Introduction
Silibinin is the main component of the silymarin
complex and is isolated from the seeds of Silybum marianum,
also known as milk thistle (1).
Silibinin has a wide range of pharmacologic effects including the
induction of apoptosis, and the inhibition of cell proliferation,
cell invasion and angiogenesis (2,3).
Recently, Kim et al reported that silibinin triggers cell
cycle arrest through the downregulation of cyclin B1 and cdc2 and
upregulation of p21 expression in triple-negative breast cancer
cells (4). In addition, silibinin
was found to effectively delay the development of spontaneous
mammary tumors and decrease the tumor mass in Her2/neu transgenic
mice (5).
Migration of cells is a dynamic process that occurs
in tissue remodeling, inflammation and wound repair and is
regulated by a variety of extracellular factors, including
extracellular matrix (ECM) proteins (6,7). Tumor
cell migration is associated with the early stages of metastasis
(8). Metastasis is considered
responsible for more than 90% of cancer-related deaths (9). During metastasis, cancer cells are
involved in numerous interactions with various factors such as the
ECM, growth factors, cytokines and basement membranes (8). Consequently, cancer cells acquire
motility and local invasive capability. In addition, cancer
cell-mediated tissue remodeling is observed to have a strong
positive correlation with matrix metalloproteinase (MMP) levels
(10).
MMPs are major critical molecules that assist tumor
cells during metastasis (11). They
play an important role in ECM degradation and cancer cell invasion.
Overexpression of MMPs contributes to tumorigenesis and tumor
progression through multiple pathways (12). MMP-9 is one of two gelatinases and
is able to degrade type IV collagen, which is abundant in basement
membranes (13). High serum levels
of MMP-9 are associated with a higher tumor grade, poor overall
survival and secondary metastasis in melanoma and breast cancer
tissue (10,14).
In the present study, we investigated the
relationship between silibinin and cell migration in thyroid and
breast cancer cells. Here, our results revealed that
12-O-tetradecanoylphorbol-13-acetate (TPA)-induced MMP-9
expression and cell migration were suppressed by silibinin in both
thyroid and breast cancer cells.
Materials and methods
Reagents and cell cultures
Dulbecco’s modified Eagle’s medium (DMEM),
antibiotics and 10% zymogram gel were purchased from Life
Technologies (Rockville, MD, USA). Fetal bovine serum (FBS) was
purchased from Hyclone (Logan, UT, USA). Silibinin was purchased
from Sigma (St. Louis, MO, USA). Mouse recombinant MMP-9 was
purchased from R&D Systems (Minneapolis, MN, USA). TPA was
purchased from Tocris (Ellisville, MO, USA). The secondary
peroxidase-conjugated antibodies and ECL Prime reagents were
purchased from Amersham (Buckinghamshire, UK).
Papillary thyroid cancer TPC-1 and breast cancer
MCF7 cells were grown in a humidified atmosphere of 95% air and 5%
CO2 at 37°C in DMEM supplemented with 10% FBS, 2 mM
glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin. Each
cell line was maintained in culture medium supplemented without FBS
for 24 h.
Drug treatment
Cells were maintained in culture medium without FBS
for 24 h, and then the culture medium was replaced with fresh
medium without FBS, and the cells were further incubated with the
indicated concentrations of silibinin for 24 h. In the experiments
involving silibinin, the cells were pretreated with 50 μM silibinin
for 60 min prior to treatment with 20 nM TPA for 24 h.
Zymography
Zymography was performed on 10% polyacrylamide gels
that had been cast in the presence of gelatin as previously
described (15). Briefly, samples
(100 μl) were resuspended in a loading buffer and run on a 10%
SDS-PAGE gel containing 0.5 mg/ml gelatin without prior
denaturation. After electrophoresis, the gels were washed to remove
SDS and incubated for 30 min at room temperature (RT) in a
renaturing buffer (50 mM Tris, 5 mM CaCl2, 0.02%
NaN3 and 1% Triton X-100). Next, the gels were incubated
for 48 h at 37°C in a developing buffer [50 mM Tris-HCl (pH 7.8), 5
mM CaCl2, 0.15 M NaCl and 1% Triton X-100]. The gels
were subsequently stained with Coomassie Brilliant Blue G-250,
destained in 30% methanol, and flooded with 10% acetic acid to
detect gelatinase secretion.
cDNA synthesis and real-time PCR
Total RNA was extracted from cells using the TRIzol
reagent (Invitrogen, Carlsbad, CA, USA), according to the
manufacturer’s protocol. Isolated RNA samples were then used for
RT-PCR. Samples (total RNA, 1 μg) were reverse-transcribed into
cDNA in 20-μl reaction volumes using a first-strand cDNA synthesis
kit for RT-PCR, according to the manufacturer’s instructions (MBI
Fermentas, Hanover, MD, USA).
Gene expression was quantified by real-time PCR
using the SensiMix SYBR kit (Bioline Ltd., London, UK) and 100 ng
of cDNA per reaction. The sequences of the primer sets used for
this analysis were: human MMP-9 (forward, 5′-CCC GGA CCA AGG ATA
CAG-3′; reverse, 5′-GGC TTT CTC TCG GTA CTG-3′), MMP-2 (forward,
5′-GGC CTC TCC TGA CAT TGA CCT T-3′; reverse, 5′-GGC CTC GTA TAC
CGC ATC AAT C-3′), and β-actin as an internal control (forward,
5′-AAA CTG GAA CGG TGA AGG TG-3′; reverse, 5′-CTC AAG TTG GGG GAC
AAA AA-3′). An annealing temperature of 60°C was used for all of
the primers. PCRs were performed in a standard 384-well plate
format with an ABI 7900HT real-time PCR detection system. For data
analysis, the raw threshold cycle (CT) value was
first normalized to the housekeeping gene for each sample to obtain
ΔCT. The normalized ΔCT was
then calibrated to control cell samples to obtain
ΔΔCT.
Cell viability
Total cell numbers following treatment with
silibinin were evaluated by Quick Cell Proliferation Assay Kit II
(Biovision, Mountain View, CA, USA) according to the manufacturer’s
protocol. Briefly, TPC-1 human papillary thyroid cancer cells
(5×104/well) were grown in a 96-well plate in 100
μl/well of culture media in the absence or presence of the
indicated concentrations of silibinin. After incubating the cells
for 24 h, 10 μl WST reagent was added to each well. Viable cells
were quantified photometrically at 480 nm.
Wound healing assay
TPC-1 thyroid cancer cells were seeded in 6-well
plates and were cultured for 24 h. A monolayer of cells was
scratched with a 200-μl pipette tip to create a wound, and then
this was washed twice in PBS to remove any suspended cells. The
cells were pretreated with silibinin (50 μM) for 60 min prior to
treatment with TPA; the monolayer of cells was then treated with 20
nM TPA for 24 h in serum-free media. The cells migrating from the
leading edge were photographed at 0 and 24 h using a CK40 inverted
microscope (Olympus, Tokyo, Japan).
Statistical analysis
Statistical significance was determined using the
Student’s t-test. The results are presented as the means ± SEM. All
p-values were two-tailed and significance was set at a p-value
<0.05.
Results
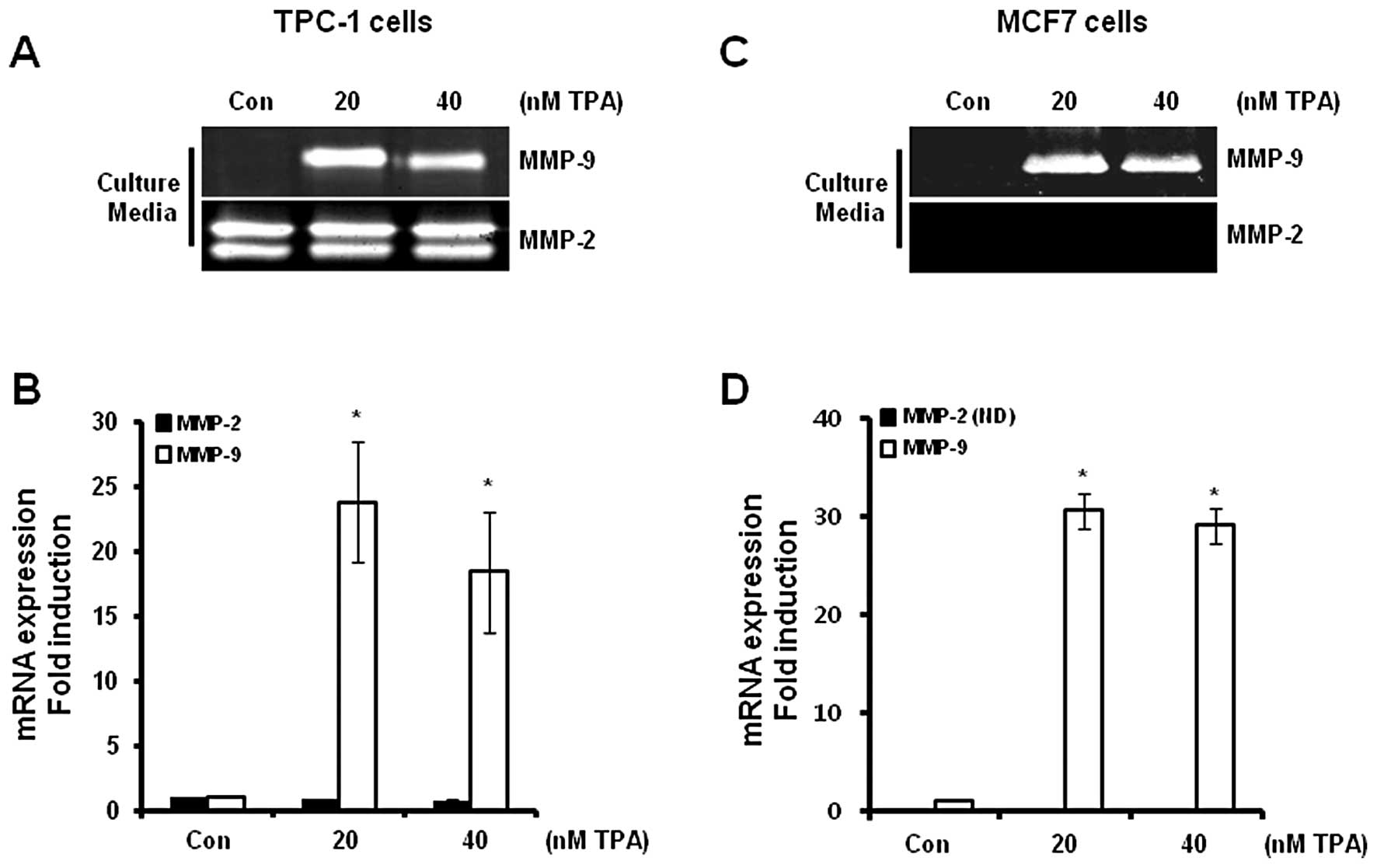
Expression of MMP-9 and MMP-2 in thyroid
and breast cancer cells following TPA treatment
The levels of MMP-9 and MMP-2 mRNA and protein
expression in the TPC-1 and MCF7 cells were determined following
treatment with TPA at the indicated concentrations for 24 h. We
analyzed the levels of MMP-9 and MMP-2 mRNA (in cell lysates) and
protein (in culture media) expression using real-time PCR and
zymography, respectively. Our results revealed that the levels of
MMP-9 protein expression were significantly increased in the TPC-1
thyroid (Fig. 1A) and MCF7 breast
(Fig. 1C) cancer cells. However,
MMP-2 protein expression was not altered following TPA treatment in
the TPC-1 cells (Fig. 1A), although
MMP-2 expression was not detected in the MCF7 cells following TPA
treatment (Fig. 1C). In addition,
the levels of MMP-9 mRNA were also increased following TPA
treatment (Fig. 1B and D). MMP-9
mRNA expression was increased by 28.8±6.6- and 30.6±2.5-fold in the
TPC-1 thyroid (Fig. 1B) and MCF7
(Fig. 1D) breast cancer cells,
respectively, following treatment with 20 nM TPA when compared with
the control level.
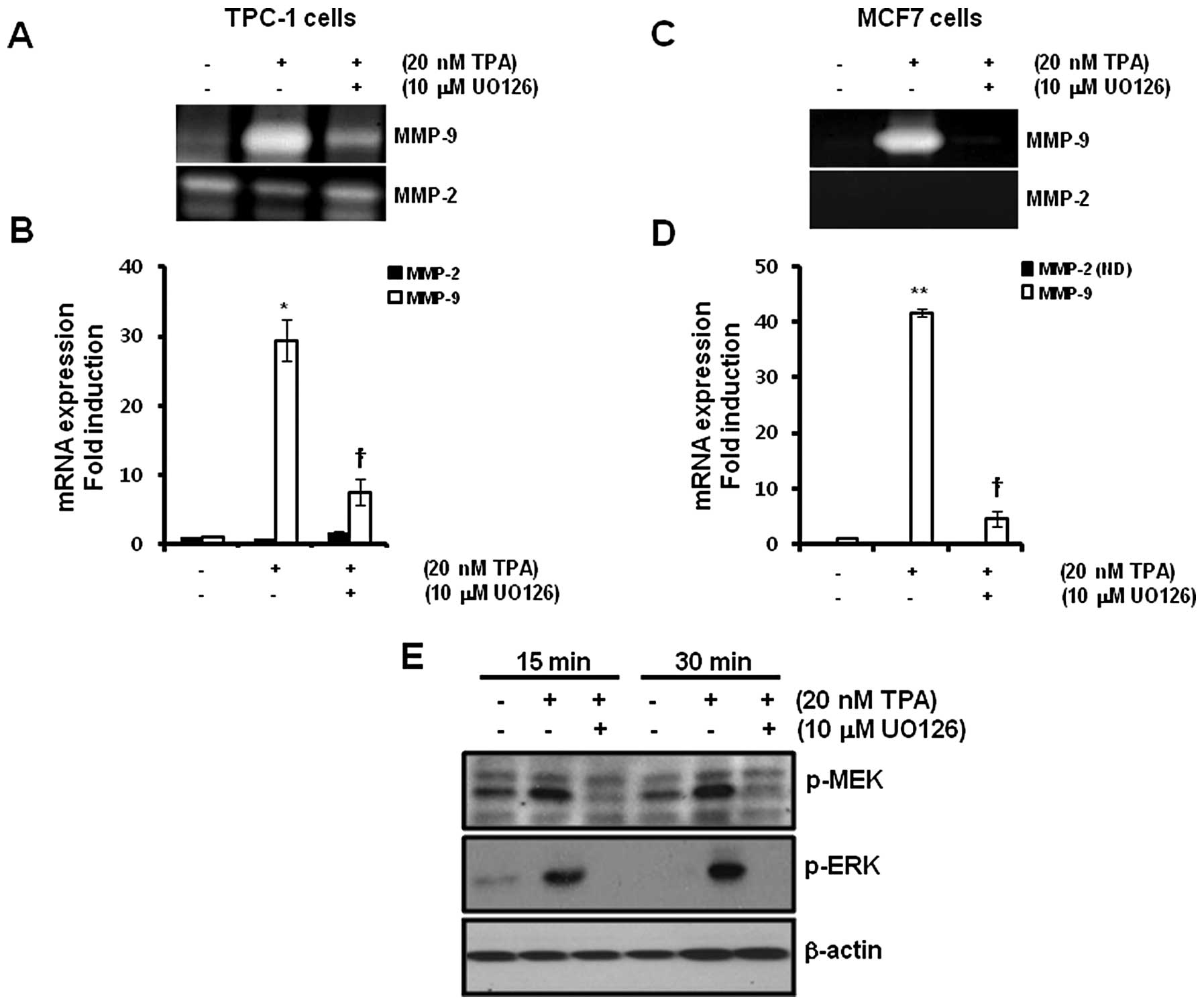
TPA-induced MMP-9 expression is inhibited
by the MEK1/2 inhibitor, UO126
To verify the regulatory mechanism of TPA-induced
MMP-9 expression, we examined the effect of the MEK1/2 inhibitor,
UO126, on TPA-induced MMP-9 expression. After pretreatment of TPC-1
and MCF7 cells with 10 μM UO126 for 30 min, we treated the cells
with 20 nM TPA for 24 h. The levels of TPA-induced MMP-9 protein
expression were significantly decreased by UO126 in the TPC-1
thyroid (Fig. 2A) and MCF7 breast
cancer cells (Fig. 2C). In
addition, TPA-induced MMP-9 mRNA expression was also decreased
following pretreatment with UO126 (Fig.
2B and D). MMP-9 mRNA expression was increased by 29.4±2.4- and
41.8±0.6-fold, respectively, in the TPC-1 thyroid (Fig. 2B) and MCF7 (Fig. 2D) breast cancer cells, following
treatment with 20 nM TPA when compared with control levels. In
contrast, TPA-induced MMP-9 expression was significantly decreased
by 7.5±1.9 and 4.5±1.3-fold in the TPC-1 thyroid (Fig. 2B) and MCF7 (Fig. 2D) breast cancer cells, respectively,
following treatment with 10 μM UO126 when compared with the control
levels.
Next, we confirmed the effect of UO126 on
TPA-induced phosphorylation of MEK and ERK in TPC-1 thyroid cancer
cells. As expected, the phosphorylation of MEK and ERK was
increased following TPA treatment (Fig.
2E). In contrast, TPA-induced phosphorylation of MEK and ERK
was significantly decreased by UO126 (Fig. 2E).
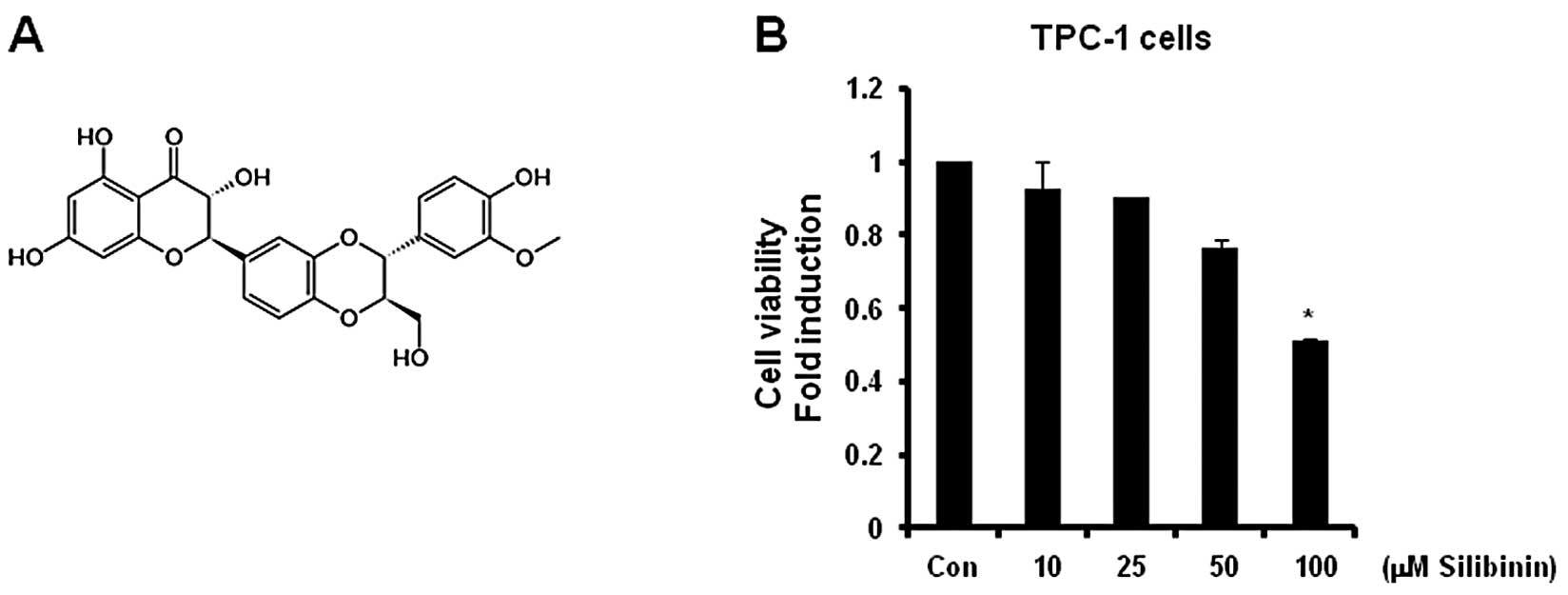
TPA-induced MMP-9 expression is
completely suppressed by silibinin
The chemical structure of silibinin is represented
in Fig. 3A. Our results revealed
that the cell viability was significantly (50% of the control
level) decreased following treatment with 100 μM silibinin
(Fig. 3B). Therefore, we treated
cells with 50 μM silibinin in the subsequent studies.
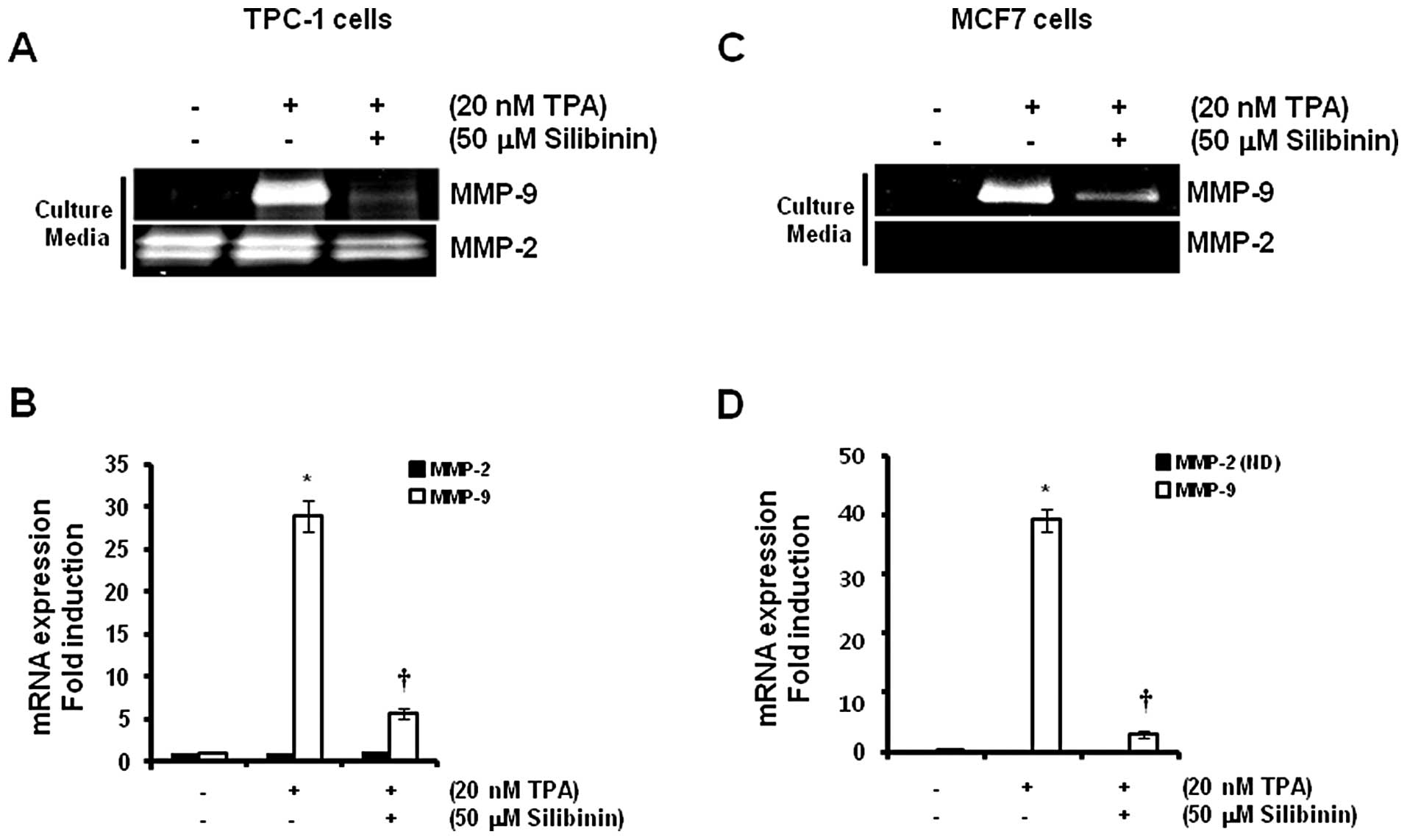
To investigate the effect of silibinin on
TPA-induced MMP-9 expression, we pretreated cells with 50 μM
silibinin for 60 min prior to treatment with 20 nM TPA. We found
that TPA-induced MMP-9 protein expression was significantly
decreased by silibinin treatment (Fig.
4A and C). In addition, the levels of expression of MMP-9 mRNA
increased significantly (29.0±2.6- and 39.1±2.5-fold) in the TPC-1
thyroid (Fig. 4B) and MCF7 breast
(Fig. 4D) cancer cells,
respectively, following treatment with a concentration of 20 nM TPA
when compared to control levels. In contrast, TPA-induced MMP-9
mRNA expression was decreased to 5.7±0.6-fold in TPC-1 thyroid
cancer cells by 50 μM silibinin (Fig.
4B). MCF7 breast cancer cells also showed similar results
(Fig. 4D) following treatment with
50 μM silibinin when compared with the control level.
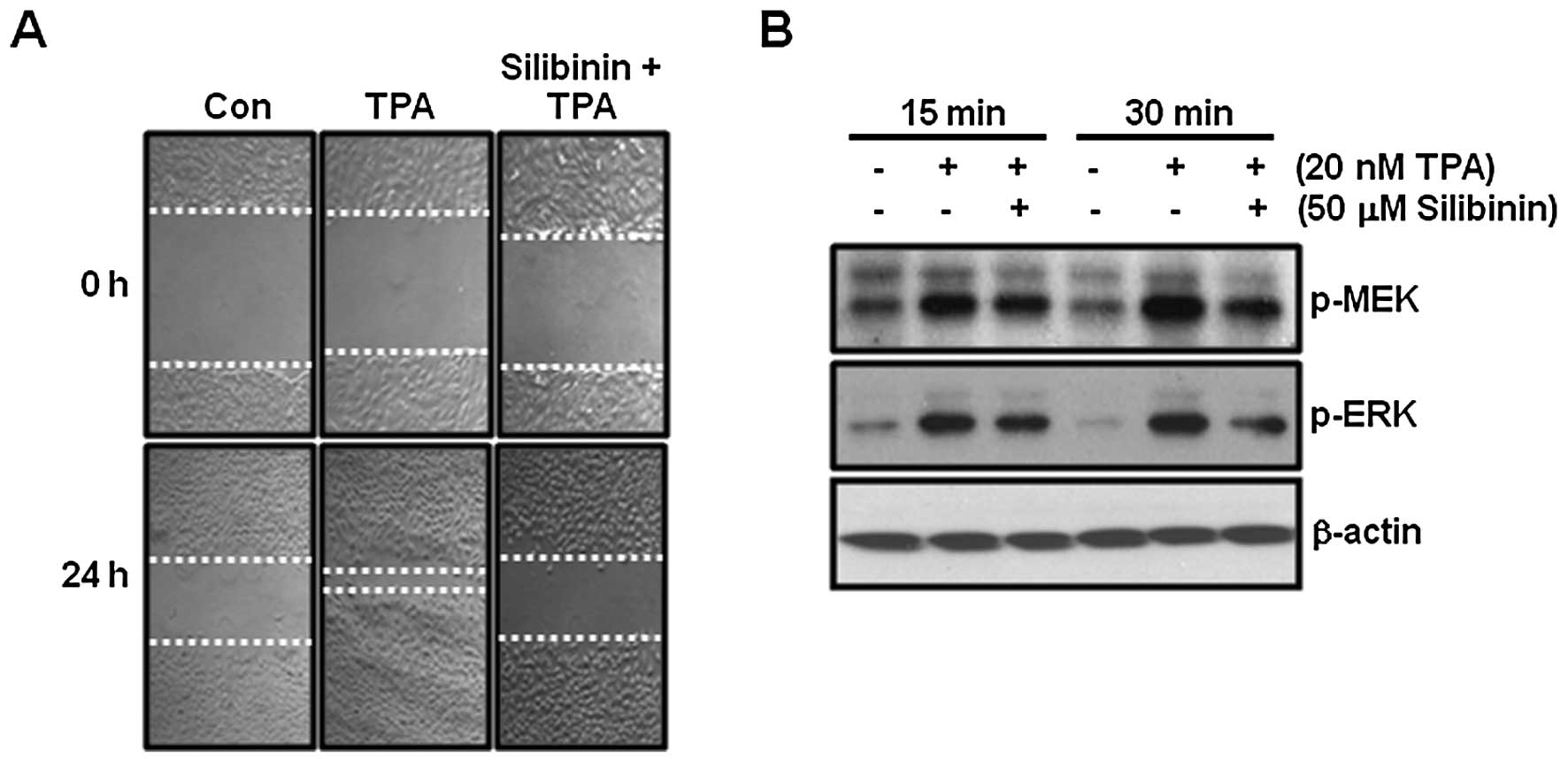
TPA-induced cell migration is completely
suppressed by silibinin
In the next experiment, we examined the effect of
silibinin on TPA-induced cell migration in TPC-1 thyroid cancer
cells. As shown in Fig. 5A,
TPA-induced cell migration was completely blocked by 50 μM
silibinin treatment.
Next, we investigated the effect of silibinin on
TPA-induced phosphorylation of MEK and ERK in TPC-1 thyroid cancer
cells. As expected, the phosphorylation of MEK and ERK was
increased following TPA treatment (Fig.
5B). In contrast, TPA-induced phosphorylation of MEK and ERK
was decreased by silibinin (Fig.
5B). Therefore, we demonstrated that silibinin suppressed
TPA-induced cell migration as well as inhibited MMP-9
expression.
Discussion
Phorbol esters, such as TPA, are natural molecules
that are recognized as potent tumor promoters and can potently
trigger multiple cellular events such as protein kinase C (16,17).
TPA was found to significantly enhance cell migration abilities of
human cancer cells including hepatoma and breast cancer cells
(18,19). In addition, TPA-induced migration
and invasion of glioblastoma cells were prevented by blocking
PKCα-dependent pathways (20).
Consistent with these reports, we found that TPA increased cell
migration in thyroid and breast cancer cells. Herein, we
investigated the inhibitory effect of silibinin on TPA-induced
cancer cell migration.
MMPs are positively associated with tumor
progression including tumor differentiation, metastasis and poor
prognosis (21,22). In addition, inhibition of MMPs
decreases cell invasion while the activation of MMPs yields
increased tumor cell invasion (23). MMP-9 is one of the gelatinases and
is expressed in a large number of cell types, including epithelial
and inflammatory cells (24). MMP-9
has been associated with the development and extent of metastases
in lymph nodes (25). Although we
did not present the data, TPA-induced cell migration was
significantly suppressed by a broad-spectrum MMP inhibitor,
PD166793, in both TPC-1 and MCF7 cells. Therefore, we demonstrated
that a broad-spectrum MMP inhibitor, PD166793, directly or
indirectly affects TPA-induced cell migration through the
regulation of MMP activity.
The region of the MMP-9 promoter contains
cis-acting regulatory elements for transcription factors,
including two AP-1 sites and an NF-κB site (26,27).
The DNA binding activity of a variety of transcription factors such
as NF-kB and AP-1 is regulated by ERK activity (26–28).
Recently, we reported that TPA-induced transcriptional activity of
AP-1 is mediated through the Raf/MEK/ERK-dependent pathway
(3). In addition, the
transcriptional expression of MMP-9 was found to be directly
regulated by AP-1 DNA binding activity (28) and was completely suppressed by the
MEK1/2 inhibitor, UO126 (3). In
accordance with these studies, TPA-induced MMP-9 expression was
significantly decreased by silibinin. TPA-induced phosphorylation
of MEK and ERK was also suppressed.
Conclusively, we found that silibinin suppresses the
TPA-induced phosphorylation of MEK and ERK in thyroid cancer cells.
In addition, cell migration and MMP-9 expression were completely
inhibited by silibinin. Therefore, silibinin may be a promising
drug for the treatment of thyroid and breast cancer.
Acknowledgements
This study was supported by a Korea Research
Foundation Grant funded by the Korean government
(KRF-2012-000493).
References
|
1
|
Davis-Searles PR, Nakanishi Y, Kim NC,
Graf TN, Oberlies NH, Wani MC, Wall ME, Agarwal R and Kroll DJ:
Milk thistle and prostate cancer: differential effects of pure
flavonolignans from Silybum marianum on antiproliferative
end points in human prostate carcinoma cells. Cancer Res.
65:4448–4457. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaur M, Velmurugan B, Tyagi A, Deep G,
Katiyar S, Agarwal C and Agarwal R: Silibinin suppresses growth and
induces apoptotic death of human colorectal carcinoma LoVo cells in
culture and tumor xenograft. Mol Cancer Ther. 8:2366–2374. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim S, Choi JH, Lim HI, Lee SK, Kim WW,
Kim JS, Kim JH, Choe JH, Yang JH, Nam SJ and Lee JE: Silibinin
prevents TPA-induced MMP-9 expression and VEGF secretion by
inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast
cancer cells. Phytomedicine. 16:573–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim S, Lee HS, Lee SK, Kim SH, Hur SM, Kim
JS, Kim JH, Choe JH, Shin I, Yang JH, Lee JE and Nam SJ:
12-O-Tetradecanoyl phorbol-13-acetate (TPA)-induced growth
arrest is increased by silibinin by the down-regulation of cyclin
B1 and cdc2 and the up-regulation of p21 expression in MDA-MB231
human breast cancer cells. Phytomedicine. 17:1127–1132. 2010.
|
|
5
|
Provinciali M, Papalini F, Orlando F,
Pierpaoli S, Donnini A, Morazzoni P, Riva A and Smorlesi A: Effect
of the silybin-phosphatidylcholine complex (IdB 1016) on the
development of mammary tumors in HER-2/neu transgenic mice. Cancer
Res. 67:2022–2029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frasca F, Vigneri P, Vella V, Vigneri R
and Wang JY: Tyrosine kinase inhibitor STI571 enhances thyroid
cancer cell motile response to hepatocyte growth factor. Oncogene.
20:3845–3856. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Webb DJ, Donais K, Whitmore LA, Thomas SM,
Turner CE, Parsons JT and Horwitz AF: FAK-Src signalling through
paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell
Biol. 6:154–161. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Geho DH, Bandle RW, Clair T and Liotta LA:
Physiological mechanisms of tumor-cell invasion and migration.
Physiology. 20:194–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mehlen P and Puisieux A: Metastasis: a
question of life or death. Nat Rev Cancer. 6:449–458. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
11
|
Fingleton B: Matrix metalloproteinases:
roles in cancer and metastasis. Front Biosci. 11:479–491. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giambernardi TA, Grant GM, Taylor GP, Hay
RJ, Maher VM, McCormick JJ and Klebe RJ: Overview of matrix
metalloproteinase expression in cultured human cells. Matrix Biol.
16:483–496. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li HC, Cao DC, Liu Y, Hou YF, Wu J, Lu JS,
Di GH, Liu G, Li FM, Ou ZL, Jie C, Shen ZZ and Shao ZM: Prognostic
value of matrix metalloproteinases (MMP-2 and MMP-9) in patients
with lymph node-negative breast carcinoma. Breast Cancer Res Treat.
88:75–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim S, Choi JH, Lim HI, Lee SK, Kim WW,
Cho S, Kim JS, Kim JH, Choe JH, Nam SJ, Lee JE and Yang JH:
EGF-induced MMP-9 expression is mediated by the JAK3/ERK pathway,
but not by the JAK3/STAT-3 pathway in a SKBR3 breast cancer cell
line. Cell Signal. 21:892–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Detjen KM, Brembeck FH, Welzel M, Kaiser
A, Haller H, Wiedenmann B and Rosewicz S: Activation of protein
kinase Calpha inhibits growth of pancreatic cancer cells via
p21(cip)-mediated G(1) arrest. J Cell Sci. 113:3025–3035.
2000.PubMed/NCBI
|
|
17
|
Kikkawa U, Takai Y, Tanaka Y, Miyake R and
Nishizuka Y: Protein kinase C as a possible receptor protein of
tumor-promoting phorbol esters. J Biol Chem. 258:11442–11445.
1983.PubMed/NCBI
|
|
18
|
Okabe K, Kato K, Teranishi M, Okumura M,
Fukui R, Mori T, Fukushima N and Tsujiuchi T: Induction of
lysophosphatidic acid receptor-3 by
12-O-tetradecanoylphorbol-13-acetate stimulates cell
migration of rat liver cells. Cancer Lett. 309:236–242. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin CW, Hou WC, Shen SC, Juan SH, Ko CH,
Wang LM and Chen YC: Quercetin inhibition of tumor invasion via
suppressing PKC delta/ERK/AP-1-dependent matrix metalloproteinase-9
activation in breast carcinoma cells. Carcinogenesis. 29:1807–1815.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin CW, Shen SC, Chien CC, Yang LY, Shia
LT and Chen YC: 12-O-tetradecanoylphorbol-13-acetate-induced
invasion/migration of glioblastoma cells through activating
PKCalpha/ERK/NF-kappaB-dependent MMP-9 expression. J Cell Physiol.
225:472–481. 2010.
|
|
21
|
Ala-aho R and Kähäri VM: Collagenases in
cancer. Biochimie. 87:273–286. 2005. View Article : Google Scholar
|
|
22
|
Bjorklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
23
|
Devy L, Huang L, Naa L, Yanamandra N,
Pieters H, Frans N, Chang E, Tao Q, Vanhove M, Lejeune A, van Gool
R, Sexton DJ, Kuang G, Rank D, Hogan S, Pazmany C, Ma YL,
Schoonbroodt S, Nixon AE, Ladner RC, Hoet R, Henderikx P, Tenhoor
C, Rabbani SA, Valentino ML, Wood CR and Dransfield DT: Selective
inhibition of matrix metalloproteinase-14 blocks tumor growth,
invasion, and angiogenesis. Cancer Res. 69:1517–1526. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mook OR, Frederiks WM and Van Noorden CJ:
The role of gelatinases in colorectal cancer progression and
metastasis. Biochim Biophys Acta. 1705:69–89. 2004.PubMed/NCBI
|
|
25
|
Nakajima M, Welch DR, Wynn DM, Tsuruo T
and Nicolson GL: Serum and plasma M(r) 92,000 progelatinase levels
correlate with spontaneous metastasis of rat 13762NF mammary
adenocarcinoma. Cancer Res. 53:5802–5807. 1993.PubMed/NCBI
|
|
26
|
Eberhardt W, Huwiler A, Beck KF, Walpen S
and Pfeilschifter J: Amplification of IL-1 beta-induced matrix
metalloproteinase-9 expression by superoxide in rat glomerular
mesangial cells is mediated by increased activities of NF-kappa B
and activating protein-1 and involves activation of the
mitogen-activated protein kinase pathways. J Immunol.
165:5788–5797. 2000.
|
|
27
|
Sen T, Dutta A and Chatterjee A:
Epigallocatechin-3-gallate (EGCG) downregulates gelatinase-B
(MMP-9) by involvement of FAK/ERK/NFkappaB and AP-1 in the human
breast cancer cell line MDA-MB-231. Anticancer Drugs. 21:632–644.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maeda-Yamamoto M, Suzuki N, Sawai Y,
Miyase T, Sano M, Hashimoto-Ohta A and Isemura M: Association of
suppression of extracellular signal-regulated kinase
phosphorylation by epigallocatechin gallate with the reduction of
matrix metalloproteinase activities in human fibrosarcoma HT1080
cells. J Agric Food Chem. 51:1858–1863. 2003. View Article : Google Scholar
|