Introduction
microRNAs (miRNAs) are molecules, ~22 nucleotides
long, that inhibit gene expression in animals and plants. Mounting
evidence indicates that miRNAs are key regulators of human diseases
such as cancer (1).
Pancreatic cancer is a deadly malignancy with a
5-year survival rate of ~5%; it is the fourth most common cause of
cancer-related mortality in the Western world (2). The molecular mechanisms responsible
for pancreatic cancer development remain unknown and there are no
established guidelines for prevention. Recent studies have revealed
a relationship between altered miRNA expression and pancreatic
cancer (3,4).
The miR-155 locus is located within a region known
as the B-cell integration cluster (BIC) (3), which was originally thought to be a
proto-oncogene associated with lymphoma (5). miR-155 is overexpressed in various
solid tumors, including breast, lung, colon and thyroid cancers,
where it functions as an oncogenic miRNA (6–9).
Reports have also shown that many miRNAs including miR-155 are
differentially expressed in pancreatic cancer (10,11).
High expression of miR-155 is correlated with poor prognoses of
pancreatic cancer (12). miR-155
promotes pancreatic cancer development and mammary gland epithelial
cell migration and invasion by targeting TP53INP1 and RhoA,
respectively (13,14). These lines of evidence are
consistent with the notion that miR-155 plays an important role in
the development of pancreatic cancer.
Suppressor of cytokine signaling 1 (SOCS1) is a
tumor suppressor that normally functions as a negative feedback
regulator of Janus activated kinase (JAK)/signal transducer and
activator of transcription-3 (STAT3) signaling (15). It is a target gene of miR-155 in
breast cancer (16). We found that
STAT3 signaling was overactivated in pancreatic cancer and that it
promoted invasion and metastasis (17,18).
However, the relationship between miR-155 overexpression and
overactivation of STAT3 signaling in pancreatic cancer is
unknown.
In the present study, we utilized miR-155 mimics and
an inhibitor to regulate miR-155 expression. Migration and invasion
in vitro were assessed, and SOCS1 expression and activation
of STAT3 were detected. In situ hybridization and
immunohistochemical analysis in tissue microarrays were performed
to analyze the correlation of miR-155 and SOCS1 expression with
various clinicopathologic factors.
Materials and methods
Cell culture and transient
transfection
Human pancreatic cancer cell lines Panc-1 and
Capan-2 were obtained from the American Type Culture Collection
(Manassas, VA, USA) and cultured with Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and
penicillin/streptomycin at 37°C in a 5% CO2 incubator.
Panc-1 and Capan-2 cells (1×106) were seeded into each
well of 6-well plates and transfected with miR-155 mimics and
anti-miR-155. Cognate control RNAs were used as negative controls.
Transfection was performed using Lipofectamine® 2000
(Invitrogen) according to the manufacturer’s instructions, and
miR-155 mimics or antisense oligonucleotides were mixed with
Lipofectamine 2000. After 48 h, the cells were assayed. The
sequences of miR-155 mimics were 5′-UUAAUGCUAAUC GUGAUAGGGGU-3′ and
5′-CCCUAUCACGAUUAGCAU UAAUU-3′; the inhibitor sequence was
5′-ACCCCUAUCACG AUUAGCAUUAA-3′.
Invasion and migration assays
The cell invasion assay was performed in a
specialized invasion chamber that included a 24-well tissue culture
plate and 12-cell culture inserts (both from Corning). The inserts
contained an 8-μm pore polycarbonate membrane. A thin layer of
basement membrane matrix (1:3 dilution; BD Biosciences) coated each
well. Briefly, medium supplemented with 10% FBS was added to the
lower chamber as a chemo-attractant. After reaching 60–70%
subconfluence, pancreatic cancer cells were trypsinized,
re-suspended in DMEM, and ~5×104 cells were added to
each upper compartment.
After 48 h of incubation at 37°C, the non-invasive
cells and membranes were removed from the upper surface using a
moist cotton swab. Invasive cells on the lower surface of the
membrane were stained for 20 min and rinsed several times with
distilled water. Invasiveness was quantified by selecting 5
different views (x400) and calculating the number of invading
cells.
The cell migration assay was performed as the
invasion assay, but the basement membrane matrix was not used and
the cell seeding number was 8×104.
Quantitative real-time reverse
transcription-polymerase chain reaction (qRT-PCR)
Total RNA was isolated from Panc-1 and Capan-2 cells
using TRIzol reagent (Invitrogen). The RNA was then purified using
an RNeasy Mini kit (Qiagen) according to the manufacturer’s
instructions. The miR-155, miR-21 and miR-210 levels were
quantified by quantitative reverse transcription-PCR (qRT-PCR)
using SYBR-Green assay kits (Genecopoeia), with U6 small nuclear
RNA as an internal normalized reference. SOCS1 and STAT3 mRNA
levels were determined using the forward and reverse primers with
β-actin as an internal reference. Specific primers for the PCR
reaction were as follows: SOCS1, 5′-GAGGGAGC GGATGGGTGTA-3′
(forward) and 5′-GAGGTAGGAGGT GCGAGTTCAG-3′ (reverse); STAT3,
5′-CCAAGGAGGAGG CATTCG-3′ (forward) and 5′-ACATCGGCAGGTCAATGG-3′
(reverse); β-actin, 5′-AGTTGCGTTACACCCTTTC-3′ (forward) and
5′-CACCTTCACCGTTCCAGT-3′ (reverse). Relative miRNA or mRNA
expression of target genes, following normalization to an
endogenous sequence, was calculated by the ΔΔCt method. miRNAs or
mRNAs upregulated or downregulated 1-fold were identified as being
significantly altered.
Protein extraction and western
immunoblotting
Cells were harvested 48 h after transfection and
lysed in radioimmunoprecipitation assay buffer (Beyotime, Haimen,
Jiangsu, China) containing 1 mmol/l phenylmethanosulfonyl fluoride
on ice for 15 min. Protein concentration was determined with a BCA
protein assay kit (Beyotime). Lysates were mixed with SDS-PAGE
sample loading buffer and boiled for 5 min. Total cellular protein
(50 μg) was resolved on 8 or 10% SDS-polyacrylamide gels and
transferred to nitrocellulose membranes. The membranes were stained
with 0.5% Ponceau S containing 1% acetic acid to verify equal
loading and transfer efficiency. The membranes were blocked in 5%
bovine skim milk overnight and with primary antibody overnight at
4°C. After washing in TBS, the membranes were incubated with
peroxidase-conjugated secondary antibody for 1.5 h at room
temperature. Enhanced chemiluminescence reagent from Millipore
(Billerica, MA, USA) was used to detect positive protein bands. The
primary antibodies were as follows: SOCS1 (1:1,000; Abcam,
Cambridge, MA, USA), STAT3 (1:1,000) and P-STAT3 (1:2,000; both
from Cell Signaling Technology Danvers, MA, USA); and β-actin
(1:1,000; Biomart, Shanghai, China). Secondary antibodies included
peroxidase-conjugated Affinipure goat anti-mouse or anti-rabbit IgG
(Jackson ImmunoResearch; West Grove, PA, USA).
Immunohistochemistry
Pancreatic cancer and tumor-adjacent tissue chips
were purchased from Shanghai Outdo Biotech Co. (Shanghai, China);
each point was 1.5 mm in diameter and 4 μm thick. The chip was
deparaffinized in xylene and rehydrated in successive washes of
ethanol, and then heated in a microwave oven at medium power for 8
min in citrate buffer (pH 6.0) for heat-induced epitope retrieval.
Endogenous peroxidase activity was blocked, followed by
non-specific binding of the primary antibody, target protein
localization with the first antibody, visualization with the
secondary antibody, and the color reaction. The primary antibodies
included SOCS1 (1:1,000).
Stained tumor cells and paraffin sections were
reviewed and scored using light microscopy performed by a
pathologist blinded to the treatment group. Positivity of the
stained tumor cells on coverslips and paraffin sections was defined
by staining intensity and the percentage of positive cells. The
staining intensity of SOCS1 expression was classified
semi-quantitatively into negative and weak, moderate, and strong
positivity (0, +, ++ and +++, respectively).
In situ hybridization of miRNAs
In situ hybridization of miR-155 was
performed on tissue chip sections. The sequence of miR-155 probe
was ACCCCTATCTCGATTAGCATT AA-HRP. Sections were deparaffinized in
xylene, rehydrated in successive washes of DEPC-treated water
through a graded series of ethanol (100, 70, 50 and 25%), and left
in PBS for 10 min. After permeabilization with 0.1% Triton X-100 in
PBS for 10 min, the sections were washed in PBS (2 × 5 min) and
treated with Proteinase K (1 μg/ml in 50 mmol/l EDTA, pH 8.0, 0.1
mol/l Tris-HCl) for 5 min at 37°C, followed by washing in PBS (3 ×
5 min). Mercury Locked Nucleic Acid (LNA) miRNA detection probes
(Fudan Biotechnology Co., Shanghai, China) were used; hsa-miR-155
(40 nM in a formamide-free ISH buffer). Probes were denatured by
heating to 95°C for 5 min and 50 ml of probe mixture was hybridized
with the tissue sections in a hybridizer at 37°C for 60 min. The
slides were then placed at RT in 5X saline-sodium citrate (SSC)
(Invitrogen) and washed for 5 min at 55°C in 5X SSC (1 wash), 1X
SSC (2 washes) and 0.5X SSC (2 washes). After washing in TBS,
sections were blocked with blocking buffer and incubated for 30
min. Slides were then incubated for 120 min in TBS with
HRP-conjugated anti-DIG (diluted 1:500 in blocking solution;
Roche). After washing in TBS (2 × 5 min), the DAB color reaction
was performed.
Positivity of stained tumor cells on coverslips and
paraffin sections was defined by staining intensity and the
percentage of positive cells as in the immunohistochemistry
experiment.
Statistical analysis
Statistical analyses were performed using SPSS 13.0
software (SPSS, Chicago, IL, USA). The data are expressed as means
± SD when possible and were analyzed with the Student-Newman-Keuls
test to determine statistical significance. P<0.05 was
considered statistically significant. Correlations were calculated
using Spearman’s r test (two-sided) unless otherwise specified.
P-values were not adjusted for multiple testing. Categorical
variables were assessed by the Chi-square test.
Results
Regulation of miR-155 expression in
Panc-1 and Capan-2 cells
miR-155, miR-210 and miR-21 have been reported to be
associated with tumor invasion and are highly expressed in
pancreatic cancers (5–9). We determined expression of these
microRNAs in Panc-1 and Capan-2 cells. No difference in miR-210
expression was noted while miR-21 expression was higher in the
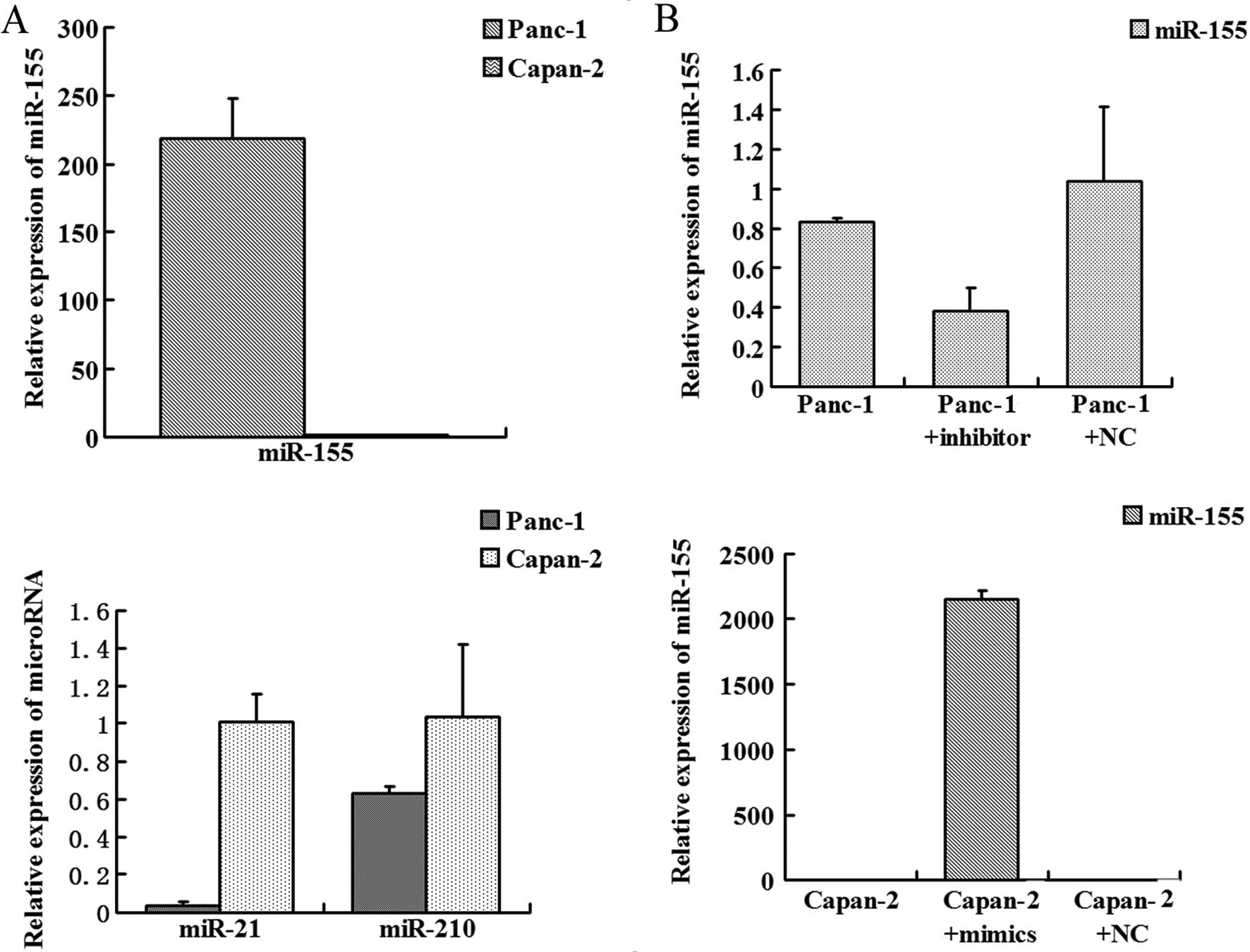
Capan-2 cells when compared to that in the Panc-1 cells. miR-155
expression was much higher in the Panc-1 cells than that in the
Capan-2 cells (Fig. 1A). qRT-PCR
revealed that miR-155 mimics upregulated miR-155 expression in
Capan-2 cells and the miR-155 inhibitor successfully knocked down
miR-155 expression in Panc-1 cells (Fig. 1B).
Invasion and migration ability and
miR-155 modulation in pancreatic cancer cells
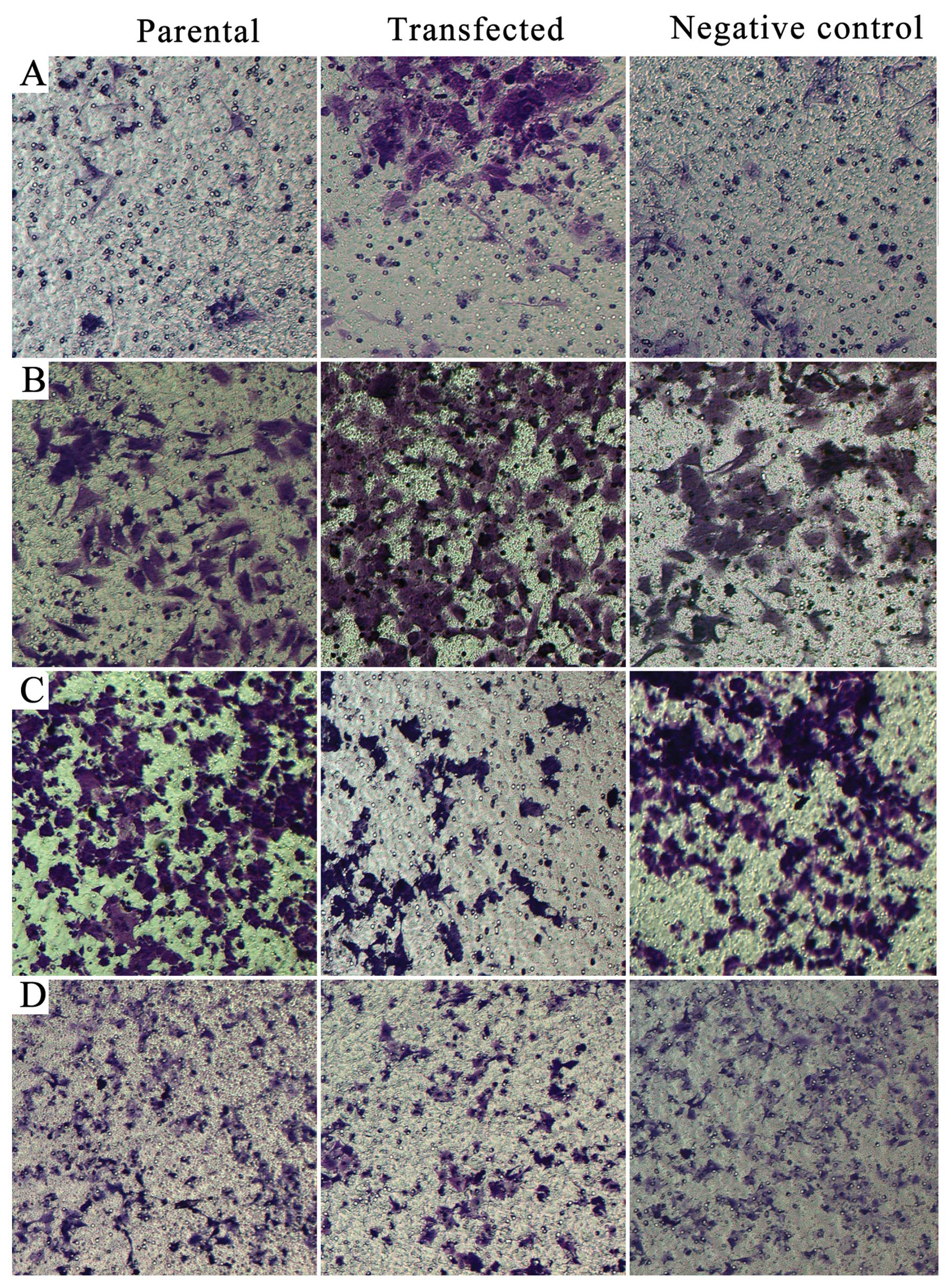
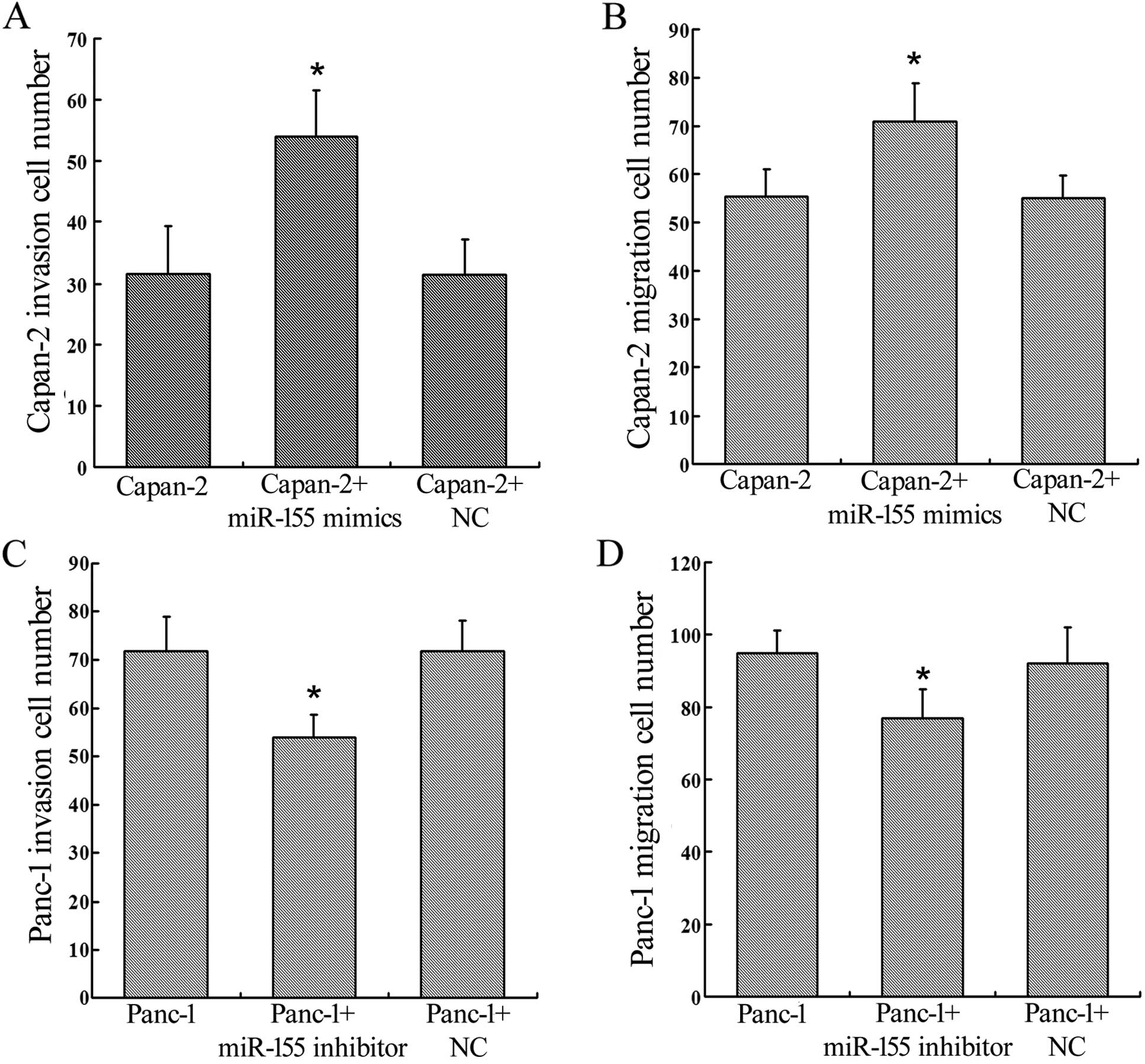
We assessed changes in invasion and migration
ability of Panc-1 and Capan-2 cells after regulation of miR-155
expression by using Transwell assays. Upregulation of miR-155
expression in Capan-2 cells enhanced invasion and migration ability
(P=0.0002, P=0.0001) (Figs. 2A and
B; 3A and B), and knockdown of
miR-155 expression in Panc-1 cells inhibited invasion and migration
ability (P=0.0005, P=0.0002) (Figs. 2C
and D; 3C and D).
Expression of SOCS1 and STAT3 and
activation of STAT3 following regulation of miR-155
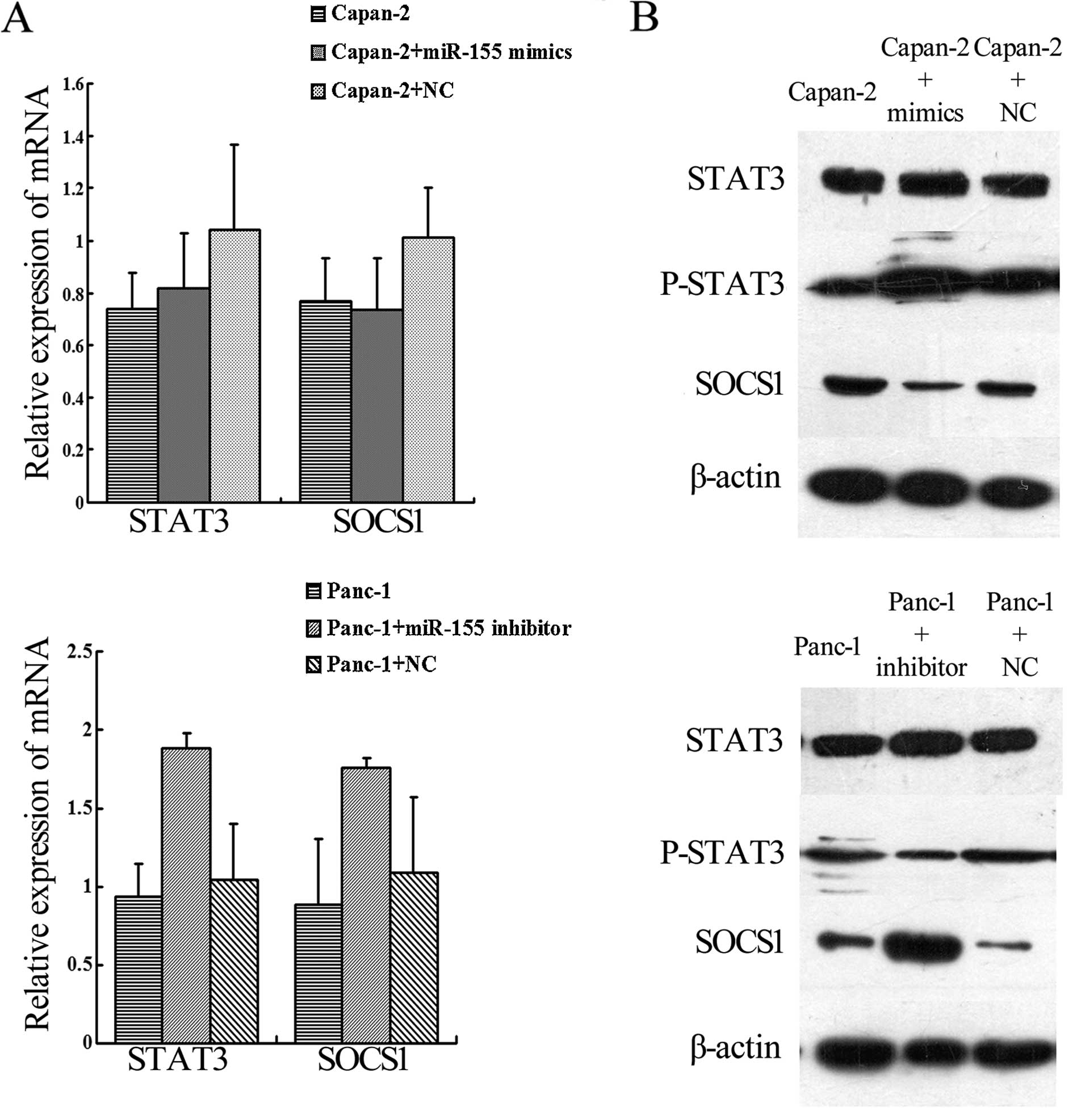
We determined SOCS1 gene expression in
miR-155-regulated cells by qRT-PCR and western blotting. The data
revealed that SOCS1 and STAT3 mRNA expression did not differ in the
transfected cells when compared with the parental and control cells
(Fig. 4A). However, at the protein
level, SOCS1 expression was increased by miR-155 knockdown and
decreased by miR-155 upregulation in Panc-1 cells. P-STAT3 protein
was decreased by miR-155 knockdown in Panc-1 cells and was
increased by miR-155 upregulation in Capan-2 cells (Fig. 4B).
Expression of miR-155 and SOCS1 in
pancreatic cancer and tumor-adjacent tissues
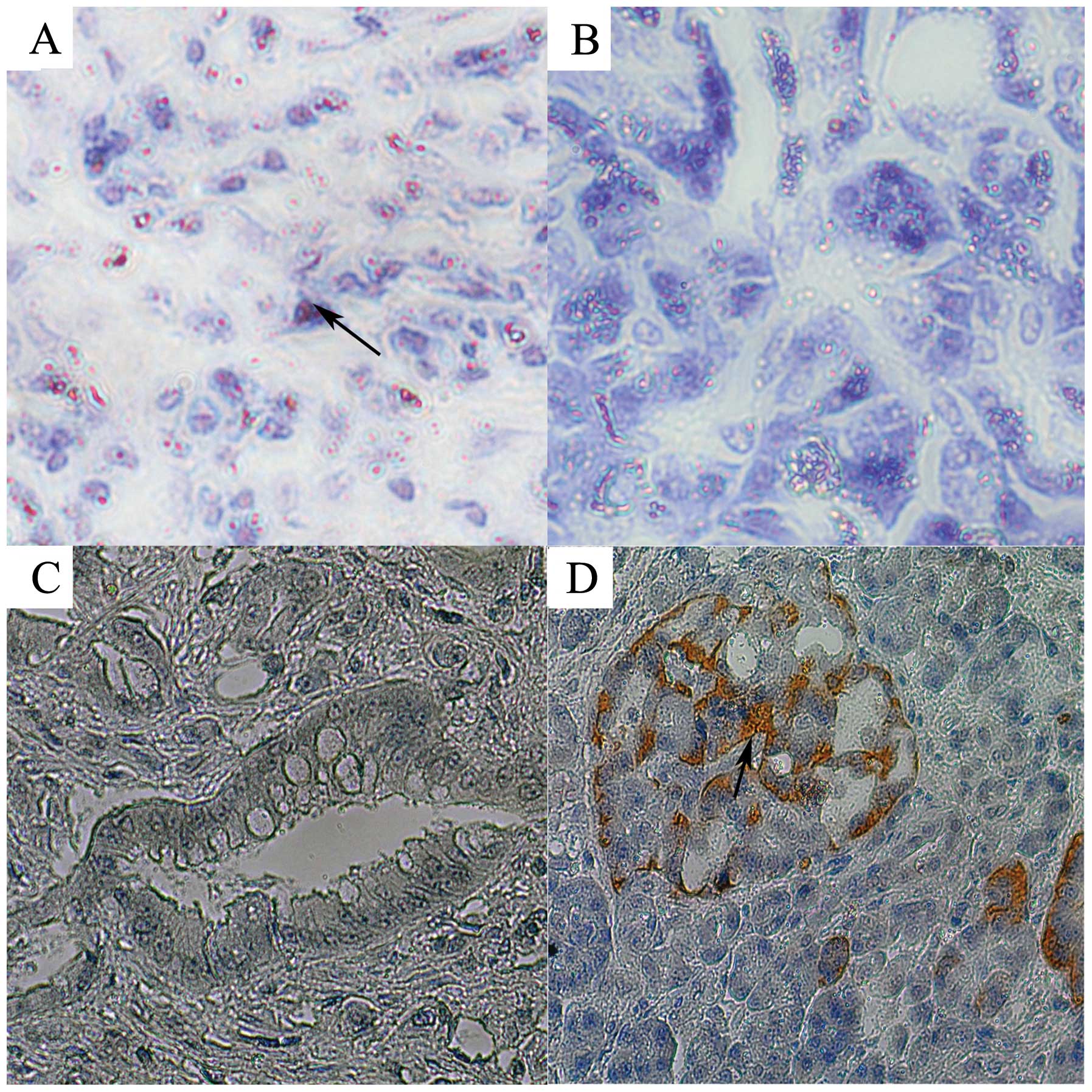
We detected expression of miR-155 and SOCS1 in
pancreatic cancer and tumor-adjacent tissues in tissue chips by
in situ hybridization and immunohistochemistry. The rate of
miR-155-positive expression in the pancreatic cancer tissues was
81.25% (65/80), and the rate of strong-positive expression was 10%
(10/80). However, in tumor-adjacent tissues, the rate of
miR-155-positive expression was 71.25% (57/80) and the rate of
strong-positive expression was 1.25% (1/80). Statistical analyses
showed that miR-155-positive expression in pancreatic cancer
tissues was significantly higher than that in tumor-adjacent
tissues (P=0.0001) (Fig. 5A)
(Table I). The positive expression
rate of SOCS1 in pancreatic cancer tissues was 37.5% (30/80) and
65% (52/80) in tumor-adjacent tissues. The rates of strong-positive
expression were 2.5% (2/80) and 25% (20/80), respectively. There
was significantly higher expression in the tumor-adjacent tissues
when compared with that in the cancer tissues (P=0.0003) (Fig. 5B) (Table II).
 | Table IExpression of miR-155 in pancreatic
tumor and tumor-adjacent tissues. |
Table I
Expression of miR-155 in pancreatic
tumor and tumor-adjacent tissues.
| miR-155 | |
|---|
|
| |
|---|
| − | + | ++ | +++ | Positive rate
(%) |
|---|
| Tissue |
| Tumor | 15 | 27 | 30 | 8 | 81.5a |
|
Tumor-adjacent | 23 | 1 | 55 | 1 | 71.25 |
 | Table IIExpression of SOCS1 protein in
pancreatic tumor and tumor-adjacent tissues. |
Table II
Expression of SOCS1 protein in
pancreatic tumor and tumor-adjacent tissues.
| SOCS1 protein | |
|---|
|
| |
|---|
| − | + | ++ | +++ | Positive rate
(%) |
|---|
| Tissue |
| Tumor | 50 | 17 | 11 | 2 | 37.5a |
|
Tumor-adjacent | 28 | 17 | 15 | 20 | 65 |
Relationship between miR-155 and SOCS1
expression in pancreatic cancer and tumor-adjacent tissues
We analyzed the relationship between miR-155 and
SOCS1 expression in pancreatic cancer and tumor-adjacent tissues.
The data revealed no relationship between miR-155 and SOCS1 in
cancer or tumor-adjacent tissues (r1=−0.178,
P1=0.115; r2=−0.002, P2=0.947)
(Tables III and IV).
 | Table IIImiR-155 and SOCS1 expression in
pancreatic cancer tumors. |
Table III
miR-155 and SOCS1 expression in
pancreatic cancer tumors.
| miR-155 | Spearman’s rank
correlation |
|---|
|
|
|
|---|
| − (n=15) | + (n=27) | ++ (n=30) | +++ (n=8) | r | P-value |
|---|
| SOCS1 | | | | | −0.178 | 0.115 |
| − (n=50) | 8 | 14 | 23 | 5 | | |
| + (n=17) | 4 | 7 | 4 | 2 | | |
| ++ (n=11) | 2 | 5 | 3 | 1 | | |
| +++ (n=2) | 1 | 1 | 0 | 0 | | |
 | Table IVmiR-155 and SOCS1 expression in
tumor-adjacent tissue. |
Table IV
miR-155 and SOCS1 expression in
tumor-adjacent tissue.
| miR-155 | Spearman’s rank
correlation |
|---|
|
|
|
|---|
| − (n=23) | + (n=1) | ++ (n=55) | +++ (n=1) | r | P-value |
|---|
| SOCS1 | | | | | −0.002 | 0.947 |
| − (n=28) | 6 | 0 | 22 | 0 | | |
| + (n=17) | 8 | 0 | 8 | 1 | | |
| ++ (n=15) | 4 | 0 | 11 | 0 | | |
| +++ (n=20) | 5 | 1 | 14 | 0 | | |
Relationship between miR-155, SOCS1 and
clinical stage of pancreatic cancer
We analyzed the relationship between miR-155, SOCS1
and the clinical stage of pancreatic cancer and found
SOCS1-positive expression in 33.3% (13/39) of the non-lymph node
metastatic pancreatic cancer tissues and in 48.4% (15/31) of the
lymph node metastatic pancreatic cancer tissues. Thus, positive
expression of SOCS1 was not related to lymph node metastasis
(P=0.767). Positive expression was noted in 34.5% (10/29), 22.22%
(2/9), 34.37% (11/32) of stage I, IIa and IIb + III + IV cases,
respectively. However, there was no relationship between positivity
and clinical stage (P=0.539) (Table
V).
 | Table VDistribution of SOCS1 expression in
pancreatic cancer tumors according to TNM stage and lymph node
metastasis. |
Table V
Distribution of SOCS1 expression in
pancreatic cancer tumors according to TNM stage and lymph node
metastasis.
| | SOCS1 |
|---|
| |
|
|---|
| n | Low | High | P-value |
|---|
| TNM stage | | | | 0.767 |
| I | 29 | 19 | 10 | |
| IIa | 9 | 7 | 2 | |
| IIb + III +
IV | 32 | 21 | 11 | |
| Lymph nodes | | | | 0.539 |
| No metastasis | 39 | 26 | 13 | |
| Metastasis | 31 | 16 | 15 | |
Positive expression of miR-155 was noted in 30.8%
(12/39) of the non-lymph node metastatic pancreatic cancer tissues
and in 67.7% (21/31) of the lymph node metastatic pancreatic cancer
tissues. Thus, miR-155 expression was related to lymph node
metastasis (P=0.0001). Positive expression was noted in 51.7%
(15/29), 55.5% (5/9) and 81.25% (26/32) of stage I, IIa and IIb +
III + IV cases, respectively. Thus, miR-155 expression was related
to clinical stage (P=0.011) (Table
VI).
 | Table VIDistribution of miR-155 expression in
pancreatic cancer tumors according to TNM stage and lymph node
metastasis. |
Table VI
Distribution of miR-155 expression in
pancreatic cancer tumors according to TNM stage and lymph node
metastasis.
| | miR-155 |
|---|
| |
|
|---|
| n | Low | High | P-value |
|---|
| TNM stage |
| I | 29 | 14 | 15 | 0.000a |
| IIa | 9 | 4 | 5 | |
| IIb + III +
IV | 32 | 6 | 26 | |
| Lymph nodes |
| No metastasis | 39 | 27 | 12 | 0.011a |
| Metastasis | 31 | 10 | 21 | |
Discussion
miR-155 mimics and inhibitor respectively
upregulated and downregulated expression of miR-155 in Panc-1 and
Capan-2 cells in comparison to the parental and negative control
cells. Invasion and migration ability of pancreatic cancer cells
was significantly reduced in vitro when miR-155 was
downregulated. The reverse was true for miR-155 upregulation.
miR-155 was previously found to be highly expressed in pancreatic
cancer tissues (19,20) and to influence invasion and
metastasis through its target genes including TP53INP1 and RhoA
(13,14). Our results are consistent with these
previous reports. However, our study was performed in vitro; in
vivo studies will follow.
miRNAs have hundreds of potential target genes.
SHIP1, C/EBPβ and CK1α are targets of miR-155 (21–23).
As these genes have different functions, miR-155 plays multiple
roles in cancer development. Reports indicate that SOCS1 is a
target gene of miR-155 in breast cancer and plays an important role
in activation of the STAT3 signaling pathway (24). In the present study, we found that
expression of SOCS1 protein (not transcription) in pancreatic
cancer cells was regulated by miR-155. This finding suggests that
miR-155 regulated SOCS1 expression only at the subtranscription
level but did not lead to its mRNA degradation. Phosphorylation of
STAT3 was also affected by miR-155, but with a reverse trend. Thus,
miR-155 may influence pancreatic cancer invasion and metastasis, at
least partly, by regulating SOCS1 through the STAT3 signaling
pathway. Our previous study showed that overactivation of the STAT3
signaling pathway in pancreatic cancer regulated MMP-2, MMP-7 and
others to mediate invasion and metastasis (18,25).
However, we do not know what induces the overactivation of STAT3 in
pancreatic cancer. We now suggest that high miR-155 expression may
be one cause of STAT3 overactivation in pancreatic cancer. Other
reports have indicated that miR-155 activates the STAT3 signaling
pathway in cancer cells (26,27).
However, these hypotheses require in vivo validation.
Reports indicate that expression of miR-155 and
SOCS1 is related to clinical stage and prognosis (28–30).
We found increased expression of miR-155 in pancreatic cancer
tissue, but not in tumor-adjacent tissues. SOCS1 was more highly
expressed in tumor-adjacent tissues than in pancreatic cancer
tissues. However, there was no relationship between these
phenomena. This may suggest some contradiction with our in
vitro results. However, we know that expression of one type of
protein may be regulated by many factors and there is a reticular
structure to the regulation of protein expression. Therefore, of
the many factors influencing SOCS1 expression in pancreatic
cancers, miR-155 is one.
Our study also showed that expression of miR-155 was
related to lymph node metastasis and clinical stage. A previous
study indicated that miR-155 levels significantly increased in
intraepithelial neoplasia grade II pancreatic ductal epithelial
cells or early-stage pancreatic cancer in comparison to normal
pancreatic tissues (31). This
result suggests that when malignant transformation occurs in
pancreatic ductal epithelial cells, miR-155 levels increase.
Several studies have shown increased miR-155 expression in
pancreatic cancer tissues in comparison to normal pancreatic
tissues or chronic pancreatitis tissues, and they showed that
miR-155 may serve as an index for diagnosis and clinical staging
(12,32,33).
These results are consistent with our study. However, other studies
have indicated that miR-155 can inhibit gastric cancer invasion and
metastasis by altering expression of smad2, acting as a type of
tumor-suppressor gene (34).
Therefore, continued investigation of the roles of miR-155 in
pancreatic cancer and its relationship with metastasis and
prognosis is warranted.
Identification of the molecular mechanisms
responsible for pancreatic cancer invasion and metastasis are
critical to successful treatment of this disease. In the present
study, we found that miR-155 can affect activation of STAT3 to
mediate invasion and metastasis through SOCS1. These findings may
be helpful to find suitable targets for microRNA-based gene therapy
and for novel approaches for the early diagnosis of pancreatic
cancer.
Acknowledgements
The present study was supported by grants 81101844
and 81210108027 (to C.H.) from the National Natural Science
Foundation of China and grants 2012040 and 13PJD024 (to C.H.) from
Shanghai Municipal Human Resources and Social Security Bureau.
References
|
1
|
Stefani G and Slack FJ: Small non-coding
RNAs in animal development. Nat Rev Mol Cell Biol. 9:219–230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Yu J, Li A, Hong SM, et al: MicroRNA
alterations of pancreatic intraepithelial neoplasias. Clin Cancer
Res. 18:981–992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Z, Chen LY, Dai HY, et al: miR-301a
promotes pancreatic cancer cell proliferation by directly
inhibiting Bim expression. J Cell Biochem. 113:3229–3235. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tam W, Ben-Yehuda D and Hayward WS: bic, a
novel gene activated by proviral insertions in avian leukosis
virus-induced lymphomas, is likely to function through its
noncoding RNA. Mol Cell Biol. 17:1490–1502. 1997.PubMed/NCBI
|
|
6
|
Kong W, He L, Coppola M, et al:
MicroRNA-155 regulates cell survival, growth, and chemosensitivity
by targeting FOXO3a in breast cancer. J Biol Chem. 285:17869–17879.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Babar IA, Czochor J, Steinmetz A, Weidhaas
JB, et al: Inhibition of hypoxia-induced miR-155 radiosensitizes
hypoxic lung cancer cells. Cancer Biol Ther. 12:908–914. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bakirtzi K, Hatziapostolou M,
Karagiannides I, et al: Neurotensin signaling activates
microRNAs-21 and -155 and Akt, promotes tumor growth in mice, and
is increased in human colon tumors. Gastroenterology.
141:1749–1761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nikiforova MN, Tseng GC, Steward D, et al:
MicroRNA expression profiling of thyroid tumors: biological
significance and diagnostic utility. J Clin Endocrinol Metab.
93:1600–1608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szafranska AE, Davison TS, John J, et al:
MicroRNA expression alterations are linked to tumorigenesis and
non-neoplastic processes in pancreatic ductal adenocarcinoma.
Oncogene. 26:4442–4452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bloomston M, Frankel WL, Petrocca F, et
al: MicroRNA expression patterns to differentiate pancreatic
adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA.
297:1901–1908. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greither T, Grochola LF, Udelnow A, et al:
Elevated expression of microRNAs 155, 203, 210 and 222 in
pancreatic tumors is associated with poorer survival. Int J Cancer.
126:73–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gironella M, Seux M, Xie MJ, et al: Tumor
protein 53-induced nuclear protein 1 expression is repressed by
miR-155, and its restoration inhibits pancreatic tumor development.
Proc Natl Acad Sci USA. 104:16170–16175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong W, Yang H, He L, et al: MicroRNA-155
is regulated by the transforming growth factor beta/Smad pathway
and contributes to epithelial cell plasticity by targeting RhoA.
Mol Cell Biol. 28:6773–6784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davey GM, Heath WR and Starr R: SOCS1: a
potent and multifaceted regulator of cytokines and cell-mediated
inflammation. Tissue Antigens. 67:1–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Zhao H, Chen J, et al:
Interferon-β-induced miR-155 inhibits osteoclast differentiation by
targeting SOCS1 and MITF. FEBS Lett. 586:3255–3262. 2012.
|
|
17
|
Huang C, Yang G, Jiang T, et al: The
effects and mechanisms of blockage of STAT3 signaling pathway on
IL-6 inducing EMT in human pancreatic cancer cells in vitro.
Neoplasma. 58:396–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li HD, Huang C, Huang KJ, et al: STAT3
knockdown reduces pancreatic cancer cell invasiveness and matrix
metalloproteinase-7 expression in nude mice. PLoS One.
6:e259412011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee EJ, Gusev Y, Jiang J, et al:
Expression profiling identifies microRNA signature in pancreatic
cancer. Int J Cancer. 120:1046–1054. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Habbe N, Koorstra JB, Mendell JT, et al:
MicroRNA miR-155 is a biomarker of early pancreatic neoplasia.
Cancer Biol Ther. 8:340–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pedersen IM, Otero D, Kao E, et al:
Onco-miR-155 targets SHIP1 to promote TNFalpha-dependent growth of
B cell lymphomas. EMBO Mol Med. 1:288–295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Costinean S, Sandhu SK, Pedersen IM, et
al: Src homology 2 domain-containing inositol-5-phosphatase and
CCAAT enhancer-binding protein β are targeted by miR-155 in B cells
of Eμ-MiR-155 transgenic mice. Blood. 114:1374–1382.
2009.PubMed/NCBI
|
|
23
|
Zhang P, Bill K, Liu J, Young E, et al:
MiR-155 is a liposarcoma oncogene that targets casein kinase-1α and
enhances β-catenin signaling. Cancer Res. 72:1751–1762.
2012.PubMed/NCBI
|
|
24
|
Jiang S, Zhang HW, Lu MH, et al:
MicroRNA-155 functions as an OncomiR in breast cancer by targeting
the suppressor of cytokine signaling 1 gene. Cancer Res.
70:3119–3127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang C, Cao J, Huang KJ, et al:
Inhibition of STAT3 activity with AG490 decreases the invasion of
human pancreatic cancer cell in vitro. Cancer Sci. 97:1417–1423.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang S, Zhang LF, Zhang HW, et al: A
novel miR-155/miR-143 cascade controls glycolysis by regulating
hexokinase 2 in breast cancer cells. EMBO J. 31:1985–1998. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su C, Hou Z, Zhang C, et al: Ectopic
expression of microRNA-155 enhances innate antiviral immunity
against HBV infection in human hepatoma cells. Virol J. 8:3542011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han ZB, Chen HY, Fan JW, et al:
Up-regulation of microRNA-155 promotes cancer cell invasion and
predicts poor survival of hepatocellular carcinoma following liver
transplantation. J Cancer Res Clin Oncol. 138:153–161. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sasi W, Jiang WG, Sharma A and Mokbel K:
Higher expression levels of SOCS 1,3,4,7 are associated with
earlier tumour stage and better clinical outcome in human breast
cancer. BMC Cancer. 10:1782010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Li H, Yu JP, et al: Role of SOCS1
in tumor progression and therapeutic application. Int J Cancer.
130:1971–1980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ryu JK, Hong SM, Karikari CA, et al:
Aberrant microRNA-155 expression is an early event in the multistep
progression of pancreatic adenocarcinoma. Pancreatology. 10:66–73.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Panarelli NC, Chen YT, Zhou XK, et al:
MicroRNA expression aids the preoperative diagnosis of pancreatic
ductal adenocarcinoma. Pancreas. 41:685–690. 2012.PubMed/NCBI
|
|
33
|
Wang J, Chen J, Chang P, et al: microRNAs
in plasma of pancreatic ductal adenocarcinoma patients as novel
blood-based biomarkers of disease. Cancer Prev Res. 2:807–813.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li CL, Nie H, Wang M, et al: microRNA-155
is downregulated in gastric cancer cells and involved in cell
metastasis. Oncol Rep. 27:1960–1966. 2012.PubMed/NCBI
|