Introduction
Gastric cancer is the fourth most frequently
diagnosed cancer and the second leading cause of cancer-related
mortality (1). An estimated 989,000
new cases of gastric cancer and 738,000 deaths due to gastric
cancer occurred worldwide in 2008 alone, which accounted for 8% of
all new cases of cancer and 10% of all cancer-related deaths
(2). The highest incidence rates of
gastric cancer have been reported in Eastern Asia, Eastern Europe,
and South America, and the lowest rates have been reported in North
America and most parts of Africa (3). In addition to surgical resection,
chemotherapy constitutes an important treatment regimen for gastric
cancer (4). However, despite major
improvements in diagnosis and treatment regimens, gastric cancer
remains one of the most lethal types of cancer, with <20% of
patients surviving up to 5 years. Thus, novel agents that are
nontoxic, efficacious, and can significantly enhance the effects of
existing chemotherapeutic drugs are urgently required.
Angiogenesis is one of the most important factors in
tumor growth and metastasis. We previously reported the critical
role of angiogenesis in tumorigenesis and metastasis in a variety
of gastrointestinal carcinomas (5–12).
Many angiogenic factors were previously demonstrated to be involved
in gastric cancer; among them, vascular endothelial growth factor
(VEGF) is one of the major cytokines involved in angiogenesis in
gastrointestinal tumors. In combination with its receptors, VEGF
promotes endothelial cell proliferation and new blood vessel
formation in in vitro models of angiogenesis (13). We also previously clarified the role
of VEGF in gastric cancer angiogenesis using an original in
vitro angiogenesis assay model, and revealed a correlation
between VEGF expression and the metastatic potential of gastric
cancer (14). Moreover, some
studies have demonstrated that VEGF expression in gastric cancer
correlates with patient survival (15,16).
In addition, the serum concentration of VEGF has been reported to
be elevated in patients with gastric cancer compared to healthy
controls (17) and has been shown
to correlate with patient survival (18). Therefore, we hypothesized that
VEGF-targeted therapy may have some therapeutic potential in the
treatment of gastric cancer.
Aberrant expression of nuclear factor-κB (NF-κB),
which belongs to the rel family of transcription factors, has been
associated with gastric carcinogenesis (19). Preliminary results have demonstrated
that NF-κB is constitutively activated in most human gastric cancer
cell lines and primary tumor specimens (20,21).
Previous studies have suggested an important role for NF-κB in the
regulation of apoptosis, cell adhesion, oncogenesis, and
angiogenesis (22). Regulation of
gene expression by NF-κB is controlled mainly by inhibitory IκB
proteins, including IκBα; on stimulation, IκBα is rapidly
phosphorylated and degraded via the ubiquitin-proteasome pathway,
permitting activation and nuclear importation of NF-κB (23,24).
Moreover, a previous study demonstrated that NF-κB plays an
important role in VEGF expression and angiogenesis in gastric
cancer (25). These data suggest
that NF-κB may be an effective therapeutic target in the treatment
of gastric cancer. Bortezomib, a proteasome inhibitor that also
inhibits NF-κB activity, is already being used for the treatment of
patients with multiple myeloma. In addition, bortezomib has been
suggested to have potential as a novel molecular targeting drug for
the treatment of unresectable advanced gastric cancer (26). However, treatment with bortezomib
has been shown to elicit some critical adverse effects, such as
peripheral neuropathy (27).
Therefore, new and more nontoxic drugs that inhibit NF-κB activity
are required.
Natural products, generally regarded as safe, have
been shown to mediate anticancer activities in a variety of cell
types (28). Since zerumbone is
derived from a subtropical ginger (Zingiber zerumbet Smith)
and should therefore have minimum toxicity, it is used routinely in
traditional medicine (29).
Moreover, zerumbone was previously reported to have antigrowth and
anti-inflammatory properties in several cancer cell lines (30–38).
However, to date, no studies have described such properties in
gastric cancer. Treatment with zerumbone has been reported to
moderate NF-κB, tumor necrosis factor-α (TNF-α), induced nitric
oxide synthase (iNOS), cyclooxygenase-2 (COX-2) and CXC chemokine
receptor 4 (CXCR4) (30,31,39).
However, the effects of zerumbone on cancer angiogenesis have yet
to be fully elucidated.
In the present study, we sought to determine the
role of zerumbone in gastric cancer angiogenesis. Initially, we
confirmed that the secretion of VEGF in AGS cells was increased
compared to secretion of these factors in other gastric cancer
cells. Zerumbone inhibited both the secretion of VEGF and the
activity of NF-κB in AGS. Consequently, treatment with zerumbone
significantly blocked gastric cancer-induced tube formation by
human umbilical vein endothelial cells (HUVECs). Coculture of
HUVECs and fibroblasts (FBs) for a total of 2 weeks with or without
AGS cells allowed us to investigate tumor-induced angiogenesis from
the viewpoint of interactions between endothelial cells and their
stromal cells, and we therefore evaluated the effects of zerumbone
on gastric cancer-induced angiogenesis in more detail in
vitro. Our data demonstrated that zerumbone suppressed
angiogenesis in gastric cancer by blocking NF-κB activity and
subsequent production of angiogenic factors. To our knowledge, this
is the first study to demonstrate the effective role of zerumbone
in gastric cancer angiogenesis. Based on our results, zerumbone may
be useful in treating gastric cancer.
Materials and methods
Cell lines and agents
Zerumbone was purchased from Wako, Japan, dissolved
in dimethyl sulfoxide (DMSO) as a 50 mM stock, and stored at 4°C.
The following gastric cancer cell lines were used: MKN1, MKN28,
MKN45, MKN74 and NUGC4 (JCRB, Japan), and AGS (ATCC, Washington,
DC, USA). AGS cells were cultured in HAM-F12 (Wako) with 10% fetal
bovine serum (FBS) and 1% antibiotics and antimycotics (10,000
units penicillin, 10 mg streptomycin, and 25 μg amphotericin B per
ml; Sigma, St. Louis, MO, USA) at 37°C in an atmosphere of 5%
CO2 and 95% air. Other cells were cultured in RPMI-1640
(Sigma) with 10% FBS and 1% antibiotics and antimycotics.
WST-1 assay
We examined the proliferation of gastric cancer
cells using the Premix WST-1 Cell Proliferation Assay System
(Takara Bio Inc., Japan). Gastric cancer cells (500 or 2,000
cells/well) were seeded into 96-well plates and incubated with
different concentrations of zerumbone for 72 h. The premix WST-1
was added to the multi-well plate, and the absorbance was measured
at 450 nm in each well using a SPECTRAmax 340 spectrophotometer
(Molecular Devices, Sunnyvale, CA, USA).
Real-time reverse transcription
polymerase chain reaction (RT-PCR)
The mRNA expression of VEGF in gastric cancer cells
was measured with real-time RT-PCR. A total of 1×105 AGS
cells were cultured with 10 ml medium in 100-mm dishes, treated
with zerumbone for 72 h, and collected. RNA was extracted from cell
pellets using an RNeasy Plus Mini kit (Qiagen, TX, USA), and RT-PCR
was performed using Superscript III First-strand Synthesis SuperMix
for qRT-PCR (Invitrogen, Carlsbad, CA, USA). The concentration of
each cDNA was measured by NanoDrop1000 (Thermo Fisher Scientific,
DE, USA) and adjusted to 40 ng/ml with diethylpyrocarbonate (DPEC)
water. We performed real-time PCR with FAM-labeled TaqMan probes
[VEGF: Hs00173625_m, GAPDH: Hs99999905_m1,
β2-microglobulin (β2M): Hs99999907_m1; Applied Biosystems,
Foster City, CA, USA] and TaqMan Universal Master Mix (Applied
Biosystems) using Chromo4 (Bio-Rad, Cambridge, MA, USA). PCR was
carried out by an initial incubation at 50°C for 2 min, followed by
denaturation at 95°C for 10 min and 50 cycles of 95°C for 15 sec
and 60°C for 1 min. The expression of VEGF mRNA was
normalized to that of β2M mRNA. We did not use GAPDH
as an internal control as zerumbone treatment in gastric cancer
cell lines resulted in alteration of GAPDH mRNA expression.
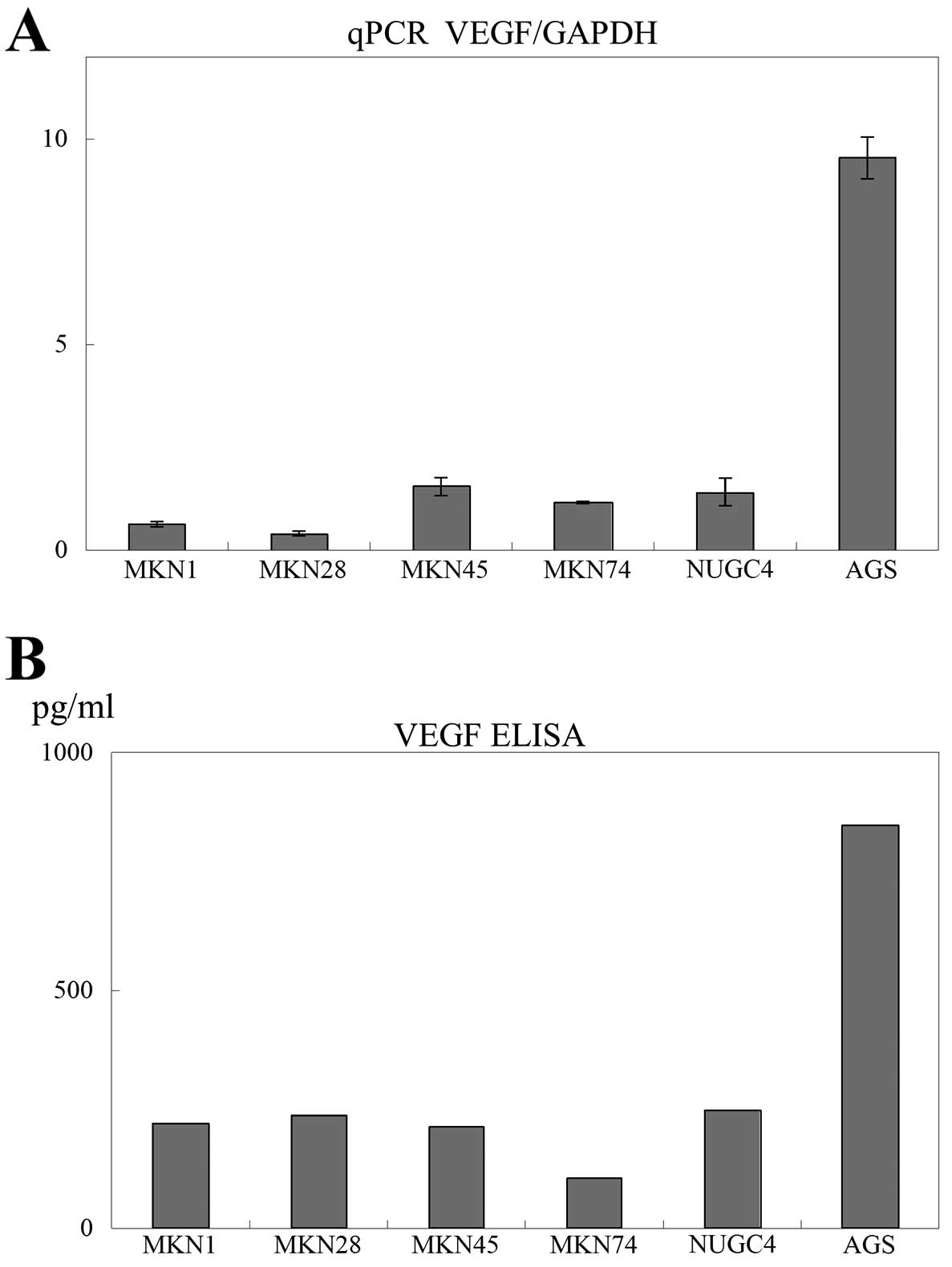
Since VEGF mRNA expression was the highest in AGS cells
compared to that in the other gastric cancer cell lines tested
(Fig. 1A), we used AGS cells in
subsequent experiments.
Enzyme-linked immunosorbent assay
(ELISA)
The secretion of VEGF was determined using
Quantikine ELISA Human VEGF Immunoassay (DVE00; R&D Systems,
Minneapolis, MN, USA). A total of 1×105 AGS cells were
seeded in 100-mm dishes and incubated with different concentrations
of zerumbone. The supernatants of gastric cancer cells were
collected after 72 h of treatment with zerumbone. The supernatants
were microfuged at 1,500 rpm for 5 min to remove particles and
frozen at −20°C until use in ELISA. According to the manufacturer’s
instructions, supernatant samples from gastric cancer cell cultures
were added to 96-well microplates coated with mouse monoclonal
antibodies targeting VEGF. After washing, horseradish peroxidase
(HRP)-conjugated polyclonal VEGF antibodies were added. The wells
were washed again, and hydrogen peroxide and tetramethylbenzidine
were added. The absorbance of each well was measured, and
quantification was carried out using diluted recombinant
VEGF165 as a standard.
NF-κB (p65) transcription factor
assay
A total of 5×105 AGS cells were cultured
with 10 ml medium in 100-mm dishes. Cells were treated with
zerumbone for 12 h, and nuclear proteins were extracted using
NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo
Scientific, IL, USA). The concentrations of nuclear proteins were
measured using a Pierce BCA Protein Assay kit (Thermo Scientific),
and protein concentrations were adjusted for equal loading. Equal
amounts of nuclear proteins were then added to 96-well plates
coated with a specific double-stranded DNA sequence containing the
NF-κB response element. NF-κB, extracted from AGS cells, was bound
with primary anti-NF-κB antibodies. After washing, secondary
antibodies conjugated to HRP were added. The absorbance of each
well of the multi-well plate was then measured at 450 nm. We used
the transcription factor NF-κB (human p65) positive control (P.C.)
from the assay kit and the positive control and transcription
factor NF-κB specific competitor dsDNA as a negative control
(N.C.).
Electrophoretic mobility shift assay
(EMSA)
To reconfirm the activity of NF-κB in AGS cells,
EMSA was carried out using gel shift assay system (Promega,
Madison, WI, USA) according to the manufacturer’s instructions. The
following sequence was used for NF-κB
(5′-AGTTGAGGGGACTTTCCCAGGC-3′). Oligonucleotide probes were labeled
using T4 polynucleotide kinase and [γ-32P]-ATP (3,000
Ci/mmol; Amersham, Piscataway, NJ, USA) and purified by ethanol
precipitation. Nuclear extracts (10 μg) were incubated with
32P-labeled oligonucleotide, binding buffer, and gel
loading buffer at room temperature. The samples were then loaded on
nondenaturing 4% acrylamide gels in 0.5X Tris-borate-EDTA buffer
and run at 350 V. The gels were dried and exposed to X-ray
film.
In vitro angiogenesis assay
In vitro angiogenesis assay was performed
using an Angiogenesis kit (Kurabo, Japan). HUVECs and neonatal
normal human dermal FBs were cultured in 24-well plates with basal
medium and 2% FBS Ham-F12 medium. AGS cells were cultured in the
upper chamber, separated from the lower chamber with a membrane
having 0.45-μm pores (2×103 cells/well). The medium was
changed on the fourth, seventh, and ninth day. The upper chamber
was changed on the seventh day (2×103 cells/well).
HUVECs were stained with anti-CD31 antibodies on the 11th day. Tube
formation areas were quantified by counting 8 random fields per
sample under a microscope (magnification, ×40) and pixelized with
an image analyzer. We used 10 ng/ml recombinant VEGF-A as a
positive control.
Statistical analysis
The data were analyzed using nonrepeated measures
ANOVA and Student-Newman-Keuls test versus the vehicle-treated
control (0.1% DMSO). For NF-κB and in vitro angiogenesis
assay, we used Student-Newman-Keuls test for multiple
comparisons.
Results
VEGF expression in gastric cancer cell
lines
First, the mRNA expression of VEGF was
measured in gastric cancer cell lines (MKN1, MKN28, MKN45, MKN74,
NUGC4 and AGS) using real-time quantitative RT-PCR and ELISA. The
mRNA expression of VEGF in AGS cells was higher than that in
other gastric cancer cell lines (Fig.
1A). The secretion of VEGF was also higher in AGS cells than in
other gastric cancer cell lines (Fig.
1B). Since AGS cells exhibited the highest expression of VEGF,
we used AGS cells in subsequent experiments.
Effects of zerumbone on gastric cancer
cell proliferation
The proliferation of gastric cancer cells was
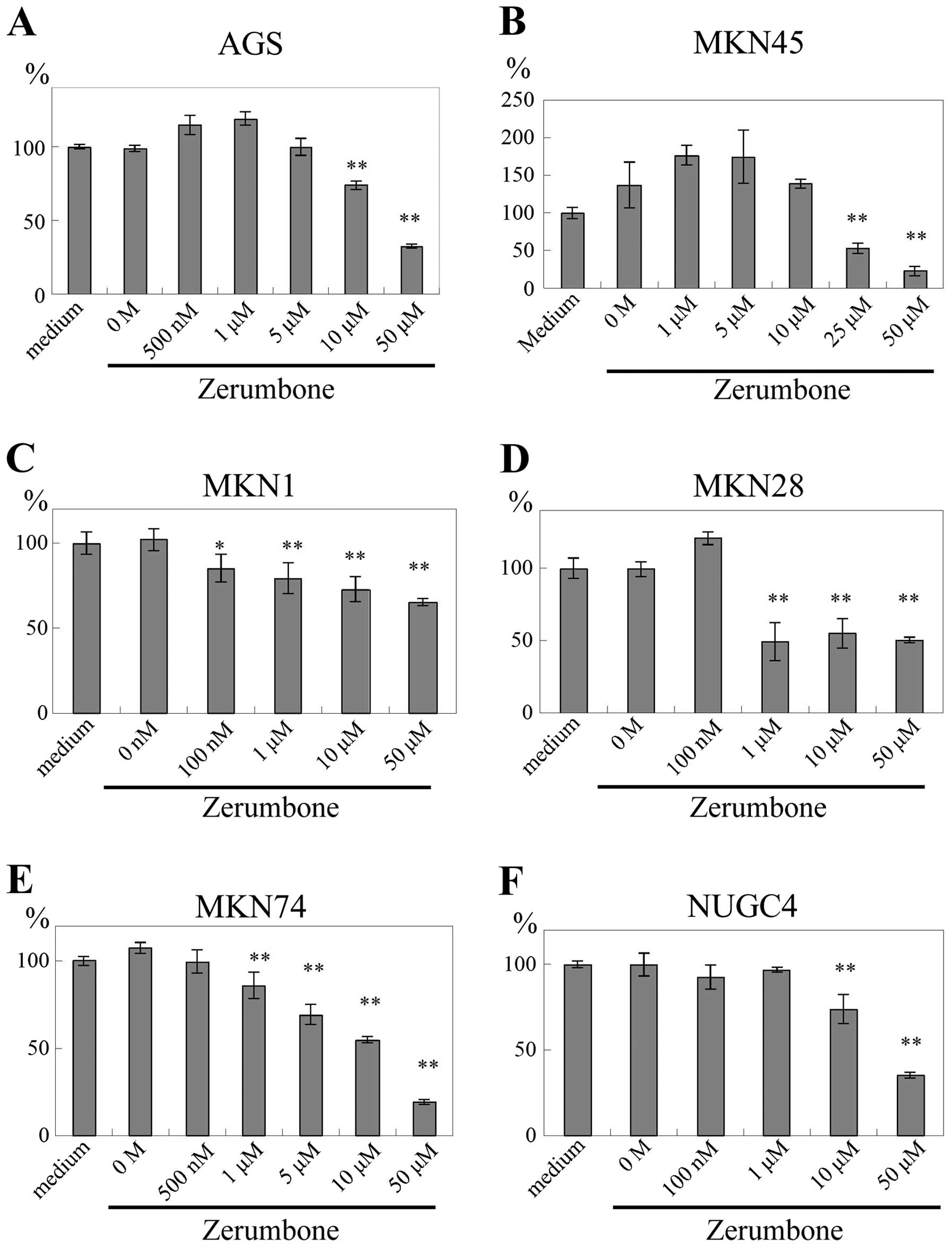
measured using WST-1 assay. AGS cell proliferation was inhibited by
zerumbone at concentrations of ≥10 μM (P<0.01; Fig. 2A). Zerumbone also inhibited the
proliferation of other gastric cancer cell lines in a
dose-dependent manner. MKN45 cells were inhibited by zerumbone at
≥25 μM (P<0.01; Fig. 2B); MKN1
cells were inhibited by zerumbone at ≥100 nM (P<0.05) and ≥1 μM
(P<0.01; Fig. 2C); MKN28 cells
were inhibited by zerumbone at ≥1 μM (P<0.01; Fig. 2D); MKN74 cells were inhibited by
zerumbone at ≥1 μM (P<0.01; Fig.
2E); and NUGC4 cells were inhibited by zerumbone at ≥10 μM
(P<0.01; Fig. 2F).
Effects of zerumbone on VEGF mRNA
expression in gastric cancer
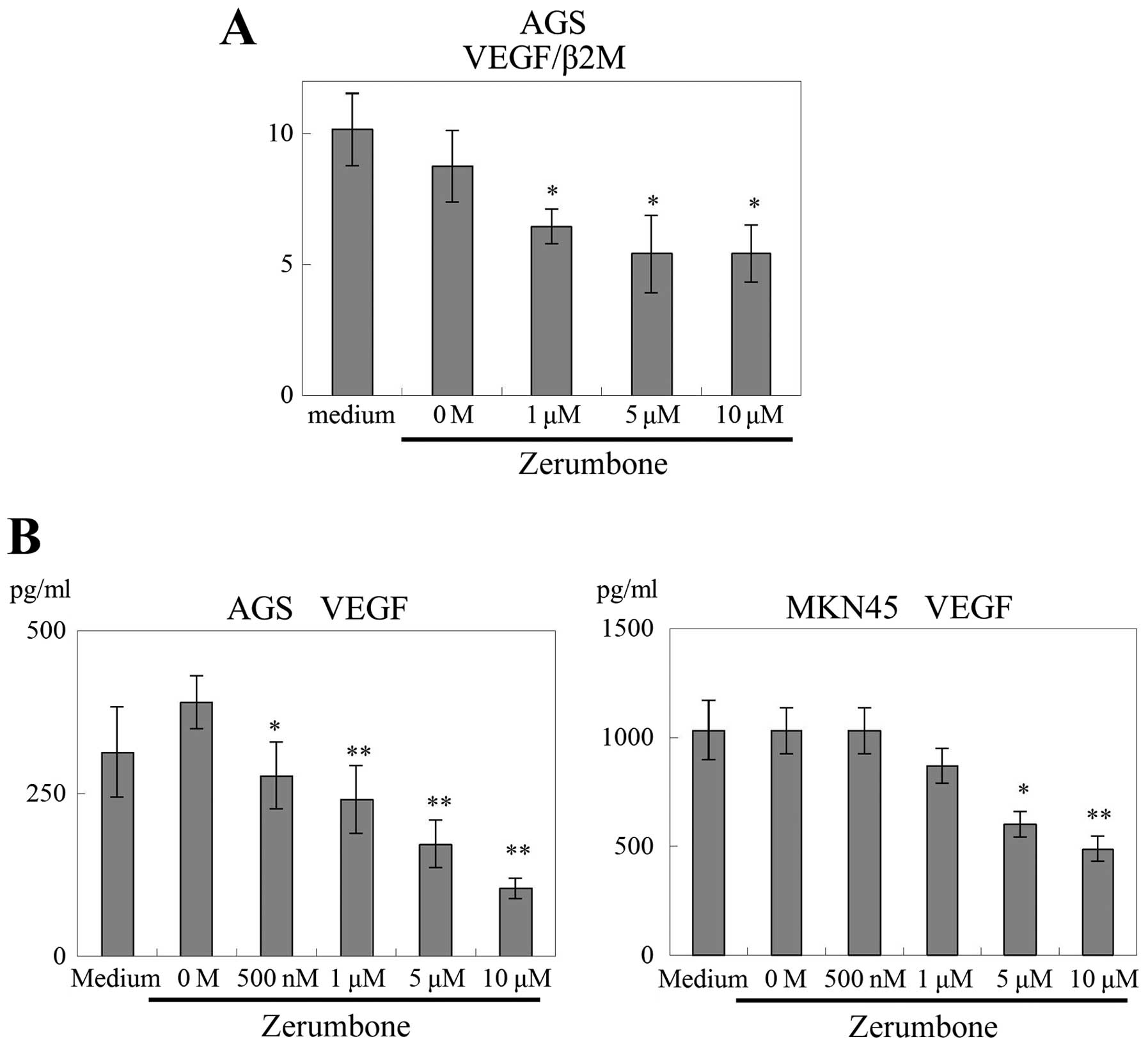
The effects of zerumbone on VEGF mRNA
expression in AGS cells were examined. The expression of
VEGF mRNA (VEGF/β2M) in AGS cells was significantly
inhibited by the addition of zerumbone in a dose-dependent manner
(≥1 μM, P<0.05; Fig. 3A).
Subsequently, the secretion of VEGF protein from
gastric cancer cells was measured using VEGF ELISA. Treatment with
zerumbone significantly reduced the secretion of VEGF from AGS
cells (500 nM, P<0.05; and ≥1 μM, P<0.01; Fig. 3B). In addition, the secretion of
VEGF from MKN45 cells was decreased by zerumbone (5 μM, P<0.05;
and 1 μM, P<0.01; Fig. 3B).
Effects of zerumbone on the activation of
NF-κB in gastric cancer cells
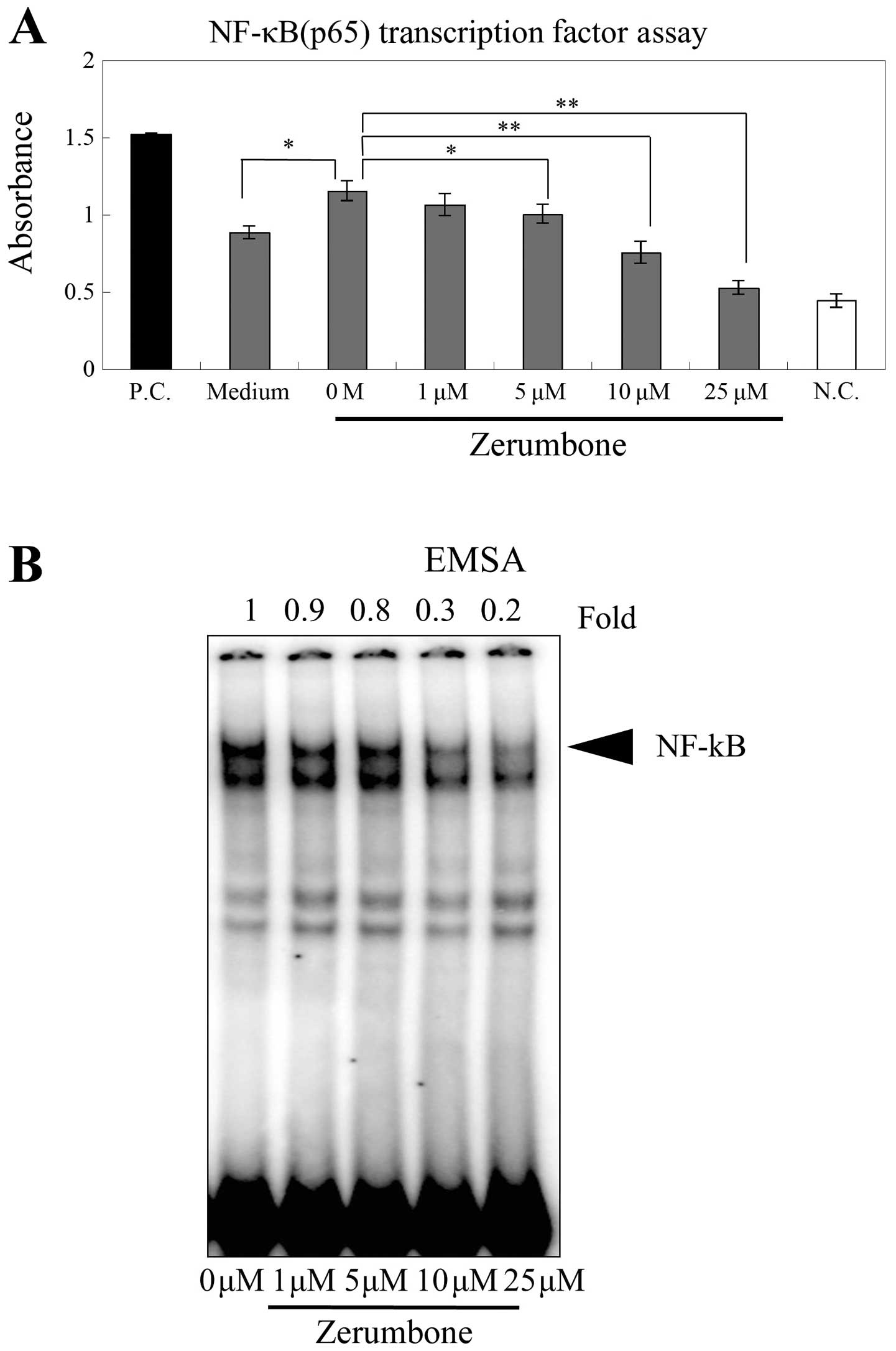
The activity of NF-κB was measured using NF-κB (p65)
transcription factor assay. The activity of NF-κB was increased by
0.1% DMSO, the solvent used to dissolve zerumbone. This increased
activity was significantly inhibited by ≥5 μM concentrations of
zerumbone (≥5 μM, P<0.05; ≥10 μM, P<0.01; Fig. 4A). We also performed EMSA to examine
changes in NF-κB expression in response to zerumbone treatment in
AGS cells. Consistent with our previous results, the activity of
NF-κB was also inhibited by zerumbone in a dose-dependent manner
(Fig. 4B).
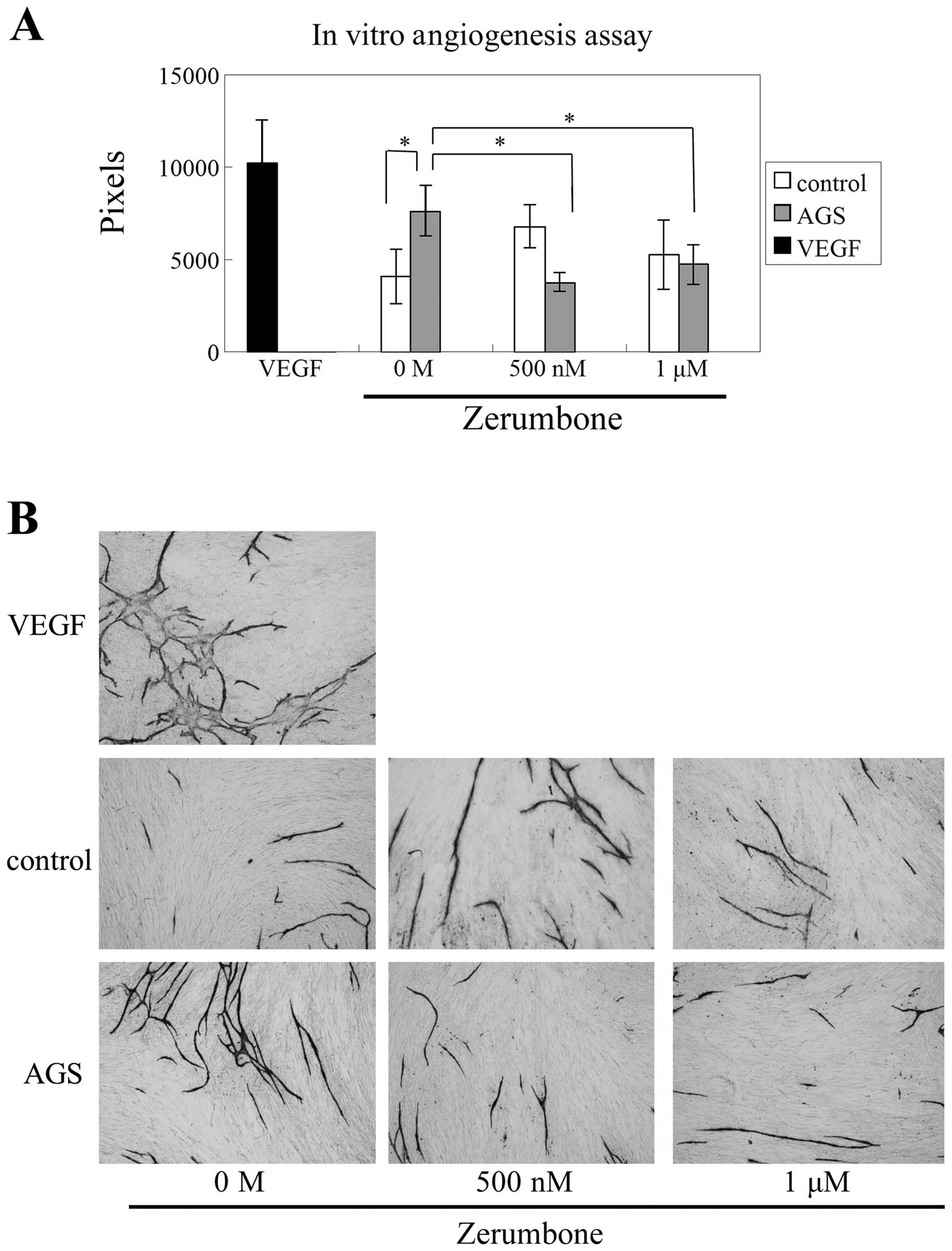
Effects of zerumbone on HUVEC tube
formation
In order to estimate the effects of zerumbone on
angiogenesis, we used in vitro angiogenesis assay. HUVECs
and FBs were cocultured with AGS cells, and the effects of
zerumbone treatment were examined. Tube formation by HUVECs was
significantly enhanced by coculture with AGS cells (P<0.05;
Fig. 5A). Moreover, the enhancement
of tube formation by AGS cells was inhibited by zerumbone (500 nM
or 1 μM, P<0.05; Fig. 5A and
B).
Discussion
The aim of the present study was to determine
whether zerumbone, a component of shampoo ginger that has been
linked to anticancer activities, could suppress NF-κB activity and
consequently reduce the production of VEGF in gastric cancer cells.
Almost all types of gastric cancer cells produced VEGF, and
zerumbone downregulated VEGF expression in various gastric cancer
cell lines. In addition to VEGF production, zerumbone suppressed
NF-κB expression in gastric cancer cells. Thus, our results showed,
for the first time, that zerumbone inhibited the production of VEGF
in gastric cancer cells via downregulation of NF-κB.
Angiogenesis is one of the most important factors in
tumor metastasis. Along with its receptors, VEGF promotes
endothelial cell proliferation and new blood vessel formation in
in vitro models of cancer associated-angiogenesis (13). VEGF expression in gastric cancer is
correlated with survival prognosis (15,16).
Moreover, serum VEGF has been reported to be elevated in gastric
cancer patients as compared with healthy individuals (17) and has also been correlated with
patient survival (18). Therefore,
these data support that VEGF-targeted therapy may have some
therapeutic potential.
Aberrant expression of NF-κB, which belongs to the
rel family of transcription factors, has been associated with
gastric carcinogenesis (19).
Previous studies have suggested an important role for NF-κB in the
regulation of apoptosis, cell adhesion, oncogenesis and
angiogenesis (22). In addition,
another study demonstrated that NF-κB plays an important role in
VEGF expression and angiogenesis in gastric cancer (25). Therefore, NF-κB may be a therapeutic
target in the treatment of gastric cancer. NF-κB inhibitors, such
as bortezomib, are already being used for patients with multiple
myeloma; however, these inhibitors commonly have adverse
side-effects that limit their widespread use (40). Thus, new and more nontoxic drugs
that inhibit NF-κB activity are needed.
In this study, we focused on the effects of natural
products, which are generally regarded as safe and nontoxic. Some
natural products have been reported to have anticancer effects.
Natural products, such as curcumin (41), baicalin (42), sesamin (43), and zerumbone (29), have been reported to regulate cell
survival, proliferation and invasion in some types of cancer.
Additionally, some studies have demonstrated that the effects of
these natural products on cancer are derived from the inhibition of
NF-κB activity (41).
Zerumbone is derived from a subtropical ginger
(Z. zerumbet Smith) and has a molecular weight of 218.33 Da.
Z. zerumbet is commonly known as the pinecone or shampoo
ginger and has several names in various countries, including
‘Lempoyang’ (Malaysia and Indonesia), ‘Awapuhi’ (Hawaii) (29,36,44–47),
‘Hana shoga’ and ‘White ukon’ (Japan). The white rhizome of Z.
zerumbet is traditionally used as a botanical medicine for the
treatment of several conditions and was previously reported to have
anti-inflammatory (45,46,48,49),
antinociceptive (45,49,50),
antimicrobial (51–54), and anti-allergic effects (55). It was also found to have anticancer
effects in colon cancer (30,32–34),
leukemia (35), myeloid cancer
(36), liver cancer (37), breast cancer (31,38),
pancreatic cancer (31), and lung
cancer (34). However, to date, no
studies have reported the inhibition of gastric cancer by
zerumbone. The molecule targets of zerumbone include cyclooxygenase
2 (COX2) (56,57), free radical generation (30), NF-κB (34,38,39),
iNOS (56), and CXCR4 (31), among others. However, the effects of
zerumbone on cancer angiogenesis have not been elucidated.
Therefore, in this study, we investigated the effects of zerumbone
on angiogenesis in gastric cancer cells.
Based on this premise, we first examined the
expression of VEGF in several gastric cancer cell lines. mRNA and
protein expression of VEGF were observed in all tested gastric
cancer cells. Since AGS cells exhibited the highest levels of VEGF
expression, we primarily used these cells in our subsequent
experiments to determine the anticancer effects of zerumbone.
Zerumbone significantly inhibited the proliferation of both AGS and
MKN45 cells. Additionally, we provided evidence supporting that
zerumbone markedly altered VEGF expression in gastric cancer cells,
including secretion of VEGF protein from both AGS and MKN45 cells.
Based on these results, we concluded that zerumbone has not only
direct pro-apoptotic effects on gastric cancer cells (at higher
concentrations), but also anti-angiogenic effects, mediated through
reduction of VEGF production by gastric cancer cells (at lower
concentration). Our data demonstrated that zerumbone partially
induced apoptosis in AGS cells at concentrations greater than 10
μM, but significantly decreased VEGF production in AGS cells at 500
nM. We did not observe any cytotoxicity (by WST-1 assay) in AGS
cells at this low concentration, indicating that zerumbone may have
potential use as a nontoxic agent in the treatment of gastric
cancer.
To clarify the molecular signaling mechanisms
through which zerumbone inhibited the production of VEGF, we
examined the effects of zerumbone on NF-κB activity. Our data
revealed that the activity of NF-κB was significantly inhibited by
zerumbone in a dose-dependent manner. In gastric cancer, many
studies have demonstrated that NF-κB is constitutively activated
and promotes tumorigenesis (19–21).
Additionally, some studies have revealed that Helicobacter
pylori activates NF-κB and consequently enhances carcinogenesis
(58,59). NF-κB activity has also been shown to
stimulate VEGF production from gastric cancer cells (60). These previous studies are consistent
with our results demonstrating that zerumbone inhibited NF-κB and
consequently decreased VEGF production in gastric cancer cells.
Finally, we examined whether zerumbone regulated the
vascularization of gastric cancer using in vitro
angiogenesis assay. Tube formation by HUVECs was significantly
enhanced by coculture with AGS cells, and this effect was
significantly inhibited by zerumbone. To our knowledge, this is the
first study to demonstrate the marked effects of zerumbone on
gastric cancer-induced angiogenesis. Since zerumbone did not
decrease HUVEC tube formation during coculture with FBs only (i.e.
without gastric cancer cells), we concluded that zerumbone
inhibited gastric cancer-induced angiogenesis by affecting the
gastric cancer cells directly. Even low concentrations of zerumbone
decreased VEGF production from gastric cancer cells. Therefore, the
natural product zerumbone may have the potential to become an
anti-angiogenic drug in the treatment of gastric cancer.
In conclusion, our results indicated that zerumbone
inhibited both proliferation and angiogenesis in gastric cancer,
and these effects were correlated with the suppression of NF-κB.
Notably, gastric cancer-induced angiogenesis was inhibited by
zerumbone, even at low concentrations. Since low-dose zerumbone did
not block angiogenesis associated with normal physiological
function, use of the natural product zerumbone may be safer and may
show reduced toxicity compared to other available treatments.
Therefore, zerumbone has potential use as a new anti-angiogenic
drug for the treatment of gastric cancer.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 13:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
13:69–90. 2011. View Article : Google Scholar
|
|
3
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 13:477–490. 2009.
View Article : Google Scholar
|
|
4
|
Wagner AD, Grothe W, Haerting J, Kleber G,
Grothey A and Fleig WE: Chemotherapy in advanced gastric cancer: a
systematic review and meta-analysis based on aggregate data. J Clin
Oncol. 24:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsuo Y, Sawai H, Funahashi H, et al:
Enhanced angiogenesis due to inflammatory cytokines from pancreatic
cancer cell lines and relation to metastatic potential. Pancreas.
28:344–352. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tong Z, Kunnumakkara AB, Wang H, et al:
Neutrophil gelatinase-associated lipocalin: a novel suppressor of
invasion and angiogenesis in pancreatic cancer. Cancer Res.
68:6100–6108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuo Y, Sawai H, Ochi N, et al:
Interleukin-1alpha secreted by pancreatic cancer cells promotes
angiogenesis and its therapeutic implications. J Surg Res.
153:274–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuo Y, Ochi N, Sawai H, et al:
CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote
invasiveness and angiogenesis in pancreatic cancer. Int J Cancer.
124:853–861. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuo Y, Sawai H, Ma J, et al: IL-1alpha
secreted by colon cancer cells enhances angiogenesis: the
relationship between IL-1alpha release and tumor cells’ potential
for liver metastasis. J Surg Oncol. 99:361–367. 2009.PubMed/NCBI
|
|
10
|
Matsuo Y, Sawai H, Ochi N, et al:
Proteasome inhibitor MG132 inhibits angiogenesis in pancreatic
cancer by blocking NF-kappaB activity. Dig Dis Sci. 55:1167–1176.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuo Y, Raimondo M, Woodward TA, et al:
CXC-chemokine/CXCR2 biological axis promotes angiogenesis in vitro
and in vivo in pancreatic cancer. Int J Cancer. 125:1027–1037.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsuo Y, Campbell PM, Brekken RA, et al:
K-Ras promotes angiogenesis mediated by immortalized human
pancreatic epithelial cells through mitogen-activated protein
kinase signaling pathways. Mol Cancer Res. 7:799–808. 2009.
View Article : Google Scholar
|
|
13
|
Carmeliet P: Angiogenesis in health and
disease. Nat Med. 9:653–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma J, Sawai H, Matsuo Y, et al:
Interleukin-1alpha enhances angiogenesis and is associated with
liver metastatic potential in human gastric cancer cell lines. J
Surg Res. 148:197–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maeda K, Chung YS, Ogawa Y, et al:
Prognostic value of vascular endothelial growth factor expression
in gastric carcinoma. Cancer. 77:858–863. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maeda K, Kang SM, Onoda N, et al: Vascular
endothelial growth factor expression in preoperative biopsy
specimens correlates with disease recurrence in patients with early
gastric carcinoma. Cancer. 86:566–571. 1999. View Article : Google Scholar
|
|
17
|
Kikuchi S, Obata Y, Yagyu K, et al:
Reduced serum vascular endothelial growth factor receptor-2
(sVEGFR-2) and sVEGFR-1 levels in gastric cancer patients. Cancer
Sci. 102:866–869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karayiannakis AJ, Syrigos KN,
Polychronidis A, et al: Circulating VEGF levels in the serum of
gastric cancer patients: correlation with pathological variables,
patient survival, and tumor surgery. Ann Surg. 236:37–42. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim KK and Kim HB: Protein interaction
network related to Helicobacter pylori infection response.
World J Gastroenterol. 15:4518–4528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Varro A, Noble PJ, Pritchard DM, et al:
Helicobacter pylori induces plasminogen activator inhibitor
2 in gastric epithelial cells through nuclear factor-kappaB and
RhoA: implications for invasion and apoptosis. Cancer Res.
64:1695–1702. 2004. View Article : Google Scholar
|
|
21
|
Keates S, Hitti YS, Upton M and Kelly CP:
Helicobacter pylori infection activates NF-kappa B in
gastric epithelial cells. Gastroenterology. 113:1099–1109. 1997.
View Article : Google Scholar
|
|
22
|
Xiong HQ, Abbruzzese JL, Lin E, Wang L,
Zheng L and Xie K: NF-kappaB activity blockade impairs the
angiogenic potential of human pancreatic cancer cells. Int J
Cancer. 108:181–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beg AA and Baldwin AS Jr: The I kappa B
proteins: multifunctional regulators of Rel/NF-kappa B
transcription factors. Genes Dev. 7:2064–2070. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Verma IM, Stevenson JK, Schwarz EM, Van
Antwerp D and Miyamoto S: Rel/NF-kappa B/I kappa B family: intimate
tales of association and dissociation. Genes Dev. 9:2723–2735.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nam SY, Ko YS, Jung J, et al: A
hypoxia-dependent upregulation of hypoxia-inducible factor-1 by
nuclear factor-κB promotes gastric tumour growth and angiogenesis.
Br J Cancer. 104:166–174. 2011.
|
|
26
|
Nakata W, Hayakawa Y, Nakagawa H, et al:
Anti-tumor activity of the proteasome inhibitor bortezomib in
gastric cancer. Int J Oncol. 39:1529–1536. 2011.PubMed/NCBI
|
|
27
|
Caballero-Velázquez T, López-Corral L,
Encinas C, et al: Phase II clinical trial for the evaluation of
bortezomib within the reduced intensity conditioning regimen (RIC)
and post-allogeneic transplantation for high-risk myeloma patients.
Br J Haematol. 162:474–482. 2013.
|
|
28
|
Gupta SC, Kim JH, Prasad S, et al:
Regulation of survival, proliferation, invasion, angiogenesis, and
metastasis of tumor cells through modulation of inflammatory
pathways by nutraceuticals. Cancer Metastasis Rev. 29:405–434.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yob NJ, Jofrry SM, Affandi MM, et al:
Zingiber zerumbet (L.) Smith: a review of its
ethnomedicinal, chemical, and pharmacological uses. Evid Based
Complement Alternat Med. 2011:5432162011.PubMed/NCBI
|
|
30
|
Murakami A, Takahashi D, Kinoshita T, et
al: Zerumbone, a Southeast Asian ginger sesquiterpene, markedly
suppresses free radical generation, proinflammatory protein
production, and cancer cell proliferation accompanied by apoptosis:
the alpha, beta-unsaturated carbonyl group is a prerequisite.
Carcinogenesis. 23:795–802. 2002. View Article : Google Scholar
|
|
31
|
Sung B, Jhurani S, Ahn KS, et al:
Zerumbone down-regulates chemokine receptor CXCR4 expression
leading to inhibition of CXCL12-induced invasion of breast and
pancreatic tumor cells. Cancer Res. 68:8938–8944. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yodkeeree S, Sung B, Limtrakul P and
Aggarwal BB: Zerumbone enhances TRAIL-induced apoptosis through the
induction of death receptors in human colon cancer cells: evidence
for an essential role of reactive oxygen species. Cancer Res.
69:6581–6589. 2009. View Article : Google Scholar
|
|
33
|
Kirana C, McIntosh GH, Record IR and Jones
GP: Antitumor activity of extract of Zingiber aromaticum and
its bioactive sesquiterpenoid zerumbone. Nutr Cancer. 45:218–225.
2003.
|
|
34
|
Kim M, Miyamoto S, Yasui Y, Oyama T,
Murakami A and Tanaka T: Zerumbone, a tropical ginger
sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int
J Cancer. 124:264–271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xian M, Ito K, Nakazato T, et al:
Zerumbone, a bioactive sesquiterpene, induces G2/M cell cycle
arrest and apoptosis in leukemia cells via a Fas- and
mitochondria-mediated pathway. Cancer Sci. 98:118–126. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang GC, Chien TY, Chen LG and Wang CC:
Antitumor effects of zerumbone from Zingiber zerumbet in
P-388D1 cells in vitro and in vivo. Planta Med. 71:219–224. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sakinah SA, Handayani ST and Hawariah LP:
Zerumbone induced apoptosis in liver cancer cells via modulation of
Bax/Bcl-2 ratio. Cancer Cell Int. 7:42007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sung B, Murakami A, Oyajobi BO and
Aggarwal BB: Zerumbone abolishes RANKL-induced NF-κB activation,
inhibits osteoclastogenesis, and suppresses human breast
cancer-induced bone loss in athymic nude mice. Cancer Res.
69:1477–1484. 2009.PubMed/NCBI
|
|
39
|
Takada Y, Murakami A and Aggarwal BB:
Zerumbone abolishes NF-κB and IκBα kinase activation leading to
suppression of antiapoptotic and metastatic gene expression,
upregulation of apoptosis, and downregulation of invasion.
Oncogene. 24:6957–6969. 2005.
|
|
40
|
Chen D, Frezza M, Schmitt S, Kanwar J and
Dou QP: Bortezomib as the first proteasome inhibitor anticancer
drug: current status and future perspectives. Curr Cancer Drug
Targets. 11:239–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Aggarwal BB, Van Kuiken ME, Iyer LH,
Harikumar KB and Sung B: Molecular targets of nutraceuticals
derived from dietary spices: potential role in suppression of
inflammation and tumorigenesis. Exp Biol Med. 234:825–849. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li-Weber M: New therapeutic aspects of
flavones: the anticancer properties of Scutellaria and its
main active constituents Wogonin, Baicalein and Baicalin. Cancer
Treat Rev. 35:57–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Harikumar KB, Sung B, Tharakan ST, et al:
Sesamin manifests chemopreventive effects through the suppression
of NF-kappa B-regulated cell survival, proliferation, invasion, and
angiogenic gene products. Mol Cancer Res. 8:751–761. 2010.
View Article : Google Scholar
|
|
44
|
Bhuiyan NI, Chowdhury JU and Begum J:
Chemical investigation of the leaf and rhizome essential oils of
Zingiber zerumbet (L.) Smith from Bangladesh. Bangladesh J
Pharmacol. 4:9–12. 2009.
|
|
45
|
Zakaria ZA, Mohamad AS, Chear CT, Wong YY,
Israf zDA and Sulaiman MR: Antiinflammatory and antinociceptive
activities of Zingiber zerumbet methanol extract in
experimental model systems. Med Princ Pract. 19:287–294.
2010.PubMed/NCBI
|
|
46
|
Zakaria ZA, Mohamad AS, Ahmad MS, et al:
Preliminary analysis of the anti-inflammatory activity of essential
oil of Zingiber zerumbet. Biol Res Nurs. 13:425–432. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tushar, Basak S, Sarma GC and Rangan L:
Ethnomedical uses of Zingiberaceous plants of Northeast India. J
Ethnopharmacol. 132:286–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Somchit MN and Shukriyah MHN:
Antiinflammatory property of ethanol and water extracts of
Zingiber zerumbet. Indian J Pharmacol. 35:181–182. 2003.
|
|
49
|
Somchit MN, Shukriyah MHN, Bustamam AA and
Zuraini A: Anti-pyretic and analgesic activity of Zingiber
zerumbet. Int J Pharmacol. 1:277–280. 2005. View Article : Google Scholar
|
|
50
|
Sulaiman MR, Tengku Mohamad TA, Shaik
Mossadeq WM, et al: Antinociceptive activity of the essential oil
of Zingiber zerumbet. Planta Med. 76:107–112. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jantan IB, Yassin MS, Chin CB, Chen LL and
Sim NL: Antifungal activity of the essential oils of nine
Zingiberaceae species. Pharm Biol. 41:392–397. 2003. View Article : Google Scholar
|
|
52
|
Voravuthikunchai SP, Phongpaichit S and
Subhadhirasakul S: Evaluation of antibacterial activities of
medicinal plants widely used among AIDS patients in Thailand. Pharm
Biol. 43:701–706. 2005. View Article : Google Scholar
|
|
53
|
Voravuthikunchai SP, Limsuwan S, Supapol O
and Subhadhirasakul S: Antibacterial activity of extracts from
family Zingiberaceae against foodborne pathogens. J Food Safety.
26:325–334. 2005. View Article : Google Scholar
|
|
54
|
Phongpaichit S, Vuddhakul V,
Subhadhirasakul S and Wattanapiromsakul C: Evaluation of the
antimycobacterial activity of extracts from plants used as
self-medication by AIDS patients in Thailand. Pharm Biol. 44:71–75.
2006. View Article : Google Scholar
|
|
55
|
Tewtrakul S and Subhadhirasakul S:
Anti-allergic activity of some selected plants in the Zingiberaceae
family. J Ethnopharmacol. 109:535–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Murakami A and Ohigashi H: Targeting NOX,
INOS and COX-2 in inflammatory cells: chemoprevention using food
phytochemicals. Int J Cancer. 121:2357–2363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Murakami A, Shigemori T and Ohigashi H:
Zingiberaceous and citrus constituents, 10-acetoxychavicol acetate,
zerumbone, auraptene, and nobiletin, suppress
lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7
murine macrophages through different modes of action. J Nutr.
135(Suppl 12): 2987–2992. 2005.
|
|
58
|
Maeda S, Yoshida H, Ogura K, et al: H.
pylori activates NF-kappaB through a signaling pathway
involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and
TRAF6 in gastric cancer cells. Gastroenterology. 119:97–108. 2000.
View Article : Google Scholar
|
|
59
|
Lamb A and Chen LF: Role of the
Helicobacter pylori-induced inflammatory response in the
development of gastric cancer. J Cell Biochem. 114:491–497.
2013.
|
|
60
|
Yin Y, Si X, Gao Y, Gao L and Wang J: The
nuclear factor-κB correlates with increased expression of
interleukin-6 and promotes progression of gastric carcinoma. Oncol
Rep. 29:34–38. 2013.
|