Introduction
Gastric cancer is the leading cause of
cancer-related mortality in China and is the third leading cause of
cancer-related mortality in North America and Western Europe
(1). Previous studies have found
that an acidic tumor microenvironment is key to managing cancer
progression and metastasis (2–4).
Vacuolar-ATPases (V-ATPases), specific proton pumps of the cell,
have an important role in maintaining a relatively neutral
intracellular pH (pHi), an acidic luminal pH, and an acidic
extracellular pH (pHe). They are overexpressed in many types of
metastatic cancers and are positively correlated with invasive and
metastatic tumor potential (5).
Furthermore, blocking the expression of V-ATPases can inhibit the
growth and metastasis of human cancer (6).
Some molecules and drugs that inhibit V-ATPases have
been identified (7), such as
bafilomycin, concanamycin and NiK-12192, but their toxic effect and
poor results in preclinical tests have limited their development as
therapeutic agents. Recent insight into the mechanism of tumor
acidification has provided new strategies for targeting V-ATPases
(8). Proton pump inhibitors (PPIs)
could represent a class of drugs suitable to this purpose (9). PPIs have demonstrated gastric acid
suppression and have been applied in acid-related diseases
generally with good safety and few side effects. A specific target
of PPIs is K+/H+-ATPase which is located in
gastric parietal cells. Moreover, our previous study found that
PPIs can inhibit the expression of V-ATPases, and reverse the
transmembrane pH gradient (10).
Among the cancer-related signaling pathways, the
canonical Wnt pathway, also known as the Wnt/β-catenin pathway, is
involved in gastrointestinal carcinogenesis. Wnt ligands engage
their receptor complex, stabilize intracellular levels of
β-catenin, and allow the nuclear accumulation of β-catenin,
together with the transcription factor lymphoid enhancer-binding
factor 1/T cell-specific factor, followed by transcriptional
activation of Wnt/β-catenin target genes such as c-Myc and cyclin
D1 (11). In this study, we
explored the effect of a PPI on gastric carcinoma by regulating
Wnt/β-catenin signaling.
Materials and methods
Drugs
Pantoprazole was purchased from Altana Pharma
(Konstanz, Germany; AGD-78467). According to our previous study, a
concentration >20 mg/ml of pantoprazole inhibited the
proliferation of SGC7901 cells. Pantoprazole was resuspended in
normal saline at 20 mg/ml immediately before use.
Cell line and cell culture
The human gastric adenocarcinoma cell line, SGC7901,
was kindly provided by the Department of Oncology, Drum Tower
Hospital of the Nanjing University Medical School. Cells were
cultured in RPMI-1640 (HyClone, Logan, UT, USA) supplemented with
10% fetal bovine serum (FBS) (Hangzhou Sijiqing Biological
Engineering Materials, China) and antibiotics (100 U/ml penicillin
and 100 μg/ml streptomycin) in humidified air with a 5%
CO2 atmosphere at 37ºC (Direct Heat CO2
incubator; Thermo Scientific, Rockford, IL, USA).
Transfection
Cells plated in 100-mm dishes were transfected at
50–80% confluence with V-ATPase expression vectors or with control
vectors, using the liposome-mediated transfection method. SGC7901
cells were transfected with an shRNA-V-ATPase or negative control
vector (GAPDH) for 2 days, then trypsinized and plated at low
density. Stable clones were selected by maintaining cells in medium
containing G418 antibiotic.
Cell viability assay
The cytotoxicity of pantoprazole was determined
using the MTT (KeyGen Biotech Co., Ltd., China) assay. Cells
(1×104/well) were plated in 96-well plates in 200 μl of
medium/well and then treated with pantoprazole at 20 mg/ml. Control
cells were treated with Dulbecco’s modified Eagle’s medium (DMEM).
At different time points, 50 μl of 5 mg/ml MTT was added, and the
cells were cultured for another 4 h. The supernatant was removed,
and 150 μl of dimethyl sulfoxide (DMSO) was added per well. The
absorbance at 570 nm was measured with a microplate reader (Tecan
Sunrise, Switzerland), using wells without cells as blanks and
untreated cells as a negative control. The viability of the
drug-treated cells was expressed as a percentage of the population
growth with standard error of the mean relative to that of the
untreated control cells.
Annexin V-fluorescein isothiocyanate
(FITC) apoptosis detection
Apoptosis detection in cells was performed by the
Annexin V-FITC and propidium iodide (PI) double staining apoptosis
detection kit (KeyGen Biotech, Co. Ltd.) using flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA). Briefly, the cells were
trypsinized, washed with phosphate-buffered saline (PBS),
centrifuged and resuspended with Annexin V binding buffer (500 μl).
The cells were incubated with 5 ml Annexin V-FITC solution for 5
min at room temperature. In the same step, PI was added at 5 μg/ml
(5 μl) for another 5 min to distinguish necrotic cells. The samples
were analyzed within 1 h by fluorescence-activated cell sorter
(FACS) with CellQuest software (version 3.3).
Colony formation assay
Cells (6×104/well) were plated in Petri
dishes with a 6-cm diameter with 10 ml of DMEM (Gibco-BRL,
Carlsbad, CA, USA) containing 10% fetal calf serum (FCS) (HyClone)
in the presence or absence of various concentrations of
pantoprazole at 37ºC. After culture for 10 days, colonies
consisting of >50 cells were counted under the microscope.
Matrigel invasion assay
For the invasion assay, a modified Boyden chamber
(Neuro Probe, Gaithersburg, MD, USA) was used. The pore size of the
polycarbonate filters was 8.0 μm. The bottom chamber of the
Transwell chamber was filled with 30 μl RPMI-1640 containing 10%
FBS. The cells were then suspended at a density of 1×105
cells/ml in 500 μl of RPMI supplemented with 0.5% FBS and
pantoprazole, and added to 8-μm porous BioCoat Matrigel chamber
inserts (BD Biosciences) and placed in wells filled with 0.7 ml of
medium supplemented with 10% FCS as a chemoattractant. After 2 days
of incubation, the upper side of the filter was scraped with a
cotton tip to eliminate cells that had not migrated through it. To
obtain the total apoptosis rate and inhibition rate by pantoprazole
for 48 h, we calculated the number of cells which did not undergo
apoptosis (Live cells = plated cells + proliferated cells -
apoptotic cells). The invasive ability of the cells was determined
by counting the cells that had migrated to the lower side of the
filter with a microscope. The relative invasion rate was obtained
using the following formula: Relative invasion rate = migrated
cells/live cells. Experiments were performed in triplicate, and at
least 10 fields were counted for each experiment.
Western blot analysis
Proteins were extracted in lysis buffer (30 mmol/l
Tris, pH 7.5, 150 mmol/l sodium chloride, 1 mmol/l
phenylmethylsulfonyl fluoride, 1 mmol/l sodium orthovanadate, 1%
Nonidet P-40, 10% glycerol and phosphatase and protease
inhibitors). The proteins were then separated by SDS-PAGE and
electrophoretically transferred onto polyvinylidene fluoride
membranes. The membranes were probed with antibodies overnight at
4ºC, and then incubated with the secondary antibody. Images of the
western blotting products were captured and analyzed by Quantity
One v4.31 (Bio-Rad, Hercules, CA, USA).
Data analysis
Statistical comparisons were performed with the
software package SPSS 13.0 using the Student’s t-test for paired
observations or one-way ANOVA with SNK, LSD and Dunnett’s methods.
All data are presented as means ± standard deviation (SD).
P<0.05 was considered statistically significant. Mean values and
SD were calculated for experiments carried out in triplicate.
Results
Growth inhibition of human SGC7901 cells
by pantoprazole
The inhibitory activity of pantoprazole on the
proliferation of human gastric cancer SGC7901 cells was
investigated. Cells were grown in the absence or presence of
pantoprazole (20 mg/ml) for 12, 24 and 48 h. MTT assays were then
performed. The growth inhibition occurred in a time-dependent
manner. The cell viability of SGC7901 cells in the PPI group
(43.1±3.9%) was lower than that in the shRNA-GAPDH group (89±4.9%)
at 48 h post treatment (Fig. 1A).
The effect of pantoprazole on colony formation of the cells was
also assessed. On day 10 post-treatment, pantoprazole suppressed
the colony formation of the cells (Fig.
1B). These results suggest that pantoprazole preferentially
inhibits the growth of SGC7901 cells.
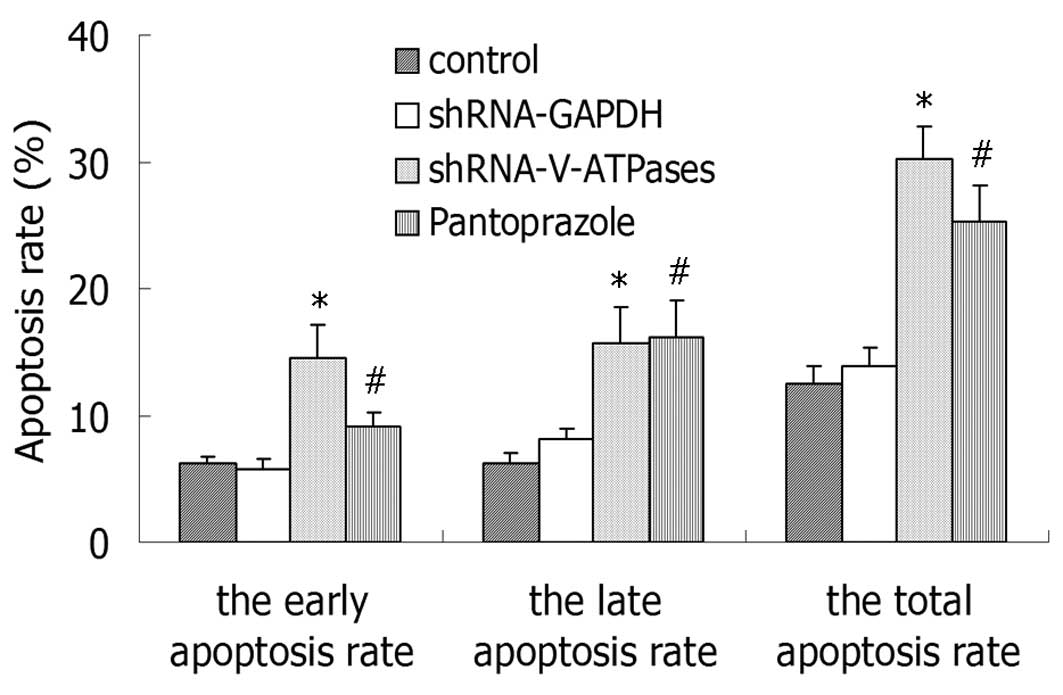
Apoptosis detection
A quantitative analysis of the fluorescent signals
was performed by FACS. As noted in Fig.
2, the total apoptosis was increased from 10.5% in the control
cells and reached 25.3% following treatment with 20 mg/ml
pantoprazole at 48 h. Treatment of SGC7901 cells showed a similar
dose-dependent response pattern for the early and late apoptosis
rates (Fig. 2).
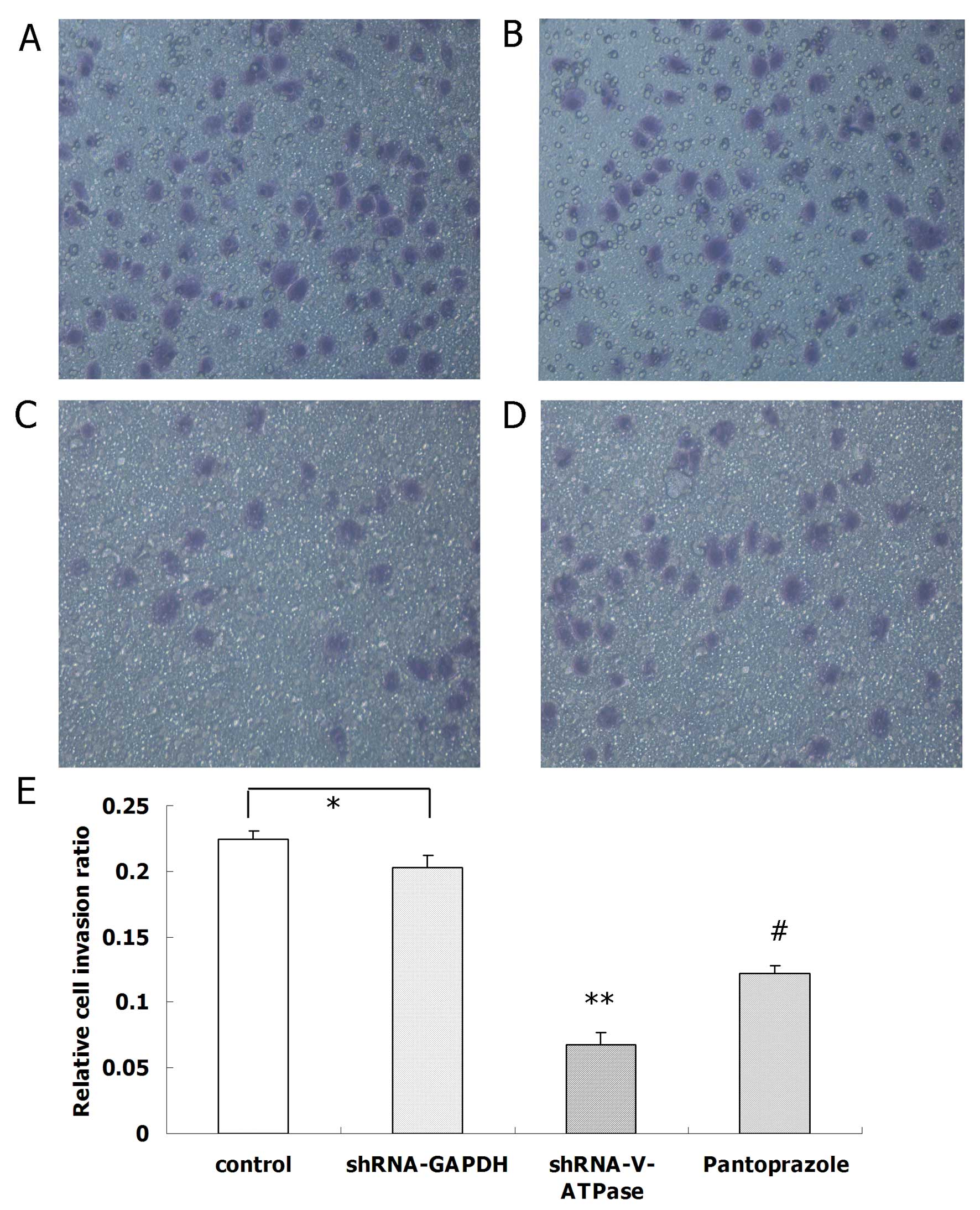
Matrigel cell invasion assay
We observed a significant difference between SGC7901
cells with and without pantoprazole treatment in the migration
assay. Moreover, SGC7901 cell invasion was reduced by pantoprazole
at 20 mg/ml (P<0.05) in the Matrigel invasion assay (Fig. 3).
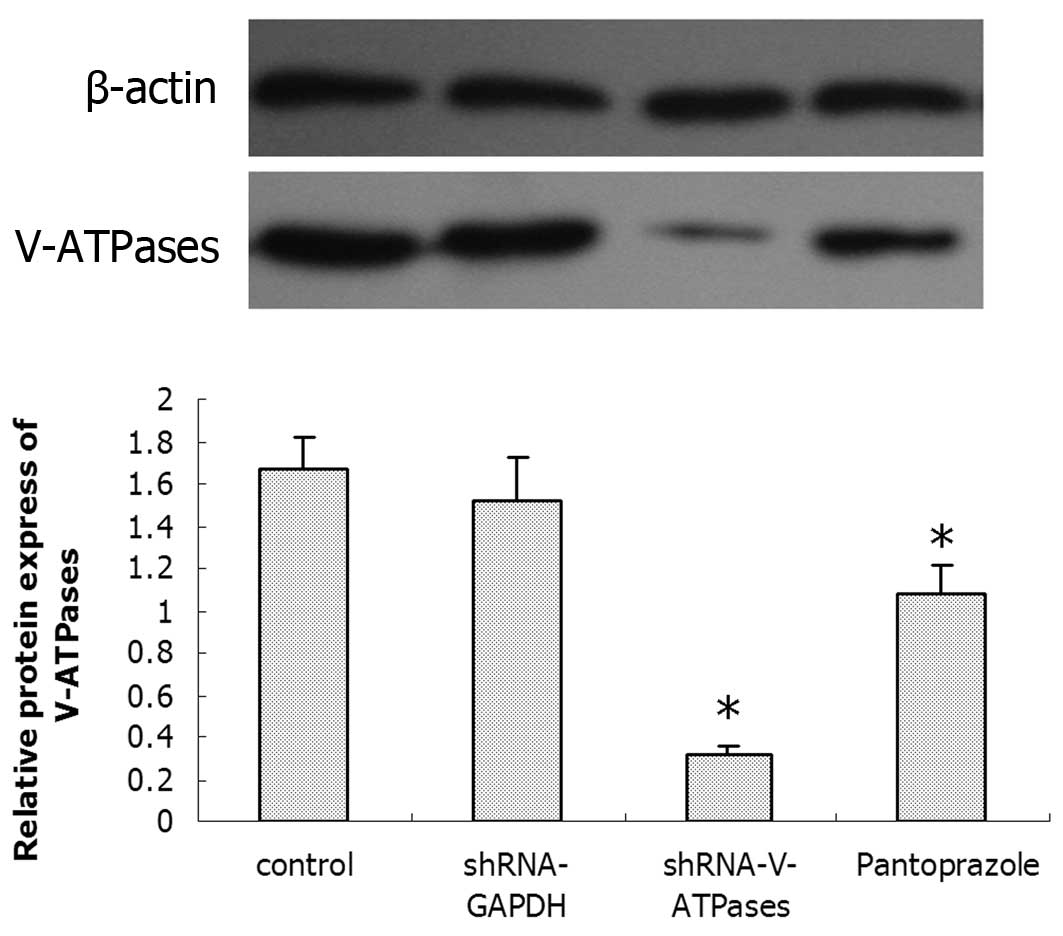
Effects of pantoprazole on V-ATPases
To determine whether pantoprazole inhibits the
expression of V-ATPases, SGC7901 cells were treated with 20 mg/ml
pantoprazole and after 48 h were assessed for the presence of
V-ATPase expression by immunofluorescence and western blotting.
After 48 h of pantoprazole treatment, the expression
of V-ATPases was altered when compared with that in the control
group (Fig. 4). The expression of
V-ATPases in the pantoprazole group was significantly less than
that in the control group (P<0.05), whereas no significant
difference was found between the control group and the shRNA-GAPDH
group.
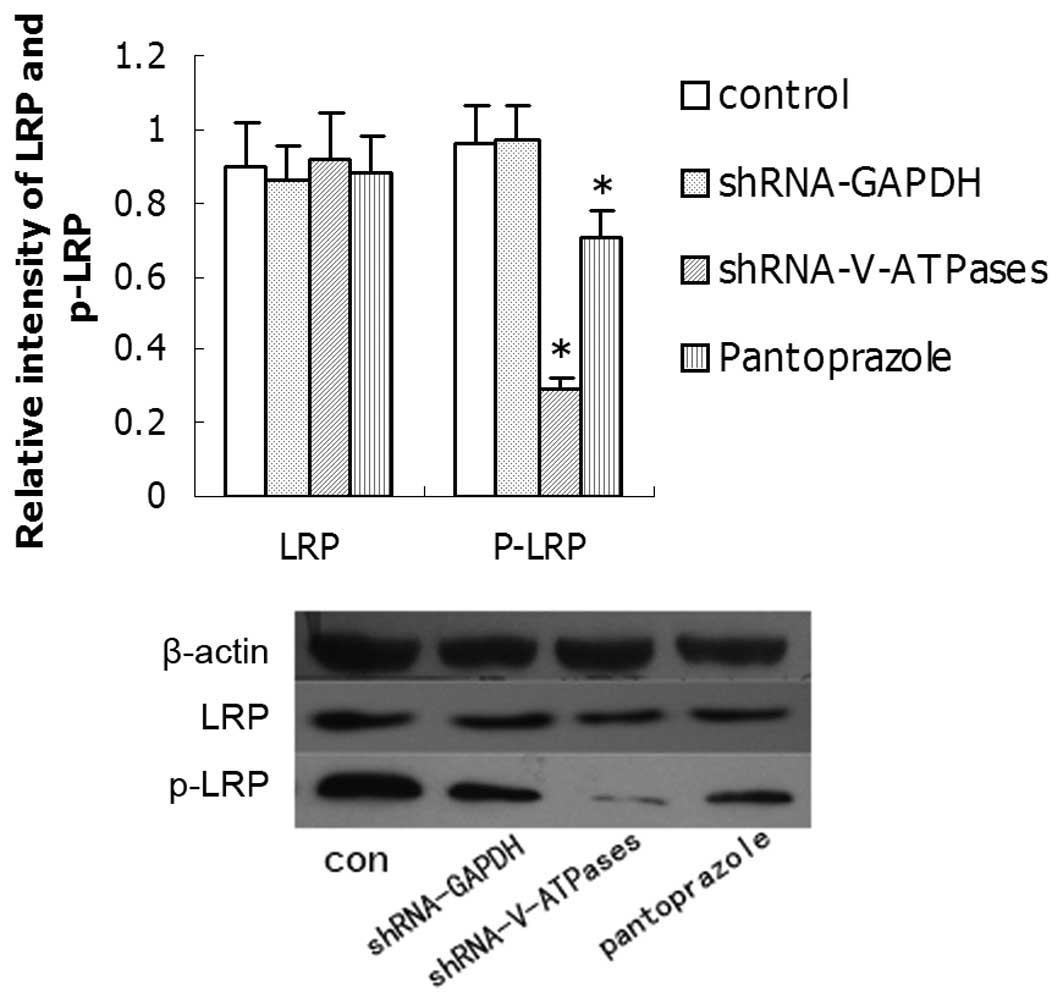
Effects on LRP6 and downstream genes by
pantoprazole
Research has demonstrated that phosphorylation of
LRP6 (which correlates with LRP6 activation) requires V-ATPase
activity, suggesting that the receptor may need to enter an acidic
intracellular compartment to become phosphorylated. We tested
whether pantoprazole inhibits the expression of phospho-LRP6 and
its downstream gene, β-catenin. The expression levels of these
proteins in SGC7901 cells treated with 20 mg/ml pantoprazole after
48 h were detected by western blotting (Fig. 5). The expression of phospho-LRP6 and
β-catenin was weak in the SGC7901 cells after pantoprazole
treatment compared with that in the control group, but LRP6 showed
no difference.
To examine the relationship between inhibition of
V-ATPases and Wnt/β-catenin signaling in gastric cancers, we also
detected expression of c-Myc and cyclin D1, which are well-known
target genes of the Wnt/β-catenin canonical pathway. c-Myc and
cyclin D1 were markedly downregulated in SGC7901 cells after
treatment with 20 mg/ml pantoprazole (Fig. 6). Therefore, we confirmed that the
inhibition of V-ATPase by pantoprazole reduced the expression of
c-Myc and cyclin D1.
Discussion
The V-ATPase is a multiprotein complex localized in
intracellular organelles and at the plasma membrane. It is involved
in diverse processes such as phagocytosis, virus entry, metastasis,
and embryonic left-right patterning. Its main mechanism is to pump
protons and acidify vesicles, thereby promoting vesicular traffic,
notably endocytosis (12,13). V-ATPases exist in various cell
types, including those of many solid tumors, and are involved in
progression and metastasis. These enzymes contribute to the acidic
pH of solid tumors, and have been proposed as a therapeutic target
for selective anticancer treatments (8,14,15) In
recent years, several studies have shown that PPIs such as
omeprazole, esomeprazole and pantoprazole have an antineoplastic
activity against human hematopoietic and solid tumors (16–18).
Our previous study also found that pantoprazole reversed the
transmembrane pH gradient and chemosensitized SGC7901 cells to
antitumor agents (10). These
results suggest that PPIs may be useful as an anticancer agent.
However, to date, no precise molecular mode of action in cancer
cells has been presented. Thus, we further studied its possible
cell targets.
Our previous study (10) found that pantoprazole inhibits the
expression of V-ATPases at concentrations of 20 mg/ml, and inhibits
the concentration of V-ATPases around the cell membrane, affecting
its role in transporting H+ out of the cell, and thereby
decreasing the intracellular pH and increasing the extracellular pH
value. Thus, in the present study, the concentration we applied was
20 mg/ml. We found that pantoprazole inhibited the proliferation,
induced apoptosis, and decreased the invasive ability of cells.
Thus, we confirmed that V-ATPase is a target of pantoprazole in
SGC7901 cells and that pantoprazole is a V-ATPase inhibitor. To
date, a few V-ATPase inhibitors have been identified, but none have
been used clinically because of their toxic effect on normal cells
(19–23). PPIs can suppress gastric acid and
treat diseases related with gastric acid with few side effects.
Therefore, we believe that PPIs, as anticancer agents, may
potentially benefit many patient groups.
The Wnt pathway is known to be involved in the
tumorigenesis of many human cancers, including colon cancer, breast
cancer, lung cancer, melanomas and hepatocellular carcinoma
(24–26). Dysregulation of this pathway can be
caused by mutations in many molecular components (e.g., CTNNB1,
AXIN or FZD7) in colon cancers, hepatocellular carcinomas and other
cancers (27–29). Recently, Cruciat et
al(30) found that V-ATPase is
involved in Wnt/β-catenin signaling, which is related to tumor
development and metastasis. LRP6 phosphorylation is accompanied by
receptor internalization in caveolin-containing vesicles and
endocytosis is essential for Wnt/β-catenin signaling (31,32).
This raised the possibility that V-ATPases may influence LRP6
endocytosis, phosphorylation and β-catenin activation. Our study
found that, consistent with the inhibition of V-ATPases by
pantoprazole, phospho-LRP6 and β-catenin simultaneously decreased,
but LRP6 was unchanged. To confirm that pantoprazole inhibits
Wnt/β-catenin signaling, we also detected c-Myc and cyclin D1,
which are well-known target genes of the Wnt canonical pathway
(33). c-Myc and cyclin D1 were
markedly downregulated after treatment with pantoprazole at
concentrations of 20 mg/ml in SGC7901 cells.
In conclusion, we report that pantoprazole as an
inhibitor of V-ATPases can induce cell death in human gastric
cancer using the Wnt/β-catenin pathway as a mechanism. Although
more careful analyses of the effects of pantoprazole on various
organs remain to be carried out, the results of this study showed
that V-ATPase is a potential cell target of pantoprazole for the
chemotherapy of gastric cancers.
Acknowledgements
This study was supported by the National Science
Foundation Grant (no. 81071816). Special thanks to Yong Liu and
Junhao Chen for their technical assistance in the flow cytometry.
We also thank Xingyun Xu for collecting the materials and
references.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Chiche J, Brahimi-Horn MC and Pouysségur
J: Tumor hypoxia induces a metatloic shift causing acidosis: a
common feature in cancer. J Cell Mol Med. 14:771–794. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trédan O, Galmarini CM, Patel K and
Tannock IF: Drug resistance and the solid tumor microenvironment. J
Natl Cancer Inst. 99:1441–1454. 2007.
|
|
4
|
Subarsky P and Hill RP: The hypoxic tumour
microenvironment and metastatic progression. Clin Exp Metastasis.
20:237–250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sennoune SR, Bakunts K, Martinez GM, et
al: Vacuolar H+-ATPase in human breast cancer cells with
distinct metastatic potential: distribution and functional
activity. Am J Physiol Cell Physiol. 286:1443–1452. 2004.
|
|
6
|
Lu X, Qin W, Li J, et al: The growth and
metastasis of human hepatocellular carcinoma xenografts are
inhibited by small interfering RNA targeting to the subunit ATP6L
of proton pump. Cancer Res. 65:6843–6849. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martinez-Zaguilan R, Lynch RM, Martinez GM
and Gillies RJ: Vacuolar-type H(+)-ATPases are functionally
expressed in the plasma membranes of human tumor cells. Am J
Physiol. 265:C1015–C1029. 1993.
|
|
8
|
Fais S, De Milito A, You HY and Qin W:
Targeting vacuolar H+-ATPases as a new strategy against
cancer. Cancer Res. 67:10627–10630. 2007.PubMed/NCBI
|
|
9
|
Barrison AF, Jarboe LA, Weinberg BM, et
al: Patterns of proton pump inhibitor use in clinical practice. Am
J Med. 111:469–473. 2011. View Article : Google Scholar
|
|
10
|
Chen M, Zou XP, Luo HS, et al: Effects and
mechanisms of proton pump inhibitors as a novel chemosensitizer on
human gastric adenocarcinoma (SGC7901) cells. Cell Biol Int.
33:1008–1019. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peifer M and Polakis P: Wnt signaling in
oncogenesis and embryogenesis - a look outside the nucleus.
Science. 287:1606–1609. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nishi T and Forgac M: The vacuolar
(H+)-ATPases - nature's most versatile proton pumps. Nat Rev Mol
Cell Biol. 3:94–103. 2002.
|
|
13
|
Swietach P, Vaughan-Jones RD and Harris
AL: Regulation of tumor pH and the role of carbonic anhydrase 9.
Cancer Metastasis Rev. 26:299–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sennoune SR, Luo D and Martínez-Zaguilán
R: Plasmalemmal vacuolar-type H+-ATPase in cancer
biology. Cell Biochem Biophys. 40:185–206. 2004. View Article : Google Scholar
|
|
15
|
De Milito A, Canese R, Marino ML, et al:
pH-dependent antitumor activity of proton pump inhibitors against
human melanoma is mediated by inhibition of tumor acidity. Int J
Cancer. 127:207–219. 2009.PubMed/NCBI
|
|
16
|
Luciani F, Spada M, De Milito A, et al:
Effect of proton pump inhibitor pretreatment on resistance of solid
tumors to cytotoxic drugs. J Natl Cancer Inst. 96:1702–1713. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yeo M, Kim DK, Kim YB, et al: Selective
induction of apoptosis with proton pump inhibitor in gastric cancer
cells. Clin Cancer Res. 10:8687–8696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Milito A, Iessi E, Logozzi M, et al:
Proton pump inhibitors induce apoptosis of human B-cell tumors
through a caspase-independent mechanism involving reactive oxygen
species. Cancer Res. 67:5408–5417. 2007.PubMed/NCBI
|
|
19
|
Bowman EJ, Siebers A and Altendorf K:
Bafilomycins: a class of inhibitors of membrane ATPases from
microorganisms, animal cells, and plant cells. Proc Natl Acad Sci
USA. 85:7972–7976. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Melikova MS, Blagoveshchenskaia AD,
Nikol’skiĭ NN and Kornilova ES: Effect of an vacuolar proton pump
inhibitor bafilomycin A1 on the intracellular processing of markers
for receptor-mediated and liquid phase endocytosis. Tsitologiia.
44:807–816. 2002.(In Russian).
|
|
21
|
Petrangolini G, Supino R, Pratesi G, et
al: Effect of a novel vacuolar-H+-ATPase inhibitor on
cell and tumor response to camptothecins. J Pharmacol Exp Ther.
318:939–946. 2006.PubMed/NCBI
|
|
22
|
Niikura K: Effect of a V-ATPase inhibitor,
FR202126, in syngeneic mouse model of experimental bone metastasis.
Cancer Chemother Pharmacol. 60:555–562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hesselink RW, Fedorov A, Hemminga MA and
Prieto M: Membrane-bound peptides from V-ATPases subunit a do not
interact with an indole-type inhibitor. J Pept Sci. 14:383–388.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.
|
|
25
|
Korinek V, Barker N, Morin PJ, et al:
Constitutive transcriptional activation by a β-catenin-Tcf complex
in APC-/-colon carcinoma. Science. 275:1784–1787. 1997.
|
|
26
|
Rubinfeld B, Robbins P, El-Gamil M, et al:
Stabilization of β-catenin by genetic defects in melanoma cell
lines. Science. 275:1790–1792. 1997.
|
|
27
|
Satoh S, Daigo Y, Furukawa Y, et al: AXIN1
mutations in hepatocellular carcinomas, and growth suppression in
cancer cells by virus mediated transfer of AXIN1. Nat Genet.
24:245–250. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
de La Coste A, Romagnolo B, Billuart P, et
al: Somatic mutations of the β-catenin gene are frequent in mouse
and human hepatocellular carcinomas. Proc Natl Acad Sci USA.
95:8847–8851. 1998.
|
|
29
|
Merle P, de la Monte S, Kim M, et al:
Functional consequences of frizzled-7 receptor overexpression in
human hepatocellular carcinoma. Gastroenterology. 127:1110–1122.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cruciat CM, Ohkawara B, Acebron SP, et al:
Requirement of prorenin receptor and vacuolar
H+-ATPase-mediated acidification for Wnt signaling.
Science. 327:459–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Blitzer JT and Nusse R: A critical role
for endocytosis in Wnt signaling. BMC Cell Biol. 7:282006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamamoto H, Komekado H and Kikuchi A:
Caveolin is necessary for Wnt-3a-dependent internalization of LRP6
and accumulation of β-catenin. Dev Cell. 11:213–223.
2006.PubMed/NCBI
|
|
33
|
Wang J, Wang X, Gong W, et al: Increased
expression of β-catenin, phosphorylated glycogen synthase kinase
3β, cyclin D1, and c-myc in laterally spreading colorectal
tumors. J Histochem Cytochem. 57:363–371. 2009.
|