Introduction
Hypothyroidism has been considered an uncommon
adverse effect of cytotoxic anticancer treatment. Recently,
however, some small-molecule tyrosine kinase inhibitors (TKIs) have
been demonstrated to induce hypothyroidism and thyroid function
test abnormalities (1,2). The oral multitarget inhibitor
sunitinib, for example, has been associated with hypothyroidism in
36–85% of patients, ranging from transient increases in
thyroid-stimulating hormone (TSH) to persistent hypothyroidism
requiring thyroxine replacement therapy (2–5).
Sunitinib also induces thyrotoxicosis due to destructive
thyroiditis (6). Sorafenib is
reported to be responsible for hypothyroidism in 18% of patients
with metastatic renal-cell carcinoma (7,8), while
imatinib worsens preexisting hypothyroidism or causes de
novo hypothyroidism (9,10). A cohort study conducted in Germany
reported incidence rates of sunitinib- and sorafenib-induced
hypothyroidism of 24.2 and 12.1 per 100 person-years, respectively
(1). However, the effects of other
molecular target agents on thyroid function are unknown.
Common symptoms of thyroid hormone deficiency
include fatigue, cold intolerance, weight gain, constipation,
myalgia and menstrual irregularities. Because these typical
clinical manifestations lack specificity, however, the diagnosis of
hypothyroidism generally relies on laboratory tests. The United
States National Health and Nutrition Examination Survey reports
that the prevalence of hypothyroidism is 4.6%, of which 0.3% is
clinical and 4.3% is subclinical (11). Cancer-related fatigue is common in
patients receiving cytotoxic chemotherapy for cancer, and
prevalence exceeds 75% in patients with metastatic disease,
suggesting that thyroid dysfunction may be underdiagnosed and
undertreated in cancer patients. The management of thyroid
dysfunction and possible related symptoms, such as fatigue,
represents a challenge to oncologists (12).
Several mechanisms of the pathogenesis of
TKI-induced hypothyroidism have been considered. The most plausible
are destructive thyroiditis, an immune-mediated thyroiditis in
which the synthesis of thyroid hormones related to inhibition of
thyroid peroxidase activity is decreased; and drug-induced
regression of the gland vascular bed with significant capillary
alteration and reduction in density via the inhibition of vascular
endothelial growth factor (VEGF) signaling (2,5,7,13–15).
Regarding thyroid autoantibodies, the presence of thyroglobulin
antibodies was not associated with either the incidence or severity
of thyroid dysfunction (4).
Although it has been suggested that VEGFR inhibitors decrease
thyroid function by interfering with VEGF function or impairing
thyroid blood flow, the role of VEGF in thyroid signaling is
unknown (16).
Bevacizumab, a monoclonal antibody for VEGF-A which
thus differs from anti-angiogenic TKIs, is now approved for the
treatment of colorectal cancer, non-squamous non-small cell lung
cancer, and breast cancer, in combination with chemotherapy
(17–19). Although bevacizumab’s
anti-angiogenic effect might also induce thyroid dysfunction, the
effect of bevacizumab on thyroid function has not been investigated
(20). Here, we evaluated thyroid
function in patients receiving bevacizumab in combination with
fluoropyrimidine-based chemotherapy and the effect of a VEGF
monoclonal antibody on thyroid function.
Materials and methods
Patients and evaluation of thyroid
function
The study was conducted under an observational
design in patients with colorectal cancer who received
fluoropyrimidine-based chemotherapy at Kobe University Hospital.
Eligibility criteria were age ≥18 years, histologically confirmed
adenocarcinoma of the colorectum, Eastern Cooperative Oncology
Group performance status of 0 to 2, and no previous systemic
chemotherapy except adjuvant therapy. A history of
fluoropyrimidine-based adjuvant chemotherapy was allowed if disease
recurrence occurred within 6 months after completion. Patients with
a history of hypothyroidism, hyperthyroidism, or thyroid hormone
replacement therapy were excluded. Administration of bevacizumab
was at the discretion of the attending physician. The study was
approved by the institutional review board of Kobe University
Hospital.
All patients were evaluated for serum TSH and free
thyroxine (fT4) levels at baseline and monthly thereafter. Fatigue,
lethargy, intolerance to cold or heat, body weight change,
disturbed sweating and menstrual irregularities possibly associated
with thyroid dysfunction were recorded by the attending physician.
The decision to initiate thyroid replacement therapy was made by
the attending physician in accordance with clinical
presentation.
Treatment schedule
Patients received fluoropyrimidine-based
chemotherapy at the discretion of the attending physician, namely
with mFOLFOX6, FOLFIRI, XELOX, or capecitabine monotherapy.
mFOLFOX6 consisted of concurrent folic acid (200 mg/m2)
and oxaliplatin (85 mg/m2) followed by a bolus injection
of 5-fluorouracil (5FU) (400 mg/m2) on day 1 and
subsequent continuous infusion of 5FU (2400 mg/m2) over
46 h, repeated every 2 weeks. XELOX consisted of oxaliplatin (130
mg/m2) on day 1 followed by oral capecitabine (1,000
mg/m2) twice daily on days 1 to 14, repeated every 3
weeks. To prevent serious peripheral sensory neuropathy,
oxaliplatin was administrated using the stop-and-go strategy in
patients with metastatic colorectal cancer and omitted in any
patient with neuropathy of grade 2 or more. FOLFIRI consisted of
concurrent folic acid (200 mg/m2) and irinotecan (150
mg/m2) followed by a bolus injection of 5FU (400
mg/m2) on day 1 and subsequent continuous infusion of
5FU (2,400 mg/m2) over 46 h, repeated every 2 weeks.
Irinotecan dose in FOLFIRI was 150 mg/m2, the approved
dose in Japan. Capecitabine monotherapy consisted of oral
capecitabine (1,250 mg/m2) twice daily on days 1 to 14,
repeated every 3 weeks.
Treatment was continued until disease progression,
death, completion of adjuvant chemotherapy for 24 weeks,
unacceptable toxicity, or patient refusal, whichever came first.
Clinical and laboratory toxicity were graded according to the
National Cancer Institute Common Terminology Criteria for Adverse
Events v3.0. Grade 3 or 4 toxic effects were managed by dose
modification and appropriate supportive care. Tumor response was
assessed with the Response Evaluation Criteria in Solid Tumor
(RECIST) v1.0 every 8 to 12 weeks, or sooner if disease progression
was suspected (21).
Definition of hypothyroidism
The biochemical diagnosis of subclinical and
clinical hypothyroidism was determined in accordance with the
guidelines of the American Thyroid Association (ATA), the American
Association of Clinical Endocrinologists (AACE) and the Endocrine
Society. Subclinical hypothyroidism was considered as a serum TSH
level above the upper limit of normal, with fT4 within normal
limits. Clinical hypothyroidism was defined as low serum fT4
together with elevated TSH. Patients with at least two consecutive
TSH measurements >10 μU/ml and those with TSH above the upper
limit of normal and symptoms compatible with hypothyroidism (e.g.,
fatigue, cold intolerance, constipation or weight gain) received
thyroid hormone replacement therapy with L-thyroxine (22). Normal ranges in our laboratory are
0.45–3.81 μU/ml for TSH and 0.82–1.22 ng/ml for fT4.
Statistical analysis
TSH and fT4 levels of patients who received
fluoropyrimidine-based chemotherapy with or without bevacizumab
were compared using the Mann-Whitney U test, and differences in
clinicopathological variables were examined using Fisher’s exact
test. All analyses were performed using the SPSS statistical
package (SPSS v19.0 for Windows; SPSS, Inc., Chicago, IL, USA).
Results
Patient demographics
A total of 27 patients with colorectal cancer were
enrolled between October 2007 and January 2010. Median follow-up
time was 51 weeks (12–192). Patient demographics and baseline
characteristics are shown in Table
I. Of the total patients, 21 received mFOLFOX6, 3 received
FOLFIRI, 2 received XELOX and 1 received capecitabine monotherapy.
Sixteen patients (59.3%) received bevacizumab in combination with
chemotherapy. Combination therapy with bevacizumab was not carried
out due to adjuvant chemotherapy in 4 patients, presence of
hemorrhagic lesions in 3, use of anticoagulants in 2 and advanced
age and Parkinson’s disease as a complication in 1 each. The median
number of chemotherapy cycles in all patients was 12 (range, 2–35),
with 12 (2–35) cycles in the bevacizumab group and 10 (5–27) in the
chemotherapy-alone group. Treatment was discontinued due to disease
progression in 12 (44.4%) patients and adverse events in 8 (29.6%),
while the remaining 7 patients completed adjuvant therapy.
 | Table IPatient demographics and baseline
characteristics. |
Table I
Patient demographics and baseline
characteristics.
| Chemotherapy +
bevacizumab | Chemotherapy | P-value |
|---|
| n=16 | n=11 | |
|---|
| Age, years | | | |
| Median (range) | 63 (42–77) | 71 (37–79) | 0.24 |
| Gender | | | |
| Male/female | 11/5 | 8/3 | 0.82 |
| ECOG PS | | | |
| 0/1/2 | 8/7/1 | 5/3/3 | 0.78 |
| Primary site | | | |
| Colon/rectum | 8/8 | 5/6 | 0.82 |
| Treatment cycles | | | |
| Median (range) | 12 (2–35) | 10 (5–27) | 0.74 |
| Reason for
discontinuation | | | |
| Progression | 7 | 5 | |
| Completion of
adjuvant chemotherapy | 4 | 3 | |
| Adverse events | 5 | 3 | |
Thyroid function
Thyroid hormone parameters are provided in Table II. No patients had fatigue or other
symptoms associated with thyroid dysfunction at baseline. At
baseline, the mean TSH level was 1.72 μU/ml in the bevacizumab
group and 2.00 μU/ml in the chemotherapy-alone group. Three (11.1%)
patients who received fluoropyrimidine-based chemotherapy developed
a TSH level >10 μU/ml and 13 (48.1%) developed an elevation
above the upper limit of the normal range. In the limited
observational period, a considerable number of patients developed
clinical and subclinical hypothyroidism irrespective of bevacizumab
use. Eight of 16 patients (50%) in the bevacizumab group and 5 of
11 (45%) in the chemotherapy-alone group experienced elevation of
TSH above the upper normal limit (P=1.00), while 2 (12.5%) in the
bevacizumab group and 1 (20%) in the chemotherapy-alone group
developed levels >10 μU/ml (P=1.00). There were no differences
in TSH and fT4 level between the bevacizumab and chemotherapy-alone
groups. One of 3 patients in the bevacizumab group who developed a
TSH level >10 μU/ml showed only a transient increase in TSH
(13.62 μU/ml) and decrease in free T4 (0.76 ng/ml), and these
levels were improved the next month without any treatment. The
remaining 2 patients received thyroid hormone replacement therapy
with L-thyroxine, as detailed below. In 12 patients with TSH levels
above the upper limit of normal, median time to exceeding this
level was 18 (4–64) weeks. Median time to highest TSH level was 25
(4–53) weeks.
 | Table IIThyroid hormone parameters. |
Table II
Thyroid hormone parameters.
| Chemotherapy +
bevacizumab | Chemotherapy | P-value | All patients | P-value |
|---|
| n=16 | n=11 | | n=27 | |
|---|
| TSH (0.45–3.81
μU/ml) |
| At baseline | 1.72±0.97 | 2.00±0.86 | 0.31 | 1.83±0.92 | |
| Two months after
treatment | 2.47±1.55 | 2.64±1.91 | 0.92 | 2.54±1.68 | 0.06a |
| Ratio of TSH at
baseline and 2 months after treatment | 1.51±0.64 | 1.31±0.52 | 0.32 | | |
| Maximum TSH | 4.54±3.56 | 6.31±8.42 | 0.81 | 5.26±5.95 | 0.005a |
| fT4 (0.82–1.22
ng/ml) |
| At baseline | 1.15±0.20 | 1.15±0.25 | 0.94 | 1.15±0.22 | |
| Two months after
treatment | 1.13±0.15 | 1.16±0.22 | 0.61 | 1.14±0.18 | 0.89a |
| Patients with TSH
above ULN, n (%) | 8 (50) | 5 (45) | 1.00 | 13 (48.1) | |
| Patients with TSH
>10 μU/ml, n (%) | 2 (12.5) | 1 (9) | 1.00 | 3 (11.1) | |
Case presentation 1 (Fig. 1A)
A 71-year-old male was diagnosed with sigmoid colon
cancer (T3N1) with solitary liver metastasis. He underwent surgical
resection for the primary tumor and liver metastasis, and then
received adjuvant chemotherapy with mFOLFOX plus bevacizumab for a
total of 12 courses. After 11 courses, he experienced an increase
in TSH (11.48 μU/ml) and decrease in fT4 (0.81 ng/ml), with the
increase in TSH maintained the next month (10.1 μU/ml). He reported
grade 1 fatigue, which resolved following thyroid hormone
replacement therapy. Two years after completing adjuvant
chemotherapy, he is alive without recurrence and is still receiving
hormone replacement therapy.
Case presentation 2 (Fig. 1B)
A 71-year-old male was diagnosed with colorectal
cancer with multiple liver and lymph node metastases. Colonoscopy
revealed an easily bleeding ulcerative lesion on the ascending
colon. After seven courses of mFOLFOX therapy, the TSH level was
10.06 μU/ml but fT4 level was normal (1.15 ng/ml). After 10
courses, he had grade 2 fatigue and grade 2 peripheral sensory
neuropathy. Because TSH was increased to 14.2 μU/ml, he received
thyroid hormone replacement therapy and continued chemotherapy but
with oxaliplatin omitted. CT at 7 months of treatment showed
progression of liver metastases, for which he received second-line
chemotherapy with irinotecan, but he died of primary disease at 11
months of treatment.
Discussion
In this observational study in patients with
colorectal cancer, we found that bevacizumab seemed to have no
effect on thyroid function, whereas fluoropyrimidines did induce
thyroid dysfunction. All cases of fluoropyrimidine-induced
hypothyroidism appeared to be controllable by thyroid hormone
replacement therapy. Further investigation is needed to clarify the
mechanism of fluoropyrimidine-induced thyroid dysfunction.
In this analysis, 3 (11.1%) patients who received
fluoropyrimidine-based chemotherapy developed TSH levels >10
μU/ml and 13 (49.3%) showed elevation above the upper limit of the
normal range. Considering the median follow-up time of 51 weeks
(range, 12–192), this high incidence of thyroid dysfunction is
unusual. To our knowledge, an association between colorectal cancer
and thyroid dysfunction has not been previously reported. One study
reported a significant increase in T4 and T3 levels in 15 patients
with advanced breast cancer during weekly treatment with 5FU, but
no change in fT4 or TSH and no clinical signs of hyperthyroidism
(23). However, administration of
fluoropyrimidines differed in this previous and our present
study.
The mechanism of this putative
fluoropyrimidine-induced thyroid dysfunction is currently unclear,
as is that of TKI-induced thyroid dysfunction. Although our study
did not measure fT3 and rT3, we hypothesize that thyroid
dysfunction associated with fluoropyrimidines may be due to altered
thyroid hormone metabolism, which also occurs with sorafenib
(7). This effect might be related
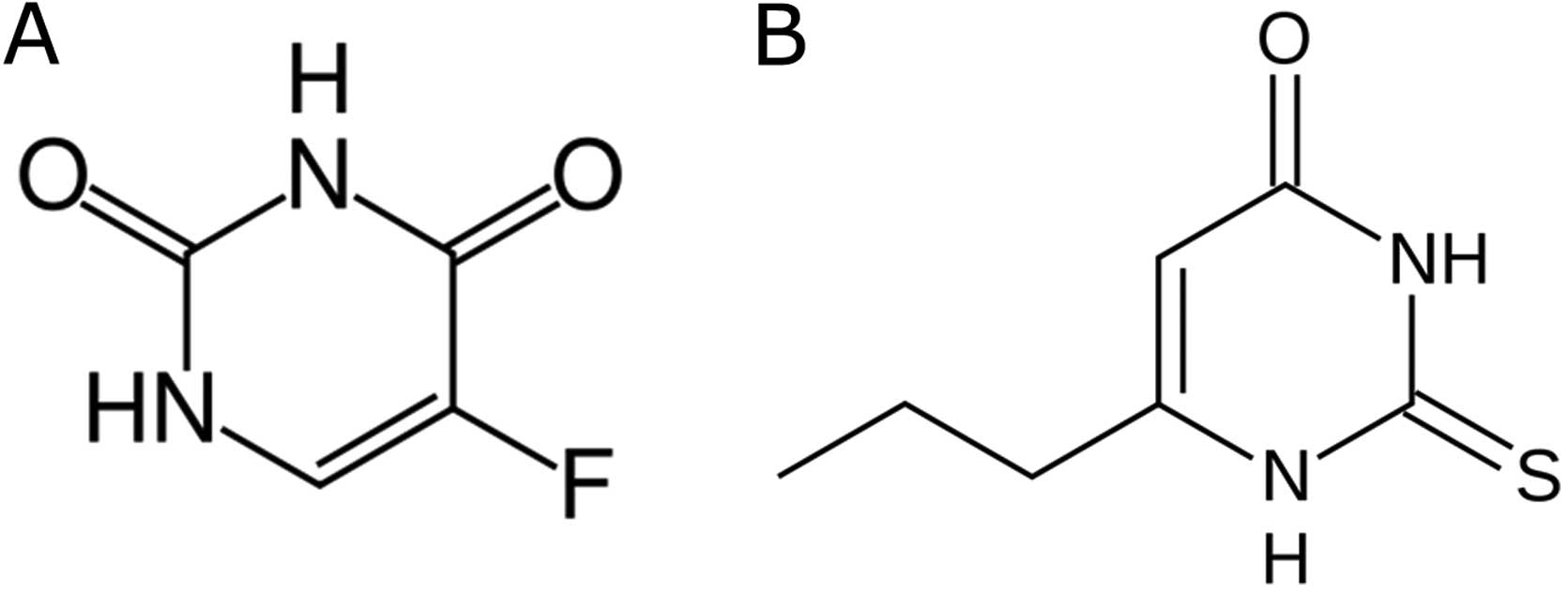
to the structural similarity between 5FU and propylthiouracil
(Fig. 2). Capecitabine is also a
prodrug that is enzymatically converted to 5FU in tumors.
Propylthiouracil, a thioamide drug used to treat hyperthyroidism,
inhibits the thyroperoxidase that liberates iodine for addition
onto tyrosine residues on thyroglobulin for the production of T4 or
T3, as well as thyroid hormones, and also inhibits the enzyme
5′-deiodinase, which converts T4 to the active form T3. Further
basic and clinical investigation is required to clarify the
mechanism of fluoropyrimidine-induced thyroid dysfunction.
Bevacizumab is classified as a monoclonal antibody
and anti-angiogenesis drug which binds to and neutralizes VEGF. In
contrast, sunitinib and sorafenib are small-molecule compounds
which inhibit cellular signaling by targeting multiple receptor
tyrosine kinases, including VEGF receptors. It is suggested that
sunitinib and sorafenib decrease thyroid function by interfering
with VEGF function or impairing thyroid blood flow (2,24). In
several reports, however, including the present analysis,
bevacizumab did not appear to be associated with altered thyroid
homeostasis (25,26). Based on this finding, the
neutralization of VEGF would therefore have no significant effect
on thyroid function.
In the present study, 2 patients who experienced
hypothyroidism were effectively treated with hormone replacement
therapy, as judged from measured TSH levels. Administration of
thyroid hormone replacement therapy reversed the
fluoropyrimidine-induced severe fatigue in these patients.
In conclusion, bevacizumab did not affect thyroid
function in these patients with colorectal cancer, whereas
fluoropyrimidines did induce thyroid dysfunction. Thyroid hormone
replacement therapy may relieve the symptoms of
fluoropyrimidine-induced hypothyroidism. Further investigation is
required to clarify the mechanism of fluoropyrimidine-induced
thyroid dysfunction.
Acknowledgements
The authors are grateful to the physicians, nurses
and other medical staff of the Medical Oncology/Hematology unit of
Kobe University Hospital and the Graduate School of Medicine for
their support.
References
|
1
|
Feldt S, Schüssel K, Quinzler R, et al:
Incidence of thyroid hormone therapy in patients treated with
sunitinib or sorafenib: a cohort study. Eur J Cancer. 48:974–981.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torino F, Corsello SM, Longo R, Barnabei A
and Gasparini G: Hypothyroidism related to tyrosine kinase
inhibitors: an emerging toxic effect of targeted therapy. Nat Rev
Clin Oncol. 6:219–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Desai J, Yassa L, Marqusee E, et al:
Hypothyroidism after sunitinib treatment for patients with
gastrointestinal stromal tumors. Ann Intern Med. 145:660–664. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rini BI, Tamaskar I, Shaheen P, et al:
Hypothyroidism in patients with metastatic renal cell carcinoma
treated with sunitinib. J Natl Cancer Inst. 99:81–83. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong E, Rosen LS, Mulay M, et al:
Sunitinib induces hypothyroidism in advanced cancer patients and
may inhibit thyroid peroxidase activity. Thyroid. 17:351–355. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Faris JE, Moore AF and Daniels GH:
Sunitinib (Sutent)-induced thyrotoxicosis due to destructive
thyroiditis: a case report. Thyroid. 17:1147–1149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abdulrahman RM, Verloop H, Hoftijzer H, et
al: Sorafenib- induced hypothyroidism is associated with increased
type 3 deiodination. J Clin Endocrinol Metab. 95:3758–3762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tamaskar I, Bukowski R, Elson P, et al:
Thyroid function test abnormalities in patients with metastatic
renal cell carcinoma treated with sorafenib. Ann Oncol. 19:265–268.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Groot JW, Zonnenberg BA, Plukker JT,
van Der Graaf WT and Links TP: Imatinib induces hypothyroidism in
patients receiving levothyroxine. Clin Pharmacol Ther. 78:433–438.
2005.PubMed/NCBI
|
|
10
|
de Groot JW, Zonnenberg BA, van
Ufford-Mannesse PQ, et al: A phase II trial of imatinib therapy for
metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab.
92:3466–3469. 2007.PubMed/NCBI
|
|
11
|
Hollowell JG, Staehling NW, Flanders WD,
et al: Serum TSH, T(4), and thyroid antibodies in the United States
population (1988 to 1994): National Health and Nutrition
Examination Survey (NHANES III). J Clin Endocrinol Metab.
87:489–499. 2002. View Article : Google Scholar
|
|
12
|
Eisen T, Sternberg CN, Robert C, et al:
Targeted therapies for renal cell carcinoma: review of adverse
event management strategies. J Natl Cancer Inst. 104:93–113. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iavarone M, Perrino M, Viganò M,
Beck-Peccoz P and Fugazzola L: Sorafenib-induced destructive
thyroiditis. Thyroid. 20:1043–1044. 2010. View Article : Google Scholar
|
|
14
|
Kappers MH, van Esch JH, Smedts FM, et al:
Sunitinib-induced hypothyroidism is due to induction of type 3
deiodinase activity and thyroidal capillary regression. J Clin
Endocrinol Metab. 96:3087–3094. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shinohara N, Takahashi M, Kamishima T, et
al: The incidence and mechanism of sunitinib-induced thyroid
atrophy in patients with metastatic renal cell carcinoma. Br J
Cancer. 104:241–247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim S, Yazici YD, Calzada G, et al:
Sorafenib inhibits the angiogenesis and growth of orthotopic
anaplastic thyroid carcinoma xenografts in nude mice. Mol Cancer
Ther. 6:1785–1792. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saltz LB, Clarke S, Díaz-Rubio E, et al:
Bevacizumab in combination with oxaliplatin-based chemotherapy as
first-line therapy in metastatic colorectal cancer: a randomized
phase III study. J Clin Oncol. 26:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sandler A: Bevacizumab in non-small cell
lung cancer. Clin Cancer Res. 13:s4613–s4616. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miller K, Wang M, Gralow J, et al:
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic
breast cancer. N Engl J Med. 357:2666–2676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van Meter ME and Kim ES: Bevacizumab:
current updates in treatment. Curr Opin Oncol. 22:586–591.
2010.PubMed/NCBI
|
|
21
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cooper DS: Clinical practice. Subclinical
hypothyroidism. N Engl J Med. 345:260–265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beex L, Ross A, Smals A and Kloppenborg P:
5-fluorouracil-induced increase of total serum thyroxine and
triiodothyronine. Cancer Treat Rep. 61:1291–1295. 1977.PubMed/NCBI
|
|
24
|
Kamba T and McDonald DM: Mechanisms of
adverse effects of anti-VEGF therapy for cancer. Br J Cancer.
96:1788–1795. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang JF, Milosveski V, Schramek C, Fong
GH, Becks GP and Hill DJ: Presence and possible role of vascular
endothelial growth factor in thyroid cell growth and function. J
Endocrinol. 157:5–12. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gordon MS, Margolin K, Talpaz M, et al:
Phase I safety and pharmacokinetic study of recombinant human
anti-vascular endothelial growth factor in patients with advanced
cancer. J Clin Oncol. 19:843–850. 2001.PubMed/NCBI
|