Introduction
Axonal regeneration depends on the environment and
the intrinsic regenerative capacity of neurons in the adult central
nervous system (CNS). After CNS injury, the regenerative ability is
reduced in comparison to embryonic neurons, which is partially
reflected in the differential regulation of regeneration-associated
proteins, such as growth associated protein-43 (GAP-43) (1,2). In
the adult CNS, poor axonal regeneration of injured neurons is in
part due to the existence of myelin-associated axonal growth
inhibitors such as myelin-associated glycoprotein (MAG), Nogo and
oligodendrocyte-myelin glycoprotein (OMgp) (3,4). These
inhibitors activate RhoA, a member of the small GTPases, which is
known to regulate the actin cytoskeleton (5,6). RhoA
acts as a molecular switch that controls various intracellular
signaling pathways by changing between an active (GTP-bound) and
inactive (GDP-bound) form. Several studies have shown that the
activation of RhoA results in growth cone collapse and neurite
outgrowth inhibition, and the inactivation of RhoA could promote
axonal regeneration and functional recovery in the injured CNS
(7–9). The downstream effector of RhoA is the
Rho-associated kinase (ROCK), including ROCK1 and ROCK2 (10,11).
Activation of ROCK1 and ROCK2 enhances phosphorylation of the
regulatory myosin light-chain phosphatase (MLCP), which is a key
step in cytoskeletal rearrangement (12). The activity of ROCK1 and ROCK2 is
inhibited by ROCK inhibitor Y-27632 (13,14)
and inactivation of ROCK can promote axonal growth (8,15).
Y-39983, a selective ROCK inhibitor, has great
potency at inhibiting the activity of ROCK1 and ROCK2 (16). Previous studies have shown that
Y-39983 promotes axonal regeneration of damaged retinal ganglion
cells (RGCs) (15,17,18).
Despite the demonstrated benefits in neuro-regeneration, there are
no data available concerning the effects of Y-39983 on RhoA/ROCK
expression during its promotion of axonal regeneration. Therefore,
the present study investigated the effects of Y-39983 on RhoA/ROCK
expression in the promotion of axonal regeneration of RGCs.
Materials and methods
Animals
Adult male Sprague-Dawley rats (200–250 g) were
obtained from the Laboratory Animal Center of Shanghai Institute of
Traumatology and Orthopedics, Shanghai Jiaotong University, China.
They were housed individually and maintained on a 12-h light/dark
cycle. Food and water were available ad libitum. All
experiments were performed in accordance with the Association for
Research in Vision and Ophthalmology (ARVO) Statement for the Use
of Animals in Ophthalmic and Vision Research. Animals were
anesthetized using a 10% chloral hydrate solution (420 mg/kg b.w.),
and sacrificed using an intraperitoneal overdose of a 30% solution.
Every effort was made to minimize suffering and limit the number of
animals used.
Retrograde labeling of RGCs
To determine densities of the RGCs, all experimental
rats were randomly chosen for the retrograde labeling of RGCs by
fluorescent tracer DiI. The retrograde labeling of RGCs was
performed as previously described (19). Briefly, animals were anesthetized,
the skin was incised mediosagitally, and the skull cartilage was
opened dorsal to the lambda fissure. DiI (1.5 μl) (10% in
dimethylsulfoxide) was then applied to both superior colliculi
(SC), respectively, using a micropipette. The optic nerve crush was
performed as described below 3 days after DiI labeling.
Optic nerve crush (ONC) model
Surgical procedures were similar to those described
previously (20). Rats were
anesthetized, and a 1- to 1.5-cm incision was made in the skin
above the right orbit. The optic nerve (ON) of the right eye was
exposed under an operating microscope, and the sheath was opened
longitudinally. Using special forceps (40 g), the ON was crushed
within the sheath at the site 1 mm behind the optic nerve head for
9 sec, avoiding injury to the ophthalmic artery. Nerve injury was
verified by the appearance of a clearing at the crush site; the
vascular integrity of the retina was verified by fundoscopic
examination after dilating the pupil with tropicamide. In the sham
operation group, the ON of the right eye was exposed, and the
sheath was opened longitudinally, but without the crush
procedure.
Drug injection into the vitreous
The drugs were administered to the vitreous via a
stereotactically positioned 30-gauge needle attached to a 10-μl
Hamilton syringe. A final volume of 3 μl was administered via the
peripheral temporal retinal site taking care to avoid contact with
the lens since its injury releases neuroregenerative factors
(21). The eye was examined
ophthalmoscopically to check that the retinal vasculature was
intact. Y-39983
(4-[(1R)-aminoethyl]-N-(1H-pyrrolo[2,3-b]pyridine-4-yl)benzamide
monohydrochloride), (MW=316.8; Tocris Bioscience, Ellisville, MO,
USA) was diluted in phosphate-buffered saline (PBS). According to
the drug administration, the animals were divided into three
groups: the sham-operated group (sham group), the ONC+PBS
intravitreal injection group (ONC+PBS group) and the ONC+ Y-39983
intravitreal injection group (ONC+Y-39983 group). Apart from the
sham group while received no intravitreal injection, the other
groups received 3 μl PBS or 20 μM [intraocular end density as mean
vitreous volume 56 μl (22)]
Y-39983 intravitreal injection, respectively, immediately after
ONC. The doses of Y-39983 used in the present study were chosen
according to a previous study (18). The second injection of 3 μl PBS or
20 μM Y-39983 was performed on day 7 after ONC. Groups of rats were
sacrificed 15 days after surgery.
Sample preparations of the retina and
optic nerves
Fifteen days after ONC, deeply anesthetized rats
were transcardially perfused with 4% paraformaldehyde. For mRNA
analysis, the rats were directly sacrificed by an intraperitoneal
overdose of anesthetic without transcardial perfusion to avoid mRNA
degradation. The eyes were enucleated with at least 5 mm of optic
nerve attached and bisected. The eyes were dissected as eye cups
without cornea and lens; the optic nerves were dissected free from
connective tissue. Retinas were dissected from the underlying
sclera for further use. The optic nerves were immediately fixed
overnight, then transferred to 30% sucrose solution overnight
(4°C), and the frozen sections were cut. Optic nerve longitudinal
sections (16 μm) were cut along the optic nerve anterior-posterior
axis. The sections were collected on gelatin-coated glass slides
and stored at −80°C for further use.
RGC densities
Retinas were dissected from the underlying sclera,
flattened by four radial cuts, and mounted vitreal side-up on
gelatin-coated slides. Labeled RGCs were examined by a confocal
microscope (Axiovert 35; Zeiss, Germany) using a rhodamine filter
(560 nm for DiI). RGC densities were determined by counting labeled
RGCs in 12 distinct areas of 62,500 μm2 each (four areas
per retinal quadrant at three different eccentricities of 1/6, 3/6
and 5/6 of the retinal radius). Cell counting was performed with a
computerized image-analysis system (Image Pro Plus version 6.0;
Media Cybernetics, Silver Spring, MD, USA) in duplicate by two
independent investigators in a blinded manner. The averages of the
number of RGCs in 12 distinct areas were taken as the mean density
of RGCs for each retina. Eighteen rat retinas were used for RGC
densities analysis and there were six rats in each group.
Immunohistochemistry
Optic nerve sections were dehydrated at 37°C for 1
h, and unspecific binding was blocked by application of 10% normal
goat serum. Primary antibodies including GAP-43 monoclonal antibody
(1:100, sc-17790), ROCK1 monoclonal antibody (1:50, sc-17794),
ROCK2 polyclonal antibody (1:50, sc-5561) (all from Santa Cruz
Biotechnology, Santa Cruz, CA, USA) were applied at 4°C overnight.
Negative controls were performed by replacing the primary antibody
with PBS or serum. Secondary antibodies including Cy-3-labeled
anti-rat IgG, FITC-labeled anti-rabbit or anti-mouse antibody (all
from Invitrogen, CA, USA) were applied (1:100) for 45 min at room
temperature. Cell nuclei were counterstained with DAPI
(4′,6-diamidino-2-phenylindole) (1:1000; Sigma-Aldrich, St. Louis,
MO, USA). Immunoreactivity was examined with a confocal microscope
(Axiovert 35, Zeiss, Germany). For evaluation of axon regeneration
of RGCs, counting of the GAP-43-positive axons was performed as
previously described (23).
Briefly, the optic nerve sections with anti-GAP-43 immunoreactivity
were photographed using a confocal microscope. Images of whole
sections were assembled from single images captured with a ×20
objective. Using a calibrated ocular to measure distance, we
counted the number of GAP-43-positive axons crossing a line at
distance d (50, 250, 500 μm) from the end of the crush site.
By measuring the cross-sectional width of the nerve at the point at
which the counts were taken, we converted axon counts into axon
crossings per unit nerve width (axons per millimeter) and obtained
the average of these over the four sections. ∑ad,
the total number of axons extending distance d in a nerve
having a radius of r, was estimated by summing over all
sections of thickness t. For all immunohistochemical
staining, three sections per eye were examined and there were six
rats in each group.
In situ active-RhoA pull-down assay
Active-RhoA pull-down assay was performed according
to a modified previous published method (24,25).
Briefly, 16-μm frozen sections of optic nerve were post-fixed in 4%
paraformaldehyde and incubated with glutathione-S-transferase
(GST)-Rho-binding domain (RBD) fushion protein (14–662; Millipore,
Billerica, MA, USA) overnight at 4°C. Sections were then washed 3
times in PBS, blocked in 10% normal goat serum for 1 h at room
temperature and incubated with an anti-GST antibody (Millipore)
overnight at 4°C. Sections were then washed in PBS, incubated with
Cy-3-labeled anti-rat secondary antibodies (Invitrogen). The
specimens were imaged with a confocal microscope (Axiovert 35,
Zeiss). Three sections per eye were examined and there were six
rats in each group.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Twenty-four rat retinas were used for RT-PCR
analysis. Each retina served as an individual sample (n=8 per
group). Total RNA was isolated from the pulverized samples using
the TRIzol® reagent (Invitrogen). The concentration and
purity of the preparations were determined by measuring the
absorbance at 260/280 nm in a spectrophotometer (Beckman). Total
RNA was reverse-transcribed into cDNA in a 20-μl reaction
containing 2 μg RNA, 4 μl 5× M-MLV buffer, 2 μl of dNTP, 1 μl
random hexamer primer, 0.5 μl of RNase inhibitor and 1 μl of M-MLV
RTase. Reactions were performed at 25°C for 10 min, at 42°C for 60
min and at 70°C for 10 min.
The following targets were analyzed by RT-PCR. The
nucleotide sequences of the primers were based on previously
published sequences (26,27). The primer sequences used for RT-PCR
were: RhoA, 5′-GTGATTGTTGGTGATGGAGC-3′ and
5′-CTCGTGGCCATCTCAAAAAC-3′; ROCK-1, 5′-TGC GGGAGTTACAAGATCAGCT-3′
and 5′-TTTCCGTCA GTCTCATCAGCAC-3′; ROCK-2, 5′-TCTGAAAGGAGG
GACCGAACC-3′ and 5′-GTTCCTGTTTGTGTCGAGCCA TCA-3′; glyceraldehyde
3-phosphate dehydrogenase (GAPDH), 5′-ATGGGGAAGGTGAAGGTCGG-3′ and
5′-CAGGAGG CATTGCTGATGAT-3′. The PCR protocol comprised an initial
incubation for 5 min at 94°C; 30 cycles (for RhoA, ROCK-1 and
ROCK-2) or 25 cycles (for GAPDH) of 45 sec at 94°C, 45 sec at 55°C,
and 2 min at 72°C and a final incubation for 7 min at 72°C. These
PCR products were separated by 2% agarose gel electrophoresis and
stained with 0.5 μg/ml ethidium bromide, and the band signals were
exposed to ultraviolet light before they were scanned and
quantified with a gel image analyzer (GelDoc Quantity One; Bio-Rad,
Hercules, CA, USA). Band intensities were quantified and normalized
against that of GAPDH.
Western blot analysis
Total retinal protein was extracted from pulverized
samples using modified radioimmunoprecipitation (modified RIPA)
buffer with a Halt™ protease and phosphatase inhibitor cocktail
(Thermo Scientific, Rockford, IL, USA). The protein concentrations
were determined using the method of the Bradford protein assay
(Bio-Rad). Each retina served as an individual sample (n=8 per
group). Equal amounts of protein (20 μg/lane) were separated on
polyacrylamide gels and then electrotransferred onto nitrocellulose
membranes (Amersham, UK). After blocking for 3 h in Tris-buffered
saline with 0.1% Tween-20 (TBST) and 3% bovine serum albumin (BSA),
membranes were incubated overnight at 4°C with the primary
antibodies [GAP-43 (1:100, sc-17790), ROCK1 (1:50, sc-17794), ROCK2
(1:50, sc-5561)] in TBST containing 3% BSA. Membranes were then
washed and incubated with alkaline phosphatase-conjugated secondary
antibodies in TBST for 2 h and developed using nitro blue
tetrazolium chloride (NBT)/5-bromo-4-chloro-3-indolylphosphate
(BCIP) substrate (Promega, Madison, WI, USA). The densities of the
bands on the membrane were scanned and analyzed with Image Pro Plus
version 6.0 (Media Cybernetics). Twenty-four rat retinas were used
for western blot analysis.
RhoA activity assay
Active RhoA was assayed from tissue lysates using a
Rho activation assay kit (Upstate Biotechnology, Milton Keynes, UK)
following the manufacturer’s instructions as described elsewhere
(25). Twenty-four rat retinas were
used for the RhoA activity assay (n=8 per group).
Statistical analyses
Data are expressed as means ± standard deviation,
unless otherwise stated. Statistical analyses were performed using
SPSS software (IBM SPSS Statistics 19.0). To compare data among the
three groups, one-way analysis of variance (ANOVA) followed by post
hoc tests was conducted. A P-value <0.05 was indicative of a
statistically significant result.
Results
Y-39983 promotes RGC survival and axonal
regeneration after ONC
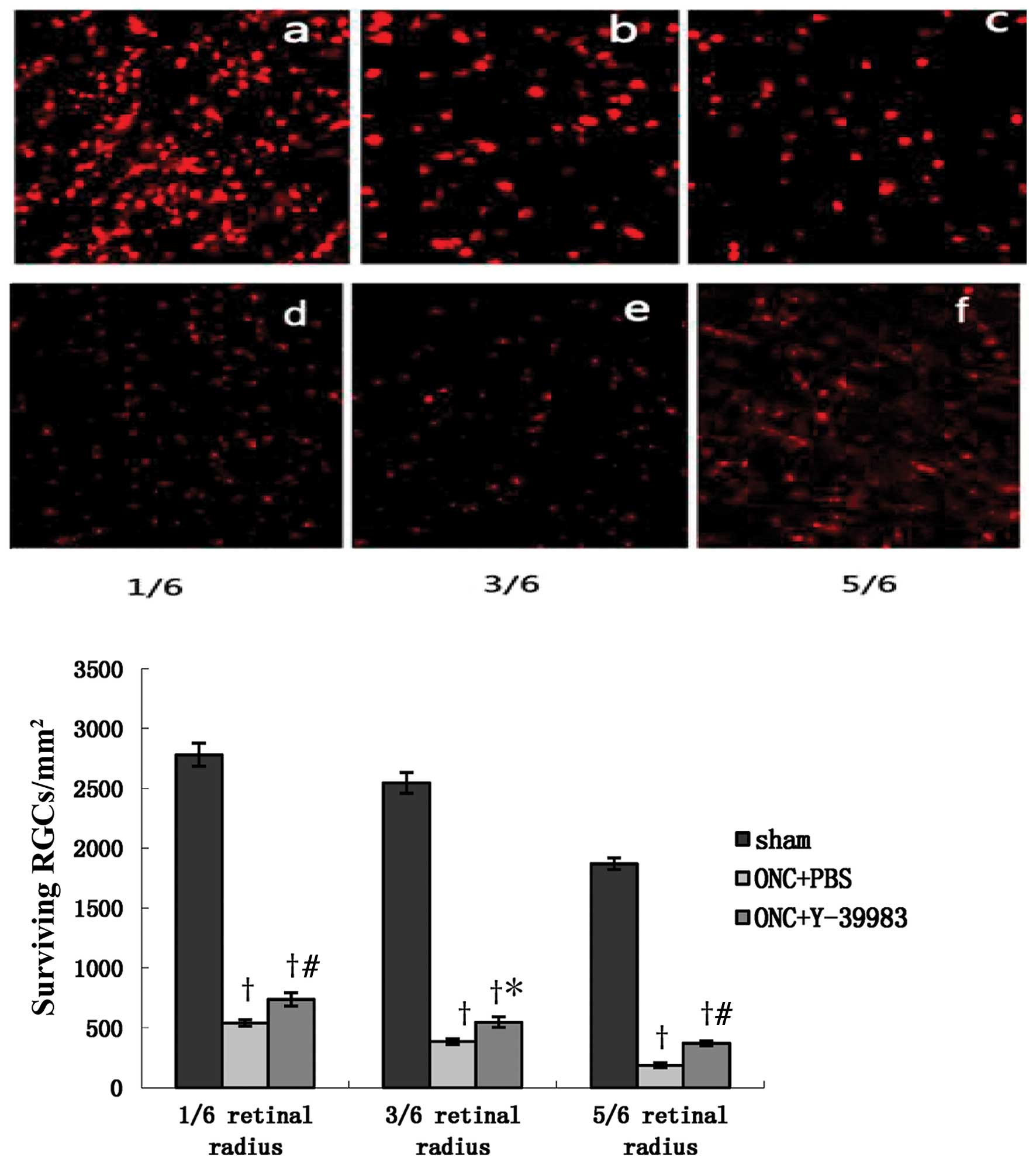
The densities of surviving RGCs in the ONC+PBS group
and ONC+Y-39983 group were significantly lower than that in the
sham group. However, the density of surviving RGCs in the
ONC+Y-39983 group was significantly higher than that in the ONC+PBS
group, and the densities of surviving RGCs at 1/6, 3/6, 5/6 of the
retinal radius in the ONC+Y-39983 group were significantly higher
than that at the corresponding regions in the ONC+PBS group,
respectively (Fig. 1). The
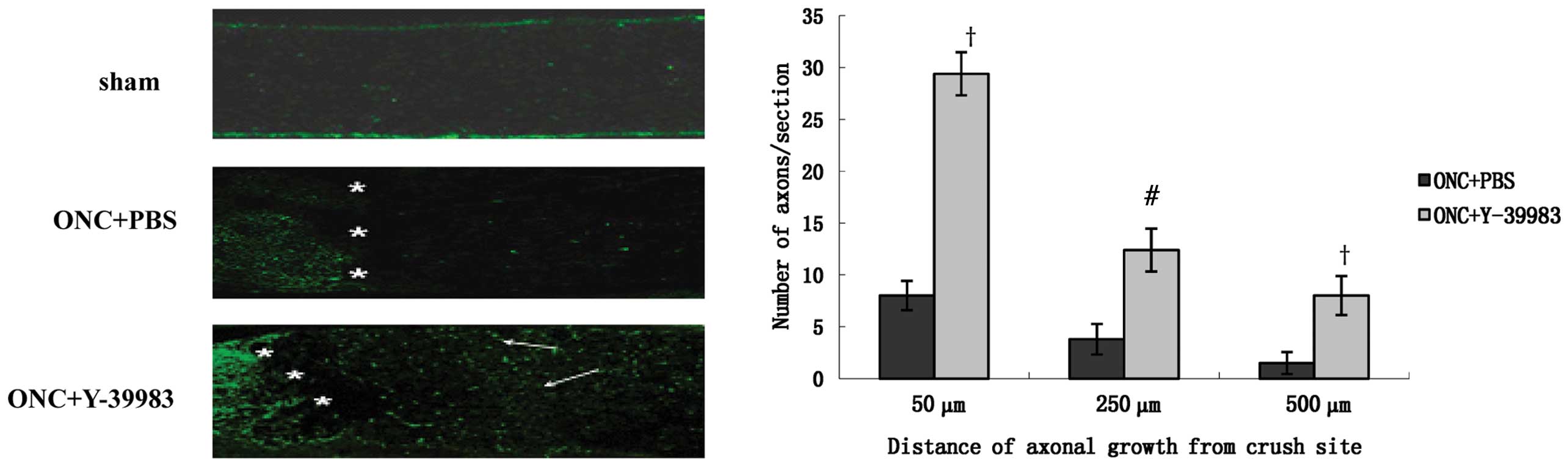
longitudinal optic nerve sections were immunostained for GAP-43 to
identify the regenerating axons. The sham group exhibited almost no
GAP-43 positive staining and the ONC+PBS group showed few
GAP-43-positive axons throughout the crush site (Fig. 2). The ONC+Y-39983 group exhibited
numerous GAP-43-positive axons throughout the crush site and distal
optic nerve segment (Fig. 2). The
number of axons 50, 250 and 500 μm from the crush site in the
ONC+Y-39983 group was significantly more than that at the
corresponding distances in the ONC+PBS group, respectively
(Fig. 2).
Y-39983 increases GAP-43 protein
expression in the retina after ONC
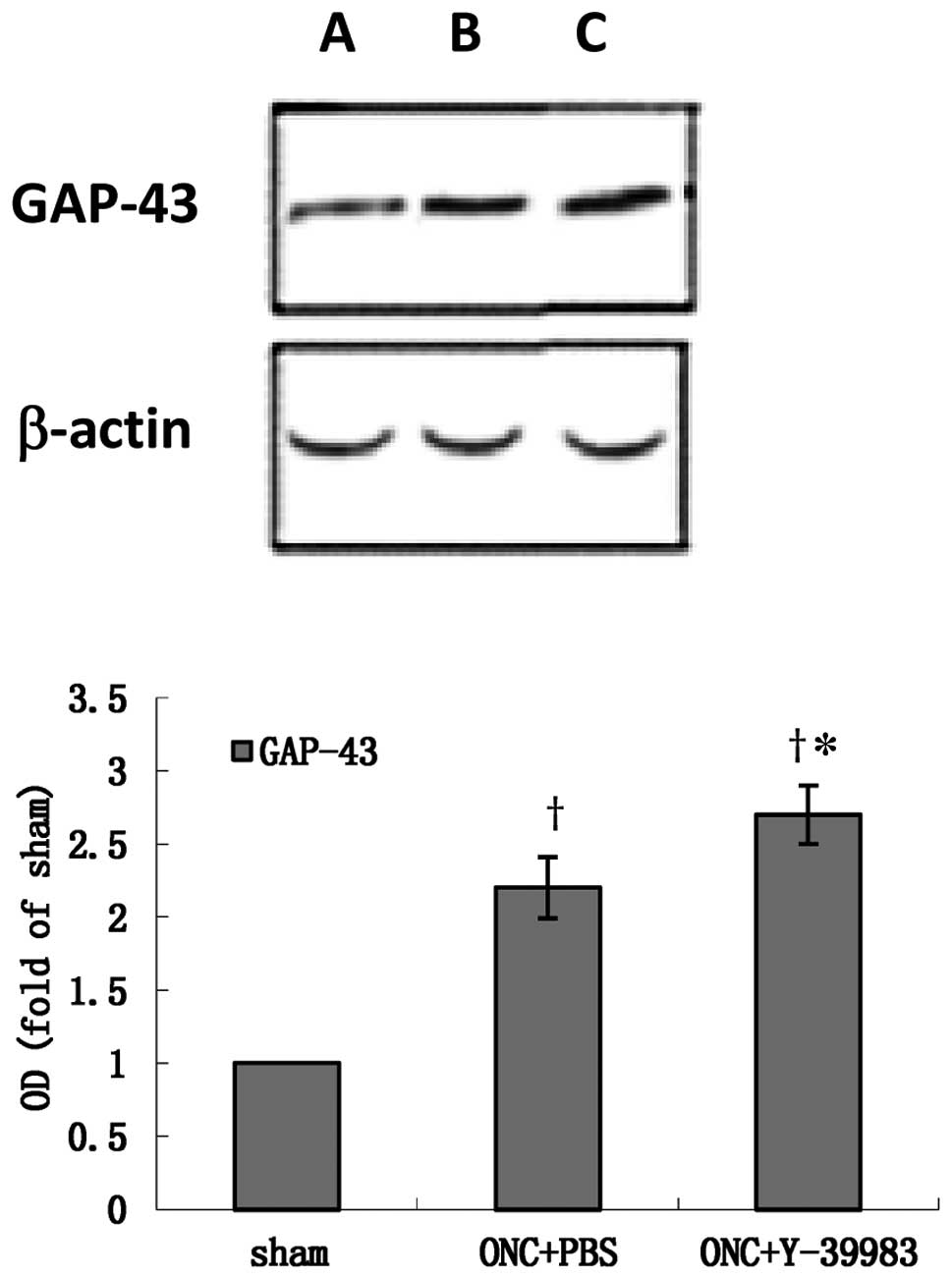
The GAP-43 protein expression in the retina was
detected by western blotting on day 15 after ONC. Compared with the
sham group, the GAP-43 expression in the retina in the ONC+PBS and
ONC+Y-39983 groups was significantly increased (Fig. 3). The GAP-43 expression in the
retina in the ONC+Y-39983 group was significant higher than that in
the ONC+PBS group (Fig. 3), which
suggested that Y-39983 upregulates the GAP-43 expression in the
retina after ONC.
Y-39983 downregulates the expression of
active-RhoA, ROCK1 and ROCK2 after ONC
RhoA, ROCK1 and ROCK2 mRNA expression in the retina
was detected by RT-PCR on day 15 after ONC. Total RhoA mRNA
expression did not differ substantially among the sham, ONC+PBS and
ONC+Y-39983 groups (Fig. 4). mRNA
expression of ROCK1 and ROCK2 in the ONC+PBS group was significant
higher than that in the sham group. Y-39983 downregulated the mRNA
expression of ROCK1 and ROCK2 in the retina (Fig. 4).
The protein expression of total-RhoA, active-RhoA,
ROCK1 and ROCK2 in the retina or optic nerve was detected by
immunohistochemistry, western blotting and affinity precipitation
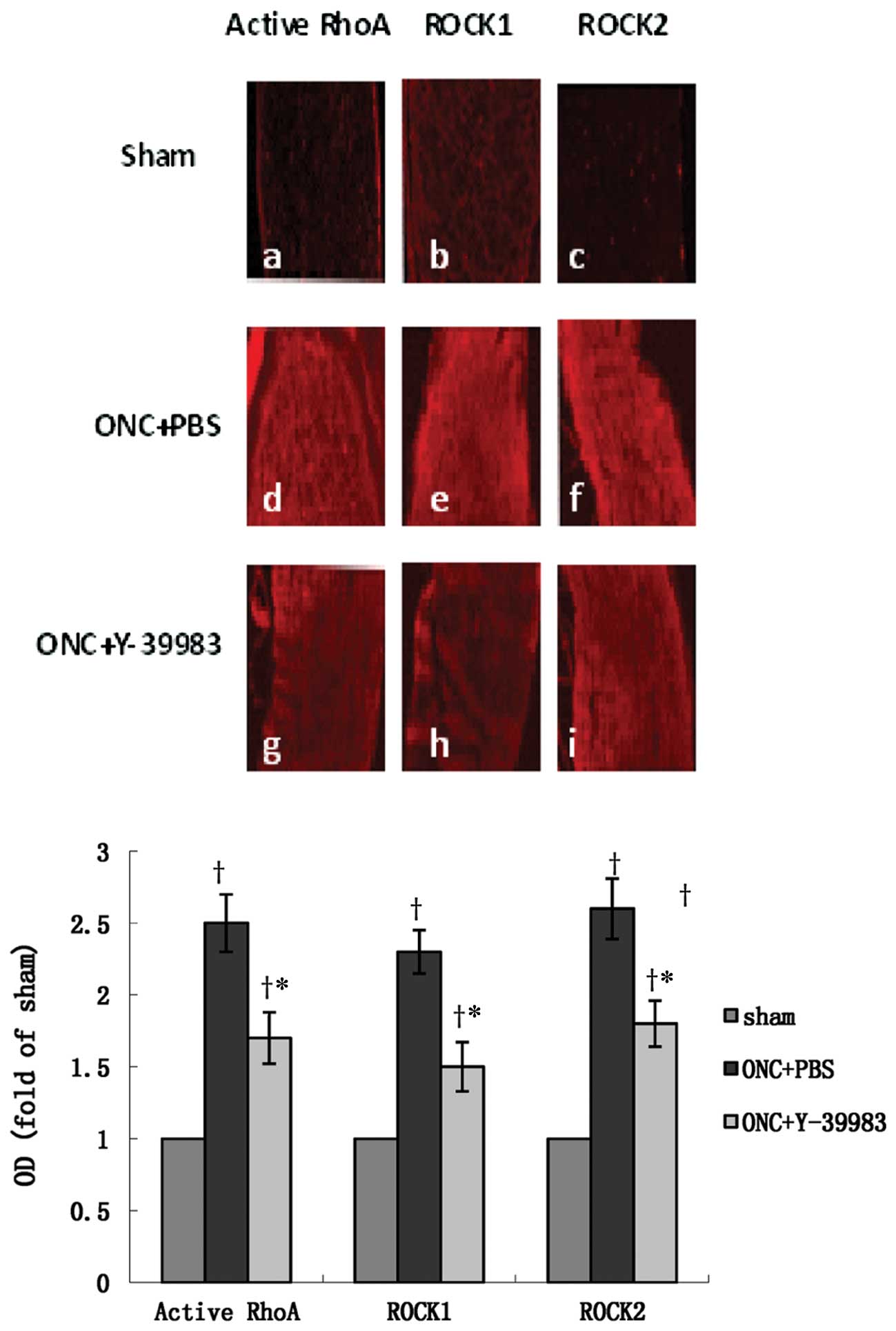
on day 15 after ONC. The optic nerve in the sham group showed
almost no visible active RhoA, ROCK1 and ROCK2 immunofluorescence
staining (Fig. 5a-c). The optic
nerve in the ONC+PBS group showed strong immunoactivity of active
RhoA, ROCK1 and ROCK2 (Fig. 5d-f),
while Y-39983 downregulated the immunoactivity of active RhoA,
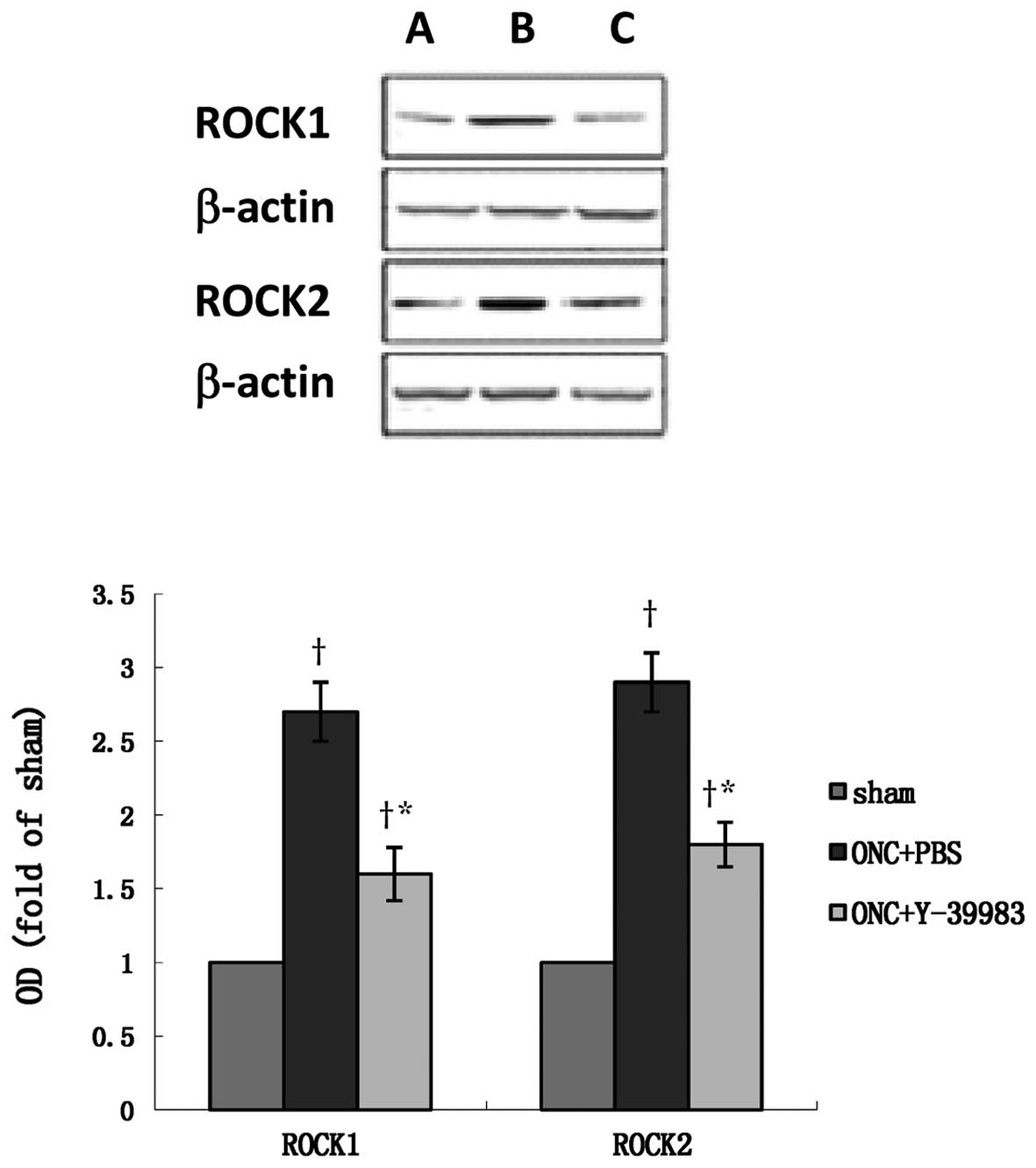
ROCK1 and ROCK2 in the optic nerve after ONC (Fig. 5g-i). Compared with the sham group,
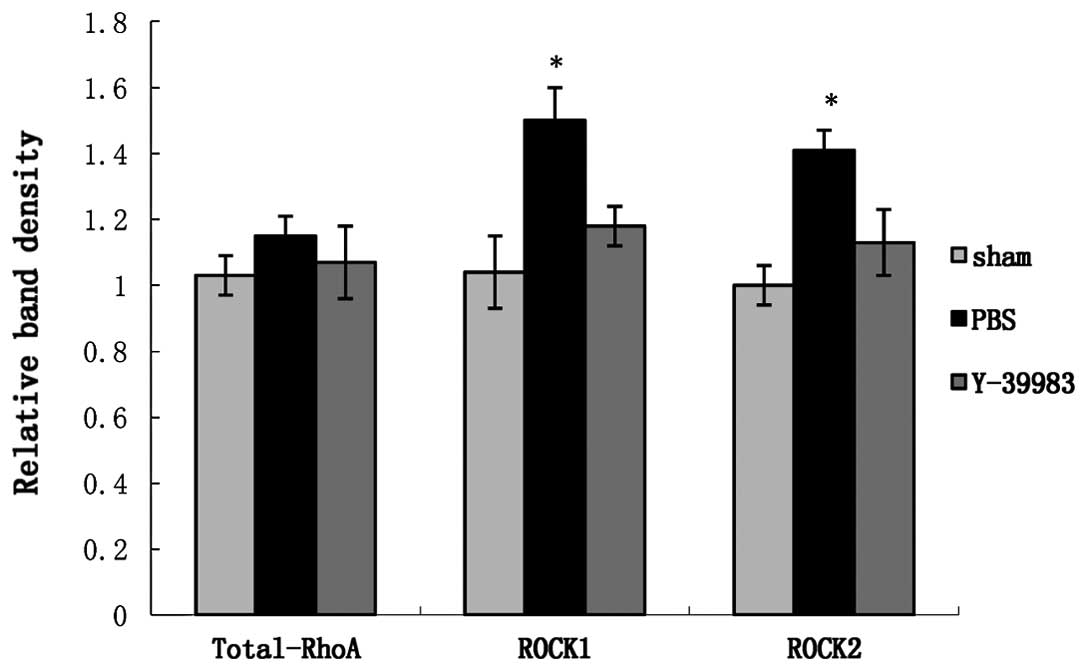
the ROCK1 and ROCK2 protein expression in the retina in the ONC+PBS
group was significantly increased (Fig.
6), while Y-39983 downregulated the ROCK1 and ROCK2 protein
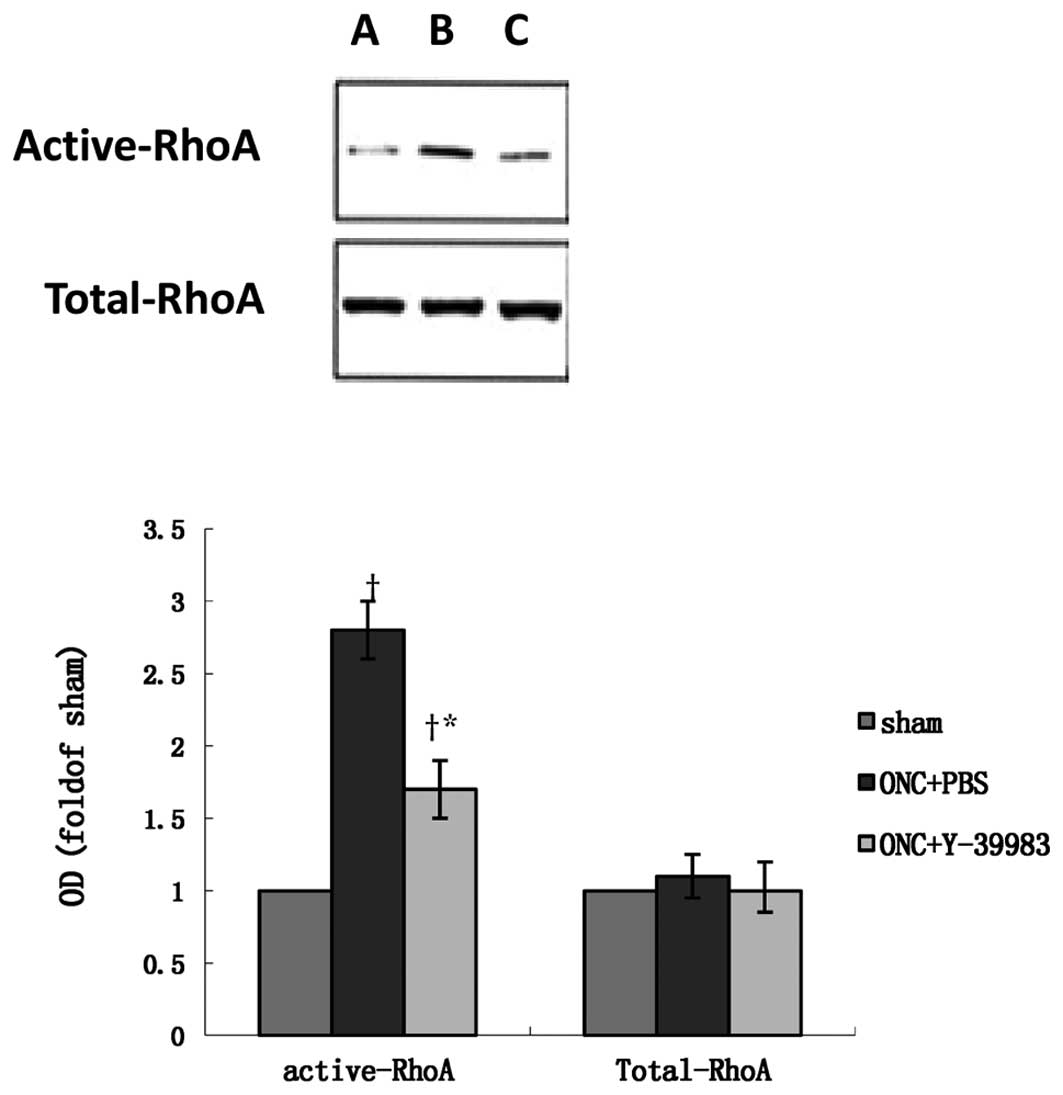
expression in the retina on day 15 after ONC (Fig. 6). The total-RhoA protein expression
in the retina remained unchanged in all groups as detected by the
western blot analysis (Fig. 7).
Compared with the sham group, the active-RhoA protein expression in
the retina in the ONC+PBS group was significantly increased, while
Y-39983 downregulated the active-RhoA protein expression in the
retina on day 15 after ONC (Fig.
7).
Discussion
The multitude of inhibitory molecules present in the
lesioned CNS requires a comprehensive strategy with which to
identify common inhibitory pathways. The Rho/ROCK pathway
represents such a promising target for pharmacological intervention
being the convergence point for the signaling of numerous
inhibitory substrates. Several ROCK inhibitors, such as Y-27632
(8,28,29),
Y-39983 (17,18,28,29),
fasudil (28) and dimethylfasudil
(28), have been used for the study
of neuroprotection and axonal regeneration. These inhibitors can
promote the neurite outgrowth of RGCs (17,28).
In terms of their intensity, the effect of Y-39983 on the promotion
of neurite outgrowth was stronger than that of Y-27632, fasudil and
dimethylfasudil (17,28). Thus, Y-39983, serving as a candidate
of ROCK inhibitors, was chosen for use in this study.
In order to clarify the biological effects of
Y-39983, we investigated the effects of Y-39983 on RhoA/ROCK
expression during its promotion of axonal regeneration. The present
study found that ONC upregulated the expression of active-RhoA,
ROCK1 and ROCK2 in the retina or optic nerve. However, Y-39983
downregulated active-RhoA, ROCK1 and ROCK2 expression in the retina
or optic nerve on day 15 after ONC and significantly promoted RGC
axonal regeneration after ONC.
In addition to its effect of promoting RGC axonal
regeneration, Y-39983 could relax isolated ciliary artery (29), increase blood flow in the optic
nerve head (18), relax the ciliary
muscle and trabecular meshwork (30) and decrease intraocular pressure
(IOP) (30–32). Thus, Y-39983 may be a candidate drug
not only for lowering IOP but also for increasing blood flow in the
optic nerve head in the treatment of glaucoma. Moreover, Y-39983
may have therapeutic potential for axonal regeneration of RGCs in
the treatment of diseases presenting with degenerating axons of
RGCs including glaucoma.
In summary, we investigated the effects of Y-39983
on RhoA/ROCK expression during its promotion of axonal
regeneration. Y-39983 promoted RGC axonal regeneration and
downregulated the expression of active-RhoA, ROCK1 and ROCK2 in the
retina or optic nerve on day 15 after ONC. The present study
further elucidated the pharmacological effects of Y-39983, making
it a promising drug for future therapeutic approaches.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (nos. 81070728 and 81000373), Shanghai
Natural Science Foundation (nos. 08ZR1413900 and 11ZR1422000),
Shanghai Leading Academic Discipline Project (no. S30205) and
Shanghai ‘Science and Technology Innovation Action Plan’ Basic
Research Key Project (nos. 11JC1407700 and 11JC1407701).
References
|
1
|
Schreyer DJ and Skene JH:
Injury-associated induction of GAP-43 expression displays axon
branch specificity in rat dorsal root ganglion neurons. J
Neurobiol. 24:959–970. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schaden H, Stuermer CA and Bahr M: GAP-43
immunoreactivity and axon regeneration in retinal ganglion cells of
the rat. J Neurobiol. 25:1570–1578. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Domeniconi M and Filbin MT: Overcoming
inhibitors in myelin to promote axonal regeneration. J Neurol Sci.
233:43–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cafferty WB, Duffy P, Huebner E and
Strittmatter SM: MAG and OMgp synergize with Nogo-A to restrict
axonal growth and neurological recovery after spinal cord trauma. J
Neurosci. 30:6825–6837. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winton MJ, Dubreuil CI, Lasko D, Leclerc N
and McKerracher L: Characterization of new cell permeable C3-like
proteins that inactivate Rho and stimulate neurite outgrowth on
inhibitory substrates. J Biol Chem. 277:32820–32829. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dergham P, Ellezam B, Essagian C,
Avedissian H, Lubell WD and McKerracher L: Rho signaling pathway
targeted to promote spinal cord repair. J Neurosci. 22:6570–6577.
2002.PubMed/NCBI
|
|
7
|
Bertrand J, Winton MJ, Rodriguez-Hernandez
N, Campenot RB and McKerracher L: Application of Rho antagonist to
neuronal cell bodies promotes neurite growth in compartmented
cultures and regeneration of retinal ganglion cell axons in the
optic nerve of adult rats. J Neurosci. 25:1113–1121. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lingor P, Tönges L, Pieper N, Bermel C,
Barski E, Planchamp V and Bähr M: ROCK inhibition and CNTF interact
on intrinsic signalling pathways and differentially regulate
survival and regeneration in retinal ganglion cells. Brain.
131:250–263. 2008.PubMed/NCBI
|
|
9
|
Zhang G, Lehmann HC, Manoharan S, Hashmi
M, Shim S, Ming GL, Schnaar RL, Lopez PH, Bogdanova N and Sheikh
KA: Anti-ganglioside antibody-mediated activation of RhoA induces
inhibition of neurite outgrowth. J Neurosci. 31:1664–1675. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Riento K and Ridley AJ: Rocks:
multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol.
4:446–456. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olson MF: Applications for ROCK kinase
inhibition. Curr Opin Cell Biol. 20:242–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brown ME and Bridgman PC: Myosin function
in nervous and sensory systems. J Neurobiol. 58:118–130. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishizaki T, Maekawa M, Fujisawa K, Okawa
K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N
and Narumiya S: The small GTP-binding protein Rho binds to and
activates a 160 kDa Ser/Thr protein kinase homologous to myotonic
dystrophy kinase. EMBO J. 15:1885–1893. 1996.PubMed/NCBI
|
|
14
|
Mehta NR, Nguyen T, Bullen JW Jr, Griffin
JW and Schnaar RL: Myelin-associated glycoprotein (MAG) protects
neurons from acute toxicity using a ganglioside-dependent
mechanism. ACS Chem Neurosci. 1:215–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watanabe M: Regeneration of optic nerve
fibers of adult mammals. Dev Growth Differ. 52:567–576. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ramachandran C, Patil RV, Combrink K,
Sharif NA and Srinivas SP: Rho-Rho kinase pathway in the actomyosin
contraction and cell-matrix adhesion in immortalized human
trabecular meshwork cells. Mol Vis. 17:1877–1890. 2011.PubMed/NCBI
|
|
17
|
Sagawa H, Terasaki H, Nakamura M, Ichikawa
M, Yata T, Tokita Y and Watanabe M: A novel ROCK inhibitor,
Y-39983, promotes regeneration of crushed axons of retinal ganglion
cells into the optic nerve of adult cats. Exp Neurol. 205:230–240.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tokushige H, Waki M, Takayama Y and
Tanihara H: Effects of Y-39983, a selective Rho-associated protein
kinase inhibitor, on blood flow in optic nerve head in rabbits and
axonal regeneration of retinal ganglion cells in rats. Curr Eye
Res. 36:964–970. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu S, Tanabe T and Yoshimura N: A rat
model of glaucoma induced by episcleral vein ligation. Exp Eye Res.
83:758–770. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leon S, Yin Y, Nguyen J, Irwin N and
Benowitz LI: Lens injury stimulates axon regeneration in the mature
rat optic nerve. J Neurosci. 20:4615–4626. 2000.PubMed/NCBI
|
|
21
|
Fischer D, Pavlidis M and Thanos S:
Cataractogenic lens injury prevents traumatic ganglion cell death
and promotes axonal regeneration both in vivo and in culture.
Invest Ophthalmol Vis Sci. 41:3943–3954. 2000.PubMed/NCBI
|
|
22
|
Berkowitz BA, Lukaszew RA, Mullins CM and
Penn JS: Impaired hyaloidal circulation function and uncoordinated
ocular growth patterns in experimental retinopathy of prematurity.
Invest Ophthalmol Vis Sci. 39:391–396. 1998.
|
|
23
|
Yin Y, Cui Q, Li Y, Irwin N, Fischer D,
Harvey AR and Benowitz LI: Macrophage-derived factors stimulate
optic nerve regeneration. J Neurosci. 23:2284–2293. 2003.PubMed/NCBI
|
|
24
|
Dubreuil CI, Winton MJ and McKerracher L:
Rho activation patterns after spinal cord injury and the role of
activated Rho in apoptosis in the central nervous system. J Cell
Biol. 162:233–243. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ahmed Z, Suggate EL, Brown ER, Dent RG,
Armstrong SJ, Barrett LB, Berry M and Logan A: Schwann cell-derived
factor-induced modulation of the NgR/p75NTR/EGFR axis disinhibits
axon growth through CNS myelin in vivo and in vitro. Brain.
129:1517–1533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakamura M, Nagano T, Chikama T and
Nishida T: Role of the Small GTP-binding protein Rho in epithelial
cell migration in the rabbit cornea. Invest Ophthalmol Vis Sci.
42:941–947. 2001.PubMed/NCBI
|
|
27
|
Kim BK, Kim HM, Chung KS, Kim DM, Park SK,
Song A, Won KJ, Lee K, Oh YK, Lee K, Song KB, Simon JA, Han G and
Won M: Upregulation of RhoB via c-Jun N-terminal kinase signaling
induces apoptosis of the human gastric carcinoma NUGC-3 cells
treated with NSC12618. Carcinogenesis. 32:254–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lingor P, Teusch N, Schwarz K, Mueller R,
Mack H, Bähr M and Mueller BK: Inhibition of Rho kinase (ROCK)
increases neurite outgrowth on chondroitin sulphate proteoglycan in
vitro and axonal regeneration in the adult optic nerve in vivo. J
Neurochem. 103:181–189. 2007.PubMed/NCBI
|
|
29
|
Watabe H, Abe S and Yoshitomi T: Effects
of Rho-associated protein kinase inhibitors Y-27632 and Y-39983 on
isolated rabbit ciliary arteries. Jpn J Ophthalmol. 55:411–417.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakajima E, Nakajima T, Minagawa Y,
Shearer TR and Azuma M: Contribution of ROCK in contraction of
trabecular meshwork: proposed mechanism for regulating aqueous
outflow in monkey and human eyes. J Pharm Sci. 94:701–708. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tokushige H, Inatani M, Nemoto S, Sakaki
H, Katayama K, Uehata M and Tanihara H: Effects of topical
administration of y-39983, a selective rho-associated protein
kinase inhibitor, on ocular tissues in rabbits and monkeys. Invest
Ophthalmol Vis Sci. 48:3216–3222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Whitlock NA, Harrison B, Mixon T, Yu XQ,
Wilson A, Gerhardt B, Eberhart DE, Abuin A and Rice DS: Decreased
intraocular pressure in mice following either pharmacological or
genetic inhibition of ROCK. J Ocul Pharmacol Ther. 25:187–194.
2009. View Article : Google Scholar : PubMed/NCBI
|