Introduction
Hepatocellular carcinoma (HCC) is a major health
problem in the world; it is the fifth most common cancer and the
third cause of cancer-related mortality in the world (1). Moreover, HCC is often diagnosed at the
advanced stage (2). Only a small
proportion of patients are candidates for curative therapies, such
as liver transplant or liver resection (3,4). In
unresectable and non-transplantable HCC, chemoembolization with
Lipiodol (iodized oil) is a common treatment. Although this method
can reduce tumor growth, it does not significantly improve survival
(5). Selective internal radiation
therapy (SIRT) is another treatment option for non-resectable HCC.
SIRT delivers the β ray radionuclides to the inner of the tumor and
the β rays kill the tumor cells, without significantly affecting
the normal hepatic cells. HCC patients have a high tolerance to
this method compared to other treatments (6).
CD147 is a member of the immunoglobulin superfamily
(7). It is a transmembrane
glycoprotein that is categorized as an immunoglobulin superfamily
of receptors (8). In the
physiological status, CD147 expression is associated with MCT1
protein expression in retinal pigment epithelia and spermatogenesis
(9). CD147 is also regarded as an
inducer of matrix metalloproteinase which is related to various
types of cancer (8,10–17).
In HCC, the expression level of CD147 is elevated. When the
expression of CD147 is blocked, HCC growth and metastasis are
significantly inhibited (15,18–20).
Almost all blood supply of the tumor in the liver
derives from the hepatic arterial system; thus, the transcatheter
arterial (TA) procedure is suitable for delivering substances to
treat HCC. Several studies have established its safety and efficacy
with long-term survival rates comparable to those of surgical
resection methods (21).
In this study we delivered the
131I-labeled CD147 monoclonal antibody
(131I-CD147-Ab) to the tumor by TA in an established
model of rabbit VX2 hepatic tumors and we studied the safety and
antitumor effects of this new approach.
Materials and methods
Animal model and groups
This study was approved by the Institutional Animal
Care and Use Committee of Zhengzhou University. New Zealand white
rabbits (4 weeks of age, 2.5–3.5 kg) were used in the
investigations. All rabbits were initially fed with standard food
and water for a week during adaptation in the animal research
facility. All rabbits were anesthetized using 3 mg/kg pentobarbital
sodium (Alvetra, Neumuenster, Germany) administered by ear vein
injection prior to tumor implantation, interventional procedures
and single-photon emission computed tomography (SPECT)-CT (Symbia T
16, Siemens, Germany) examination.
An orthotopic model of hepatocarcinoma was created
using VX2 carcinoma as previously described (22). All rabbit models were randomly
assigned to the 3 groups (n=14 each group) according to treatment;
saline (group A), 131I (group B),
131I-CD147-Ab (group C). The treatments were performed
by TA at 14 days after the animal models were established. Five
rabbits in each group were sacrificed for histopathological
examination at Day 7 after TA infusion (TAI) in all groups, and the
rest of the animals were kept for survival study.
Treatment procedure and tumor response
measurement
TAI was performed 14 days after tumor implantation
using a digital subtraction angiography system (Allura Xper FD20,
Philips Medical Systems, Best, The Netherlands) according to a
method previously described (23).
Briefly, a 4F vascular sheath was inserted into the right femoral
artery of the anesthetized rabbits (1% sodium pentobarbital at 3
mg/kg intravenous infusion) (Terumo, Tokyo, Japan). Selective
catheterization of the proper hepatic artery feeding the VX2
carcinoma was carried out by a 3F microcatheter (GP, Terumo), which
was coaxially inserted through a 4F Cobra catheter (Cook Inc.,
Bloomington, IN, USA). Hepatic angiography was obtained with hand
injection of 3 ml of contrast medium (Omnipaque 300; Ansheng
Pharmaceutical Co., Shanghai, China) at a rate of ~0.5 ml/sec. The
131I-CD147-Ab solution was infused carefully through the
3F microcatheter into the tumor-feeding artery. Then, the femoral
artery was ligated and the wound was closed. All procedures were
performed under DSA monitor and guaranteed the
131I-CD147-Ab flow through the liver tumor.
The animals were scanned by SPECT-CT at Days 1, 7
and 14 following TAI. Images were acquired 300 sec for static
images and at the speed of 25 sec per image for tomo-images.
The objective responses to treatment were evaluated
according to the criteria by Therasse et al(24) including complete response (CR), a
partial response (PR), stable disease (SD) and progressive disease
(PD) as judged by the longest dimension or the sum of the longest
dimensions of all measured target lesions or any new lesion. All
SPECT-CT images were analyzed by two radiologists who were blinded
to the experimental design.
131I-labeled CD147
antibody
CD147-Ab was labeled according to Mather’s method
(25), Briefly, we dissolved 10 mg
CD147 Ab powder in phosphate-buffered saline (0.1 mol/l, pH 7.4);
antibody solution was added with 1700 MBq 131I sodium
iodine solution which was based on the weight of the rabbits (27
MBq/kg). N-bromosuccinimide (2 mg) was dissolved in 2 ml of 0.1 M
PBS. NBS (200 μl) was added to the antibody/iodine mixture. The
vial was gently swirled and the reaction was quenched by 10 mg/ml
of human serum albumin (HSA) in 0.5 ml of 0.1 ml PBS. The reaction
mixture was purified on a Sephadex-G25 column according to the
manufacturer’s instructions. The final production was taken into
the sterile penicillin vial for the research. The iodine of the
final production was >95% bound as demonstrated by high-pressure
liquid chromatography (HPLC) and its immunoreactivity was
>40%.
Histopathological and immunohistochemical
evaluation
The animals were sacrificed by intravenous injection
of an overdose of sodium pentobarbital at Day 14 following TAI. The
whole liver and lung were resected and fixed in 10% formalin and
the specimens were cut into 4-μm sections in the axial positions
corresponding to the section of the SPECT-CT scan. Hematoxylin and
eosin (H&E)-stained sections were examined microscopically.
To evaluate TUNEL and MMP2 expression, the slides
were stained by a TUNEL or MMP2 monoclonal antibody (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) according to the
manufacturer’s instructions. Ten random non-necrotic areas (x200)
from each specimen were evaluated. TUNEL and MMP2 expression were
semiquantitatively evaluated at three levels: positive staining in
<10% was regarded as negative (−), positive staining in 10–50%
as weakly positive (±) and positive staining in ≥50% as positive
(+) (26).
For determination of microvessel density (MVD), the
paraffin-embedded sections were stained using anti-CD31 rabbit
monoclonal antibody (Dako Corp., Carpinteria, CA, USA) following a
standard SABC procedure, according to a method previously described
(27). The MVD was determined by
CD31 staining densities.
Briefly, five fields of ‘vascular hot spots’ with a
200-fold magnification in each tumor section obtained at 7 days
after TAI were examined and the mean MVD value was recorded in a
blinded fashion. The percentage of the necrotic area in the entire
tumor area was calculated from H&E sections according to a
previously described method (28).
Biochemical studies
Peripheral blood samples (2.0 ml) were collected for
biochemical examination at Days 0, 1, 3, 7, 10, 14 and 21 after
treatment. Plasma aspartate aminotransferase (AST), alanine
aminotransferase (ALT), blood urea nitrogen (BUN), serum creatinine
(Cr) and total bilirubin (TBIL) levels were measured using a
biochemical autoanalyzer (Model LX 20; Beckman, CA, USA). Free
triiodothyronine (FT3), free (unbound) thyroxin (FT4),
thyrotropic-stimulating hormone (TSH) and white blood cells (WBCs)
were also measured. Each sample was measured in triplicate.
Statistical analysis
Statistical evaluation was performed using SPSS
software (ver.13.0; PSS Inc., Chicago, IL, USA). Numerical data
were expressed as the means ± SD. P<0.05 was considered to
indicate a statistically significant difference. The Kruskal-Wallis
and Mann-Whitney U tests were performed among groups. Survival
rates were assessed using the Kaplan-Meier method.
Results
One animal in group B died 10 days after the tumor
implantation. This animal was excluded from further analysis. The
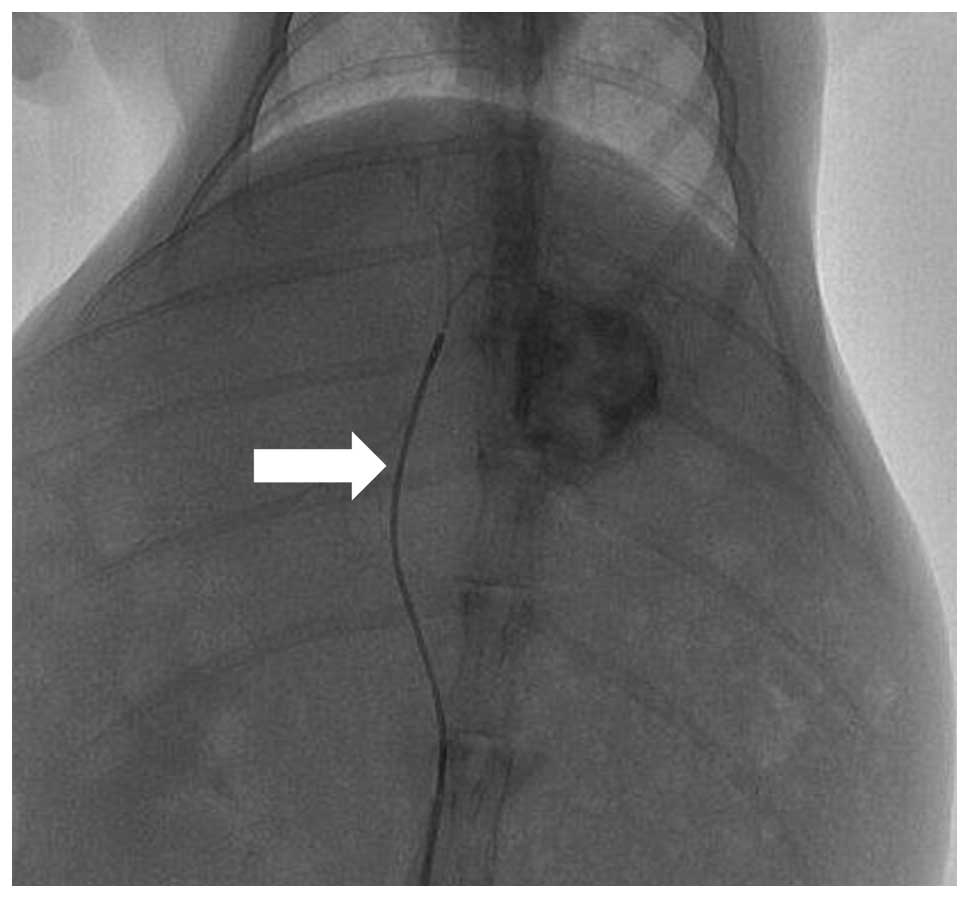
remaining surviving animals all successfully underwent TAI
(Fig. 1) and SPECT-CT
procedures.
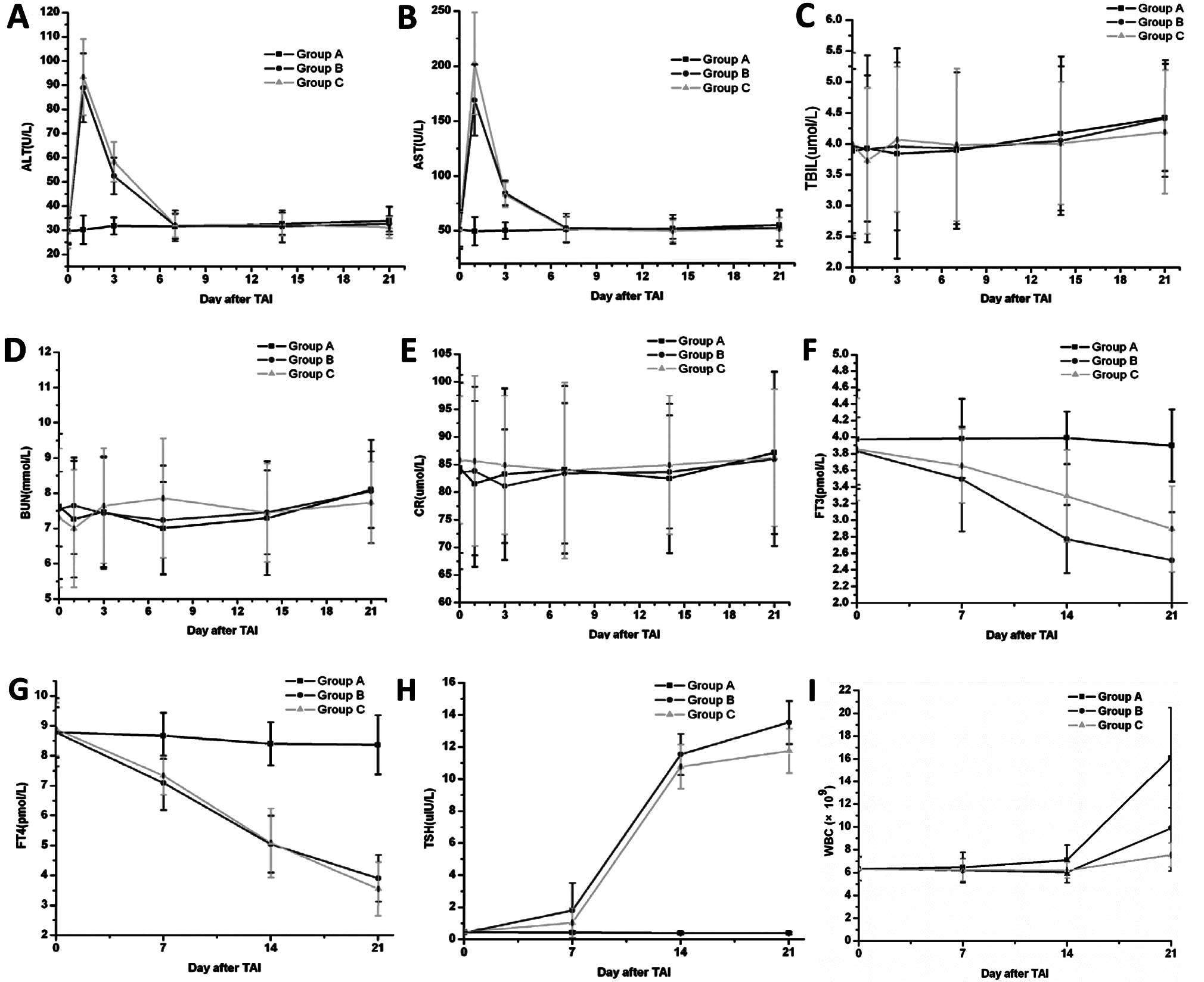
Biochemical tests
Liver function tests showed that AST and ALT levels
increased transiently 1 day after intra-arterial infusion with
131I or 131I-CD147-Ab and lasted ~7 days,
before returning to normal levels. The elevation of AST and ALT
levels was significantly different (P<0.05) at Day 1 in the
treatment groups compared with the saline group (Fig. 2A and B). The plasma TBIL (Fig. 2C) remained apparently unaltered in
the three groups. For the renal functions, BUN (Fig. 2D) and Cr (Fig. 2E) levels did not significantly
change in any group at any day.
To test the thyroid functions, we measured the FT3,
FT4 and TSH levels in all groups at Days 0, 7, 14 and 21 after TAI.
The value of FT3 and FT4 tended to decrease, whereas TSH increased;
significant differences were observed only at Days 14 and 21
(P<0.05), and not at Day 7 (P>0.05) (Fig. 2F-H). The WBC did not change
significantly until Day 21 following treatment (Fig. 2I).
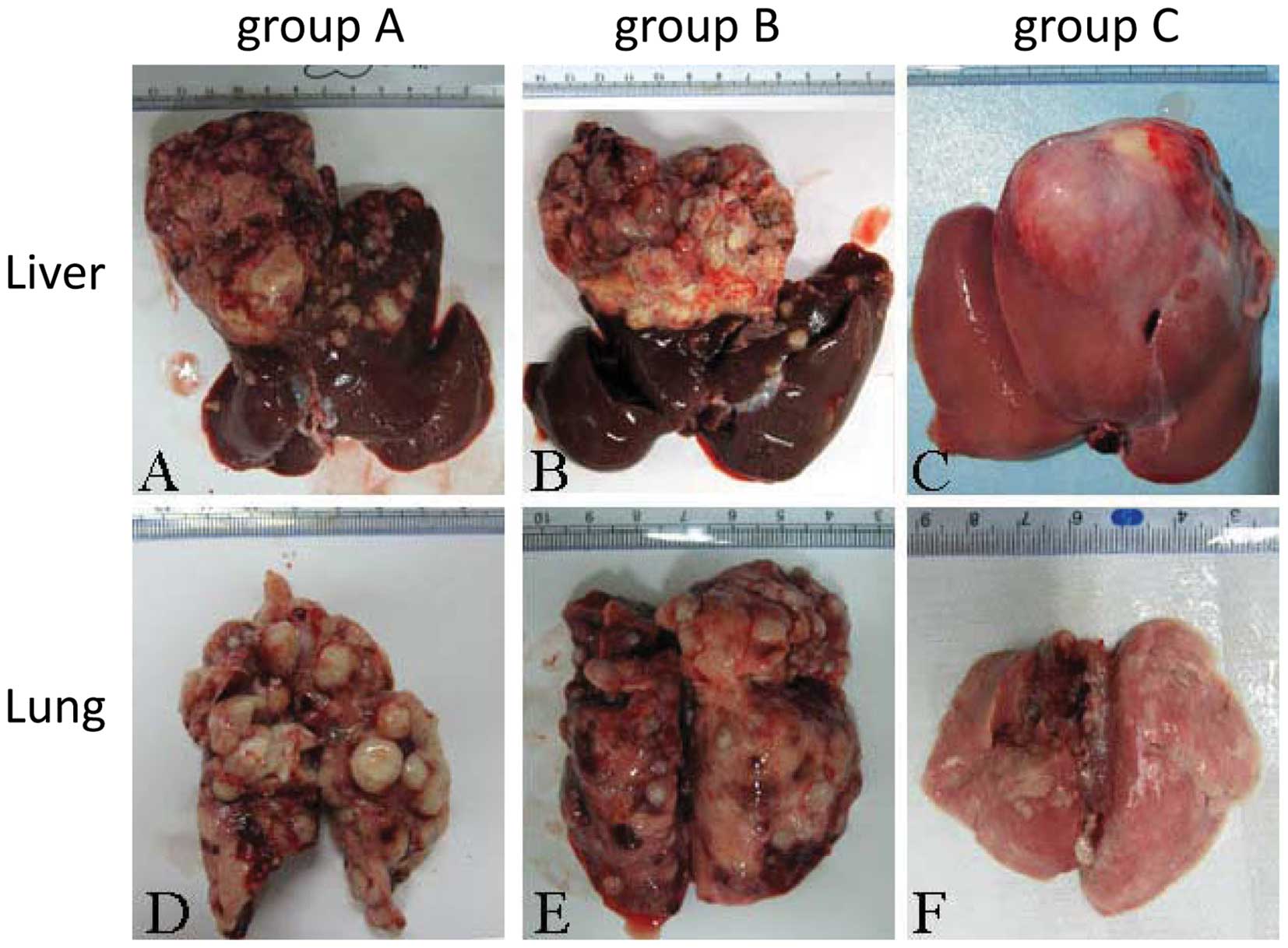
Lung and liver tumor measurement
The gross size of the tumor measured by the ruler in
group C was significantly smaller than in groups A and B.
Intrahepatic metastasis was the least extensive in group C, as was
lung metastasis (Fig. 3), with the
fewest nodes observed in group C.
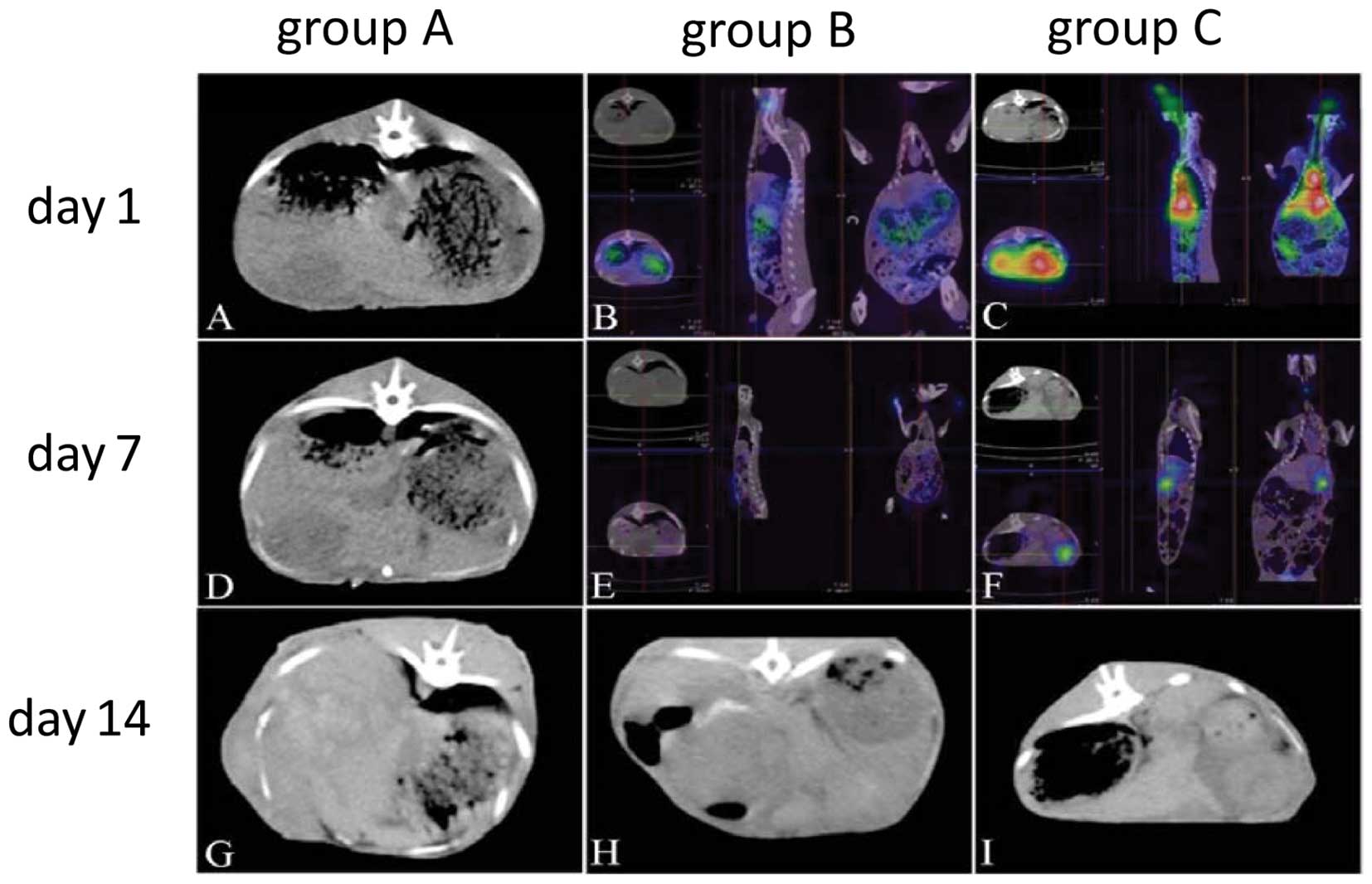
SPECT-CT inspection
The liver tumors increased quickly in groups A and
B, but not in group C (Fig. 4). The
radionuclide uptake was markedly higher in group C than in B at
Days 1 and 7 after treatment (Fig. 4B,
C, E and F). This suggested that 131I-CD147-Ab in
group C binds to the tumor cell more effectively than the
131I alone. At Day 14, the radioactivity had almost
disappeared in all groups.
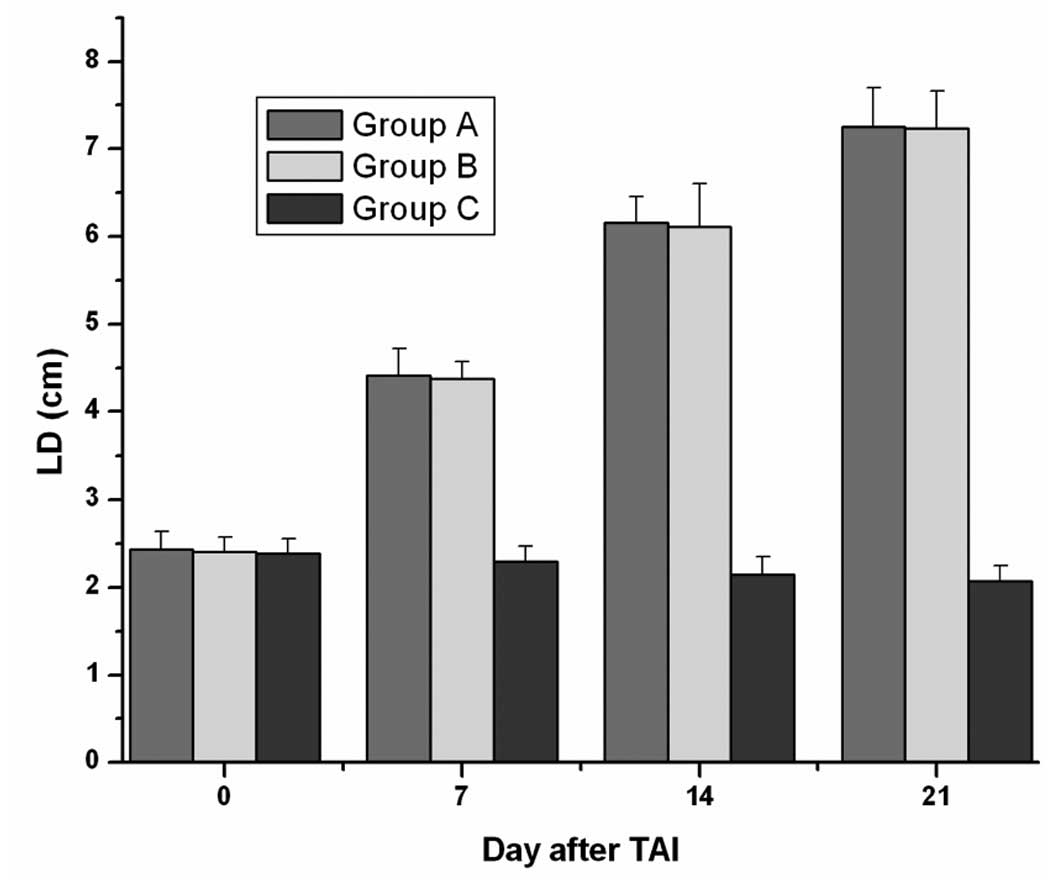
Treatment-associated tumor response
The tumor-inhibited effect as evaluated by the
Response Evaluation Criteria in Solid Tumors (RECIST) methodology
at Day 14 is shown in Table I. A
gradual decrease of the tumor’s LD on SPECT-CT scanning was
observed during the following 2 weeks in all group C rabbits
treated by 131I-CD147-Ab. The mean LD in group C at 1
and 2 weeks post-treatment was significantly smaller than in the
other two groups (P<0.05, each; Fig.
5).
 | Table ITumor response by RECIST
criteria. |
Table I
Tumor response by RECIST
criteria.
| Tumor response |
|---|
|
|
|---|
| Group | Complete response
(%) | Partial response
(%) | Stable disease
(%) |
|---|
| Group A | 0 | 0 | 0 |
| Group B | 0 | 3 (37.5) | 4 (50) |
| Group C | 0 | 8 (88.9) | 1 (11.1) |
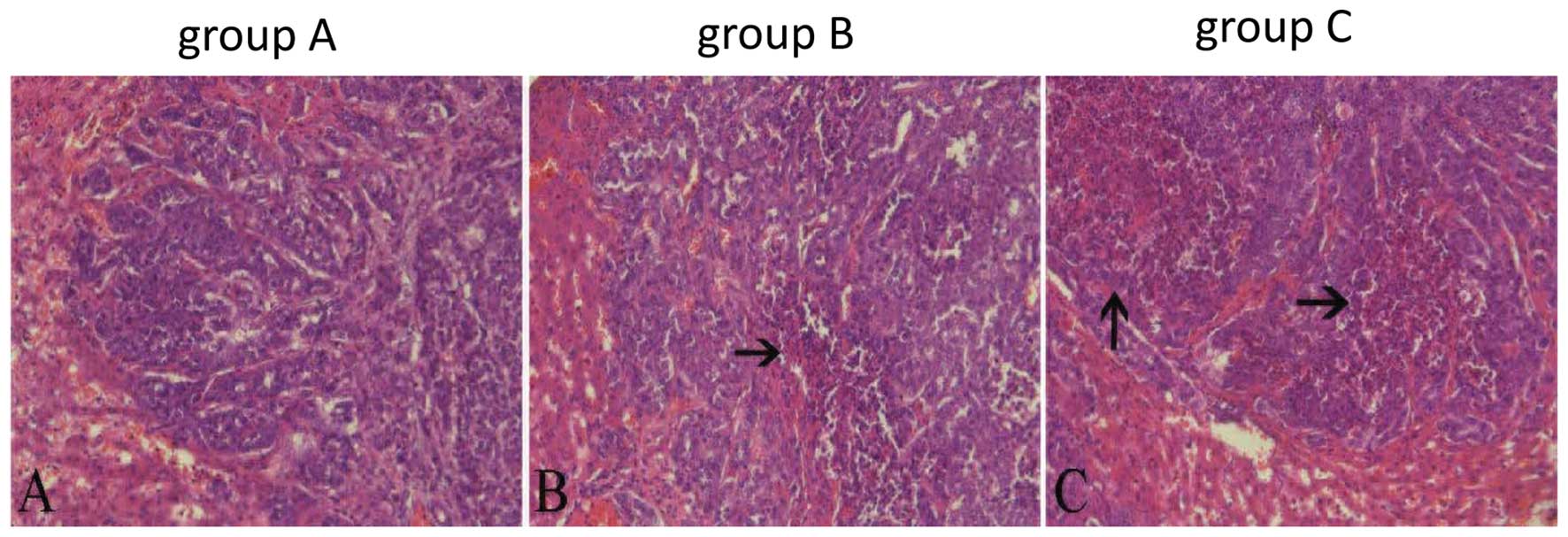
Histological findings
H&E staining
The necrosis of the tumor was confirmed by H&E
staining. The necrosis of group C was much larger than in the other
two groups (Fig. 6). The overall
necrosis rate of group C was the highest (P<0.01) (Table II).
 | Table IIMean tumor necrosis ratios in the
different groups. |
Table II
Mean tumor necrosis ratios in the
different groups.
| Group | Mean necrotic area
ratios (%) (mean ± SD) |
|---|
| Group A | 45.09±13.72a,b |
| Group B | 47.64±7.55c |
| Group C | 83.56±5.38 |
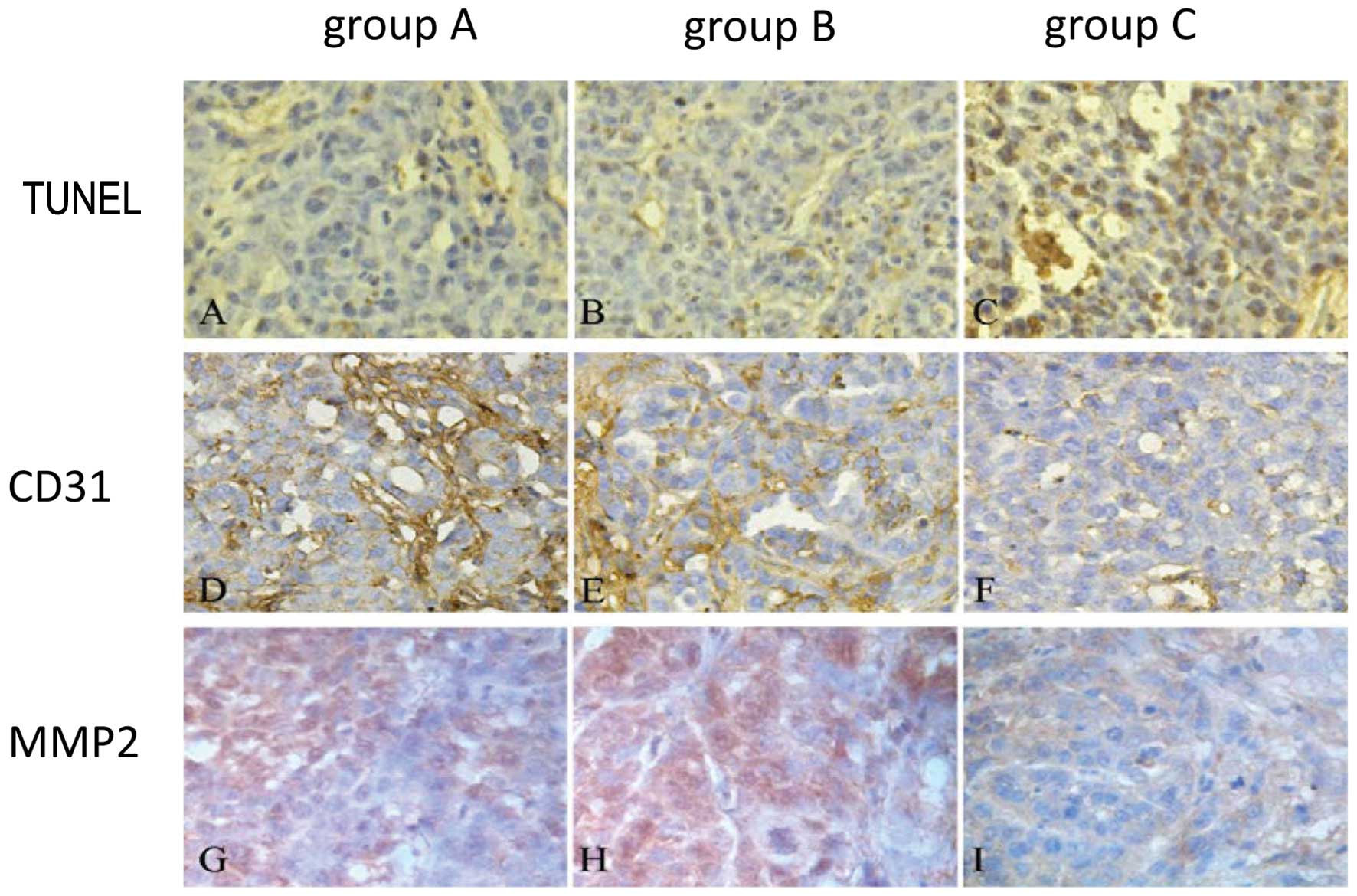
TUNEL, CD31 and MMP2 expression
The TUNEL expression was markedly high in group C
compared to the other 2 groups (Fig.
7A-C), while CD31 (Fig. 7D-F)
and MMP2 (Fig. 7G-I) expression
were much lower than in the other 2 groups; there were no
significant differences between groups A and B.
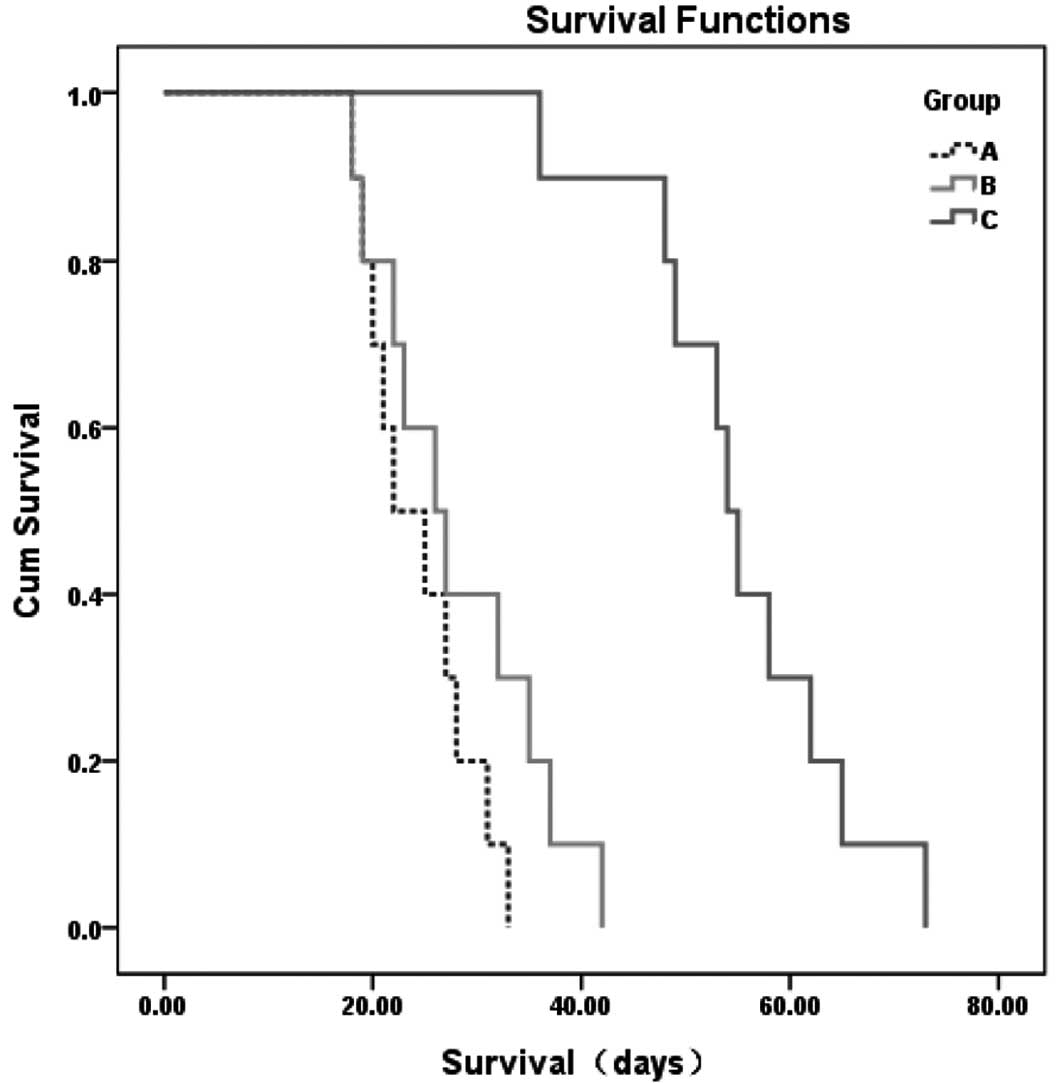
Survival
All rabbits died of hepatic and/or respiratory
failure secondary to extensive tumor burden within 73 days. The
animals treated by 131I-CD147-Ab lived significantly
longer than the animals in the other two groups (Kaplan-Meier
method with log-rank test, P<0.001) (Fig. 8).
Discussion
Although transcatheter arterial chemoembolization
(TACE) can extend the survival time of HCC patients, the overall
survival rate of these patients remains poor (29,30).
Xu et al used the Licartin (131I labeled human
CD147 monoclonal Ab) to treat HCC patients following liver
transplantation (19). They found
that Licartin decreases the recurrence rate and improves the
survival rate. Although the survival time and metastasis of the
131I-CD147-Ab treatment group were better than the other
groups in our experiment, all the rabbits eventually died. However,
the animals in the 131I-CD147-Ab treatment group lived
significantly longer than the animals in the other two groups, as
we only gave one treatment to the animals in our study. If
131I-CD147-Ab was given 2 or 3 times, as Xu et al
reported in the clinic, the survival time would be much longer.
SPECT-CT and tumor size resected from the animals
confirmed the inhibition of metastasis and growth in the treatment
group. From the SPECT-CT examination, we found that the
131I-CD147-Ab in the treatment group remained
significantly longer in the tumor than groups A and B, indicating
that the CD147 monoclonal antibody can combine its Ag effectively
by TAI, thus, the 131I and the CD147 monoclonal antibody
can work collaboratively. Administration of the
131I-CD147-Ab via TA was suitable for the delivery of
this drug without any side-effects. 131I-CD147-Ab
decreases the MVD and MMP2 expression in the tumor, partly since
the Ab blocked the expression of CD147 which can affect its
downstream signaling. Also, the 131I can kill tumor
cells. All these work together to inhibit the tumor growth and
metastasis. In this experiment, delivering the
131I-CD147-Ab by TA was more specific for the tumor and
caused fewer side-effects in the surrounding normal liver. This
method is suitable treatment for unresectable HCC patients, with
fewer side-effects.
Several groups have found that CD147 is related to
tumor invasion and metastasis (12,13,15,20,31–33).
Our data also showed that 131I-CD147-Ab was closely
associated with multiple processes of HCC invasion and metastasis.
We found that the expression levels of CD31 and MMP2 were
significantly decreased following treatment compared to the other
two groups, as reported by previous studies (34–36).
This may be one of the mechanisms by which 131I-CD147-Ab
prolongs survival time and decreases the tumor size in the animal
model.
This report is the first to demonstrate that
131I-CD147-Ab inhibits the tumor growth and metastasis
in the animal model of VX2 carcinoma, a suitable model for the
study of HCC. Although we obtained some encouraging results in our
preliminary study, a lot remains to be elucidated and further
studies into the treatment of HCC are required. However, our study
has some limitations; first, we did not compare the therapeutic
effects of 131I-CD147-Ab with those of other
chemotherapy drugs or 131I-Lipiodol treatment which is
already used for liver tumors. We used 14 rabbits per group in our
study; future studies should include larger groups. Also, although
131I-CD147-Ab can decrease tumor growth and metastasis,
the specific mechanisms were not fully investigated; further
studies into the underlying mechanisms are warranted.
Despite these limitations, this preliminary study
demonstrated that 131I-CD147 is a promising drug for HCC
and may ultimately be used for clinical practice in the future.
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
TAI
|
transcatheter arterial infusion
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
TBIL
|
total bilirubin
|
|
BUN
|
blood urea nitrogen
|
|
Cr
|
creatinine
|
|
FT3
|
free triiodothyronine
|
|
FT4
|
free (unbound) thyroxin
|
|
TSH
|
thyrotropic-stimulating hormone
|
|
MMP2
|
matrix metalloproteinase-2
|
|
MVD
|
microvessel density
|
|
SPECT-CT
|
single-photon emission computed
tomography
|
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
3
|
Jelic S: Hepatocellular carcinoma: ESMO
clinical recommendations for diagnosis, treatment and follow-up.
Ann Oncol. 20(Suppl 4): 41–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lai EC, Fan ST, Lo CM, Chu KM, Liu CL and
Wong J: Hepatic resection for hepatocellular carcinoma. An audit of
343 patients. Ann Surg. 221:291–298. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
No authors listed. A comparison of
lipiodol chemoembolization and conservative treatment for
unresectable hepatocellular carcinoma. Groupe d’Etude et de
Traitement du Carcinome Hepatocellulaire. N Engl J Med.
332:1256–1261. 1995.
|
|
6
|
Schwartz JD and Beutler AS: Therapy for
unresectable hepatocellular carcinoma: review of the randomized
clinical trials-II: systemic and local non-embolization-based
therapies in unresectable and advanced hepatocellular carcinoma.
Anticancer Drugs. 15:439–452. 2004. View Article : Google Scholar
|
|
7
|
Biswas C: Tumor cell stimulation of
collagenase production by fibroblasts. Biochem Biophys Res Commun.
109:1026–1034. 1982. View Article : Google Scholar
|
|
8
|
Biswas C, Zhang Y, DeCastro R, et al: The
human tumor cell-derived collagenase stimulatory factor (renamed
EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res.
55:434–439. 1995.PubMed/NCBI
|
|
9
|
Philp NJ, Ochrietor JD, Rudoy C, Muramatsu
T and Linser PJ: Loss of MCT1, MCT3, and MCT4 expression in the
retinal pigment epithelium and neural retina of the
5A11/basigin-null mouse. Invest Ophthalmol Vis Sci. 44:1305–1311.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo H, Zucker S, Gordon MK, Toole BP and
Biswas C: Stimulation of matrix metalloproteinase production by
recombinant extracellular matrix metalloproteinase inducer from
transfected Chinese hamster ovary cells. J Biol Chem. 272:24–27.
1997. View Article : Google Scholar
|
|
11
|
Zhu S, Li Y, Mi L, et al: Clinical impact
of HAb18G/CD147 expression in esophageal squamous cell carcinoma.
Dig Dis Sci. 56:3569–3576. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong WD, Liang YX, Lin SX, et al:
Expression of CD147 is associated with prostate cancer progression.
Int J Cancer. 130:300–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu F, Cui L, Zhang Y, et al: Expression
of HAb18G is associated with tumor progression and prognosis of
breast carcinoma. Breast Cancer Res Treat. 124:677–688. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He HC, Han ZD, Dai QS, et al: Expression
and significance of CD147 protein in prostate cancer. Zhonghua Yi
Xue Za Zhi. 89:1844–1846. 2009.(In Chinese).
|
|
15
|
Xu J, Xu HY, Zhang Q, et al: HAb18G/CD147
functions in invasion and metastasis of hepatocellular carcinoma.
Mol Cancer Res. 5:605–614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Redondo P, Lloret P, Idoate M and Inoges
S: Expression and serum levels of MMP-2 and MMP-9 during human
melanoma progression. Clin Exp Dermatol. 30:541–545. 2005.
View Article : Google Scholar
|
|
17
|
Kawamura K, Kamiya N, Suyama T, et al: In
situ gelatinolytic activity correlates with tumor progression and
prognosis in patients with bladder cancer. J Urol. 172:1480–1484.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun J and Hemler ME: Regulation of MMP-1
and MMP-2 production through CD147/extracellular matrix
metalloproteinase inducer interactions. Cancer Res. 61:2276–2281.
2001.PubMed/NCBI
|
|
19
|
Xu J, Shen ZY, Chen XG, et al: A
randomized controlled trial of Licartin for preventing hepatoma
recurrence after liver transplantation. Hepatology. 45:269–276.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanekura T, Chen X and Kanzaki T: Basigin
(CD147) is expressed on melanoma cells and induces tumor cell
invasion by stimulating production of matrix metalloproteinases by
fibroblasts. Int J Cancer. 99:520–528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Llovet JM, Real MI, Montana X, et al:
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma: a
randomised controlled trial. Lancet. 359:1734–1739. 2002.
View Article : Google Scholar
|
|
22
|
Hansler J, Neureiter D, Wasserburger M, et
al: Percutaneous US-guided radiofrequency ablation with perfused
needle applicators: improved survival with the VX2 tumor model in
rabbits. Radiology. 230:169–174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoon CJ, Chung JW, Park JH, et al:
Transcatheter arterial chemoembolization with paclitaxel-lipiodol
solution in rabbit VX2 liver tumor. Radiology. 229:126–131. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
25
|
Zhang Z, Bian H, Feng Q, et al:
Biodistribution and localization of iodine-131-labeled metuximab in
patients with hepatocellular carcinoma. Cancer Biol Ther.
5:318–322. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamaguchi R, Yano H, Iemura A, Ogasawara
S, Haramaki M and Kojiro M: Expression of vascular endothelial
growth factor in human hepatocellular carcinoma. Hepatology.
28:68–77. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hamaguchi S, Tohnai I, Ito A, et al:
Selective hyperthermia using magnetoliposomes to target cervical
lymph node metastasis in a rabbit tongue tumor model. Cancer Sci.
94:834–839. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marelli L, Stigliano R, Triantos C, et al:
Transarterial therapy for hepatocellular carcinoma: which technique
is more effective? A systematic review of cohort and randomized
studies. Cardiovasc Intervent Radiol. 30:6–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Biolato M, Marrone G, Racco S, et al:
Transarterial chemoembolization (TACE) for unresectable HCC: a new
life begins? Eur Rev Med Pharmacol Sci. 14:356–362. 2010.PubMed/NCBI
|
|
31
|
Yang Y, Lu N, Zhou J, Chen ZN and Zhu P:
Cyclophilin A up-regulates MMP-9 expression and adhesion of
monocytes/macrophages via CD147 signalling pathway in rheumatoid
arthritis. Rheumatology. 47:1299–1310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dai JY, Dou KF, Wang CH, et al: The
interaction of HAb18G/CD147 with integrin alpha6beta1 and its
implications for the invasion potential of human hepatoma cells.
BMC Cancer. 9:3372009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou J, Zhu P, Jiang JL, et al:
Involvement of CD147 in overexpression of MMP-2 and MMP-9 and
enhancement of invasive potential of PMA-differentiated THP-1. BMC
Cell Biol. 6:252005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stewart CJ and Crook ML: CD147 (EMMPRIN)
and matrix metalloproteinase-2 expression in uterine endometrioid
adenocarcinoma. Pathol Res Pract. 207:30–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Afonso J, Longatto-Filho A, Baltazar F, et
al: CD147 overexpression allows an accurate discrimination of
bladder cancer patients’ prognosis. Eur J Surg Oncol. 37:811–817.
2011.PubMed/NCBI
|
|
36
|
Voigt H, Vetter-Kauczok CS, Schrama D,
Hofmann UB, Becker JC and Houben R: CD147 impacts angiogenesis and
metastasis formation. Cancer Invest. 27:329–333. 2009. View Article : Google Scholar : PubMed/NCBI
|