Introduction
Colorectal cancer (CRC) is one of the leading
cancers in Western countries and is responsible for ~500,000
deaths/year worldwide (1,2). Inflammation is a key factor in the
development and progression of CRC. The close interaction between
inflammatory processes and the development of malignancy is
documented by a high incidence of CRC in inflammatory bowel disease
as well as a decreased CRC incidence in patients under ingestion of
COX-2 inhibitors (3–5). Moreover, it is increasingly recognized
that tumor initiation, growth and progression are results of
complex interactions between cancer cells and the surrounding
tissue, mainly consisting of fibroblasts, called the tumor
microenvironment (6).
Tumor-associated fibroblasts (TAFs) interact strongly with cancer
cells and appear to enforce tumor progression e.g. by remodeling of
the extracellular matrix, their pro-inflammatory gene signature and
by guidance of cancer cells during invasion (6).
Intercellular adhesion molecule-1 (ICAM-1) is a
member of the immunoglobulin superfamily and is expressed as a
single cell surface glycoprotein by different cell types including
fibroblasts, endothelial cells, epithelial cells, cardiomyocytes,
smooth muscle cells, lymphocytes and monocytes (7–9). This
molecule typically participates in cell-cell as well as cell-matrix
adhesion and is also involved in cancer cell adhesion and in the
immune response of tumors (9–12).
Several studies suggest that ICAM-1 plays a major
role in CRC (13–17). Interestingly, the impact of ICAM-1
depends on whether the protein is expressed in a membrane-bound or
a soluble form (13).
Immunohistochemical staining of CRC revealed a lower incidence of
lymph node and liver metastases in primary tumors expressing
membrane-bound ICAM-1, whereas a lower number of ICAM-1-positive
cells are noted in primary tumors that metastasize (14,16,17).
Taglia et al(16) showed
that membrane-bound ICAM-1 mediates tumor cell attachment to the
extracellular matrix. Thus, ICAM-1 is expected to prevent cells
from detaching from the primary tumor and to attenuate metastases
(16). Furthermore, membrane-bound
ICAM-1 expression by cancer cells in CRC correlates with
differentiation. Higher amounts of ICAM-1 were found in
well-differentiated compared to lower levels in poorly
differentiated tumors (16).
In contrast, the soluble form of ICAM-1 (sICAM-1) is
significantly correlated with tumor stage and the development of
metastasis, not only in CRC (13,15,18–20)
but also in other cancers such as gastric cancer (21,22),
breast cancer (23–25), urological malignancies (26), malignant melanoma (27), lung cancer (28), hepatocellular cancer (29) and chronic B-lymphocytic leukemia
(30). At present, the source of
sICAM-1 is unclear (13,22) but it may be produced by proteolytic
cleavage of membrane-bound ICAM-1 (31–33).
Interestingly, sICAM-1 levels decrease significantly after curative
surgery for CRC (13,15).
In order to investigate the impact of the fibroblast
phenotype on CRC progression, we isolated viable pure fibroblasts
from human CRC and corresponding healthy colon mucosa of 14
patients to compare potential differences regarding their adhesive
capacity mediated by ICAM-1. We demonstrated for the first time
that TAFs isolated from human CRC exhibit an increased affinity for
monocytic cells. Moreover, increased ICAM-1 expression was
confirmed in stromal fibroblasts in CRC tissues in vivo.
Materials and methods
Tissues from the tumor and the corresponding healthy
colon were obtained from 14 patients (6 females and 8 males; mean
age: 68 years; range: 55–80 years) undergoing elective standard
surgical procedure for primarily diagnosed CRC at the Department of
Surgery, University Medical Center Erlangen between February 2008
and July 2010. None of the patients received pretreatment prior to
surgery. Tumors were histopathologically characterized according to
the Union International Contre le Cancer (UICC). Patients with UICC
stage I/II (n=8) and with UICC stage III/IV CRC (n=6) were
included. Due to the limited life span of the isolated primary
cells, not all of the tests were performed for each patient. The
patient characteristics and analyses that were performed are
documented in Table I. The
procedure was approved by the local ethics committee, and all
patients provided written informed consent.
 | Table ICharacteristics of the study patients
and the performed analyses. |
Table I
Characteristics of the study patients
and the performed analyses.
| | | Included in
analysis |
|---|
| | |
|
|---|
| Patient no. | UICC stage | Gender | Vimentin/CK-20/CD45
immunocytochemistry | Adhesion assay | ICAM-1
immunocytochemistry | ICAM-1
immunohistochemistry |
|---|
| 1 | IV | M | ● | | ● | ● |
| 2 | III | F | ● | ● | ● | ● |
| 3 | I | F | ● | ● | ● | ● |
| 4 | II | M | ● | | ● | ● |
| 5 | II | M | ● | | ● | ● |
| 6 | III | M | ● | ● | | ● |
| 7 | III | F | ● | ● | | ● |
| 8 | I | M | ● | ● | ● | ● |
| 9 | IV | M | ● | ● | ● | ● |
| 10 | III | F | ● | ● | ● | |
| 11 | II | M | ● | ● | ● | ● |
| 12 | I | M | ● | ● | ● | ● |
| 13 | I | F | ● | | ● | ● |
| 14 | II | F | ● | ● | ● | ● |
| | | n=14 | n=10 | n=12 | n=13 |
Fibroblast isolation and cell
culture
According to our published protocol (34), small pieces from the non-necrotic
center of the CRC as well as from the healthy tissue at a minimum
distance of 10 cm from the visible tumor margin were used for the
generation of a single-cell suspension (34). Approximately 5 to 7 days after
initial seeding, endothelial cells were removed by magnetic cell
separation. The resulting negative fraction was cultivated in
Dulbecco's modified Eagle's medium (DMEM) (Life Technologies,
Darmstadt, Germany) supplemented with 10% fetal bovine serum (FBS)
(Cambrex, Verviers, Belgium) and antibiotics (1X
penicillin/streptomycin) in a 8.5% CO2 humid atmosphere
at 37°C. Cells were grown until confluence, and were routinely
tested for mycoplasma contamination. Medium replacement was
performed every 3 days.
Immunocytochemistry
Cells were cultured on LabTek chamber slides (Nunc,
Wiesbaden, Germany) until confluent and subsequently fixed and
stained. For ICAM-1 staining, the cells were either starved for 14
h in DMEM-0.5% FBS and stimulated in the same medium enriched with
200 U/ml interleukin-1β (IL-1β; Roche, Mannheim, Germany) for 14 h
or received control medium without cytokine before fixation. All
slides were washed once with PBS and fixed in ice-cold ethanol for
at least 20 min. The cells were rehydrated and incubated for 2 h
with anti-human CD45 antibody (1:10; clone T29/33), anti-human
cytokeratin-20 antibody (CK-20; 1:10; both from DakoCytomation,
Hamburg, Germany; clone KS 20.8) or anti-human ICAM-1 antibody
(1:20; R&D Systems, Wiesbaden-Nordenstadt, Germany; clone
BBIG-I1 11C8). Subsequently, rabbit anti-mouse immunoglobulin
(1:50) was added for 45 min followed by APAAP mouse (1:50; both
from DakoCytomation) for another 45 min. Staining was developed
using Fuchsin chromogen (DakoCytomation). Nuclei were
counterstained with Gill-III hematoxylin (Merck KGaA, Darmstadt,
Germany). Slides were mounted with Aqueous Mount (Zytomed Systems,
Berlin, Germany). Stained sections and cells were photographed
using a Sony 3CCD color video camera (Sony Corporation, Munich,
Germany) mounted on a Leitz Aristoplan microscope (Leica, Solms,
Germany).
Stained sections were evaluated by 2 independent
individuals. The ratio of ICAM-1-positive/unstained fibroblasts was
evaluated for each slide, and ICAM-1 expression in TAFs and
corresponding normal tissue-associated fibroblasts (NAFs) was
categorized as follows: TAFs>NAFs; TAFs=NAFs; TAFs<NAFs. In
case of inconsistent results, a third evaluation was performed.
Adhesion assay
For the adhesion assay, 50,000 fibroblasts/well were
cultivated in DMEM-10% FBS (Life Technologies) on 1.5%
gelatin-precoated LabTek 4-chamber slides (Nunc). After 6 h, the
cells were starved for 14 h in DMEM-0.5% FBS, stimulated with or
without tumor necrosis factor-α (TNF-α; 1000 U/ml; Roche, Grenzach,
Germany) for 24 h, and U937 cells (500,000/well) were placed in
RPMI-0.5% FBS (PAA, Cölbe, Germany) for 15 min at 37°C in a 8.5%
CO2 atmosphere. Afterwards the slides were washed with
RPMI-0.5% FBS and fixed in paraformaldehyde (Sigma-Aldrich, Munich,
Germany) for at least 30 min. Three different optical
fields/chamber were documented with a Sony 3CCD color video camera
mounted on a Leitz Aristoplan microscope. TAFs and corresponding
NAFs with equal confluence rates were included in the final
evaluation. Adherent U937 cells were counted manually, and a mean
value/culture was calculated.
Immunohistochemistry
ICAM-1 staining of formalin-fixed, paraffin-embedded
sections (4 μm) from the tumor and normal tissues was performed as
follows. Antigen retrieval was carried out using Target Retrieval
Solution citrate buffer pH 6.0 (DakoCytomation). The primary rabbit
anti-human ICAM-1 antibody (DakoCytomation) was diluted (1:25) in
Antibody Diluent (Zytomed Systems) and incubated for 1 h in a wet
chamber at room temperature. After a washing step with
Tris-buffered saline (0.05 M, pH 7.6) the Rabbit Vectastain Elite
ABC kit (Linaris, Dossenheim, Germany) was used as a detection
system. The staining was developed using the NovaRED Substrate kit
(Linaris) for 30 min. After counterstaining of nuclei with Gill-III
hematoxylin, the slides were dehydrated and mounted with VectaMount
Permanent Mounting Medium (Vector Laboratories, Burlingame, CA,
USA).
The stained sections were evaluated by 3 independent
investigators. First, the ratio of ICAM-1-positive/negative
fibroblasts (in %) was evaluated for each slide and second, the
slides of the normal and tumor tissue for each patient were
compared with each other. Statistical differences were evaluated by
the Student's t-test (SPSS Statistic Program, IBM, version 18.0).
Stained tissue sections were photographed using an AxioCam MRc
mounted on a Zeiss Imager A.2 Axio (Zeiss, Jena, Germany).
Results
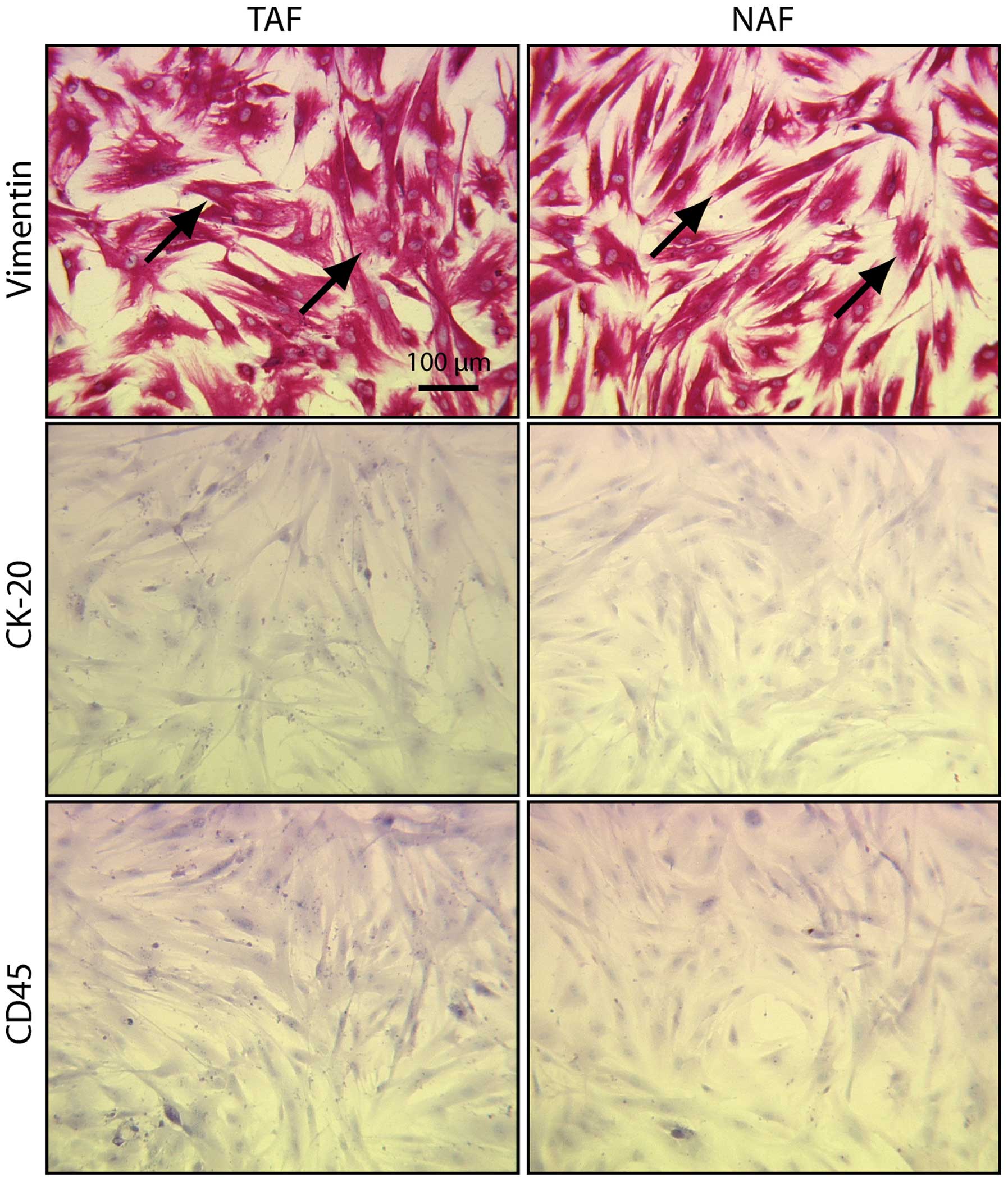
TAFs and NAFs are vimentin-positive and
CK-20/CD45-negative
Viable fibroblasts were isolated from the surgical
specimens of 14 patients and cultivated for several passages. The
isolated fibroblasts presented with the typical spindle-shaped
morphology (Fig. 1). All cells were
positive for vimentin (Fig. 1,
vimentin). Contamination by epithelial and hematopoietic cells was
excluded by negative CK-20 (epithelial cells) and CD45
(hematopoietic cells) staining (Fig.
1, CK-20 and CD45). Due to the limited life span of the
isolated cells, ICAM-1 staining and the adhesion assays were
performed with 12 (ICAM-1 staining) and 10 (adhesion assay) out of
the 14 TAF/NAF pairs (Table I).
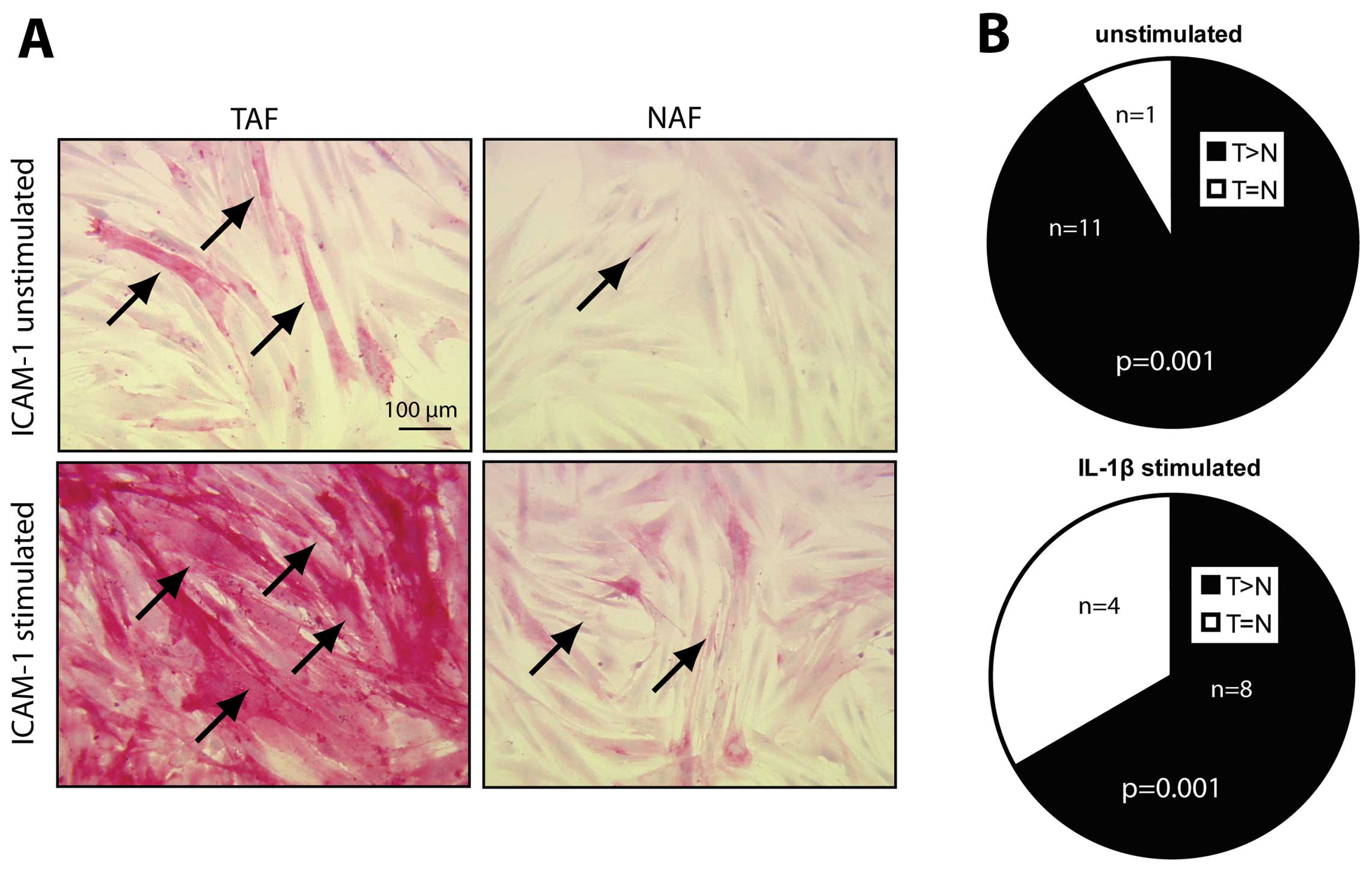
ICAM-1 expression is increased in
unstimulated and stimulated TAFs when compared to ICAM-1 expression
in NAFs
Immunocytochemical staining of ICAM-1 was performed
in 12 out of 14 TAF/NAF pairs. In 11 out of 12 cultures, a higher
number of ICAM-1-positive cells was detected in TAF when compared
to the corresponding NAF cultures (Fig.
2A, compare TAF vs. NAF, unstimulated; Fig. 2B, unstimulated). After stimulation
of the fibroblast cultures with interleukin-1β (IL-1β), ICAM-1
expression was increased in all of the cultures when compared to
the basal levels (Fig. 2A, compare
unstimulated vs. stimulated). When comparing stimulated TAF with
the corresponding stimulated NAF cultures in 8 out of the 12
cultures, TAFs presented with a higher number of ICAM-1-positive
cells (Fig. 2A, compare stimulated
TAF vs. NAF; Fig. 2B, IL-1β
stimulated, p=0.001). The amount of ICAM-1-positive cells in TAFs
and NAFs was equal after IL-1β stimulation in 4 out of the 12
cultures (Fig. 2B, IL-1β
stimulated, p=0.001).
TAFs display a higher adhesion capacity
for U937 monocytic cells when compared to NAFs
In order to estimate whether the increased ICAM-1
expression leads to increased adhesive capacity, adhesion assays
using U937 monocytic cells were performed with 10 TAF/NAF pairs
(Fig. 3A). In 8 out of 10
unstimulated TAF/NAF pairs, the number of adherent U937
cells/optical field was increased in the TAFs when compared to the
number in the NAFs (Fig. 3B,
patient no. 2, 3, 6, 7, 8, 9, 12, 14). Of note, in 3 of the pairs,
the differences were statistically significant (Fig. 3B, asterisks, p<0.05). Comparing
the average number of U937 cells/optical field in all unstimulated
TAFs vs. NAFs, the number was increased by 2.33-fold (Fig. 3D, compare TAF/NAF, p=0.051).
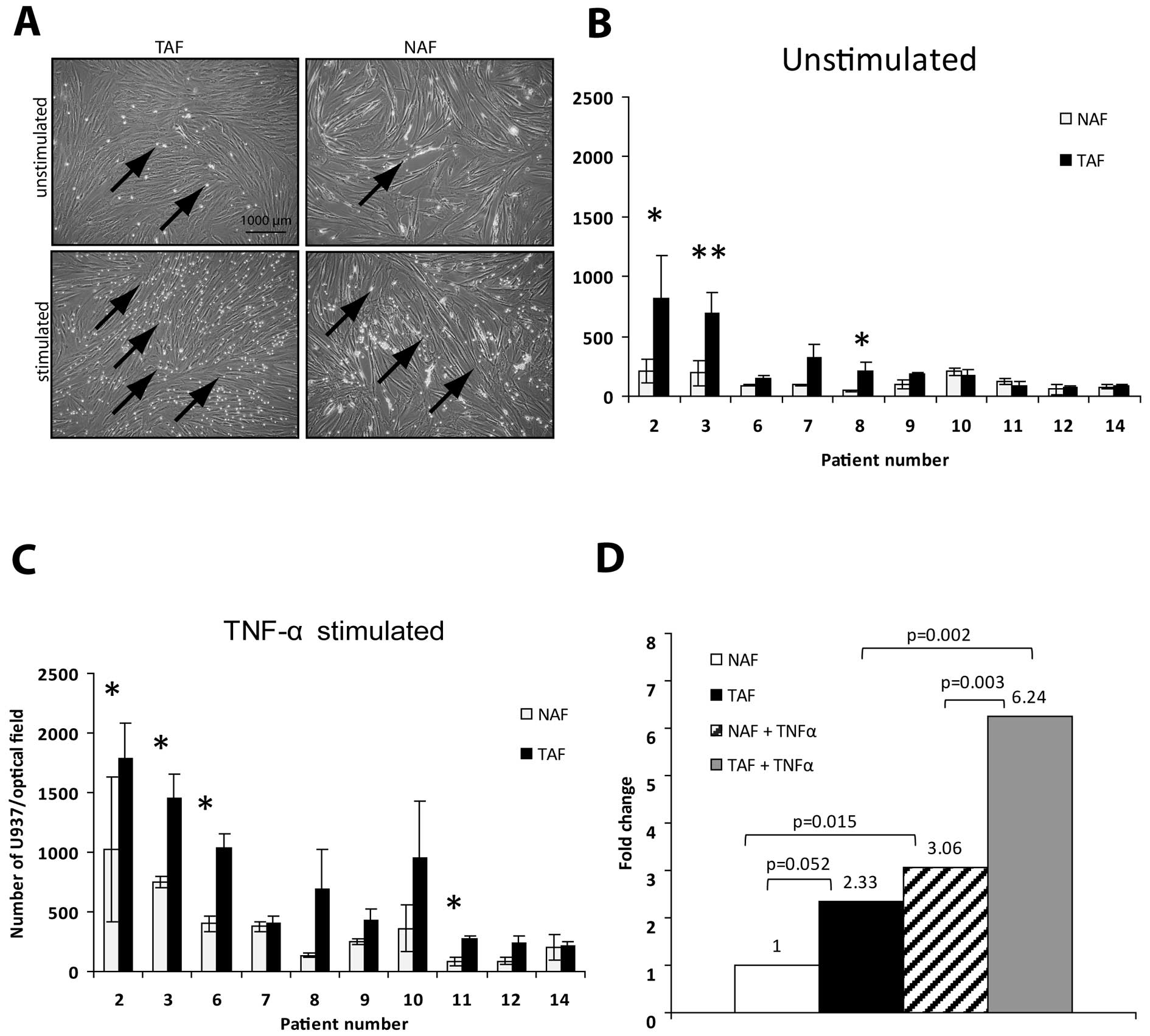 | Figure 3Unstimulated as well as stimulated
(TNF-α) TAF cultures showed a higher adhesion capacity for U937
when compared with the capacity of NAFs. (A) Starved fibroblast
cultures (unstimulated or stimulated with TNF-α for 24 h) were
incubated with U937 monocytic cells for 15 min. After fixation,
adherent U937 cells were counted manually. Arrows indicate adherent
U937 monocytic cells. Scale bar, 1000 μm. (B) In 8 out of 10
unstimulated TAF/NAF pairs, the mean number of adherent U937 cells
in 3 different optical fields was increased in TAFs when compared
to the number in the NAFs (patient no. 2, 3, 6, 7, 8, 9, 12, 14).
In 3 of the pairs, the differences were statistically significant
(*p<0.05, **p<0.01). (C) After TNF-α
stimulation in all of the 10 cultures, the adhesion capacity of
TAFs was higher when compared to this capacity in the NAF cultures.
In 4 of the pairs, the differences between TAFs and NAFs were
statistically significant (*p<0.05). (D) Average
fold-changes are given for unstimulated and stimulated TAFs vs.
NAFs. The respective p-values are indicated in the graph (Student's
t-test). TNF-α, tumor necrosis factor-α; TAFs, tumor-associated
fibroblasts; NAFs, normal tissue-associated fibroblasts. |
After stimulation of the cultures with TNF-α, in all
of the 10 cultures, the adhesion capacity of TAFs was higher when
compared to the NAF cultures (Fig.
3C). Of note, in 4 of the pairs, the differences between TAF
and NAF were statistically significant (Fig. 3C, asterisks, p<0.05). The average
number of U937 cells/optical field was increased in the stimulated
TAFs when compared to the number in the stimulated NAFs by 2-fold
(Fig. 3D, compare TAF + TNF-α vs.
NAF + TNF-α, 6.24- vs. 3.06-fold, p=0.002), was increased in the
stimulated TAFs when compared to the number in the unstimulated
TAFs by 2.67-fold (Fig. 3D, compare
6.24- vs. 2.33-fold, p=0.002) and was also increased in the
stimulated when compared to the unstimulated NAFs by 3.06-fold
(Fig. 3D, compare 1 vs. 3.06,
p=0.015).
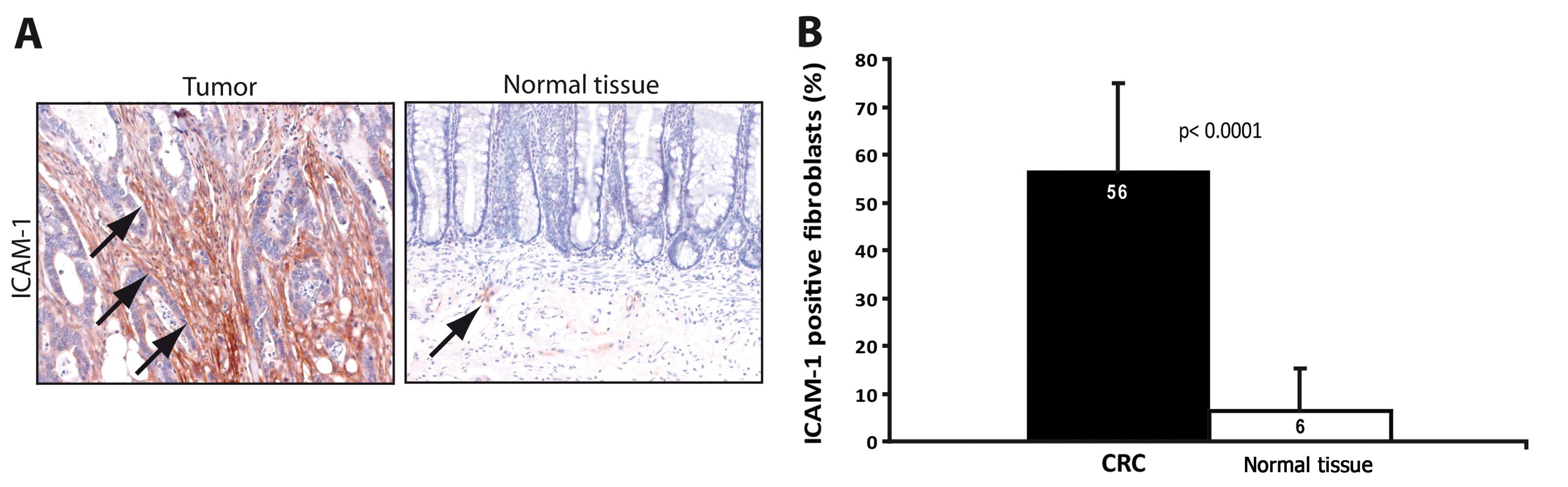
Increased ICAM-1 expression in TAFs is
reflected by a corresponding increase in ICAM-1 expression in
fibroblasts of the tumor tissue
In order to determine whether the increased
expression of ICAM-1 in TAFs was an effect that was already present
in the original tumor tissue, immunohistochemical staining was
performed in 13 out of 14 tumor/normal tissue pairs (Fig. 4A). In one TAF/NAF pair, the
corresponding tumor section presented only parts of an adenoma
without invasive carcinoma, therefore the tumor and normal tissues
of this patient were excluded from the evaluation. When counting
ICAM-1-positive and -negative fibroblasts, the frequency of
ICAM-1-positive fibroblasts was significantly increased in the
tumor tissue as compared to the normal tissue (Fig. 4B, p<0.001). This indicates that
the increased ICAM-1 expression of TAFs was a tumor-specific effect
stably maintained in the isolated cultures.
Discussion
Recently, the understanding of fibroblast function
in tumor development is gaining renewed attention. Tumor
progression and the development of metastasis appear to be
influenced by the tumor microenvironment. The so-called
tumor-associated fibroblasts, fibroblasts activated by
tumor-derived mediators such as transforming growth factor β1 and
platelet-derived growth factor, play a crucial role in these
processes (35,36).
Cellular adhesion molecules manage the interactions
between cells and are therefore essential during the development
and maintenance of the tissue architecture (15). ICAM-1 has received increased
attention in cancer biology. Notably, membrane-bound ICAM-1 appears
to protect tumor cells from separation from the tumor mass by tumor
cell attachment to the extracellular matrix. Therefore, patients
with increased membrane-bound ICAM-1 are found to have decreased
lymph node and liver metastases as well as better differentiated
tumors (14,16,17).
In this report, we isolated and characterized
tumor-associated-fibroblasts and compared them with corresponding
normal tissue-derived fibroblasts. Both cell types were isolated
from fresh CRC surgical specimens. Notably, TAFs presented with
higher ICAM-1 expression when compared to the expression in NAFs,
despite their separation from malignant cells during in
vitro cultivation. These results were obtained by
immunocytochemical staining of isolated fibroblast cultures for
ICAM-1 and by performing adhesion assays with U937 cells to
demonstrate the functional capacity of increased ICAM-1 expression.
The performed tests revealed higher expression levels of ICAM-1 in
11 out of 12 CRC fibroblast pairs and a higher adhesion capacity of
TAFs compared to corresponding NAFs in 8 out of 10 pairs. These
results were reproducible after stimulation of cultures by IL-1β
and subsequent ICAM-1 detection or with TNF-α and subsequent
measurement of the cell adhesive capacity. In the paraffin-embedded
tumor and mucosal tissue sections, we demonstrated a corresponding
significant increase in the number of ICAM-1-positive fibroblasts
in tumor tissue when compared to that in fibroblasts within the
normal mucosa. This indicates, that i) the pro-inflammatory
micromilieu of CRC leads to a higher expression of ICAM-1-positive
cells in the tumor-surrounding tissue and that ii) these
alterations are stable in fibroblasts when isolated from the
surgical specimens and cultured in vitro.
These results support the theory that inflammation,
as detected here by increased ICAM-1 expression in tumor-associated
fibroblasts may i) enhance intratumoral adhesion of infiltrating
antitumor immune cells and thereby foster a host reaction against
the tumor. Moreover, fibroblasts stimulated by such an inflammatory
reaction within the tumor do show stable expression of ICAM-1 in
vitro and in vivo and ii) may stabilize the tumor by
reducing tumor cell dissemination and consequently tumor
progression may be reduced. As yet it is unclear how this reaction
by TAFs influences the survival of CRC patients. Therefore, TAFs
isolated from CRC will be an important tool for further elucidation
of tumor microenvironment-associated processes in human CRC.
In conclusion, we demonstrated the successful
isolation and cultivation of fibroblasts from fresh surgical
specimens of patients with CRC. Importantly, the isolated cells
preserved their commemoration which is affected by the
microenvironment of the tumor. These fibroblasts are the main
cellular component of the desmoplastic tumor stroma but their
function in tumor progression is not well understood. Further
elucidation of these cells and their interactions may open new
options for non-surgical antitumor therapy.
Acknowledgements
We thank Christina von Kleinsorgen and Ingrid Mons
for their technical support. The present study was supported by
grants from ‘Dr Robert Pfleger Stiftung’ to V.S./E.N. and ‘Deutsche
Krebshilfe’ (AZ 109510) to E.N./M.S., and IZKF-B20 to
R.S.C./M.S./E.N.
Abbreviations:
|
CRC
|
colorectal cancer
|
|
ICAM-1
|
intercellular adhesion molecule-1
|
|
IL-1β
|
interleukin-1β
|
|
NAF
|
normal tissue-associated
fibroblasts
|
|
sICAM-1
|
soluble intercellular adhesion
molecule-1
|
|
TAF
|
tumor-associated fibroblasts
|
|
TNF-α
|
tumor necrosis factor-α
|
|
UICC
|
Union International Contre le
Cancer
|
References
|
1
|
Saunders M and Iveson T: Management of
advanced colorectal cancer: state of the art. Br J Cancer.
95:131–138. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
3
|
Din FV, Theodoratou E, Farrington SM,
Tenesa A, Barnetson RA, Cetnarskyj R, et al: Effect of aspirin and
NSAIDs on risk and survival from colorectal cancer. Gut.
59:1670–1679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang D and Dubois RN: The role of COX-2 in
intestinal inflammation and colorectal cancer. Oncogene.
29:781–788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lakatos PL and Lakatos L: Risk for
colorectal cancer in ulcerative colitis: changes, causes and
management strategies. World J Gastroenterol. 14:3937–3947. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Strell C, Rundqvist H and Ostman A:
Fibroblasts - a key host cell type in tumor initiation,
progression, and metastasis. Ups J Med Sci. 117:187–195. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Benson V, McMahon AC and Lowe HC: ICAM-1
in acute myocardial infarction: a potential therapeutic target.
Curr Mol Med. 7:219–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niessen HW, Lagrand WK, Visser CA, Meijer
CJ and Hack CE: Upregulation of ICAM-1 on cardiomyocytes in
jeopardized human myocardium during infarction. Cardiovasc Res.
41:603–610. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang WC, Chan ST, Yang TL, Tzeng CC and
Chen CC: Inhibition of ICAM-1 gene expression, monocyte adhesion
and cancer cell invasion by targeting IKK complex: molecular and
functional study of novel α-methylene-γ-butyrolactone derivatives.
Carcinogenesis. 25:1925–1934. 2004.PubMed/NCBI
|
|
10
|
van de Stolpe A and van der Saag PT:
Intercellular adhesion molecule-1. J Mol Med. 74:13–33. 1996.
|
|
11
|
Ksiazek K, Mikula-Pietrasik J, Catar R,
Dworacki G, Winckiewicz M, Frydrychowicz M, et al: Oxidative
stress-dependent increase in ICAM-1 expression promotes adhesion of
colorectal and pancreatic cancers to the senescent peritoneal
mesothelium. Int J Cancer. 127:293–303. 2010.
|
|
12
|
Arteta B, Lasuen N, Lopategi A,
Sveinbjörnsson B, Smedsrød B and Vidal-Vanaclocha F: Colon
carcinoma cell interaction with liver sinusoidal endothelium
inhibits organ-specific antitumor immunity through
interleukin-1-induced mannose receptor in mice. Hepatology.
51:2172–2182. 2010. View Article : Google Scholar
|
|
13
|
Alexiou D, Karayiannakis AJ, Syrigos KN,
Zbar A, Kremmyda A, Bramis I and Tsigris C: Serum levels of
E-selectin, ICAM-1 and VCAM-1 in colorectal cancer patients:
correlations with clinicopathological features, patient survival
and tumour surgery. Eur J Cancer. 37:2392–2397. 2001. View Article : Google Scholar
|
|
14
|
Maeda K, Kang SM, Sawada T, Nishiguchi Y,
Yashiro M, Ogawa Y, et al: Expression of intercellular adhesion
molecule-1 and prognosis in colorectal cancer. Oncol Rep.
9:511–514. 2002.PubMed/NCBI
|
|
15
|
Mantur M, Snarska J, Koper O, Dzieciol J,
Plonski A and Lemancewicz D: Serum sICAM, sVCAM and sE-selectin
levels in colorectal cancer patients. Folia Histochem Cytobiol.
47:621–625. 2009.PubMed/NCBI
|
|
16
|
Taglia L, Matusiak D, Matkowskyj KA and
Benya RV: Gastrin-releasing peptide mediates its morphogenic
properties in human colon cancer by upregulating intracellular
adhesion protein-1 (ICAM-1) via focal adhesion kinase. Am J Physiol
Gastrointest Liver Physiol. 292:G182–G190. 2007. View Article : Google Scholar
|
|
17
|
Wimmenauer S, Keller H, Rückauer KD,
Rahner S, Wolff-Vorbeck G, Kirste G, et al: Expression of CD44,
ICAM-1 and N-CAM in colorectal cancer. Correlation with the tumor
stage and the phenotypical characteristics of tumor-infiltrating
lymphocytes. Anticancer Res. 17:2395–2400. 1997.
|
|
18
|
Kitagawa T, Matsumoto K and Iriyama K:
Serum cell adhesion molecules in patients with colorectal cancer.
Surg Today. 28:262–267. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanchez-Rovira P, Jimenez E, Carracedo J,
Barneto IC, Ramirez R and Aranda E: Serum levels of intercellular
adhesion molecule 1 (ICAM-1) in patients with colorectal cancer:
inhibitory effect on cytotoxicity. Eur J Cancer. 34:394–398. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang X, Wang F, Xie JD, Cao J and Xian PZ:
Clinical evaluation of serum concentrations of intercellular
adhesion molecule-1 in patients with colorectal cancer. World J
Gastroenterol. 11:4250–4253. 2005.PubMed/NCBI
|
|
21
|
Alexiou D, Karayiannakis AJ, Syrigos KN,
Zbar A, Sekara E, Michail P, et al: Clinical significance of serum
levels of E-selectin, intercellular adhesion molecule-1, and
vascular cell adhesion molecule-1 in gastric cancer patients. Am J
Gastroenterol. 98:478–485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakata B, Hori T, Sunami T, Ogawa Y,
Yashiro M, Maeda K, et al: Clinical significance of serum soluble
intercellular adhesion molecule 1 in gastric cancer. Clin Cancer
Res. 6:1175–1179. 2000.PubMed/NCBI
|
|
23
|
Zhang GJ and Adachi I: Serum levels of
soluble intercellular adhesion molecule-1 and E-selectin in
metastatic breast carcinoma: Correlations with clinicopathological
features and prognosis. Int J Oncol. 14:71–77. 1999.
|
|
24
|
O'Hanlon DM, Fitzsimons H, Lynch J, Tormey
S, Malone C and Given HF: Soluble adhesion molecules (E-selectin,
ICAM-1 and VCAM-1) in breast carcinoma. Eur J Cancer. 38:2252–2257.
2002. View Article : Google Scholar
|
|
25
|
Silva HC, Garcao F, Coutinho EC, De
Oliveira CF and Regateiro FJ: Soluble VCAM-1 and E-selectin in
breast cancer: relationship with staging and with the detection of
circulating cancer cells. Neoplasma. 53:538–543. 2006.PubMed/NCBI
|
|
26
|
Perabo F, Sharma S, Gierer R, Wirger A,
Fimmers R, Steiner G, et al: Circulating intercellular adhesion
molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and
E-selectin in urological malignancies. Indian J Cancer. 38:1–7.
2001.PubMed/NCBI
|
|
27
|
Harning R, Mainolfi E, Bystryn JC, Henn M,
Merluzzi VJ and Rothlein R: Serum levels of circulating
intercellular adhesion molecule 1 in human malignant melanoma.
Cancer Res. 51:5003–5005. 1991.PubMed/NCBI
|
|
28
|
Grothey A, Heistermann P, Philippou S and
Voigtmann R: Serum levels of soluble intercellular adhesion
molecule-1 (ICAM-1, CD54) in patients with non-small-cell lung
cancer: correlation with histological expression of ICAM-1 and
tumour stage. Br J Cancer. 77:801–807. 1998. View Article : Google Scholar
|
|
29
|
Shimizu Y, Minemura M, Tsukishiro T,
Kashii Y, Miyamoto M, Nishimori H, et al: Serum concentration of
intercellular adhesion molecule-1 in patients with hepatocellular
carcinoma is a marker of the disease progression and prognosis.
Hepatology. 22:525–531. 1995.PubMed/NCBI
|
|
30
|
Christiansen I, Sundström C and Tötterman
TH: Elevated serum levels of soluble vascular cell adhesion
molecule-1 (sVCAM-1) closely reflect tumour burden in chronic
B-lymphocytic leukaemia. Br J Haematol. 103:1129–1137. 1998.
View Article : Google Scholar
|
|
31
|
Jackson AM, Alexandrov AB, Gribben SC,
Esuvarnathan K and James K: Expression and shedding of ICAM-1 in
bladder cancer and its immunotherapy. Int J Cancer. 55:921–925.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Budnik A, Grewe M, Gyufko K and Krutmann
J: Analysis of the production of soluble ICAM-1 molecules by human
cells. Exp Hematol. 24:352–359. 1996.PubMed/NCBI
|
|
33
|
Franzke A, Probst-Kepper M, Buer J,
Duensing S, Hoffmann R, Wittke F, et al: Elevated pretreatment
serum levels of soluble vascular cell adhesion molecule 1 and
lactate dehydrogenase as predictors of survival in cutaneous
metastatic malignant melanoma. Br J Cancer. 78:40–45. 1998.
View Article : Google Scholar
|
|
34
|
Schellerer VS, Croner RS, Weinländer K,
Hohenberger W, Stürzl M and Naschberger E: Endothelial cells of
human colorectal cancer and healthy colon reveal phenotypic
differences in culture. Lab Invest. 87:1159–1170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Desmoulière A, Guyot C and Gabbiani G: The
stroma reaction myofibroblast: a key player in the control of tumor
cell behavior. Int J Dev Biol. 48:509–517. 2004.PubMed/NCBI
|
|
36
|
Gonda TA, Varro A, Wang TC and Tycko B:
Molecular biology of cancer-associated fibroblasts: can these cells
be targeted in anti-cancer therapy? Semin Cell Dev Biol. 21:2–10.
2010. View Article : Google Scholar : PubMed/NCBI
|