5 (1:200; Sigma-Aldrich) and a sheep
antiserum against synaptophysin (ab72242, 1:100; Abcam). As a
negative control, the primary antisera were replaced with a
species-specific IgG isotype control at the same concentration
(Abcam). After washing, tissue sections were incubated with 1:200
FITC-labeled anti-rabbit IgG and rhodamine-labeled anti-sheep IgG
(Vector Laboratories) for 1 h at RT. Specific immunostaining was
detected by immunofluorescence imaging with a Zeiss A2 microscope
(Zeiss).
Results
INSL5 and RXFP4 in the normal colorectal
epithelium
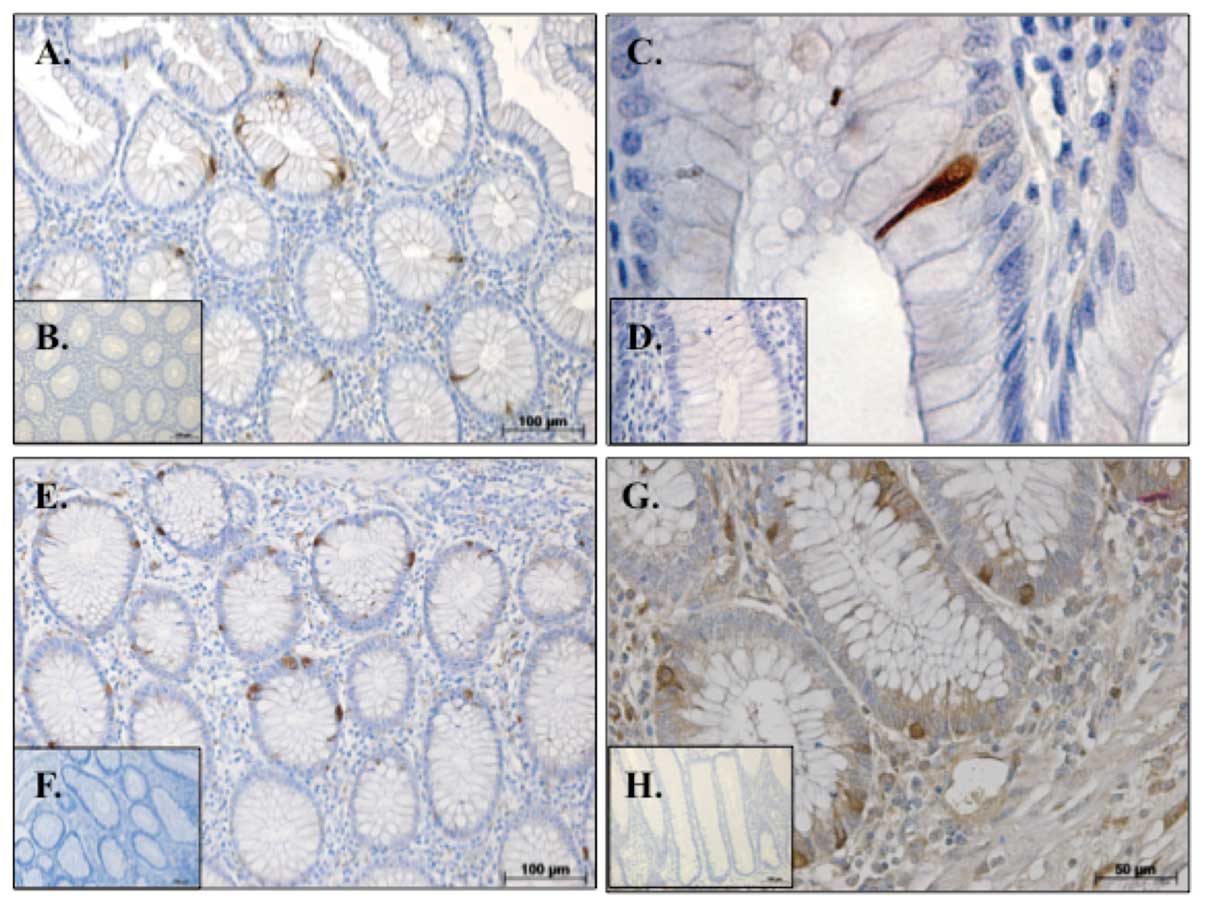
We observed the presence of individual INSL5
immunoreactive cells within the mucosa of normal human colorectal
tissue (Fig. 1A and B). To further
characterize these INSL5+ intra-epithelial cells, we
immunostained serial sections for the EEC markers synaptophysin and
chromogranin A. Intra-epithelial cells of similar appearance to the
INSL5+ cells were also immunopositive for synaptophysin
(Fig. 1E) and chromogranin A
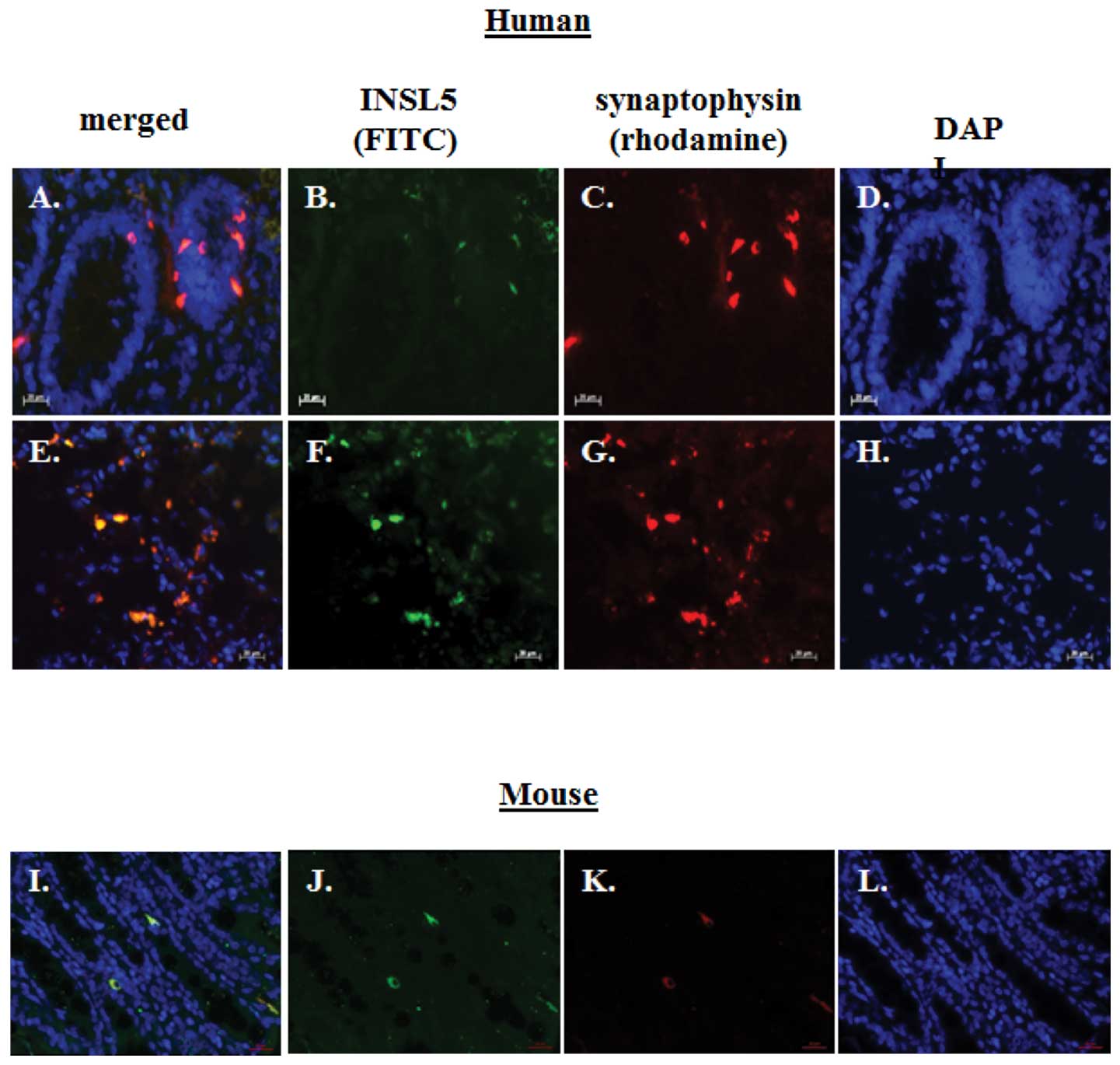
(Fig. 1G). Double immunofluorescent
staining on cryo-sectioned human normal colon tissues demonstrated
the co-localization of INSL5 and synaptophysin within the same
intra-epithelial cells (Fig. 2A–D).
We aimed to ascertain whether the expression of INSL5 was required
for the presence of synaptophysin+ EECs within the large
intestine. Employing double immunofluorescent staining on
cryosections of normal mouse colon tissues revealed the
co-localization of Insl5 and synaptophysin in EECs (Fig. 2I–L). We also employed a newly
derived mouse model in which we deleted the Insl5 gene
resulting in a complete loss of Insl5 expression (RNA and
protein) in mouse tissues, including colorectal tissues (29). In agreement with our recent mouse
data, we failed to detect the Insl5 peptide but did observe
immunoreactive synaptophysin in mouse colonic EECs of
Insl5-deficient mice indicating that in our mouse model the
Insl5 peptide is not an essential requirement for the formation of
synaptophysin+ EECs in colorectal tissues (29). We were unable to co-localize INSL5
and chromogranin A since the available primary antisera had been
generated in the same species and precluded the specific detection
of both markers with different sets of labelled secondary
antisera.
Insl5 in a mouse model of inflammatory
bowel disease (IBD)
IBD, consisting of Crohn’s disease (CD) and
ulcerative colitis (UC), are characterized by a chronic relapsing
and remitting course as a result of intestinal inflammation
(30). Mucosal changes in IBD as
characterized by ulcerative or infection-induced lesions are
accompanied by an alteration of EECs (31–33).
In the experimental acute models of colitis induced by DNBS and DSS
mimicking CD and UC, respectively, EC hyperplasia has been
described. We employed both mouse models to study the early dynamic
changes provoked by induced inflammatory responses on the
expression of INSL5 in EECs in the large intestine. Histological
analysis of the large intestine revealed the frequent presence of
submucosal inflammatory sites in both IBD models and we detected
the presence of immunoreactive INSL5 and synaptophysin in the
overlaying epithelial cells. However, we failed to observe
differences in either the frequency and/or staining intensity of
EECs at these inflammatory sites when compared to the adjacent
colon without obvious signs of inflammation or colon tissues of
untreated mice (Fig. 1A and C).
This suggested that during the acute phase of inflammation there
was no obvious change in the number of
INSL5+/synaptophysin+ EECs within the mucosal
layer.
INSL5 and RXFP4 in human
neuroendocrine/carcinoid tumours
Members of the INSL-like family of peptides and
their receptors have specific functions in cancer (34). Immunofluorescent analysis revealed
the presence of INSL5+/synaptophysin+ cells
in normal colon tissue (Fig. 2A-D)
and both markers also were co-localized in cells of
neuroendocrine/carcinoid tumours (Fig.
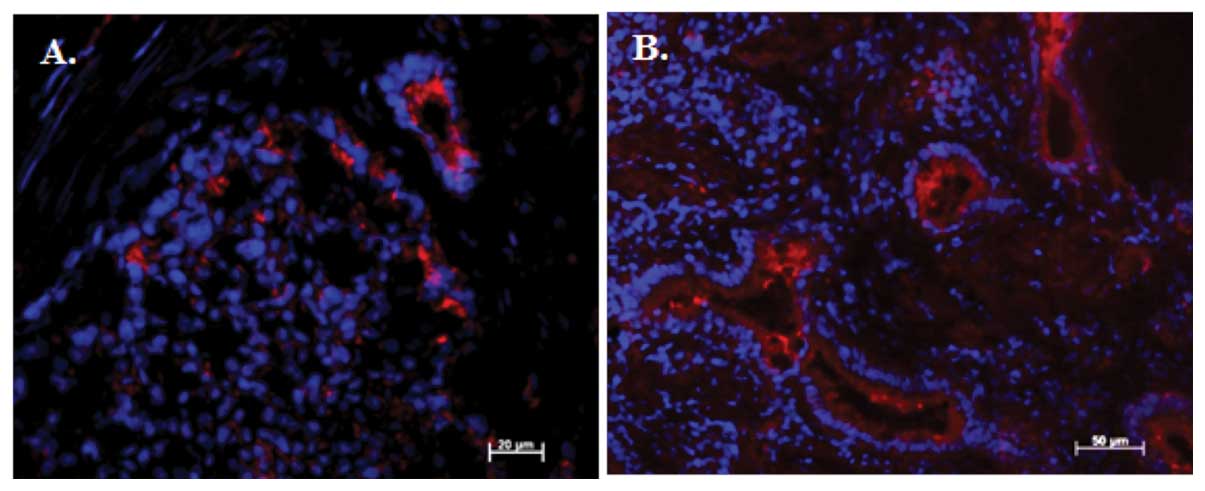
2E–H). Immunofluorescence for RXFP4+ cells was also
detected in neuroendocrine/carcinoid tumours (Fig. 3A) and normal colon tissue (Fig. 3B). Secreted INSL5 can bind with high
affinity to its G protein-coupled receptor RXFP4 (3) and the proximity of INSL5-expressing
EECs and RXFP4+ colonocytes suggest the presence of a
novel autocrine/paracrine INSL5-RXFP4 ligand receptor system within
the mucosa of the human large intestine and in NETs.
Discussion
INSL5 is among the most recently identified member
of the insulin-like peptides and has been shown to bind with high
affinity to the class-A neuropeptide-like Gi/o
protein-coupled receptor RXFP4/GPCR-142 (2–4).
Transcripts for both INSL5 and RXFP4 have been detected in the
human colon, and increasing copy numbers for INSL5 were reported in
the mid- and distal-colon (2,3). In
the present study we identified human INSL5 as a novel marker of an
EEC population within the mucosa of the normal colon. We recently
employed a novel Insl5-deficient mouse model and showed a
depletion of these Insl5-expressing mucosal EECs without apparent
alterations to the remaining colon mucosa in these mice (29). Our data confirm and extend a
previous report on the high lacZ activity in discrete EEC
populations present exclusively in the colorectal mucosa of a mouse
model that had the Insl5 gene replaced by an in vivo lacZ
reporter system (35). The human
large intestine contains EECs specifically staining for
synaptophysin, a major integral glycoprotein and component of small
synaptic-like microvesicles (SLMV) that is also present in
chromaffin cells of the adrenal medulla, endocrine cells of the
pancreas and neuroendocrine cells of carcinoid tumours (16,36–38).
Here we demonstrated by immunofluorescent imaging the co-expression
of INSL5 in EECs immunopositive for synaptophysin. The EEC lineage
derives from the same pluripotent stem cells, similar to the other
3 lineages of the intestinal epithelium: absorptive enterocytes,
goblet and Paneth cells (13). To
determine whether the presence of INSL5 is essential for EEC
development in the large intestine, we utilized mice with a
deletion of the Insl5 gene (29). Despite the lack of Insl5 and similar
to wild-type mice, the colonic mucosa of Insl5-deficient
mice contained synaptophysin+ EECs. Thus, we concluded
that Insl5 was not essential for the development of
synaptophysin+ EECs or the development of other colonic
epithelial cell types. Intriguingly, INSL5+ EECs were
embedded within an epithelium largely composed of colonocytes
immunopositive for the INSL5 receptor RXFP4, suggesting a potential
intraepithelial autocrine/paracrine biological role for the
INSL5-RXFP4 ligand-receptor system in the colon. Little is known
about the biological roles of either INSL5 or its receptor RXFP4.
However, reports on the presence of both INSL5 and RXFP4 in
multiple peripheral tissues including the colon, placenta, heart,
spleen, brain, prostate, kidney, bone marrow and liver implicate
the involvement of this ligand receptor system in a broad spectrum
of biological functions (3,4). In vitro experiments revealed
that INSL5 is capable of activating RXFP4 at EC50 values
as low as 1.2 nM and this resulted in Ca2+ mobilization
in HEK-293 cells expressing RXFP4 and Gα16 protein (3). Insl5-deficient mice displayed
impaired fertility in males and females as a result of a marked
reduction in sperm motility and alterations in the estrus cycle.
Intriguingly, Insl5-deficient mice also had impaired glucose
homeostasis and elevated serum glucose levels in aging mice
(29). This glucose intolerance
resulted from reduced insulin secretion and coincided with markedly
reduced average islet size and β-cell mass in
Insl5-deficient mice. This suggests a novel signaling role
of the Insl5-RXFP4 system by affecting the balance of pancreatic
α-cells to β-cells in favor of the former cell type, resulting in
altered regulation of insulin secretion and β-cell homeostasis. It
is clear from these data that the INSL5-RXFP4 pathway is emerging
as a novel autocrine/paracrine mucosal and systemic regulatory
signaling system with intriguing potential to impact on important
physiological/metabolic functions within the neuro-gastrointestinal
endocrine network.
The large intestine is a site of inflammatory bowel
disease (IBD), including Crohn’s disease (CD) and ulcerative
colitis (UC) and is associated with changes in gut sensation,
motility and secretion. Patients suffering from IBD also have a
higher risk of developing neuroendocrine tumours (NETs) (18). IBD is associated with alterations in
the number of EECs, and complete lack of mucosal EECs
(anendocrinosis) as a result of a point mutation in Ngn3, the key
transcription factor for enteroendocrine lineage differentiation,
causes life-threatening malabsorptive congenital diarrhea syndrome
(39–41). Increased levels of EECs have been
reported in human Crohn’s ileitis (42) and in animal models of colitis
(43–45). To address whether INSL5+
EECs may be affected by acute IBD, we chose 2 mouse models of
dextran sodium sulphate (DSS)- and dinitrobenzenesulphonic acid
(DNBS)-induced colitis. DSS induced IBD symptoms resembling UC and
DNBS induced Crohn’s-like pathology 5 days after the application of
these compounds. The presence of mucosal INSL5+ EECs was
confirmed at inflammatory sites but their number remained unchanged
when compared to normal colon in the untreated animals. Thus, the
acute phase of DSS- and DNBS-induced IBD did not coincide with
changes in the number of Insl5+ EECs at the inflammatory
sites. The Insl5-immunopositive EECs behaved similar to
enteroendocrine cell populations reported to be immunopositive for
cholecystokinin, glucagon-like peptide 2, glucose-dependent
insulinotropic peptide and peptide YY which also showed no
significant numerical change in experimental colitis (45). This is in contrast to increased
numbers of neurotensin, somatostatin and serotonin immunopositive
EECs observed in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced
colitis in guinea pigs and DSS- and DNBS-induced colitis in mice
(45,46). Additional studies will reveal
whether the number of Insl5-immunopositive EECs is affected during
the chronic phase of IBD.
Here, we provide the first report on the presence of
human INSL5 and RXFP4 in carcinoid tumours. All
neuroendocrine/carcinoid tumours showed aggressive invasive
behavior and were immunopositive for the neuroendocrine markers
synaptophysin and/or chromogranin A. Carcinoid cells co-expressed
immunopositive INSL5 and synaptophysin and the presence of RXFP4
suggests a possible autocrine/paracrine INSL5-RXFP4 signaling
system active within human carcinoid tissues. Recent evidence from
relaxin 2, INSL3 and their receptors RXFP1 and RXFP2, respectively,
have shown that members of the relaxin-like peptide family engage
in similar local signaling systems to promote growth, survival and
migration/invasion of tumour cells (34,47–49).
The characterization of essential structural and signaling
parameters of the INSL5-RXFP4 interaction (50,51)
may provide the basis to explore molecular mechanisms engaged by
the INSL5-RXFP4 system in cancer.
Acknowledgements
The authors are grateful to Ms. Maike Bossert for
her expert assistance. C.H.V. and T.K. thank the German Research
Council (DFG HO 1813/12-1) for financial support. S.H.K. and T.K.
are grateful to their respective Natural Sciences and Engineering
Council of Canada (NSERC) for financial support. Dr Del Bigio holds
the Canada Research Chair in Developmental Neuropathology. J.E.G.
was supported by the Manitoba Health Research Council (MHRC).
References
|
1
|
Wilkinson TN, Speed TP, Tregear GW and
Bathgate RA: Evolution of the relaxin-like peptide family. BMC Evol
Biol. 5:142005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Conklin D, Lofton-Day CE, Haldeman BA, et
al: Identification of INSL5, a new member of the insulin
superfamily. Genomics. 60:50–56. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu C, Kuei C, Sutton S, et al: INSL5 is a
high affinity specific agonist for GPCR142 (GPR100). J Biol Chem.
280:292–300. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu C and Lovenberg TW: Relaxin-3, INSL5,
and their receptors. Results Probl Cell Differ. 46:213–237. 2008.
View Article : Google Scholar
|
|
5
|
Liu C, Chen J, Kuei C, et al:
Relaxin-3/insulin-like peptide 5 chimeric peptide, a selective
ligand for G protein-coupled receptor (GPCR)135 and GPCR142 over
leucine-rich repeat-containing G protein-coupled receptor 7. Mol
Pharmacol. 67:231–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen J, Kuei C, Sutton SW, et al:
Pharmacological characterization of relaxin-3/INSL7 receptors
GPCR135 and GPCR142 from different mammalian species. J Pharmacol
Exp Ther. 312:83–95. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu C, Eriste E, Sutton S, et al:
Identification of relaxin-3/INSL7 as an endogenous ligand for the
orphan G-protein-coupled receptor GPCR135. J Biol Chem.
278:50754–50764. 2003. View Article : Google Scholar
|
|
8
|
Field BC, Chaudhri OB and Bloom SR: Bowels
control brain: gut hormones and obesity. Nat Rev Endocrinol.
6:444–453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khan WI and Ghia JE: Gut hormones:
emerging role in immune activation and inflammation. Clin Exp
Immunol. 161:19–27. 2010.PubMed/NCBI
|
|
10
|
Moran GW, Leslie FC, Levison SE,
Worthington J and McLaughlin JT: Enteroendocrine cells: neglected
players in gastrointestinal disorders? Therap Adv Gastroenterol.
1:51–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rindi G, Leiter AB, Kopin AS, Bordi C and
Solcia E: The ‘normal’ endocrine cell of the gut: changing concepts
and new evidences. Ann NY Acad Sci. 1014:1–12. 2004.
|
|
12
|
Andrew A, Kramer B and Rawdon BB: The
origin of gut and pancreatic neuroendocrine (APUD) cells - the last
word? J Pathol. 186:117–118. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gordon JI: Understanding gastrointestinal
epithelial cell biology: lessons from mice with help from worms and
flies. Gastroenterology. 105:315–324. 1993.PubMed/NCBI
|
|
14
|
Schonhoff SE, Giel-Moloney M and Leiter
AB: Minireview: Development and differentiation of gut endocrine
cells. Endocrinology. 145:2639–2644. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
May CL and Kaestner KH: Gut endocrine cell
development. Mol Cell Endocrinol. 323:70–75. 2010. View Article : Google Scholar
|
|
16
|
Wiedenmann B, Franke WW, Kuhn C, Moll R
and Gould VE: Synaptophysin: a marker protein for neuroendocrine
cells and neoplasms. Proc Natl Acad Sci USA. 83:3500–3504. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gunawardene AR, Corfe BM and Staton CA:
Classification and functions of enteroendocrine cells of the lower
gastrointestinal tract. Int J Exp Pathol. 92:219–231. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
West NE, Wise PE, Herline AJ, Muldoon RL,
Chopp WV and Schwartz DA: Carcinoid tumors are 15 times more common
in patients with Crohn’s disease. Inflamm Bowel Dis. 13:1129–1134.
2007.PubMed/NCBI
|
|
19
|
Kang H, O’Connell JB, Leonardi MJ, Maggard
MA, McGory ML and Ko CY: Rare tumors of the colon and rectum: a
national review. Int J Colorectal Dis. 22:183–189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soga J: Carcinoids and their variant
endocrinomas. An analysis of 11842 reported cases. J Exp Clin
Cancer Res. 22:517–530. 2003.PubMed/NCBI
|
|
21
|
Modlin IM and Sandor A: An analysis of
8305 cases of carcinoid tumors. Cancer. 79:813–829. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Modlin IM, Lye KD and Kidd M: A 5-decade
analysis of 13,715 carcinoid tumors. Cancer. 97:934–959. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soga J: Early-stage carcinoids of the
gastrointestinal tract: an analysis of 1914 reported cases. Cancer.
103:1587–1595. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grabowski P, Schonfelder J, Ahnert-Hilger
G, et al: Heterogeneous expression of neuroendocrine marker
proteins in human undifferentiated carcinoma of the colon and
rectum. Ann NY Acad Sci. 1014:270–274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okayasu I, Hatakeyama S, Yamada M, Ohkusa
T, Inagaki Y and Nakaya R: A novel method in the induction of
reliable experimental acute and chronic ulcerative colitis in mice.
Gastroenterology. 98:694–702. 1990.PubMed/NCBI
|
|
26
|
Sturiale S, Barbara G, Qiu B, et al:
Neutral endopeptidase (EC 3.4.24.11) terminates colitis by
degrading substance P. Proc Natl Acad Sci USA. 96:11653–11658.
1999. View Article : Google Scholar
|
|
27
|
Ghia JE, Blennerhassett P, Deng Y, Verdu
EF, Khan WI and Collins SM: Reactivation of inflammatory bowel
disease in a mouse model of depression. Gastroenterology.
136:2280–2288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
29
|
Burnick-Turek O, Mohamed BA, Shirneshan K,
et al: INSL5-deficient mice display an alteration in glucose
homeostasis and impaired fertility. Endocrinology. 153:4655–4665.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bernstein CN, Papineau N, Zajaczkowski J,
Rawsthorne P, Okrusko G and Blanchard JF: Direct hospital costs for
patients with inflammatory bowel disease in a Canadian tertiary
care university hospital. Am J Gastroenterol. 95:677–683. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
El-Salhy M, Danielsson A, Stenling R and
Grimelius L: Colonic endocrine cells in inflammatory bowel disease.
J Intern Med. 242:413–419. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Steeds J, Motomura Y, et al:
CD4+ T cell-mediated immunological control of
enterochromaffin cell hyperplasia and 5-hydroxytryptamine
production in enteric infection. Gut. 56:949–957. 2007.
|
|
33
|
Ahonen A, Kyosola K and Penttila O:
Enterochromaffin cells in macrophages in ulcerative colitis and
irritable colon. Ann Clin Res. 8:1–7. 1976.PubMed/NCBI
|
|
34
|
Klonisch T, Bialek J, Radestock Y,
Hoang-Vu C and Hombach-Klonisch S: Relaxin-like ligand-receptor
systems are autocrine/paracrine effectors in tumor cells and
modulate cancer progression and tissue invasiveness. Adv Exp Med
Biol. 612:104–118. 2007. View Article : Google Scholar
|
|
35
|
Jaspers S, Lok S, Lofton-Day CE, et al:
The Genomics of Insulin 5. Kluwer Academic Publishers; Dordrecht:
2001
|
|
36
|
Jahn R, Schiebler W, Ouimet C and
Greengard P: A 38,000-dalton membrane protein (p38) present in
synaptic vesicles. Proc Natl Acad Sci USA. 82:4137–4141. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gould VE, Lee I, Wiedenmann B, Moll R,
Chejfec G and Franke WW: Synaptophysin: a novel marker for neurons,
certain neuroendocrine cells, and their neoplasms. Hum Pathol.
17:979–983. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Buffa R, Rindi G, Sessa F, et al:
Synaptophysin immunoreactivity and small clear vesicles in
neuroendocrine cells and related tumours. Mol Cell Probes.
1:367–381. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bjerknes M and Cheng H: Neurogenin 3 and
the enteroendocrine cell lineage in the adult mouse small
intestinal epithelium. Dev Biol. 300:722–735. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cortina G, Smart CN, Farmer DG, et al:
Enteroendocrine cell dysgenesis and malabsorption, a
histopathologic and immunohistochemical characterization. Hum
Pathol. 38:570–580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Skipper M and Lewis J: Getting to the guts
of enteroendocrine differentiation. Nat Genet. 24:3–4. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bishop AE, Pietroletti R, Taat CW,
Brummelkamp WH and Polak JM: Increased populations of endocrine
cells in Crohn’s ileitis. Virchows Arch A Pathol Anat Histopathol.
410:391–396. 1987.PubMed/NCBI
|
|
43
|
Linden DR, Chen JX, Gershon MD, Sharkey KA
and Mawe GM: Serotonin availability is increased in mucosa of
guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest
Liver Physiol. 285:G207–G216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oshima S, Fujimura M and Fukimiya M:
Changes in number of serotonin-containing cells and serotonin
levels in the intestinal mucosa of rats with colitis induced by
dextran sodium sulfate. Histochem Cell Biol. 112:257–263. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
O’Hara JR, Ho W, Linden DR, Mawe GM and
Sharkey KA: Enteroendocrine cells and 5-HT availability are altered
in mucosa of guinea pigs with TNBS ileitis. Am J Physiol
Gastrointest Liver Physiol. 287:G998–G1007. 2004.PubMed/NCBI
|
|
46
|
Ghia JE, Li N, Wang H, et al: Serotonin
has a key role in pathogenesis of experimental colitis.
Gastroenterology. 137:1649–1660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Feng S, Agoulnik IU, Truong A, et al:
Suppression of relaxin receptor RXFP1 decreases prostate cancer
growth and metastasis. Endocr Relat Cancer. 17:1021–1033. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vinall RL, Mahaffey CM, Davis RR, et al:
Dual blockade of PKA and NF-κB inhibits H2 relaxin-mediated
castrate-resistant growth of prostate cancer sublines and induces
apoptosis. Horm Cancer. 2:224–238. 2011.
|
|
49
|
Hombach-Klonisch S, Bialek J, Radestock Y,
et al: INSL3 has tumor-promoting activity in thyroid cancer. Int J
Cancer. 127:521–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Akhter Hossain M, Bathgate RA, Kong CK, et
al: Synthesis, conformation, and activity of human insulin-like
peptide 5 (INSL5). Chembiochem. 9:1816–1822. 2008.PubMed/NCBI
|
|
51
|
Belgi A, Hossain MA, Shabanpoor F, et al:
Structure and function relationship of murine insulin-like peptide
5 (INSL5): free C-terminus is essential for RXFP4 receptor binding
and activation. Biochemistry. 50:8352–8361. 2011. View Article : Google Scholar : PubMed/NCBI
|