Introduction
Idiopathic pulmonary fibrosis (IPF), accounting for
>50% of cases with idiopathic interstitial pneumonia (IIP), is a
progressive, lethal disease, whose etiology remains enigmatic
(1,2). The majority of IPF cases are sporadic,
while 2–20% of cases are familial, inherited in an autosomal
dominant pattern with incomplete penetration (2). The current hypothesis supports the
notion that IPF is not an inflammatory disorder, but a complex
process characterized by abnormal pneumocyte apoptosis and the
profound disruption of the renewal of the alveolar epithelium,
making it, at least in many aspects, quite similar to malignant
lung disease (2–4).
IPF and lung cancer have several striking
similarities. Both are fatal lung diseases, whose main event is
aberrant cell proliferation and they share a number of pathogenetic
pathways (4–6). Genetic alterations, response to growth
and inhibitory signals, resistance to apoptosis, myofibroblast
origin and behavior, altered cellular communication and
intracellular signaling pathways are all fundamental pathogenic
hallmarks of both IPF and cancer (4–6).
Furthermore, both diseases are characterized by the lack of
effective treatment and a poor survival rate, a combination that
underlines the need for further research for novel information and
perspectives (2,4–6).
Mutations of various genes have emerged in search of
the etiology of both diseases, such as the ‘aging’ gene, telomerase
(3,4). Telomeres are repetitive DNA sequences
at the end of chromosomes, which protect them from degradation,
irregular recombination and end-to-end fusions (3). Telomeres decrease in length with every
cell division until they reach a critically short size and signal
the arrest of cell proliferation, senescence and apoptosis
(5). The active telomerase
ribonucleoprotein complex contains 3 subunits: the telomerase
reverse transcriptase (h-TERT), the RNA subunit (h-TERC) and
dyskerin (6).
The catalytic activity of this enzyme resides in the
h-TERT component, and thus the regulation of h-TERT mRNA expression
seems to be the most important step for telomerase activation
(7). Although h-TERT seems to be
the key component in telomerase regulation and telomere synthesis,
h-TERC is also required to maintain cell growth, particularly when
h-TERT is overexpressed (8). It
appears that h-TERC may play a stabilizing role in the telomerase
complex. h-TERT is highly expressed in germ cells, cells with
proliferative potential and in immortalized cancer cells (9–11),
although in most other cells, telomerase activity is restricted in
humans (12). In addition, in IPF,
25% of individuals who have either familial or sporadic pulmonary
fibrosis, without h-TERT or h-TERC mutation, have shorter telomeres
in their circulating leukocytes (2).
Since there are indications of common pathogenetic
pathways between IPF and lung cancer, in this study, we aimed to
evaluate the expression levels of both telomerase subunits (h-TERT
and h-TERC) in lung tissue and bronchoalveolar lavage fluid (BALF)
from patients with IPF and non-small cell lung cancer (NSCLC).
Patients and methods
Patients
Consecutive patients with IPF and NSCLC from the
Department of Thoracic Medicine, University Hospital of Heraklion,
Crete, Greece were enrolled in this study. The diagnosis for IPF
was based on internationally accepted clinical and imaging
criteria, using video-assisted thoracoscopic surgery (VATS), where
needed (13). All patients were
sporadic IPF cases. The diagnosis for NSCLC was based on
histopathological criteria from bronchial biopsies and cytology
from bronchial washings. The patients included in this study were
classified as NSCLC according to the WHO criteria (1997). All IPF
and NSCLC patients were newly diagnosed and treatment naive at the
time of either bronchoscopy or surgery.
The lung tissue samples were obtained from 29
patients with IPF, 10 patients with NSCLC and 21 controls. NSCLC
samples were obtained from sections of the lung with verified
positive histology. Control lung tissue specimens were obtained
from patients undergoing lobectomy or pneumonectomy for
bronchogenic carcinoma, at a macroscopically healthy site distant
from the malignancy. The samples were further verified
histologically as free of malignancy before being classified as
control samples, as previously described by ours and other groups
(14,15). The BALF samples were obtained from
23 patients with IPF, 31 patients with NSCLC and 12 controls.
Control subjects were patients undergoing bronchoscopy for the
investigation of haemoptysis, without any pulmonary comorbidities
and with normal bronchoscopic findings and cytology results.
Subjects who had experienced respiratory infections during the 6
weeks prior to bronchoscopy or surgery were excluded from this
study. All patients were of comparable age. The patients with NSCLC
and the control subjects exhibited near normal pulmonary function
tests, whereas the patients with IPF had a mild restriction with
decreased pulmonary volumes. Demographics and pulmonary function
tests of the patients and controls are presented in Table I.
 | Table IClinical characteristics and
pulmonary function tests of all patients studied. |
Table I
Clinical characteristics and
pulmonary function tests of all patients studied.
| A, Lung tissue
samples |
|---|
|
|---|
|
Characteristics | Controls | IPF | NSCLC | p-value |
|---|
| No. | 21 | 29 | 10 | |
| Agea | 63.41±4.12 | 67.8±3.93 | 59.38±6.81 | p1, p2, p3=NS |
| Gender (M/F) | 17/4 | 21/8 | 9/1 | |
| Non-smoker | 9 | 13 | 0 | |
| Smokers | 6 | 5 | 8 | |
| Ex-smokers | 6 | 11 | 2 | |
| FEV1a | 85.31±8.49 | 75.64±4.23 | 83.78±5.74 | p1, p2, p3=NS |
| FVCa | 93.22±7.50 | 74.85±3.30 | 92.19±8.25 | p1<0.05, p2,
p3=NS |
| FEV1/FVCa | 73.33±4.29 | 82.14±1.85 | 71.89±4.91 | p1, p3<0.05,
p2=NS |
| DLCOa | 74.43±11.37 | 50.73±4.30 | - | p1<0.05 |
|
| B, BALF
samples |
|
|
Characteristics | Controls | IPF | NSCLC | p-value |
|
| No. | 12 | 23 | 31 | |
| Agea | 61.75±3.51 | 69.17±1.23 | 66.62±1.74 | p1, p2, p3=NS |
| Gender (M/F) | 10/2 | 18/5 | 27/4 | |
| Non-smoker | 5 | 9 | 2 | |
| Smokers | 4 | 3 | 18 | |
| Ex-smokers | 3 | 11 | 11 | |
| FEV1a | 86.78±8.71 | 77.88±3.45 | 82.34±4.71 | p1, p2, p3=NS |
| FVCa | 91.88±6.90 | 71.68±4.52 | 91.13±8.92 | p1, p3<0.05,
p2=NS |
| FEV1/FVCa | 75.41±5.19 | 85.31±1.79 | 72.14±5.23 | p1, p3<0.05,
p2=NS |
| DLCOa | 72.28±9.71 | 52.69±4.13 | - | p1<0.05 |
Ethics statement
Informed consent was obtained from all patients and
control subjects who participated in this study. The study protocol
was approved by the Ethics Committee of the University Hospital of
Heraklion.
Biological samples and processing
BALF was obtained from all patients at room
temperature as previously described (16). After filtering through a sterile
gauze (Thompson, Ontario, Canada) to remove debris, BALF from each
patient was centrifuged at 400 × g for 15 min at 4°C and the
supernatant and pellet were stored at −80°C. Lung tissue biopsy
specimens were obtained at room temperature, immediately frozen in
liquid nitrogen and stored at −80°C.
Gene expression
BALF pellets and homogenized lung tissue samples
were processed using the TRIzol reagent (Invitrogen, Carlsbad, CA,
USA) protocol for total RNA extraction according to the
manufacturer’s instructions. RNA concentration and purity were
evaluated using a spectrophotometer. Aliquots of RNA were stored at
−80°C until use. cDNA from each sample was derived by reverse
transcription of 2 μg of total RNA using the AffinityScript™ Multi
Temperature cDNA synthesis kit, (Stratagene, La Jolla, CA, USA).
Random hexamers were used as amplification primers. To remove the
RNA template, cDNA was incubated with E. coli RNaseH and
stored at −20°C until use.
Transcript levels of h-TERT, h-TERC and transforming
growth factor (TGF)-β were determined using the Mx3000P Real-Time
PCR system (Stratagene) and SYBR-Green I Master Mix (Stratagene)
according to the manufacturer’s instructions, as previously
described (17–19). All primers were designed to span at
least one intron in order to avoid amplification of contaminating
genomic DNA. β-globin was used as an internal control to normalize
mRNA expression levels, as previously described by our study group
(17–19). To verify the results of the melt
curve analysis, PCR products were analyzed by electrophoresis on 2%
agarose gels stained with ethidium bromide and photographed on a UV
light transilluminator. Primer sequences and annealing temperatures
for all the genes analyzed, as well as for β-globin are presented
in Table II.
 | Table IIPrimer sequences used for real-time
RT-PCR. |
Table II
Primer sequences used for real-time
RT-PCR.
| Gene | Primer pair
sequence (5′→3′) | Annealing
temperature (°C) |
|---|
| TGF-β | Forward
AAGGACCTCGGCTGGAAGTGC
Reverse CCGGGTTATGCTGGTTGTA | 62 |
| h-TERT | Forward
TGACACCTCACCTCACCCAC
Reverse CACTGTCTTCCGCAAGTTCAC | 51 |
| h-TERC | Forward
GCCTGCCGCCTTCCACCGTTCATT
Reverse GACTCGCTCCGTTCCTCTTCCTG | 59 |
| β-globin | Forward
GCTTCTGACACAACTGTGTTCACTAGC
Reverse CACCAACTTCATCCACGTTCACC | 58 |
Statistical analysis
The one sample Kolmogorov-Smirnov test was employed
to assess normality. Data were compared using Kruskal-Wallis ANOVA
with the Mann-Whitney test for post-hoc comparisons. The
percentages of patients expressing hTERT, h-TERC and TGF-β were
compared using the χ2 test. Values are expressed as the
median (lower-upper quartiles) and a value of p<0.05 was
considered to indicate a statistically significant difference.
Linear regression analysis (Spearman’s rank correlation
coefficient) was used to assess TGF-β expression in the lung
tissue. Statistical calculations were performed using Statistica 7
software (StatSoft, Tulsa, OK, USA).
Results
Gene expression in lung tissue
As regards the h-TERT subunit, the telomerase gene
was expressed in 52.4% of the controls, 13.8% of the patients with
IPF and 60% of the NSCLC population (Table III). As regards the h-TERC
subunit, the telomerase gene was expressed in a significantly lower
number of patients with IPF compared with the controls and patients
with NSCLC (Table III).
 | Table IIIPercentage of subjects expressing
h-TERT and h-TERC mRNA in lung tissue and BALF samples. |
Table III
Percentage of subjects expressing
h-TERT and h-TERC mRNA in lung tissue and BALF samples.
| A, Lung tissue
samples |
|---|
|
|---|
| Control (n=21) | IPF (n=29) | NSCLC (n=10) | p-valuea |
|---|
| h-TERT | 52.4% | 13.8% | 60% | p1=0.003
p2=NS
p3=0.004 |
| h-TERC | 61.9% | 17.3% | 90% | p1=0.001
p2=NS
p3=0.0001 |
|
| B, BALF
samples |
|
| Control (n=12) | IPF (n=23) | NSCLC (n=31) | p-valuea |
|
| h-TERT | 33% | 65.2% | 25.8% | p1=0.07
p2=NS
p3=0.004 |
| h-TERC | 50% | 73.9% | 59.1% | p1, p2, p3=NS |
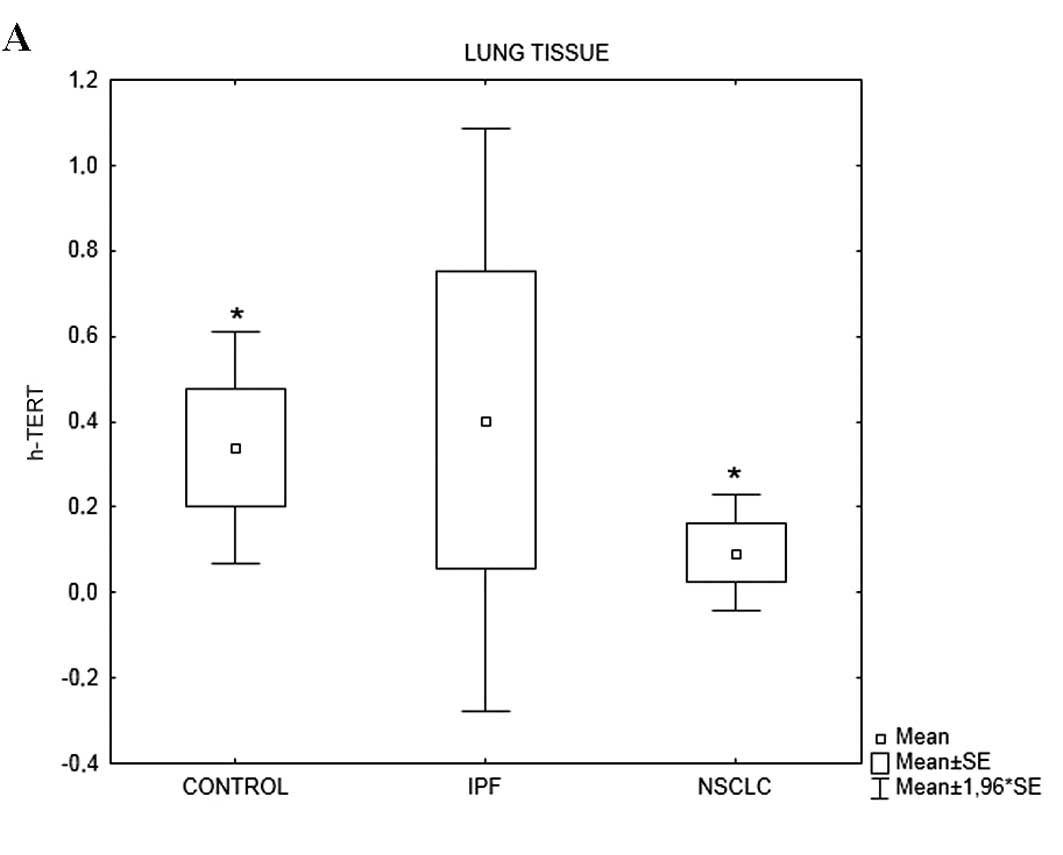
h-TERT mRNA expression levels differed among the 3
groups (p=0.036). h-TERT mRNA levels in the patients with IPF were
significantly lower compared with those in the controls (p=0.009)
and patients with NSCLC (p=0.004). The majority of subjects in all
3 groups expressed h-TERC mRNA (Table
IVA). h-TERC mRNA expression levels differed among the 3 groups
(p=0.002). Again, h-TERC mRNA levels in the patients with IPF were
significantly lower compared with those in the controls (p=0.0005)
and patients with NSCLC (p=0.0004) (Table IVA, Fig. 1).
 | Table IVh-TERT and h-TERC relative mRNA
expression levels in lung tissue and BALF samples from patients
with IPF and NSCLC and the control subjects. |
Table IV
h-TERT and h-TERC relative mRNA
expression levels in lung tissue and BALF samples from patients
with IPF and NSCLC and the control subjects.
| A, Lung tissue
samples |
|---|
|
|---|
| Control (n=21) | IPF (n=29) | NSCLC (n=10) | p-value |
|---|
| h-TERT | 0.01 [0–0.35] | 0.00 [0–0] | 0.695 [0–2.34] | 0.036 |
| h-TERC | 1.12 [0–2.66] | 0.00 [0–0] | 0.97
[0.68–2.88] | 0.002 |
| TGF-β | 0.7390
[0.1280–3.220] | 194
[0.0015–3070] | 0.00
[0.00–0.03] | <0.0001 |
|
| B, BALF
samples |
|
| Control (n=12) | IPF (n=23) | NSCLC (n=31) | p-value |
|
| h-TERT | 0.00 [0–0.02] | 0.09 [0–0.23] | 0.00 [0–0.01] | 0.012 |
| h-TERC | 0.005
[0–0.385] | 0.44 [0–1.21] | 0.06 [0–0.73] | 0.07 |
| TGF-β | 100.3
[1.51–533.6] | 199.2
[1.873–11200000] | 0.0020
[0–2.7933] | <0.0001 |
TGF-β mRNA expression levels differed among the 3
groups (p<0.0001). TGF-β mRNA levels in the patients with IPF
were significantly higher compared with those in the controls and
patients with NSCLC (Table IVA).
Using linear regression analysis (Spearman’s rank correlation
coefficient), we assessed that TGF-β expression in the lung tissue
positively correlated with h-TERT and h-TERC expression in a very
small percentage of patients with IPF and NSCLC (Table V).
 | Table VCorrelation of h-TERT and h-TERC
relative mRNA expression with TGF-β relative mRNA expression in
patients with IPF and NSCLC. |
Table V
Correlation of h-TERT and h-TERC
relative mRNA expression with TGF-β relative mRNA expression in
patients with IPF and NSCLC.
| Group |
|---|
|
|
|---|
| Telomerase gene per
biological sample | IPF | NSCLC |
|---|
| TGF-β relative mRNA
expression in BALF correlated with h-TERT |
r2=0.015, r=0.3012, p=NS |
r2=0.006, r=0.055, p=NS |
| TGF-β relative mRNA
expression in BALF correlated with h-TERC |
r2=0.022, r=0.070, p=NS |
r2=0.016, r=0.126, p=NS |
| TGF-β relative mRNA
expression in lung tissue correlated with h-TERT |
r2=0.106, r=−0.074, p=NS |
r2=0.006, r=0.077, p=NS |
| TGF-β relative mRNA
expression in lung tissue correlated with h-TERC |
r2=0.089, r=−0.065, p=NS |
r2=0.343, r=0.585, p=0.028 |
Gene expression in BALF
The percentage of the patients with NSCLC and
control subjects expressing h-TERT was lower than that of patients
with IPF (p<0.05, Table III).
The percentages of h-TERC mRNA expression did not differ
significantly among the groups (Table
III).
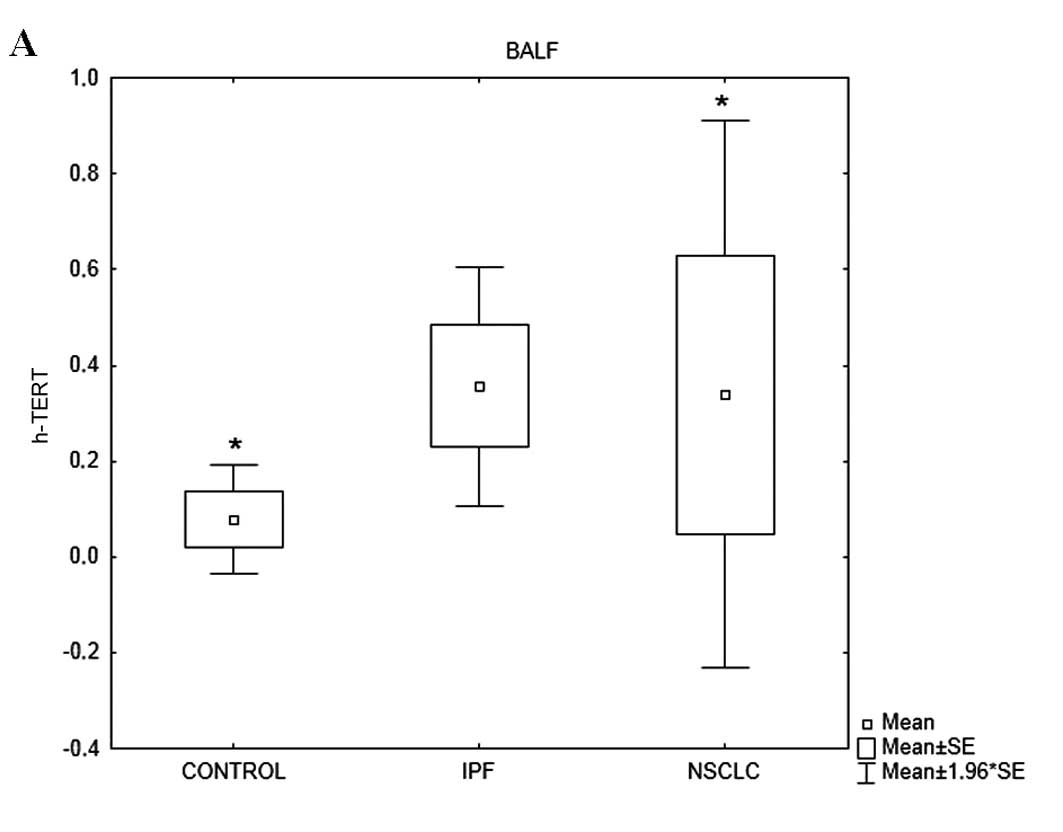
h-TERT mRNA expression levels varied among the
groups (p=0.012) (Table IVB). More
specifically, h-TERT mRNA levels in the patients with IPF were
significantly higher compared with those in the controls (p=0.03)
and patients with NSCLC (p=0.007). h-TERC mRNA expression levels
varied among the 3 groups (p=0.07). Post-hoc analysis revealed that
h-TERC mRNA levels in the patients with IPF tended to be higher
compared with those in the control subjects (p=0.08) (Table IVB, Fig. 2).
TGF-β mRNA expression levels differed among the 3
groups (p<0.0001) (Table IVB).
Using linear regression analysis (Spearman’s rank correlation
coefficient), we assessed that TGF-β expression in the BALF samples
negatively correlated with h-TERT and h-TERC expression in a very
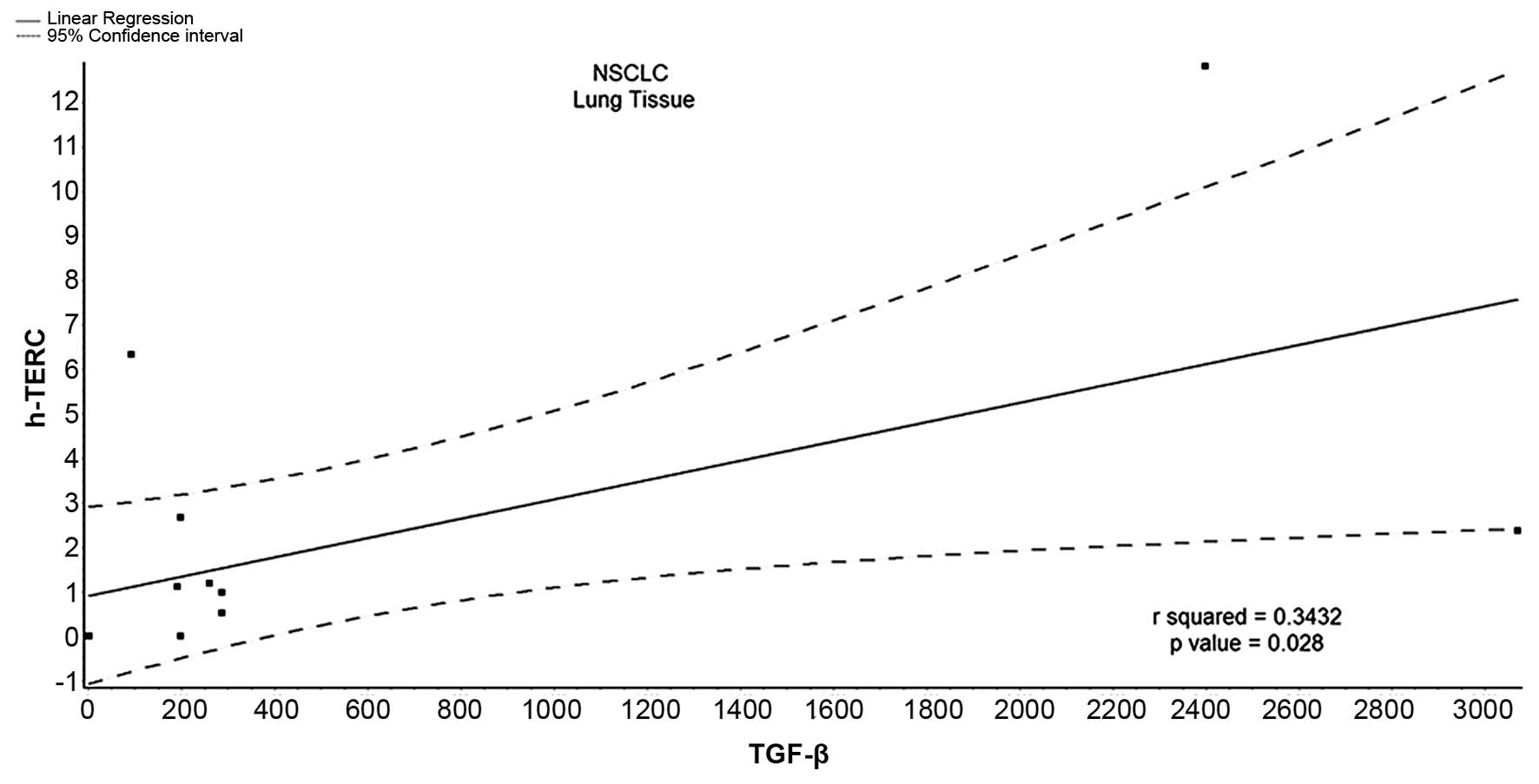
small percentage of patients with IPF (Table V). On the contrary, TGF-β expression
positively correlated with h-TERC in the BALF samples from patients
with NSCLC with a statistically significant p-value
(r2=0.343, p=0.028), while the positive correlation
between TGF-β expression and h-TERT was very weak and statistically
insignificant (Table V, Fig. 3).
Discussion
The main finding of this study is the attenuated
expression of both telomerase subunits measured in lung tissue
obtained from patients with IPF, compared with lung tissue obtained
from patients with NSCLC and control subjects. To our knowledge,
this is the first time that h-TERT/h-TERC expression levels have
been measured in human lung tissue and BALF.
Fibrogenesis and carcinogenesis are attractive
research topics, as although therapeutic efforts have increased
over the past decade, few advances have been made (4–6,20).
Certain studies have suggested a link between IPF and lung cancer
through different pathogenetic mechanisms, such as viral
implications, inflammation, coagulation, dysregulated apoptosis,
focal hypoxia, activation of oncogenes, genetics and the
accumulation of myofibroblasts, as well as extracellular matrix
accumulation (21,22). However, diversities in the
expression of molecular pathways have also been recently described
by ours (14,23) and other study groups (4–6).
Previous studies have demonstrated that mutations in telomerase
h-TERT and h-TERC genes cause a shortening of telomere lengths, and
account for approximately 10% of familial IPF cases (24–26).
In this study, h-TERT mRNA expression was
downregulated in the lung tissue samples obtained from patients
with IPF, which is the most novel finding of our study. Our study
was not designed to unravel the mechanism of h-TERT downregulation
in this disease. Nevertheless, some speculations are worth
pursuing. Decreased h-TERT expression levels may represent
decreased h-TERT mRNA transcription due to downregulation by a
variety of factors, such as p53, TGF-β, Wilms tumor-1 (Wt-1) and
murine double minute 2 (Mdm2) (27), which have been implicated in the
pathogenesis of IPF. These factors have been shown to negatively
regulate h-TERT, thus limiting telomerase activation (28). However, it should be acknowledged
that h-TERT activity may also be regulated by alternative splicing
and post-translational modifications (27). As h-TERT is the catalytic subunit of
the telomerase complex it can be hypothesized that the attenuated
expression also involves the downregulation of telomerase activity.
Another possible explanation for the attenuated h-TERT expression
measured in our patients compared with the healthy controls is the
existence of mutations in the h-TERT gene in patients with IPF,
which would inhibit telomerase activity. However, telomerase
mutations in sporadic IPF cases are rare, detected in only 1 out of
100 patients in the general population (29).
In contrast to the lung tissue samples, h-TERT
expression was augmented in the BALF samples compared with the
healthy controls. This suggests that BALF is not representative of
telomerase expression in tissue and cannot be recommended as
surrogate material of telomerase expression determination in
patients with IPF. The reason for the increased expression in BALF
is not clear and cannot be addressed by the results of our study.
It is highly possible that macrophages and neutrophils which
constitute the major cell subpopulations in BALF from patients with
IPF (30) exhibit increased h-TERT
expression, which is in accordance with previous results from our
group, where bone marrow-derived mesenchymal stem cells from
patients with IPF showed a trend for increased h-TERT expression
compared with the healthy controls (31). h-TERC mRNA expression levels
exhibited the same pattern as the h-TERT levels in patients with
IPF.
The assessment of h-TERT/h-TERC mRNA expression in
lung tissue and BALF from patients with NSCLC revealed a profile
similar to that of the control group for both subunits. In the lung
tissue samples, a trend for increased h-TERC expression was
observed compared with the controls, although statistical
significance was not achieved. However, when comparing NSCLC with
IPF, significant differences were observed. More explicitly, both
h-TERT and h-TERC expression levels were significantly decreased in
the tissue samples from patients with IPF compared with the
patients with NSCLC, suggesting that telomerase genes play a
differential role in fibrogenesis and carcinogenesis. An analysis
of the BALF samples revealed increased h-TERT expression levels in
patients with IPF compared with patients with NSCLC, again
suggesting differences in the telomerase pathway between these two
diseases.
This study further explored the correlation between
a known fibrogenic gene, namely TGF-β, and h-TERT, as well as
h-TERC gene expression. The results revealed no significant
correlation between fibrotic gene expression, represented by TGF-β,
and telomerase gene expression in the IPF samples. Of note, TGF-β
expression in the lung tissue samples from patients with NSCLC was
found to significantly correlate with h-TERC gene expression.
Alveolar epithelial cells exposed to TGF-β have been shown to
gradually lose epithelial markers, such as cytokeratin, and acquire
specific mesenchymal markers, such as α smooth muscle actin
(α-SMA), vimentin and type I collagen (6). In pulmonary fibrosis, epithelial cells
surrounding fibroblast foci express both epithelial and mesenchymal
markers, suggesting that epithelial-mesenchymal transition (EMT)
occurs in those areas of lung tissue, supporting an active role for
EMT in lung fibrogenesis. As EMT is a form of metaplasia, it is
also involved in the early steps of carcinogenesis and cancer cell
invasion (6). Recent data from an
in vitro study indicate that hTERT overexpression promotes
the EMT of cancer cells, thereby contributing to lung cancer
progression through a TGF-β- and β-catenin-mediated pathway
(32).
Implications and limitations
In this study, we measured h-TERT/h-TERC expression
in both BALF and lung tissue samples. Thus, although we did not
determine the cells of origin, combining both tissue and lavage
fluid allowed us to provide complementary information that
elucidates the expression pattern of telomerase in these diseases.
Possible cells of origin of the h-TERT/h-TERC expression from our
BALF and lung tissue samples are alveolar epithelial cells
(30), alveolar macrophages and
leukocytes infiltrating within the lung vessels or the
interstitium. Our group has previously demonstrted that BALF
epithelial cells exhibit genetic instability [microsatellite
instability (MSI)/loss of heterozygosity (LOH)] compared with
leukocytes/macrophages (33), which
suggests that the reduced telomerase expression in epithelial cells
may contribute to the attenuated telomerase expression in the lungs
of patients with IPF. One further limitation of the present study
was that we did not confirm these findings on the protein level in
order to exclude the possibility of non-coding RNA at the
post-transcriptional level. Further studies are required in order
to clarify the cell(s) of origin of telomerase expression in the
healthy and diseased human lung. We did not measure telomere
length, as we did not study separate cell subpopulations of BALF or
lung tissue samples, which is a limitation of our study. Certain
data indicate that shorter telomeres in peripheral blood
lymphocytes positively correlate with telomere length measured in
alveolar epithelial cells from the same individuals (29). Yet, it is not certain that the
degree of telomere shortening observed in circulating leukocytes is
representative of the telomere length of resident lung cells of the
same subjects (34).
In conclusion, in the present study, we demonstrate
that both h-TERT and h-TERC mRNA expression is downregulated in
lung tissue from patients with IPF compared with healthy controls.
The activation of attenuated telomerase genes in IPF has been
implicated as a potential therapeutic strategy (31,35,36).
Moreover, h-TERT and h-TERC expression levels were found to be
significantly decreased in tissue samples from patients with IPF
compared with the patients with NSCLC. These results do not provide
support for a common pathway hypothesis concerning the telomerase
pathway, but rather reveal distinct telomerase activation profiles
between NSCLC and IPF. However, further studies are required to
evaluate telomere length in both diseases.
References
|
1
|
Raghu G: Idiopathic pulmonary fibrosis:
guidelines for diagnosis and clinical management have advanced from
consensus-based in 2000 to evidence-based in 2011. Eur Respir J.
37:743–746. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wuyts WA, Agostini C, Antoniou K, et al:
The pathogenesis of pulmonary fibrosis: a moving target. Eur Respir
J. 41:1207–1218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calado RT and Young NS: Telomere diseases.
N Engl J Med. 361:2353–2365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vancheri C, Failla M, Crimi N and Raghu G:
Idiopathic pulmonary fibrosis: a disease with similarities and
links to cancer biology. Eur Respir J. 35:496–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vancheri C and Du Bois RM: A
progression-free end-point for idiopathic pulmonary fibrosis
trials: lessons from cancer. Eur Respir J. 41:262–269. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vancheri C: Idiopathic pulmonary fibrosis:
an altered fibroblast proliferation linked to cancer biology. Proc
Am Thorac Soc. 9:153–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Collins K and Mitchell JR: Telomerase in
the human organism. Oncogene. 21:564–579. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fernandez-Garcia I, Ortiz-de-Solorzano C
and Montuenga LM: Telomeres and telomerase in lung cancer. J Thorac
Oncol. 3:1085–1088. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Broccoli D, Young JW and De Lange T:
Telomerase activity in normal and malignant hematopoietic cells.
Proc Natl Acad Sci USA. 92:9082–9086. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wright WE, Piatyszek MA, Rainey WE, Byrd W
and Shay JW: Telomerase activity in human germline and embryonic
tissues and cells. Dev Genet. 18:173–179. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim NW, Piatyszek MA, Prowse KR, et al:
Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Diaz de Leon A, Cronkhite JT, Katzenstein
AL, et al: Telomere lengths, pulmonary fibrosis and telomerase
(TERT) mutations. PLoS One. 5:e106802010.PubMed/NCBI
|
|
13
|
American Thoracic Society. Idiopathic
pulmonary fibrosis: diagnosis and treatment. International
consensus statement. American Thoracic Society (ATS) and the
European Respiratory Society (ERS). Am J Respir Crit Care Med.
161:646–664. 2000. View Article : Google Scholar
|
|
14
|
Margaritopoulos GA, Antoniou KM, Soufla G,
Vassalou E, Spandidos DA and Siafakas NM: Yin Yang-1 (YY-1)
expression in idiopathic pulmonary fibrosis. J Recept Signal
Transduct Res. 31:188–191. 2011.PubMed/NCBI
|
|
15
|
Mehrad B, Burdick MD, Zisman DA, et al:
Circulating peripheral blood fibrocytes in human fibrotic
interstitial lung disease. Biochem Biophys Res Commun. 353:104–108.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Samara KD, Antoniou KM, Karagiannis K, et
al: Expression profiles of Toll-like receptors in non-small cell
lung cancer and idiopathic pulmonary fibrosis. Int J Oncol.
40:1397–1404. 2012.PubMed/NCBI
|
|
17
|
Antoniou KM, Soufla G, Proklou A, et al:
Different activity of the biological axis VEGF-Flt-1 (fms-like
tyrosine kinase 1) and CXC chemokines between pulmonary sarcoidosis
and idiopathic pulmonary fibrosis: a bronchoalveolar lavage study.
Clin Dev Immunol. 2009:5379292009. View Article : Google Scholar
|
|
18
|
Margaritopoulos GA, Antoniou KM,
Karagiannis K, et al: Investigation of Toll-like receptors in the
pathogenesis of fibrotic and granulomatous disorders: a
bronchoalveolar lavage study. Fibrogenesis Tissue Repair. 3:202010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Antoniou KM, Soufla G, Lymbouridou R, et
al: Expression analysis of angiogenic growth factors and biological
axis CXCL12/CXCR4 axis in idiopathic pulmonary fibrosis. Connect
Tissue Res. 51:71–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Margaritopoulos GA, Romagnoli M, Poletti
V, Siafakas NM, Wells AU and Antoniou KM: Recent advances in the
pathogenesis and clinical evaluation of pulmonary fibrosis. Eur
Respir Rev. 21:48–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lasithiotaki I, Antoniou KM, Derdas SP, et
al: The presence of merkel cell polyomavirus is associated with
deregulated expression of BRAF and Bcl-2 genes in non-small cell
lung cancer. Int J Cancer. 133:604–611. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lasithiotaki I, Antoniou KM, Vlahava VM,
et al: Detection of herpes simplex virus type-1 in patients with
fibrotic lung diseases. PLoS One. 6:e278002011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Antoniou KM, Margaritopoulos GA, Soufla G,
et al: Expression analysis of Akt and MAPK signaling pathways in
lung tissue of patients with idiopathic pulmonary fibrosis (IPF). J
Recept Signal Transduct Res. 30:262–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Kuan PJ, Xing C, et al: Genetic
defects in surfactant protein A2 are associated with pulmonary
fibrosis and lung cancer. Am J Hum Genet. 84:52–59. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsakiri KD, Cronkhite JT, Kuan PJ, et al:
Adult-onset pulmonary fibrosis caused by mutations in telomerase.
Proc Natl Acad Sci USA. 104:7552–7557. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Diaz de Leon A, Cronkhite JT, Yilmaz C, et
al: Subclinical lung disease, macrocytosis, and premature graying
in kindreds with telomerase (TERT) mutations. Chest. 140:753–763.
2011.PubMed/NCBI
|
|
27
|
Flores I, Benetti R and Blasco MA:
Telomerase regulation and stem cell behaviour. Curr Opin Cell Biol.
18:254–260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin SY and Elledge SJ: Multiple tumour
suppressor pathways negatively regulate telomerase. Cell.
113:881–889. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alder JK, Chen JJ, Lancaster L, et al:
Short telomeres are a risk factor for idiopathic pulmonary
fibrosis. Proc Natl Acad Sci USA. 105:13051–13056. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meyer KC, Raghu G, Baughman RP, et al; for
the American Thoracic Society Committee on BAL in Interstitial Lung
Disease. An official American Thoracic Society clinical practice
guideline: the clinical utility of bronchoalveolar lavage cellular
analysis in interstitial lung disease. Am J Respir Crit Care Med.
185:1004–1014. 2012. View Article : Google Scholar
|
|
31
|
Antoniou KM, Margaritopoulos GA, Proklou
A, et al: Investigation of telomerase/telomeres system in bone
marrow mesenchymal stem cells derived from IPF and RA-UIP. J
Inflamm (Lond). 9:272012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Z, Li Q, Li K, et al: Telomerase
reverse transcriptase promotes epithelial-mesenchymal transition
and stem cell-like traits in cancer cells. Oncogene. 32:4203–4213.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Samara KD, Tzortzaki EG, Neofytou E, et
al: Somatic DNA alterations in lung epithelial barrier cells in
COPD patients. Pulm Pharmacol Ther. 23:208–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cronkhite JT, Xing C, Raghu G, et al:
Telomere shortening in familial and sporadic pulmonary fibrosis. Am
J Respir Crit Care Med. 178:729–737. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Antoniou KM, Papadaki HA, Soufla G and
Siafakas NM: Short telomeres and treatment of pulmonary fibrosis:
implications for early intervention. Am J Respir Crit Care Med.
179:9702009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Garcia CK: Idiopathic pulmonary fibrosis:
update on genetic discoveries. Proc Am Thorac Soc. 8:158–162. 2011.
View Article : Google Scholar : PubMed/NCBI
|