Introduction
Head and neck squamous cell carcinoma (HNSCC) is a
malignancy with a high rate of recurrence, largely because it is
usually diagnosed at a late stage. Tobacco and alcohol exposure are
the main risk factors and account for ~85% of HNSCC cases (1). Despite advances in surgical and
non-surgical therapy, the mortality rate from this disease has
remained constant over the last few years, mainly due to the
development of therapy-resistant local and regional recurrences.
Antineoplastic treatments such as chemotherapy or radiation can
efficiently eradicate a majority of proliferating malignant cells
within malignant tumors. However, there is increasing evidence that
there is a subpopulation of resistant tumor cells that cannot be
eradicated by these regimens. These cancer stem cells (CSCs) have
distinct features of somatic stem cells such as self-renewal,
extensive proliferation and differentiation. Therefore, these cells
are essential and responsible for initiation, but also maintenance
and recurrence of malignant disease. In recent years, the CSC
hypothesis has been coined for HNSCC as well (2,3).
Prince et al(2) showed that
CD44+ cancer cells, which typically comprise <10% of
the cells in an HNSCC tumor, but not CD44− cancer cells,
give rise to new tumors in vivo. Since then,
CD44+ cells in tumors of the head and neck are referred
to as CSCs of HNSCC.
CD44 is an integral cell membrane glycoprotein and
it comprises different isoforms that arise from alternative
splicing of a region of variable exons. They differ in the primary
amino acid sequence as well as the amount of N- and O-glycosylation
(4), thereby its apparent molecular
mass ranges from 85 to 250 kDa (5).
At least 20 variants of CD44 have been reported due to the
alternative splicing of 10 exons that encode the membrane’s
proximal portion of the extracellular domain (6–8).
Originally, it was described as a receptor on circulating
lymphocytes involved in cell homing, adhesion and migration
(9,10).
In 1991 Günthert et al(11) showed that the expression of CD44
conferred metastatic potential to a non-metastatic cell line in a
rat carcinoma model (12). Since
then, several analyses have indicated that there is a correlation
between the expression of CD44 and progression, metastasis and
prognosis of malignant disease. This has also been shown in
different types of epithelial carcinoma, in addition to HNSCC, such
as colorectal carcinoma (13,14),
breast cancer (15) and certain
types of gastric carcinoma (5,16). The
in-depth analysis of expression markers such as CD44 in tissue
samples of HNSCC patients may reveal their role as potential
prognostic biomarkers or therapeutic targets, e.g., for
antigen-directed immunotherapy.
The analysis of the so-called CSC niche theory may
provide information on cell trafficking and underlying mechanisms,
such as tumor expansion and metastatic progression. The interaction
between stromal cell-derived factor-1α (SDF-1α) and its receptor
CXCR4 may play an important role in this field. SDF-1α is a
multifunctional cytokine that is constitutively expressed and
secreted by several types of tissues, including endothelium and
stromal cells (17–19). It has a single open reading frame of
282 nucleotides encoding a polypeptide of 93 amino acids. SDF-1
consists of two forms, SDF-1α (amino acids 24-88) and SDF-1β (amino
acids 24-93), by alternative splicing (18,20,21).
SDF-1α is the only proven chemoattractant for primitive
hematopoietic progenitor cells (HPCs), to date (18,22–24).
Accordingly, SDF-1α is considered to be one of the key regulators
for HPC trafficking between the peripheral circulation and the bone
marrow (25). Faber et
al(18) and others demonstrated
that SDF-1α induces polarization and formation of podia of HPCs and
leukemic cells (26), two
properties that represent prerequisites for directed locomotion.
SDF-1α alone showed a moderate effect on cell proliferation in
CD34+ cells (18,27),
and its effect on survival or apoptosis of HPCs remains
controversial (18,27–29).
Furthermore the SDF-1-CXCR4 axis plays a crucial role in the
regulation of cell homing and adhesion to the supportive cellular
microenvironment in the hematopoietic stem cell niche (30).
The receptor for SDF-1α has been identified as the
7-transmembrane receptor CXCR4. SDF-1α/CXCR4 interaction was
reported to play an important role during embryonic development,
especially in hematopoiesis, vascular development and
cardiogenesis. CXCR4 expression on bone marrow endothelial cells is
important for internalization of circulating SDF-1α, resulting in
its translocation into the bone marrow (17). CXCR4 is also expressed on primitive
CD34+ HPCs (27). Signal
transduction pathways initiated by the binding of SDF-1α to CXCR4
are not fully understood. Mechanisms involved in CXCR4 signaling
include Gi-protein-mediated activation of PI3K and the
phospholipase C cascade (24,31).
The function of SDF-1α can be mimicked by small peptide agonists
(18,32). Such molecules have several
advantages compared to the natural one such as the ease of
manufacturing. Interference of the signal transduction pathways
followed by the SDF-1-CXCR4 axis by these agonists/antagonists
could open new possibilities for therapeutic intervention.
Here, we monitored the effects of SDF-1α on
polarization, migration and proliferation of the CD44+
CXCR4+ HNSCC cell line UM-SCC 11A.
Materials and methods
Cell line and cell culture
The HNSCC cell line 11A (UM-SCC 11A) was obtained
from Dr T.E. Carey (University of Michigan, Ann Arbor, MI, USA). It
originated from a primary human HNSCC of the larynx of a male
patient without prior treatment (33).
Cell cultures were carried out at 37°C in a 5%
CO2 fully humidified atmosphere using Dulbecco’s
modified minimum essential medium (DMEM) (Fisher Scientific Co.,
Pittsburgh, PA, USA) supplemented with 10% fetal calf serum (FCS)
and antibiotics (Life Technologies, Inc., Gaithersburg, MD,
USA).
Immunofluorescence labeling
To detect the expression of CD44 and CXCR4 in UM-SCC
11A, cells were incubated with CD44−/CXCR4−
antibody (mouse monoclonal, 1:100; Abcam, Cambridge, UK) for 1 h at
37°C followed by incubation with a second biotinylated antibody
(anti-mouse, 1:100) for 30 min. After further washing steps with
PBS, cells were treated with streptavidin-Cy3
(1:1,000)/streptavidin-Alexia 488 (1:500) for 30 min at room
temperature. Finally, cells were covered in FluorSave™ reagent and
dried to be evaluated by fluorescence microscopy. Cell nuclei were
stained with DAPI.
Proliferation assay
Proliferation of HNSCC cells was measured by the
Alamar Blue® (Invitrogen, Darmstadt, Germany)
proliferation assay. Proliferation was measured on Days 1, 2, 3 and
4 by measurement of the fluorescence at a wavelength of 540 nm
(exitation) and 590 nm (emission). Absorbance was monitored at 590
nm. Three independent experiments were performed (n=3).
Microscopy
Analysis of cell morphology under the influence of
SDF-1α was carried out as follows. CD44+ HNSCC cells
were seeded in DMEM (Fisher Scientific Co.) supplemented with 10%
FCS and antibiotics and then incubated with SDF-1α (0, 10, 100 and
500 ng/ml) for 24 h.
Cell morphology was assessed via light microscopy.
At least 5 fields of view in each well were evaluated in each of 3
(n=minimum 3) independent experiments.
The ratio of polarized cells with filopodia or a
prominent uropod compared to round cells was determined for the
different concentrations of SDF-1α (10, 100 and 500 ng/ml).
Migration assay
Chemotaxis was assessed by an in vitro
2-chamber Transwell-assay. Different concentrations of SDF-1α (0,
10, 100 and 500 ng/ml) were added to the lower section of a
Transwell chamber (8.0-μm pore size, 6.5-mm diameter inserts;
Costar Inc.). Equal cell numbers of UM-SCC 11A were seeded in the
upper chamber in medium without SDF-1α. After 24 h, the Transwells
were removed, and the number of cells that had migrated through the
micropores was calculated. As the cell line used (UM-SCC 11A) is
adherent, cells turned out to migrate through the pores and stick
to the bottom of the Transwell membrane. The width of the cell ring
measured in nm served as a dimension for the number of cells that
migrated (n=3).
Statistical analysis
All results were plotted as means ± standard
deviation. To estimate the probability of differences, we adopted
the Student’s t-test (two-tailed distribution, 2-sample equal
variance). Probability value of P<0.05 denoted statistical
significance.
Results
Expression of CD44 and CXCR4 in the HNSCC
cell line
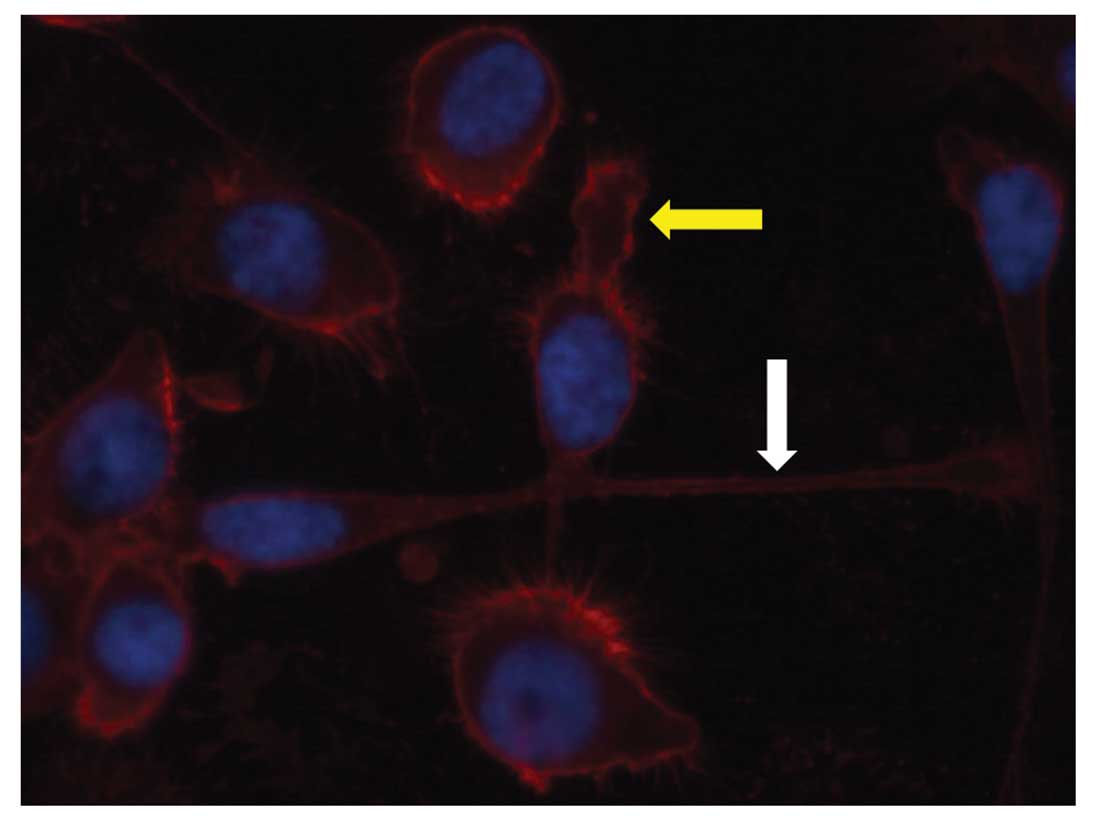
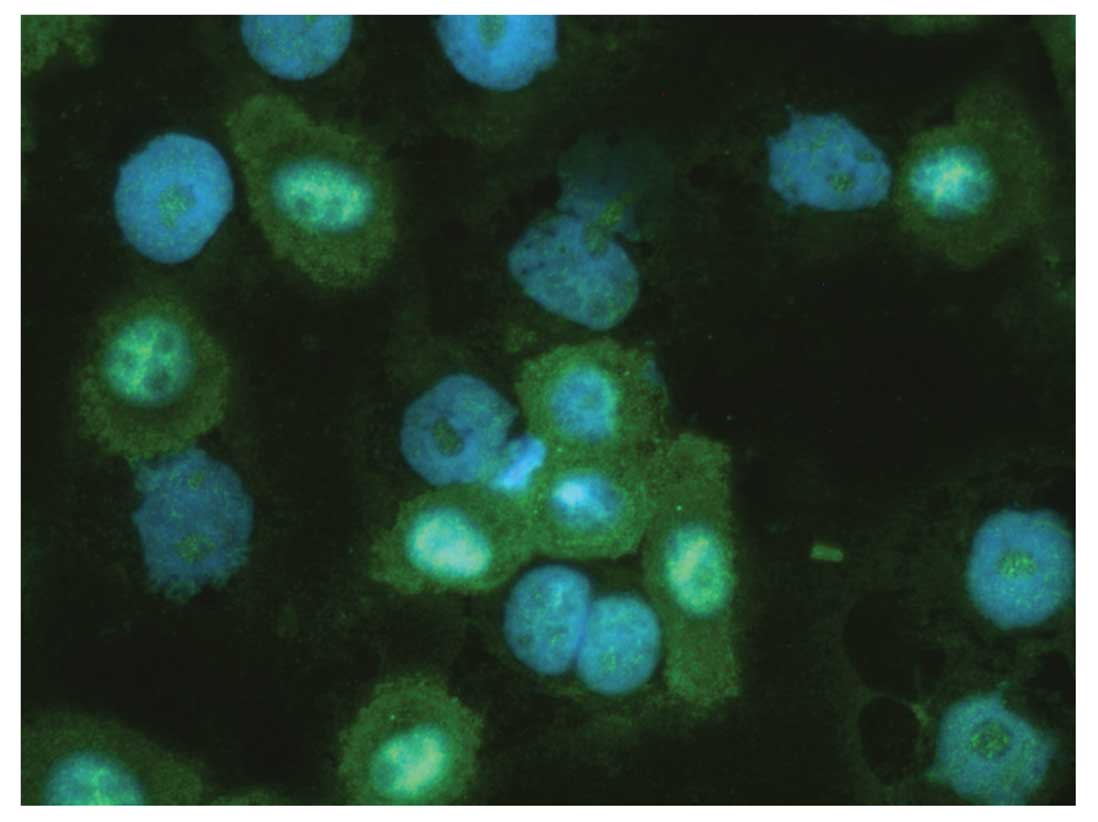
Immunofluorescence labeling of UM-SCC 11A cells was
performed. CD44 was visualized in red color by immunofluorescence
labeling via Cy3. CXCR4 was detected in green color by Alexia 488.
In all cell lines an intense red fluorescence signal of all cells
was detected by marking CD44 (Fig.
1). CD44 was mainly expressed on the cell surface in all
samples stained. Most cells were also CXCR4+. CXCR4
showed a cytoplasmatic staining pattern (Fig. 2).
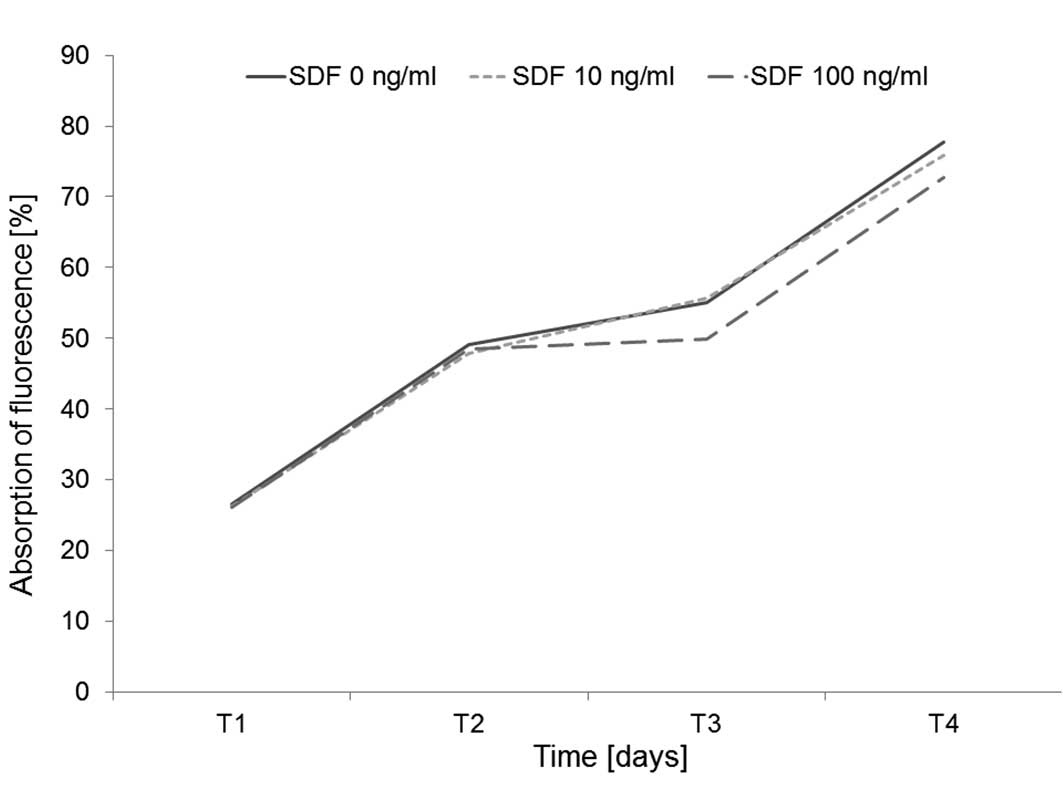
Effects on cell proliferation
CD44+ CXCR4+ UM-SCC 11A cells
were cultured at 37°C in a 5% CO2 fully humidified
atmosphere using DMEM (Fisher Scientific Co.) supplemented with 10%
FCS and antibiotics supplemented with SDF-1α in concentrations of
0, 10 or 100 ng/ml. Proliferation of HNSCC cell line under SDF-1α
was measured by the Alamar Blue proliferation assay on Days 1, 2, 3
and 4 as described above. The addition of SDF-1α did not have any
significant impact on the proliferation or viability of HNSCC cells
(Fig. 3).
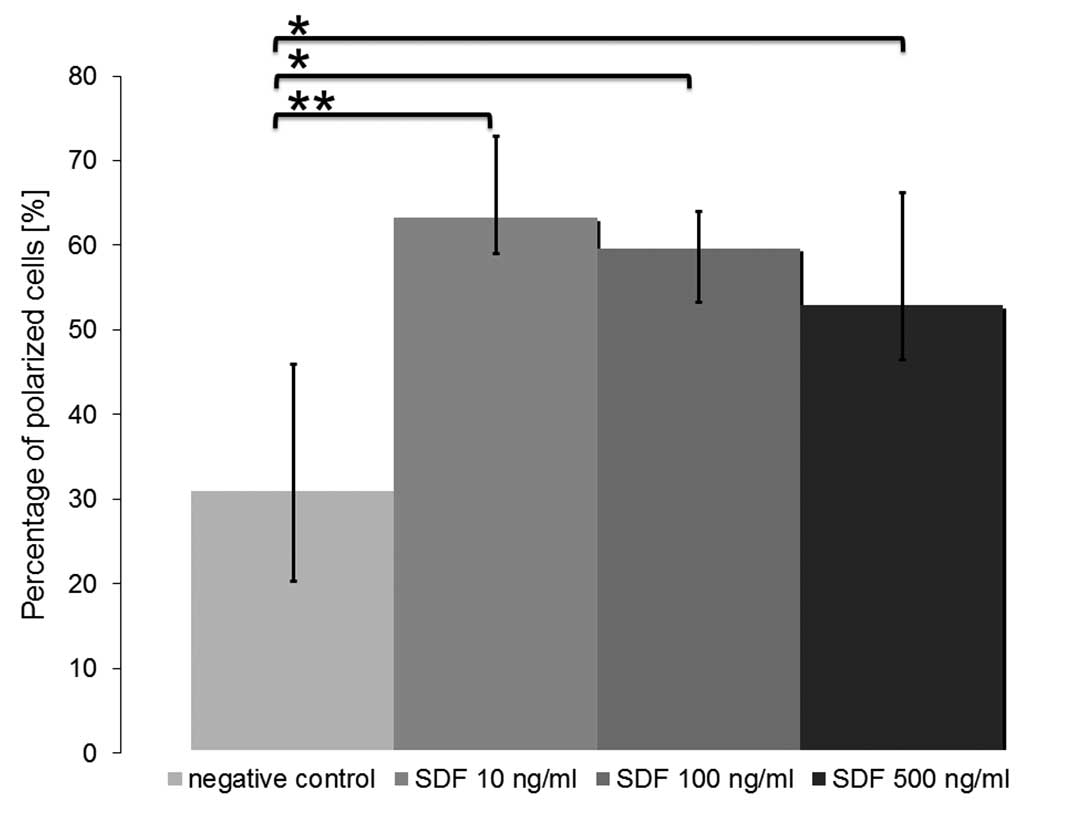
Effects on the formation of podia
Polarization and formation of podia are
prerequisites for directed locomotion of cells. We analyzed podia
formation of CD44+ CXCR4+ HNSCC cells
following a 4-h treatment with SDF-1α. In our control experiments
(0 ng/ml SDF-1α) 31.01±14.97 (10.68%) of the cells demonstrated an
elongated morphology with a prominent uropod of filopodia. The
percentage of polarized cells increased following treatment with
SDF-1α in a concentration-dependent manner (up to 63.20±9.73
(4.15%) at 10 ng/ml SDF-1α; P=5.3×10−6). Notably, the
highest increase in the formation of podia was not achieved by the
highest concentration tested (500 ng/ml SDF-1α). Thus the main
impact on HNSCC cells might be observed under the influence of
lower concentrations than those tested in our experiments. The
concentration-dependent effect on the formation of podia in UM-SCC
11A cells is shown in Fig. 4
(means: 0 ng/ml SDF-1α, 31.01±14.97 (10.68%); 10 ng/ml SDF-1α,
63.20±9.73 (4.15%), P=5.3×10−6; 100 ng/ml SDF-1α,
59.36±4.35 (6.39%), P=0.0017; 500 ng/ml SDF-1α, 52.88±13.33
(9.75%), P=0.00022).
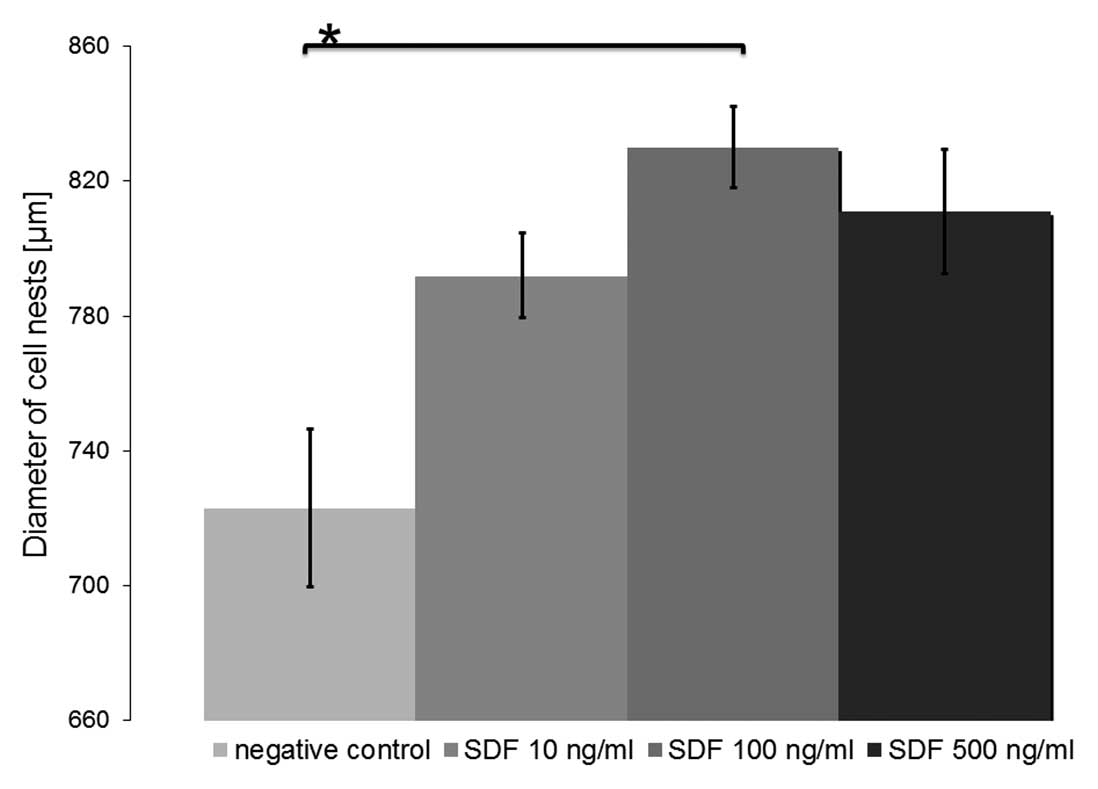
Effects on migration
Chemotaxis of CD44+ CXCR4+
UM-SCC 11A cells towards a gradient of SDF-1α was assessed in a
Transwell migration assay (Fig. 5).
The number of migrating cells increased continuously with
increasing concentrations of SDF-1α up to 100 ng/ml, but decreased
with concentrations higher than that (500 ng/ml). However,
induction of significant migration was observed under the influence
of all concentrations tested (means: 0 ng/ml SDF-1α, 734±23.5 μm;
10 ng/ml SDF-1α, 792±12.5 μm, P=0.094; 100 ng/ml SDF-1α, 830±12 μm,
P=0.027; 500 ng/ml SDF-1α, 812±18.5 μm, P=0.067).
Discussion
To examine the potential role of SDF-1α in HNSCC, we
monitored its effect on directed migration, the formation of podia
and proliferation of CD44+ CXCR4+ UM-SCC 11A.
We showed that the HNSCC cell line UM-SCC 11A may be a target for
SDF-1α by expressing CXCR4 (34)
and also shows characteristics of head and neck squamous cell
carcinoma cancer stem cells by expressing CD44 (2,35). It
should be mentioned that ‘the real cancer stem cells’ in HNSCC are
defined by a combination of membrane markers that have been found
to be characteristic for cells that have the capability to build an
entire new bulk of a tumor (36,37),
e.g., in mouse models (2). These
cells are said to have the properties of self-renewal,
differentiation and unlimited proliferation. The cancer stem cell
theory postulates these cells to be resistant to available
therapeutical options to date, such as chemotherapy and radiation
(38). Unfortunately, the marker
used as a CSC marker in our experiments (CD44) is also expressed in
ordinary cells (36). It is the
current challenge of research to find a combination of surface
markers that identifies more precisely the subgroup of potent CSCs
from the bulk of the tumor. CD44 cannot be sufficiently used as a
defining CSC marker alone, since as a cell surface glycoprotein it
takes part in many cell-cell interactions (36,39)
and in humans it is present in a multitude of splice variants
(36) and can be found
ubiquitously. Approaches for the investigation of the ‘real cancer
stem cell’ in HNSCC requires research using human tumor material
with isolation of a small subpopulation of cells out of the bulk of
the tumor using complicated cell separation steps including
recurring washing steps, lysis by DNAses and FACS sorting. Based on
the current knowledge, a combination of lineage markers must be
negative in order to separate CSCs out of the entire population of
HNSCC cells (CD3, CD3, CD10, CD18, CD31, CD64 and CD140) (2,35,36).
Other markers have been postulated as CSC markers yet only a small
amount of cells carrying these markers are said to be able to
initiate entirely new HNSCC tumors, e.g., in mouse models (CD44,
ALDH1) (2,40). The remaining cell amount after
separation as described above is often quite small and remaining
cells are often not useful for further experiments. Thus, it is
often necessary to revert to cell lines to perform experiments.
SDF-1α acts on the CXCR4 receptor and we
demonstrated that the functional effects of SDF-1α varied with
concentration. The maximum formation of podia in UM-SCC 11A cells
was achieved at a concentration of 10 ng/ml SDF-1α, while most
cells showed directed migration against a gradient of 100 ng/ml
SDF-1α. Generally, the formation of podia is considered to be a
pre-stage condition to directed locomotion (18). It is possible that the concentration
of SDF-1α that transforms elongated cells into migrating cells is
found between 10 and 100 ng/ml SDF-1α. It is also possible that a
maximum percentage of elongated UM-SCC 11A cells could be found
using lower concentrations of SDF-1α than those tested in our
experiments. Faber et al(18) used hematopoietic progenitor cells,
and various concentrations of the SDF-1α analogue (CTCE-0214) in
the range of μl/ml (18).
CD34+ cells showed an effect on the formation of podia
and directed migration under these conditions, which was comparable
to SDF-1α in a range of ng/ml (18).
Although a marked effect of SDF-1α was noted in
UM-SCC 11A cell morphology and directed migration, we did not note
any effect on cell proliferation and survival. Despite the fact
that SDF-1α acts on the same CXCR4 receptor, it is possible that
its effects are not only increased in a dose-dependent manner but
also depend on the influence of a particular dose of the agent.
These results also suggest that the signal cascade induced by
SDF-1α is not a monocausal succession (18) but rather a complex network. In
previous experiments we showed that the binding of SDF-1α analogues
has selective effects on hematopoietic progenitor cells (18). This indicates that the signal
cascade induced by SDF-1α is not a monocausal succession (SDF-1α
binding to CXCR4 activating G-proteins further activating
downstream mediators) but rather a complex network (18,41,42).
Analysis of the downstream targets in SDF-1α signal cascades such
as G-protein-associated activation of intracellular Ca-stores
(42,43) or Akt-triggered activation of IL-8
secretion (41,42) are only a few examples for the
complexity of intracellular SDF-1α signaling.
It is well-known that SDF-1α is a powerful
chemoattractant for primitive hematopoietic cells (18). Its effect as a chemoattractant has
also been shown in cholangiocarcinoma (44) and breast cancer (45). The SDF-CXCR4 axis is involved in
several aspects of tumor progression, such as angiogenesis,
metastasis and survival (42,46).
The microenvironment of the bone marrow has been said to support
survival, differentiation and proliferation of hematopoietic
progenitor cells (47), but also
malignant progenitor cells of the hematopoietic system, e.g.,
B-cell acute lymphoblastic leukemia (B-ALL) (47,48).
The pathway including the SDF-1-CXCR4 axis is postulated to be
responsible for retention of lymphoid and myeloid leukemia cells in
the bone marrow (42,49,50).
The importance of the SDF-1-CXCR4 axis has been well discussed in
the hematopoietic system, but this is the first time that we
provide evidence that the SDF-1-CXCR4 axis may also play a crucial
role in the development and particularly the progression, invasion
and metastasis of HNSCC.
In this study, we showed that polarization and
formation of filopodia and a prominent uropod were increased in
CD44+ CXCR4+ UM-SCC 11A cells in a
dose-dependent manner by SDF-1α. This effect can probably be
attributed to cytoskeleton rearrangements of actin-containing
protrusions (51,52) and this also might be influenced by
extracellular factors such as matrix metalloproteinases (52–54).
In general, the formation of podia is said to be concomitant with
cell adhesion to the microenvironment, especially the cellular
microenvironment surrounding them (18). If there is evidence that the
formation of podia and adhesion to their cell cancer stem cell
niche also play a role in HNSCC CD44+ cells,
understanding of these interactions might offer insights into new
strategies of cancer-directed therapy in HNSCC. Thus,
small-molecule agonists or antagonists (18) could be used to influence the cancer
stem cell niche of HNSCC resulting in inhibition or ideally
attenuation of tumor invasion and metastasis. Further experiments
must be carried out in human tissue to expand and focus our insight
into the cell-cell-interactions in the so-called cancer stem cell
niche of solid tumors. Thus, it may be possible to develop
therapeutic strategies specifically aimed at CSCs or targeted to
the SDF-1-CXCR4 axis, thus affecting the interaction of CSCs with
their cancer stem cell niche.
Acknowledgements
We gratefully thank Petra Prohaska for the excellent
technical support.
References
|
1
|
Vokes EE, Weichselbaum RR, Lippman SM and
Hong WK: Head and neck cancer. N Engl J Med. 328:184–194. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prince ME, Sivanandan R, Kaczorowski A, et
al: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang P, Zuo H, Ozaki T, Nakagomi N and
Kakudo K: Cancer stem cell hypothesis in thyroid cancer. Pathol
Int. 56:485–489. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Franzmann EJ, Reategui EP, Pedroso F, et
al: Soluble CD44 is a potential marker for the early detection of
head and neck cancer. Cancer Epidemiol Biomarkers Prev.
16:1348–1355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saito H, Tsujitani S, Katano K, Ikeguchi
M, Maeta M and Kaibara N: Serum concentration of CD44 variant 6 and
its relation to prognosis in patients with gastric carcinoma.
Cancer. 83:1094–1101. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ponta H, Wainwright D and Herrlich P: The
CD44 protein family. Int J Biochem Cell Biol. 30:299–305. 1998.
View Article : Google Scholar
|
|
7
|
Screaton GR, Bell MV, Bell JI and Jackson
DG: The identification of a new alternative exon with highly
restricted tissue expression in transcripts encoding the mouse
Pgp-1 (CD44) homing receptor. Comparison of all 10 variable exons
between mouse, human, and rat. J Biol Chem. 268:12235–12238.
1993.
|
|
8
|
Screaton GR, Bell MV, Jackson DG, Cornelis
FB, Gerth U and Bell JI: Genomic structure of DNA encoding the
lymphocyte homing receptor CD44 reveals at least 12 alternatively
spliced exons. Proc Natl Acad Sci USA. 89:12160–12164. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Picker LJ, Nakache M and Butcher EC:
Monoclonal antibodies to human lymphocyte homing receptors define a
novel class of adhesion molecules on diverse cell types. J Cell
Biol. 109:927–937. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stamenkovic I, Amiot M, Pesando JM and
Seed B: A lymphocyte molecule implicated in lymph node homing is a
member of the cartilage link protein family. Cell. 56:1057–1062.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gunthert U, Hofmann M, Rudy W, et al: A
new variant of glycoprotein CD44 confers metastatic potential to
rat carcinoma cells. Cell. 65:13–24. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hofmann M, Rudy W, Zoller M, et al: CD44
splice variants confer metastatic behavior in rats: homologous
sequences are expressed in human tumor cell lines. Cancer Res.
51:5292–5297. 1991.PubMed/NCBI
|
|
13
|
Thenappan A, Li Y, Shetty K, Johnson L,
Reddy EP and Mishra L: New therapeutics targeting colon cancer stem
cells. Curr Colorectal Cancer Rep. 5:2092009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zlobec I, Gunthert U, Tornillo L, et al:
Systematic assessment of the prognostic impact of membranous CD44v6
protein expression in colorectal cancer. Histopathology.
55:564–575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park SY, Lee HE, Li H, Shipitsin M, Gelman
R and Polyak K: Heterogeneity for stem cell-related markers
according to tumor subtype and histologic stage in breast cancer.
Clin Cancer Res. 16:876–887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okayama H, Kumamoto K, Saitou K, et al:
CD44v6, MMP-7 and nuclear Cdx2 are significant biomarkers for
prediction of lymph node metastasis in primary gastric cancer.
Oncol Rep. 22:745–755. 2009.PubMed/NCBI
|
|
17
|
Dar A, Goichberg P, Shinder V, et al:
Chemokine receptor CXCR4-dependent internalization and resecretion
of functional chemokine SDF-1 by bone marrow endothelial and
stromal cells. Nat Immunol. 6:1038–1046. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Faber A, Roderburg C, Wein F, et al: The
many facets of SDF-1alpha, CXCR4 agonists and antagonists on
hematopoietic progenitor cells. J Biomed Biotechnol.
2007:260652007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Imai K, Kobayashi M, Wang J, et al:
Selective secretion of chemoattractants for haemopoietic progenitor
cells by bone marrow endothelial cells: a possible role in homing
of haemopoietic progenitor cells to bone marrow. Br J Haematol.
106:905–911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De La Luz Sierra M, Yang F, Narazaki M, et
al: Differential processing of stromal-derived factor-1alpha and
stromal-derived factor-1beta explains functional diversity. Blood.
103:2452–2459. 2004.PubMed/NCBI
|
|
21
|
Shirozu M, Nakano T, Inazawa J, et al:
Structure and chromosomal localization of the human stromal
cell-derived factor 1 (SDF1) gene. Genomics. 28:495–500. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim CH and Broxmeyer HE: In vitro behavior
of hematopoietic progenitor cells under the influence of
chemoattractants: stromal cell-derived factor-1, steel factor, and
the bone marrow environment. Blood. 91:100–110. 1998.PubMed/NCBI
|
|
23
|
Mohle R, Bautz F, Rafii S, Moore MA,
Brugger W and Kanz L: The chemokine receptor CXCR-4 is expressed on
CD34+ hematopoietic progenitors and leukemic cells and
mediates transendothelial migration induced by stromal cell-derived
factor-1. Blood. 91:4523–4530. 1998.PubMed/NCBI
|
|
24
|
Petit I, Goichberg P, Spiegel A, et al:
Atypical PKC-zeta regulates SDF-1-mediated migration and
development of human CD34+ progenitor cells. J Clin
Invest. 115:168–176. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chute JP: Stem cell homing. Curr Opin
Hematol. 13:399–406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fruehauf S, Srbic K, Seggewiss R, Topaly J
and Ho AD: Functional characterization of podia formation in normal
and malignant hematopoietic cells. J Leukoc Biol. 71:425–432.
2002.PubMed/NCBI
|
|
27
|
Lataillade JJ, Clay D, Dupuy C, et al:
Chemokine SDF-1 enhances circulating CD34(+) cell proliferation in
synergy with cytokines: possible role in progenitor survival.
Blood. 95:756–768. 2000.
|
|
28
|
Broxmeyer HE, Kohli L, Kim CH, et al:
Stromal cell-derived factor-1/CXCL12 directly enhances
survival/antiapoptosis of myeloid progenitor cells through CXCR4
and G(alpha)i proteins and enhances engraftment of competitive,
repopulating stem cells. J Leukoc Biol. 73:630–638. 2003.
View Article : Google Scholar
|
|
29
|
Kijowski J, Baj-Krzyworzeka M, Majka M, et
al: The SDF-1-CXCR4 axis stimulates VEGF secretion and activates
integrins but does not affect proliferation and survival in
lymphohematopoietic cells. Stem Cells. 19:453–466. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hattori K, Heissig B, Tashiro K, et al:
Plasma elevation of stromal cell-derived factor-1 induces
mobilization of mature and immature hematopoietic progenitor and
stem cells. Blood. 97:3354–3360. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goichberg P, Kalinkovich A, Borodovsky N,
et al: cAMP-induced PKCzeta activation increases functional CXCR4
expression on human CD34+ hematopoietic progenitors.
Blood. 107:870–879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tudan C, Willick GE, Chahal S, et al:
C-terminal cyclization of an SDF-1 small peptide analogue
dramatically increases receptor affinity and activation of the
CXCR4 receptor. J Med Chem. 45:2024–2031. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grenman R, Burk D, Virolainen E, Wagner
JG, Lichter AS and Carey TE: Radiosensitivity of head and neck
cancer cells in vitro. A 96-well plate clonogenic cell assay for
squamous cell carcinoma. Arch Otolaryngol Head Neck Surg.
114:427–431. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Domanska UM, Kruizinga RC, Nagengast WB,
et al: A review on CXCR4/CXCL12 axis in oncology: No place to hide.
Eur J Cancer. June 8–2012.(Epub ahead of print).
|
|
35
|
Pries R, Wittkopf N, Hasselbacher K and
Wollenberg B: Constitutive expression of the potential stem cell
marker CD44 in permanent HNSCC cell lines. Hno. 56:461–466.
2008.(In German).
|
|
36
|
Wollenberg B: Implication of stem cells in
the biology and therapy of head and neck cancer.
Laryngorhinootologie. 90(Suppl 1): S110–S119. 2011.(In German).
|
|
37
|
Zhang Z, Filho MS and Nor JE: The biology
of head and neck cancer stem cells. Oral Oncol. 48:1–9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
La Porta CA: Thoughts about cancer stem
cells in solid tumors. World J Stem Cells. 4:17–20. 2012.PubMed/NCBI
|
|
39
|
Jaggupilli A and Elkord E: Significance of
CD44 and CD24 as Cancer Stem Cell Markers: An Enduring Ambiguity.
Clin Dev Immunol. 2012:7080362012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Clay MR, Tabor M, Owen JH, et al:
Single-marker identification of head and neck squamous cell
carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck.
32:1195–1201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li KC, Huang YH, Ho CY, et al: The role of
IL-8 in the SDF-1alpha/CXCR4-induced angiogenesis of laryngeal and
hypopharyngeal squamous cell carcinoma. Oral Oncol. 48:507–515.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Stenvik J, Sletta H, Grimstad O, et al:
Alginates induce differentiation and expression of CXCR7 and
CXCL12/SDF-1 in human keratinocytes-The role of calcium. J Biomed
Mater Res A. 100:2803–2812. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gentilini A, Rombouts K, Galastri S, et
al: Role of the stromal-derived factor-1 (SDF-1)-CXCR4 axis in the
interaction between hepatic stellate cells and cholangiocarcinoma.
J Hepatol. June 19–2012.(Epub ahead of print).
|
|
45
|
Chen J and Gallo KA: MLK3 regulates
paxillin phosphorylation in chemokine-mediated breast cancer cell
migration and invasion to drive metastasis. Cancer Res.
72:4130–4140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jin F, Brockmeier U, Otterbach F and
Metzen E: New insight into the SDF-1/CXCR4 axis in a breast
carcinoma model: Hypoxia-induced endothelial SDF-1 and tumor cell
CXCR4 are required for tumor cell intravasation. Mol Cancer Res.
10:1021–1031. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Flores-Figueroa E, Varma S, Montgomery K,
Greenberg PL and Gratzinger D: Distinctive contact between
CD34+ hematopoietic progenitors and CXCL12+
CD271+ mesenchymal stromal cells in benign and
myelodysplastic bone marrow. Lab Invest. 92:1330–1341. 2012.
|
|
48
|
Purizaca J, Meza I and Pelayo R: Early
lymphoid development and microenvironmental cues in B-cell acute
lymphoblastic leukemia. Arch Med Res. 43:89–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Juarez J, Dela Pena A, Baraz R, et al:
CXCR4 antagonists mobilize childhood acute lymphoblastic leukemia
cells into the peripheral blood and inhibit engraftment. Leukemia.
21:1249–1257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nervi B, Ramirez P, Rettig MP, et al:
Chemosensitization of acute myeloid leukemia (AML) following
mobilization by the CXCR4 antagonist AMD3100. Blood. 113:6206–6214.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Faber A, Barth C, Hormann K, et al: CD44
as a stem cell marker in head and neck squamous cell carcinoma.
Oncol Rep. 26:321–326. 2011.PubMed/NCBI
|
|
52
|
Geutskens SB, Andrews WD, van Stalborch
AM, et al: Control of human hematopoietic stem/progenitor cell
migration by the extracellular matrix protein Slit3. Lab Invest.
92:1129–1139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ghosh MC, Makena PS, Gorantla V, Sinclair
SE and Waters CM: CXCR4 regulates migration of lung alveolar
epithelial cells through activation of Rac1 and matrix
metalloproteinase-2. Am J Physiol Lung Cell Mol Physiol.
302:L846–L856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Faber A, Sauter A, Hoedt S, et al:
Alteration of MMP-2 and -14 expression by imatinib in HPV-positive
and -negative squamous cell carcinoma. Oncol Rep. 28:172–178.
2012.PubMed/NCBI
|