Introduction
Ovarian cancer has the highest mortality rate of all
the gynecological cancers (1). The
standard treatment is aggressive surgery followed by platinum and
taxane-based chemotherapy. Due to the continuous improvement in
surgical and diagnostic techniques, response rates have improved;
however, the overall survival remains low (31%) (2). Poor prognosis is mainly due to the
late diagnosis of stage III and IV ovarian cancer and acquired
chemoresistance from standard therapy. Therefore, a better
understanding of the molecular mechanism of ovarian carcinogenesis
and metastasis may provide insights for the development of novel
therapeutic strategies.
The human epidermal growth factor receptor-2 (HER-2)
gene is a member of the epidermal growth factor receptor (EGFR)
family and encodes a 185-kDa protein with tyrosine kinase activity
(3,4). The HER-2 protein can dimerize with
other members of the EGFR family. Additionally, the overexpression
of the HER-2 gene has been shown to enhance the kinase-mediated
activation of downstream signaling pathways, such as
mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3
kinase (PI3K) (5). Experimental and
clinical data have demonstrated that the overexpression or
amplification of HER-2 correlate with tumorigenesis in a variety of
tumors (6). The blockade of the
HER-2 signaling pathway represents an opportunity for the
development of novel and selective anticancer therapies (7,8).
Although previous studies have demonstrated that HER-2-blocking
antibodies are somewhat effective, the clinical application of
these treatments is controversial (9). HER-2 is one of the most widely
characterized oncogenes linked with poor prognosis for ovarian
cancer (10). Previous studies have
shown that the overexpression of the HER-2 gene is found in
approximately 30% of human ovarian cancers and correlates with a
more advanced tumor stage and increased chemoresistance (11).
RNA interference (RNAi) has become an excellent
research tool for the silencing of target genes (12). Short interfering RNAs (siRNAs),
21–23 nucleotides (nt) in length, can silence the targeted gene by
binding to complementary mRNA and interfering with gene expression
(13). A previous study
demonstrated that the transfection of synthetic 21-nt siRNA
duplexes into mammalian cells efficiently inhibited endogenous gene
expression in a sequence-specific manner (14). However, studies on the RNAi
silencing of HER-2 expression and its role in ovarian cancer are
limited.
In the present study, we designed siRNAs that
targeted different regions within the open reading frame of HER-2
mRNA. We examined their ability to interfere with HER-2 gene
expression, utilizing the HER-2-positive ovarian cancer cell line,
SKOV-3. Specifically, we assessed HER-2 inhibition by examining
cell proliferation, apoptosis, cell invasion and markers of
epithelial-mesenchymal transition (EMT) in SKOV-3 cells.
Materials and methods
Cell lines and cell culture
The SKOV-3 ovarian cancer cell line was obtained
from the China Center for Type Culture Collection (Wuhan, China).
Cell culture was performed according to the manufacturer's
instructions. The cells were routinely maintained in McCoy's 5A
medium (Gibco-BRL, Invitrogen, Carlsbad, CA) supplemented with 10%
fetal bovine serum (FBS) (Gibco-BRL) and 1% penicillin in a
well-humidified incubator of 5% CO2 at 37°C.
siRNA preparation
HER-2 cDNA sequences were selected based on HER-2
target sites (obtained from GenBank) using siRNA design software
from Invitrogen Life Technologies (Grand Island, NY). The siRNA
target design tools from Ambion® were used to design the
HER-2 siRNA, as well as non-specific siRNA sequences corresponding
to the HER-2 gene, with 3′ overhanging UU dinucleotides. The
sequences were designed for the regions of HER-2 mRNA that were the
least homologous to the other HER-2 family members to ensure
affinity. Briefly, the siRNAs were reconstituted in annealing
buffer, as per the manufacturer's instructions, to obtain a 20-μM
solution. Three different regions within the HER-2 gene (NCBI
accession no. M11730) were used in this study: siRNA-1 (positions
2215–2237), siRNA-2 (positions 2644–2666) and siRNA-3 (positions
2734–2756). siRNA-3S (scrambled) was constructed from random
sequences of siRNA-3 and served as the control (depending on the
experimental results). All siRNAs contained a 19-bp double-stranded
(ds) sequence and symmetric 3′ overhangs of two deoxythymidines.
HER-2 siRNA-specific sequences were as follows: i) HER-2 siRNA-1
sense, 5′-GAUCC GGAAGUACACGAUGUU-3′ and antisense, 5′-CAUCGUGU
ACUUCCGGAUCUU-3′; ii) HER-2 siRNA-2 sense, 5′-GAGU
CCCAACCAUGUCAAAUU-3′ and antisense, 5′-UUUGACA UGGUUGGGACUCUU-3′;
iii) HER-2 siRNA-3 sense, 5′-CUG GUGUAUGCAGAUUGCCUU-3′ and
antisense, 5′-GGCAA UCUGCAUACACCAGUU-3′; siRNA-3S sense, 5′-CUUGU
AUGGGCAGAUUGCCUU-3′ and antisense, 5′-GGCAAUC UGCCCAUACAAGUU-3′;
and T7DNA template, 5′-CCTG TCTC-3′. All siRNAs were synthesized
in vitro using T7 transcription.
siRNA transfection
Cells were plated in growth medium without
antibiotics for ~24 h prior to transfection. Transient transfection
of siRNA was performed with Lipofectamine 2000 (Invitrogen)
according to the manufacturer's instructions. The transfected cells
were then cultured for 6 h and the culture medium was replaced with
fresh medium supplemented with 10% FBS. The cells were harvested 24
h after transfection.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted using TRIzol (Invitrogen)
according to the manufacturer's instructions. For RT-PCR analysis,
5 μg of total RNA was reverse-transcribed using RT-PCR kits
(Promega, Madison, WI). PCR products were analyzed using standard
agarose gel electrophoresis with ethidium bromide. The primer
sequences used were as follows: forward primer,
5′-CCTGCTGAACTGGTGTATGCA-3′ and reverse primer,
5′-TCAGAGTCAATCATCCAACATTTG-3′.
Western blot analysis
Total protein was extracted using
radioimmunoprecipitation assay (RIPA) buffer. Protein (50 μg) was
loaded onto SDS-PAGE gels (8–10%) and electrophoresed (200 V, 2 h),
transferred onto PVDF membranes (BD Biosciences) and hybridized
with primary antibodies, against HER-2, GAPDH, β-actin (1:1000,
Zhongshan, Beijing, China), E-cadherin, vimentin, N-cadherin and
matrix metallopeptidase-9 (MMP-9) (1:1000, Santa Cruz
Biotechnology, Santa Cruz, CA), followed by incubation with the
appropriate goat anti-rabbit secondary antibodies conjugated with
alkaline phosphatase (1:2000, Zhongshan). Antibody binding was
detected using a BCIP/NBT kit (Zhongshan).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
MTT assay (Sigma, St. Louis, MO) was performed to
assess the silencing effect of HER-2 on SKOV-3 cell proliferation.
Cells (1,000/well) were plated in 96-well plates. The growth was
monitored at various time-points (24, 48, 72 and 96 h) and cell
viability was measured using the MTT assay. Cell viability was
expressed as optical density (OD). Absorbance was measured at 490
nm using a microplate reader (VersaMax, Molecular Devices,
Sunnyvale, CA).
Annexin V apoptosis assay
The Annexin V-FITC-labeled Apoptosis Detection kit
(Beijing Biosea Biotechnology) was used to assess apoptosis by flow
cytometry, according to the manufacturer's instructions. At 48 h
after transfection, a total of 5×105 SKOV-3 cells were
transferred to a sterile tube, in which 5 μl of Annexin V and 5 μl
of propidium iodide were added. The cells were then allowed to
incubate at room temperature for 15 min and analyzed by flow
cytometry (FACSCalibur, BD Biosciences, San Jose, CA).
Apoptosis in situ
The terminal deoxynucleotidyltransferase-mediated
dUTP-biotin nick end-labeling (TUNEL) assay was performed according
to the manufacturer's instructions using the Promega DeadEnd™
fluorometric TUNEL system. Briefly, 1.5×106 SKOV-3 cells
were plated using chamber slides and transfected with 2 μg of
siRNA-3. The cells were then washed with phosphate-buffered saline
(PBS), fixed in 40 mg/ml paraformaldehyde solution for 25 min and
then treated with 2 mg/ml Triton X-100 at 4°C for 5 min for
permeabilization. The cells were then washed and labeled with TUNEL
reaction mixture at 37°C for 60 min. After washing with
saline-sodium citrate (SSC) buffer, cells were treated with DAPI.
The chamber slides were evaluated using a fluorescence microscope
(BD Biosciences) to assess for apoptotic cells indicated by strong
green nuclear fluorescence. Apoptotic cells were determined by
counting TUNEL-positive cells in five random fields for each
sample. The apoptotic index (AI) was the number of apoptotic cells
counted in ten high-powered (x400) fields (~1,000 cells) that were
randomly picked for quantification from the most intensely stained
area of each section.
Invasion and migration assays
For the migration assay, 5×104 cells were
suspended in serum-free McCoy's 5A medium and plated on chambers
(8-μm pore size, Corning Costar, Cambridge, MA) that were not
coated with Matrigel. For the invasion assay, the upper chamber was
pre-coated with 5 mg/ml Matrigel (Sigma Aldrich) prior to the
addition of 5×105 cells in serum-free McCcoy's 5A
medium. For both assays, medium containing 10% FBS was added to the
lower chamber as a chemoattractant. Following incubation for 18 h
at 37°C, non-invaded cells on the upper surface of the filter were
wiped out with a cotton swab. The invading cells on the lower
surface of the filter were fixed and stained with hematoxylin and
eosin. The cell motility and invasiveness were determined by
counting cells in five microscopic fields per well and the extent
of invasion was expressed as the average number of cells per
field.
Statistical analyses
All experiments represent an average of at least
triplicate samples or as indicated. The data are presented as the
means ± SEM. All statistical analyses were carried out using Sigma
Plot 13.0 software (SPSS, Chicago, IL). Differences between groups
were assessed by one-way analysis of variance (ANOVA)
(nonparametric statistics) and unpaired Student's t-tests. A value
of P<0.05 was considered to indicate a statistically significant
difference.
Results
Screening and identification of the
target site of HER-2 gene by T7 in vitro transcription system
We examined the ability of specific HER-2 siRNAs to
reduce the endogenous level of the HER-2 protein in SKOV-3 cells.
We selected the SKOV-3 cell line since it is known to express high
levels of endogenous HER-2 (15).
We transfected SKOV-3 cells with three different HER-2-specific
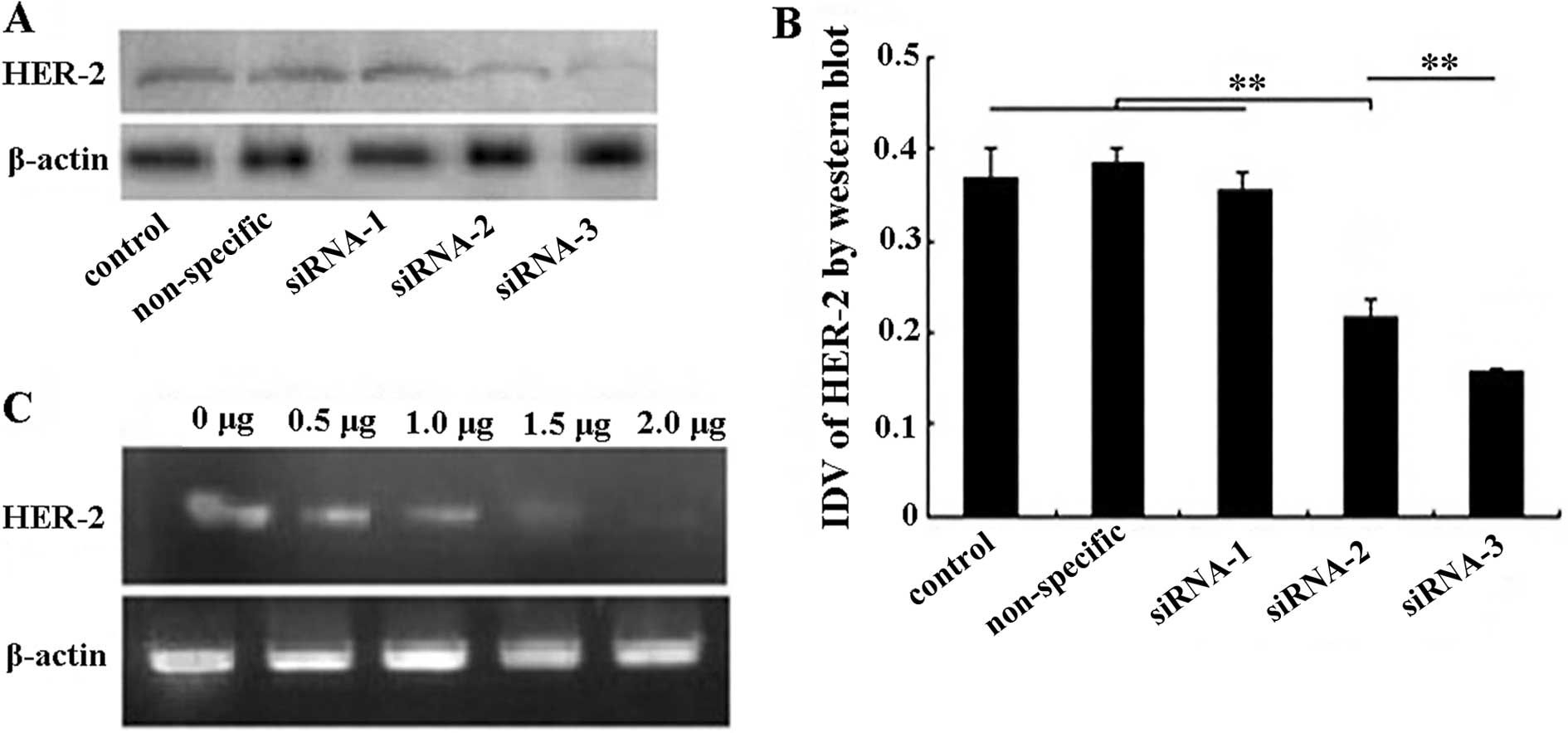
siRNAs. The western blot analysis results showed that the three
HER-2-specific siRNAs had different levels of efficiency in
downregulating the expression of the HER-2 protein (Fig. 1A). The greatest interference in
HER-2 protein expression was achieved by transfection with siRNA-3
(57.7%) and, to a lesser extent, with siRNA-2 (41.2%). The control
cells, or cells transfected with siRNA-1, exhibited no reduction in
HER-2 protein levels (Fig. 1B).
Thus, HER-2 siRNA-3 was chosen for the experimental studies.
Furthermore, a non-specific siRNA-3 was designed to serve as the
non-specific control group. Accordingly, the results showed that
the non-specific siRNA-3 had no effect on HER-2 protein expression
(Fig. 1B).
RT-PCR was used to compare the relative expression
value of each siRNA-transfected group (0.5, 1.0, 1.5 and 2.0 μg),
which demonstrated significant changes in HER-2 mRNA expression
(P<0.01) (Fig. 1C). The
expression of HER-2 mRNA correlated with the transfection amount of
HER-2 siRNA-3. As shown in Fig. 1C,
the expression of HER-2 mRNA was maximal with 2.0 μg of transfected
HER-2 siRNA-3. The siRNA interference was corroborated by western
blot analysis of the HER-2 protein. Thus, 2.0 μg of transfected
siRNA-3 was used to investigate the characteristic changes of
SKOV-3 cells in further experiments.
Silencing of HER-2 by siRNA-3 results in
the inhibition of proliferation and the induction of apoptosis of
SKOV-3 cells
The HER-2 gene is important for tumor development.
Therefore, we investigated the role of HER-2 inhibition in SKOV-3
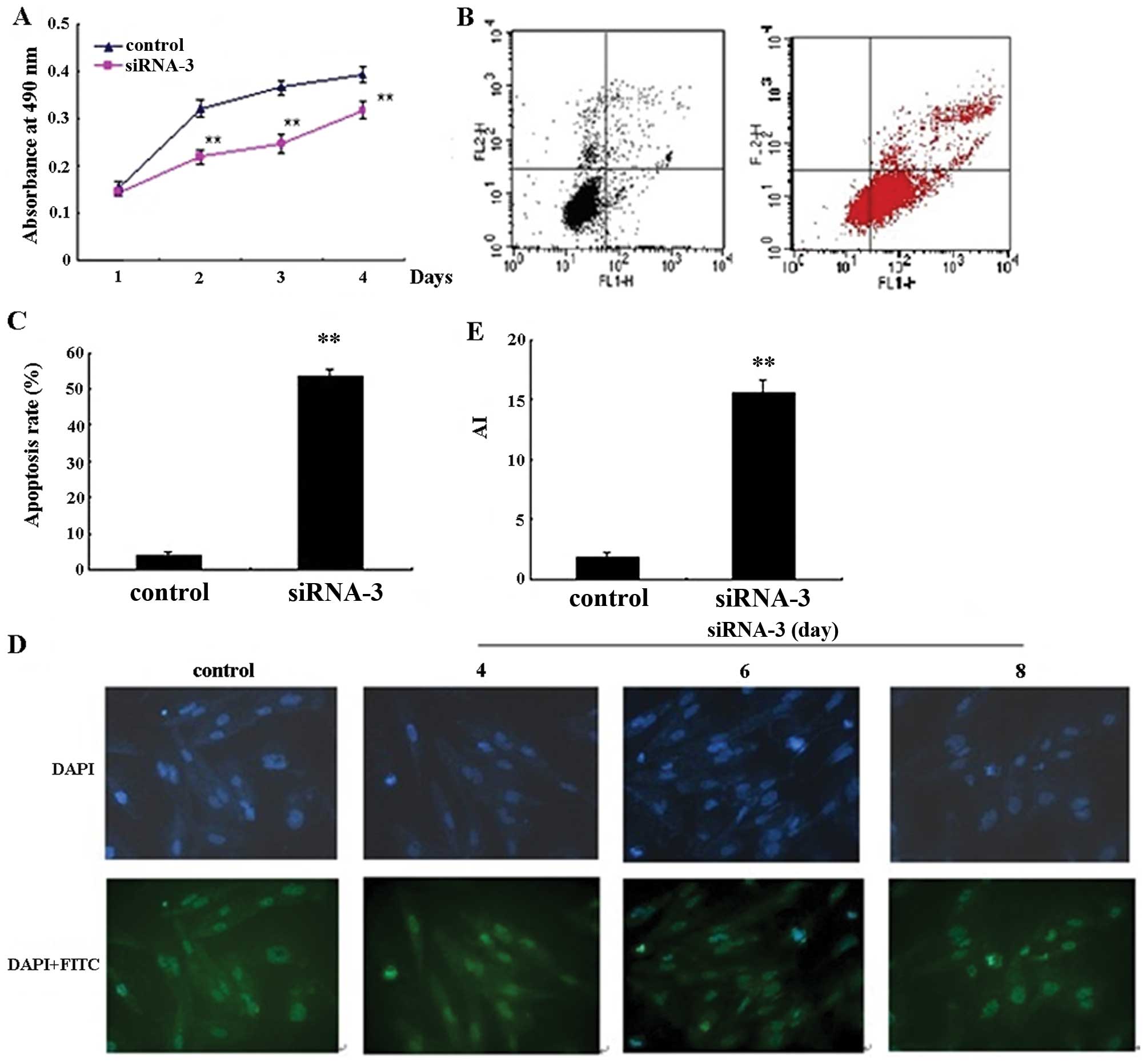
cell proliferation. As illustrated in Fig. 2A, HER-2 siRNA-3 exhibited a
statistically significant growth-inhibitory effect on SKOV-3 cells
when evaluated by MTT assay. Additionally, HER-2 siRNA-3 resulted
in a significant decrease in cell growth compared to the control
cells at various time-points (24, 72 and 96 h) (P<0.01). To
determine whether apoptosis could be induced by HER-2 siRNA-3, we
assessed apoptosis by Annexin V staining and flow cytometry. As
illustrated in Fig. 2B, the
percentage of apoptotic SKOV-3 cells increased following
transfection with HER-2 siRNA-3 and significantly increased
compared to the control cells (P<0.01, Fig. 2C).
To further evaluate apoptosis and morphological
changes caused by HER-2 siRNA-3 transfection, we analyzed SKOV-3
cells using TUNEL assay at different time-points (96, 144, and 192
h). The number of apoptotic cells after siRNA-3 transfection was
the highest at 192 h compared to the control group (P<0.01)
(Fig. 2D). Collectively, the data
from Annexin V and TUNEL analysis demonstrate that siRNA-3
targeting of HER-2 induced apoptosis in SKOV-3 cells.
Effects of HER-2 siRNA-3 on SKOV-3 cell
migration and invasion
Enhanced cell migration is characteristic of cancer
cells during EMT. Invasion and migration assays were performed to
assess cell invasion and the migration ability of HER-2
siRNA-transfected SKOV-3 cells.
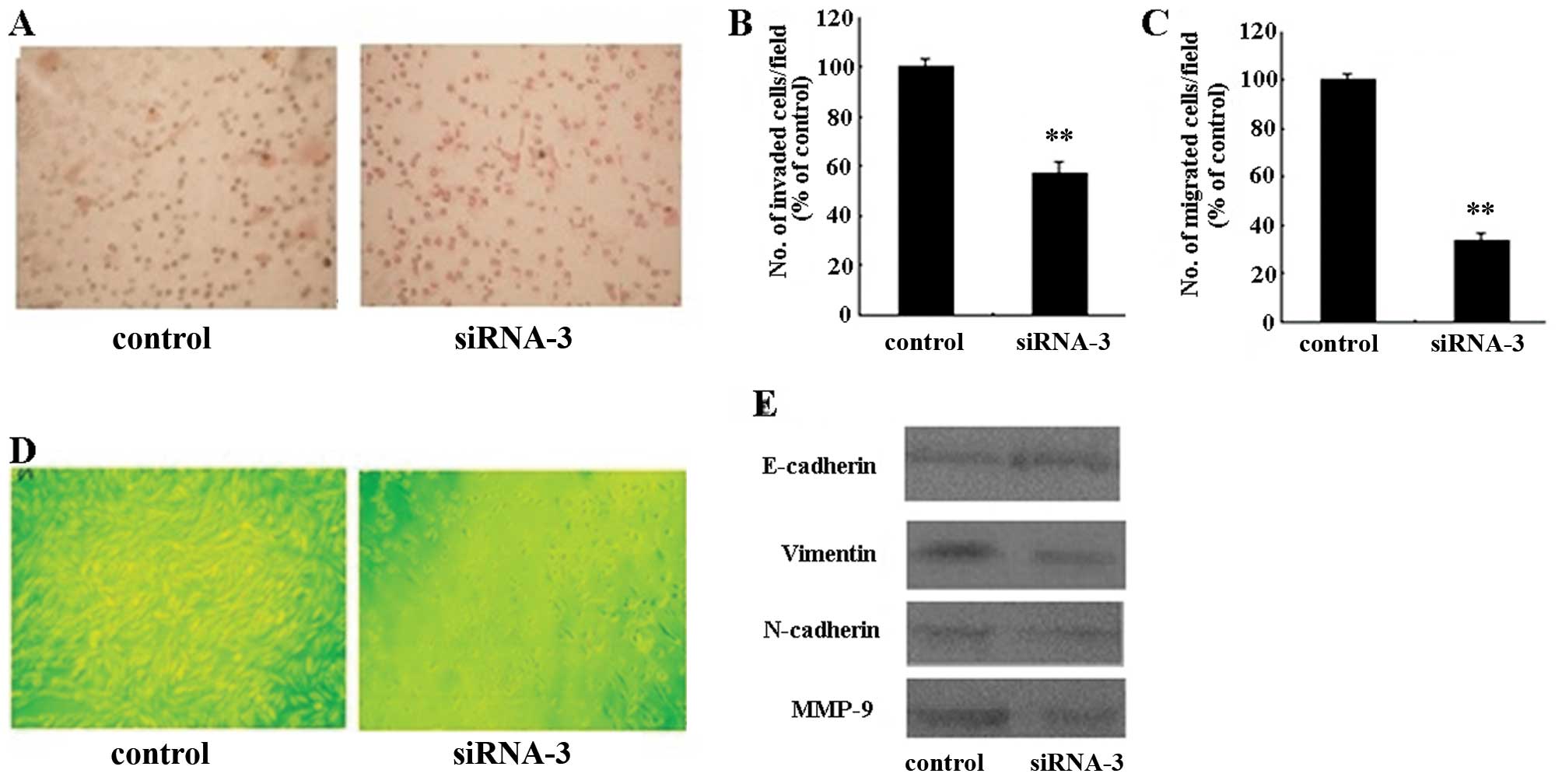
As shown in Fig. 3A,
HER-2 siRNA-3 induced a significant decrease in the number of cells
that invaded through a reconstituted basement membrane. The
invasion and chemotactic capacity were decreased in HER-2
siRNA-transfected SKOV-3 cells (P<0.01) (Fig. 3B and C). We also examined the effect
of HER-2 siRNA-3 on the expression of MMP-9 by western blot
analysis, which showed a marked decrease (Fig. 3E). Based on these results, the
silencing of HER-2 may inhibit the cell migration and invasion of
SKOV-3 cells via the downregulation of MMP-9.
Effects of HER-2 siRNA-3 on expression of
EMT markers in SKOV-3 cells
Silencing HER-2 expression can induce morphological
changes in SKOV-3 cells. As shown in Fig. 3D the shape of the cells changed from
an elongated spindle-like shape to a cuboidal one, a morphology
typical of epithelial-like cells. To determine whether HER-2 siRNA
affected specific molecular changes of EMT in SKOV-3 cells, we
assessed the protein levels of E-cadherin, vimentin and N-cadherin.
As illustrated in Fig. 3E,
E-cadherin levels were increased, while vimentin and N-cadherin
levels were decreased in the transfected cells. These results
indicate that the HER-2 siRNA-3-induced morphological changes may
involve EMT in SKOV-3 cells (Fig.
3E).
Discussion
The amplification and overexpression of the HER-2
gene have been associated with many human cancers, such as breast
and ovarian cancers, as well as with the poor prognosis of ovarian
carcinoma (7,8). In this study, we used the T7 in
vitro transcription system to silence the HER-2 gene with
siRNAs in SKOV-3 cells. Three specific pairs of HER-2-targeted
sequences were devised using the coding region of HER-2 mRNA. We
found that all three siRNAs were efficient in downregulating the
expression of HER-2. However, HER-2 siRNA-3 was the most effective
suppressor of HER-2 protein expression.
HER-2 is involved in numerous cellular functions,
including apoptosis and cell cycle progression (5,6). In
the present study, the inhibition of HER-2 resulted in a
significant decrease in the growth of SKOV-3 cells. Apoptosis has
been reported to play an important role during the malignant
transformation of normal cells (16,17).
These findings, in conjunction with ours, suggest that the
dysregulation of HER-2 disrupts the balance between proliferation
and apoptosis and may represent an important pathogenic step in the
development of ovarian cancer. These changes were observed when the
oncogene was neutralized in HER-2-overexpressing tumors or
antisense oligonucleotides (8,18–20).
Metastasis is an important characteristic of
malignant tumors. An essential part of invasion and metastasis is
the degradation of the basement membrane by members of the MMP
family (21). MMPs belong to a
family of zinc-dependent proteinases that have the ability to
degrade extracellular matrix components. Accumulating evidence has
suggested a strong correlation exists between high levels of MMPs
and malignant cancer invasiveness (22). Recent clinical studies have
indicated that there is an association between HER-2 expression and
MMP-9 production in cancer patients (23,24).
Kim et al, as well as others, demonstrated that the
overexpression of the HER-2 gene induced the invasion of human
breast epithelial cells via MMP-9 (24,25).
In this study, we showed that the suppression of HER-2 by siRNA
inhibited the migration and invasiveness of ovarian carcinoma
cells. Decreased MMP-9 protein expression was also detected by
western blot analysis. The results of the present study suggest
that overexpression of the HER-2 gene may promote the metastatic
potential of SKOV-3 cells expressing MMP-9.
EMT, which has been recognized as a crucial feature
of embryogenesis, has recently been shown to be closely related
with carcinoma cell migration and invasion (26). Previous studies have focused on the
mechanisms and regulation of HER-2-driven EMT of mammary tumor
cells (27,28). In addition, recent studies have
shown that EGFR causes EMT in cervical, ovarian and liver cancers
(29–31). During EMT, the polarized and basal
membrane-anchored epithelial cells undergo a number of biochemical
changes to acquire a mesenchymal, fibroblastoid phenotype. In our
study, silencing HER-2 expression induced morphological changes in
SKOV-3 cells, whose shape changed from an elongated spindle-like to
a cuboidal shape, a morphology typical of epithelial-like cells.
The strongest evidence for the significance of EMT relates to the
cell adhesion molecule, E-cadherin (epithelial cadherin).
Abolishing E-cadherin function in vitro confers invasive
properties to non-invasive cells and, conversely, the introduction
of E-cadherin into invasive epithelial cell lines abrogates their
invasive potential. In this regard, E-cadherin is considered a
broadly acting suppressor of invasion and metastasis and its
functional inactivation represents a critical step in the
acquisition of this capability (16). Not surprisingly, loss of E-cadherin
expression is a defining characteristic of EMT (32). N-cadherin (neural cadherin), another
adhesion molecule, is associated with a heightened invasive
potential in cancer. In addition, vimentin, a mesenchymal marker,
is significantly associated with the expression of N-cadherin
(33). In this study, E-cadherin
levels were increased and the expression of vimentin and N-cadherin
was decreased following transfection with HER-2 siRNA-3, indicating
that the HER-2 siRNA-3-induced morphological changes may involve
EMT in SKOV-3 cells. Considering the emerging importance of EMT in
invasive cell behavior, the detailed mechanisms for the role of EMT
in HER-2-driven malignancy in SKOV-3 cells require further
elucidation.
The HER-2 gene has been identified to play important
roles in chemoresistance in a number of tumors. In a previous study
conducted by our laboratory, we investigated the inhibition of
HER-2 by siRNA in SKOV-3 cells and its role in potentiating the
efficacy of cisplatin. Our findings demonstrated that cisplatin
inhibited cellular proliferation in a dose-dependent manner
(34). These results are consistent
with growing evidence suggesting that the activation of the HER-2
signal transduction pathway may induce chemoresistance in SKOV-3
cells (35).
In conclusion, the siRNA-mediated interference of
HER-2 expression inhibited the proliferation, induced apoptosis and
inhibited invasive and migratory phenotypes of SKOV-3 cells. Given
that HER-2 is one of the most important oncogenes in human ovarian
cancer and an attractive therapeutic target, our findings provide a
molecular basis for further evaluation of the role of HER-2 in
ovarian cancer progression.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81001163), the Liaoning Education
Department for Scientific and Technological Research Project funds
(2009A781) and the Scientific Research Foundation of Shengjing
Hospital of China Medical University.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
3
|
Olayioye MA, Neve RM, Lane HA and Hynes
NE: The ErbB signaling network: receptor heterodimerization in
development and cancer. EMBO J. 19:3159–3167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baselga J and Arteaga CL: Critical update
and emerging trends in epidermal growth factor receptor targeting
in cancer. J Clin Oncol. 23:2445–2459. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hynes NE and Lane HA: ERBB receptors and
cancer: the complexity of targeted inhibitors. Nat Rev Cancer.
5:341–354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duchnowska R, Biernat W, Szostakiewicz B,
et al: Correlation between quantitative HER-2 protein expression
and risk for brain metastases in HER-2+ advanced breast
cancer patients receiving trastuzumab-containing therapy.
Oncologist. 17:26–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Howe LR and Brown PH: Targeting the
HER/EGFR/ErbB family to prevent breast cancer. Cancer Prev Res.
4:1149–1157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Modjtahedi H and Essapen S: Epidermal
growth factor receptor inhibitors in cancer treatment: advances,
challenges and opportunities. Anticancer Drugs. 20:851–855. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Serrano-Olvera A, Dueñas-González A,
Gallardo-Rincón D, Candelaria M and De la Garza-Salazar J:
Prognostic, predictive and therapeutic implications of HER2 in
invasive epithelial ovarian cancer. Cancer Treat Rev. 32:180–190.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rubin SC, Finstad CL, Wong GY, Almadrones
L, Plante M and Lloyd KO: Prognostic significance of HER-2/neu
expression in advanced epithelial ovarian cancer: a multivariate
analysis. Am J Obstet Gynecol. 168:162–169. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar
|
|
13
|
Szweykowska-Kulińska Z, Jarmołowski A and
Figlerowicz M: RNA interference and its role in the regulation of
eucaryotic gene expression. Acta Biochim Pol. 50:217–229.
2003.PubMed/NCBI
|
|
14
|
Elbashir SM, Harborth J, Lendeckel W,
Yalcin A, Weber K and Tuschl T: Duplexes of 21-nucleotide RNAs
mediate RNA interference in cultured mammalian cells. Nature.
411:494–498. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ueno NT, Bartholomeusz C, Herrmann JL, et
al: E1A-mediated paclitaxel sensitization in
HER-2/neu-overexpressing ovarian cancer SKOV3.ip1 through apoptosis
involving the caspase-3 pathway. Clin Cancer Res. 6:250–259.
2000.PubMed/NCBI
|
|
16
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chuang TC, Hsu SC, Cheng YT, Shao WS, Wu
K, Fang GS, Ou CC and Wang V: Magnolol down-regulates HER2 gene
expression, leading to inhibition of HER2-mediated metastatic
potential in ovarian cancer cells. Cancer Lett. 311:11–19. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamashita-Kashima Y, Iijima S, Yorozu K,
Furugaki K, Kurasawa M, Ohta M and Fujimoto-Ouchi K: Pertuzumab in
combination with trastuzumab shows significantly enhanced antitumor
activity in HER2-positive human gastric cancer xenograft models.
Clin Cancer Res. 17:5060–5070. 2011. View Article : Google Scholar
|
|
20
|
Tanizaki J, Okamoto I, Fumita S, Okamoto
W, Nishio K and Nakagawa K: Roles of BIM induction and survivin
downregulation in lapatinib-induced apoptosis in breast cancer
cells with HER2 amplification. Oncogene. 30:4097–4106. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Decock J, Thirkettle S, Wagstaff L and
Edwards DR: Matrix metalloproteinases: protective roles in cancer.
J Cell Mol Med. 15:1254–1265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hua H, Li M, Luo T, Yin Y and Jiang Y:
Matrix metalloproteinases in tumorigenesis: an evolving paradigm.
Cell Mol Life Sci. 68:3853–3868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luukkaa H, Klemi P, Leivo I, et al:
Expression of matrix metalloproteinase-1, -7, -9, -13, Ki-67, and
HER-2 in epithelial- myoepithelial salivary gland cancer. Head
Neck. 32:1019–1027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim IY, Yong HY, Kang KW and Moon A:
Overexpression of ErbB2 induces invasion of MCF10A human breast
epithelial cells via MMP-9. Cancer Lett. 275:227–233. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan M, Yao J and Yu D: Overexpression of
the c-erbB-2 gene enhanced intrinsic metastasis potential in human
breast cancer cells without increasing their transformation
abilities. Cancer Res. 57:1199–1205. 1997.
|
|
26
|
Chua KN, Poon KL, Lim J, Sim WJ, Huang RY
and Thiery JP: Target cell movement in tumor and cardiovascular
diseases based on the epithelial-mesenchymal transition concept.
Adv Drug Deliv Rev. 63:558–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sethi S, Sarkar FH, Ahmed Q, et al:
Molecular markers of epithelial-to-mesenchymal transition are
associated with tumor aggressiveness in breast carcinoma. Transl
Oncol. 4:222–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jenndahl LE, Isakson P and Baeckström D:
c-erbB2-induced epithelial-mesenchymal transition in mammary
epithelial cells is suppressed by cell-cell contact and initiated
prior to E-cadherin downregulation. Int J Oncol. 27:439–448.
2005.
|
|
29
|
Ricono JM, Huang M, Barnes LA, et al:
Specific cross-talk between epidermal growth factor receptor and
integrin alphavbeta5 promotes carcinoma cell invasion and
metastasis. Cancer Res. 69:1383–1391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahmed N, Maines-Bandiera S, Quinn MA,
Unger WG, Dedhar S and Auersperg N: Molecular pathways regulating
EGF-induced epithelio-mesenchymal transition in human ovarian
surface epithelium. Am J Physiol Cell Physiol. 290:C1532–C1542.
2006. View Article : Google Scholar
|
|
31
|
Fuchs BC, Fujii T, Dorfman JD, Goodwin JM,
Zhu AX, Lanuti M and Tanabe KK: Epithelial-to-mesenchymal
transition and integrin-linked kinase mediate sensitivity to
epidermal growth factor receptor inhibition in human hepatoma
cells. Cancer Res. 68:2391–2399. 2008. View Article : Google Scholar
|
|
32
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vergara D, Merlot B, Lucot JP, Collinet P,
Vinatier D, Fournier I and Salzet M: Epithelial-mesenchymal
transition in ovarian cancer. Cancer Lett. 291:59–66. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu YM, Zhang SL, Meng LR and Zhao YY:
Influence of human epidermal growth factor receptor-2 siRNA on
chemosensitivity to cisplatin of human ovarian carcinoma cells: an
in vitro experiment. Zhonghua Yi Xue Za Zhi. 88:909–913. 2008.(In
Chinese).
|
|
35
|
Abuharbeid S, Apel J, Zugmaier G, et al:
Inhibition of HER-2 by three independent targeting strategies
increases paclitaxel resistance of SKOV-3 ovarian carcinoma cells.
Naunyn Schmiedebergs Arch Pharmacol. 371:141–151. 2005. View Article : Google Scholar : PubMed/NCBI
|