Introduction
Erythropoietin (Epo) is a glycoprotein that
regulates the growth, maturation, and survival of erythroid
progenitor cells. Epo has received attention for its role(s)
outside of hematopoiesis (1).
Several groups have demonstrated the presence of the Epo receptor
(EpoR) expressed by ovarian cancer cells (2–5) with
differing results regarding its localization and functionality. Our
previous studies revealed a weak surface EpoR signal in A2780 cells
with most of EpoR found in the cytoplasm, more abundantly as an
intracellular membrane-associated protein than a soluble one.
Silencing EpoR expression resulted in reduced A2780 proliferation
as well as a reduction in Epo-induced phosphorylation of Erk1/2
(6). The presence and the
functionality of EpoR have been confirmed in several other cancer
cells. Unlike hematopoietic cells, where Epo/EpoR signaling is
associated with increased cell proliferation and/or survival, in
tumor cells the Epo/EpoR axis does not always lead to increased
proliferation but may increase the resistance of cancer cells to
different therapies. Szenajch et al(7) provide a critical review of this.
In 1990, Anagnostou et al(8) found that recombinant human Epo (rhEpo)
enhances the proliferation and migration of human umbilical vein
endothelial cells (HUVECs) and bovine adrenal capillary endothelial
cells (9,10) and demonstrated the presence of EpoR
mRNA in HUVECs as well as strong positive EpoR protein staining of
the vascular endothelium in vivo(11). The presence of EpoR was also shown
by Yamaji et al(12), who
suggested that brain capillary endothelial cells express not only
an authentic form of EpoR but also a soluble one and that Epo acts
directly on brain capillary endothelial cells as a competence
factor. In vivo angiogenic potential of Epo was originally
demonstrated by Yasuda et al(13) who found that injection of Epo into
the ovariectomized mouse uterine cavity promoted blood vessel
formation of the endometrium. Similarly, Ribatti et
al(14) demonstrated that Epo
induced a potent in vivo angiogenic response of the chick
embryo chorioallantoic membrane. Furthermore, the role of Epo in
the physiological angiogenesis was described during wound healing
and in the developing of mouse embryo (15,16).
The study of Yasuda et al(17) revealed that normal human cervix,
endometrium as well as ovary malignant tumors of female
reproductive organs produce Epo and EpoR, and that the tumor cells
themselves and capillary endothelial cells are sites responsive to
the Epo signal. Based on the mitogenic action of Epo as well as the
finding that injection of soluble EpoR or Epo-monoclonal antibody
into the tumor was followed by apoptosis of tumor cells, Yasuda
et al(18) proposed the
presence of a paracrine or autocrine Epo/EpoR loop and its
contribution to tumorigenesis in female reproductive organs.
Subsequently, Epo-induced angiogenesis was demonstrated on
chemically induced murine hepatic tumors (19), glioma tumor cells in chick embryo
chorioallantoic membrane (20) and
melanoma cells in Matrigel plug assay (21). Epo also accelerated the growth of
EpoR negative Lewis lung carcinoma cells by promoting tumor
angiogenesis in vivo(22).
Notably, Epo/EpoR levels correlated well with angiogenesis and
progression of patients with hepatocellular carcinoma,
neuroblastoma, squamous cell carcinoma of the tongue, melanoma and
gastric adenocarcinoma (23–27).
Although studies have not confirmed a direct
stimulatory effect of rhEpo on tumor cells, there is ample evidence
of this effect on endothelial cell proliferation and/or
angiogenesis of tumors. We have now carried out a series of
experiments to explore mechanisms of such an endothelial cell
stimulation induced by conditioned media of diverse ovarian
adenocarcinoma cells A2780 and SKOV-3. Both cell lines were
incubated either in normoxic or hypoxic conditions and their
conditioned media were investigated in order to test the
proliferation of HUVECs. The potential pro-angiogenic effect of
rhEpo, which remains in the conditioned media of rhEpo-treated
A2780 and SKOV-3 cells, was also studied.
Materials and methods
Cell culture conditions
Human ovarian adenocarcinoma cell lines A2780 and
SKOV-3 were obtained from the American Tissue Culture Collection.
Both cell lines were grown in RPMI-1640 with L-glutamine (Life
Technologies, Carlsbad, CA, USA). Media was supplemented with 10%
FCS (Life Technologies) and antibiotic/antimycotic solution (100
U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml
amphotericin B; Life Technologies). The cells were maintained under
standard tissue culture conditions of 37°C, 95% air/5%
CO2.
HUVECs were isolated, cultured, and characterized as
previously described (28,29). Cells were cultured on gelatin-coated
dishes in M199 media supplemented with 10% heat-inactivated human
serum (PAA, Piscataway, NJ, USA), 10% heat-inactivated new born
calf serum (Cambrex, East Rutherford, NJ, USA), 150 μg/ml crude
endothelial cell growth factor (ECGF), 5 U/ml heparin, 100 IU/ml
penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich, St. Louis,
MO, USA) at 37°C under a 5% CO2/95% air atmosphere.
Twenty-four hours prior to the experiments, the endothelial cell
cultures were refreshed with a media without crude ECGF and
heparin.
Cell culture treatments
A2780 and SKOV-3 cells were seeded at a density of
1×106 cells/Petri dish in RPMI-1640 media with 10% FCS
for 24 h. Subsequently, cells were washed with PBS and serum-free
media with or without 50 U/ml of rhEpo (Eprex®;
Janssen-Cilag, Beerse, Belgium) was added. Half of the experimental
Petri dishes were then moved to a hypoxic chamber with 1%
O2 and the other half were kept under standard tissue
culture conditions, both for 24 h. Conditioned media were then
immediately added to HUVECs for 48-h cell proliferation assay.
HUVEC proliferation assay
Endothelial cells were seeded at a density of
8×103 cells/cm2 in 24-well plates.
Twenty-four hours after seeding, the conditioned media obtained
from A2780 and SKOV-3 cells were added to endothelial cells in
triplicate wells. Vascular endothelial growth factor (VEGF)-treated
cells (R&D Systems, Minneapolis, MN, USA) (25 ng/ml) were used
as a positive control. After 48 h of culturing, the proliferation
activity was determined using Coulter Counter (Model ZF; Coulter
Electronics Ltd., Luton, Bedfordshire, UK) and the total viability
was analyzed by staining cells with 0.15% eosine via light
microscopy. Cell pellets, after being washed extensively with PBS,
were dispersed in anti-fade mounting fluid, placed on glass slides
SuperFrost Plus (Menzel Gläser, Braunschweig, Germany) and used for
light microscopy using a Leica DMI6000 microscope at ×400
magnification.
RNA isolation, reverse transcription and
quantitative RT-PCR
Total RNA was isolated using TRIzol (Gibco,
Invitrogen, Grand Island, NY, USA) and purified using RNeasy Mini
kit (Qiagen, Hamburg, Germany). The RNA concentration was
quantified at 260 nm and 1 μg of RNA was transcribed using
SuperScript II (Invitrogen, Carlsbad, CA, USA) reverse
transcriptase and oligo(dT) primers (Invitrogen). Quantitative
RT-PCRs were performed in duplicates by iCycler iQ™ Real-Time PCR
Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) in
30 μl reaction volume containing 1X iQ™ SYBR Green Supermix (0.2 mM
dNTP, 3 mM MgCl2), 0.5 μM forward and reverse primer and
2 μl of cDNA. The reaction conditions were: 95°C 3 min, 40 cycles
(94°C 30 sec, 55°C 50 sec, 72°C 50 sec), 72°C 7 min followed by
melting curve analysis to confirm amplification of the desired
single and specific product. The relative expression levels of
hypoxia-inducible factor-1α (HIF-1α), Epo, VEGF and β-actin genes
(primers designed by SABiosciences) were evaluated using the
standard curve method. Standard curves for HIF-1α, Epo, VEGF and
β-actin were obtained by amplification of serially-diluted mixtures
of cDNA samples (four-fold dilutions), with four to five dilution
points, each in duplicate. The calculated resulting relative
expression of HIF-1α, Epo, VEGF genes were normalized to relative
β-actin expression. The results are presented as mean ± standard
deviation of three independent experiments.
Western blot analysis
Western blot analyses were performed according to
the standard protocol. HUVECs were incubated with conditioned media
of A2780 cells for 30 min. Then, the cells were washed twice with
ice-cold PBS and scraped into RIPA buffer (PBS, 1% Nonidet P-40,
0.5% sodium deoxycholate, 0.1% SDS; all Sigma) containing freshly
added protease and phosphatase inhibitor cocktail (Roche
Diagnostics GmbH, Penzberg, Germany). Scraped lysates were
transferred into a microcentrifuge tube and passed through a
21-gauge needle to shear the DNA. Following incubation of the
lysates on ice for 45 min, the samples were centrifuged at 10,000 ×
g for 10 min at 4°C and the supernatant was transferred into a new
microcentrifuge tube. The protein samples were separated on 10%
SDS-PAGE gels, electroblotted onto Immobilon-P transfer membrane
(Millipore Co., Billerica, MA, USA) and incubated using the
following primary antibodies: anti-STAT5 (AF2168, 1:200; R&D
Systems), anti-phospho-STAT5 (Y694/Y699) (AF4190, 1:100; R&D
Systems), anti p44/42 MAP kinase (#9102, 1:1,000; Cell Signaling
Technology, Danvers, MA, USA), anti-phospho-p44/42 MAP kinase
(#9272, 1:1,000; Cell Signaling Technology) and anti-β-actin (clone
AC-74, 1:10,000; Sigma). The membranes were then incubated with
secondary horseradish peroxidase-conjugated antibodies [goat
anti-rabbit immunoglobulin G (IgG) F(AB9) 2, 1:10,000, PI-31461,
goat anti-mouse IgG F(AB9) 2, 1:10,000, PI-31436 or rabbit
anti-goat IgG F(AB9) 2, 1:10,000, PI-31403; Pierce, Rockford, IL,
USA] for 1 h, and the antibody reactivity was visualized with ECL
western blotting substrate (PI-32106; Pierce) using Kodak BioMax
film (#1788207; Sigma).
ELISA assay
ELISA assay with Epo and VEGF detection was
performed according to the manufacturer’s instructions (R&D
Systems). Briefly, 96-well plates with diluent, standards, positive
control and experimental samples were incubated for 2 h at room
temperature and then each well was washed three times with washing
solution. After the addition of VEGF or Epo conjugate, the plates
were incubated for another 2 h at room temperature. Subsequently,
substrates were added after repeated washing and plates were
incubated protected from light for a further 20 min. The enzymatic
reaction was stopped by addition of stop solution and absorbance
was measured at λ =450 nm using a FLUOStar Optima universal
microplate reader (BMG Labtech, Inc., Offenburg, Germany). The
concentrations of Epo and VEGF in conditioned media were calculated
based on standard curves.
Multi-analyte ELISA assay
The Mix-N-Match Multi-Analyte ELISArray kit (Qiagen)
was identical to the common ELISA assay in protocol and development
or incubation time. We used this assay to profile the levels of the
following 12 cytokines: IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12,
IL-13, GM-CSF, interferon-γ (IFN-γ), tumor necrosis factor-α
(TNF-α) and transforming growth factor-β1 (TGF-β1).
Statistical analysis
Data were processed using scientific graphing and
Origin analysis software (OriginLab Co., Northampton, MA, USA) and
statistically analyzed using one-way ANOVA followed by Tukey’s
multiple comparison tests.
Results
We observed the effect of conditioned media of
untreated as well as rhEpo-treated A2780 and SKOV-3 cells mainly
due to changes in gene expression of HIF-1α, Epo and VEGF as well
as in connection with the change of VEGF165 secretion in
both A2780 and SKOV-3 cell lines. Markedly, we found that the
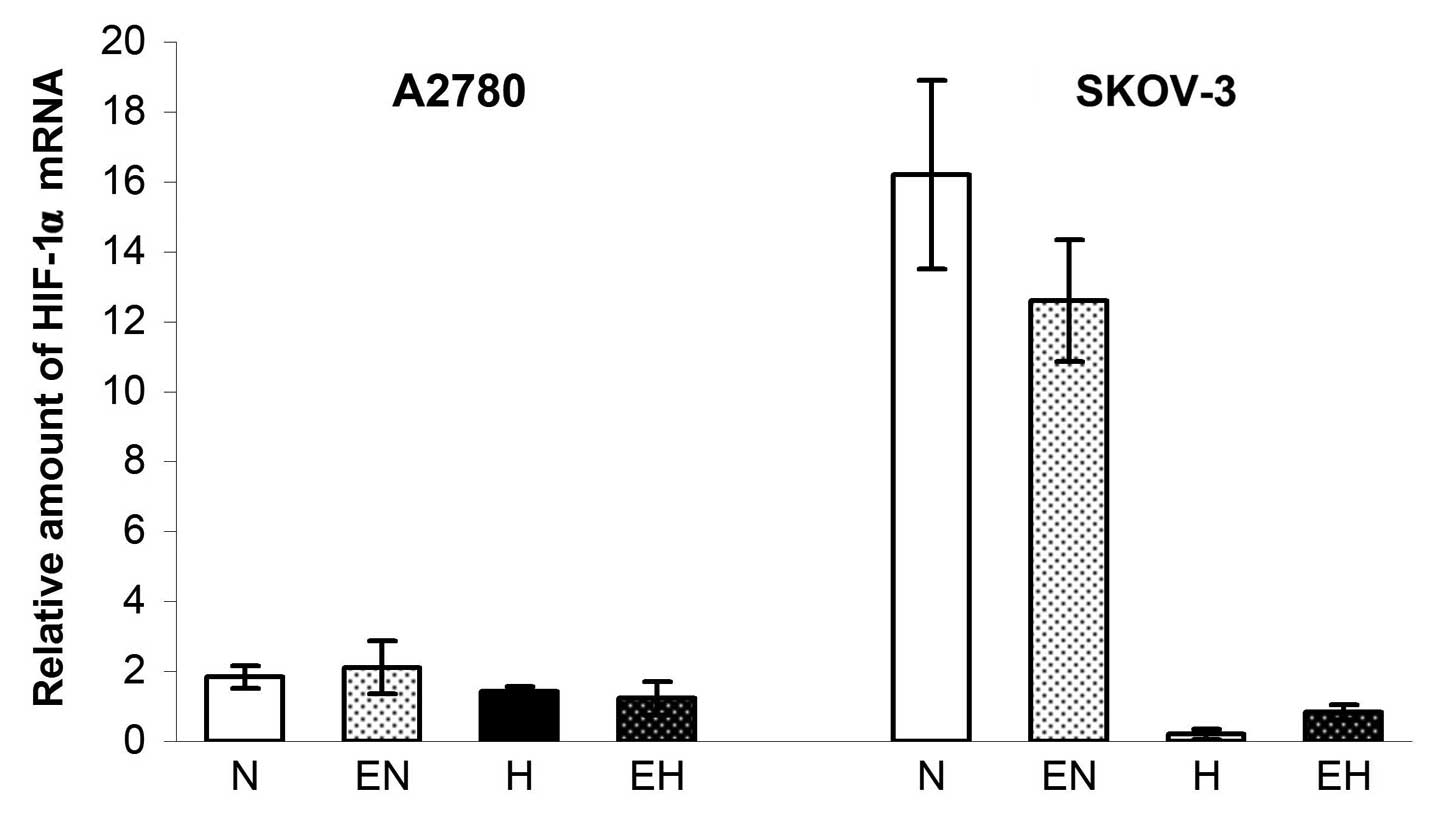
expression of HIF-1α did not increase in A2780 cells after 24 h of
incubation in hypoxia and, in the case of SKOV-3 cells, the
expression of HIF-1α even significantly decreased (probably by the
mechanism of feedback regulation). Both A2780 and SKOV-3 cells
showed >95% viability after hypoxia incubation (data not shown).
The administration of rhEpo, however, had no effect on the
expression of HIF-1α in both cell lines in either normoxic or
hypoxic conditions (Fig. 1).
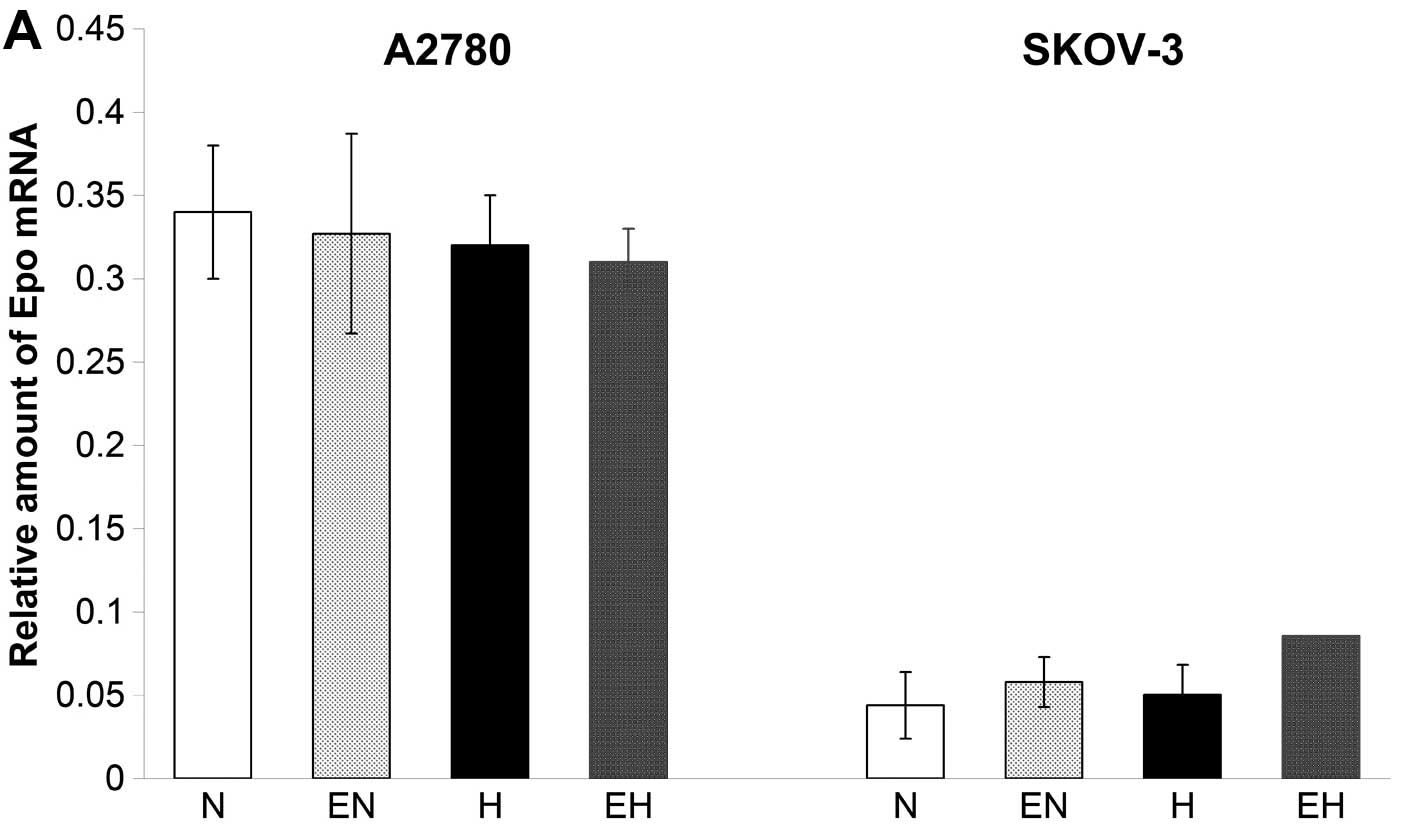
We analyzed the expression of the Epo gene and
measured the amount of secreted endogenous Epo in conditioned media
of A2780 and SKOV-3 cells under normoxic as well as hypoxic
conditions. The expression of Epo was monitored by real-time PCR
and clearly showed the presence of low concentrations of mRNA for
Epo in both cell lines. The Epo expression was higher in A2780
compared to SKOV-3 cells (Fig. 2A).
Furthermore, hypoxic conditions did not result in any stimulatory
effect on the expression of Epo in A2780 and SKOV-3 cells. In
addition, ELISA test results clearly showed low production of a
secreted form of Epo protein under normoxia in both A2780 as well
as SKOV-3 cells (Fig. 2B). Although
the Epo gene expression remained unchanged in both cell lines under
conditions of hypoxia (Fig. 2A),
the concentration of secreted Epo protein increased from 5.3 to
38.9 mU/ml in conditioned media of hypoxic A2780 cells compared to
normoxic ones. On the other hand, the concentration of secreted Epo
in the conditioned media of SKOV-3 cells did not change under
hypoxic conditions compared with normoxic ones (Fig. 2B). With regard to the application of
rhEpo, we did not find any significant effect of 50 U/ml of rhEpo
on the expression of Epo gene in A2780 and SKOV-3 cells (Fig. 2A).
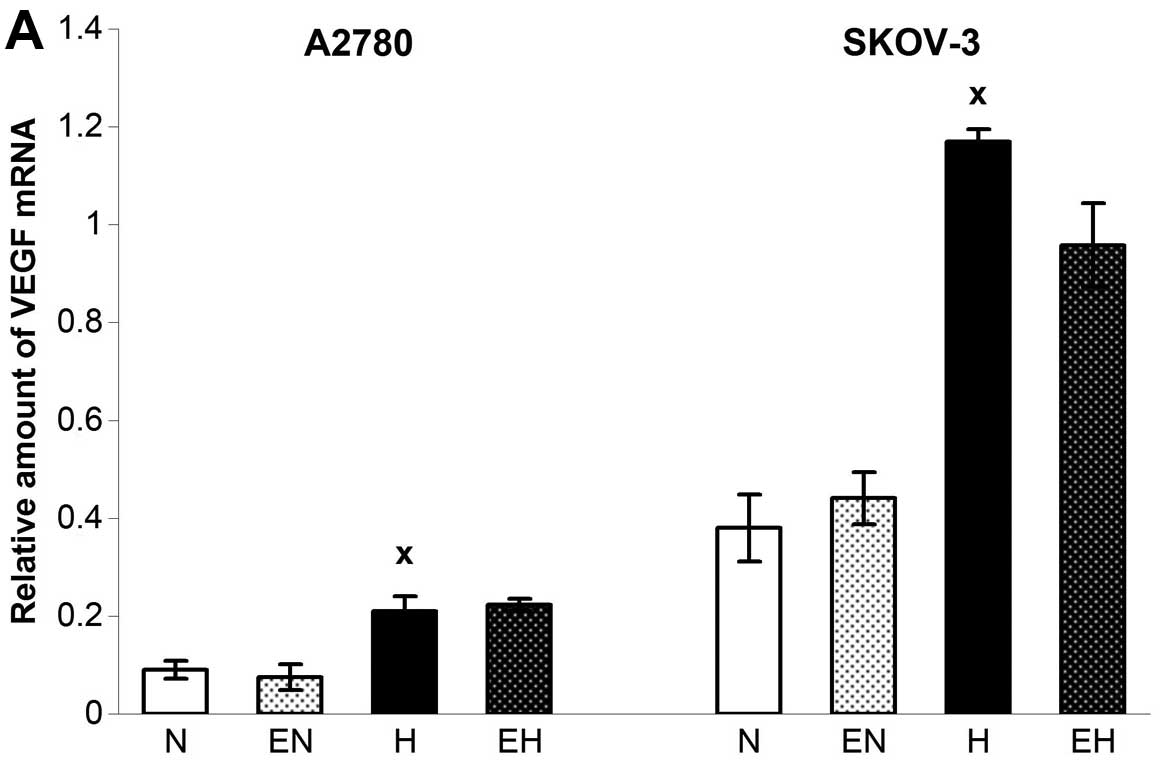
In contrast to Epo and HIF-1α, the expression of
VEGF was upregulated through the hypoxic conditions in both A2780
as well as SKOV-3 cells (Fig. 3A)
without additional upregulation using rhEpo. The only change in
VEGF expression after administration of rhEpo occurred when A2780
cells were incubated with a higher dose of rhEpo under normoxic
conditions. Indeed, 150 U/ml enhanced the expression of VEGF in
A2780 under normoxia, which was accompanied paradoxically by a
decrease in VEGF165 secretion. Based on the application
of anti-Epo antibody or soluble EpoR and observing even stronger
decline of VEGF165 secretion, we hypothesized that it
was a non-specific effect of such a high concentration of rhEpo
(data not shown). However, enhanced mRNA level of VEGF under the
hypoxia correlated well with higher secretion of VEGF165
in the SKOV-3 cell line only (Fig.
3B). On the other hand, rhEpo upregulated VEGF secretion in
SKOV-3 cells under normoxic conditions without altering the gene
expression for VEGF (Fig. 3).
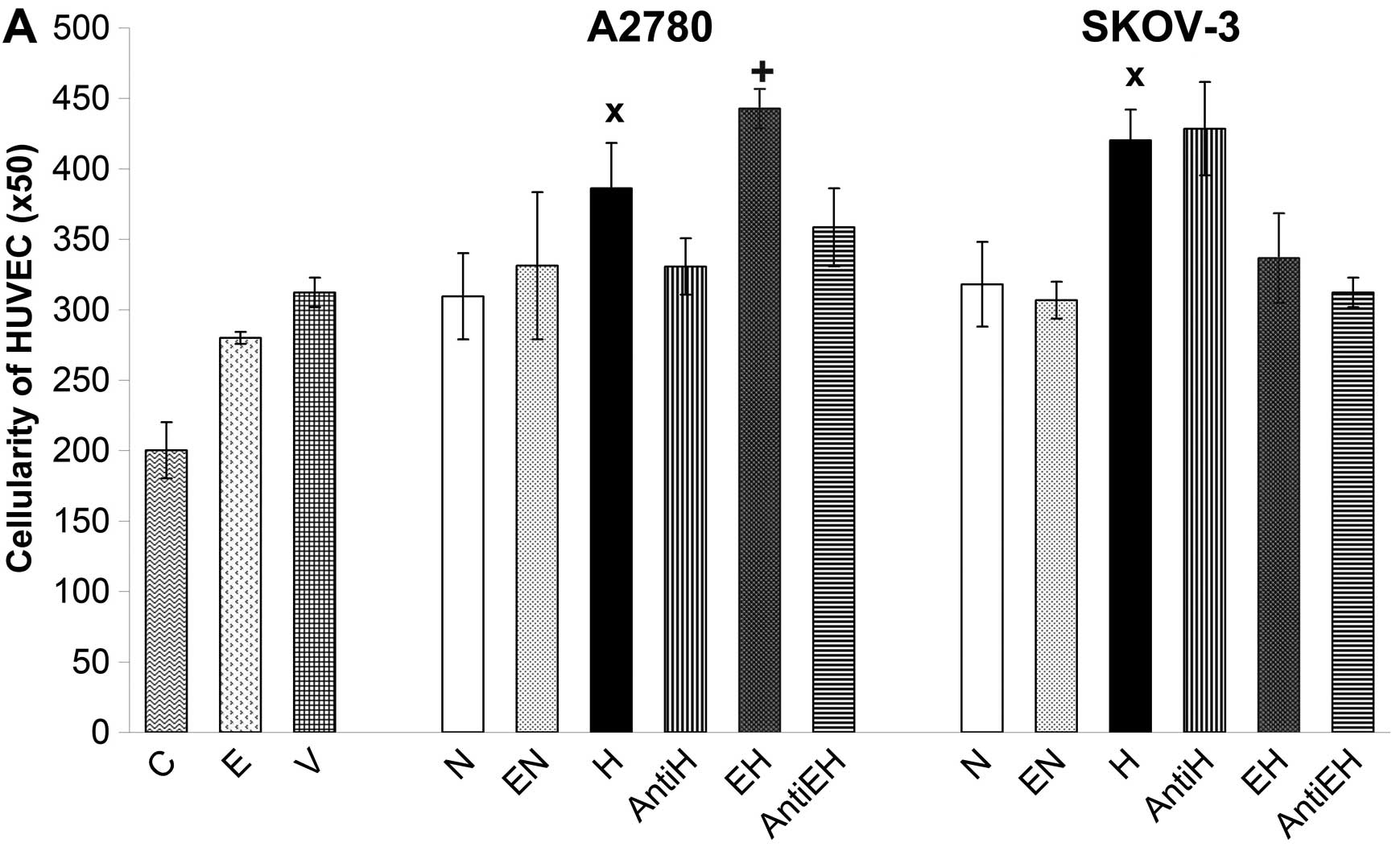
The pro-angiogenic potential of conditioned media
from A2780 and SKOV-3 cells was monitored by HUVEC proliferation.
All conditioned media, including those originating from A2780 and
SKOV-3 cells incubated with 50 U/ml of rhEpo as well as control
with rhVEGF and rhEpo were added to HUVECs for 48 h. The result of
stimulation and/or proliferation was evaluated by simple counting
of HUVECs using the Coulter Counter. In this regard, conditioned
media of A2780 and SKOV-3 cells kept under normoxic conditions had
no significant effect on the proliferation of HUVECs compared with
rhVEGF control (Fig. 4A). The
opposite was observed when HUVECs were incubated with conditioned
media of hypoxic A2780 and SKOV-3 cells. These media significantly
increased the proliferation of HUVECs compared with rhVEGF control
and compared with conditioned media of A2780 and SKOV-3 cells
incubated under normoxic conditions (Fig. 4A). A marked finding was that
conditioned media collected from A2780 cells incubated under
hypoxic conditions with 50 U/ml of rhEpo had a more pronounced
stimulatory effect on HUVECs than the media of hypoxic A2780 cells
without rhEpo. Paradoxically, conditioned media of SKOV-3 cells
incubated under hypoxic conditions with the same concentration of
rhEpo inhibited the proliferation of HUVECs compared to media of
hypoxic SKOV-3 cells without rhEpo (Fig. 4). In order to establish the actual
proportion of Epo to stimulate HUVECs, we used neutralizing
anti-Epo monoclonal antibody. The stimulation of HUVECs by
conditioned media of hypoxic A2780 cells as well as by hypoxic
media of rhEpo-treated A2780 cells was blocked using anti-Epo
antibody. Contrary to A2780, we did not observe the same blocking
effect of anti-Epo antibody in the case of conditioned media of
SKOV-3 cells incubated under hypoxic conditions with or without
rhEpo (Fig. 4). In this regard,
inhibition of HUVECs by hypoxic media of SKOV-3 cells with rhEpo
was probably not related to Epo but was due to alternative factors.
On the other hand, it appears that endogenous Epo secreted by A2780
cells under hypoxic conditions but also rhEpo added into media of
hypoxic A2780 cells could play an important role in the stimulation
of endothelial cells represented in our experiment by HUVECs. The
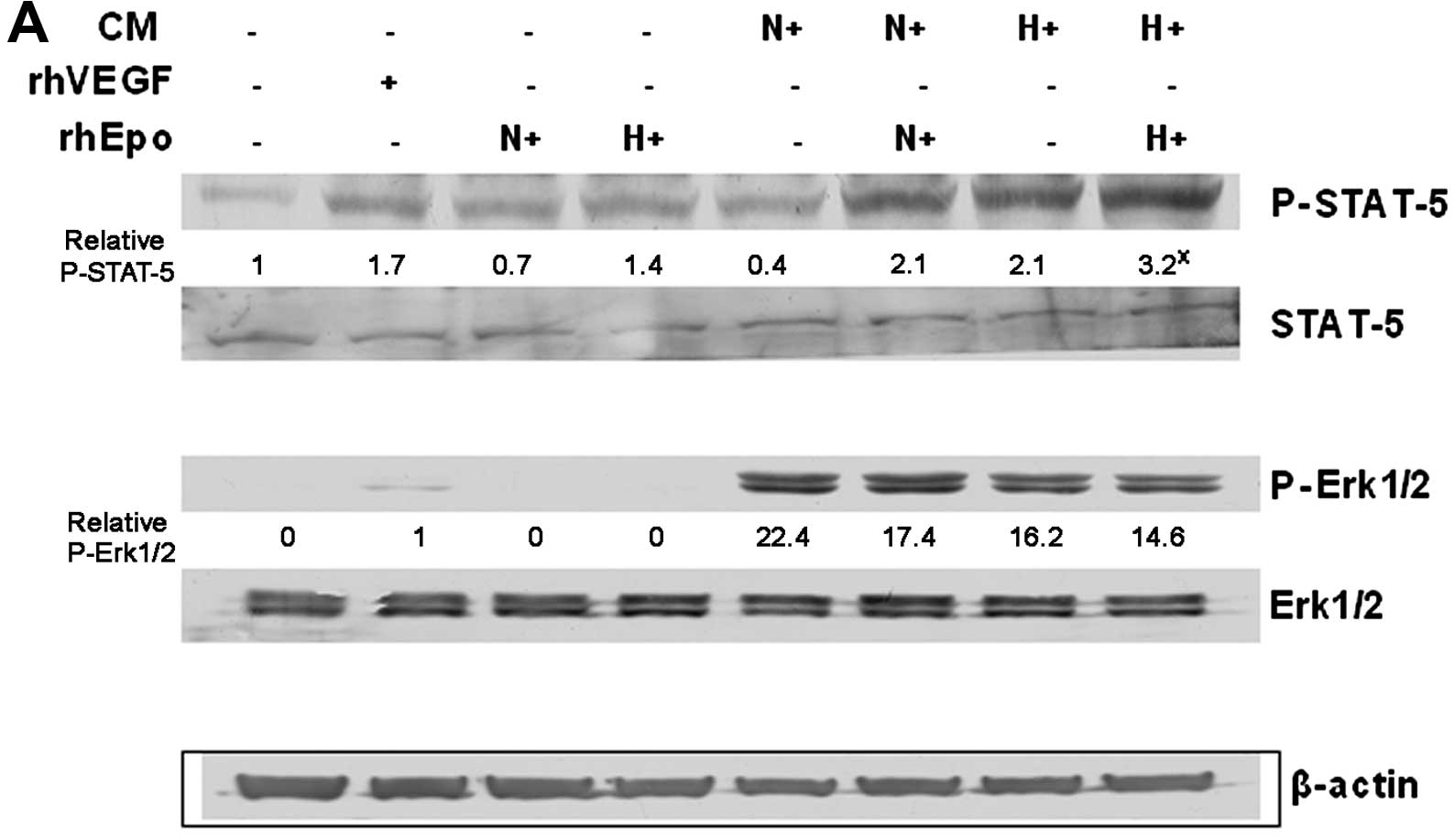
effect of such a ‘hypoxic’ Epo stimulation was also confirmed by
increased phosphorylation of STAT-5 protein in HUVECs and by
blocking of such signal using anti-Epo antibody (Fig. 5). We also monitored the
phosphorylation of Erk1/2 proteins, but there was no marked
difference induced by hypoxia or due to rhEpo application; we only
observed the difference in Erk1/2 phosphorylation between the
conditioned media in general compared with rhVEGF or rhEpo controls
(Fig. 5A).
To better understand A2780 conditioned media and its
composition in terms of pro- and anti-angiogenic factors, we tested
the alterations in the levels of selected cytokines using multi
ELISA assay. Based on the results of HUVEC stimulation by
conditioned media, we focused more on the content of selected
cytokines in conditioned media of A2780 cells treated with rhEpo
under hypoxic conditions. It is of note that in this hypoxic media
of A2780 cells with rhEpo we found elevated concentrations of IL-4,
IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, GM-CSF and IFN-γ compared
with conditioned media of A2780 hypoxic cells without rhEpo
treatment as well as compared with conditioned media of normoxic
A2780 cells with or without rhEpo (Fig.
6). The role of rhEpo-induced secretion of the mentioned
cytokines in A2780 cells under hypoxic conditions as well as the
role of these cytokines in HUVEC stimulation will be studied in our
future studies.
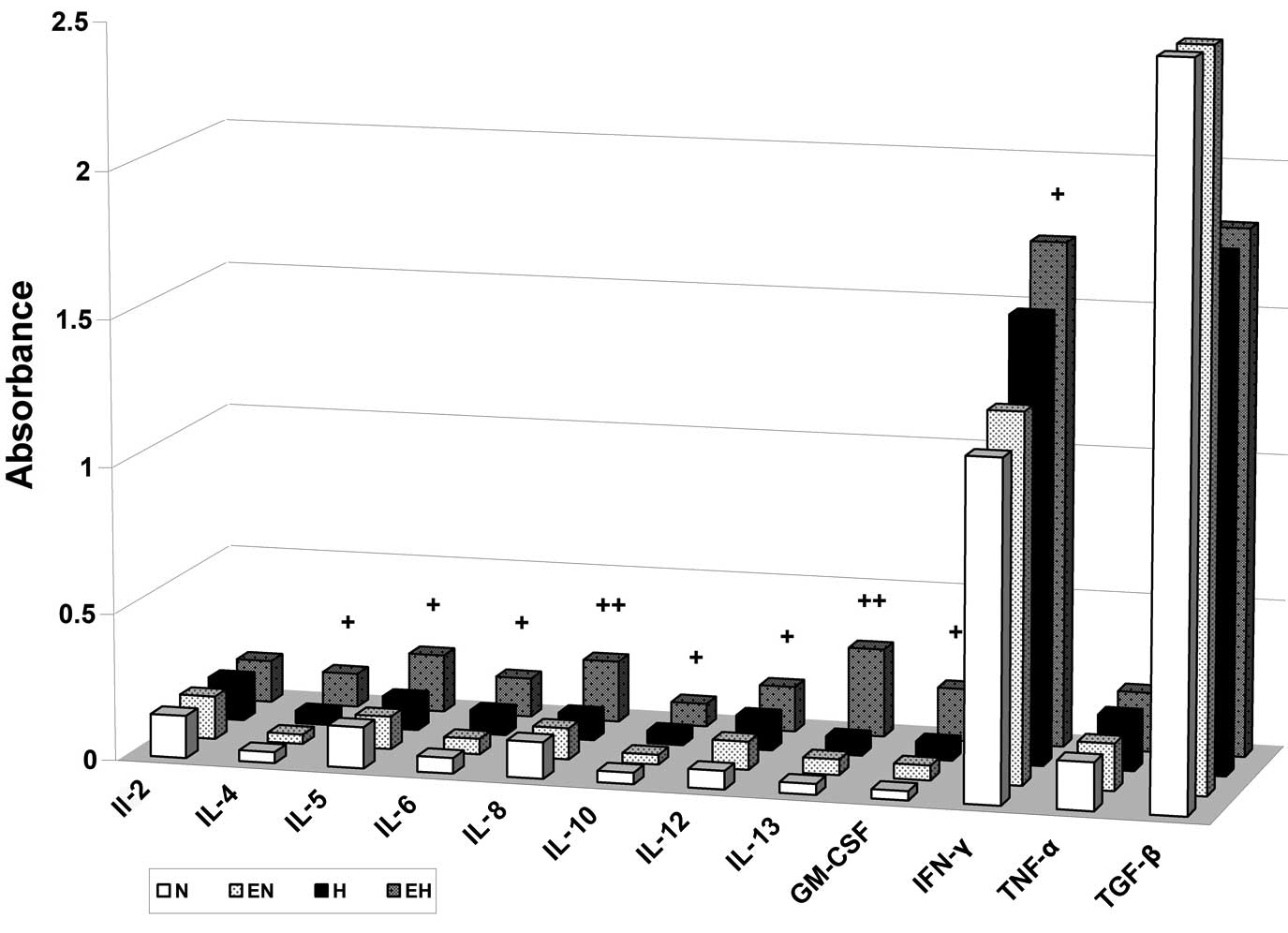 | Figure 6Erythropoietin (Epo) induces
secretion of interleukin (IL)-4, IL-5, IL-6, IL-8, IL-10, IL-12,
IL-13, GM-CSF and interferon (IFN)-γ cytokines in A2780 cells under
hypoxic conditions. A2780 cells were treated with or without 50
U/ml of recombinant Epo (E) under normoxic (N) or hypoxic (H)
conditions for 24 h. The levels of IL-2, IL-4, IL-5, IL-6, IL-8,
IL-10, IL-12, IL-13, GM-CSF, IFN-γ, tumor necrosis factor (TNF)-α
and transforming growth factor (TGF)-β1 in conditioned media are
presented as means of the absorbance of three independent
experiments. The statistical significance is designated as follows:
EH vs. H, +P<0.05 or ++P<0.01. |
Discussion
Epo stimulates cells through its interaction with
the EpoR on the cell surface. The EpoR has been identified not only
in the hematopoietic cells, but also in a number of
non-hematopoietic cells and tissues (30). Despite the fact that several of the
tumor cells were confirmed by the presence of the EpoR, the
discrepancy in the stimulatory effect of Epo on these cells
remains. On the one hand, there are published reports that indicate
the proliferative response of cancer cells following rhEpo
treatment (31–33), on the other hand, tumor cells, in
spite of evidence of EpoR functionality (4,6), did
not exhibit a growth Epo response (4,5,34).
Although Paragh et al(5)
suggested the Epo-independent, EpoR-mediated pathway in the growth
of some types of human cancer, additional experiments aimed at
typing EpoR are necessary to clarify this contradiction.
There is increasing experimental evidence on the
stimulatory effect of Epo on endothelial cell proliferation and/or
angiogenesis of tumors. Epo secreted by glioma tumor cells affected
glioma vascular endothelial cells via its receptor and promoted
angiogenesis in a paracrine manner (20); recently, Epo induced angiogenesis in
Matrigel plug assays, and neutralization of Epo secreted by
melanoma cells resulted in decreased angiogenesis (21).
Our results provide evidence of the effect of
conditioned media of different ovarian adenocarcinoma A2780 and
SKOV-3 cells on the proliferation of HUVECs. We hypothesized a
stimulatory effect of conditioned media of A2780 and SKOV-3 cells
that grew under hypoxic conditions on HUVECs. Our new finding is
the fact that pro-stimulatory effect of hypoxic A2780 media, but
not SKOV-3, was partly mediated by Epo protein. This was confirmed
by increased secretion of Epo by A2780 cells under hypoxia but
mainly via the blocking effect of anti-Epo on HUVEC proliferation.
Nevertheless, the key drivers of angiogenesis are VEGF molecules
(35), at least in A2780 cells Epo
appears to also be an important stimulus. An interesting fact
remains that despite increased A2780 cell secretion of Epo
molecules, neither HIF-1α or Epo gene overexpression in these cells
was observed. This result was probably due to increased
stabilization of Epo protein. On the other hand, SKOV-3 cells with
eight times higher mRNA levels for HIF-1α than A2780 cells, respond
to hypoxia by increased VEGF expression associated with enhanced
secretion of VEGF into the media. Increase of VEGF secretion was
observed in SKOV-3 cells also after incubation with rhEPO in
normoxic conditions without altering VEGF gene expression. Our
previous study showed that SKOV-3 compared to A2780 cells
constitutive expression of Akt and basal level of phosphorylated
Ser473 (activated by overexpression of HER2), which was even
enhanced after 24 h incubation of SKOV-3 cells with rhEpo in
normoxic conditions (36). Taking
into account that activation of the PI3K/Akt/mTOR pathway can
increase (37) and considering our
previous results (36) rhEpo now
increased secretion of VEGF in SKOV-3 cells under normoxia probably
by activation of the Akt pathway followed by increased
stabilization of VEGF mRNA or higher translation of VEGF
protein.
The mechanism of tumor growth in the context of Epo
is not completely clarified, and it remains unclear whether there
is a direct effect of Epo in tumor cells as opposed to exogenous
effect on angiogenesis (26). We
previously demonstrated that each of four human ovarian cancer cell
lines, A2780, CaOV, SKOV-3 and OVCAR-3, expresses the EpoR but none
of the cell lines exhibited a growth response in culture to
exogenous Epo in normoxic conditions (38). Presently, rhEpo (50 U/ml) did not
affect the proliferation of hypoxic A2780 and SKOV-3 cells measured
by incorporation of BrdU (data not shown). rhEpo did not even
affect the expression of selected genes associated with tumor
angiogenesis. If there were any changes in the expression of
HIF-1α, Epo and VEGF, they were induced by lower oxygen pressure
not by exogenous Epo. This finding is in contrast with results of
Hale et al(39) where Epo
significantly reduced hypoxia-induced VEGF expression in SKOV-3 and
MCF-7 cells. However, the authors did not use anti-Epo or soluble
EpoR to confirm specificity of Epo effect. Our result with
stimulatory effects of conditioned media of hypoxic A2780 cells
treated with rhEpo on HUVECs is also in contrast to the findings of
Hale et al(39). This effect
was, however, the specific effect of Epo due to its blocking via
anti-Epo antibody.
Epo signaling as a mitogen of endothelial cells was
conducted via tyrosine phosphorylation of proteins including
phosphorylation of transcription factor STAT-5, which is similar to
that occurring in erythroid cells (40). Moreover, experiments performed in
cultured vascular cells demonstrated that Epo strongly induced
phosphorylation of STAT-5 in HUVECs, but only very weakly in smooth
muscle cells (41). We observed
STAT-5 signalization in HUVECs after their 30-min incubation with
either conditioned media of hypoxic A2780 cells with or without
addition of rhEpo or conditioned media of normoxic A2780 cells with
rhEpo. Notably, we found the association between increased
phosphorylation of STAT-5 and HUVEC stimulation only in hypoxic
media of A2780 cells with or without rhEpo, not in normoxic media
with rhEpo. On the other hand, anti-Epo antibody significantly
reduced A2780 hypoxic media with or without rhEpo induced STAT-5
phosphorylation as well as proliferation of HUVECs. The stimulative
effect of secreted as well as rhEpo on HUVECs could be explained by
different levels of pro- and anti-angiogenic cytokines secreted by
A2780 cells under the hypoxic conditions compared with normoxic
ones.
Cancer cells secrete several angiogenic factors,
such as VEGF, bFGF, PDGF, IL-6 and IL-8 (42–45)
and cytokines, such as MCP-1, G-CSF, M-CSF, TNFα, IL-1α and IL-1β
(46–49). These cytokines could bind to their
receptors expressed by endothelial and hematopoietic/lymphoid cells
and induce production of additional types of cytokines (50). In this regard, IL-6 produced by
human ovarian epithelial cells (43) can stimulate inflammatory cytokine
production, tumor angiogenesis and the tumor macrophage infiltrate
in ovarian cancer (51). On the
other hand, IL-10 had suppressive effects on angiogenesis, tumor
growth, and peritoneal dissemination of VEGF-producing ovarian
cancer cells (52). IL-4 as well as
IL-13 inhibited the migration of cultured bovine or human
microvascular cells, showing unusual dose-response curves that were
sharply stimulatory at a concentration of 0.01 ng/ml but inhibitory
over a wide range of higher concentrations (53). Similarly, IL-12, a cytokine with
both immunostimulatory and anti-angiogenic effects, suppressed both
tumor-associated angiogenesis and growth of canine hemangiosarcoma
in vivo(54). The treatment
of A2780 cells with rhEpo under hypoxic conditions led to increased
secretion of IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, GM-CSF
and IFN-γ cytokines. The role of individual pro- and
anti-angiogenic cytokines in HUVEC stimulation will be examined in
future studies. It is critical to determine which cytokines are
secreted by HUVECs in response to conditioned media with or without
Epo. The final effect of Epo on HUVECs will probably depend on the
balance between pro- and anti-angiogenic cytokines in an
environment of HUVECs.
Our results revealed that conditioned media of
rhEpo-treated A2780 cells under hypoxic conditions induced
significant STAT-5 phosphorylation as well as proliferation of
HUVECs. Furthermore, rhEpo increased secretion of IL-4, IL-5, IL-6,
IL-8, IL-10, IL-12, IL-13, GM-CSF and IFN-γ by A2780 cells in
hypoxic conditions.
Acknowledgements
This study was supported by the Slovak Research and
Development Agency under contract no. VVCE-0001-07 and LPP-0062-09
and the Scientific Grant Agency of the Ministry of Education of the
Slovak Republic under contract nos. VEGA 1/0296/09, VEGA 1/0733/12
and the NEXO (Network of Excellence in Oncology) under contract no.
ITMS 26220120024.
References
|
1
|
Sytkowski AJ: Erythropoietin: Blood, Brain
and Beyond. Wiley-VCH; Weinheim: 2004, View Article : Google Scholar
|
|
2
|
Miller CP, Lowe KA, Valliant-Saunders K,
et al: Evaluating erythropoietin-associated tumor progression using
archival tissues from a phase III clinical trial. Stem Cells.
27:2353–2361. 2009. View
Article : Google Scholar
|
|
3
|
Swift S, Ellison AR, Kassner P, et al:
Absence of functional EpoR expression in human tumor cell lines.
Blood. 115:4254–4263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeong JY, Feldman L, Solar P, Szenajch J
and Sytkowski AJ: Characterization of erythropoietin receptor and
erythropoietin expression and function in human ovarian cancer
cells. Int J Cancer. 122:274–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paragh G, Kumar SM, Rakosy Z, Choi SC, Xu
X and Acs G: RNA interference-mediated inhibition of erythropoietin
receptor expression suppresses tumor growth and invasiveness in
A2780 human ovarian carcinoma cells. Am J Pathol. 174:1504–1514.
2009. View Article : Google Scholar
|
|
6
|
Solar P, Hrckova G, Varinska L, et al:
Location and the functionality of erythropoietin receptor(s) in
A2780 cells. Oncol Rep. 28:141–146. 2012.PubMed/NCBI
|
|
7
|
Szenajch J, Wcislo G, Jeong JY, Szczylik C
and Feldman L: The role of erythropoietin and its receptor in
growth, survival and therapeutic response of human tumor cells From
clinic to bench - a critical review. Biochim Biophys Acta.
1806:82–95. 2010.PubMed/NCBI
|
|
8
|
Anagnostou A, Lee ES, Kessimian N,
Levinson R and Steiner M: Erythropoietin has a mitogenic and
positive chemotactic effect on endothelial cells. Proc Natl Acad
Sci USA. 87:5978–5982. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carlini RG, Dusso AS, Obialo CI, Alvarez
UM and Rothstein M: Recombinant human erythropoietin (rHuEPO)
increases endothelin-1 release by endothelial cells. Kidney Int.
43:1010–1014. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carlini RG, Reyes AA and Rothstein M:
Recombinant human erythropoietin stimulates angiogenesis in vitro.
Kidney Int. 47:740–745. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anagnostou A, Liu Z, Steiner M, et al:
Erythropoietin receptor mRNA expression in human endothelial cells.
Proc Natl Acad Sci USA. 91:3974–3978. 1994.PubMed/NCBI
|
|
12
|
Yamaji R, Okada T, Moriya M, et al: Brain
capillary endothelial cells express two forms of erythropoietin
receptor mRNA. Eur J Biochem. 239:494–500. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yasuda Y, Masuda S, Chikuma M, Inoue K,
Nagao M and Sasaki R: Estrogen-dependent production of
erythropoietin in uterus and its implication in uterine
angiogenesis. J Biol Chem. 273:25381–25387. 1998.PubMed/NCBI
|
|
14
|
Ribatti D, Presta M, Vacca A, et al: Human
erythropoietin induces a pro-angiogenic phenotype in cultured
endothelial cells and stimulates neovascularization in vivo. Blood.
93:2627–2636. 1999.PubMed/NCBI
|
|
15
|
Haroon ZA, Amin K, Jiang X and Arcasoy MO:
A novel role for erythropoietin during fibrin-induced wound-healing
response. Am J Pathol. 163:993–1000. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kertesz N, Wu J, Chen TH, Sucov HM and Wu
H: The role of erythropoietin in regulating angiogenesis. Dev Biol.
276:101–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yasuda Y, Fujita Y, Musha T, et al:
Expression of erythropoietin in human female reproductive organs.
Ital J Anat Embryol. 106(Suppl 2): S215–S222. 2001.PubMed/NCBI
|
|
18
|
Yasuda Y, Fujita Y, Masuda S, et al:
Erythropoietin is involved in growth and angiogenesis in malignant
tumours of female reproductive organs. Carcinogenesis.
23:1797–1805. 2002. View Article : Google Scholar
|
|
19
|
Nakamatsu K, Nishimura Y, Suzuki M,
Kanamori S, Maenishi O and Yasuda Y:
Erythropoietin/erythropoietin-receptor system as an angiogenic
factor in chemically induced murine hepatic tumors. Int J Clin
Oncol. 9:184–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nico B, Annese T, Guidolin D, Finato N,
Crivellato E and Ribatti D: Epo is involved in angiogenesis in
human glioma. J Neurooncol. 102:51–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumar SM, Zhang G, Bastian BC, et al:
Erythropoietin receptor contributes to melanoma cell survival in
vivo. Oncogene. 31:1649–1660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okazaki T, Ebihara S, Asada M, Yamanda S,
Niu K and Arai H: Erythropoietin promotes the growth of tumors
lacking its receptor and decreases survival of tumor-bearing mice
by enhancing angiogenesis. Neoplasia. 10:932–939. 2008.PubMed/NCBI
|
|
23
|
Ribatti D, Marzullo A, Gentile A, et al:
Erythropoietin/erythropoietin-receptor system is involved in
angiogenesis in human hepatocellular carcinoma. Histopathology.
50:591–596. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ribatti D, Nico B, Perra MT, et al:
Erythropoietin is involved in angiogenesis in human primary
melanoma. Int J Exp Pathol. 91:495–499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li HG, Li JS, Chen WL, Wang L, Wu DH and
Lin ZY: Prognostic significance of erythropoietin and
erythropoietin receptor in tongue squamous cell carcinoma. Br J
Oral Maxillofac Surg. 47:470–475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ribatti D: Erythropoietin and tumor
angiogenesis. Stem Cells Dev. 19:1–4. 2010. View Article : Google Scholar
|
|
27
|
Wang L, Li HG, Xia ZS, Wen JM and Lv J:
Prognostic significance of erythropoietin and erythropoietin
receptor in gastric adenocarcinoma. World J Gastroenterol.
17:3933–3940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Van Hinsbergh VW, Sprengers ED and
Kooistra T: Effect of thrombin on the production of plasminogen
activators and PA inhibitor-1 by human foreskin microvascular
endothelial cells. Thromb Haemost. 57:148–153. 1987.PubMed/NCBI
|
|
29
|
Defilippi P, van Hinsbergh V, Bertolotto
A, Rossino P, Silengo L and Tarone G: Differential distribution and
modulation of expression of alpha 1/beta 1 integrin on human
endothelial cells. J Cell Biol. 114:855–863. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arcasoy MO, Jiang X and Haroon ZA:
Expression of erythropoietin receptor splice variants in human
cancer. Biochem Biophys Res Commun. 307:999–1007. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Westenfelder C and Baranowski RL:
Erythropoietin stimulates proliferation of human renal carcinoma
cells. Kidney Int. 58:647–657. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lai SY, Childs EE, Xi S, et al:
Erythropoietin-mediated activation of JAK-STAT signaling
contributes to cellular invasion in head and neck squamous cell
carcinoma. Oncogene. 24:4442–4449. 2005. View Article : Google Scholar
|
|
33
|
Feldman L, Wang Y, Rhim JS, Bhattacharya
N, Loda M and Sytkowski AJ: Erythropoietin stimulates growth and
STAT5 phosphorylation in human prostate epithelial and prostate
cancer cells. Prostate. 66:135–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Selzer E, Wacheck V, Kodym R, et al:
Erythropoietin receptor expression in human melanoma cells.
Melanoma Res. 10:421–426. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ferrara N: VEGF and the quest for tumour
angiogenesis factors. Nat Rev Cancer. 2:795–803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Solar P, Koval J, Mikes J, et al:
Erythropoietin inhibits apoptosis induced by photodynamic therapy
in ovarian cancer cells. Mol Cancer Ther. 7:2263–2271. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Solar P, Feldman L, Jeong JY, Busingye JR
and Sytkowski AJ: Erythropoietin treatment of human ovarian cancer
cells results in enhanced signaling and a paclitaxel-resistant
phenotype. Int J Cancer. 122:281–288. 2008. View Article : Google Scholar
|
|
39
|
Hale SA, Wong C and Lounsbury KM:
Erythropoietin disrupts hypoxia-inducible factor signaling in
ovarian cancer cells. Gynecol Oncol. 100:14–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Haller H, Christel C, Dannenberg L, Thiele
P, Lindschau C and Luft FC: Signal transduction of erythropoietin
in endothelial cells. Kidney Int. 50:481–488. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Janmaat ML, Heerkens JL, de Bruin AM,
Klous A, de Waard V and de Vries CJ: Erythropoietin accelerates
smooth muscle cell-rich vascular lesion formation in mice through
endothelial cell activation involving enhanced PDGF-BB release.
Blood. 115:1453–1460. 2010. View Article : Google Scholar
|
|
42
|
Sonoda T, Kobayashi H, Kaku T, Hirakawa T
and Nakano H: Expression of angiogenesis factors in monolayer
culture, multicellular spheroid and in vivo transplanted tumor by
human ovarian cancer cell lines. Cancer Lett. 196:229–237. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lidor YJ, Xu FJ, Martinez-Maza O, et al:
Constitutive production of macrophage colony-stimulating factor and
interleukin-6 by human ovarian surface epithelial cells. Exp Cell
Res. 207:332–339. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Di Blasio AM, Carniti C, Vigano P and
Vignali M: Basic fibroblast growth factor and ovarian cancer. J
Steroid Biochem Mol Biol. 53:375–379. 1995.PubMed/NCBI
|
|
45
|
Versnel MA, Haarbrink M, Langerak AW, et
al: Human ovarian tumors of epithelial origin express PDGF in vitro
and in vivo. Cancer Genet Cytogenet. 73:60–64. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Negus RP, Stamp GW, Relf MG, et al: The
detection and localization of monocyte chemoattractant protein-1
(MCP-1) in human ovarian cancer. J Clin Invest. 95:2391–2396. 1995.
View Article : Google Scholar
|
|
47
|
Savarese TM, Mitchell K, McQuain C, et al:
Coexpression of granulocyte colony stimulating factor and its
receptor in primary ovarian carcinomas. Cancer Lett. 162:105–115.
2001.PubMed/NCBI
|
|
48
|
Glezerman M, Mazot M, Maymon E, et al:
Tumor necrosis factor-alpha and interleukin-6 are differently
expressed by fresh human cancerous ovarian tissue and primary cell
lines. Eur Cytokine Netw. 9:171–179. 1998.PubMed/NCBI
|
|
49
|
Gorelik E, Landsittel DP, Marrangoni AM,
et al: Multiplexed immunobead-based cytokine profiling for early
detection of ovarian cancer. Cancer Epidemiol Biomarkers Prev.
14:981–987. 2005. View Article : Google Scholar
|
|
50
|
Muller L and Pawelec G: Cytokines and
antitumor immunity. Technol Cancer Res Treat. 2:183–194. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Coward J, Kulbe H, Chakravarty P, et al:
Interleukin-6 as a therapeutic target in human ovarian cancer. Clin
Cancer Res. 17:6083–6096. 2011. View Article : Google Scholar
|
|
52
|
Kohno T, Mizukami H, Suzuki M, et al:
Interleukin-10-mediated inhibition of angiogenesis and tumor growth
in mice bearing VEGF-producing ovarian cancer. Cancer Res.
63:5091–5094. 2003.PubMed/NCBI
|
|
53
|
Volpert OV, Fong T, Koch AE, et al:
Inhibition of angiogenesis by interleukin 4. J Exp Med.
188:1039–1046. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Akhtar N, Padilla ML, Dickerson EB, et al:
Interleukin-12 inhibits tumor growth in a novel angiogenesis canine
hemangiosarcoma xenograft model. Neoplasia. 6:106–116. 2004.
View Article : Google Scholar : PubMed/NCBI
|