Introduction
Selection of patients based on either epidermal
growth factor receptor (EGFR) mutations or clinical
characteristics appears to be an effective approach to optimize
EGFR-tyrosine kinase inhibitor (TKI) treatment for
chemotherapy-pretreated non-small cell lung cancer (NSCLC) patients
(1). In multivariate analyses,
EGFR mutations found in female East Asian never-smokers with
adenocarcinoma were associated with an objective response (2). Most of the mutations found in the
EGFR catalytic domain are located in a frame deletion in
exon 19 and in point mutations in exon 21. The mutations alter the
ATP/inhibitor binding site, stabilize the binding of drugs or
intensify their inhibitory effect (3). However, patients with activating
EGFR mutations, for whom tumor progression is observed
despite treatment based on TKIs, may acquire resistance to
gefitinib or erlotinib (4,5). Mutation analysis employing cytological
material sampled using fine needle aspiration (FNA) or cell block
of the pleural fluid proved advantageous for the detection of
inhibiting mutations in such material (6). Material obtained via endobronchial
ultrasound (EBUS)-transbronchial needle aspiration (TBNA) may also
be useful for detecting EGFR mutations in patients with lung
adenocarcinoma, since positive results were previously demonstrated
in ~1/10 Spanish patients (7,8).
To the best of our knowledge, no prior studies have
described a cytological scale and its correlation with the analysis
of EGFR and KRAS mutations studied in cytological
samples obtained from a Polish population. Therefore, the aim of
the present study was to analyze the EGFR, KRAS and
BRAF status in limited cytological material from Polish
patients.
Materials and methods
Selection and processing of
pathomorphological samples
Cytological material from 78 patients with NSCLC was
collected at the Department of Tumor Pathology and Pathomorphology,
Oncology Center, Franciszek Lukaszczyk Memorial Hospital in
Bydgoszcz, Poland. Informed consent for mutation testing was
obtained from all the patients.
A total of 78 specimens were obtained following 67
FNA procedures, 7 bronchial brush procedures, 3 EBUS-TBNA
procedures and 1 pleural liquid sampling. In the 78 patients, 75
adenocarcinoma subtypes were determined, and all were tested for
the presence of EGFR mutations and the majority of samples
were tested for KRAS mutations. Four cytological samples did
not pass quality control steps (pathomorphological qualification or
qualitative and quantitative DNA analysis). Biopsy samples were
also stained with hematoxylin and eosin (H&E) for qualitative
and quantitative analysis of tumor cells in the analyzed material
(including macrodissection in marked out samples). Representative
cytological smears were subjected to molecular oncological and
genetic assessment of mutations in the EGFR and KRAS
genes. Two clinical pathomorphologists (W.J. and C.J.), who were
unaware of the patient characteristics, examined the cytological
smears as follows. In each smear, two elements were evaluated by
the number of neoplastic and non-neoplastic nucleated cells, using
a subjective method of microscopic counting of 10 neighboring cells
as the smallest virtual ‘decimal cell group’, dispersed uniquely
within each tissue section, then 10-folded to the ‘100-fold’ and
‘1,000-fold’ cell groups in the two following steps, yielding the
‘10-fold’, ‘100-fold’ or ‘1,000-fold’ cell measures, respectively,
to perform an approximate cell number estimation, when needed, with
an accuracy of ~10%. Qualification of cytological material for
molecular analysis was based on the following quantitative scale
(QS): C1+, ≤100 tumor cells; C2+, >100–1,000 tumor cells; C3+,
>1,000–5,000 tumor cells; C4+, >5,000–10,000 tumor cells;
C5+, >10,000 (countless) tumor cells) (Table I). Two samples were not qualified
due to the small number of cells (no more than 20 tumor cells)
(Table II). The percentage of
tumor cells (PTCs) was analyzed based on the number of neoplastic
cells compared to all nucleated cells in the cytological
specimens.
 | Table IQuantitative scale (QS) and sample
numbers of detected EGFR and KRAS mutations. |
Table I
Quantitative scale (QS) and sample
numbers of detected EGFR and KRAS mutations.
| QS | No. of tumor
cells | No. of EGFR
mutations detected | No. of KRAS
mutations detected |
|---|
| C1+ | >20–100 | None | None |
| C2+ | >100–1,000 | None | 1 |
| C3+ |
>1,000–5,000 | 1 | 7 |
| C4+ |
>5,000–10,000 | 4 | 8 |
| C5+ | >10,000
(countless) | 2 | 3 |
 | Table IIEGFR mutation status in the
studied lung adenocarcinomas. |
Table II
EGFR mutation status in the
studied lung adenocarcinomas.
| EGFR | Exon | No. of
patients | Percentage of
patients | Mutation (presence
or absence)/no. of tests performed |
|---|
| EGFR
wild-type | 18, 19, 20 and
21 | 64 | 64/71 (90%) | 64/71
(90%) |
| Exon 19
deletion | 19 | 4 | 4/7 (57.1%) | 7/71
(10%) |
| Exon 20
insertion | 20 | 1 | 1/7 (14.3%) | |
| c.2582T>A
(L861Q) | 21 | 1 | 1/7 (14.3%) | |
| c.2369C>T
(T790M)/c.2573T>G (L858R) | 18 and 21 | 1 | 1/7 (14.3%) | |
| No. of disqualified
analyses | - | 4 | 4/75 (5.3%) | - |
DNA isolation from biopsy smears (after H&E
evaluation and image acquisition) was conducted by immersing
cytological samples in xylene and by incubation at room temperature
overnight or until cover slips were removed (up to 5 days). Once
cover slips were taken off, the samples were incubated for 5 min in
100% ethanol, and then washed with 70% ethanol to rehydrate the
tissue before the cells were scraped with a sterile scalpel into a
tube. Subsequently, DNA was isolated using the QIAamp DNA FFPE
tissue kit.
All isolation procedures were performed according to
the manufacturer’s instructions. DNA was subjected to qualitative
and quantitative analysis (NanoDrop; Thermo Scientific) and DNA
isolates with an A260/A280 ratio ranging from 1.8 to 2.0 were
tested for the presence of mutations in the EGFR and
KRAS genes.
Detection of EGFR, KRAS and BRAF
mutations
All 29 EGFR mutations in exons 18, 19, 20 and
21 were evaluated via the real-time polymerase chain reaction (PCR)
methodology using mutation-specific oligonucleotides (EntroGen). A
DNA quantity of 600–650 ng was adequate for the detection of 29
mutations in the samples of interest; however, in a few cases the
total amount of DNA was reduced to 250 ng for the detection of the
29 mutations due to the high quality of DNA, even though a lower
DNA yield was obtained from biopsy samples. Such amount of DNA was
in accordance with the instructions for the detection of the 29
EGFR mutations, in which the recommended minimum DNA
quantity was 50 ng. Mutation analysis was identical to the
manufacturer’s protocol for EGFR mutation analysis using
real-time PCR (EntroGen) performed using LightCycler®
480 II (Roche). For the purpose of validation, direct Sanger
sequencing was performed, followed by real-time PCR of the most
common mutations in exons 19 and 21. This assay reports the
presence of the following EGFR mutations: 3 mutations in
codon 719 in exon 18; 19 deletions in exon 19; 3 insertions, as
well as p.T790M and p.S768I mutations in exon 20; p.L858R and
p.L861Q mutations in exon 21. Each analysis was initiated by
checking the endogenous control amplification plot of every sample
(VIC detector). When endogenous Ctrl CT ranged from 22 to 32, FAM
detector was used. When no FAM signal was present, the sample did
not contain the selected mutation. Normal positive CT values
(mutation detected) may range from 20 to 37. When the CT value was
>38, the reaction alone or the entire procedure including DNA
isolation was repeated. The EGFR mutation assay was
established to have an analytical sensitivity of 1% based on
dilution studies prepared by EntroGen.
In order to investigate the mutation status in
codons 12 and 13 of the KRAS gene in 54 cytological samples,
HybProbe and melting curve analysis (LightMix®
Diagnostic kits; TIB MolBiol) were performed.
LightCycler® FastStart DNA Master HybProbe was prepared
according to the manufacturer’s instructions, along with the
controls and the CTRL, LOW and HIGH reactions with the tested DNA.
This assay reports the presence of KRAS mutations located in
codons 12 and 13: c.34G>C, c.34G>A, c.34G>T, c.35G>A,
c.35G>T, c.35G>C, c.37G>T and c.38G>A. Following
real-time PCR analyses, melting curve analysis was performed
according to the following protocol: the 13C reaction exhibits a
peak at ~56°C, the 12C reaction exhibits a peak at ~68–70°C, the
NTC reaction exhibits baseline value, the WT reaction exhibits a
peak at ~64–65°C, the cWT reaction exhibits baseline value or a
maximum of 10% value of the WT reaction signal. Reaction for a
sample was analyzed when the control reaction met specific
criteria. Wild-type was reported when a sample displayed no peak in
the HIGH reaction and the LOW reaction displayed a
wild-type-specific peak at the same time. The CTRL reaction also
had to demonstrate a wild-type-specific peak. A mutation was
reported when a sample displayed a clear peak in the HIGH reaction.
The LOW reaction might return the same result or display two peaks
corresponding to the wild-type and mutation. A mutation was also
reported when a sample returned no peak in the HIGH reaction but
had a clear peak at any temperature between 50 and 65°C in the LOW
reaction. The CTRL reaction had to show a wild-type-specific peak
or display two peaks corresponding to the wild-type and
mutation.
Selected samples were confirmed using additional
methods: EGFR mutation-positive samples via Sanger
sequencing (ABI Prism 3130xl Genetic Analyzer), and
KRAS-BRAF mutation-positive samples via Strip Assay,
which also allowed the analysis of BRAF status in codon 600
(ViennaLab Diagnostics GmbH) according to the manufacturer’s
instructions (30).
Results
Characteristics of the patients and
specimens
Clinical information
Clinical characteristics, such as gender, and
procedures used for cytological material collection are provided in
Table III in correlation with the
EGFR, KRAS and BRAF c.1799T>A (V600E)
mutation status. Generally, 100% of the patients were Caucasians,
among whom most of the EGFR mutations were observed in women
(85.7%), while most of the KRAS mutations were observed in
men (65%). EGFR, KRAS and BRAF mutations were
detected in material collected using FNA (47 patients), brushing
procedure (4 patients) and EBUS-TBNA (3 patients).
 | Table IIIClinicopathological characteristics
of 54 lung adenocarcinomas according to the EGFR and
KRAS mutation status. |
Table III
Clinicopathological characteristics
of 54 lung adenocarcinomas according to the EGFR and
KRAS mutation status.
| Gender, n | Procedure, n |
|---|
|
|
|
|---|
| Group | Female | Male | FNA | EBUS and TBNA | Brushing |
|---|
|
EGFR+/KRAS−
(n=7) | 6 | 1 | 6 | 0 | 1 |
|
EGFR−/KRAS+/BRAF−
(n=18) | 5 | 13 | 16 | 1 | 1 |
|
EGFR−/KRAS+/BRAF+
(n=1) | 0 | 1 | 1 | 0 | 0 |
|
EGFR−/KRAS−
(n=28) | 20 | 8 | 24 | 2 | 2 |
Specimen evaluation
Every cytological sample that qualified for
EGFR mutation analysis was carefully analyzed by
pathomorphologists at the Oncology Center, Franciszek Lukaszczyk
Memorial Hospital. Pathomorphologists selected representative
biopsy samples among several collected in each case for further
molecular analysis. In order to determine whether mutations could
be detected in the cytological material with a small number of
tumor cells, identification was performed with samples meeting the
following criteria: i) the sample was identified as adenocarcinoma,
and ii) the pathological material was available for PTCs and QS in
tumor analysis prior to DNA isolation. The real-time PCR
methodology was validated using mutated and WT EGFR samples
previously confirmed by sequencing. Some cytological specimens gave
a low yield of genomic DNA extraction but their quality was high
enough to perform mutation analysis. All samples were evaluated for
several parameters, such as PTCs and QS (Table IV).
 | Table IVEGFR, KRAS and
BRAF mutations detected in cytological material qualified
for molecular analysis using quantitative scale (QS) and the
percentage of tumor cells (PTCs). |
Table IV
EGFR, KRAS and
BRAF mutations detected in cytological material qualified
for molecular analysis using quantitative scale (QS) and the
percentage of tumor cells (PTCs).
| Mutation
detected | C+ (QS) | % (PTCs) | Procedure |
|---|
| EGFR exon 19
deletion | C4+ | Unknown | FNA |
| EGFR exon 19
deletion | C4+ | 80 | FNA |
| EGFR exon 19
deletion | C4+ | 80 | FNA |
| EGFR exon 19
deletion | C4+ | 50 | Brushing |
| EGFR exon 20
insertion | C5+ | 90 | FNA |
| EGFR
c.2582T>A (L861Q) | C3+ | 95 | FNA |
| EGFR
c.2369C>T (T790M) and EGFR c.2573T>G (L858R) | C5+ | Unknown | FNA |
| KRAS
c.34G>T (G12C) | Unknown | Unknown | FNA-CT |
| KRAS
c.35G>T (G12V) | C3+ | 50 | TBNA |
| KRAS
c.35G>T (G12V) | C3+ | Unknown | FNA |
| KRAS
c.34G>T (G12C) | C3+ | 30 | Brushing |
| KRAS
c.34G>T (G12C) | C3+ | 60 | FNA |
| KRAS
c.35G>A (G12D) | C4+ | 80 | FNA |
| KRAS
c.34G>T (G12C) | C4+ | 75 | FNA |
| KRAS
c.35G>A (G12D) | C4+ | 80 | FNA |
| KRAS
c.35G>T (G12V) | C3+ | 60 | FNA |
| KRAS
c.34G>T (G12C) | C4+ | 80 | FNA |
| KRAS
c.35G>T (G12V) | C2+ | 80 | FNA |
| KRAS
c.34G>T (G12C) | C3+ | 40 | Brushing |
| KRAS
c.34G>T (G12C) | C5+ | 90 | FNA |
| KRAS
c.35G>T (G12V) | C4+ | 80 | FNA |
| KRAS
c.34G>T (G12C) | C4+ | 90 | FNA |
| KRAS
c.34G>T (G12C) | C5+ | 80 | FNA |
| KRAS
c.35G>A (G12D) | C4+ | 70 | FNA |
| KRAS
c.34G>T (G12C) | C5+ | 80 | FNA |
| KRAS
c.34G>T (G12C) | C4+ | 80 | FNA |
| BRAF
c.1799T>A (V600E) | C3+ | 60 | FNA |
All detected mutations were derived from specimens
with >1,000 tumor cells. One EGFR mutation in exon 21 was
detected in cytological material that qualified for the C3 group
with >1,000 tumor cells. Another 4 mutations were detected in
specimens that qualified for the C4 group (with >5,000 tumor
cells) and 2 mutations qualified for the C5 group (with countless
tumor cells). No mutations were detected in C1 and C2 specimens
(Table I).
EGFR mutation analysis
Among the 75 samples that qualified for evaluation
of the presence of EGFR mutations in exons 18, 19, 20 and 21
in adenocarcinoma cytological samples, 4 samples did not qualified
for molecular analysis since few tumor cells were found in the
cytological material (<20) or due to the fact that the low yield
and poor quality of the extracted DNA material prevented the use of
real-time PCR (Table II).
In the 71 samples analyzed, 7 mutations were
detected, mostly regarding a deletion in exon 19, followed by
substitutions in exon 21 and a single insertion in exon 20, while 1
sample carried two mutations of inhibiting EGFR c.2369C>T
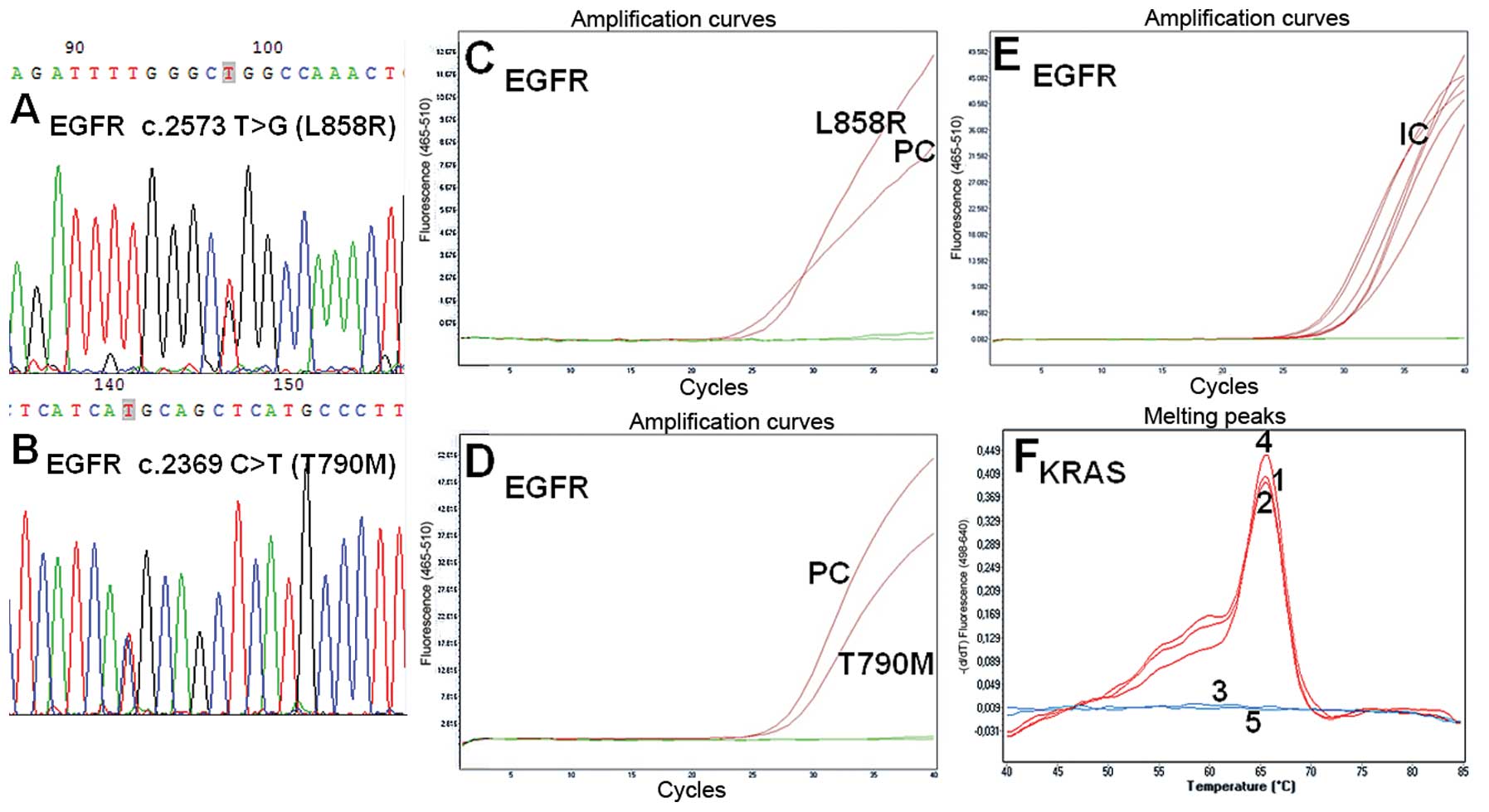
(T790M) (Fig. 2B) and activating
EGFR c.2573T>G (L858R) types in exon 21 (Fig. 2A and Table II). Six EGFR mutations were
found in the cytological material collected via the FNA procedure
and 1 deletion in exon 19 was found in the material obtained using
brushing (Table III).
The real-time PCR method allowed detection of an
EGFR c.2582T>A (L861Q) mutation in cytological material
with the number of tumor cells ranging from 1,000 to 5,000 (QS=C3+,
PTS=95%), obtained using BAC/CT (Fig.
1III). However, most of the mutations were detected with the
minimum number of adenocarcinoma cells exceeding 5,000. The
EGFR mutation detection rate was 10%, taking into
consideration the tested cytological samples with a minimum of
1,000 tumor cells (Tables I and
IV).
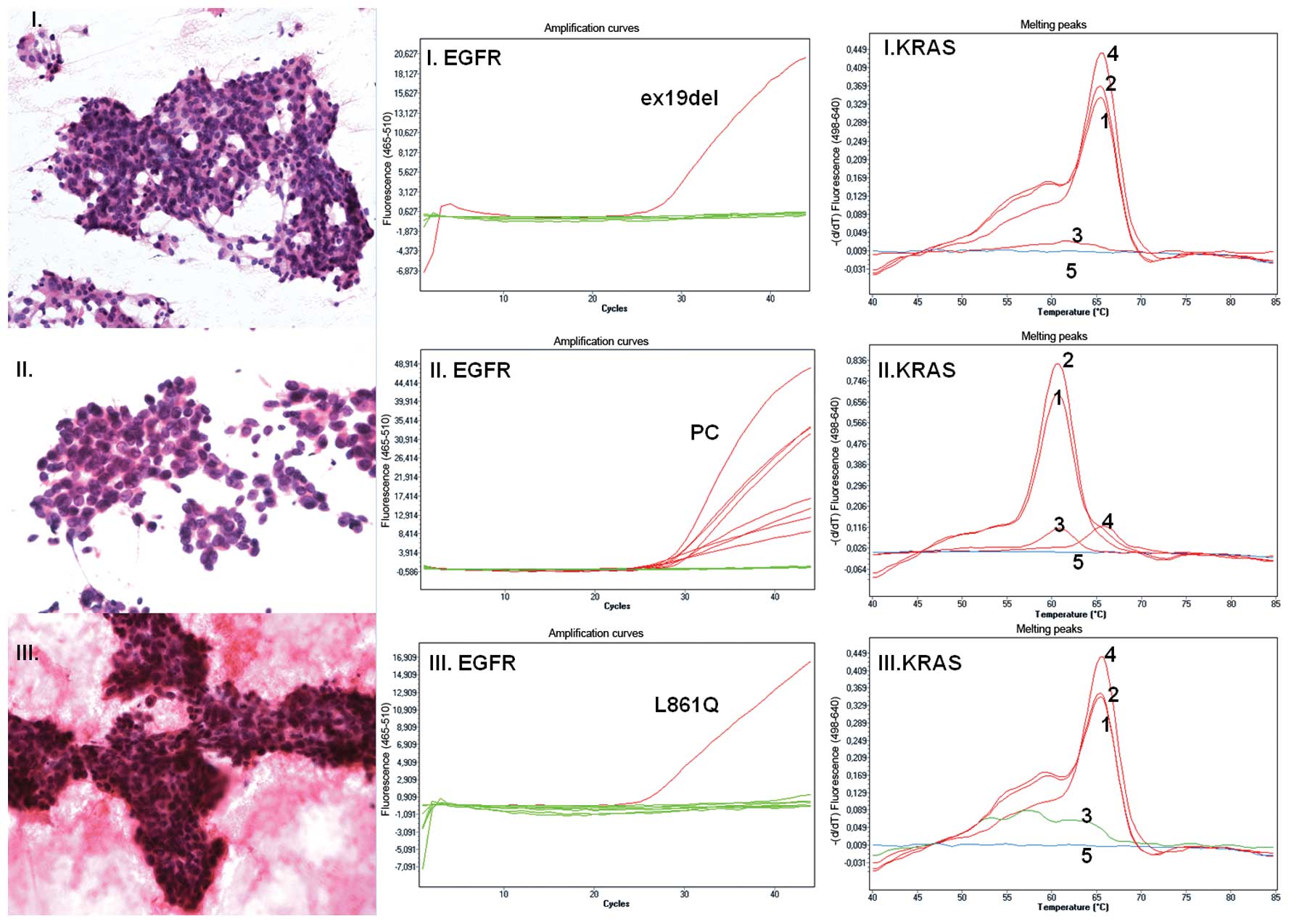 | Figure 1Lung adenocarcinoma. (I–III) Cytology
of lung adenocarcinoma following H&E staining (magnification,
×200, ×400 and ×200 for samples I, II and III, respectively).
(I–III EGFR) EGFR status detected using mutation-specific
oligonucleotides. Green lines represent baseline value with no
mutation detected in exons 18, 19, 20 and 21; red lines represent
amplification curves as follows: (I. EGFR) EGFR exon 19
deletion detected in sample I; (II. EGFR) all positive controls in
detection of 29 mutations in sample II, no mutation detected in
sample II; (III. EGFR) detection of EGFR c.2582T>A
(L861Q) in sample III. (I–III KRAS) KRAS status detection. Nos. 1–5
represent melting curves for the CTRL reaction: 1, LOW reaction; 2,
HIGH reaction; 3; and for the controls: 4, WT and 5, NTC. Melting
curve analysis detected KRAS WT (I. KRAS), KRAS
c.35G>A (G12D) (II. KRAS) and KRAS WT (III. KRAS). |
KRAS and BRAF mutation analysis
Out of the 71 samples qualified for real-time PCR
analysis and tested for EGFR mutations (Table II), only 54 samples were selected
for KRAS mutation analysis (Table III). Seventeen samples were not
used for KRAS analysis due to the limited quantity of DNA
obtained via extraction from the cytological material, which was
depleted for EGFR mutation analysis. The presence or absence
of the 10th most common KRAS mutation in codons 12 and 13
was also determined. We found 11 KRAS c.34G>T (G12C), 4
KRAS c.35G>T (G12V), 2 KRAS c.35G>A (G12D)
mutations (Fig. 1II) and a single
mutation in codon 13, KRAS c.38G>A (G13D). Every
KRAS mutation analysis was confirmed using different PCR
methods: HRM analysis (TIB MolBiol) and biotinylated sequences
detected using streptavidin-alkaline phosphatase (ViennaLab
Diagnostics GmbH). The BRAF c.1799T>A (V600E) mutation
was analyzed in only 19 samples (due to limited cytological
material), in which a KRAS mutation was detected (Table IV). Notably, the real-time PCR
method allowed detection of the KRAS c.35G>T (G12V)
mutation in cytological material characterized by QS=C2+ and
PTS=80% (Table IV). No cases of
concurrent presence of KRAS and EGFR mutations were
observed in the same tumor sample (Table III). The majority of samples
(n=28) were EGFR wild-type and KRAS wild-type. Seven
patients with an EGFR mutation lacked KRAS mutations
in codons 12 and 13 (Fig. 2F) and
vice versa. One patient with wild-type EGFR and a
KRAS mutation in codon 12 (G12C) had a mutation in
BRAF c.1799T>A (V600E).
Discussion
The presence of an EGFR mutation in the
tyrosine kinase domain constitutes an important predictive factor
for the response to treatment with TKIs (9). Therefore, it is crucial to analyze the
EGFR mutation status using the most reliable method, which
permits detection of activating and inhibiting EGFR
mutations.
In the real-time PCR and new generation sequencing
era, small quantities of input material may be used for the
analysis of numerous mutations. However, the heterogeneous
character of tumors should not be underestimated. Therefore,
quantitative estimation of cytological specimens should be
performed in the preanalytical step, in order to determine
mutations in as many tumor cells as possible. The findings of the
present study suggest that EGFR mutations are detected in
DNA isolated from heterogeneous cytological material containing
≥1,001 tumor cells. Therefore, the EGFR mutation analysis in
cytological specimens may be performed as a routine evaluation in
patients with inoperable NSCLC. In contrast, unequal cytological
sampling could be potentially misleading. Any EGFR WT result
should be carefully assessed as it might reflect sampling bias,
particularly when the analysis was performed using DNA isolated
from a small number of tumor cells (21–1,000). Taking into
consideration the histological heterogeneity of NSCLC, genetic
differentiation implied as local divergence of mutation status
within a tumor may also be possible. Thus, the fact that
cytological samples become less representative with a decreasing
number of tumor cells cannot be excluded. In such a case, potential
EGFR WT results obtained following analysis of cytological
specimens with a small number of tumor cells might be considered as
potentially containing false negatives, and different results might
be obtained following the analysis of an additional FNA sample from
the same patient. Cytological material may be analyzed using
smeared slides or cytological cell blocks. This leads to the
serious drawback of the time-consuming digital archiving of smear
slides which is ‘sacrificed’ for DNA isolation.
Cytological smears constitute material more
difficult to handle when compared to paraffin-embedded tissues;
therefore, it is highly important to use well-validated, sensitive
methods which do not provide false-positive results, and also
decrease any discrepancies and false-negative results of the
EGFR mutation status analysis, when DNA material derived
from cytological samples is limited. Currently, different methods
of EGFR status assessment are used, ranging from
immunocytochemistry (10) and
qualitative or quantitative PCR methods (11) to sequencing (including either Sanger
or new generation sequencing methods). Taking into consideration
that in most cases of inoperable NSCLC the existing cytological
material is critical, we evaluated the real-time PCR detection of
EGFR mutations in exons 18, 19, 20 and 21 of adenocarcinoma
cells obtained via FNA, EBUS, TBNA, brushing procedures, and
thoracentesis. In the present study, we found that 10% of the 77
analyzed adenocarcinoma samples harbored EGFR mutations, 35%
of which carried KRAS mutations, while 51% of the 54 samples
analyzed for KRAS mutations were negative regarding both
mutation types. KRAS mutation results were moderately high
for the detection of KRAS mutations with cytomorphological
features of adenocarcinomas (12,13),
but are in accordance with the recently reported prevalence of
NSCLC patients with KRAS mutations (27%) detected using
COLD-PCR (14) and 36.9%
KRAS mutations detected in malignant pleural effusion of
lung adenocarcinoma in a Dutch population (15).
EGFR mutations detected using an assay based
on amplifying mutant-specific sequences (EGFR-RT52; EntroGen) were
confirmed by Sanger sequencing. KRAS mutations were detected
using high melting temperature (LightMix® kit; TIB
MolBiol), a method known to provide false-positive results
(16). Therefore all samples with
detected KRAS mutations were verified using another method,
KRAS Strip Assay. In fact, in the case of the two samples, the use
of high melting temperature yielded borderline results [c.35G>A
(G12D)], while following verification with a second method
(ViennaLab Diagnostics GmbH), the samples were evaluated as KRAS-WT
(data not shown). It should be taken into consideration that direct
sequencing is not always sensitive enough to detect mutant DNA
(17), and that high-resolution
melting analysis may also give false-negative results (18). Notably, two false-negative results
were found in cytological material with a small proportion of tumor
cells, indicating that cytologists should not only distinguish
benign and malignant samples, but they should also conduct proper
qualification of the material for molecular analysis (18). The real-time PCR analysis of
EGFR and KRAS mutations conducted in the present
study, led to identification of EGFR c.2582T>A (L861Q)
and KRAS c.35G>T (G12V), detected in 1,000–5,000 and
100–1,000 tumor cells, respectively. Thus, our results confirm that
cytological samples characterized by QS=C3+ or even C2+ should not
be evaluated as inadequate for EGFR mutation analysis using
real-time PCR, but should be carefully selected by a
cytopathologist.
Mutations in EGFR and KRAS detected in
tumors embedded in paraffin blocks and fresh-frozen tumor specimens
appear to be mutually exclusive (19). Our results obtained following
analysis of cytological material are consistent with this
observation (Table I and Fig. 1). Usually, EGFR mutations are
characteristic of tumors in the non-smoker groups of patients,
particularly among Asian women, while KRAS mutations are
often detected in smoking-associated cancer types. Therefore,
KRAS mutations have been suggested to constitute a primary
mechanism of resistance to gefitinib or erlotinib in lung
adenocarcinoma. This finding does not clarify whether this
insensitivity is due to the presence of the mutated KRAS or
the absence of the mutated EGFR(20). In contrast, the acquired resistance
to TKIs has been studied to a great extent, particularly due to
mutations in exon 20. The most frequent TKI mutation is EGFR
c.2369C>T (T790M) (20),
followed by insertions in exon 20 which may render the epidermal
receptor approximately 100-fold less sensitive to erlotinib or
gefitinib (21). Concerning the
findings of the present study, 1 adenocarcinoma patient was found
to carry both EGFR activating c.2573T>G and inhibiting
c.2369C>T mutations following analysis of material isolated via
the FNA procedure, indicating that the mutations were of activating
and inhibiting types, respectively. The structure of the wild-type
EGFR kinase domain has been published in both active and
inactive conformations, while its crystal structure has been
reported alone and in complex with erlotinib (22). Along with the discovery of the
EGFR c.2573T>G (L858R) EGFR mutant crystal structure,
which is TKI-sensitive, it was indicated that the substitution of
arginine for leucine at position 858 activates the kinase.
Comparison of the fold activity between the wild-type and the L858R
mutant enzyme demonstrated an approximately 50-fold higher activity
of the mutant conformation (23).
Further studies of gefitinib revealed that this
4-anilinoquinazoline inhibitor, structurally similar to erlotinib,
binds the EGFR c.2573T>G (L858R) mutant with a 20-fold
higher affinity compared to the wild-type enzyme. This higher
affinity for the EGFR c.2573T>G (L858R) mutant was
explained by tighter binding to the active conformation of the
kinase compared to the inactive conformation (23). In contrast, some patients were
reported to become resistant following TKI treatment due to
mutation in the ‘gatekeeper’ residue of threonine 790 (24,25).
Another inhibiting mutation in exon 21 is the A→G change, which
leads to the substitution of alanine for threonine at position 854
(5). Both mutations, the activating
EGFR c.2573T>G (L858R) and the inhibiting EGFR
c.2369C>T (T790M) were found in our cytological assessment.
The cytological samples of the present study
obtained via FNA, were found to carry both mutations EGFR
c.2573T>G and c.2369C>T (L858R/T790M), demonstrating the
advantage of the FNA procedure and the ability of performing serial
sampling of a given tumor to assess the efficacy of targeted
therapy or identify genetic shifts of adenocarcinoma with EGFR
mutations (3). This recommendation
does not exclude patients from targeted therapies, since patients
with the EGFR c.2369C>T (T790M) mutation of EGFR might
still respond to erlotinib after gefitinib treatment (6). In fact, the patient with EGFR
c.2573T>G (L858R) and EGFR c.2369C>T (T790M) was
treated with erlotinib and a short-term improvement was observed.
However, 7 months after the detection of the L858R/T790M mutation,
a CT scan of the chest and abdomen revealed metastases to both lung
and liver, resulting in the death of the patient.
According to a recent anticancer drug discovery
using a high-throughput cell-based assay, inhibitors of the
L858R/T790M mutant EGFR pathway were identified after screening
60,000 compounds (26). Those
findings may also allow classification of novel inhibitors which
suppress mutant EGFR c.2369C>T (T790M) signaling
(26). An additional study on a
classical protein kinase C inhibitor, revealed high potency against
the mutated EGFR and significantly reduced tumor growth in
an in vivo xenograft model employing an EGFR-mutant NSCLC
cell line containing the EGFR c.2369C>T (T790M) mutation
(27). Moreover, novel EGFR TKIs
which bind irreversibly to EGFR-TK and form covalent cross-links
with EGFR, such as afatinib (BIBW2992), have been demonstrated to
be active against tumors resistant to reversible EGFR TKI (28,29).
In conclusion, cytological material is useful for
the assessment of the EGFR mutation status for assessment of
personalized therapy with EGFR-TKIs. We demonstrated that the
real-time PCR methods used here may allow detection of activating
and inhibiting mutations. Furthermore, in cytology material with
>1,000 tumor cells in samples from a Polish Caucasian
population, there was a frequency of 10% EGFR mutations,
while in samples with a minimum of 5,000 tumor cells the frequency
was 12,24%. Our data suggest the presence of false-negative cases
within the EGFR WT results, obtained from the cytological
specimens with a small number of tumor cells (even up to 1,000).
Molecular EGFR and KRAS testing of cytological material could
provide also further information to oncologists and
pathomorphologists since the presence of EGFR mutations is
mutually exclusive of EML4-ALK transcript and low percentage
of KRAS mutation (11%) is found in NSCLC specimens carrying the
EML4-ALK transcripts (31).
Acknowledgements
We thank Andrzej Tysarowski and Jaroslaw Starzynski
for the technical support, the medical and nursing staff involved
in the daily care of the patients, as well as the staff involved in
the conduction of this study.
References
|
1
|
Milella M, Nuzzo C, Bria E, Sperduti I,
Visca P, Buttitta F, Antoniani B, Merola R, Gelibter A, Cuppone F,
D’Alicandro V, Ceribelli A, Rinaldi M, Cianciulli A, Felicioni L,
Malatesta S, Marchetti A, Mottolese M and Cognetti F: EGFR
molecular profiling in advanced NSCLC: a prospective phase II study
in molecularly/clinically selected patients pretreated with
chemotherapy. J Thorac Oncol. 7:672–680. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsao MS, Sakurada A, Cutz JC, Zhu CQ,
Kamel-Reid S, Squire J, Lorimer I, Zhang T, Liu N, Daneshmand M,
Marrano P, da Cunha Santos G, Lagarde A, Richardson F, Seymour L,
Whitehead M, Ding K, Pater J and Shepherd FA: Erlotinib in lung
cancer - molecular and clinical predictors of outcome. N Eng J Med.
353:133–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malapelle U, Bellevicine C, Zeppa P,
Palombini L and Troncone G: Cytology-based gene mutation tests to
predict response to anti-epidermal growth factor receptor therapy:
a review. Diagn Cytopathol. 39:703–710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bean J, Brennan C, Shih JY, Riely G, Viale
A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, Balak M, Chang
WC, Yu CJ, Gazdar A, Pass H, Rusch V, Gerald W, Huang SF, Yang PC,
Miller V, Ladanyi M, Yang CH and Pao W: MET amplification occurs
with or without T790M mutations in EGFR mutant lung tumors with
acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci
USA. 104:20932–20937. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bean J, Riely GJ, Balak M, Marks JL,
Ladanyi M, Miller VA and Pao W: Acquired resistance to epidermal
growth factor receptor kinase inhibitors associated with a novel
T854A mutation in a patient with EGFR-mutant lung adenocarcinoma.
Clin Cancer Res. 14:7519–7525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu SG, Shih JY, Yu CJ and Yang PC: Lung
adenocarcinoma with good response to erlotinib after gefitinib
treatment failure and acquired T790M mutation. J Thorac Oncol.
3:451–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garcia-Olivé I, Monsó E, Andreo F,
Sanz-Santos J, Taron M, Molina-Vila MA, Llatjós M, Castellà E,
Moran T, Bertran-Alamillo J, Mayo-de-Las-Casas C, Queralt C and
Rosell R: Endobronchial ultrasound-guided transbronchial needle
aspiration for identifying EGFR mutations. Eur Respir J.
35:391–395. 2010.PubMed/NCBI
|
|
8
|
Lewandowska MA, JóŸwicki W, Starzynski J
and Kowalewski J: Analysis of EGFR mutation frequency and
coexistence of KRAS and EGFR mutations using RT-PCR in lung
adenocarcinoma: may a clinical and pathological model of a
patient’s qualification for targeted therapy have an impact on time
to obtain genetic results? Pol J Cardiothorac Surg. 9:443–451.
2012.
|
|
9
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J and
Haber DA: Activating mutations in the epidermal growth factor
receptor underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Eng J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawahara A, Azuma K, Sumi A, Taira T,
Nakashima K, Aikawa E, Abe H, Yamaguchi T, Takamori S, Akiba J and
Kage M: Identification of non-small-cell lung cancer with
activating EGFR mutations in malignant effusion and cerebrospinal
fluid: rapid and sensitive detection of exon 19 deletion E746-A750
and exon 21 L858R mutation by immunocytochemistry. Lung Cancer.
74:35–40. 2011. View Article : Google Scholar
|
|
11
|
Yung TK, Chan KC, Mok TS, Tong J, To KF
and Lo YM: Single-molecule detection of epidermal growth factor
receptor mutations in plasma by microfluidics digital PCR in
non-small cell lung cancer patients. Clin Cancer Res. 15:2076–2084.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marotti JD, Schwab MC, McNulty NJ, Rigas
JR, Delong PA, Memoli VA, Tsongalis GJ and Padmanabhan V:
Cytomorphologic features of advanced lung adenocarcinomas tested
for EGFR and KRAS mutations: a retrospective review of 50 cases.
Diagn Cytopathol. 41:15–21. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dacic S, Shuai Y, Yousem S, Ohori P and
Nikiforova M: Clinicopathological predictors of EGFR/KRAS
mutational status in primary lung adenocarcinomas. Mod Pathol.
23:159–168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pennycuick A, Simpson T, Crawley D, Lal R,
Santis G, Cane P, Tobal K and Spicer J: Routine EGFR and KRAS
mutation analysis using COLD-PCR in non-small cell lung cancer. Int
J Clin Pract. 66:748–752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smits AJ, Kummer JA, Hinrichs JW, Herder
GJ, Scheidel-Jacobse KC, Jiwa NM, Ruijter TE, Nooijen PT,
Looijen-Salamon MG, Ligtenberg MJ, Thunnissen FB, Heideman DA, de
Weger RA and Vink A: EGFR and KRAS mutations in lung carcinomas in
the Dutch population: increased EGFR mutation frequency in
malignant pleural effusion of lung adenocarcinoma. Cell Oncol.
35:189–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Whitehall V, Tran K, Umapathy A, Grieu F,
Hewitt C, Evans TJ, Ismail T, Li WQ, Collins P, Ravetto P, Leggett
B, Salto-Tellez M, Soong R, Fox S, Scott RJ, Dobrovic A and
Iacopetta B: A multicenter blinded study to evaluate KRAS mutation
testing methodologies in the clinical setting. J Mol Diagn.
11:543–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smouse JH, Cibas ES, Janne PA, Joshi VA,
Zou KH and Lindeman NI: EGFR mutations are detected comparably in
cytologic and surgical pathology specimens of nonsmall cell lung
cancer. Cancer. 117:67–72. 2009.PubMed/NCBI
|
|
18
|
Nomoto K, Tsuta K, Takano T, Fukui T,
Yokozawa K, Sakamoto H, Yoshida T, Maeshima AM, Shibata T, Furuta
K, Ohe Y and Matsuno Y: Detection of EGFR mutations in archived
cytologic specimens of non-small cell lung cancer using
high-resolution melting analysis. Am J Clin Pathol. 126:608–615.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pao W, Wang TY, Riely GJ, Miller VA, Pan
Q, Ladanyi M, Zakowski MF, Heelan RT, Kris MG and Varmus HE: KRAS
mutations and primary resistance of lung adenocarcinomas to
gefitinib or erlotinib. PLoS Med. 2:e172005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Greulich H, Chen TH, Feng W, Jänne PA,
Alvarez JV, Zappaterra M, Bulmer SE, Frank DA, Hahn WC, Sellers WR
and Meyerson M: Oncogenic transformation by inhibitor-sensitive and
-resistant EGFR mutants. PLoS Med. 2:e3132005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stamos J, Sliwkowski MX and Eigenbrot C:
Structure of the epidermal growth factor receptor kinase domain
alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol
Chem. 277:46265–46272. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yun CH, Boggon TJ, Li Y, Woo MS, Greulich
H, Meyerson M and Eck MJ: Structures of lung cancer-derived EGFR
mutants and inhibitor complexes: mechanism of activation and
insights into differential inhibitor sensitivity. Cancer Cell.
11:217–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kwak EL, Sordella R, Bell DW,
Godin-Heymann N, Okimoto RA, Brannigan BW, Harris PL, Driscoll DR,
Fidias P, Lynch TJ, Rabindran SK, McGinnis JP, Wissner A, Sharma
SV, Isselbacher KJ, Settleman J and Haber DA: Irreversible
inhibitors of the EGF receptor may circumvent acquired resistance
to gefitinib. Proc Natl Acad Sci USA. 102:7665–7670. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kobayashi S, Boggon TJ, Dayaram T, Janne
PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG and Halmos
B: EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Eng J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin WH, Song JS, Lien TW, Chang CY, Wu SH,
Huang YW, Chang TY, Fang MY, Yen KJ, Chen CH, Chu CY, Hsieh HP,
Chen YR, Chao YS and Hsu JT: A high-throughput cell-based screening
for L858R/T790M mutant epidermal growth factor receptor inhibitors.
Anticancer Res. 32:147–151. 2012.PubMed/NCBI
|
|
27
|
Taube E, Jokinen E, Koivunen P and
Koivunen JP: A novel treatment strategy for EGFR mutant NSCLC with
T790M-mediated acquired resistance. Int J Cancer. 131:970–979.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li D, Ambrogio L, Shimamura T, Kubo S,
Takahashi M, Chirieac LR, Padera RF, Shapiro GI, Baum A,
Himmelsbach F, Rettig WJ, Meyerson M, Solca F, Greulich H and Wong
KK: BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective
in preclinical lung cancer models. Oncogene. 27:4702–4711. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gately K, O’Flaherty J, Cappuzzo F, Pirker
R, Kerr K and O’Byrne K: The role of the molecular footprint of
EGFR in tailoring treatment decisions in NSCLC. J Clin Pathol.
65:1–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lewandowska MA, JóŸwicki W and Zurawski B:
KRAS and BRAF mutation analysis in colorectal adenocarcinoma
specimens with a low percentage of tumor cells. Mol Diagn Ther.
17:193–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martelli MP, Sozzi G, Hernandez L,
Pettirossi V, Navarro A, Conte D, Gasparini P, Perrone F, Modena P,
Pastorino U, Carbone A, Fabbri A, Sidoni A, Nakamura S, Gambacorta
M, Fernández PL, Ramirez J, Chan JKC, Grigioni WF, Campo E, Pileri
SA and Falini B: EML4-ALK rearrangement in non-small cell lung
cancer and non-tumor lung tissues. Am J Pathol. 174:661–670. 2009.
View Article : Google Scholar : PubMed/NCBI
|