Introduction
Ovarian cancer is the third most common gynecologic
malignancy and is the most lethal form of gynecological cancer.
Aberrations of metabolic pathways are the characteristic
physiological changes occurring in cancer cells. In the 1920s,
Warburg (1) discovered that cancer
cells reprogram energy production mechanism through high aerobic
glycolysis. Furthermore, it was found that cancer cells derive most
fatty acids from de novo synthesis (2). Cancer is a complex blend of genetic
and metabolic perturbations and de novo lipogenesis is
believed to be active in oncogenesis. Expression of most enzymes
required for the synthesis of fatty acids, cholesterol,
triacylglycerols and phosphoglycerides is regulated by the SREBPs
which have now been established as global lipid synthetic
regulators (3,4). The SREBPs have many targets such as
fatty acid synthase (FASN), ATP citrate lyase (ACLY), acetyl-CoA
carboxylase (ACAC) and stearoyl-CoA desaturase-1 (SCD1) (5–8). The
regulatory function of sterol regulatory element-binding protein 1
(SREBP1) indicates a role in sensing and regulating
cancer-associated lipogenesis. Altered expression of SREBP1 has
been reported in various human cancers, such as hepatocellular
carcinoma, colorectal carcinoma, breast and prostate cancers
(9–12). However, the functional studies on
the role of SREBP1 in human ovarian cancer are limited to date.
In this study, we investigated the relationship
between SREBP1 expression levels and ovarian cancer. We studied the
association between expression of SREBP1 in ovarian cancer and its
effect on ovarian cancer progression and metabolic changes in
vitro and in vivo.
Materials and methods
Ovarian cancer specimen and
immunohistochemical (IHC) staining
Human ovarian tumor specimens were obtained from the
primary ovarian sites of previously untreated patients. All of the
specimens were anonymous and tissues were obtained in compliance
with institutional review board regulations. Patient features and
tumor characteristics for this study are reported in Tables I and II. On follow-up, 26 patients survived, 59
patients were deceased, and 11 patients were lost, adding up to a
follow-up rate of 88.54%. Follow-up records until December 2012
(from 9 to 120 months) are presented, with a mean follow-up time of
60.03 months.
 | Table ICorrelation between SREBP1 expression
and different phenotypes of ovarian tissues. |
Table I
Correlation between SREBP1 expression
and different phenotypes of ovarian tissues.
| Characteristics | No. of patients | SREBP1 n+ (%) | SREBP1 n− (%) | P-value |
|---|
| Benign ovarian
tumor | 15 | 2 | 13 | <0.001 |
| Borderline ovarian
tumor | 16 | 5 | 11 | |
| Ovarian cancer | 96 | 65 | 31 | |
 | Table IICorrelation between
clinicopathological parameters and SREBP1 expression in ovarian
cancer. |
Table II
Correlation between
clinicopathological parameters and SREBP1 expression in ovarian
cancer.
|
Characteristics | No. of
patients | SREBP1 n+ (%) | SREBP1 n− (%) | P-value |
|---|
| Age (years) |
| ≤50 | 35 | 20 (20.83%) | 15 (15.63%) | 0.094 |
| >50 | 61 | 45 (46.88%) | 16 (16.67%) | |
| FIGO stage |
| I–II | 25 | 7 (7.29%) | 18 (18.75%) | <0.001 |
| III–IV | 71 | 58 (60.42%) | 13 (13.54%) | |
| Histologic
grade |
| G1–G2 | 36 | 16 (16.67%) | 14 (14.58%) | 0.042 |
| G3 | 60 | 49 (51.04%) | 17 (17.71%) | |
| Ascites/peritoneal
washings |
| Negative | 25 | 15 (15.63%) | 10 (10.42%) | 0.335 |
| Positive | 71 | 50 (52.08%) | 21 (21.88%) | |
| Lymph node
metastasis |
| Negative | 70 | 51 (53.13%) | 19 (19.79%) | 0.582 |
| Positive | 26 | 14 (14.58%) | 12 (12.50%) | |
| Pathologic
type |
| Ovarian serous
carcinoma | 70 | 48 (50.00%) | 22 (22.92%) | 0.944 |
| Mucinous ovarian
cancer | 15 | 10 (10.42%) | 5 (5.21%) | |
| Endometrioid
adenocarcinoma | 11 | 7 (7.29%) | 4 (4.17%) | |
| FASN
expression |
| Positive | 63 | 60 (62.50%) | 3 (3.12%) | <0.001 |
| Negative | 32 | 5 (5.21%) | 28 (29.17%) | |
The immunohistochemical staining was carried out by
cutting out 4-μm sections from the formaldehyde-fixed and
paraffin-embedded tissue specimens. The tissue sections were baked,
deparaffinized with xylene, and rehydrated through a graded alcohol
series. Endogenous peroxidase activity was blocked with a 3%
hydrogen peroxide solution. After antigen retrieval and blocking
the nonspecific binding of the primary antibodies, the slides were
then incubated with the anti-SREBP1 rabbit polyclonal antibody
(ab93638, Abcam, Cambridge, MA, USA) and the anti-FASN rabbit
polyclonal antibody (ab96866 Abcam) overnight. The slides were
incubated in biotinylated secondary antibody (horseradish
peroxidase-conjugated anti-mouse/rabbit IgG) and the avidin biotin
peroxidase complex. 3,3′-Diaminobenzidine (DAB) substrate chromogen
solution was applied subsequently for visualization. For each
specimen, immunoreactive score (H-score) was analyzed according to
the total percentage of positive cells and the intensity of the
cytoplasmic staining (1+, 2+, or 3+), where H = (%1+ ×1) + (%2+ ×2)
+ (%3+ ×3). A minimum of 100 cells were evaluated in calculating
the H-score.
Cancer cell lines, plasmids and cell
culture
Human ovarian cancer cell lines HO8910PM, A2780,
3AO, SKOV3 were obtained from Shanghai Institute for Biological
Sciences, Chinese Academy of Sciences. Three shRNAs targeting human
SREBF-1 and empty vector were from Open Biosystems (catalogue no.
RHS4533, Lafayette, CO, USA). A2780, SKOV3, 3AO, HO8910PM cell
lines were cultured in RPMI-1640 medium. The human embryonic kidney
293T cells (HEK293T) were maintained in DMEM medium. For lipid-free
culture condition, the basal medium was supplemented with 10%
lipid-depleted FBS purchased from Cocalico Biological (Reamstown,
PA, USA) (catalogue no. 55-0116).
Transfection and constructing stable cell
lines
Superfect Transfection Reagent (Qiagen, Valencia,
CA, USA) was used for transient transfection according to
manufacturer’s instructions. Lenti-vira were prepared for cell
transduction through Trans-Lentiviral shRNA packaging kit (Open
Biosystems). The lenti-viral vector expressing shRNA were then
introduced into HEK 293T cells by transient co-transfection with
helper virus with calcium phosphate precipitation. After the viral
production, the medium was filtered through a 0.45-μm filter. The
ovarian cancer cells were infected at ~70% confluence in DMEM
medium containing 8 μg/ml of polybrene. After a 24-h culture, the
medium was replenished with fresh medium. Then the stable cell
lines were selected using 2 μg/ml puromycin for two weeks. The
knockdown efficiency was determined by both western blotting and
qRT-PCR.
Gene expression analysis (RNA isolation,
quantitative real-time PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). The cDNAs were synthesized from
total RNA using the SuperScript™ II Reverse Transcriptase kit
(Invitrogen). PCR was carried out using SYBR® Green
Real-time PCR master mix (code no. QPK-201, Toyobo Co., Ltd.). The
thermal cycling conditions were maintained at an initial
denaturation at 95°C for 30 sec, followed by 40 cycles of PCR using
the following profile: 94°C, 5 sec; 60°C, 10 sec; and 72°C, 15 sec.
GAPDH was used as a control of normalization, the primers used for
qRT-PCR are listed in Table
III.
 | Table IIIThe primers used for real-time
PCR. |
Table III
The primers used for real-time
PCR.
| Gene symbol | Forward primer
sequence | Reverse primer
sequence | Amplicon size
(bp) |
|---|
| SREBP1a |
5′-CGGCGCTGCTGACCGACATC |
5′-CCCTGCCCCACTCCCAGCAT | 104 |
| SREBP1c |
5′-GCGCAGATCGCGGAGCCAT |
5′-CCCTGCCCCACTCCCAGCAT | 116 |
| FASN |
5′-CACAGGGACAACCTGGAGTT |
5′-ACTCCACAGGTGGGAACAAG | 97 |
| ACLY |
5′-GCCCATCCCCAACCAGCCAC |
5′-TTGCAGGCGCCACCTCATCG | 137 |
| ACACa |
5′-CGGAAGGGACAGTAGAAATCA |
5′-AGTCGCTCAGCCAAGTGGA | 94 |
| SCD1 |
5′-CGACGTGGCTTTTTCTTCTC |
5′-CCTTCTCTTTGACAGCTGGG | 70 |
| GAPDH |
5′-GAGTCAACGGATTTGGTCGT |
5′-TTGAGGTCAATGAAGGGGTC | 103 |
Western blot analysis
The cells were collected and lysed in lysis buffer,
the boiled lysates were resolved by SDS-PAGE and then blotted onto
PVDF membrane. Afer incubating with primary antibodies against
SREBP1, FASN, ACLY, SCD, ACAC (Abcam) and β-actin (#IE9A3
ZSGB-BIO). The membranes were incubated with appropriate
horseradish peroxidase-labeled secondary antibodies and visualized
by ECL Plus system (Amersham Life Sciences, Piscataway, NJ, USA).
Quantitative data were assigned using a computing densitometer with
Image-Pro Plus software.
Cell apoptosis and cell cycle assays
Cell apoptosis was performed by Annexin V-FITC/PI
apoptosis detection kit (BD Biosciences Clontech) following
manufacturer’s instructions. Cell cycle was determined by RNase A
and PI. The mixture was immediately subjected to cell apoptosis
analysis and cell cycle assays on FACS calibur flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA).
Cell proliferation, transwell migration
and invasion assay
For cell proliferation assay using the Cell Counting
Kit-8 (CCK-8), cells (2×103 cells per well) were seeded
on 96-well plates for 5-day incubation. Then cells were harvested
and numbered by enzyme-linked immunosorbent assay (ELISA) reader.
The 24-well transwell cell culture chambers were utilized to
examine in vitro cell migration and invasion of ovarian
cancer cells. Briefly, the undersides of the upper chambers were
precoated with extracellular matrix (ECM) gel (1:4 dilution, for
invasion assay) or not (for migration assay). Cells
(1×105) were seeded inside the precoated upper chambers.
After 24 h of incubation, the numbers of migrated or invading cells
were measured by the crystal violet staining method.
Mouse xenograft experiments
In order to analyze the tumorigenic potential of
SREBP1, Female (4–6 weeks old) SCID mice were purchased from
Medical College of Peking University and all animal work was
performed in conformity with the National Institutes of Health
Guide for Care and Use of Laboratory Animals (publication no.
85–23, revised 1985). The mice were subcutaneously inoculated with
highly metastatic HO8910PM cells in the axillary fossa. Comparisons
were made among control-shRNA, shRNA-#1 and shRNA-#3 groups
(3×106 cells/mouse) with 9 mice in each group. The tumor
volume was calculated using the following equation: V = a ×
b2/2, where a and b represented the longest and shortest
diameter of the tumor, respectively. At the time of sacrifice,
tumors were excised, weighed and preserved in order to perform
further experiments.
Statistical analysis
For in vitro experiments, results are
presented as means ± standard deviation (SD). Least significant
difference t test (LSD-t) and t-test were performed for group
comparison. An exponential regression model was performed for
estimate of in vivo experiment. Fisher’s exact tests for
contingency tables were used in Tables
I and II. The relative risk of
death from ovarian cancer was evaluated through the Cox
proportional hazards regression model. Kaplan-Meier survival curves
were used and significance was assessed by the Log-rank test. The
overall survival was also compared by Log-rank test. For all of the
statistical tests, all P-values were 2-sided and P<0.05 was
defined as statistically significant. In all cases, SPSS 17.0
software (SPSS Inc., Chicago, IL) was used.
Results
Increased SREBP1 protein expression in
ovarian cancer
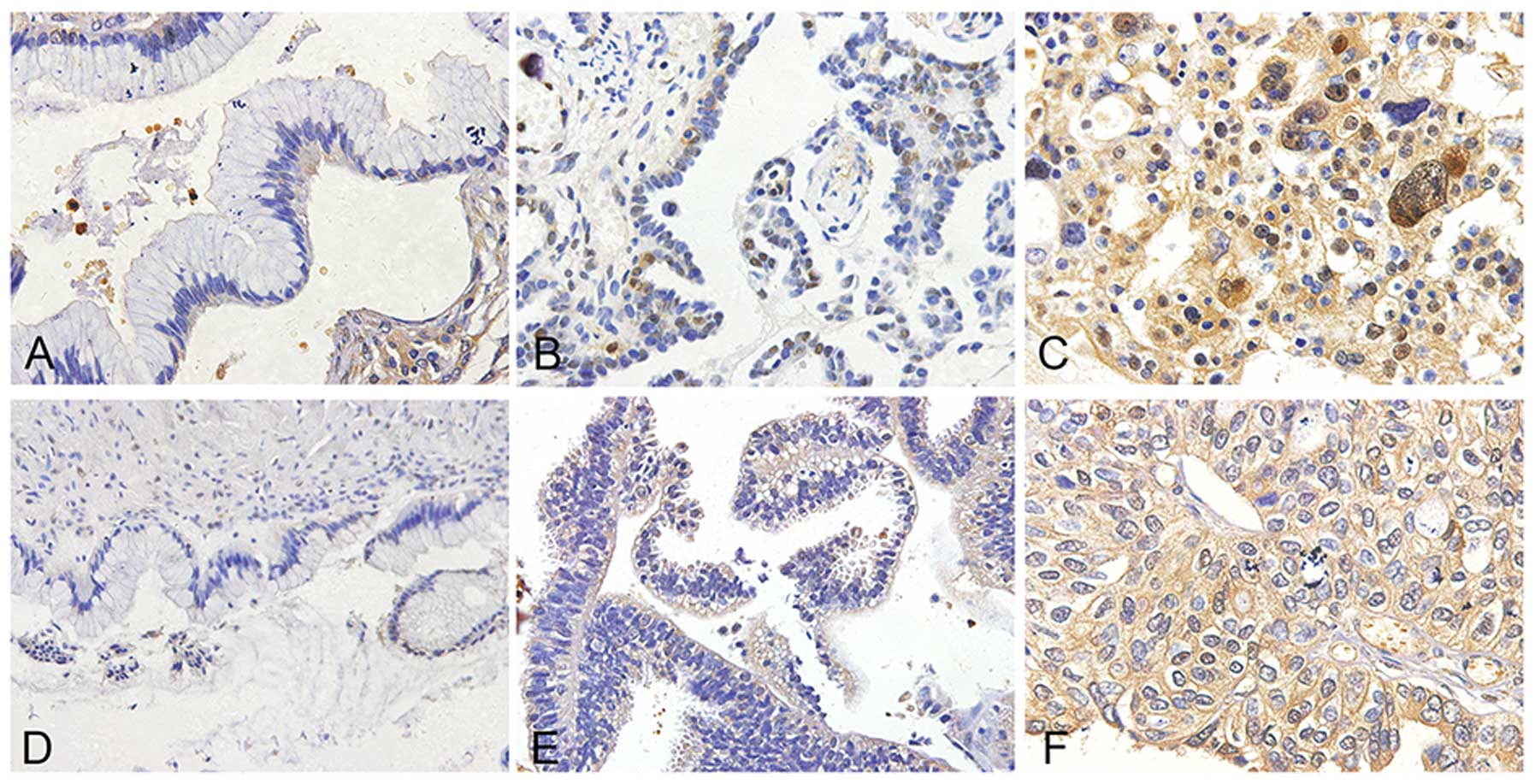
We performed immunohistochemical (IHC) staining on
different subtypes of ovarian cancer specimens and observed that
SREBP1 expression was detected in epithelial cells but not in
stromal cells. Cytoplasmic distribution of SREBP1 was enhanced in
ovarian cancer when compared to benign and borderline ovarian tumor
(P<0.001), (Figs. 1A–C and
3A; Table I). The detection implies SREBP1
maybe active in ovarian cancer genesis.
Correlation between SREBP1 expression and
clinicopathological parameters
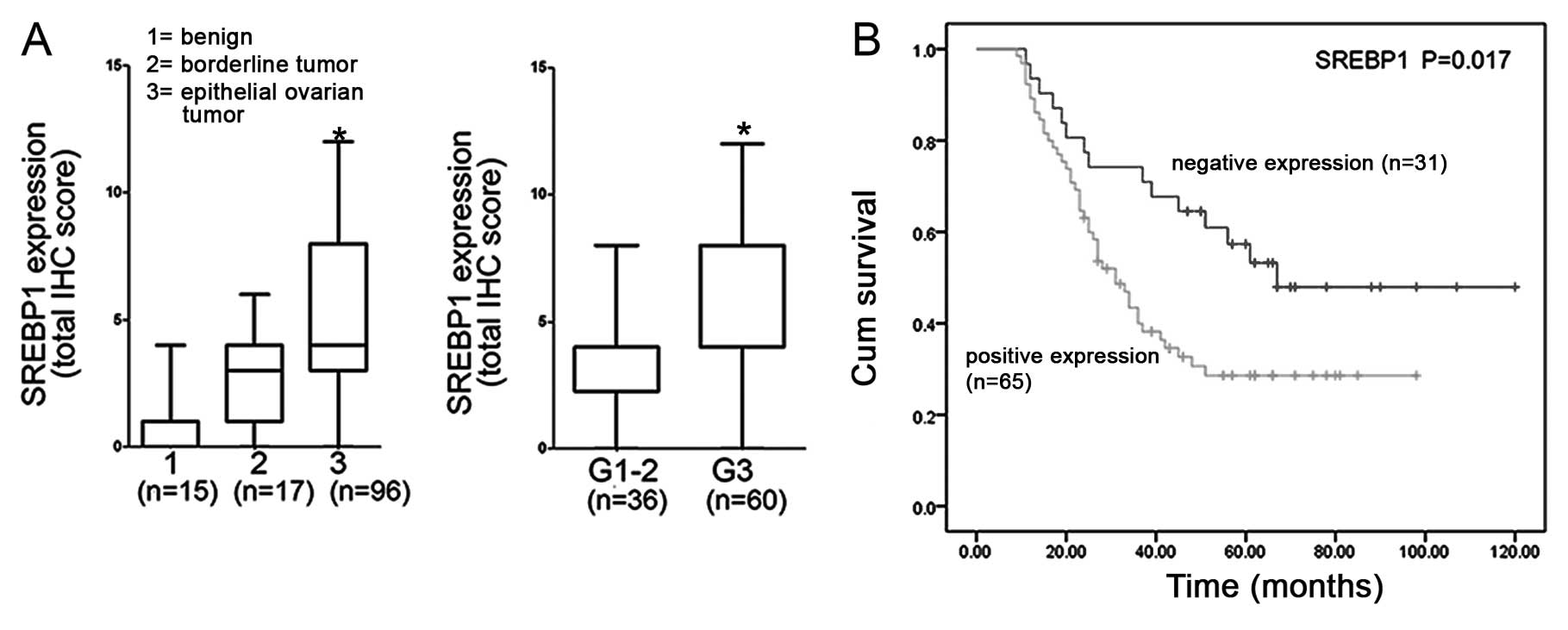
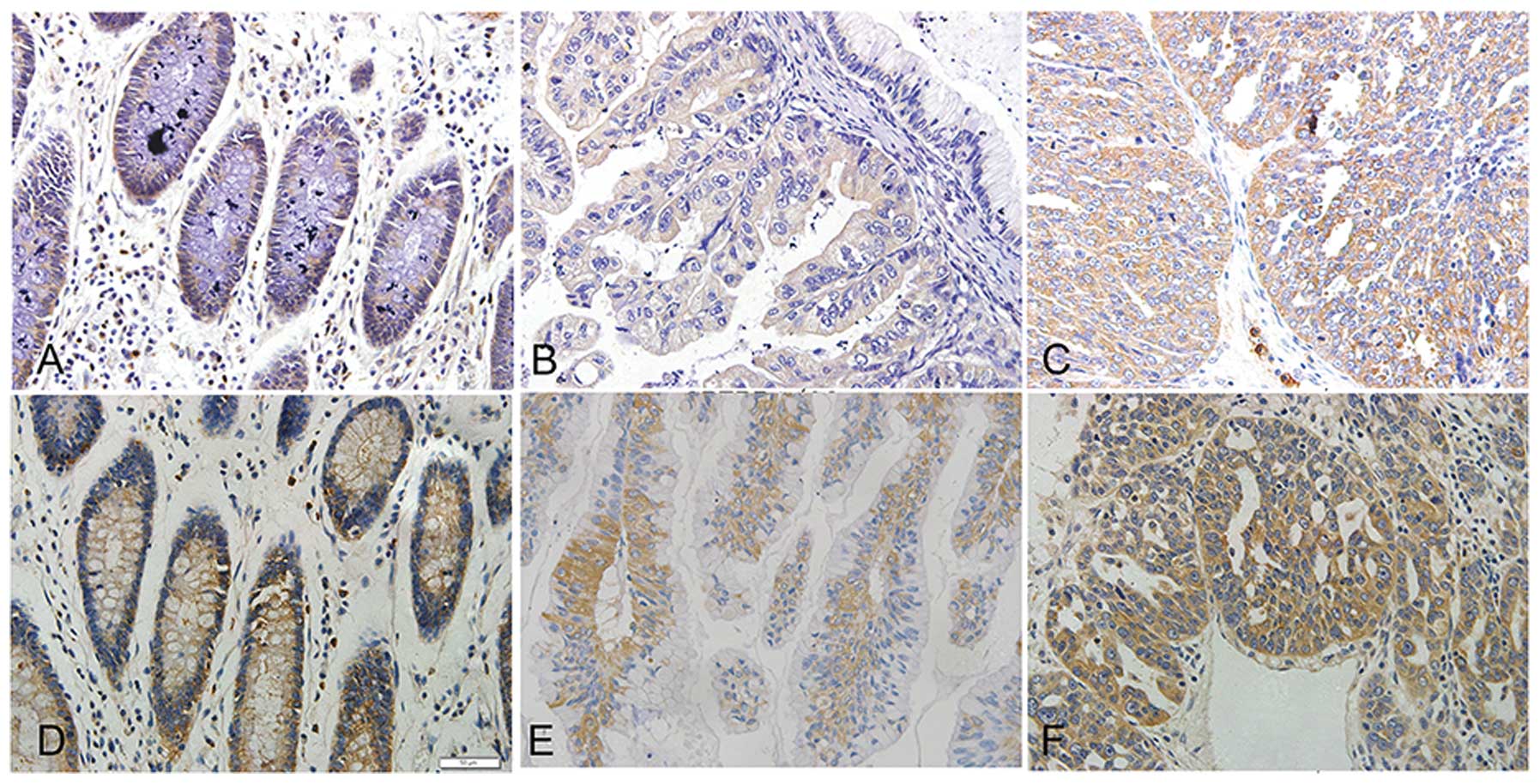
SREBP1 expression levels were significantly
correlated with FIGO surgical stage, histological grade and FASN
expression of ovarian cancer (Table
II, Figs. 1 and 2). Expression of SREBP1 protein increased
with higher FIGO surgical stage and histological grade of disease
[from 16.05% (stage I–II) to 73.95% (stage III–IV) (P<0.001);
from 37.50% (G1–G2) to 62.50% (G3) (P=0.042); Table I]. The more aggressive phenotypes of
ovarian cancer have more intensive staining of SREBP1 (Fig. 2A–C). However, SREBP1 expression did
not show any significant correlation with pathological type,
patient age, lymph node metastasis or ascitic cytology (Table II). Elevated expression of nuclear
SREBP1 in ovarian cancer was observed, however it did not show any
significant correlation with the clinicopathological parameters,
which may be due to the size of the patient cohort. These results
suggest that SREBP1 is overexpressed in ovarian cancer, which may
contribute to cancer progression.
Prognostic factor analysis for ovarian
cancer and predictive value of SREBP1 in patient survival
We examined the relationship between SREBP1
expression and survival in patients with ovarian cancer (n=96). The
mean survival for patients, tested positive for SREBP1, was
significantly shorter than the mean survival for those tested
negative (46.06 months; 95%CI, 37.44–54.68 months vs. 75.90 months;
95%CI, 59.67–92.12 months; P=0.017, Fig. 3B). Univariate survival analysis
showed that FIGO stage and SREBP1 expression were significantly
associated with survival (P<0.05). Furthermore, Cox proportional
hazards regression model indicated the FIGO stages to be the
definitive prognostic factor (P<0.001). Histological
classification, pathologic stage, ascitic cytology, lymphatic
metastasis, age, FASN and SREBP1 expression appeared to be
independent factor in predicting the overall survival in ovarian
cancer patients.
SREBP1 is responsible for lipogenic gene
expression in ovarian cancer cells
We performed the initial screening for SREBP1
expression in four aforementioned ovarian cancer cell lines. SREBP1
expression was almost undetectable in the 3AO cells, moderately
expressed in SKOV3 cells and highly expressed in A2780 and HO8910PM
cells (Fig. 4A). SREBP1 downstream
target genes FASN, ACLY, ACAC and SCD1 were also highly expressed
in HO8910PM. HO8910PM cell line was selected for most of the
experiments in this study (13). To
verify whether the endogenous SREBP1 is required for lipogenic gene
expression, we constructed four shRNA expression vectors (#1–3 and
control-vector) targeting SREBP1, and transfected them into HO8910M
cells. A high knockdown efficiency of SREBP1 expression was
observed by Western blot and real-time PCR in vectors #2 and #3
(Fig. 4B–D). Simultaneous reduction
in the expression of SREBP1 target genes indicated an
SREBP1-dependent expression of these genes (Fig. 4B–D).
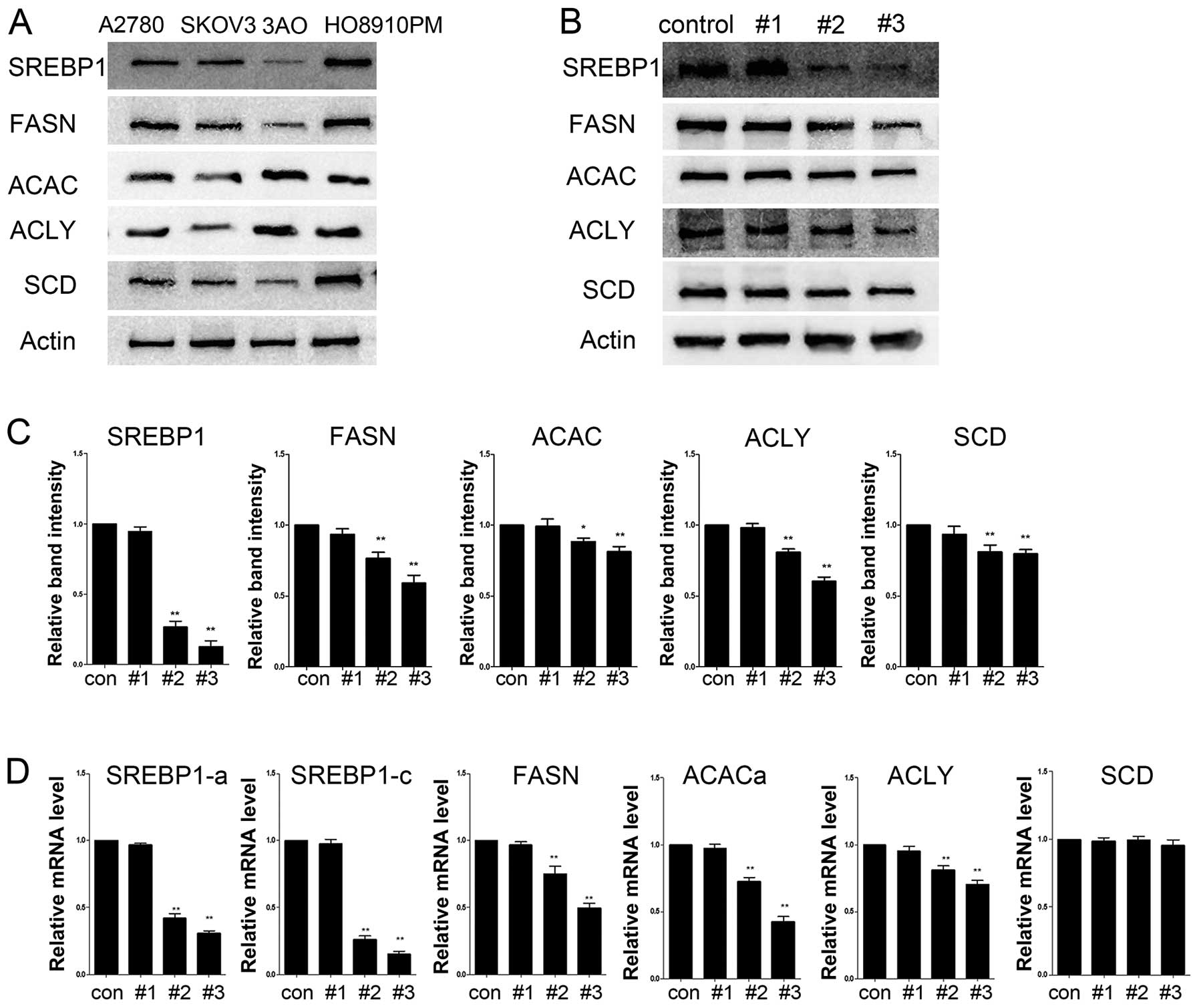 | Figure 4SREBP1 is required for lipogenic genes
in ovarian cancer cells. (A) Western blot analysis of lipogenic
gene expression in commonly used ovarian cancer cell lines. (B)
HO8910PM cells were transduced with a set of lentiviral vector
expressing shRNA targeting SREBF1 (#1, #3 and control-vector).
Western blot analysis of SREBP1, FASN, ACACα, ACLY and SCD1 were
performed. (C) Western blot analysis showed a successful knockdown
of SREBP1 and reduced expression of FASN, ACLY, ACAC, SCD as
transcriptional targets of SREBP1. *P<0.05,
**P <0.001, compared with the control-shRNA group.
(D) Quantitative RT-PCR analysis of mRNA abundance of SREBP1 and
their targets in transduced cells. *P<0.05,
**P<0.001, compared with the SREBP1 group. |
SREBP1 regulation of cellular
proliferation, migration, invasion and apoptosis
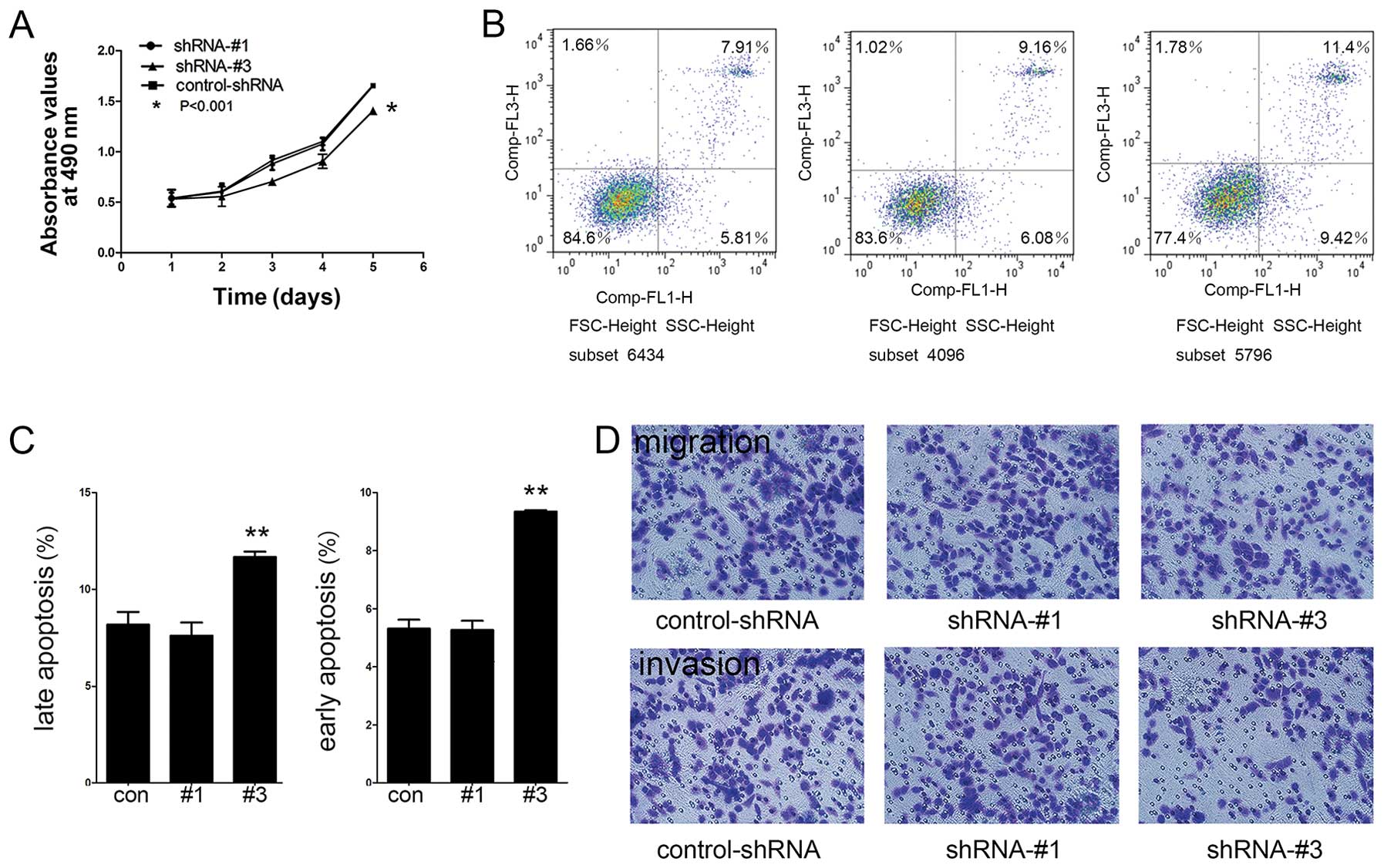
In order to compare cell proliferation rate among
cells with knockdown of SREBP1, lipid-free culture medium was used
to culture shRNA-#1, shRNA-#3 and control-shRNA cells. Statistical
analysis indicated a significant reduction of cell proliferation in
shRNA-#3 group compared to control-shRNA (P<0.05) (Fig. 5A). We also found that knockdown of
SREBP1, though attenuating cell growth, had no significant
influence on a specific phase of the cell cycle. These observations
suggested that the depletion of SREBP1 may increase the overall
cell cycle duration.
As shown in Fig. 5B,
the rate of late apoptosis in the shRNA-#3 group (11.68±0.27%) was
significantly higher than that of the control-shRNA group
(8.17±0.66%) (P<0.001) (Fig.
5C). Cells in early apoptosis were low in all three cell lines,
which showed an increase from 5.27% in control-shRNA group to 9.34%
in shRNA-#3 group (P<0.01) (Fig.
5C). Next, we analyzed the migratory and invasive properties of
SREBP1 knockdown cells. We found a significantly reduced number of
migrating cells in shRNA-#3 group (128.34±6.72), compared with
control-shRNA group (170.45±5.64) and invading cells in shRNA-#3
group (91.32±5.26), compared with control-shRNA group (137.36±8.72)
(Fig. 5D). These data revealed that
SREBP1 functions in regulating cell migration and invasion of
ovarian cancer cells.
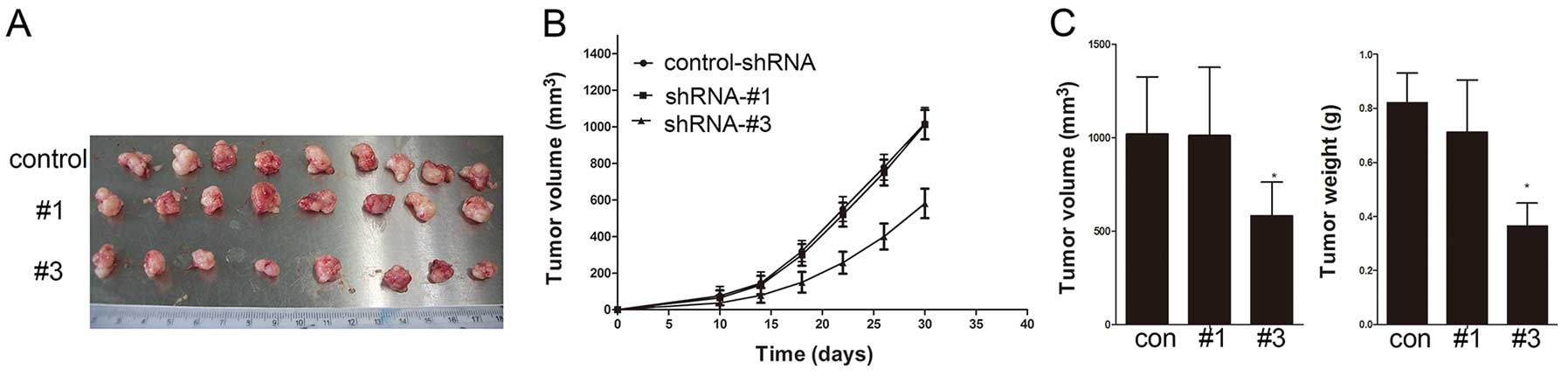
Knockdown of SREBP1 impairs ovarian tumor
growth in vivo
The xenograft female SCID mouse model exhibited a
markedly slowed growth pattern at 10 days after tumor cell
inoculation of shRNA-#3 group (Fig. 6A
and B). Control-shRNA and shRNA-#1 groups were consistent with
comparatively larger tumors (Fig.
6C). The shRNA-#3 group showed a significant inhibition of
tumor growth (P<0.001). These results demonstrate that SREBP1
plays an important role in tumor growth in vivo.
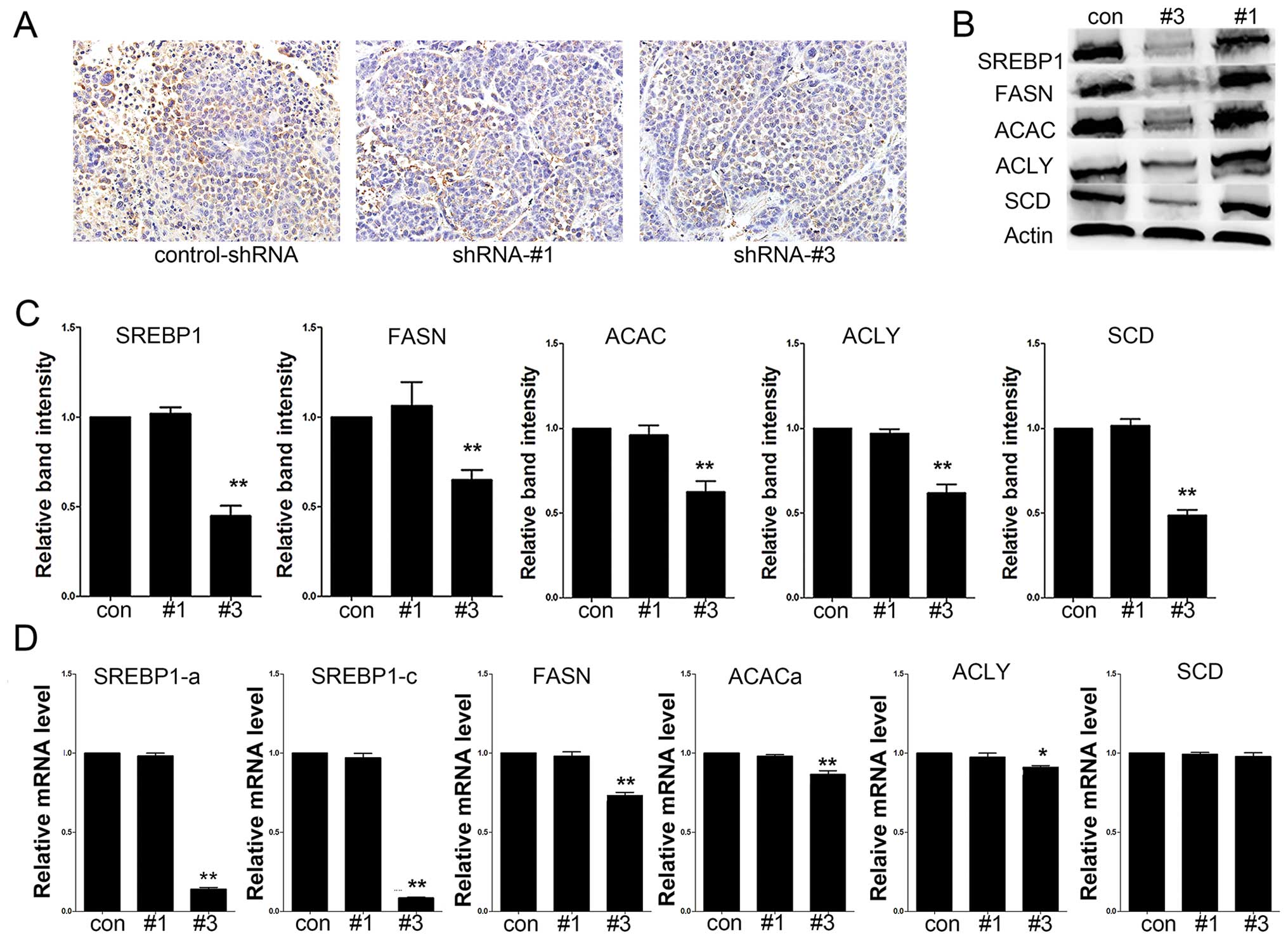
Immunohistochemical analysis of tumor sections showed a decrease of
the SREBP1 expression in tumors from shRNA-#3 groups compared to
control-shRNA group (Fig. 7A). As
shown in Fig. 7B–D, the expression
levels of SREBP1 and its downstream target genes were decreased in
the tumors in #3 group.
Discussion
In cancer cells, de novo lipogenesis is
closely related to increased synthesis of membranes, energy
production and activation of intracellular signaling pathways
during cell proliferation and division as well as cancer
development and progression (14–16).
SREBPs are major transcriptional regulators of genes involved in
de novo lipid synthesis and are critical for maintaining
lipid homeostasis. SREBP1 overexpression or activation has been
reported in hepatocellular carcinoma, colorectal carcinoma, breast,
and prostate tumors (9–12). Despite this elaborate research, this
is the first report on SREBP1 expression in ovarian cancer. In our
study, we found that SREBP1 promoted ovarian cancer growth. SREBP1
expression levels were associated with clinicopathological
parameters and with FASN expression. A previous study showed that
the lipogenic gene, FASN, was overexpressed in ovarian cancers
(17). We anticipated that SREBP1
overexpression in ovarian cancer may contribute to enhanced FASN
expression. The survival of patients who expressed high levels of
SREBP1 was significantly reduced compared to the patients negative
for SREBP1 expression. The cell culture and in vivo
experimental data collectively suggest that SREBP1 expression plays
an important role in regulation of ovarian tumor growth. The
observation of SREBP1 overexpression in ovarian cancer further
supports the notion that de novo lipid synthesis is required
in tumor growth.
To further investigate potential function of SREBP1
in ovarian cancer, we conducted several biophysiological
experiments using the shRNA knockdown approach targeting SREBP1.
Our results confirmed that SREBP1 silencing in ovarian cancer cells
inhibited cell growth, migration and invasion. Enhanced cell
apoptosis further implies the mechanisms by which SREBP1 is
required for tumor growth. The observation of cell proliferation
was only made in cell culture in the presence of fat-depleted
serum; where the cultured cells completely rely on the de
novo lipogenesis to satisfy the demand for cholesterol and
phospholipids of rapidly proliferating cells. Re-activation of
de novo fatty acid synthesis emerges in many cancers,
suggesting that lipogenesis may be a rate-limiting stage in rapidly
growing tissues (18). Induction of
de novo lipid synthesis in response to Akt activation
requires the activation of SREBP (19). Furthermore, Akt acts as an
anti-apoptotic signaling molecule and PI3K/Akt/TOR pathway
regulates lipid and protein biosynthesis. Both of these processes
are critically required for cell growth (20). Overactive Akt (often found in
cancer) activates SREBP and increases lipid synthesis, promoting
Akt signaling (21). Thus SREBP1
expression by stimulating lipogenic genes, promotes cancer cell
proliferation. This is the first report that systematically
investigated the function of SREBP1 in ovarian cancer cell
proliferation and tumor growth and may ultimately provides a novel
therapeutic strategy in the therapeutic aspect of this disease.
Several proteins including FASN, ACAC, ACLY and SCD
inlipogenic pathway have been reported to play important roles in
tumor progression and potentially targeted in cancer therapy.
Inhibition of the expression and activity of these genes with
either interfering RNA or small molecule inhibitors restrain tumor
cell proliferation in cell culture in vitro and tumor growth
in vivo(22–29). Furthermore, chemical inhibitors of
FASN have been shown to be effective in repressing tumor growth
(30). However, the efficacy of
these genes in cancer therapy requires further investigation. Out
results showed that silencing SREBP1 blocked the expression of
SREBP1 target genes FASN, ACAC and ACLY at both protein and mRNA
levels in cell culture. The effect of shRNA targeting SREBP1 was
more significant in repressing the expression of FASN, ACAC and
ACLY in mouse xenograft model in vivo. Our results
demonstrated that as a master regulator of lipogenic gene
transcription, SREBP1 regulates ovarian cancer cell growth and
survival through accommodating downstream de novo lipogenic
genes. The expression and activation of FASN, ACAC, ACLY and SCD
could be regulated not only by transcription factor SREBP1 but also
by other signal regulating pathways such as PI3K/Akt and MAPK
pathway in human (31). Our study
verified the significance of the lipogenic enzymes in ovarian
cancer pathogenesis, however, the underlying mechanisms, through
which SREBP1 regulates the downstream lipogenic genes in ovarian
cancer cells, are still poorly understood.
In summary, we have confirmed that SREBP1 plays
important roles in regulating lipid biosynthesis and homeostasis,
promoting ovarian tumor growth. Although further investigations are
needed to elucidate the molecular mechanisms underlying the
overexpression of SREBP1 and its ancillary regulatory signaling
pathways in cancers, our study suggests that SREBP1 may serve as a
therapeutic target for antitumor therapy.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China [81173614 (J.J.)] and [81173614
(Q.T.L)] and also funded by Science and Technology Development
planning of Shandong [2011GSF12122 (X.Z.)] and [2012G0021823
(J.J)].
References
|
1
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Medes G, Thomas A and Weinhouse S:
Metabolism of neoplastic tissue. IV A study of lipid synthesis in
neoplastic tissue slices in vitro. Cancer Res. 13:27–29.
1953.PubMed/NCBI
|
|
3
|
Horton JD: Sterol regulatory
element-binding proteins: transcriptional activators of lipid
synthesis. Biochem Soc Trans. 30:1091–1095. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X, Sato R, Brown MS, Hua X and
Goldstein JL: SREBP-1, a membrane-bound transcription factor
released by sterol-regulated proteolysis. Cell. 77:53–62. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Horton JD and Shimomura I: Sterol
regulatory element-binding proteins: activators of cholesterol and
fatty acid biosynthesis. Curr Opin Lipidol. 10:143–150. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown MS and Goldstein JL: The SREBP
pathway: regulation of cholesterol metabolism by proteolysis of a
membrane-bound transcription factor. Cell. 89:331–340. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Osborne TF: Sterol regulatory
element-binding proteins (SREBPs): key regulators of nutritional
homeostasis and insulin action. J Biol Chem. 275:32379–32382. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edwards PA, Tabor D, Kast HR and
Venkateswaran A: Regulation of gene expression by SREBP and SCAP.
Biochim Biophys Acta. 1529:103–113. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang YA, Morin PJ, Han WF, Chen T, Bornman
DM, Gabrielson EW and Pizer ES: Regulation of fatty acid synthase
expression in breast cancer by sterol regulatory element binding
protein-1c. Exp Cell Res. 282:132–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li JN, Mahmoud MA, Han WF, Ripple M and
Pizer ES: Sterol regulatory element-binding protein-1 participates
in the regulation of fatty acid synthase expression in colorectal
neoplasia. Exp Cell Res. 261:159–165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Swinnen JV, Heemers H, van de Sande T, de
Schrijver E, Brusselmans K, Heyns W and Verhoeven G: Androgens,
lipogenesis and prostate cancer. J Steroid Biochem Mol Biol.
92:273–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calvisi DF, Wang C, Ho C, Ladu S, Lee SA,
Mattu S, Destefanis G, Delogu S, Zimmermann A, Ericsson J,
Brozzetti S, Staniscia T, Chen X, Dombrowski F and Evert M:
Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling,
promotes development of human hepatocellular carcinoma.
Gastroenterology. 140:1071–1083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shenhua X, Lijuan Q, Hanzhou N, Xinghao N,
Chihong Z, Gu Z, Weifang D and Yongliang G: Establishment of a
highly metastatic human ovarian cancer cell line (HO-8910PM) and
its characterization. J Exp Clin Cancer Res. 18:233–239.
1999.PubMed/NCBI
|
|
14
|
Yamashita T, Honda M, Takatori H, Nishino
R, Minato H, Takamura H, Ohta T and Kaneko S: Activation of
lipogenic pathway correlates with cell proliferation and poor
prognosis in hepatocellular carcinoma. J Hepatol. 50:100–110. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Swinnen JV, Brusselmans K and Verhoeven G:
Increased lipogenesis in cancer cells: new players, novel targets.
Curr Opin Clin Nutr Metab Care. 9:358–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mukherjee A, Wu J, Barbour S and Fang X:
Lysophosphatidic acid activates lipogenic pathways and de novo
lipid synthesis in ovarian cancer cells. J Biol Chem.
287:24990–25000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uddin S, Jehan Z, Ahmed M, Alyan A,
Al-Dayel F, Hussain A, Bavi P and Al-Kuraya KS: Overexpression of
fatty acid synthase in Middle Eastern epithelial ovarian carcinoma
activates AKT and its inhibition potentiates cisplatin-induced
apoptosis. Mol Med. 17:635–645. 2011. View Article : Google Scholar
|
|
18
|
Menendez JA and Lupu R: Fatty acid
synthase and the lipogenic phenotype in cancer pathogenesis. Nat
Rev Cancer. 7:763–777. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Porstmann T, Santos CR, Griffiths B, Cully
M, Wu M, Leevers S, Griffiths JR, Chung YL and Schulze A: SREBP
activity is regulated by mTORC1 and contributes to Akt-dependent
cell growth. Cell Metab. 8:224–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: size matters. Oncogene.
22:8983–8998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krycer JR, Sharpe LJ, Luu W and Brown AJ:
The Akt-SREBP nexus: cell signaling meets lipid metabolism. Trends
Endocrinol Metab. 21:268–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Migita T, Narita T, Nomura K, Miyagi E,
Inazuka F, Matsuura M, Ushijima M, Mashima T, Seimiya H, Satoh Y,
Okumura S, Nakagawa K and Ishikawa Y: ATP citrate lyase: activation
and therapeutic implications in non-small cell lung cancer. Cancer
Res. 68:8547–8554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zu XY, Zhang QH, Liu JH, Cao RX, Zhong J,
Yi GH, Quan ZH and Pizzorno G: ATP citrate lyase inhibitors as
novel cancer therapeutic agents. Recent Pat Anticancer Drug Discov.
7:154–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo DX, Tong DJ, Rajput S, Wang C, Liao
DF, Cao D and Maser E: Targeting acetyl-CoA carboxylases: small
molecular inhibitors and their therapeutic potential. Recent Pat
Anticancer Drug Discov. 7:168–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C, Rajput S, Watabe K, Liao DF and
Cao D: Acetyl-CoA carboxylase-a as a novel target for cancer
therapy. Front Biosci. 2:515–526. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tong L and Harwood HJ Jr: Acetyl-coenzyme
A carboxylases: versatile targets for drug discovery. J Cell
Biochem. 99:1476–1488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roongta UV, Pabalan JG, Wang X, Ryseck RP,
Fargnoli J, Henley BJ, Yang WP, Zhu J, Madireddi MT, Lawrence RM,
Wong TW and Rupnow BA: Cancer cell dependence on unsaturated fatty
acids implicates stearoyl-CoA desaturase as a target for cancer
therapy. Mol Cancer Res. 9:1551–1561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Igal RA: Stearoyl-CoA desaturase-1: a
novel key player in the mechanisms of cell proliferation,
programmed cell death and transformation to cancer. Carcinogenesis.
31:1509–1515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fritz V, Benfodda Z, Rodier G, Henriquet
C, Iborra F, Avances C, Allory Y, de la Taille A, Culine S, Blancou
H, Cristol JP, Michel F, Sardet C and Fajas L: Abrogation of de
novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes
with oncogenic signaling and blocks prostate cancer progression in
mice. Mol Cancer Ther. 9:1740–1754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu H, Liu JY, Wu X and Zhang JT:
Biochemistry, molecular biology, and pharmacology of fatty acid
synthase, an emerging therapeutic target and diagnosis/prognosis
marker. Int J Biochem Mol Biol. 1:69–89. 2010.PubMed/NCBI
|
|
31
|
Mashima T, Seimiya H and Tsuruo T: De novo
fatty-acid synthesis and related pathways as molecular targets for
cancer therapy. Br J Cancer. 100:1369–1372. 2009. View Article : Google Scholar : PubMed/NCBI
|