Introduction
Hepatocellular carcinoma (HCC) still remains a
severe health issue in China. More than 402,000 new cases of HCC
(14.3% of all cancer cases) and 372,000 deaths (19.0% of all
cancer-related deaths) were estimated to have occurred in 2008 in
China. HCC is the third most common type of cancer and the third
leading cause of cancer-related mortality among the Chinese
(1). Although the poor outcome of
HCC is mainly attributed to the high rate of advanced stage disease
at diagnosis, the poor response of HCC cells to cytotoxic drugs
also plays a critical role. The chemoresistance of HCC cells,
particularly in its multiple form (multidrug resistance, MDR),
either intrinsic or acquired, is a major obstacle to the successful
management of HCC (2–4).
To improve the efficacy of cytotoxic drugs for HCC,
a few compounds have been utilized as MDR reversal agents to
overcome the chemoresistance of HCC cells (5). However, these compounds frequently
showed intolerable toxicity when they were administered at an
effective dose in clinical trials (6,7). These
defects have limited their application in clinical settings. Thus,
it is urgent to develop novel reversal agents with higher efficacy
and lower toxicity to overcome MDR of HCC.
‘Chansu’, a traditional Chinese medicine composed of
dried toad venom or dried secretion from the skin glands of Bufo
gargarizans or B. melanostictus, has been used to treat
liver cancer in China for several hundreds of years (8). Bufalin, a toxic ligand isolated from
‘Chansu’, has been confirmed as one of the most active components
for the treatment of liver cancer. Moreover, bufalin was found to
exhibit activity for inducing differentiation and apoptosis in
vitro for leukemia (9,10) and cancers of the prostate (11) and stomach (12). Our previous study revealed that
bufalin exhibited significant antitumor activity in an orthotopic
transplantation model of human HCC in nude mice with no marked
toxicity and was able to induce apoptosis of transplanted HCC cells
(13). However, little is known
concerning the possible effect of bufalin on MDR of HCC. Thus, in
the present study, the potential anti-MDR activity of bufalin was
evaluated by using a multidrug-resistant human HCC cell line, and
the possible mechanisms involving the reversal effect of bufalin
were determined.
Materials and methods
Drugs and reagents
Bufalin was purchased from Sigma Chemical Co. (St.
Louis, MO, USA) and initially dissolved in anhydrous alcohol before
serial dilution with RPMI-1640 medium. 5-Fluorouracil (5-FU) was
obtained from Shanghai Xudong Haipu Pharmaceutical Co. (Shanghai,
China). Verapamil was purchased from Shanghai Harvest
Pharmaceutical Co. (Shanghai China), and it was used as a positive
control reversal agent. The primary antibodies against human
thymidylate synthase (TS), P-glycoprotein (P-gp), multidrug
resistance protein 1 (MRP1), B-cell lymphoma-extra large (Bcl-xL)
and Bcl-2-associated X protein (Bax) were all purchased from Santa
Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Cell lines and cell culture
BEL-7402/5-FU, a 5-FU-resistant human HCC cell line
with a moderate MDR phenotype (resistance index = 18), was
successfully established by imitating the administration pattern of
clinical chemotherapy in our previous study (14). The BEL-7402/5-FU cells were able to
grow and passage steadily in medium containing 5-FU at a
concentration of 50 μM and were used as the cell model in the
present study. The cells were cultured in RPMI-1640 medium
(HyClone), supplemented with 10% fetal bovine serum (FBS; Sijiqing,
China) at 37ºC in a humidified atmosphere containing 5%
CO2.
Cell viability assay
The effect of drugs on cell viability was quantified
using the 3-[4,5-dimethyl-2-thiazol]-2,5-diphenyltetrazolium
bromide (MTT) assay. Cells in the exponential growth phase were
trypsinized and seeded in 96-well plates and then treated with the
scheduled treatment of drugs. Medium without drug was added to the
control and blank wells. After incubation for the scheduled time,
10 μl MTT (Amresco, Boston, MA, USA) at a concentration of 5 mg/ml
was added for 4 h at 37ºC. Then culture medium was removed, and the
insoluble formazan crystals were dissolved in 150 μl of dimethyl
sulfoxide (DMSO). The absorbance (optical density, OD) was measured
at 490 nm of the wavelength using a microplate reader. The cell
growth inhibition rate was calculated using the following formula:
Growth inhibition rate = (OD value of control - OD value of
test)/(OD value of control - OD value of blank) × 100%. The 10 and
50% inhibitory concentrations (IC10 and IC50)
were estimated by probit analysis.
MDR reversal assay
A reversal agent should exhibit neither an
inhibitory nor a toxic effect to tumor cells when administered. In
the present study, the IC10 value of bufalin alone for
BEL-7402/5-FU cells was calculated, and a concentration (1 nM)
lower than the IC10 value was designated as the reversal
concentration of bufalin in the following experiments.
BEL-7402/5-FU cells were plated in 96-well plates
and allowed to grow for 12 h. The cells were then pretreated with 1
nM bufalin for an incubation of 2 h, followed by treatment with
serial dilutions of 5-FU for 48 h. Cells treated with 5-FU alone
and verapamil alone were considered as the controls. By using the
MTT assay as described above, the IC50 of each treatment
was calculated. The reversal fold (RF) was defined as the ratio
between the IC50 value of 5-FU alone to that of 5-FU
combined with bufalin, and RF was used as the index of MDR-reversal
activity in the present study.
Cell cycle assay
BEL-7402/5-FU cells were seeded into 6-well plates
and incubated for 24 h. Cells were then treated with bufalin at a
final concentration of 1 nM for 48 h. The cells treated with medium
without bufalin were regarded as the control. After treatment,
cells were trypsinized, pelleted, washed, diluted and then fixed by
suspending the cells in ethanol at 4ºC overnight. After washing and
centrifugation, cells were incubated with 400 μl propidium iodide
(PI) (50 μg/ml; Sigma) and 10 μl DNase-free RNase (1 mg/ml; Sigma)
in phosphate-buffered saline (PBS) in the dark for 30 min. The
cells were measured using a FACScan flow cytometer (BD Biosciences,
San Jose, CA, USA), and the data were analyzed using ModFit LT for
Mac version 3.0 software (Verity Software House, Topsham, ME,
USA).
Apoptosis assay
Quantitative assessment of apoptosis was carried out
using the Annexin V-FITC apoptosis detection kit (Bender
MedSystems, San Bruno, CA, USA). Briefly, BEL-7402/5-FU cells were
seeded directly into 6-well plates and then incubated for 36 h.
Cells were then treated with bufalin at a final concentration of 1
nM for 24 h. Following bufalin treatment, cells were trypsinized,
pelleted, washed and diluted. Annexin V-FITC was added to the cell
suspension at a dilution of 1:40, and the mixture was incubated for
10 min at room temperature. The cells were washed, resuspended and
incubated with propidium iodide (PI) at a final concentration of 1
μg/ml. The cells were then analyzed by using flow cytometry.
Qualitative observation of apoptosis was also
carried out using the Annexin V-FITC apoptosis detection kit as
mentioned above, and the cells coated on coverslips were observed
using a Leica TCS SPE confocal microscope (Leica Microsystems GmbH,
Mannheim, Germany).
Intracellular ADM accumulation assay
Intracellular accumulation of adriamycin (ADM) was
determined by flow cytometry as an index of drug efflux pump
activity (14). Cells were plated
in 6-well plates and cultured for 36 h. Bufalin was then added at a
final concentration of 1 nM for 2 h followed by the addition of ADM
at a final concentration of 20 μg/ml for 2 h. Cells were harvested
and subjected to flow cytometry with excitation measured at 488 nm
and emission measured at 575 nm. The data were analyzed using
CellQuest software (BD Biosciences, San Jose, CA, USA).
Rhodamine 123 retention assay
Intracellular retention of Rhodamine 123 (Rh-123)
was determined by flow cytometry as a functional index of P-gp
activity (14). Cells were plated
in 6-well plates followed by culture for 36 h. Bufalin was then
added at a final concentration of 1 nM for 2 h and subsequently
Rh-123 (Sigma) was added at a final concentration of 0.25 μg/ml for
30 min at 37ºC. Cells were harvested and immediately subjected to
flow cytometric analysis to measure the Rhodamine fluorescence at
an excitation wavelength of 488 nm and an emission wavelength of
530 nm.
Fluorescein accumulation assay
Intracellular accumulation of fluorescein (FLU) was
measured by flow cytometry as a functional index of MRP1 activity
(14). Cells were plated in 6-well
plates and cultured for 36 h. Bufalin was then added at a final
concentration of 1 nM for 2 h, followed by the addition of FLU
(Sigma) at a final concentration of 100 μM for 3 h at 37ºC. Cells
were harvested and intracellular fluorescence of FLU was
immediately measured with flow cytometry at an excitation
wavelength of 488 nm and an emission wavelength of 520 nm.
Reverse transcription and real-time
quantitative PCR
BEL-7402/5-FU cells were plated in 6-well plates and
cultured for 24 h. Bufalin was added at a final concentration of 1
nM for 48 h. Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s protocol. The concentration and purity of the
extracted total RNA were computed by the OD values at 260 and 280
nm. Reverse transcription (RT) was carried out using a PrimeScript™
RT reagent kit (Takara, Shiga, Japan) following the instructions of
the manufacturer. The reaction was run in an ABI 2720 thermal
cycler (Applied Biosystems, Foster City, CA, USA) for 60 min at
37ºC and for 5 sec at 85ºC.
Real-time quantitative PCR was performed with an ABI
PRISM® 7900HT sequence detection system (Applied
Biosystems) using the SYBR® Premix Ex Taq™ II (Perfect
Real-Time) kit (Takara) as described by the manufacturer. The total
volume of reaction was 50 μl, which contained 5 μl of cDNA template
(100 ng), 25 μl of 2X Power SYBR® Green PCR Master Mix,
1 μl forward primer of 10 μM, 1 μl reverse primer of 10 μM and 18
μl ddH2O. The following primers were used to amplify the
target genes: TS, 5′-CCAGTGGAGGCAT TTTGGG-3′ (forward) and
5′-CGTCAGGGTTGGTTTTGA TGG-3′ (reverse); MDR1, 5′-ATATCAGCAGCCCACAT
CAT-3′ (forward) and 5′-GAAGCACTGGGATGTCCGGT-3′ (reverse); MRP1,
5′-CTTGGCCACGTACATTAACATGAT-3′ (forward) and
5′-CCGATTGTCTTTGCTCTTCATG-3′ (reverse); Bcl-xL,
5′-TGAATCGGAGATGGAGACCC-3′ (forward) and 5′-TCAAACTCGTCGCCTGCC-3′
(reverse); Bax, 5′-CTTTTGCTTCAGGGTTTCATC-3′ (forward) and
5′-ATCATCCTCTGCAGCTCCAT-3′ (reverse); and β-actin,
5′-TGTTACAGGAAGTCCCTTGC-3′ (forward) and 5′-AAG
CAATGCTATCACCTCCC-3′ (reverse). All primers were synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China). After an initial
denaturation at 95ºC for 30 sec, PCR was performed for 40 cycles of
95ºC for 5 sec and 60ºC for 30 sec. All samples were run in
triplicate, and data were analyzed by use of the corresponding
software. The specificity of amplification was confirmed by
analyzing the dissociation curves. The relative expression of each
target gene was normalized to β-actin expression and estimated as
values of 2−ΔΔCt.
Protein extraction and western blot
analysis
BEL-7402/5-FU cells were plated in 6-well plates and
cultured for 24 h. Bufalin was added at a final concentration of 1
nM for 48 h. The cells were lysed with RIPA lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) containing 1 mM
phenylmethylsulfonyl fluoride (PMSF) for 15 min, followed by
centrifugation at 20,000 × g for 15 min at 4ºC. The concentrations
of these protein samples were determined using an enhanced BCA
protein assay kit (Beyotime Institute of Biotechnology) according
to the manufacturer’s protocol. The protein samples were mixed with
loading buffer, boiled for 10 min and then separated through sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Proteins were transferred to PVDF membranes (Millipore, Bedford,
MA, USA) and blocked with 5% non-fat dry milk in TBST buffer for 1
h at room temperature. The membranes were then immunoblotted with
mouse anti-human TS monoclonal antibody (sc-33679; 1:500), mouse
anti-human P-gp monoclonal antibody (sc-55510; 1:500), goat
anti-human MRP1 polyclonal antibody (sc-7773; 1:500), mouse
anti-human Bcl-xL monoclonal antibody (sc-8392; 1:500), mouse
anti-human Bax monoclonal antibody (sc-20067; 1:500) and goat
anti-human β-actin polyclonal antibody (sc-1616; 1:1000) in 5%
milk/TBST at 4ºC overnight. The membranes were then washed and
incubated with horseradish peroxidase (HRP)-conjugated anti-mouse
or anti-goat secondary antibodies (Beyotime Institute of
Biotechnology) at a dilution of 1:5,000 for 1 h at room
temperature. The membranes were washed extensively and the signals
were detected using an enhanced chemiluminescence detection system
(Beyotime Institute of Biotechnology). Imaging was performed with a
ChemiDoc™ XRS+ system (Bio-Rad Laboratories), and the bands were
quantified using Image Lab™ software (version 2.0; Bio-Rad
Laboratories).
Statistical analysis
Each experiment was performed independently at least
three times and an individual experiment was performed at least in
triplicate. Data are expressed as means ± standard deviation (SD).
The Student’s t-test was used to compare the difference between two
groups. P<0.05 was considered to indicate a statistically
significant result. All analyses were run using SPSS 15.0
statistical package (SPSS Inc., Chicago, IL, USA).
Results
Bufalin enhances the chemosensitivity of
BEL-7402/5-FU cells to 5-FU
The cytotoxicity of bufalin alone to both parental
BEL-7402 cells and multidrug-resistant BEL-7402/5-FU cells was
initially determined by MTT assay. Bufalin displayed an apparent
cytotoxic effect on both cell lines in a dose- and time-dependent
manner. The IC50 values of bufalin for the parental and
resistant cells were 0.75±0.23 and 0.82±0.37 μM at 24 h, 0.15±0.06
and 0.17±0.04 μM at 48 h and 0.06±0.03 and 0.08±0.05 μM at 72 h,
respectively. The IC50 values of bufalin for both cell
lines at the three time-points were so similar that no significant
difference was detected (P>0.05). The results indicated that
BEL-7402/5-FU cells were not cross-resistant to bufalin. The
IC10 value of bufalin for BEL-7402/5-FU cells at 48 h
was 1.7 nM and consequently a concentration of 1 nM was designated
as the reversal concentration of bufalin in the following
experiments of the present study.
The reversal assay showed that, for BEL-7402/5-FU
cells, the IC50 values of 5-FU alone, 5-FU combined with
1 nM bufalin, and 5-FU combined with 1 μM verapamil at 48 h were
427.6±110.5, 112.4±25.3 and 138.7±33.7 μM, respectively. The RFs of
1 nM bufalin and 1 μM verapamil for BEL-7402/5-FU cells were
3.8-fold (P=0.008) and 3.1-fold (P=0.012), respectively. The
results revealed that 1 nM bufalin increased the sensitivity of
BEL-7402/5-FU cells to 5-FU presenting a similar reversal activity
to that of 1 μM verapamil.
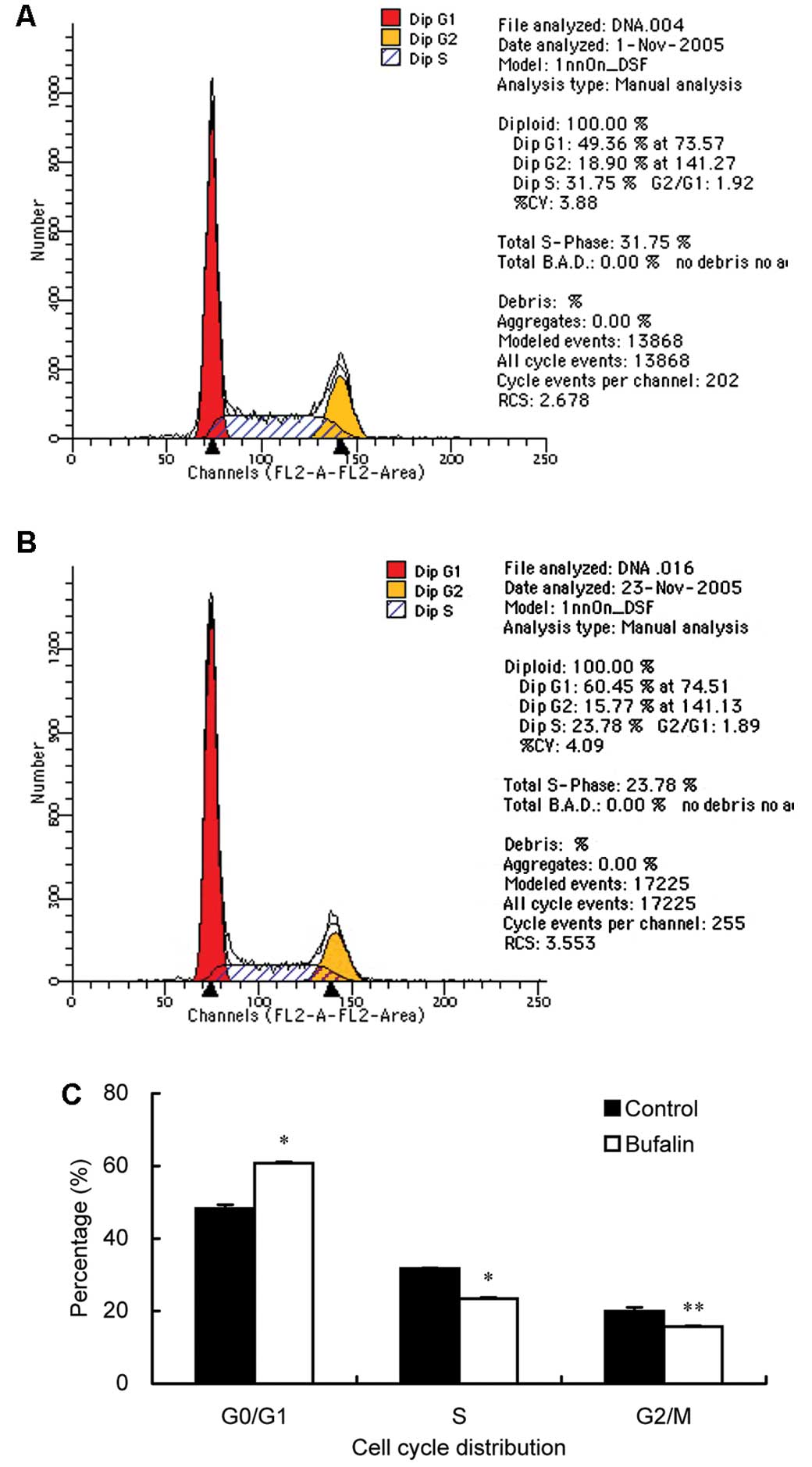
Bufalin arrests cell cycle progression in
BEL-7402/5-FU cells
After incubation with 1 nM bufalin for 48 h, the
proportion of BEL-7402/5-FU cells in the
G0/G1, S and G2/M phases was 60.8,
23.4 and 15.8%, respectively. Compared to BEL-7402/5-FU cells
without bufalin treatment (48.3, 31.7 and 20.0% for
G0/G1, S and G2/M phases,
respectively), bufalin resulted in an obvious cell cycle arrest at
the G0/G1 phase with a concomitant decrease
in the proportion of cells in the S and G2/M phases in
cell cycle distribution (Fig.
1).
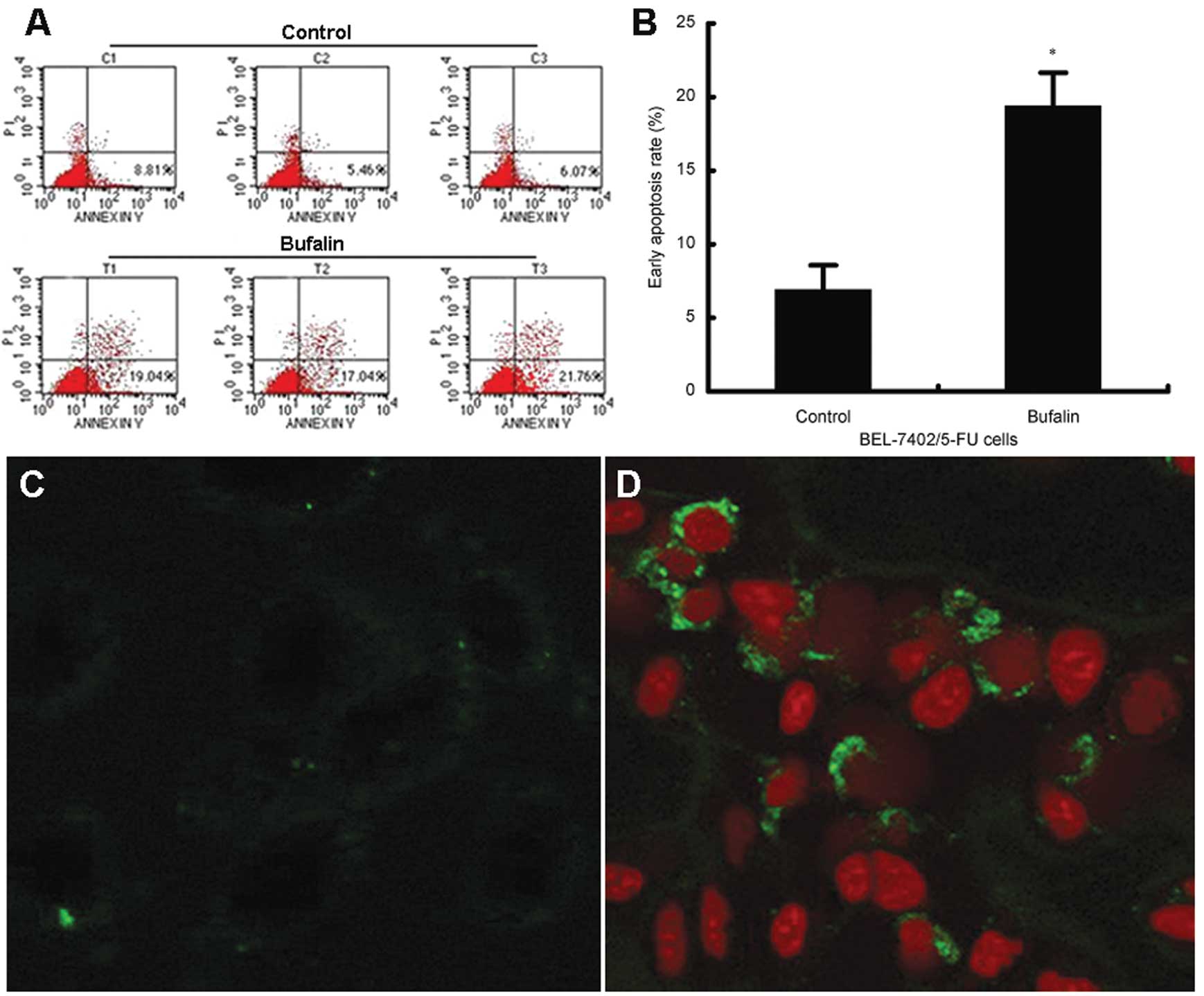
Bufalin induces cell apoptosis in
BEL-7402/5-FU cells
Induction of apoptosis in BEL-7402/5-FU cells by
bufalin at a nanomolar concentration was confirmed in the present
study. Quantitative assessment of apoptosis by Annexin V/PI
staining showed that treatment with 1 nM bufalin for 24 h
significantly increased the early apoptosis rates from 6.8 to 19.3%
(P=0.002) in BEL-7402/5-FU cells (Fig.
2A and B). Obvious apoptosis was also observed using confocal
microscopy following bufalin treatment (Fig. 2C and D).
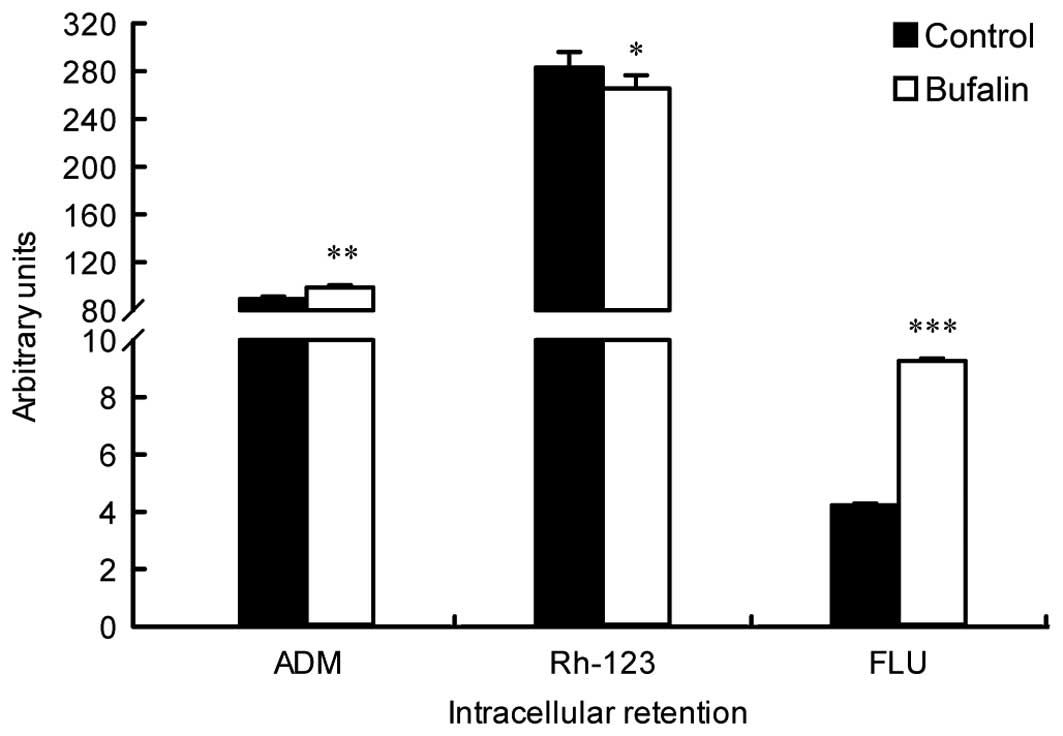
Bufalin decreases the drug efflux pump
activity in BEL-7402/5-FU cells
The effect of bufalin on drug efflux pump activity
in BEL-7402/5-FU cells was evaluated by quantification of
intracellular ADM fluorescence. As shown in Fig. 3, the intracellular ADM intensity in
BEL-7402/5-FU cells after bufalin treatment increased significantly
when compared to the control (P=0.004), which indicated that the
drug efflux pump activity was attenuated by bufalin. The
intracellular retention of Rh-123, a functional index of P-gp
activity, was also determined. Although the Rh-123 intensity in
bufalin-treated BEL-7402/5-FU cells was slightly lower than that in
the control, no significant difference was found between them
(P>0.05). The results suggest that the activity of P-gp may be
of little importance in the reversal effect of bufalin.
Additionally, the intracellular accumulation of FLU, a functional
index of MRP1 activity, was analyzed. As opposed to Rh-123, the
intracellular FLU was significantly enhanced in bufalin-treated
BEL-7402/5-FU cells when compared to the control (P<0.001) which
indicated that activity of MRP1 was markedly inhibited by bufalin
(Fig. 3). Bufalin decreased the
drug efflux pump activity in BEL-7402/5-FU cells via inhibition of
MRP1 but not P-gp.
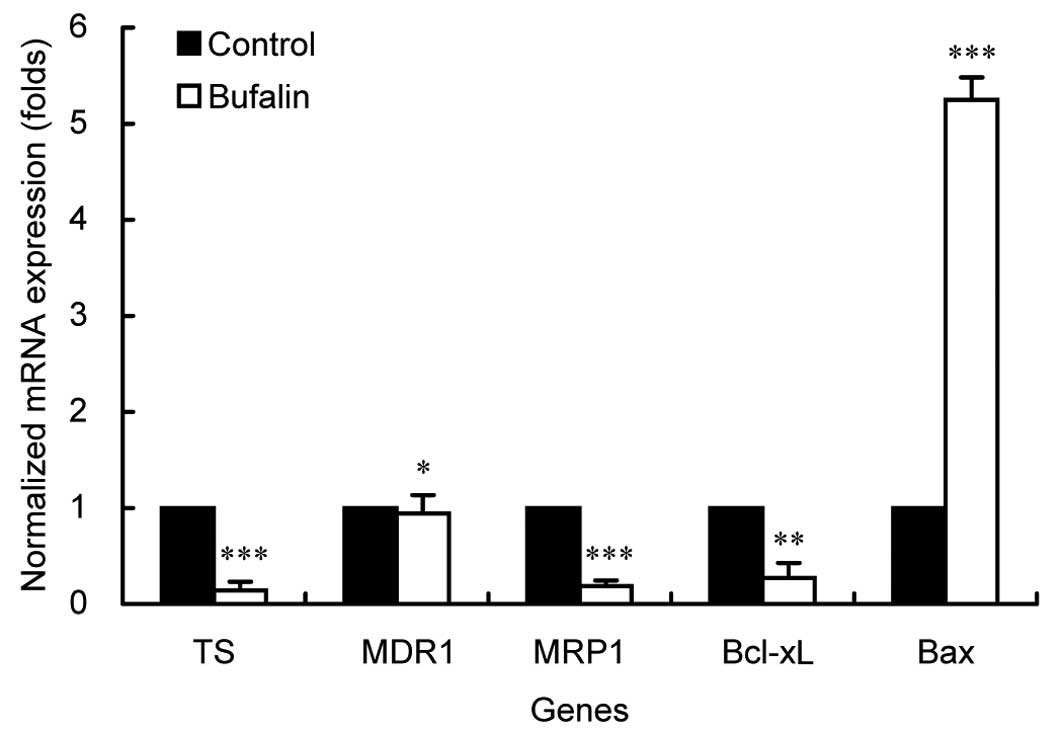
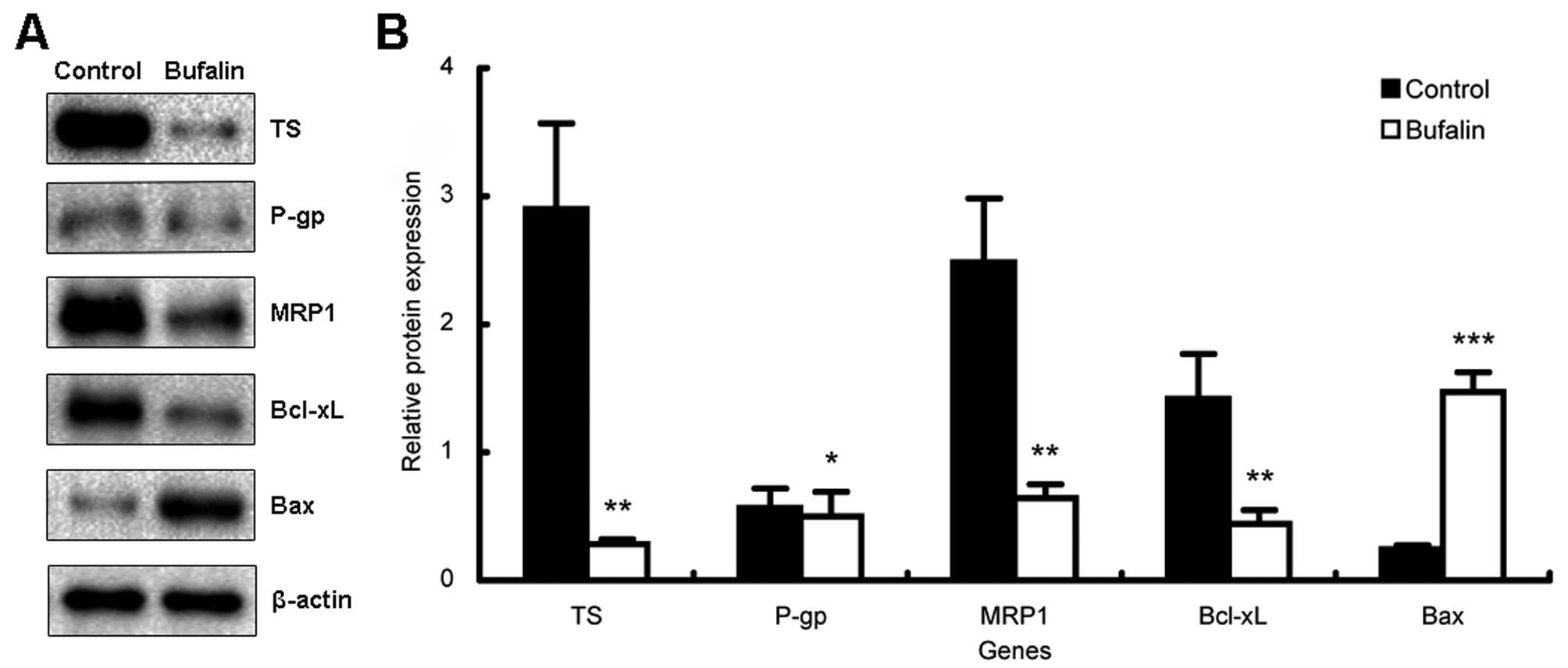
Bufalin downregulates the expression of
TS, MRP1 and the ratio of Bcl-xL/Bax
To further clarify the possible mechanisms related
to the reversal effect on MDR by bufalin, the expression of several
genes involved with MDR of BEL-7402/5-FU cells was quantified by
both real-time PCR and western blot analysis. As shown in Fig. 4, the expression levels of TS, MRP1
and Bcl-xL mRNAs, were significantly downregulated while Bax mRNA
was greatly upregulated by bufalin (P<0.05). Particularly, the
ratio of Bax/Bcl-xL in bufalin-treated BEL-7402/5-FU cells
exhibited an increase of nearly 20-fold compared to the control
cells. However, no change in the mRNA expression of MDR1 was noted
after bufalin treatment (P>0.05). Similar findings were found in
our results using western blot analysis on protein levels (Fig. 5). Collectively, these results
suggest that bufalin strengthens the efficacy of 5-FU through
inhibiting expression of TS, increasing the intracellular drug
concentration via reducing the expression/activity of MRP1,
inducing apoptosis by regulating apoptosis-related genes and
increasing the ratio of Bax/Bcl-xL consequently resulting in an
extensive enhancement of the chemosensitivity of BEL-7402/5-FU
cells to 5-FU.
Discussion
Chemotherapy with cytotoxic drugs plays an essential
role in the management of most malignant tumors. However,
chemotherapy of HCC often results in intolerable toxicity to HCC
patients who usually suffer from concomitant cirrhosis; and thus
has been of limited use in clinical practice (3). Moreover, even for HCC patients with
satisfactory liver function, only a small percentage of patients
gain benefits due to intrinsic or acquired chemoresistance. In
particular, HCC cells universally present an MDR phenotype
immediately after initial chemotherapy; and therefore, HCC is
generally deemed refractory to cytotoxic drugs (4). Therefore, reversing MDR has been a
major challenge for the success of chemotherapy for HCC. Screening
effective chemosensitizers or MDR reversal agents, and combining
them with cytotoxic drugs (fluoropyrimidines, anthracyclines,
platinum complexes) has been a promising strategy to overcome MDR
and thereby successful management of HCC may be achieved (15).
In the past several decades, great efforts have been
made to develop and screen compounds or substances to reverse MDR.
A few compounds, such as verapamil, cyclosporins, quinidine and
tamoxifen, have shown MDR reversal activity to some extent in
vitro(16). However, these
compounds often cause severe toxicity such as heart failure and
immunosuppression resulting in disappointing clinical results
(16). In recent years, several
natural products extracted from Chinese traditional medicine have
been proposed as MDR reversal agents for multidrug resistant
leukemia (17), and cancers of the
breast (18), lung (19), colon (17,20)
and pancreas (21). For HCC,
pheophorbide A from Scutellaria barbata(22), dioscin from Dioscorea
nipponica Makino (23),
schizandrin A from the Fructus schizandrae(24), and Schisandrol A from Schisandra
chinensis(25), have shown
encouraging reversal activity in vitro or in vivo.
These findings indicate that natural products isolated from Chinese
traditional medicine are promising sources of novel MDR reversal
agents and warrant further research.
Bufalin, an active ingredient of the traditional
Chinese medicine ‘Chansu’, has been proven to be a potent inducer
of differentiation in human leukemia cells (9). Moreover, it can induce apoptosis in
human leukemia and a large range of solid tumor cells (10–13).
Recently, several studies have reported that bufalin suppresses the
proliferation of prostate and bladder cancer, osteosarcoma,
choriocarcinoma, endometrial and ovarian cancer, and colon cancer
in vitro, and this activity has been associated with potent
cytotoxicity to human tumor cells through pathways related to
apoposis, cell cycle arrest and autophagy, following treatment
alone or in combination (11,26–31).
However, these findings were observed when fubalin was administered
at a cytotoxic dose, yet, the effect of bufalin on MDR when it is
administered at a non-cytotoxic concentration remains unknown. The
present study was, therefore, designed to examine the potential
reversal activity of bufalin and reveal the possible
mechanisms.
In the present study, a concentration of 1 nM which
was much less than the IC10 at 48 h (1.7 nM) was used as
the reversal concentration of bufalin. Based on the MTT assay,
treatment with 1 nM bufalin did not show any significant cytotoxic
effect on BEL-7402/5-FU cells. Under such a low dosage, bufalin
indeed enhanced the chemosensitivity of BEL-7402/5-FU cells to 5-FU
with a RF of 3.8-fold which was similar to that of 1 μM verapamil
(3.1-fold). To the best of our knowledge, this is the first study
to demonstrate the reversal effect of bufalin on MDR in cancer
chemotherapy.
To explore the mechanisms involved in the reversal
effects of bufalin on MDR of BEL-7402/5-FU cells, alterations in
cell cycle distribution, apoptosis rate, drug efflux pump activity,
and the expression of TS, P-gp, MRP1, Bcl-xL and Bax, were
determined following treatment with 1 nM bufalin. Firstly, our
results showed that bufalin significantly arrested the
BEL-7402/5-FU cells at the G0/G1 phase and
the cells could not enter cell cycle progression and died via the
apoptosis pathway. This finding was supported by previous findings
that bufalin at the same concentration (1 nM) arrested the cell
cycle in endometriotic stromal cells at the
G0/G1 phase and in human leukemia cells at
the G2/M phase (32,33).
It has been reported that the persistent activation of MAP kinase
in response to bufalin was one of the signal transduction pathways
involved in bufalin-induced apoptosis (34). Particularly, bufalin induced marked
apoptosis in human HCC HepG2 cells via both the Fas- and
mitochondrial-mediated pathways, and a Fas-mediated
caspase-10-dependent pathway may play a crucial role (35). However, in the BEL-7402/5-FU cells
treated with bufalin, downregulation of Bcl-xL and the upregulation
of Bax and a large increase in the ratio of Bax/Bcl-xL were
confirmed in the present study, which contributed to the
resensitivity of BEL-7402/5-FU cells to 5-FU. These results were
similar to the findings in human gastric cancer MGC803 cells and
our previous study in vivo(12,13).
Secondly, the drug efflux pump activity of BEL-7402/5-FU cells was
significantly attenuated by 1 nM bufalin. As shown in Figs. 3–5,
the activity and expression of MRP1 but not P-gp was greatly
inhibited. The successful inhibition of the specific ATP-binding
cassette transporter in the present study led to a significant
reversal of MDR. Additionally, bufalin markedly downregulated the
expression of TS, a rate-limiting enzyme in DNA biosynthesis and
the primary target of 5-FU (36),
which also contributed to the enhanced chemosensitivity of
BEL-7402/5-FU cells.
In conclusion, the present study confirmed the
reversal effect of bufalin on MDR in multidrug-resistant HCC cells
at a low nanomolar concentration. Bufalin at a non-cytotoxic dose
arrested the cell cycle at the G0/G1 phase,
induced apoptosis through an increase in the Bax/Bcl-xL ratio,
inhibited the drug efflux pump activity via downregulation of MRP1
and reduced the expression of TS in BEL-7402/5-FU cells. Our
results suggest that bufalin can effectively reverse the MDR in HCC
cells through multiple pathways. Thus, the combination of bufalin
with cytotoxic drugs may serve as a promising strategy for the
chemotherapy of HCC.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 30600814) and the
‘Qimingxing’ Plan for Young Scientists, Shanghai Municipal Science
and Technology Commission (no. 08QA14003).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: GLOBOCAN 2008. Cancer Incidence and
Mortality Worldwide: IARC CancerBase No. 10 [Internet].
International Agency for Research on Cancer; Lyon, France: 2010,
[http://globocan.iarc.fr].
|
|
2
|
Marin JJ, Romero MR and Briz O: Molecular
bases of liver cancer refractoriness to pharmacological treatment.
Curr Med Chem. 17:709–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu AX: Systemic therapy of advanced
hepatocellular carcinoma: how hopeful should we be? Oncologist.
11:790–800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas MB, O’Beirne JP, Furuse J, Chan AT,
Abou-Alfa G and Johnson P: Systemic therapy for hepatocellular
carcinoma: cytotoxic chemotherapy, targeted therapy and
immunotherapy. Ann Surg Oncol. 15:1008–1014. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JH, Chung JB, Park IS, et al: Combined
use of tamoxifen, cyclosporin A, and verapamil for modulating
multidrug resistance in human hepatocellular carcinoma cell lines.
Yonsei Med J. 34:35–44. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferry DR, Traunecker H and Kerr DJ:
Clinical trials of P-glycoprotein reversal in solid tumours. Eur J
Cancer. 32A:1070–1081. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thomas H and Coley HM: Overcoming
multidrug resistance in cancer: an update on the clinical strategy
of inhibiting p-glycoprotein. Cancer Control. 10:159–165.
2003.PubMed/NCBI
|
|
8
|
Meng Z, Yang P, Shen Y, et al: Pilot study
of huachansu in patients with hepatocellular carcinoma,
non-small-cell lung cancer, or pancreatic cancer. Cancer.
115:5309–5318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang LS, Nakaya K, Yoshida T and Kuroiwa
Y: Bufalin as a potent inducer of differentiation of human myeloid
leukemia cells. Biochem Biophys Res Commun. 178:686–693. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watabe M, Ito K, Masuda Y, Nakajo S and
Nakaya K: Activation of AP-1 is required for bufalin-induced
apoptosis in human leukemia U937 cells. Oncogene. 16:779–787. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yeh JY, Huang WJ, Kan SF and Wang PS:
Effects of bufalin and cinobufagin on the proliferation of
androgen-dependent and -independent prostate cancer cells.
Prostate. 54:112–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li D, Qu X, Hou K, et al: PI3K/Akt is
involved in bufalin-induced apoptosis in gastric cancer cells.
Anticancer Drugs. 20:59–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han KQ, Huang G, Gu W, Su YH, Huang XQ and
Ling CQ: Anti-tumor activities and apoptosis-regulated mechanisms
of bufalin on the orthotopic transplantation tumor model of human
hepatocellular carcinoma in nude mice. World J Gastroenterol.
13:3374–3379. 2007.
|
|
14
|
Gu W, Fang FF, Li B, Cheng BB and Ling CQ:
Characterization and resistance mechanisms of a
5-fluorouracil-resistant hepatocellular carcinoma cell line. Asian
Pac J Cancer Prev. 13:4807–4814. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi LX, Ma R, Lu R, et al: Reversal effect
of tyroservatide (YSV) tripeptide on multi-drug resistance in
resistant human hepatocellular carcinoma cell line BEL-7402/5-FU.
Cancer Lett. 269:101–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robert J and Jarry C: Multidrug resistance
reversal agents. J Med Chem. 46:4805–4817. 2003. View Article : Google Scholar
|
|
17
|
Ramachandran C, Rabi T, Fonseca HB,
Melnick SJ and Escalon EA: Novel plant triterpenoid drug amooranin
overcomes multidrug resistance in human leukemia and colon
carcinoma cell lines. Int J Cancer. 105:784–789. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ravizza R, Gariboldi MB, Molteni R and
Monti E: Linalool, a plant-derived monoterpene alcohol, reverses
doxorubicin resistance in human breast adenocarcinoma cells. Oncol
Rep. 20:625–630. 2008.PubMed/NCBI
|
|
19
|
Xu M, Sheng LH, Zhu XH, Zeng SB and Zhang
GJ: Reversal effect of Stephania tetrandra-containing Chinese herb
formula SENL on multidrug resistance in lung cancer cell line
SW1573/2R120. Am J Chin Med. 38:401–413. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han Y, Bu LM, Ji X, Liu CY and Wang ZH:
Modulation of multidrug resistance by andrographolid in a
HCT-8/5-FU multidrug-resistant colorectal cancer cell line.
Chin J Dig Dis. 6:82–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Borska S, Sopel M, Chmielewska M, Zabel M
and Dziegiel P: Quercetin as a potential modulator of
P-glycoprotein expression and function in cells of human pancreatic
carcinoma line resistant to daunorubicin. Molecules. 15:857–870.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang PM, Chan JY, Zhang DM, et al:
Pheophorbide a, an active component in Scutellaria barbata,
reverses P-glycoprotein-mediated multidrug resistance on a human
hepatoma cell line R-HepG2. Cancer Biol Ther. 6:504–509. 2007.
|
|
23
|
Sun BT, Zheng LH, Bao YL, Yu CL, Wu Y,
Meng XY and Li YX: Reversal effect of Dioscin on multidrug
resistance in human hepatoma HepG2/adriamycin cells. Eur J
Pharmacol. 654:129–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang M, Jin J, Sun H and Liu GT: Reversal
of P-glycoprotein-mediated multidrug resistance of cancer cells by
five schizandrins isolated from the Chinese herb Fructus
Schizandrae. Cancer Chemother Pharmacol. 62:1015–1026. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fong WF, Wan CK, Zhu GY, Chattopadhyay A,
Dey S, Zhao Z and Shen XL: Schisandrol A from Schisandra
chinensis reverses P-glycoprotein-mediated multidrug resistance
by affecting Pgp-substrate complexes. Planta Med. 73:212–220.
2007.
|
|
26
|
Yu CH, Kan SF, Pu HF, Jea Chien E and Wang
PS: Apoptotic signaling in bufalin-and cinobufagin-treated
androgen-dependent and -independent human prostate cancer cells.
Cancer Sci. 99:2467–2476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hong SH and Choi YH: Bufalin induces
apoptosis through activation of both the intrinsic and extrinsic
pathways in human bladder cancer cells. Oncol Rep. 27:114–120.
2012.PubMed/NCBI
|
|
28
|
Yin JQ, Shen JN, Su WW, et al: Bufalin
induces apoptosis in human osteosarcoma U-2OS and U-2OS
methotrexate 300-resistant cell lines. Acta Pharmacol Sin.
28:712–720. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takai N, Ueda T, Ishii T, et al: Effects
of bufalin on the proliferation of human choriocarcinoma cells. Int
J Gynecol Cancer. 21:1105–1109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takai N, Ueda T, Nishida M, Nasu K and
Narahara H: Bufalin induces growth inhibition, cell cycle arrest
and apoptosis in human endometrial and ovarian cancer cells. Int J
Mol Med. 21:637–643. 2008.PubMed/NCBI
|
|
31
|
Xie CM, Chan WY, Yu S, Zhao J and Cheng
CH: Bufalin induces autophagy-mediated cell death in human colon
cancer cells through reactive oxygen species generation and JNK
activation. Free Radic Biol Med. 51:1365–1375. 2011. View Article : Google Scholar
|
|
32
|
Nasu K, Nishida M, Ueda T, Takai N, Bing
S, Narahara H and Miyakawa I: Bufalin induces apoptosis and the
G0/G1 cell cycle arrest of endometriotic stromal cells: a promising
agent for the treatment of endometriosis. Mol Hum Reprod.
11:817–823. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jing Y, Watabe M, Hashimoto S, Nakajo S
and Nakaya K: Cell cycle arrest and protein kinase modulating
effect of bufalin on human leukemia ML1 cells. Anticancer Res.
14:1193–1198. 1994.PubMed/NCBI
|
|
34
|
Watabe M, Masuda Y, Nakajo S, Yoshida T,
Kuroiwa Y and Nakaya K: The cooperative interaction of two
different signaling pathways in response to bufalin induces
apoptosis in human leukemia U937 cells. J Biol Chem.
271:14067–14072. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qi F, Inagaki Y, Gao B, et al: Bufalin and
cinobufagin induce apoptosis of human hepatocellular carcinoma
cells via Fas- and mitochondria-mediated pathways. Cancer Sci.
102:951–958. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang N, Yin Y, Xu SJ and Chen WS:
5-Fluorouracil: mechanisms of resistance and reversal strategies.
Molecules. 13:1551–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|