Introduction
Photodynamic therapy (PDT) has been used in clinical
application for many years, either systemically, or locally or
topically applied to a patient bearing a lesion (cancerous or not).
PDT with systemic photosensitizers has been used to treat a range
of internal malignancies (lung and brain cancers) and for skin
cancers (1). This cancer treatment
is approved by many countries for the treatment of non-melanotic
skin cancers such as basal cell carcinomas and Bowen's disease. PDT
does not seem to be an appropriate treatment of melanotic melanoma
in vivo, and several reasons can justify this low use.
Almost all melanomas overexpress the classical ATP-binding cassette
transporters (ABC protein family), leading to drug release into the
extracellular medium and to the resistance by tumor cells. The
antioxidant activity of melanoma is much higher than observed in
other skin cancers (basal and squamous carcinomas) reducing the
cytotoxic effect of ROS generated by PDT (2). Nearly all photosensitizers (PS) show a
significant absorbance in ultraviolet (UV) wavelengths, while
melanin absorbs a large portion of the light intended to activate
PS. The light absorption by pigment strongly reduces its
penetration into the tumor. Studies have been carried out with
pigmented or amelanotic melanoma cells suggesting that amelanotic
cells may be more susceptible to PDT (2). Among the molecules used in PDT,
5-aminolevulinic acid (5-ALA) is not itself a photosensitizer and
instead it is used as a prodrug leading to the accumulation of
protoporphyrin IX in the mitochondria inducing damage and
subsequently cell death after light irradiation (3).
It is well-known that exposure of a photosensitizing
agent to appropriate light leads to a photophysical reaction and
the production of reactive oxygen species (ROS). These constitute
the main driving force to initiate three types of cell death
(necrosis, apoptosis or autophagy). Nevertheless, the signaling
pathways involved in PDT-mediated cell death are not completely
understood.
In this study, we examined the photocytotoxicity of
5-ALA on two variants of melanoma cell lines (pigmented B16F10 and
amelanotic B16G4F cells), the cell death and autophagic pathways
were investigated. One day after the induction of PDT with 5-ALA,
the pan-caspase activity differed between the two melanoma cell
lines. The recruitment of p53 and PUMA led to a caspase-dependent
cell death in the pigmented cells whereas an autophagic cell death
was activated in the non-pigmented (amelanotic) melanoma cells.
Materials and methods
Source and preparation of the
photosensitizer
5-ALA (5-δ-aminolevulinic acid hypochloride) was
obtained from Sigma-Aldrich Chimie (Saint-Quentin Fallavier,
France) and prepared as a stable 30 mM stock solution (stored in
aliquots at −80°C) from the lyophilized powder and was dissolved in
Dulbecco's modified Eagle's medium (DMEM, Invitrogen-Fisher,
Illkirch, France) containing 10% fetal bovine serum (FBS)
(Invitrogen-Fisher). Other reagents are specified below.
Cell lines and culture conditions
The B16F10 pigmented melanoma cells were a gift from
Dr Perron (Oncology Center, Toulouse, France). The amelanotic
B16G4F melanoma cells (without Mc1r expression, the receptor of
α-melanocyte stimulating hormone) were kindly provided by Professor
A. Oulmouden (UMR INRA 1061, Limoges, France). Cells were grown to
confluence in DMEM containing 10% FBS and antibiotics (100 U/ml
penicillin and 100 μg/ml streptomycin) in a humidified 5%
CO2 atmosphere at 37°C. The culture medium was renewed
every 3 days. Cells were subcultured by dispersal with trypsin-EDTA
and replated at 2×105/ml.
Light source
Exposure of melanoma cells to light was performed
using either a red lamp composed of fluorescent tubes or light
emitting diodes (LED) (Aktilite™ 128 Lamp, Galderma). The light
fluence rate used by the red lamp was 37 mW/cm2 at 631
nm on the surface of the plate.
Irradiation protocol
Cells were seeded at 105 cells/ml in
96-well plates after trypsinization. After 24 h, the cells were
washed twice with PBS and incubated with different concentration of
5-ALA (0, 2.5, 5, 10 or 20 mM) for 2 h with medium, in darkness and
in a humidified 5% CO2 atmosphere at 37°C. The cells
were then irradiated with the Aktilite Lamp™ at room temperature
(25°C). The exposure time was adjusted to obtain 37
J/cm2 fluence (corresponding to ~8 min). In previous
experiments, during cell illumination the temperature monitored at
the top of the microplate was unchanged. Two controls were
realized, one 96-well plate without photosensitizer and one with
photosensitizer and without irradiation.
Cytotoxic and photocytotoxic studies
Cytotoxicity and photocytoxicity were measured using
the XTT (3-(4,5-dimethyl- thiazol-2-yl)-2,5-diphenyltetrazolium
bromide) colorimetric assay (Cell Proliferation Kit II, Roche
Diagnostics, Meylan, France). Optical density is proportional to
relative mitochondrial dehydrogenase activity and reflects cell
viability. Three hours after the addition of 5-ALA, assays were
performed at 0, 24 and 48 h either without irradiation or after the
end of the irradiation. At the time of counting, 50 μl of DMEM
without phenol red-XTT solution (0.3 mg/ml) was added and
incubation was carried out for 3 h at 37°C. Optical density (OD) at
490 nm was determined (the OD reference at 690 nm was subtracted)
in an MRX II (Dynex) absorbance reader. Cytotoxicity was evaluated
by the OD ratio of treated vs. non-treated cells. Each value of XTT
assay was obtained from cultures in triplicate.
Pan-caspase activity assay
Both melanoma cell lines were plated in a 24-well
dish (Nunc) at 25×104 cells/well. After a 24-h culture,
5-ALA was added or not to the cells and irradiation was carried out
according to the protocol. After incubation, cells were pooled and
lysed in Chaps buffer. The assays were performed as previously
described (4). Peptide substrates
(Bachem, Germany) for pan-caspase (Z-VAD-AMC) were added to each
well to a final concentration of 5×10−5 M. Pan-caspase
(Z-VAD-CHO) inhibitors at 2.5 mM were added 30 min before the
substrate. The assay plates were incubated at 37°C for 2 h and
fluorescence was measured with a microplate reader (Twinkle LB 970,
Berthold, France), then pan-caspase activities were expressed in
relative fluorescence units (RFU). Background fluorescence was
determined in wells containing Z-VAD-AMC assay mixture and
substrate without cell lysates, and pan-caspase activity was
expressed in relative units.
Western blotting
Protein lysates (30 μg) from ALA-treated or control
B16F10 and B16G4F cells (2×106 cells/dish) were
separated by SDS-PAGE. After electrophoretic migration, proteins
were electroblotted onto cellulose membranes. After the blocking
step (TBS-1X containing 0.1% Tween 20 and 5% non-fat dry milk)
membranes were incubated with primary specific antibodies,
recognizing the following antigens: rabbit anti-PUMA obtained from
Cell Signaling (Ozyme, France), anti-cathepsin B (CA10) purchased
from Abcam (France); tubulin (clone TU-02), beclin-1 (E-8) and
MAP-LC3-II (H-50) and p53 (Bp53-12) obtained from Santa Cruz
Biotechnology (Tebu-bio, France). They were revealed with
appropriate secondary HRP-conjugated antibodies purchased from
DakoCytomation (France). Blots were developed with Immobilon
Western chemiluminescent HRP substrate from Millipore and analyzed
with the G-Box (Ozyme). Each set of experiments was repeated
independently for at least 3 times.
Results
Different levels of photocytotoxicity of
5-aminolevulinic in B16F10 and B16G4F murine melanoma cell
lines
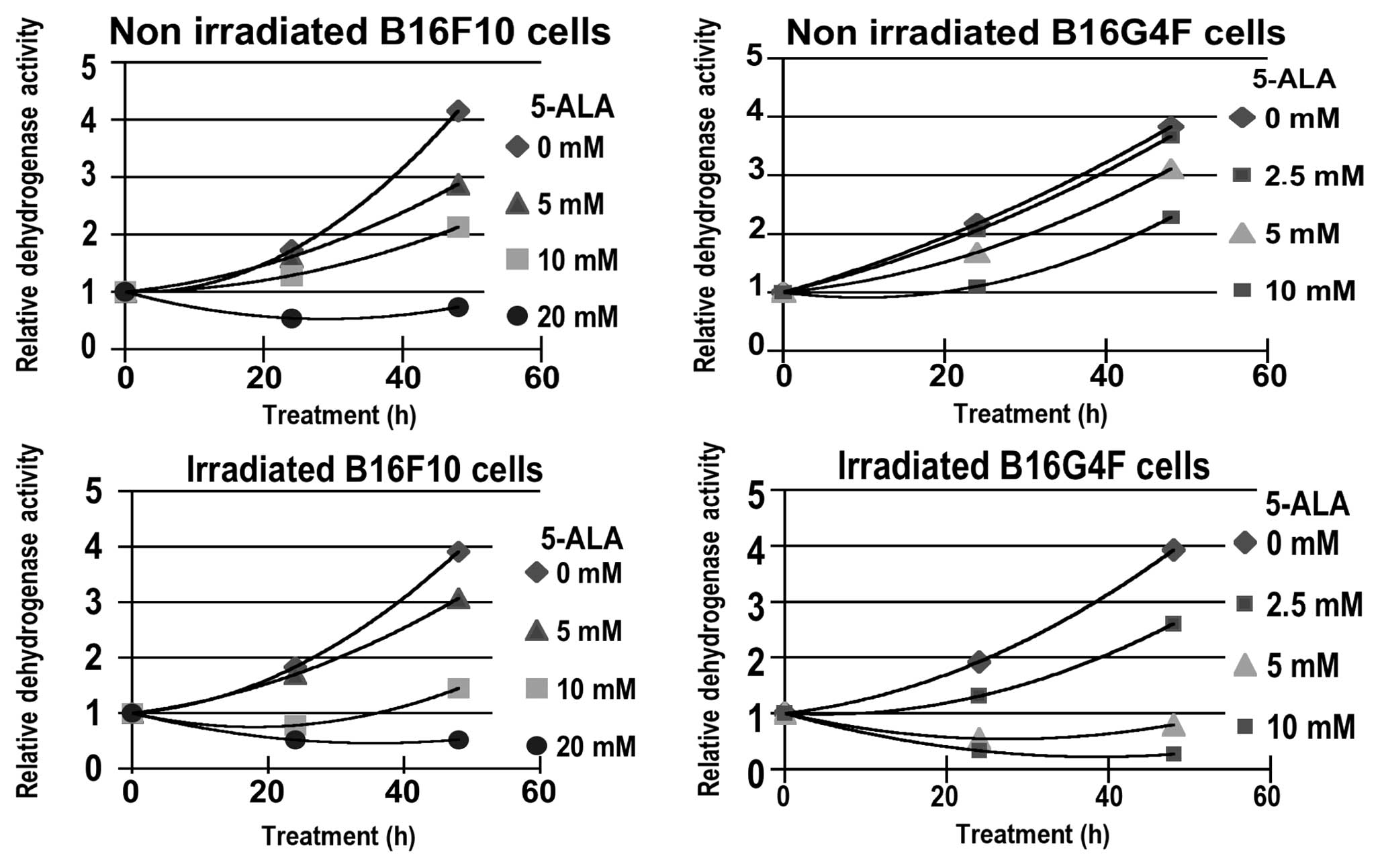
A time kinetic (0–48 h) study of cytotoxicity,
measured with the XTT assay, corresponding to relative
dehydrogenase activity (RDA), was carried out. Two series of
experiments without or after red illumination were performed using
5, 10 and 20 mM of 5-ALA, respectively for the pigmented B16F10
melanoma cell line and with 2.5, 5 and 10 mM of 5-ALA for the
amelanotic B16G4F melanoma cell line (Fig. 1). In both cell lines a similar
cytostatic effect occured in relationship with 5-ALA
concentrations. Nevertheless, after 48 h, the relative
dehydrogenase activity increased in the non-irradiated cells. At 20
mM 5-ALA in the B16F10 cells the slowing of growth was not
effective.
After photodynamic treatment (PDT) a moderate and
transitional reduction in RDA was observed when compared to control
cells at low 5-ALA concentrations; 5 mM for pigmented B16F10 and
2.5 mM for non-pigmented B16G4F cells, respectively (Fig. 1). For higher 5-ALA concentrations
(10 mM for B16F10 and 5 mM for B16G4F cells, respectively) a
significant photocytotoxicity was observed.
Pan-caspase activity in B16F10 and B16G4F
cell lines after photodynamic treatment
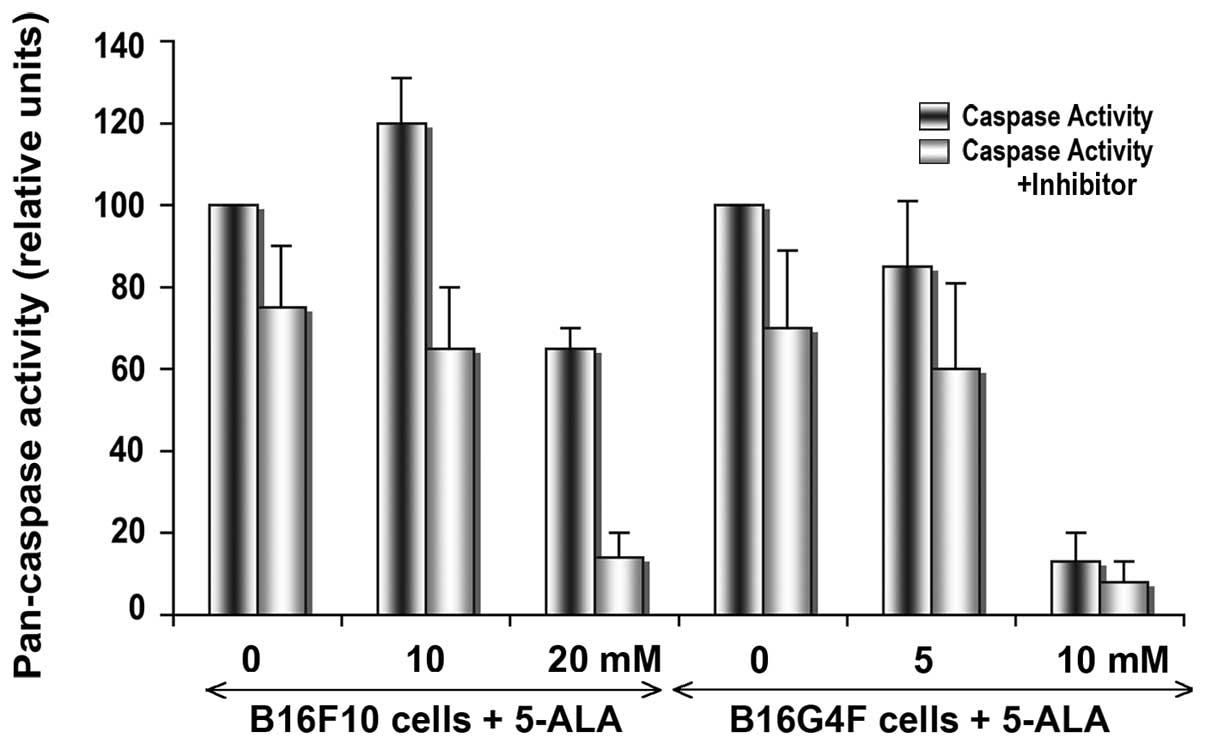
In order to detect the apoptotic process after a
24-h cell irradiation, total caspase activity was measured with a
peptide substrate recognized by all caspases (pan-caspase), and
compared with the same experiments in the presence of a specific
inhibitor (ZVAD-CHO). In pigmented B16F10 cells, the pan-caspase
activity was significantly increased following addition of 10 mM
5-ALA in comparison with the action in the control cells (Fig. 2). In the B16G4F cells, no
significant change was observed between the control conditions and
cells after PDT in combination with 5 mM 5-ALA. At high 5-ALA
concentrations (20 mM for B16F10 cells and 10 mM for B16G4F cells,
respectively), a drastic disappearance in caspase activity was
detected corresponding to cell death at 24 h.
Expression of p53 and PUMA in B16F10 and
B16G4F cell lines after PDT
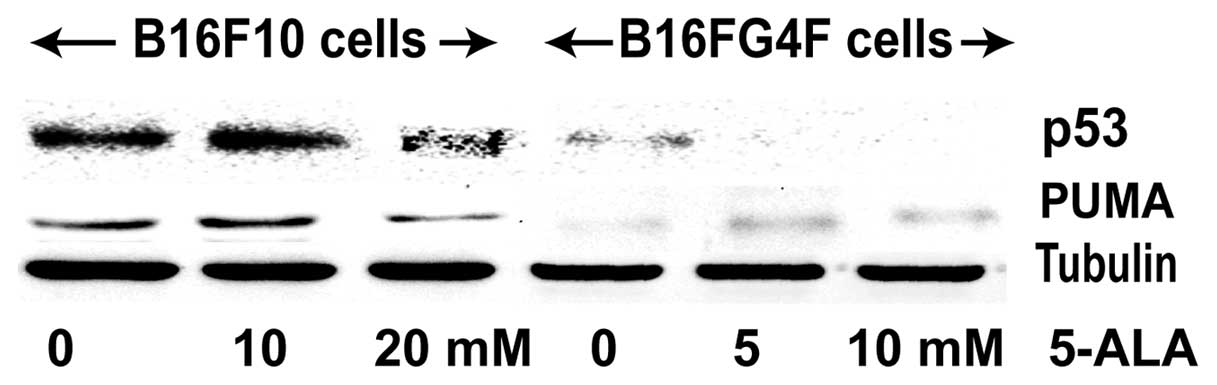
We studied the well-known tumor suppressor p53
protein involved in cell cycle control and apoptosis induction upon
stress stimuli (Fig. 3). A
significant p53 expression was observed in the control B16F10 cell
line; 24 h after PDT treatment, the p53 signal was enhanced with
the addition of 10 mM 5-ALA but its level was reduced in the
presence of 20 mM 5-ALA. In contrast, no or a faint p53 expression
was observed in the non-pigmented B16G4F cells regardless of the
conditions (control, 5 and 10 mM 5-ALA treatment). Results of the
PUMA analysis, one of the p53 transcriptional targets implicated in
executive apoptosis, confirmed the findings concerning p53. PUMA
expression in the pigmented B16F10 cells was correlated to that of
p53 and in non-pigmented B16G4F cells only a faint PUMA band was
detected.
Expression of beclin-1, LC3-I, LC3-II and
cathepsin-B after photodynamic treatment with 5-ALA
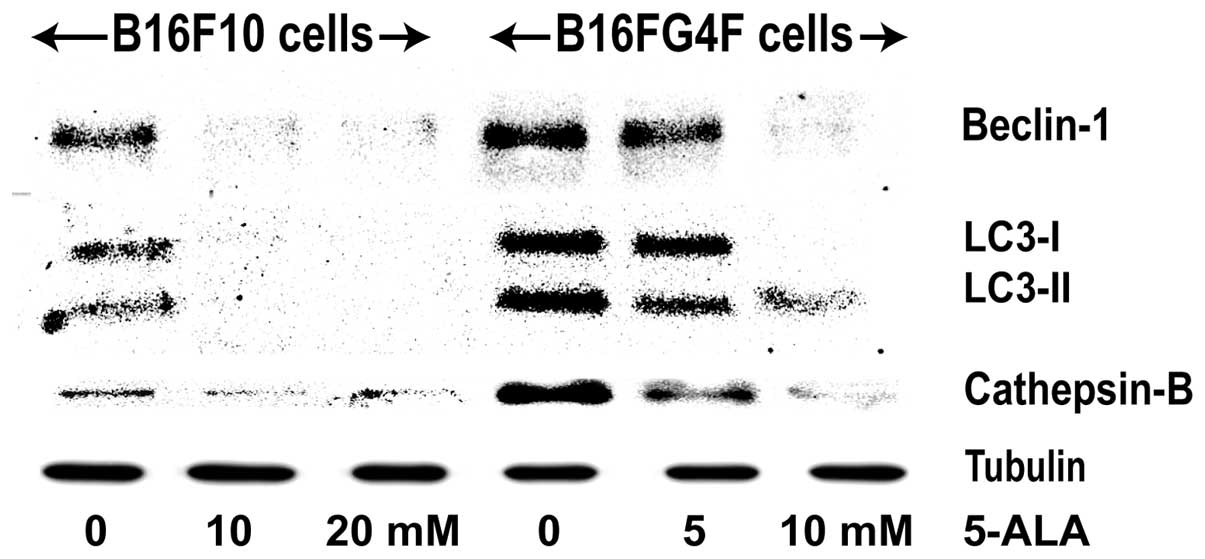
We studied the eventual activation of an autophagic
signaling, through the expression of beclin-1, a regulator
activated upstream, LC3-I and LC3-II which is implied in the
nucleation of autophagosome and a lysosomal protein cathepsin B
which concludes the autophagic process (Fig. 4). In control pigmented B16F10 cells,
a moderate signal was detected for both beclin-1, LC3-I and II and
cathepsin-B, which disappeared in the treated cells. Conversely,
their expression was significantly increased in the control
non-pigmented B16G4F cells and was reduced at a higher 5-ALA
concentration (10 mM).
Discussion
Photodynamic therapy (PDT) is now a well-established
treatment modality for cutaneous carcinomas camptothecin (1,5), but
melanotic melanomas generally have a poor response to PDT owing to
several characteristics that confer chemoresistance such as high
antioxidant activity (2). The
presence of melanin permits the absorption of UV and spectral light
by pigmented melanoma, reducing photosensitizer excitation. The
cell death pathway induced appears to differ in both according to
photosensitizer, the cell type and in the case of melanoma with the
presence of melanin. Thus, we studied the type of signaling pathway
induced (apoptosis and autophagy) by 5-ALA, a physiologic compound
currently used in vivo for various types of tumors, excited
with a red illumination on the well-known mouse melanoma cell line
B16F10 and its amelanotic variant B16G4F cells.
As expected, the 5-ALA molecule showed a similar and
moderate toxicity in both pigmented and amelanotic cells. At almost
all 5-ALA concentrations and after a 48-h treatment, cell growth
was reinitiated concomitant with a recycling process. After 24 h,
the 5-ALA photocytotoxicity varied as a function of the presence of
melanin in the melanoma cells, and when the decrease in
mitochondrial activity was moderate due to a repair process, this
permitted a partial restoration of cell growth after 48 h. The
slight reduction in dehydrogenase activity in the presence of low
5-ALA concentrations was noted in the two melanoma cell lines
(pigmented or not), then the restart of cell growth was observed at
48 h, which was consistent with an autophagic process. Due to the
high reactivity of photogenerated ROS, autophagy is initiated to
remove oxidative damaged organelles such as mitochondria which are
targets of 5-ALA (5,6). The 10 mM concentration of 5-ALA
induced photocytotoxicity in B16F10 cells slightly weaker than that
observed in the B16G4F amelanotic cells treated with 5 mM 5-ALA.
Several studies have previously demonstrated that the presence of
melanin in melanoma reduces drug photocytotoxicity. Ma et
al(7) showed that violet light
PDT was able to bleach melanin in melanotic tumors and therefore
increase their sensitivity to red light PDT. The hypericin
photocytoxicity toward pigmented UCT MEL-1 melanoma cells was found
to be lower than that in unpigmented A375 melanoma, due to the
protective properties of melanin on ROS (8). Recently, Mroz et al(9) reported the effectiveness of Photofrin
and bacteriochlorin 3 in relation to the melanin content of B16
melanoma cells. The effectiveness of Photofrin to induce cell death
was completely abolished in B16F10 cells with a high pigment
content.
Many pathways have been discovered whereby mammalian
cells execute cell death in response to PDT with different
photosensitizers (10). The
porphyrin family molecules such as 5-ALA are localized in
mitochondria. After PDT treatment they induce ROS generation as
described in numerous studies (6,11) and
mainly produce a mitochondrial apoptosis with caspase activation.
The moderate pan-caspase activity in pigmented B16F10 cells treated
with 10 mM 5-ALA was compatible with mitochondrial apoptosis with
caspase activation such as described by Buytaert et
al(5). Radzi et
al(12) in the same-pigmented
cells irradiated in the presence of indocyanine green observed a
caspase-independent apoptosis. This is similar to the type of death
that we observed in amelanotic variant B16G4F cells by 5-ALA.
Western blot analysis of p53 and PUMA (one of its
transcriptional targets) confirmed that in the presence of melanin
in the B16F10 melanoma cells a caspase-dependent apoptosis was
recruited. Data support the contribution of a p53-regulated
response to PDT in cells expressing wild-type p53 as described
(13). In the amelanotic variant
B16G4F, no p53 and PUMA signals were detected, suggesting that the
mitochondrial pathway was not activated. Prasad et
al(14) showed that in 7
patients with desmoplastic melanoma (0.1% of cutaneous melanoma)
characterized by the presence of amelanotic invasive spindle cells,
6 patients had an aberrant or null p53 expression. The absence of
p53 and PUMA expression with caspase-independent signaling was
supported by results indicating a role of a p53-independent
mechanism in response to PDT in p53-deficient cells (13). Both apoptosis and autophagy can
occur after PDT (15), and recent
studies described a non-apoptotic cell death associated with the
induction of autophagy (2,3,15,16).
In the B16F10 melanoma cell line our results showed
that the absence of expression of an autophagic marker (beclin-1,
LC3-I and LC3-II, cathepsin B) was in line with a mitochondrial
caspase-dependent apoptosis. This mitochondrial death has been
described in several melanoma cell lines treated with PDT and
molecules of 5-ALA (6,17). On the other hand, in the amelanotic
variant B16G4F cells, PDT treatment with 5-ALA led to the
activiation of autophagy as described using the same
photosensitizer in non-pigmented cells such as skin keratinocytes
(18), PC12 and CL1-0 cells
(3). The recycling process can lead
to cell death when cells attempt to recycle damaged constituents
beyond their capacity for recovery or in apoptosis-incompetent
cells (15,19).
In summary, we demonstrated that 5-ALA and PDT with
red illumination induced a differential susceptibility in melanoma
cells based on the presence of melanin. In addition, mitochondrial
apoptotic signaling was activated in the pigmented B16F10 cells,
whereas an autophagic process leading to caspase-independent cell
death occurred in amelanotic B16G4F cells. Further studies may
confirm the attribution of PDT sensitivity of B16G4F cells to the
lack of melanin and/or a p53 gene mutation.
Acknowledgements
We would like to thank Dr Cornelia Wilson-Whelan
(University of Limoges) for the critical language editing of the
manuscript, Tan Ouk and Anne-Sophie Rigaudeau for their technical
advice.
References
|
1
|
Choudhary S, Nouri K and Elsaie ML:
Photodynamic therapy in dermatology: a review. Lasers Med Sci.
24:971–980. 2009. View Article : Google Scholar
|
|
2
|
Davids LM and Kleemann B: Combating
melanoma: the use of photodynamic therapy as a novel, adjuvant
therapeutic tool. Cancer Treat Rev. 37:465–475. 2011.PubMed/NCBI
|
|
3
|
Ji HT, Chien LT, Lin YH, et al:
5-ALA-mediated photodynamic therapy induces autophagic cell death
via AMP-activated protein kinase. Mol Cancer. 9:912010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zuliani T, Duval R, Jayat C, et al:
Sensitive and reliable JC-1 and TOTO-3 double staining to assess
mitochondrial transmembrane potential and plasma membrane
integrity: interest for cell death investigations. Cytometry A.
54:100–108. 2003. View Article : Google Scholar
|
|
5
|
Buytaert E, Dewaele M and Agostinis P:
Molecular effectors of multiple cell death pathways initiated by
photodynamic therapy. Biochim Biophys Acta. 1776:86–107.
2007.PubMed/NCBI
|
|
6
|
Krestyn E, Kolarova H, Bajgar R, et al:
Photodynamic properties of ZnTPPS(4), ClAlPcS(2) and ALA in human
melanoma G361 cells. Toxicol In Vitro. 24:286–291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma LW, Nielsen KP, Iani V, et al: A new
method for photodynamic therapy of melanotic melanoma - effects of
depigmentation with violet light photodynamic therapy. J Environ
Pathol Toxicol Oncol. 26:165–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davids LM, Kleemann B, Kacerovská D, et
al: Hypericin phototoxicity induces different modes of cell death
in melanoma and human skin cells. J Photochem Photobiol B.
91:67–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mroz P, Huang YY, Szokalska A, et al:
Stable synthetic bacteriochlorins overcome the resistance of
melanoma to photodynamic therapy. FASEB J. 24:3160–3170. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robertson CA, Evans DH and Abrahamse H:
Photodynamic therapy (PDT): a short review on cellular mechanisms
and cancer research applications for PDT. J Photochem Photobiol B.
96:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kästle M, Grimm S, Nagel R, et al:
Combination of PDT and inhibitor treatment affects melanoma cells
and spares keratinocytes. Free Radic Biol Med. 50:305–312.
2011.PubMed/NCBI
|
|
12
|
Radzi R, Osaki T, Tsuka T, et al:
Photodynamic hyperthermal therapy with indocyanine green (ICG)
induces apoptosis and cell cycle arrest in B16F10 murine melanoma
cells. J Vet Med Sci. 74:545–551. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zawacka-Pankau J, Krachulec J, Grulkowski
I, et al: The p53-mediated cytotoxicity of photodynamic therapy of
cancer: recent advances. Toxicol Appl Pharmacol. 232:487–497. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prasad ML, Patel SG and Busam KJ: Primary
mucosal desmoplastic melanoma of the head and neck. Head Neck.
26:373–377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kessel D and Oleinick NL: Initiation of
autophagy by photodynamic therapy. Methods Enzymol. 431:1–16. 2009.
View Article : Google Scholar
|
|
16
|
François A, Marchal S, Guillemin F, et al:
mTHPC-based photodynamic therapy induction of autophagy and
apoptosis in cultured cells in relation to mitochondria and
endoplasmic reticulum stress. Int J Oncol. 39:1537–1543.
2011.PubMed/NCBI
|
|
17
|
Tsai T, Ji HT, Chiang PC, et al: ALA-PDT
results in phenotypic changes and decreased cellular invasion in
surviving cancer cells. Lasers Surg Med. 41:305–315. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silva JN, Galmiche A, Tomé JP, et al:
Chain-dependent photocytotoxicity of tricationic porphyrin
conjugates and related mechanisms of cell death in proliferating
human skin keratinocytes. Biochem Pharmacol. 80:1373–1385. 2010.
View Article : Google Scholar
|
|
19
|
Reiners JJ, Agostinis P, Berg K, et al:
Assessing autophagy in the context of photodynamic therapy.
Autophagy. 6:7–18. 2010. View Article : Google Scholar : PubMed/NCBI
|