Introduction
Hepatocellular carcinoma (HCC) accounts for ~95% of
the primary liver cancer and is one of the malignant tumors of high
malignancy and poor prognosis. As shown in the epidemic disease
statistics, the incidence rate of liver cancer in the world ranks
no. 8 among all kinds of cancers, and its mortality ranks no. 4
with ~0.5–1 million deaths each year. Resection is universally
recognized as the best approach to cure liver cancer, but the total
5-year survival rate after the surgery is low with only 34.6% in
terms of large hepatocellular carcinoma and 62.9% in terms of small
hepatocellular carcinoma (1–4).
μ-opioid receptor (MOR) is the main member of the
opioid receptor super-family that is extensively distributed in the
body. It is found in studies that MOR activation promotes the
proliferation of many kinds of cells (5–8) and
facilitates the growth of some malignant tumors (9–12) such
as lung cancer, breast cancer, neuroblastoma and colon cancer. This
means MOR plays an important role in the proliferation and
differentiation of various kinds of cells. MOR is found to have
apparently higher expression in HCC cells than in the normal liver
cells. However, the role that MOR plays in HCC progress has not
been reported. In addition, little is known about the mechanism by
which MOR promotes tumor growth.
C-jun N-Terminal Kinase (JNK) signal pathway is one
of three parallel pathways in the center of the pathway of
mitogen-activated protein kinase (MAPK) that plays an important
role in regulating the tissue cell reaction, such as cell
proliferation and differentiation (13–15).
It has been demonstrated by previous studies that MKK7 and MKK4 are
two kinases at the upstream of JNK pathway which can activate the
JNK signal pathway via the phosphorylation of Tyr and Thr residue
(16). However, different from the
subtypes of other MAPK, MKK7 on the Thr residue is sufficient and
specific to activate JNK pathway which, in turn, activates
substrates such as transcription factors or pro-apoptotic proteins
(17). Moreover, various studies
have demonstrated that MKK7 is indispensable for JNK activation
(18). Because of the important
role MKK7 plays in JNK pathway activation, it is necessary to
explain its role in MOR against HCC.
The purpose of this study was to investigate the
impact of downregulating MOR on in vivo and in vitro
human liver cancer progress and to explore its possible molecular
mechanism. We found the downregulation of MOR was able to inhibit
the proliferation of hepatoma cells and significantly retarded the
tumor growth. Moreover, it was found that MKK7 phosphorylation
level was increased thus activating JNK. In summary, we have found
that downregulation of MOR was able through MKK7-JNK pathway to
inhibit the progress of human liver cancer in vivo and in
vitro.
Materials and methods
Experimental specimen
Sixty cases of HCC tissue excised in the surgery of
First Hospital of Jilin University from 2009 to 2010 were selected
for the experiment which had all been pathologically confirmed to
be HCC and the patients had not received any treatment prior to the
surgery. At the same time, 20 cases of normal liver tissues from
liver trauma and next to the hepatic hemangioma were selected for
comparative study. All the specimens have been discussed and
approved by the ethics committee of The First Hospital of Jilin
University.
Cells, antibodies and reagents
Human liver cancer HepG2 cells and LO2 cells were
purchased from ATCC (ATCC, USA), BEL7402 cells were a gift from
Laboratory of Molecular Biology of Chinese Academy of Sciences
(Shanghai, China). Lipofectamine 2000 was purchased from
Invitrogen, USA, siRNA was synthesized by GenePharma (Shanghai,
China), and RT-PCR kit [Takara RNA PCR kit (AMV) version 3.0] was
purchased from Takara Biotechnology Co., Ltd. (Japan). PI was
purchased from Sigma (USA), MOR phosphorylated MKK7, MKK7,
phosphorylated JNK and JNK antibody were all purchased from Cell
Signaling Technology (USA).
Cell culture
Cells after cell passage was inoculated in the DMEM
culture medium (Gibco-BRL, NY, USA) containing 10% fetal calf serum
(Hyclone Laboratories, UT, USA), 100 U/ml penicillin and 100 U/ml
streptomycin. Then it was cultured in the incubator containing 5%
CO2 and 95% oxygen at 37°C.
Cell viability
Cells of various experimental groups in logarithmic
growth phase were taken and 6 duplicate wells were set for each
group with a negative control group. All the cells were put into 5%
CO2 incubator for further culture prior to color
reaction. Each well was added with 20 μl of MTT (5 mg/ml) and
cultured in CO2 incubator for 4 h before the culture
solution was disposed of. DMSO (200 μl) was added to each well for
room temperature oscillation for 10 min and the OD values were
measured with microplate reader at a wavelength of 490 nm.
Immunohistochemical analysis
Paraffin specimen section was conventionally dewaxed
to water and dehydrated with gradient alcohol, for antigen
retrieval, incubated for 10 min in 3% H2O2
solution at room temperature. Drowise 5 μl of 10% goat serum was
added and then sealed for 10 min at room temperature. Rabbit
anti-human (50 μl) MOR monoclonal antibody (1:50) was added and
placed at 4°C overnight. Biotin labeled goat anti-human antibody
(50 μl) was added and incubated for 30 min at room temperature. DAB
stain with haematin was applied for 2 min with hydrochloric alcohol
differentiation. Dehydration was performed with gradient alcohol.
Evaluation was performed by microscopy and images were taken.
siRNA transfection
The cells were inoculated in the 6-well culture
plate at the density of 5×105/ml. Cells were cultured in
medium free of antibiotics to >60% confluence. The culture
medium free of serum and antibiotics was replaced. Purified cells
were transfected for 24 h with siRNAs to μ-opioid receptor (MOR
siRNAs; Santa Cruz Biotechnology, CA) at a concentration of 20 nM.
Transfected cells were placed into the CO2 incubator to
cultured for 4 h, then the culture medium with serum was used to
continue the culture. Non-specific Control siRNA was used as the
control group. siRNA interfering efficiency was tested with RT-PCR
and western blotting.
RNA extraction and RT-PCR
Instructions of the RNAiso™ Plus (Takara,
Japan) for total RNA extraction were followed, employing the RT-PCR
reagent (Takara) for RT-PCR reaction with the steps as shown in the
instructions. MOP forward primer: 5′-TCTGGCTCCAAAGAAAAGGA-3′,
reverse primer: 5′-CAATGCAGAAGTGCCAAGAA-3′. MKK7 forward primer:
5′-GCCAGACTGGGAAGAAATCTG-3′, reverse primer:
5′-GGCGGACACACACTCATAAA ACAGA-3′. β-actin forward primer:
5′-CTGGGACGACATGGAGA AAA-3′, reverse primer: 5′-AAGGAAGGCTGGAAGAG
TGC-3′. PCR reaction system 50 μl at the following reaction
conditions: 94°C 2 min, 94°C degeneration 30 sec, 58°C anneal 30
sec, 72°C extension 30 sec, total 31 cycles. The PCR product was
electrophoresed with 1.0% agarose gel, then scanned and analyzed
with gel imaging system.
Analysis of apoptosis
Trypsin was digested to collect the cells, and the
cells were washed twice with pre-cooled PBS, and single cell
suspension were prepared. Annexin V and PI staining fluid were
added, respectively, in accordance with the steps of the
instructions for apoptosis reagent kit (Annexin V-FITC kit, Biosea
Biotechnology Co., Beijing, China) for 15 min at room temperature
before the analysis with flow cytometry for apoptosis.
Analysis of cell cycle
Trypsin (0.25%) was digested to collect the cells
and washed with PBS 2 times, then fixed at 4°C with 70% cold
ethanol overnight. The ethanol was discarded the following day, and
2 more washes with PBS followed. PI comprehensive dye medium (1 ml)
(with 10 μg of RNase, and 5 μl of Triton X-100) was added, then
stored in a dark for 30 min at 4°C before analysis with flow
cytometry (Becton-Dickinson, USA).
Western blot assay
Total protein was separated with SDS-PAGE. Proteins
were placed to PVDF membranes, with the semidry method, and seal
with 5% skim milk powder at 4°C overnight. The membrane was washed
with TBST and the first antibody added at 37°C for hybridization
for 1 h. The second antibody was then applied at 37°C for
hybridization for 1 h, before the use of TBST and color reaction
for 5 min with autoradiography. Quantity One for optical density
value analysis and measurement was used. The results are shown as
specimen optical density value/β-actin optical density.
Nude mouse vaccination
Ten male nude mice, aged 6–8 weeks and weighing ~20
g were used. The anumals were cared for by the Animal Experimental
Center of Jilin University (Changchun, China). The animal
experimental plan was approved by the medical ethics committee of
Jilin University. The mice were randomly divided into two groups of
5 mice each. The animals were vaccinated with the cells of control
group and MOR siRNA group, respectively. Each mouse was injected
with 1×105 cells. The growth of the nude mice were
observed every day, and after four weeks the mice were sacrificed.
The subcutaneous transplanted tumor tissue was cut, under aseptic
condition, for index analysis.
Statistical analysis
SPSS 17.0 statistical software was used for the
statistical analysis. The values are shown as the mean ± SD. The
statistical analysis was performed using the Student’s t-test, and
the differences between the groups were considered to be
statistically significant at p<0.05.
Results
MOR expression in human hepatoma
carcinoma cells and tissues
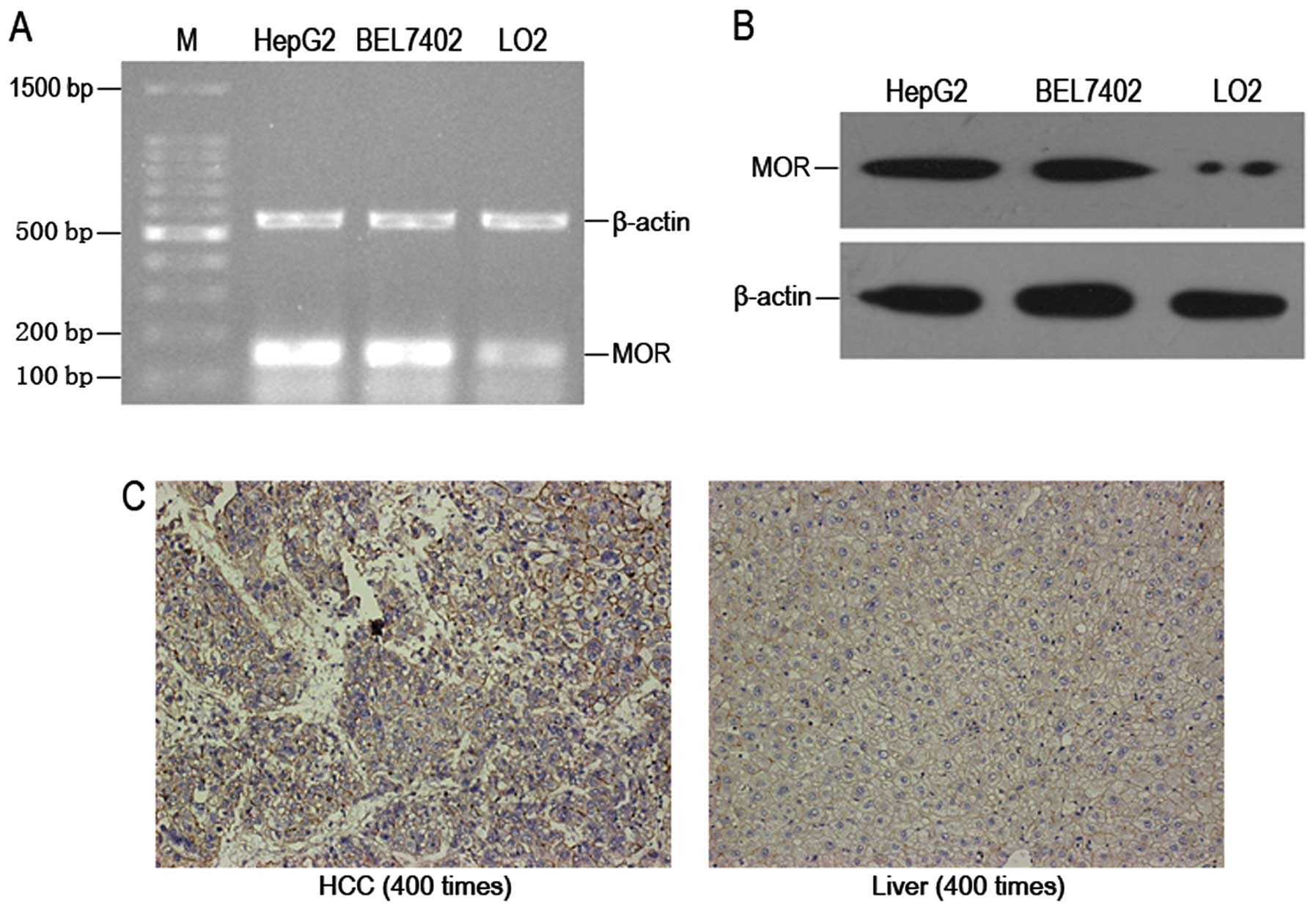
RT-PCR and western blotting were employed by us to
analyze the expression of MOR mRNA and protein in human hepatoma
carcinoma cells. It was found that there was high expression of MOR
mRNA in HepG2 cells and BEL7402 cells (Fig. 1A). The expression of MOR protein in
HepG2 cells and BEL7402 cells was similar to that of mRNA (Fig. 1B). Moreover, it was found by the
immunohistochemical analysis that MOR expression in human hepatoma
carcinoma tissues was obviously higher than that in normal human
liver tissues (Fig. 1C).
RNA interference to silence MOR gene
expression
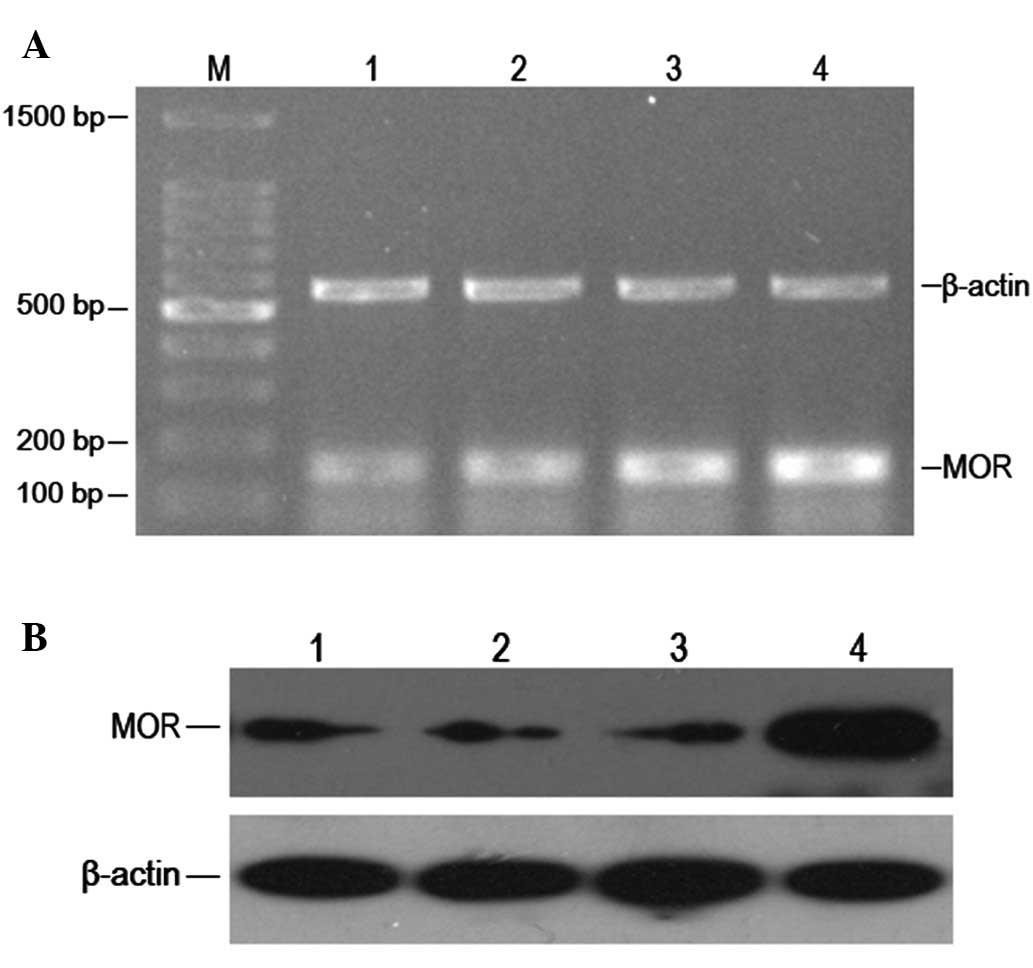
We used MOR siRNA and negative control
oligonucleotide to transfect HepG2 cells and observed the MOR mRNA
and protein expression of transfections under various conditions.
We found that 45 nM of MOR siRNA transfection for 24 h could
significantly reduce the expression level of MOR mRNA, and the
expression level of MOR protein declined accordingly. This
efficiency can endure at least for 72 h (Fig. 2A and B). This indicates that MOR
siRNA transfection could effectively silence the MOR gene and
affect the expression of MOR expression in turn.
Downregulating MOR to inhibit human
hepatoma carcinoma cell growth
In order to verify the impact of downregulating MOR
on human hepatoma carcinoma cell growth, Naloxone of various
concentrations (0, 0.1, 1.0, 10, 100 μM) and MOR siRNA of various
concentrations (0, 25, 35, 45, 55 nM) were administered to treat
human hepatoma carcinoma HepG2 cells for 24, 48 and 72 h before MTT
analysis on cell viability (Fig. 3A and
B). As indicated in the results, with the increase of the
concentrations of Naloxone and MOR siRNA, A490 value of HepG2 cells
reduced. Such a result means that downregulating MOR can inhibit
the growth of human hepatoma carcinoma cells.
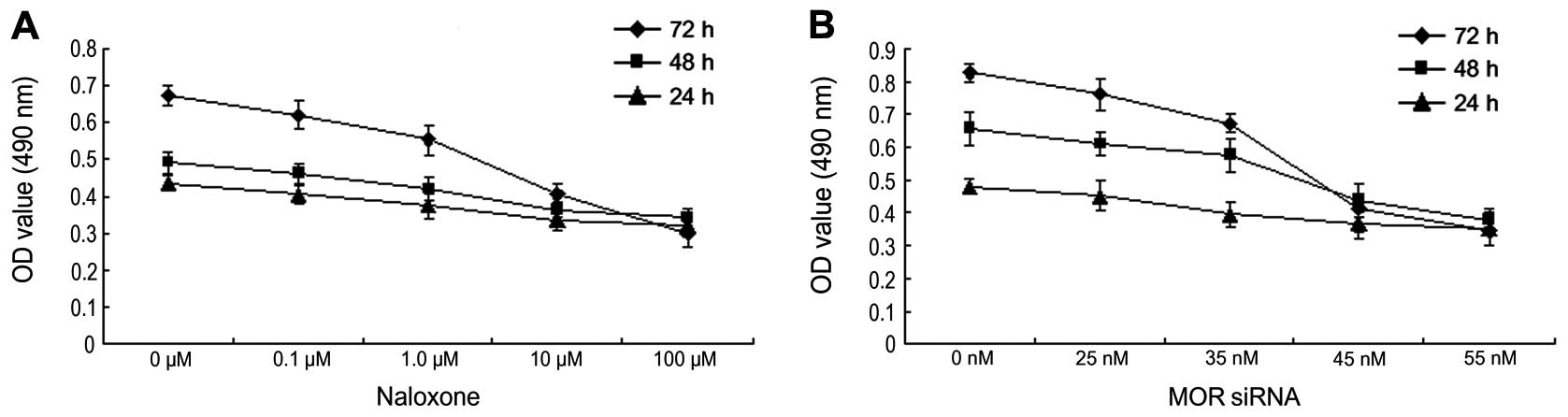 | Figure 3Downregulating MOP to inhibit human
hepatoma carcinoma cell growth. The results were repeated three
times. (A) MTT method to test cell viability after various
concentrations of Naloxone (0, 0.1, 1.0, 10, 100 μM) on HepG2 cells
for 24, 48 and 72 h. (B) MTT method to test cell viability after
various concentrations of MOP siRNA (0, 25, 35, 45, 55 nM) on HepG2
cells for 24, 48 and 72 h. |
Downregulation of MOR to promote
apoptosis of human hepatoma carcinoma cells
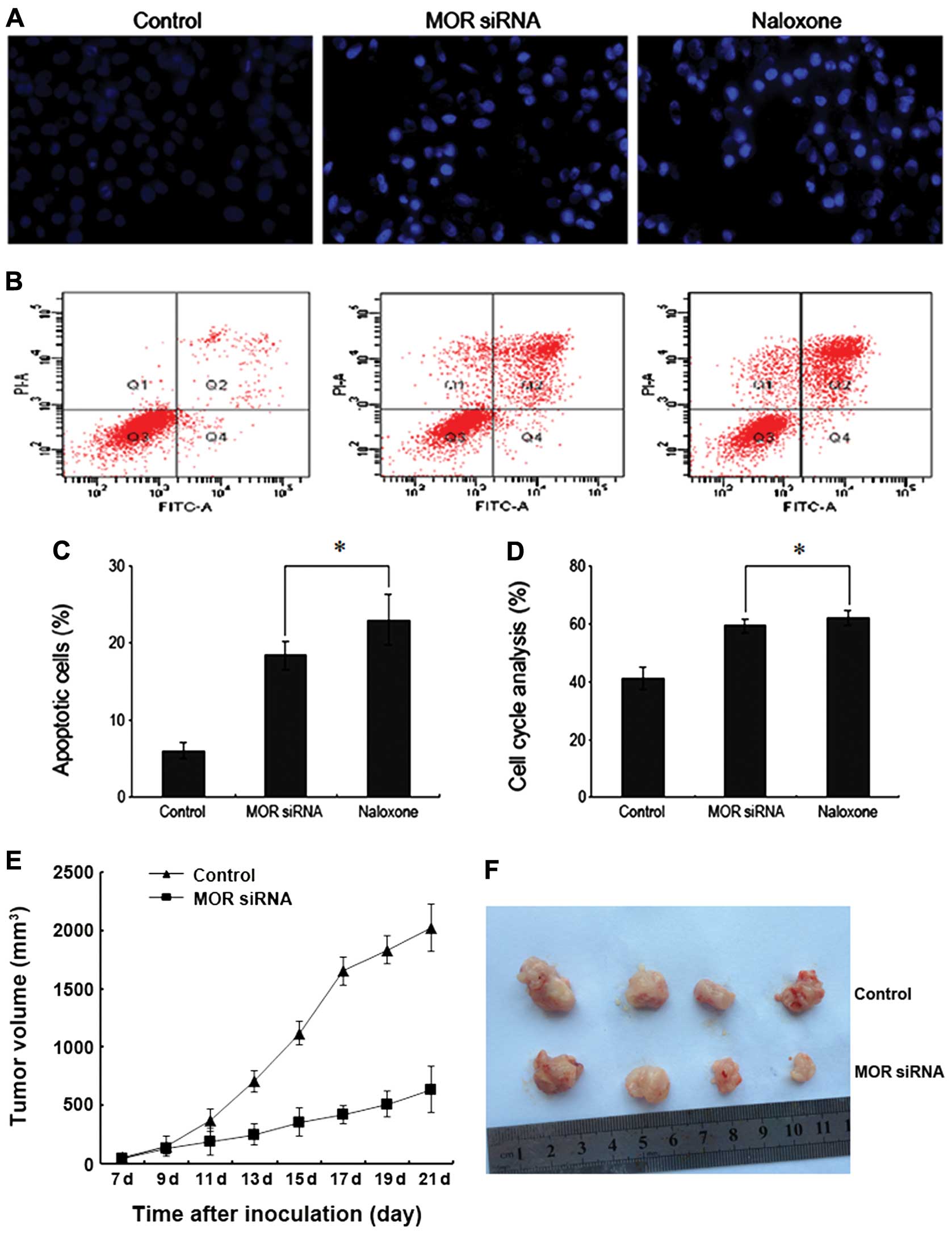
In order to explore whether downregulating MOR
results in apoptosis of human hepatoma carcinoma cells, we used
Hoechst 33342 stain and flow cytometry to observe apoptosis of
human hepatoma carcinoma cells. The results indicated that after
the administration of Naloxone and MOR siRNA to treat human liver
cancer HepG2 cells, the cell’s chromatin concentrated with the
brightness obviously higher than the normal control group (Fig. 4A). The apoptosis rate was 18.36 and
22.99%, respectively, which are both higher than the 6.6% of the
control group (Fig. 4B and C). This
indicates that downregulation of MOR can promote apoptosis of human
hepatoma carcinoma cell to inhibit the growth of human hepatoma
carcinoma cells in turn.
Downregulation of MOR to block the cycle
of human hepatoma carcinoma cells
In order to study whether downregulating MOR has any
impact on the progress of the cycle of human hepatoma carcinoma
cells, we used flow cytometry to analyze the cell cycle. The
results indicated that after HepG2 cells were treated with the
administration of Naloxone and MOR siRNA, the number of cells
remaining in the G0/G1 stage increased significantly and was higher
than that of the control group (Fig.
4D). Therefore, downregulation of MOR functions inhibiting the
progress of the human hepatoma carcinoma cell cycle, so as to
inhibit cell growth.
Downregulation of MOR to inhibit nude
mouse tumor growth
We used the nude mouse model to establish the in
vitro tumors. After 4 weeks, the five nude mice of the normal
control group all had tumors with the tumor formation rate of 100%.
Four nude mice in the MOR siRNA group had tumors with the tumor
formation rate of 80%. In addition, the tumors of the nude mice in
the control group grew faster than those in the MOR siRNA group
(Fig. 4E and F). These results
evidenced that downregulation of MOR can distinctly inhibit the
tumor progress in vivo.
Downregulation of MOR to increase
phosphorylated MKK7 expression
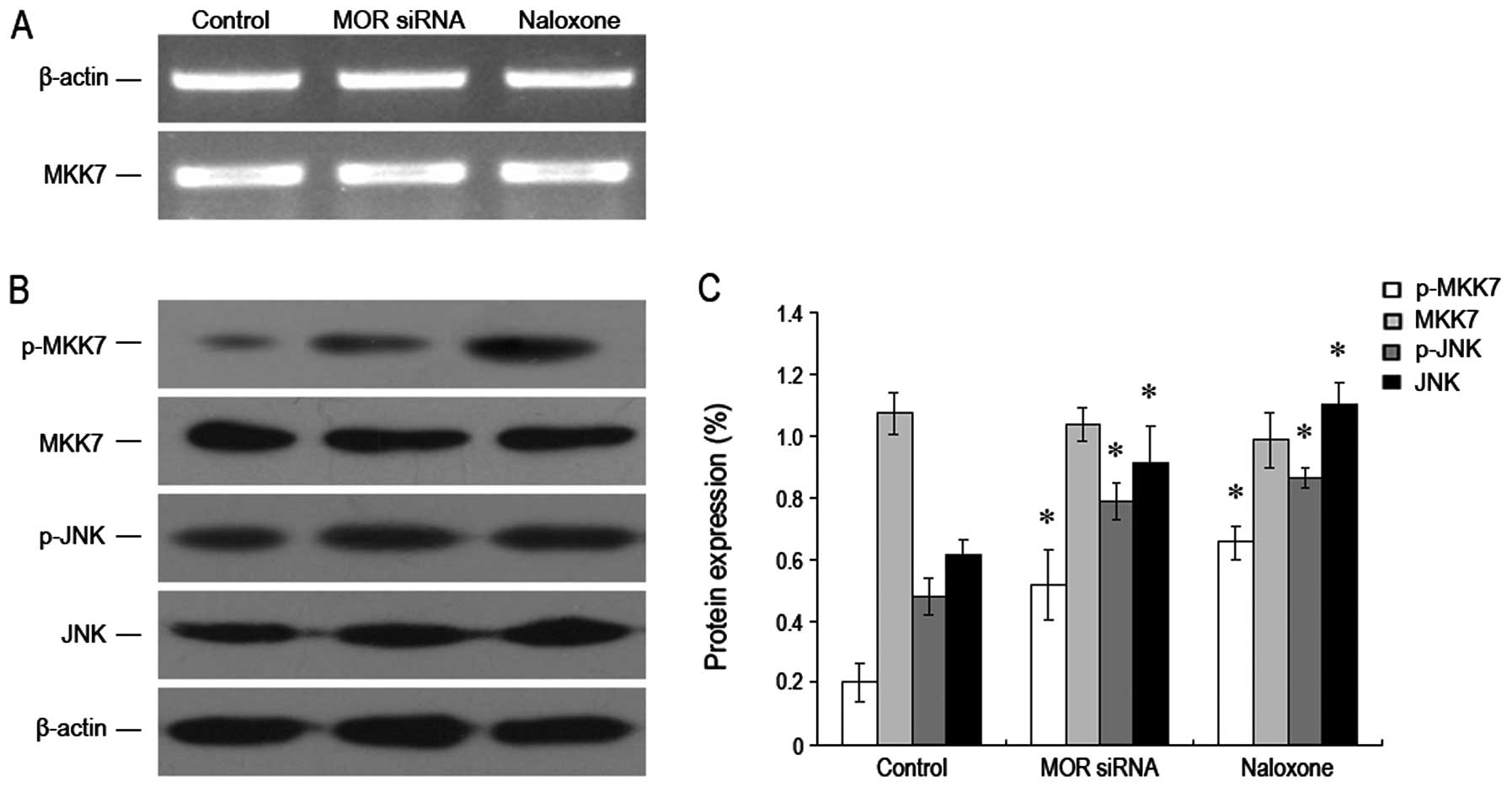
In order to verify the molecular mechanism for
downregulating MOR to inhibit the growth of human hepatoma
carcinoma cells, we used RT-PCR and western blotting, respectively,
to test the MKK7 mRNA and MKK7 proteins and their phosphorylation
levels. We found that after MOR was downregulated, the total
protein expression level of MKK7 mRNA and MKK7 was not obviously
changed, while the MKK7 phosphorylation level was significantly
increased and was higher than that of the control group. Moreover,
we found JNK and its phosphorylation level also increased
drastically (Fig. 5A–C). These
results show that downregulating MOR can increase the expression
level of phosphorylation MKK7 (but not for all MKK7), which results
in JNK and its phosphorylation level. This may be its molecular
mechanism to inhibit the growth of hepatoma carcinoma cells.
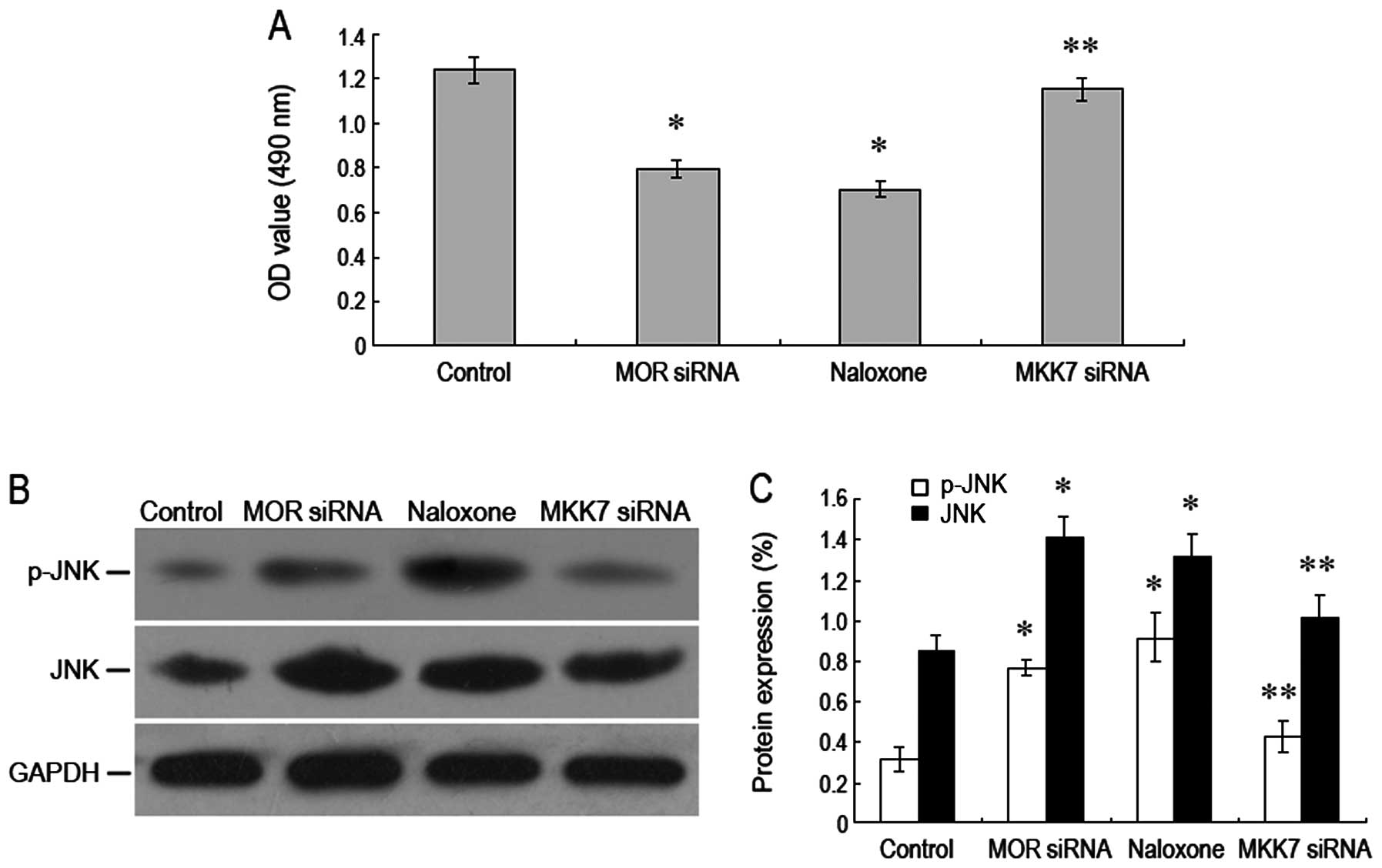
RNA interference role to silence MKK7 for
reversal of MOR to inhibit growth of human hepatoma carcinoma
cells
In order to demonstrate whether MKK7 plays a key
role in the inhibition of the growth of human hepatoma carcinoma
cells via downregulation of MOR, we used RNA interference to
silence MKK7 expression and observed the growth of human hepatoma
carcinoma cell. The results showed that after MKK7 was silenced,
A490-nm value of the hepatoma carcinoma cells was apparently on the
increase as compared to that in the group with downregulating MOR.
At the same time, JNK and its phosphorylation level dropped
significantly (Fig. 6A–C). Our data
indicated that the inhibition of the growth of human hepatoma
carcinoma cells via downregulation of MOR may be through the JNK
pathway mediated by the MKK7 pathway.
Discussion
μ-opioid receptor (MOR) is a member of the opioid
receptor super-family, and its role in antitumor has gained a great
deal of attention. Previous studies have shown that MOR can
participate in the progress of many solid tumors via affecting the
proliferation and apoptosis of tumor cells (9,10,12).
In this study, we mainly focused on exploring the impact of
downregulation of MOR on the progress of human liver cancer and its
molecular mechanism. Our study findings were similar to the above
reports. We found the expression level of MOR in human hepatoma
carcinoma cells and tissue were far higher than that in the normal
liver cells and tissue, and they were mainly on the surface of the
cell membrane. This indicated that MOR may participate, playing a
key role, in the human liver cancer development.
The impact of MOR on liver cancer was previously
unknown. However, MOR plays a significant role in protecting
neuronal cells, myocardial cells and intestinal mucosa cells.
Iglesias et al(19) found in
their studies, that MORactivation may prevent apoptosis of SH-SY5Y
cells and cerebral cortex neurons caused by serum withdrawal via
PI3-K/AKT signal pathway. Maslov et al(20) found in the cardiac
ischemia-reperfusion injury that MOR agonist plays a significant
role in protecting acute IRI and it is closely related to KATP
pathway. Gross et al(21)
reported that a neotype enkephalin derivative EP94 can reduce the
MI area of the rat via activating MOR. In addition, the studies of
Goldsmith et al(22)
demonstrated that MOR may promote the healing of the mouse acute
intestinal injury via activating the Stat3 pathway. Although there
might be a specific difference between the liver tumor cells and
the normal cells such as myocardial cells in our study, our study
findings are similar to those in the studies mentioned above. It is
indicated in our findings that the expression level of MOR in human
hepatoma carcinoma cells increased significantly, which means MOR
played a key role in the proliferation and differentiation of
hepatoma carcinoma cells. However, its specific role and relevant
mechanism deserve further verification.
To date, there have been few studies on the MOR in
the generation and progress of human tumors and many questions
remain to be effectively explained. Lennon et al(9) found in their studies that the
overexpression of MOR could promote the activation of Akt and mTOR
resulting in the tumor growth and metastasis. Mathew et
al(23) found high expression
of MOR in human lung cancer and MOR promotion of tumor progress. In
our study, we found downregulation of MOR could obviously inhibit
the proliferation of human hepatoma carcinoma cells with increased
cell apoptosis rate and the blockade of cell cycle in the G0/G1
stage. Moreover, in in vivo experiments, the growth of the
tumors of the mice retarded greatly indicating that MOR
overexpression in human hepatoma carcinoma cells and tissue plays a
positive regulation role in the progress of liver cancer.
Mitogen activated protein kinase (MAPK) signal
transduction pathway consists of a large family of kinases for the
cell to respond to exogenous and endogenous stimuli (24,25).
These three main MAPKs, JNK, p38 MAPK and ERK play important roles
in regulating the response of tissue cells (26). In addition, JNK is widely recognized
as the intracellular signal transduction enzyme associated with
cell proliferation, differentiation and apoptosis. The high
expression of JNK1 and JNK2 in tissues affect the development of
many types of cancer (27,28). As an important member of a
three-level cascade reaction, phosphorylated MKK7 may specifically
activate the JNK signal pathway, and regulate cell growth,
differentiation and apoptosis, as previously indicated (29). MKK7 (SEK2) is a component of MAPK
signal transduction pathway and a direct upstream kinase that
phosphorylates Thr-183 and Tyr-185 in JNK VIII area to activate JNK
to transduce extracellular stimulation signal to the cell and
nucleus for the cells to have a series of biological reactions.
Thus, their inactivation can block the JNK signal transduction
pathway and is a good approach to control cell damage.
Tang et al(30) found in the in vitro
experiments and studies that Alpinetin can inhibit the
proliferation of human hepatoma carcinoma cells and increase its
sensitivity to cisplatin, a chemotherapy drug via activating the
MKK7/JNK signal pathway. Therefore, the activation of MKK7 is a
crucial factor in our study. Previous studies demonstrated that MOR
can play its role via PTX-senstive G protein signal transduction
pathway (31) and KATP signal
transduction pathway (32).
Moreover, Wang et al(33)
found that MOR plays its regulating role via PKC/MAPK pathway. Our
results show that downregulation of MOR may result in the increase
of MKK7 phosphorylation to trigger the increase of JNK protein and
its phosphorylation level to inhibit the proliferation of cells. In
addition, we saw that after MKK7 was blocked by RNA interference,
degardless of downregulation of MOR or not, as the cell
proliferation was not affected. Therefore, our conclusion is that
the main function of downregulating MOR is to inhibit the
proliferation of human hepatoma carcinoma cells, while activated
MKK7 provides an important strategy for this.
Collectively, it is demonstrated by our study that
downregulation of MOR may activate JNK signal transduction pathway
via regulating MKK7 phosphorylation level so as to obviously
inhibit the proliferation of hepatoma carcinoma cells and cause
tumor growth retardation. MOR may make important contributions by
being a new approach to prevent and treat liver tumors.
Acknowledgements
We thank Dr Zhenran Wang for advice on the
manuscript preparation.
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rampone B, Schiavone B, Martino A, Viviano
C and Confuorto G: Current management strategy of hepatocellular
carcinoma. World J Gastroenterol. 15:3210–3216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rahbari NN, Mehrabi A, Mollberg NM, Müller
SA, Koch M, Büchler MW and Weitz J: Hepatocellular carcinoma:
current management and perspectives for the future. Ann Surg.
253:453–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Merle P and Mornex F: Medical therapies
for hepatocellular carcinoma. Cancer Radiother. 15:28–31. 2011.(In
French).
|
|
5
|
Kim E, Clark AL, Kiss A, Hahn JW,
Wesselschmidt R, Coscia CJ and Belcheva MM: Mu- and kappa-opioids
induce the differentiation of embryonic stem cells to neural
progenitors. J Biol Chem. 281:33749–33760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brillet K, da Conceição MM, Pattus F and
Pereira CA: Bioprocess parameters of cell growth and human mu
opioid receptor expression in recombinant Drosophila S2 cell
cultures in a bioreactor. Bioprocess Biosyst Eng. 28:291–293. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gein SV, Simonenko TA and Chereshnev VA:
Effect of beta-endorphin and DAGO, a selective agonist of mu-opioid
receptors, on the proliferative activity of lymphocytes. Dokl Biol
Sci. 391:289–291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harburg GC, Hall FS, Harrist AV, Sora I,
Uhl GR and Eisch AJ: Knockout of the mu opioid receptor enhances
the survival of adult-generated hippocampal granule cell neurons.
Neuroscience. 144:77–87. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lennon FE, Mirzapoiazova T, Mambetsariev
B, Salgia R, Moss J and Singleton PA: Overexpression of the
μ-opioid receptor in human non-small cell lung cancer promotes Akt
and mTOR activation, tumor growth, and metastasis. Anesthesiology.
116:857–867. 2012.
|
|
10
|
Bortsov AV, Millikan RC, Belfer I,
Boortz-Marx RL, Arora H and McLean SA: μ-Opioid receptor gene A118G
polymorphism predicts survival in patients with breast cancer.
Anesthesiology. 116:896–902. 2012.
|
|
11
|
Yuen JW, So IY, Kam AY and Wong YH:
Regulation of STAT3 by mu-opioid receptors in human neuroblastoma
SH-SY5Y cells. Neuroreport. 15:1431–1435. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nylund G, Pettersson A, Bengtsson C,
Khorram-Manesh A, Nordgren S and Delbro DS: Functional expression
of mu-opioid receptors in the human colon cancer cell line, HT-29,
and their localization in human colon. Dig Dis Sci. 53:461–466.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Molton SA, Todd DE and Cook SJ: Selective
activation of the c-Jun N-terminal kinase (JNK) pathway fails to
elicit Bax activation or apoptosis unless the phosphoinositide
3′-kinase (PI3K) pathway is inhibited. Oncogene. 22:4690–4701.
2003.
|
|
15
|
Dérijard B, Raingeaud J, Barrett T, Wu IH,
Han J, Ulevitch RJ and Davis RJ: Independent human MAP-kinase
signal transduction pathways defined by MEK and MKK isoforms.
Science. 267:682–685. 1995.
|
|
16
|
Kishimoto H, Nakagawa K, Watanabe T,
Kitagawa D, Momose H, Seo J, Nishitai G, Shimizu N, Ohata S,
Tanemura S, Asaka S, Goto T, Fukushi H, Yoshida H, Suzuki A, Sasaki
T, Wada T, Penninger JM, Nishina H and Katada T: Different
properties of SEK1 and MKK7 in dual phosphorylation of
stress-induced activated protein kinase SAPK/JNK in embryonic stem
cells. J Biol Chem. 278:16595–16601. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tournier C, Dong C, Turner TK, Jones SN,
Flavell RA and Davis RJ: MKK7 is an essential component of the JNK
signal transduction pathway activated by proinflammatory cytokines.
Genes Dev. 15:1419–1426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Destrument A and Tournier C:
Physiological roles of MKK4 and MKK7: insights from animal models.
Biochim Biophys Acta. 1773:1349–1357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iglesias M, Segura MF, Comella JX and
Olmos G: Mu-opioid receptor activation prevents apoptosis following
serum withdrawal in differentiated SH-SY5Y cells and cortical
neurons via phosphatidylinositol 3-kinase. Neuropharmacology.
44:482–492. 2003. View Article : Google Scholar
|
|
20
|
Maslov LN, Lasukova TV, Solenkova NV,
Lishmanov AIu, Bogomaz SA, Tam SV and Gross GJ: Participation of
K(ATP)-channels in cardioprotective effect of mu-opioid receptor
agonists in acute ischemia and reperfusion of the isolated heart.
Eksp Klin Farmakol. 64:23–27. 2001.(In Russian).
|
|
21
|
Gross GJ, Hsu A, Nithipatikom K, Bobrova I
and Bissessar E: Eribis peptide 94 reduces infarct size in rat
hearts via activation of centrally located μ opioid receptors. J
Cardiovasc Pharmacol. 59:194–197. 2012.PubMed/NCBI
|
|
22
|
Goldsmith JR, Uronis JM and Jobin C: Mu
opioid signaling protects against acute murine intestinal injury in
a manner involving Stat3 signaling. Am J Pathol. 179:673–683. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mathew B, Lennon FE, Siegler J,
Mirzapoiazova T, Mambetsariev N, Sammani S, Gerhold LM, LaRiviere
PJ, Chen CT, Garcia JG, Salgia R, Moss J and Singleton PA: The
novel role of the mu opioid receptor in lung cancer progression: a
laboratory investigation. Anesth Analg. 112:558–567. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galeotti N and Ghelardini C: Regionally
selective activation and differential regulation of ERK, JNK and
p38 MAP kinase signalling pathway by protein kinase C in mood
modulation. Int J Neuropsychopharmacol. 20:1–13. 2011.PubMed/NCBI
|
|
25
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bermudez O, Pagès G and Gimond C: The
dual-specificity MAP kinase phosphatases: critical roles in
development and cancer. Am J Physiol Cell Physiol. 299:C189–C202.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang P, Han J and Hui L: MAPK signaling
in inflammation-associated cancer development. Protein Cell.
1:218–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haeusgen W, Herdegen T and Waetzig V: The
bottleneck of JNK signaling: molecular and functional
characteristics of MKK4 and MKK7. Eur J Cell Biol. 90:536–544.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang B, Du J, Wang J, Tan G, Gao Z, Wang Z
and Wang L: Alpinetin suppresses proliferation of human hepatoma
cells by the activation of MKK7 and elevates sensitization to
cis-diammined dichloridoplatium. Oncol Rep. 27:1090–1096. 2012.
|
|
31
|
Giaroni C, Zanetti E, Vanti A, Canciani L,
Lecchini S and Frigo G: Sympathetic denervation-induced changes in
G protein expression in enteric neurons of the guinea pig colon.
Life Sci. 71:1961–1973. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen ZC, Shieh JP, Chung HH, Hung CH, Lin
HJ and Cheng JT: Activation of peripheral opioid μ-receptors in
blood vessel may lower blood pressure in spontaneously hypertensive
rats. Pharmacology. 87:257–264. 2011.
|
|
33
|
Wang Q and Traynor JR: Modulation of
μ-opioid receptor signaling by RGS19 in SH-SY5Y cells. Mol
Pharmacol. 83:512–520. 2013.
|