Introduction
Colorectal cancer (CRC) is one of the most
frequently diagnosed malignancies in both men and women worldwide,
and CRC is the second most common cause of cancer-related mortality
in developed countries such as the USA (1). Traditional and combined therapeutic
approaches (surgery, chemotherapy and radiation) for curing CRC
give rise to improvements in progression-free and overall survival,
yet, the outcome of this common malignancy remains unsatisfactory
mainly due to metastasis or recurrent disease, poor understanding
of the mechanism of CRC development and the lack of specific target
gene therapy (2,3). In recent years, multiple studies have
been conducted to investigate the genes and their products that are
involved in the progression of CRC (4–6).
The synucleins (α-, β- and γ-synuclein) are a small,
soluble, highly conserved group of neuronal proteins that have
attracted considerable attention due to their involvement in
neurodegenerative disorders such as Alzheimer’s and Parkinson’s
disease. Synuclein expression is commonly highly tissue-specific
and is restricted to brain tissue and presynaptic terminals
(7,8). However, aberrant expression of
synucleins beyond the neuronal system has been highly associated
with human malignancies. The γ-synuclein gene was initially cloned
from infiltrating breast carcinoma cells by using the expressed
sequence tag-based differential cDNA sequencing approach (9), and stage-specific expression of
γ-synuclein has been found in advanced breast cancers and other
malignancies, including ovarian, pancreatic and bladder cancers
(10–13). The α-synuclein gene was identified
in patients with bladder cancer by oligonucleotide arrays, and
α-synuclein protein is significantly associated with tumor staging
and clinical outcome (14). α- and
β-synucleins have also been found to be expressed in several
nervous system cancers and breast and ovarian cancer (15,16).
In our previous study, we investigated expression
patterns of synucleins in CRC tissues and cell lines, and found
that among α-, β- and γ-synucleins, γ-synuclein was most
significantly correlated with CRC (17). We also provided clinical evidence
suggesting that γ-synuclein expression is upregulated in CRC, which
is primarily attributed to the demethylation of the CpG island in
exon 1, and that aberrant expression and demethylation of
γ-synuclein are closely correlated with advanced clinical stage,
lymph node involvement and distant metastasis (18). Experimental data from previous
studies have also revealed a tumor phenotype of γ-synuclein in
other types of cancer. Overexpression of γ-synuclein was found to
lead to a significant increase in motility and invasiveness in
breast cancer cell culture and to a profound enhancement in
metastasis in nude mice (19,20).
γ-synuclein has been shown to compromise normal mitotic checkpoint
controls, resulting in multinucleation as well as more rapid breast
cancer cell growth (21,22). γ-synuclein has been implicated in
late stage ovarian cancer metastasis by enhancing cell motility
through activation of the RHO family small-GTPases and ERK1/2
(23).
Collectively, these findings suggest a potential
role of γ-synuclein in the process of tumorigenesis and metastasis.
However, little is known concerning the biological effects of
γ-synuclein in CRC cell line culture and in animal models. In the
present study, we investigated the effects of γ-synuclein on the
biological features of colon cancer cell line SW1116 by cell growth
curve, soft agar assay, cell migration, invasion and adhesion
assays in vitro, and by a tumor xenograft model of
tumorigenicity and a liver metastasis assay in vivo.
Materials and methods
Cell cultures
The colon cancer cell line SW1116 (ATCC no. CCL-233)
was grown in RPMI-1640 medium (Gibco-BRL, Life Technologies Inc.,
Gaithersburg, MD, USA) supplemented with 10% heat inactivated fetal
bovine serum (FBS) (Summit Biotechnology, Fort Collins, CO, USA).
The colon cancer cell line HT-29 (ATCC no. HTB-38) was cultured in
McCoy’s 5a medium (Gibco-BRL) supplemented with 10% FBS. Human
liver sinusoidal endothelial cells (HLSECs) were purchased from
Cell Systems (Kirkland, WA, USA) and grown in Dulbecco’s modified
Eagle’s medium (DMEM) (Gibco-BRL) supplemented with 10% FBS. Human
umbilical vein endothelial cells (HUVECs) (ATCC no. PCS-100-013)
were obtained from ATCC and maintained in F12K supplemented with
endothelial cell growth supplement (ECGS) (0.05 mg/ml) (BD
Biosciences, Bedford, MA, USA) and heparin (Sigma-Aldrich,
Victoria, Australia) (0.1 mg/ml). Cells were maintained in a
humidified incubator at 37°C with 5% CO2, fed every 3
days with complete medium and subcultured when confluence was
reached.
RT-PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). Synthesis of cDNA from 1 μg RNA
was performed with a reverse transcription system kit (Promega,
Madison, WI, USA). PCR reactions were carried out in a thermal
cycler (model PTC-225; M J Research, Watertown, MA, USA) using
HotStarTaq DNA polymerase (Qiagen GmbH, Hilden, Germany) as
follows: 95°C for 15 min, 34 cycles of 94°C for 30 sec, 60°C for 30
sec, and 72°C for 30 sec, and finally at 72°C for 5 min. The
expression of GAPDH mRNA was taken as an internal loading control.
The PCR products were separated on 1.5% agarose gels and stained
with ethidium bromide. Table I
provides the sequences of the primers used in the study.
 | Table ISequences of γ-synuclein
gene-specific primers and shRNA. |
Table I
Sequences of γ-synuclein
gene-specific primers and shRNA.
|
Oligonucleotides | Sequence
(5′-3′) |
|---|
| γ-synuclein-5′
(RT-PCR) |
CAAGAAGGGCTTCTCCATCGCCAAGG |
| γ-synuclein-3′
(RT-PCR) |
CCTCTTTCTCTTTGGATGCCACACCC |
| GAPDH-5′ |
GAAGGTGAAGGTCGGAGTC |
| GAPDH-3′ |
GAAGATGGTGATGGGATTTC |
| γ-synuclein-5′
(full-length PCR) |
CCCTCGAGGGATGGATGTCTTCAAGAAGG |
| γ-synuclein-3′
(full-length PCR) |
CGGGATCCCGCTAGTCTCCCCCACTCG |
| γ-synuclein-5′
(shRNA) |
GATCCGGAGAATGTTGTACAGAGCTTCAAGAGAGCTCTGTACAACATTCTCCTTTTTTGGAAA |
| γ-synuclein-5′
(shRNA) |
AGCTTTTCCAAAAAAGGAGAATGTTGTACAGAGCTCTCTTGAAGCTCTGTACAACATTCTCCG |
Plasmid construction
Total RNA was extracted from HT29 cells, and the
full-length cDNA of γ-synuclein was amplified using RT-PCR. The
digested fragment of cDNA was inserted between the XhoI and
BamHI sites of the pIRES2-EGFP plasmid to generate the
pIRES2-γ-synuclein construct. The sequences (as shown in Table I) unique to the coding region of
γ-synuclein were chemically synthesized and inserted between the
BamHI and HindIII sites of the pGCsi-U6/neo/GFP
plasmid to generate the pGCsi-γ-synuclein construct. The positive
clones of pIRES2-γ-synuclein and pGCsi-γ-synuclein were confirmed
by sequencing.
Plasmid transfection and selection of the
SW1116 stable transfectants
One day before transfection, SW1116 cells were
plated in a 6-well plate at 1×105 cells/well using
RPMI-1640 medium without antibiotics. The cells were transfected
with 3.0 μg/well of the recombinant plasmids and empty plasmids as
control, respectively, using Lipofectamine (Invitrogen). Fresh
growth medium was replaced after 4 h of transfection. Cells were
passaged at a 1:10 dilution at 24 h after transfection and cultured
in medium supplemented with G418 (Promega) at 1,000 μg/ml for 4
weeks. Stably transfected polyclones were selected from several
cell islands with green fluorescent protein (GFP or EGFP) and were
maintained in medium containing 400 μg/ml G418 for further study.
Meanwhile, GFP or EGFP fluorescence was detected by flow cytometry
to confirm that the purity of transfectants was >95%.
Western blot analysis
Cells were harvested and lysed with mammalian
protein extraction reagent (Pierce, Rockford, IL, USA). The lysated
proteins were quantified by bicinchoninic acid (BCA) protein assay
kit (Pierce). Total protein (50 μg) was subjected to 15% SDS-PAGE
and electrophoretically transferred to PVDF membranes (Millipore,
Bedford, MA, USA). Membranes were blocked overnight with 5% skimmed
milk in TBS buffer containing 0.05% Tween-20 at 4°C, and incubated
with a 1:500 dilution of mouse anti-γ-synuclein monoclonal antibody
(sc-65979; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and goat
anti-mouse IgG-AP antibody (Santa Cruz Biotechnology) (1:5,000),
respectively. Membranes incubated with ECL (Pierce) for 1 min were
exposed to the film for 1–5 min. GAPDH served as a loading
control.
Cell proliferation analysis
Cells were plated in five copies onto 96-well plates
at a density of 2×103 cells/well in 100 μl RPMI-1640
medium containing 10% FBS at 37°C with 5% CO2. The
number of viable cells was determined daily with the WST-8
cytotoxicity assay using the Cell Counting Kit-8 (Dojindo, Japan).
Briefly, 10 μl of the CCK-8 solution was added to each well of the
microplate, and the absorbance at 490 nm was measured by a
microplate reader (μQuant; Bio-Tek, Winooski, VT, USA) after a 4-h
incubation.
Soft agar colony formation assay
Cells (1×103) were trypsinized to a
single-cell suspension and then plated in triplicate onto 6-well
plates in complete culture medium containing 0.3% agar on top of
0.6% agar in the same medium. Cultures were maintained at 37°C with
5% CO2 incubator for 14 days. The colonies were fixed
with 70% ethanol and stained with 0.2% crystal violet. The colonies
containing at least 50 cells were counted. Colony formation rates
were calculated as the number of colonies relative to that of cells
initially plated in a well (1×103), and were expressed
as mean ± SD.
Wound healing assay
Cells were plated in 35-mm dishes to form a
monolayer one day before assay. After making a straight scratch
with a pipette tip, cells were incubated in RPMI-1640 medium
containing 10% FBS at 37°C with 5% CO2 for 36 h. The
motile cells were photographed under light microscopy to detect the
speed of wound closure at various intervals. Assays were repeated
three times.
Cell invasion assay
Boyden chambers with 8-μm polycarbonate membranes in
24-well dishes (Nucleopore, Pleasanton, CA, USA) were coated with 4
mg/ml growth factor-reduced Matrigel (50 μg; Collaborative
Biomedical, Becton Dickinson Labware, Bedford, MA, USA). Cells
(1×105) were resuspended in serum-free RPMI-1640 and
added to the upper chamber in triplicate. Consecutively, RPMI-1640
with 10% FBS was added to the lower chamber. Chambers were
incubated at 37°C with 5% CO2 incubator. After a 48-h
incubation, the chambers were fixed with 70% ethanol, and cells
were stained with 0.2% crystal violet. Cells on the surface of the
upper chamber were removed by swiping with cotton swabs. The number
of invasive cells in the lower chamber was determined under light
microscopy. The data are expressed as means ± SD of cell counts in
10 random fields of vision.
Cell adhesion assay
For the adhesion assay with HLSECs or HUVECs, a
96-well plate, coated by 2% gelatin, was seeded with
1×104 endothelial cells/well and cultured until
confluent. SW1116 cells (5×104) were then transferred to
each well in triplicate and left to adhere to the endothelial layer
for 1 h at 37°C. The endothelial layer was washed with PBS to
remove the non-adherent cells. Since γ-synuclein expression
plasmids contained green fluorescence protein (GFP or EGFP), the
attached cells were quantified by measuring the fluorescence
intensity using a microplate reader (model Safire 2; Tecan,
Männedorf, Switzerland).
Tumor xenograft model of tumorigenicity
and liver metastasis assay
Six-week-old male BALB/c nu/nu nude mice were
purchased from the Experimental Animal Centre of Shanghai
Institutes for Biological Sciences (SIBS, Shanghai, China) and
housed in a specific pathogen-free environment. The pIRES2-EGFP,
pIRES2-γ-synuclein, pGCsi-U6/neo/GFP, and pGCsi-γ-synuclein cells
were harvested, washed and re-suspended in PBS, respectively. For
the tumorigenicity assay, 1×106 cells suspended in 100
μl PBS were injected subcutaneously into the right flank regions
using a 27-gauge needle. Mice were checked every 3 days for tumor
appearance, and tumor size was determined by measuring two
diameters with a caliper. Tumor volume (V) was estimated by using
the equation: V = 4/3π × L/2 × (W/2)2, where L is the
mid-axis length and W is the mid-axis width. For liver metastasis
assay, mice were anesthetized intraperitoneally with 2.5% Avertin
(Sigma). The spleen was exteriorized through a left lateral flank
incision, and 1×106 cells in 50 μl of PBS were injected
into the distal tip of spleen parenchyma using a 27-gauge needle.
The injection site on the spleen was pressed with a cotton stick
wet in iodine-polividone solution in order to destroy extravasated
cells and ensure hemostasis. The peritoneum and skin were closed in
a single layer with surgical thread. Each group for the
tumorigenicity assay included 10 mice and each group for the liver
metastasis assay included 7 mice. All animals were sacrificed on
day 30 after tumor cell implantation. Tumor specimens (in the right
flank, liver and spleen) were collected, fixed in 10% neutral
buffered formalin, embedded in paraffin and subjected to
hematoxylin and eosin (H&E) staining and immunohistochemistry
(IHC). The Ethics Committee of the Fujian Medical University
approved the animal usage in the research, and all surgical
procedures and care administered to the animals were in accordance
with institutional guidelines.
Histology and immunohistochemistry
Unstained 4-mm sections were cut from tissue blocks
and stained with H&E and subjected to IHC analysis. Sections
subjected to IHC were cleared with xylene and rehydrated with
ethanol, and the slides were bathed in 0.01 mol/l sodium citrate
and heated in a microwave oven for 12 min. The sections were
incubated with anti-γ-synuclein antibody (sc-65979; Santa Cruz
Biotechnology) and kept at 4°C overnight. Negative control slides
were treated only with non-immunized mouse immunoglobulin fraction
under equivalent conditions. For the secondary developing reagents,
a labeled streptavidin-biotin kit (Dako, Carpinteria, CA, USA) was
used. Slides were developed with diaminobenzaminidine and
counterstained with hematoxylin.
Statistical analysis
The results are presented as means ± SD. Differences
between groups were compared by two-way ANOVA, and were considered
significant at P<0.05. Data analysis was carried out using SSPS
13.0 software.
Results
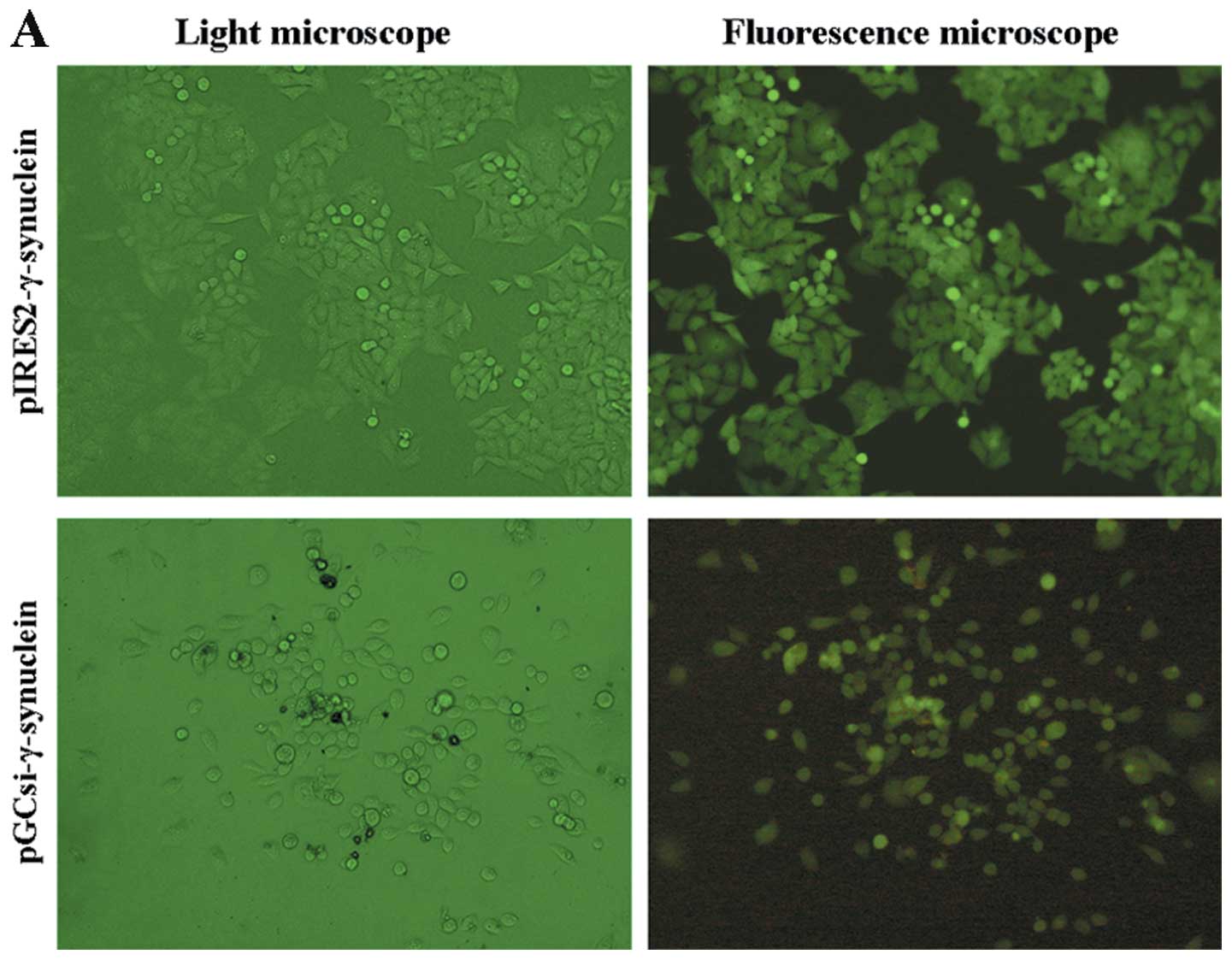
Identification of SW1116 stable
transfectants
After identification by sequence analysis,
γ-synuclein gene eukaryotic expression plasmids and siRNA plasmids
were successfully constructed. The recombinant plasmids and empty
plasmids were transfected into SW1116 cells. After 4 weeks of
selection with G418, stable transfected clones were successfully
established, and the purity of the transfectants was >95%
(Fig. 1A). To confirm the
γ-synuclein expression in the stable transfectants, we examined the
expression of γ-synuclein by RT-PCR and western blot analysis. The
expression of γ-synuclein mRNA was upregulated in pooled
pIRES2-γ-synuclein cells and downregulated in pooled
pGCsi-γ-synuclein cells (Fig. 1B);
these finding paralleled those of the western blot analysis
(Fig. 1C).
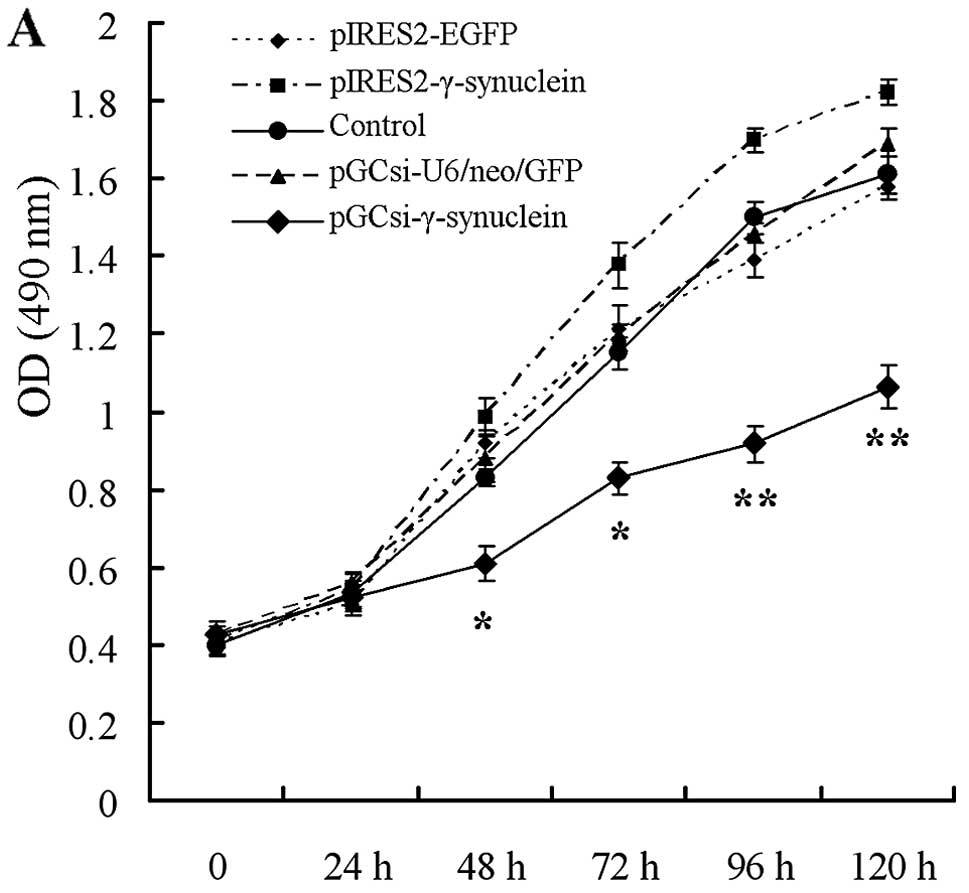
Effects of γ-synuclein on SW1116 cell
proliferation and colony formation in vitro
In vitro cell proliferation assay showed that
γ-synuclein knockdown significantly suppressed cell growth, and the
number of pGCsi-γ-synuclein cells was significantly reduced by 48,
72, 96 and 120 h after plating, respectively, compared with the
corresponding empty plasmid cells and parental (control) cells
(Fig. 2A; P<0.05). The number of
pIRES2-γ-synuclein cells was increased, but not significantly
(Fig. 2A; P>0.05), compared with
the control and pIRES2-EGFP cells, as well as the pGCsi-U6/neo/GFP
and pGCsi-γ-synuclein cells, which in general presented the effects
in a γ-synuclein expression quantity-dependent manner. Subsequent
soft agar colony formation assay was conducted to evaluate the
tumorigenicity of cells in vitro. Colony formation rates
were 32.6±.1, 42.2±7.5, 30.8±5.9, 34.2±6.2 and 12.8±4.6% in the
pIRES2-EGFP, pIRES2-γ-synuclein, control, pGCsi-U6/neo/GFP and
pGCsi-γ-synuclein cells. The colony formation rate of the
pGCsi-γ-synuclein cells was significantly reduced, compared with
the empty vector and control cells (Fig. 2B; P<0.05). The size of colonies
formed by the pGCsi-γ-synuclein cells was smaller than that of the
two control cells. Whereas in parallel with the results of the
proliferation assay, overexpression of γ-synuclein moderately
enhanced colony formation of the SW1116 cells (Fig. 2B; P>0.05), which also exhibited a
γ-synuclein expression quantity-dependent trend.
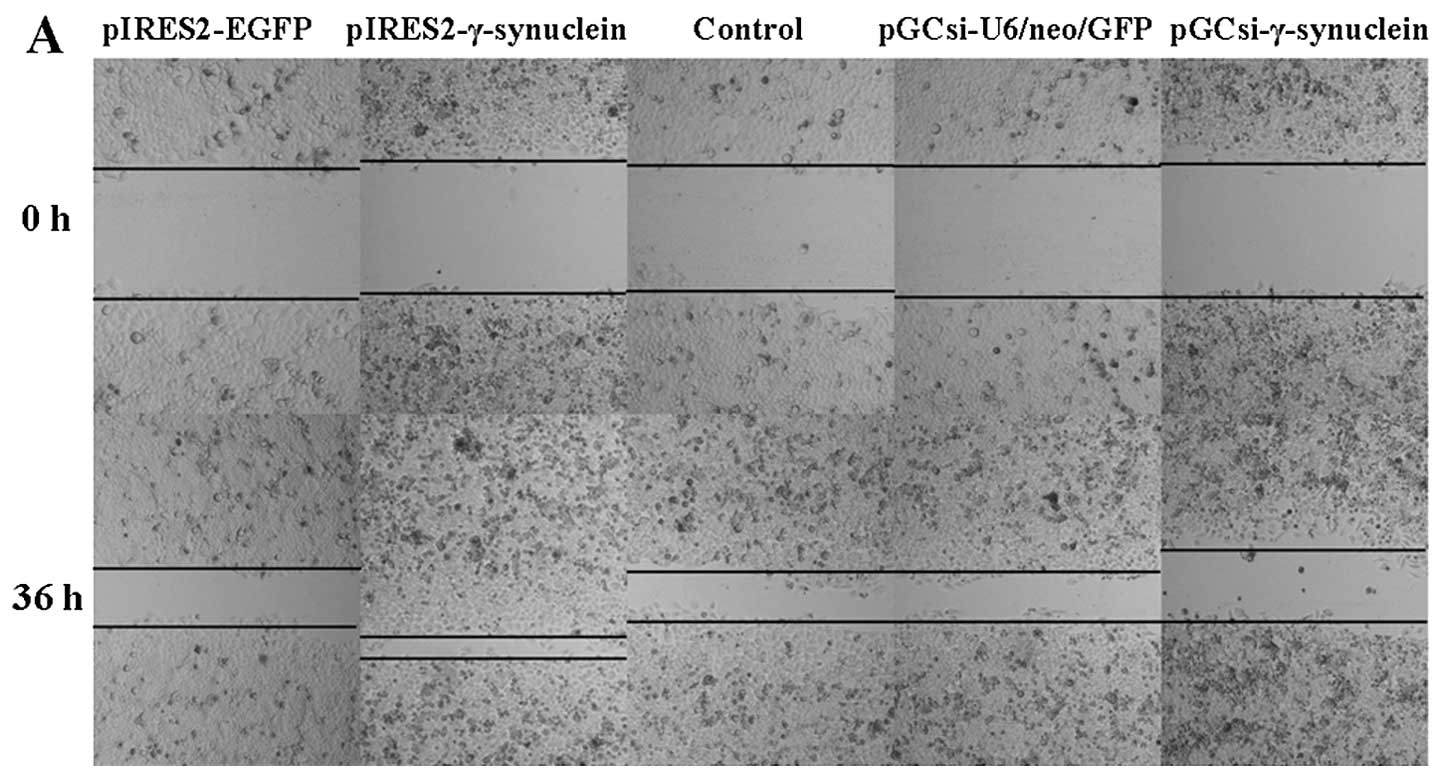
Effects of γ-synuclein on SW1116 cell
migration, invasion and adhesion in vitro
The results of the wound healing assay demonstrated
that γ-synuclein is closely involved in cell motility. SW1116 cells
overexpressing γ-synuclein moved more rapidly towards the gap,
compared with the empty vector and control cells, and γ-synuclein
knockdown decreased the healing rate of the SW1116 cell injury
(Fig. 3A). A subsequent
reconstituted basement membrane (Matrigel) invasion assay was
carried out. As expected, γ-synuclein facilitated SW1116 cell
invasion through Matrigel and a filter membrane, and the number of
invasive pIRES2-γ-synuclein cells was increased by 93.1 and 81.5%,
respectively; a higher number than that of the control and empty
vector cells (Fig. 3B; P<0.05).
However, γ-synuclein knockdown did not significantly attenuate
invasion of SW1116 cells, compared with the two corresponding
control cells (Fig. 3B; P>0.05).
Generally speaking, γ-synuclein promoted SW1116 cell migration and
invasion in a γ-synuclein expression quantity-dependent trend,
which was observed in the following in vivo assays.
We carried out adhesion assays to investigate
whether γ-synuclein influences the adhesion of SW1116 cells to
endothelial cells. In the HLSEC adhesion assay, the average
fluorescence intensity of EGFP was 1.07±0.25 in the pIRES2-EGFP
cells and 1.73±0.32 in the pIRES2-γ-synuclein cells; γ-synuclein
significantly facilitated SW1116 cell adhesion to HLSECs (Fig. 3C; P<0.05). There was no
difference in fluorescence intensity of GFP between the
pGCsi-U6/neo/GFP (0.94±0.26) and pGCsi-γ-synuclein (0.78±0.15)
cells (Fig. 3C; P>0.05).
Meanwhile, the results of the HUVEC adhesion assay revealed no
effect of γ-synuclein on the adhesive ability of SW1116 cells to
HUVECs (Fig. 3D; P>0.05), which
suggests that SW1116 cells overexpressing γ-synuclein interact
specifically with HLSECs.
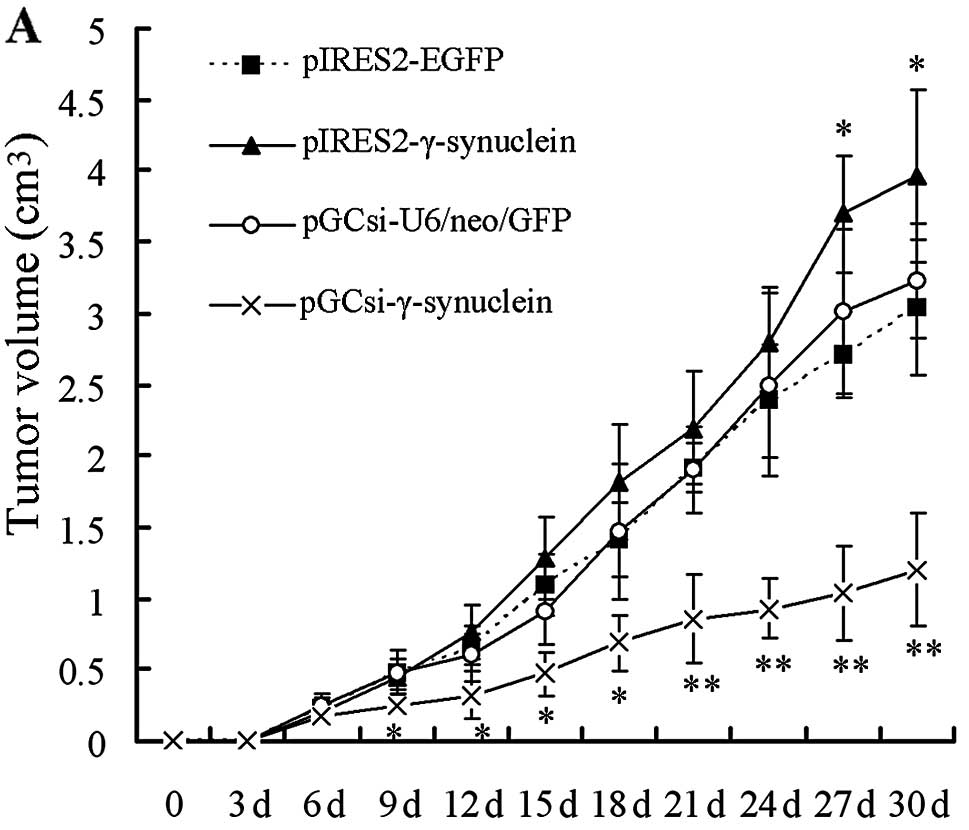
Effects of γ-synuclein on SW1116 cell
tumorigenicity in vivo
We examined the in vivo tumorigenicity of
SW1116 cells by injecting pIRES2-EGFP, pIRES2-γ-synuclein,
pGCsi-U6/neo/GFP and pGCsi-γ-synuclein cells subcutaneously into
the right flank regions of nude mice. The xenograft tumor growth
rates in the different groups were compared. Xenograft tumors
appeared in all mice of the three groups except for the
pGCsi-γ-synuclein group on day 6, and pIRES2-γ-synuclein group mice
exhibited a faster tumor growth rate from day 27 to the end of the
expreriment, compared to the other three groups (Fig. 4A; P<0.05). However, only 6 of the
10 mice injected with pGCsi-γ-synuclein cells presented with tumors
on day 6, and the other 4 mice remained with no tumor formation
until day 9. All pGCsi-γ-synuclein cell tumors maintained a slow
rate of growth, compared to the other three groups, within the time
remaining until sacrifice (Fig. 4A;
P<0.05). All animals were sacrificed on day 30 and tumor tissue
specimens were collected as shown in Fig. 4B. The expression of γ-synuclein in
tumor tissues was detected by IHC staining. The results showed that
γ-synuclein protein was upregulated in tumors derived from
pIRES2-γ-synuclein transfectants and downregulated in tumors
derived from pGCsi-γ-synuclein transfectant, when compared with the
two empty vector transfectants. (Fig.
4C).
Effects of γ-synuclein on SW1116 cell
liver metastasis in vivo
We adopted an experimental model that entailed the
injection of tumor cells into the spleen of nude mice, followed by
assessment of their ability for tumorigenesis in the spleens and
invasion via the portal vein into the liver and potential
intraperitoneal dissemination. All animals were sacrificed by
laparotomy on day 30, and tumor progression was observed
macroscopically. All mice developed primary tumors in the spleen,
and all mice injected with pIRES2-γ-synuclein cells developed
metastases on the liver surface. Meanwhile, mice infected with
pIRES2-γ-synuclein developed multiple intraperitoneal dissemination
nodules in the mesentery, gastrointestinal serosa and peritoneum,
whease less tumor nodules were found in the peritoneal cavity of
mice in the other three groups (Table
II). Liver and spleen specimens were collected for further
evaluation. Liver replacement by metastases was too extensive in
the liver of 71.4% (n=5 of 7) of pIRES2-γ-synuclein mice, which
displayed enlarged livers covered with numerous tumor nodules
throughout and with a whitish and irregular surface caused by
extensive tumor growth, whereas the livers of the other groups were
smaller in size, displaying a macroscopically normal appearance
with a few surface nodules, and no liver surface metastases were
found in 2 of 7 pIRES2-EGFP, 3 of 7 pGCsi-U6/neo/GFP and 3 of 7
pGCsi-γ-synuclein mice (Fig. 5A).
The results paralleled the weight of the livers (Fig. 5B), which also exhibited a
γ-synuclein expression quantity-dependent trend; the more
γ-synuclein protein expressed, the more obvious the liver
metastasis. As shown in Fig. 5C,
the growth of these cells in the spleens was almost identical in
the pIRES2-γ-synuclein and two empty vector groups, and was
significantly decreased in the pGCsi-γ-synuclein group (P<0.05).
Liver specimens were subsequently subjected to histologic
examination by H&E staining (Fig.
5D). The results showed that liver metastases were found
microscopically in all mice including micrometastases in mice
without macroscopic nodules. Large regions of liver tissues were
replaced by metastases with formation of neovessels, which was
reported to be required for tumors to grow beyond a critical size,
whereas livers containing pGCsi-γ-synuclein cells were mainly
occupied by small-sized metastases with central necrosis.
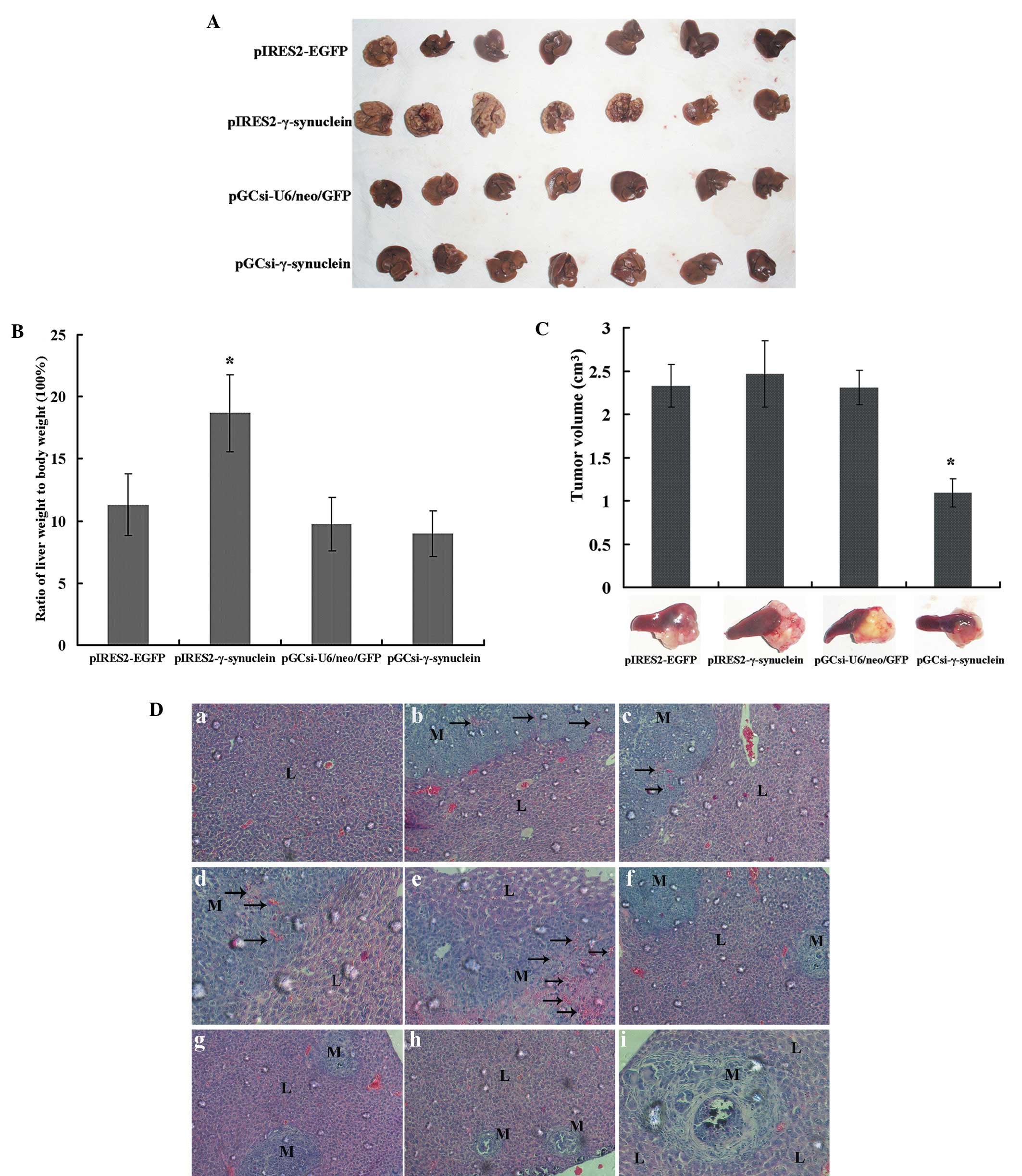 | Figure 5Effects of γ-synuclein on SW1116 cell
liver metastasis in vivo. (A) Macroscopic images of liver
metastases at 30 days after intrasplenic injection of tumor cells.
(B) Quantitative analysis of the weights of tumor-bearing livers on
day 30 and expressed as the mean ± SD from 7 mice;
*P<0.05. (C) Representative images of primary tumors
in the spleen. The tumor volume was determined on day 30 and
expressed as mean ± SD from 7 mice; *P<0.05. (D)
Representative photomicrographs of H&E-stained sections of
liver tissues from mice following intrasplenic injection of tumor
cells (original magnification, a–c, f–h, ×200; d, e, i, ×400). (a)
Normal liver tissues of pGCsi-γ-synuclein mice. (b–e) Liver tissues
from pIRES2-γ-synuclein mice, which presented large regions of
liver metastases with formation of neovessels. (f) Liver metastases
from pIRES2-EGFP mice. (g) Liver metastases from pGCsi-U6/neo/GFP
mice. (h and i) Liver tissues from pGCsi-γ-synuclein mice, which
presented small-sized metastases with central necrosis. L, liver;
M, metastases; arrows, neovessels. |
 | Table IIIntraperitoneal dissemination nodules
of the tumors. |
Table II
Intraperitoneal dissemination nodules
of the tumors.
| | No. of nodules |
|---|
| |
|
|---|
| Transfectants | Tumor incidence
(%) | Mesentery | Gastrointestinal
serosa | Peritoneum |
|---|
| pIRES2-EGFP | 4/7 (57.1) | 11 | 5 | 3 |
|
pIRES2-γ-synuclein | 7/7 (100) | 28 | 8 | 4 |
|
pGCsi-U6/neo/GFP | 3/7 (42.9) | 8 | 3 | 4 |
|
pGCsi-γ-synuclein | 2/7 (28.6) | 6 | 2 | 1 |
Discussion
Colon carcinogenesis and metastasis is a multistage
process involving alterations of various tumor-suppressor genes and
oncogenes (24,25). In recent years, multiple studies
have been conducted to investigate the genes and proteins
correlated with progression of CRC; however, no internationally
recognized molecular marker has been identifed for potential
clinical implications (4–6). γ-synuclein is a promising biomarker,
belonging to the synuclein protein family, which was initially
investigated in the field of neurodegenerative diseases (7,8).
Although one study showed downregulation of γ-synuclein in human
esophageal squamous cell carcinoma (26), additional studies including ours
support the overexpression and the oncological role of γ-synuclein
in a variety of cancers (10–13,18).
The prognostic impact of γ-synuclein overexpression is impressive,
and it was found to be the only independent predictor of reduced
overall survival and the strongest negative indicator of
disease-free survival by multivariate analysis in breast cancer
(10).
On the basis of our previous study on expression and
demethylation of γ-synuclein in CRC tissues and cell lines
(17,18), we constructed the γ-synuclein gene
eukaryotic expression and siRNA vectors, and established permanent
transfected SW1116 cells to investigate the biological functions of
γ-synuclein. Our data showed that silencing of γ-synuclein in
SW1116 cells contributed to suppression of cellular proliferation
and colony formation in vitro. These results were consistent
with observations of the γ-synuclein siRNA vector in HCT116 cells
in our previous study and as well as in other reports of breast and
ovarian cancer (21–23,27).
The overexpression of γ-synuclein moderately facilitated
proliferation and colony formation of SW1116 cells, but not
significantly compared with the other groups of cells, which
presented a promotive effect in a γ-synuclein expression
quantity-dependent manner. Similar findings were also found in the
tumorigenicity assay in vivo. In the in vivo
tumorigenicity assay, γ-synuclein-knockdown SW1116 cells formed
significantly smaller tumor masses than the empty vector cells on
day 6 over a 30-day period, whereas tumors derived from
overexpressed γ-synuclein cells did not significantly exhibit
faster tumor growth until day 27. The probable reasons are as
follow. Upregulation of γ-synuclein frequently contributes to
cancer cell survival but not proliferation, and moderate expression
of γ-synuclein is enough for cell proliferation through an HSP- or
ER-based multiprotein chaperone complex. However, overexpression of
γ-synuclein may cause occupation of all binding sites in the
multiprotein chaperone complex, with no site left for remaining
γ-synuclein proteins (10,28,29).
Metastasis leads to ~90% of CRC-related mortality,
yet, the underlying mechanisms remain largely unclear (30). Metastasis is a sequential process
that includes detachment from the primary site, invasion and
adhesion to vasculature, translocation through the systemic
circulation, extravasation into the parenchyma of distant tissues
(liver and lungs) and colonization of a distant site (31). In order to model this process, we
therefore, developed migration, invasion and adhesion assays in
vitro, and a liver metastasis assay in vivo. The results
of these assays also exhibited a γ-synuclein expression
quantity-dependent trend; the more γ-synuclein protein expressed,
the more obvious was the promotion of these malignant phenotypes.
In vitro, upregulation of γ-synuclein promoted SW1116 cell
migration through a Matrigel and a filter membrane, and adherence
of SW1116 cells to HLSECs but not HUVECs. Similar effects of
γ-synuclein on cell migration and invasion have been reported in
breast and ovarian cancer, as well as effects on adhesion as in the
report of Hu et al who found that SW1116 subpopulation,
SW1116p21, with high potential for liver metastasis that highly
express γ-synuclein, exhibited enhanced adhesion to HLSECs
(19,23,32),
whereas downregulation of γ-synuclein did not significantly inhibit
SW1116 cell ability for migration, invasion and adhesion to HLSECs.
Our previous experimental data revealed that downregulation of
γ-synuclein significantly inhibited the migration and invasion
ability of HCT116 cells. The discordant results may be due to the
difference in metastatic features between HCT116 and SW1116 cells,
and the effects of γ-synuclein siRNA vector were not apparent in
relative weak metastatic SW1116 cells. The similar magnitude of
migration and invasion-stimulating activity, and special
interaction with HLSECs suggests that γ-synuclein enhances the
SW1116 cell capacity for invasion and metastasis, which were later
confirmed by an experimental model of liver metastasis in
vivo. Overexpression of γ-synuclein facilitated SW1116 cell
liver metastases, and apparent differences were found not only in
macroscopic appearance but also in size and the weight of livers,
which further confirmed the positive role of γ-synuclein in the
invasion and liver metastasis potential of colon cancer cells.
Livers containing pGCsi-γ-synuclein cells had less and small-sized
metastases with central necrosis, consistent with observations of
the effects of γ-synuclein siRNA on cell proliferation and colony
formation. It is likely that survival and proliferation in systemic
circulation and colonization at a distant site are also important
steps in the sequential process of metastasis. On the other hand,
except for the pGCsi-γ-synuclein group, the growth rate of tumors
in the spleens was almost the same, whereas the intraperitoneal
dissemination tumor rate was different, which collectively suggests
that liver metastases occurred independent of a direct effect on
primary tumor growth in the study.
In summary, we used in vitro and in
vivo assays to study the effects of γ-synuclein on
tumorigenicity and metastasis of the colon cancer cell line SW1116.
Through construction of γ-synuclein gene eukaryotic expression and
siRNA vectors, and selection of SW1116 stable transfectants,
respectively, we found that downregulation of γ-synuclein inhibited
the proliferation and colony formation of SW1116 cells in
vitro and tumorigenicity in nude mice. Meanwhile, upregulation
of γ-synuclein enhanced SW1116 cell ability of migration, invasion
and adhesion to HLSECs in vitro and liver metastasis in nude
mice. Furthermore, γ-synuclein promoted these malignant phenotypes
of SW1116 cells in a γ-synuclein expression quantity-dependent
manner not only in vitro but also in vivo. This is
also the first report of the in vivo evidence of γ-synuclein
effects on a colon cancer cell line, which further suggests that
γ-synuclein plays a positive role in the progression of CRC, and
may serve as a novel molecular target for potential clinical
application.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (81101897) and the Science
Foundation of Fujian Province, China (2011J05061).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics. CA Cancer J Clin. 63:11–30. 2013.
|
|
2
|
Mayo SC and Pawlik TM: Current management
of colorectal hepatic metastasis. Expert Rev Gastroenterol Hepatol.
3:131–144. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berri RN and Abdalla EK: Curable
metastatic colorectal cancer: recommended paradigms. Curr Oncol
Rep. 11:200–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hartwell KA, Muir B, Reinhardt F,
Carpenter AE, Sgroi DC and Weinberg RA: The Spemann organizer gene,
Goosecoid, promotes tumor metastasis. Proc Natl Acad Sci USA.
103:18969–18974. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mani SA, Yang J, Brooks M, et al:
Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is
associated with aggressive basal-like breast cancers. Proc Natl
Acad Sci USA. 104:10069–10074. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang J, Mani SA, Donaher JL, et al: Twist,
a master regulator of morphogenesis, plays an essential role in
tumor metastasis. Cell. 117:927–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Surguchov A: Molecular and cellular
biology of synucleins. Int Rev Cell Mol Biol. 270:225–317. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahmad M, Attoub S, Singh MN, Martin FL and
El-Agnaf OM: γ-Synuclein and the progression of cancer. FASEB J.
21:3419–3430. 2007.
|
|
9
|
Ji H, Liu YE, Jia T, et al: Identification
of a breast cancer-specific gene, BCSG1, by direct differential
cDNA sequencing. Cancer Res. 57:759–764. 1997.PubMed/NCBI
|
|
10
|
Guo J, Shou C, Meng L, et al: Neuronal
protein synuclein γ predicts poor clinical outcome in breast
cancer. Int J Cancer. 121:1296–1305. 2007.
|
|
11
|
Hibi T, Mori T, Fukuma M, et al:
Synuclein-γ is closely involved in perineural invasion and distant
metastasis in mouse models and is a novel prognostic factor in
pancreatic cancer. Clin Cancer Res. 15:2864–2871. 2009.
|
|
12
|
Dokun OY, Florl AR, Seifert HH, Wolff I
and Schulz WA: Relationship of SNCG, S100A4, S100A9 and LCN2 gene
expression and DNA methylation in bladder cancer. Int J Cancer.
123:2798–2807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu H, Liu W, Wu Y, et al: Loss of
epigenetic control of synuclein-γ gene as a molecular
indicator of metastasis in a wide range of human cancers. Cancer
Res. 65:7635–7643. 2005.PubMed/NCBI
|
|
14
|
Sanchez-Carbayo M, Socci ND, Lozano J,
Saint F and Cordon-Cardo C: Defining molecular profiles of poor
outcome in patients with invasive bladder cancer using
oligonucleotide microarrays. J Clin Oncol. 24:778–789. 2006.
View Article : Google Scholar
|
|
15
|
Fung KM, Rorke LB, Giasson B, Lee VM and
Trojanowski JQ: Expression of alpha-, beta-, and gamma-synuclein in
glial tumors and medulloblastomas. Acta Neuropathol. 106:167–175.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bruening W, Giasson BI, Klein-Szanto AJ,
Lee VM, Trojanowski JQ and Godwin AK: Synucleins are expressed in
the majority of breast and ovarian carcinomas and in preneoplastic
lesions of the ovary. Cancer. 88:2154–2163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye Q, Wang TF, Peng YF, et al: Expression
of α-, β- and γ-synuclein in colorectal cancer, and potential
clinical significance in progression of the disease. Oncol Rep.
23:429–436. 2010.
|
|
18
|
Ye Q, Zheng MH, Cai Q, et al: Aberrant
expression and demethylation of γ-synuclein in colorectal cancer,
correlated with progression of the disease. Cancer Sci.
99:1924–1932. 2008.
|
|
19
|
Jia T, Liu YE, Liu J and Shi YE:
Stimulation of breast cancer invasion and metastasis by synuclein
γ. Cancer Res. 59:742–747. 1999.PubMed/NCBI
|
|
20
|
Surgucheva IG, Sivak JM, Fini ME, Palazzo
RE and Surguchov AP: Effect of γ-synuclein overexpression on matrix
metalloproteinases in retinoblastoma Y79 cells. Arch Biochem
Biophys. 410:167–176. 2003.
|
|
21
|
Gupta A, Inaba S, Wong OK, Fang G and Liu
J: Breast cancer-specific gene 1 interacts with the mitotic
checkpoint kinase BubR1. Oncogene. 22:7593–7599. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inaba S, Li C, Shi YE, Song DQ, Jiang JD
and Liu J: Synuclein gamma inhibits the mitotic checkpoint function
and promotes chromosomal instability of breast cancer cells. Breast
Cancer Res Treat. 94:25–35. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pan ZZ, Bruening W and Godwin AK:
Involvement of RHO GTPases and ERK in synuclein-γ enhanced cancer
cell motility. Int J Oncol. 29:1201–1205. 2006.PubMed/NCBI
|
|
24
|
Gennari L, Russo A and Rossetti C:
Colorectal cancer: what has changed in diagnosis and treatment over
the last 50 years? Tumori. 93:235–241. 2007.PubMed/NCBI
|
|
25
|
Samoha S and Arber N: Cyclooxygenase-2
inhibition prevents colorectal cancer: from the bench to the bed
side. Oncology. 69:33–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou CQ, Liu S, Xue LY, et al:
Down-regulation of γ-synuclein in human esophageal squamous cell
carcinoma. World J Gastroenterol. 9:1900–1903. 2003.
|
|
27
|
Pan ZZ, Bruening W, Giasson BI, Lee VM and
Godwin AK: γ-synuclein promotes cancer cell survival and inhibits
stress- and chemotherapy drug-induced apoptosis by modulating MAPK
pathways. J Biol Chem. 277:35050–35060. 2002.
|
|
28
|
Liu YE, Pu W, Jiang Y, Shi D, Dackour R
and Shi YE: Chaperoning of estrogen receptor and induction of
mammary gland proliferation by neuronal protein synuclein gamma.
Oncogene. 26:2115–2125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang Y, Liu YE, Goldberg ID and Shi YE:
γ-synuclein, a novel heat-shock protein-associated chaperone,
stimulates ligand-dependent estrogen receptor α signaling and
mammary tumorigenesis. Cancer Res. 64:4539–4546. 2004.
|
|
30
|
Gupta GP and Massague J: Cancer
metastasis: building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fidler IJ: The pathogenesis of cancer
metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003.
|
|
32
|
Hu H, Sun L, Guo C, et al: Tumor
cell-microenvironment interaction models coupled with clinical
validation reveal CCL2 and SNCG as two predictors of colorectal
cancer hepatic metastasis. Clin Cancer Res. 15:5485–5493. 2009.
View Article : Google Scholar : PubMed/NCBI
|