Introduction
Esophageal cancer is one of the most aggressive and
lethal malignancies. Surgical treatment is considered the standard
management approach for esophageal cancer. However, despite recent
advances in surgical technique, the prognosis of patients who
undergo surgery alone is poor (1–3). Thus,
multimodal treatment such as surgery following neoadjuvant
chemotherapy or chemoradiotherapy is advocated. In fact, several
clinical trials have shown that such multimodal therapies prolonged
survival of patients with esophageal cancer (4–7).
However, the reported response rate to chemotherapy in esophageal
cancer is only 19–40% (1,2,4,8–10)
and chemoresistance has emerged as a serious problem. Thus, there
is a need to understand the underlying mechanism of chemoresistance
in esophageal cancer.
Epithelial-mesenchymal transition (EMT) is a
biologic process that allows a polarized epithelial cell, which
normally interacts with the basement membrane via its basal
surface, to undergo multiple biochemical changes that enable it to
assume a mesenchymal phenotype. The latter phenotype is
characterized by enhanced migratory capacity, invasiveness, high
resistance to apoptosis and enhanced production of components of
the extracellular matrix (ECM) (11). EMT and the reverse process, termed
mesenchymal-epithelial transition (MET), play a central role in
embryogenesis (type 1 EMT). EMT is also associated with wound
healing, tissue regeneration and organ fibrosis (type 2 EMT)
(12–14). Moreover, EMT occurs in neoplastic
cells that have previously undergone genetic and epigenetic
changes, specifically in genes that favor clonal outgrowth and the
development of localized tumors (type 3 EMT). Upon undergoing EMT,
cancer cells acquire migratory and invasiveness properties that
allow them to migrate through the ECM, resulting in increased
metastatic potential (15,16).
Accumulating evidence suggests a direct link between
EMT and acquisition of stem cell characteristics (17). Induction of EMT confers many of the
properties of self-renewing stem cells (17,18).
These findings suggest that EMT plays an important role in
resistance to chemotherapy, because cancer stem cells are
considered responsible for resistance to anticancer treatment, such
as chemotherapy and radiotherapy (19–21). A
possible association between EMT and chemotherapy resistance is
suggested by recent studies on various cancer cells. However, there
is virtually no direct clinical evidence that links mesenchymal
phenotype to chemoresistance in human malignancies. Moreover, the
association between EMT and chemoresistance has not been elucidated
in esophageal cancers.
The present study was designed to determine the
expression of EMT-related markers, including E-cadherin, snail,
ZEB1 and vimentin, in residual tumors after chemotherapy using
samples obtained from patients who underwent preoperative
chemotherapy for esophageal cancers. The study also investigated
the relationship between the expressions of such EMT markers with
prognosis of patients who underwent chemotherapy.
Materials and methods
Patients and tissue samples
The 185 tissue samples were obtained from patients
who underwent radical esophagectomy with lymph node dissection for
thoracic esophageal cancer between 1999 and 2007 at the Department
of Gastroenterological Surgery, Graduate School of Medicine, Osaka
University (Osaka, Japan). Informed consent was obtained from each
patient prior to participation in the study. Of these patients, 93
received preoperative chemotherapy followed by surgery while the
remaining 92 patients underwent surgery without preoperative
therapy. In 65 of the 93 patients who underwent preoperative
chemotherapy followed by surgery, endoscopic biopsy samples were
obtained before treatment and used for immunohistochemical
analysis. Two courses of 4-week preoperative chemotherapy with
cisplatin at 70 mg/m2, adriamycin at 35 mg/m2
by rapid intravenous infusion on Day 1 and 5-FU at 700
mg/m2 by continuous intravenous infusion on Days 1–7
followed by 3-weeks off were scheduled before surgical treatment
(6,22). The median duration of the follow-up
period was 46 months (range, 18–78 months). Furthermore, 107
patients (57.8%) died during the follow-up.
Immunohistochemistry and evaluation
Resected tumor specimens were fixed with 10%
formalin in phosphate-buffered saline (PBS). The paraffin-embedded
tissue blocks were sectioned at 4-μm slices. The sections were
deparaffinized in xylene and dehydrated in graded ethanol. For
antigen retrieval, they were incubated in 10 mM citrate buffer at
95°C water bath for 40 min. The endogenous peroxidase activity in
the tissue specimens was blocked by incubating the slides in 3%
hydrogen peroxide (H2O2) solution in methanol
at room temperature for 20 min. After treatment of the sections
with 1% bovine serum albumin for 30 min at room temperature to
block nonspecific reactions, all sections were incubated with a
primary antibody at working dilution in a humidified chamber at 4°C
overnight. The antibodies used in the study were anti-E-cadherin
monoclonal antibody (mAb, dilution 1:100, buffer pH 9.0; Dako,
Corp., Carpinteria, CA), anti-Snail polyclonal antibody (pAb,
dilution 1:100, buffer pH 9.0; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA), anti-vimentin mAb (dilution 1:100, buffer pH 9.0;
Santa Cruz Biotechnology, Inc.), anti-ZEB1 mAb (dilution 1:500,
buffer pH 6.0; Dako, Corp.), anti-β-catenin mAb (dilution 1:100,
buffer pH 9.0; Dako, Corp.), anti-N-cadherin pAb (dilution 1:200,
buffer pH 9.0; Millipore, Bedford, MA). After incubation with
secondary antibodies for 20 min, the reactions were visualized
using Vectastain ABC immunoperoxidase kit (Vector Laboratories,
Burlington, VT) with 3,3′-diaminobenzidine, which stained the
antigen brown, and hematoxylin counterstaining.
Two investigators (J.H. and H.M.) independently
evaluated the immunohistochemical sections. The deepest invaded
area, called the invasive front, was recorded. The degree of
E-cadherin and β-catenin immunostaining was graded as reduced,
negative or cytoplasmic immunoreactivity; preserved, strong linear
immunoreactivity on the cell membrane (23). The expression levels of
nuclear-Snail and cytoplasmic-vimentin, cytoplasmic-ZEB1, membrane-
or cytoplasmic-N-cadherin were scored as negative, ≤10% positive
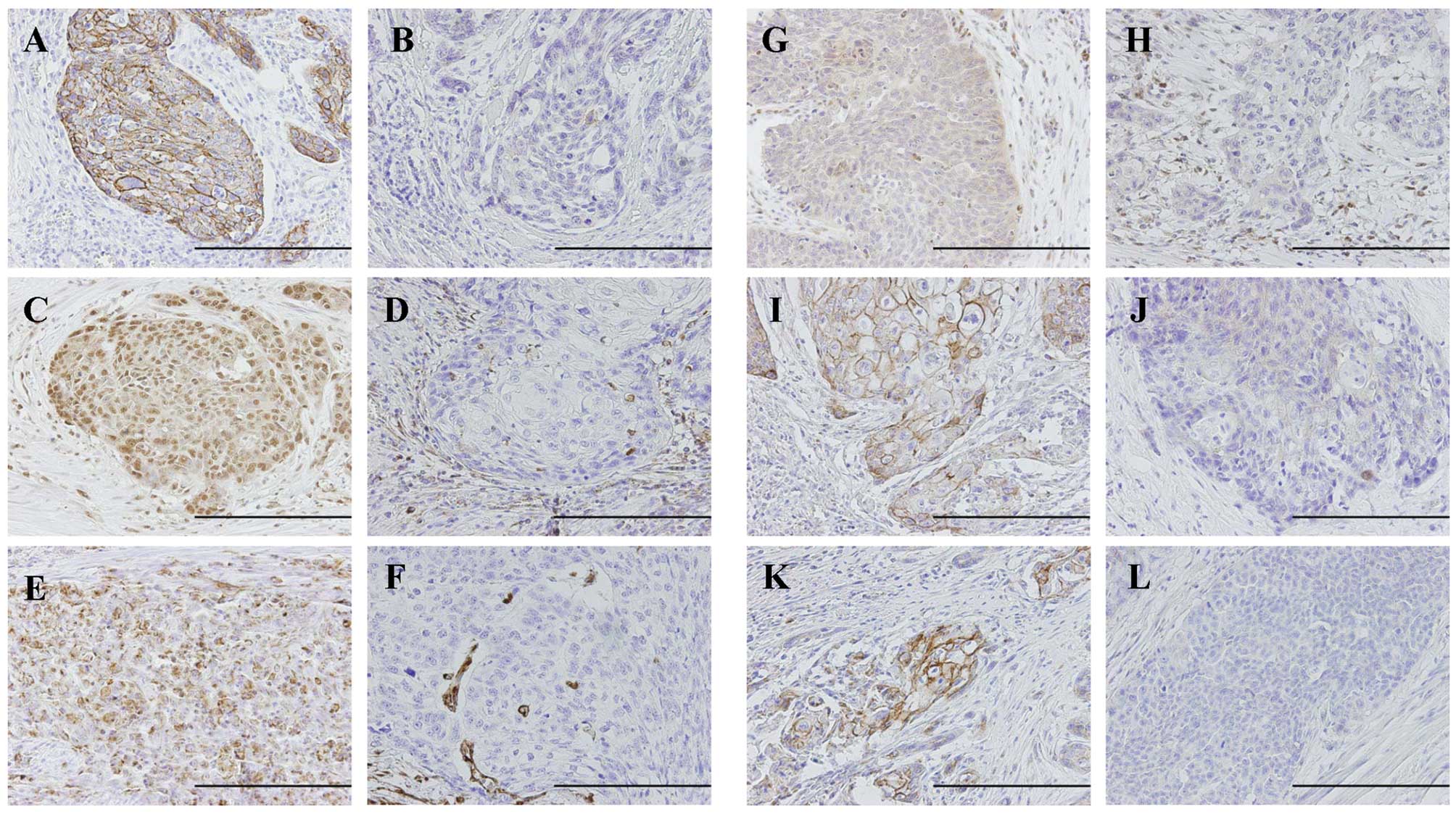
tumor cells; positive, >10% positive tumor cells (Fig. 1).
Clinical and histopathological evaluation
of response to chemotherapy
Two weeks after 2 cycles of neoadjuvant
chemotherapy, all patients were re-assessed to evaluate the
clinical response to chemotherapy by endoscopy, computed tomography
(CT) and positron emission tomography (PET). The World Health
Organization response criteria for measureable disease and the
criteria of the Japanese Society for Esophageal Diseases were used
to assess clinical response (24,25). A
complete response (CR) was defined as disappearance of all lesions.
A CR of the primary tumor represented disappearance of the tumor on
CT scan and/or PET scan and endoscopy. A partial response (PR) was
defined as >50% reduction in primary tumor size and lymph node
metastasis, as confirmed by CT scan. Progressive disease (PD) was
defined as >25% increase in the primary tumor or the appearance
of new lesions. Stable disease (SD) was defined as neither
sufficient decrease to qualify for PR nor sufficient increase to
qualify for PD.
Based on the percentage of viable residual tumor
cells at the primary site after neoadjuvant chemotherapy, curative
effect was classified into five categories. Briefly, the percentage
of viable residual tumor cells within the entire cancer tissue was
assessed as follows: grade 3, no viable residual tumor cells are
evident; grade 2, viable residual tumor cells account for less than
one-third of tumor tissue; grade 1b, viable residual tumor cells
account for less than one-third or more but less than two-thirds of
tumor tissue; grade 1a, viable residual tumor cells account for
two-thirds or more tumor tissue; and grade 0, no recognizable
histlogical chemotherapy effect (6,25).
Statistical analysis
Statistical analysis of group differences was
performed using the χ2 test, Fisher’s exact test or
Mann-Whitney U test. For survival analysis, the Kaplan-Meier method
was used to assess survival distribution according to EMT-marker
expression and differences in survival were estimated using the
log-rank test. The Cox proportional hazards regression model was
used to analyze the simultaneous influence of prognostic factors.
Wilcoxon signed-ranks test was used to assess the change in
E-cadherin and Snail expression after chemotherapy. A P-value of
<0.05 denoted the presence of statistically significant
difference between groups. All statistical analyses were performed
using the software package JMP 8 for Windows (SAS Institute, Inc.,
Cary, NC).
Results
Expression of EMT makers in residual and
chemo-naive tumors
Of the 195 tumors, 93 tumors were residual tumors
after preoperative chemotherapy and 92 tumors were chemo-naive
tumors without preoperative therapy. There was no significant
difference between residual tumors and chemo-naive tumors in
differentiation, tumor depth and lymph node metastasis (Table I).
 | Table ICharacteristics of 185 patients with
esophageal cancer. |
Table I
Characteristics of 185 patients with
esophageal cancer.
| Chemotherapy | |
|---|
|
| |
|---|
| Residual (n=93) | Naive (n=92) | P-value |
|---|
| Gender
(male/female) | 79/14 | 83/9 | 0.276 |
| Age (mean) | 64.0 | 63.7 | 0.512 |
| Tumor location
(upper/middle/lower) | 22/36/35 | 12/47/33 | 0.236 |
| Differentiation
(G1,2/G3,4) | 65/28 | 75/17 | 0.418 |
| Depth of invasion
(pT1-2/3-4) | 32/61 | 41/51 | 0.157 |
| Lymph node metastasis
(pN0/1) | 27/65 | 33/59 | 0.345 |
| Lymphatic permeation
(positive/negative) | 77/16 | 70/22 | 0.258 |
| Venous permeation
(positive/negative) | 52/41 | 43/49 | 0.212 |
We quantitated the expression of the epithelial
marker E-cadherin and mesenchymal markers snail, vimentin, ZEB1,
and N-cadherin in residual tumors and chemo-naive tumors (Table II). Fifty percent (46/92) of
chemo-naive tumors stained strongly for E-cadherin, while 71% of
residual tumors stained weakly for E-cadherin. Statistical analysis
indicated significant underexpression of E-cadherin, as a marker of
epithelial cells, in residual tumors compared with chemo-naive
tumors (P=0.003). Snail expression was significantly higher in
residual tumors than in chemo-naive tumors (P=0.028). Similarly,
the expression levels of ZEB1 and N-cadherin were significantly
higher in residual tumors than in chemo-naive tumors (P<0.001
and P=0.001, respectively). However, there were no significant
differences in the expression levels of vimentin and β-catenin
between the two types of tumors. Taken together, higher expression
of mesenchymal markers and lower expression of epithelial markers
characterize residual tumors after chemotherapy.
 | Table IIExpression of mesenchymal and
epithelial markers in residual tumors after chemotherapy and
chemo-naive tumors. |
Table II
Expression of mesenchymal and
epithelial markers in residual tumors after chemotherapy and
chemo-naive tumors.
| Chemotherapy | | |
|---|
|
| | |
|---|
| Residual
(n=93) | Naive (n=92) | Total (n=185) | P-value |
|---|
| E-cadherin |
| Preserved | 27 (29.0) | 46 (50.0) | 73 (39.5) | 0.003 |
| Reduced | 66 (71.0) | 46 (50.0) | 112 (60.1) | |
| Snail |
| Positive | 66 (71.0) | 51 (55.4) | 117 (63.4) | 0.028 |
| Negative | 27 (29.0) | 41 (44.6) | 68 (36.6) | |
| Vimentin |
| Positive | 11 (11.8) | 8 (8.7) | 19 (10.3) | 0.482 |
| Negative | 82 (88.2) | 84 (91.3) | 166 (89.7) | |
| ZEB1 |
| Positive | 36 (38.7) | 14 (15.2) | 50 (27.0) | <0.001 |
| Negative | 57 (61.3) | 78 (84.8) | 135 (73.0) | |
| β-catenin |
| Preserved | 32 (34.4) | 27 (29.3) | 59 (31.9) | 0.460 |
| Reduced | 61 (65.6) | 65 (70.1) | 126 (68.1) | |
| N-cadherin |
| Positive | 51 (54.8) | 29 (31.5) | 80 (43.2) | 0.001 |
| Negative | 42 (45.2) | 63 (68.5) | 105 (66.8) | |
We examined the relationship between E-cadherin
expression, as an epithelial marker, and the expression of several
mesenchymal markers (N-cadherin, vimentin, Snail and ZEB1) in the
residual group. E-cadherin expression correlated inversely with
Snail expression (Table III).
 | Table IIIRelationship between expression of
E-cadherin and EMT markers in the residual group. |
Table III
Relationship between expression of
E-cadherin and EMT markers in the residual group.
| E-cadherin | | |
|---|
|
| | |
|---|
| Preserved
(n=27) | Reduced (n=66) | Total (n=93) | P-value |
|---|
| Snail |
| Positive | 10 (37.0) | 56 (84.8) | 66 (71.0) | <0.001 |
| Negative | 17 (63.0) | 10 (15.2) | 27 (29.0) | |
| Vimentin |
| Positive | 2 (3.7) | 9 (13.6) | 11 (11.8) | 0.379 |
| Negative | 25 (96.3) | 57 (86.4) | 82 (88.1) | |
| ZEB1 |
| Positive | 8 (29.6) | 28 (42.4) | 36 (38.7) | 0.245 |
| Negative | 19 (70.4) | 38 (57.6) | 57 (61.3) | |
| β-catenin |
| Preserved | 12 (44.4) | 20 (30.3) | 32 (34.4) | 0.197 |
| Reduced | 15 (55.6) | 46 (69.7) | 61 (65.6) | |
| N-cadherin |
| Positive | 17 (63.0) | 34 (51.5) | 51 (54.8) | 0.311 |
| Negative | 10 (27.0) | 32 (48.5) | 42 (45.2) | |
Relationship between EMT markers and
response to chemotherapy
Next, we examined the relationship between the
expression of EMT markers and the response to chemotherapy in the
residual tumors. With regard to the clinical response, weak
E-cadherin expression correlated significantly with clinically poor
response (SD/PD), but not with clinically good response (PR)
(P=0.009, Table IV). On the other
hand, positive staining for Snail expression in tumors correlated
significantly with SD/PD, but not PR (P=0.009).
 | Table IVRelationship between response to
chemotherapy and immunohistochemical expression of E-cadherin,
Snail, vimentin, ZEB1, β-catenin and N-cadherin in residual
tumors. |
Table IV
Relationship between response to
chemotherapy and immunohistochemical expression of E-cadherin,
Snail, vimentin, ZEB1, β-catenin and N-cadherin in residual
tumors.
| | Clinical
response | Pathological
response |
|---|
| |
|
|
|---|
| Total (n=93) | PD/SD (n=47) | PR (n=46) | P-value | Grade 0/1a
(n=67) | Grade 1b/2
(n=26) | P-value |
|---|
| E-cadherin |
| Preserved | 27 (29) | 8 (17) | 19 (41) | 0.009 | 13 (19) | 14 (54) | 0.001 |
| Reduced | 66 (71) | 39 (83) | 27 (59) | | 54 (81) | 12 (46) | |
| Snail |
| Positive | 66 (71) | 39 (83) | 27 (59) | 0.009 | 52 (72) | 14 (54) | 0.027 |
| Negative | 27 (29) | 8 (17) | 19 (41) | | 15 (22) | 12 (46) | |
| Vimentin |
| Positive | 11 (12) | 5 (11) | 6 (13) | 0.719 | 8 (12) | 3 (12) | 0.957 |
| Negative | 82 (88) | 42 (89) | 40 (87) | | 59 (88) | 23 (88) | |
| ZEB1 |
| Positive | 36 (39) | 15 (31) | 21 (46) | 0.173 | 26 (39) | 10 (38) | 0.976 |
| Negative | 57 (61) | 32 (68) | 25 (54) | | 41 (41) | 16 (62) | |
| β-catenin |
| Preserved | 32 (34) | 17 (36) | 15 (33) | 0.717 | 23 (34) | 9 (35) | 0.979 |
| Reduced | 61 (66) | 30 (64) | 31 (67) | | 44 (66) | 17 (65) | |
| N-cadherin |
| Positive | 51 (55) | 28 (60) | 23 (50) | 0.353 | 40 (60) | 11 (42) | 0.131 |
| Negative | 42 (45) | 19 (40) | 23 (50) | | 27 (40) | 15 (58) | |
Similar to the clinical response, negative
E-cadherin expression and positive staining for Snail expression
correlated with histopathologically minor response (Grade 0/1a),
but not with major response Grade 1b/2 (P=0.001 and P=0.027,
respectively) (Table V).
 | Table VUnivariate and multivariate analyses
of prognostic factors. |
Table V
Univariate and multivariate analyses
of prognostic factors.
| Univariate | Multivariate |
|---|
|
|
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender
(male/female) | 0.84 | 0.46–1.71 | 0.619 | | | |
| Age
(≤65/>65) | 1.22 | 0.74–2.02 | 0.422 | | | |
| Tumor location
(upper, middle/lower) | 0.73 | 0.43–1.21 | 0.225 | | | |
| Differentiation
(G1,2/G3,4) | 0.97 | 0.55–1.74 | 0.920 | | | |
| Depth of invasion
(pT1-2/pT3-4) | 2.49 | 1.35–5.05 | 0.003 | 2.13 | 1.12–4.37 | 0.018 |
| Lymph node
metastasis (pN0/1) | 3.12 | 2.32–4.21 | <0.001 | 2.12 | 1.21–4.24 | 0.009 |
| Lymphatic invasion
(positive/negative) | 2.09 | 0.81–3.99 | 0.181 | | | |
| Venous invasion
(positive/negative) | 1.21 | 0.74–2.02 | 0.437 | | | |
| E-cadherin
(preserved/reduced) | 0.56 | 0.30–0.98 | 0.043 | 1.21 | 0.63–2.21 | 0.551 |
| Snail
(positive/negative) | 3.31 | 1.78–6.71 | <0.001 | 3.83 | 1.96–8.11 | <0.0001 |
| Vimentin
(positive/negative) | 0.86 | 0.38–1.70 | 0.679 | | | |
| ZEB1
(positive/negative) | 0.88 | 0.51–1.45 | 0.617 | | | |
| β-catenin
(preserved/reduced) | 1.41 | 0.85–2.33 | 0.179 | | | |
| N-cadherin
(positive/negative) | 0.93 | 0.56–1.53 | 0.760 | | | |
| Clinical response
(PD-SD/PR) | 2.29 | 1.38–3.87 | 0.001 | 1.68 | 0.99–2.92 | 0.052 |
Relationship between EMT markers and
survival
We also examined relationship between the expression
of EMT markers and prognosis of patients who underwent preoperative
chemotherapy for esophageal cancer. Low expression of E-cadherin
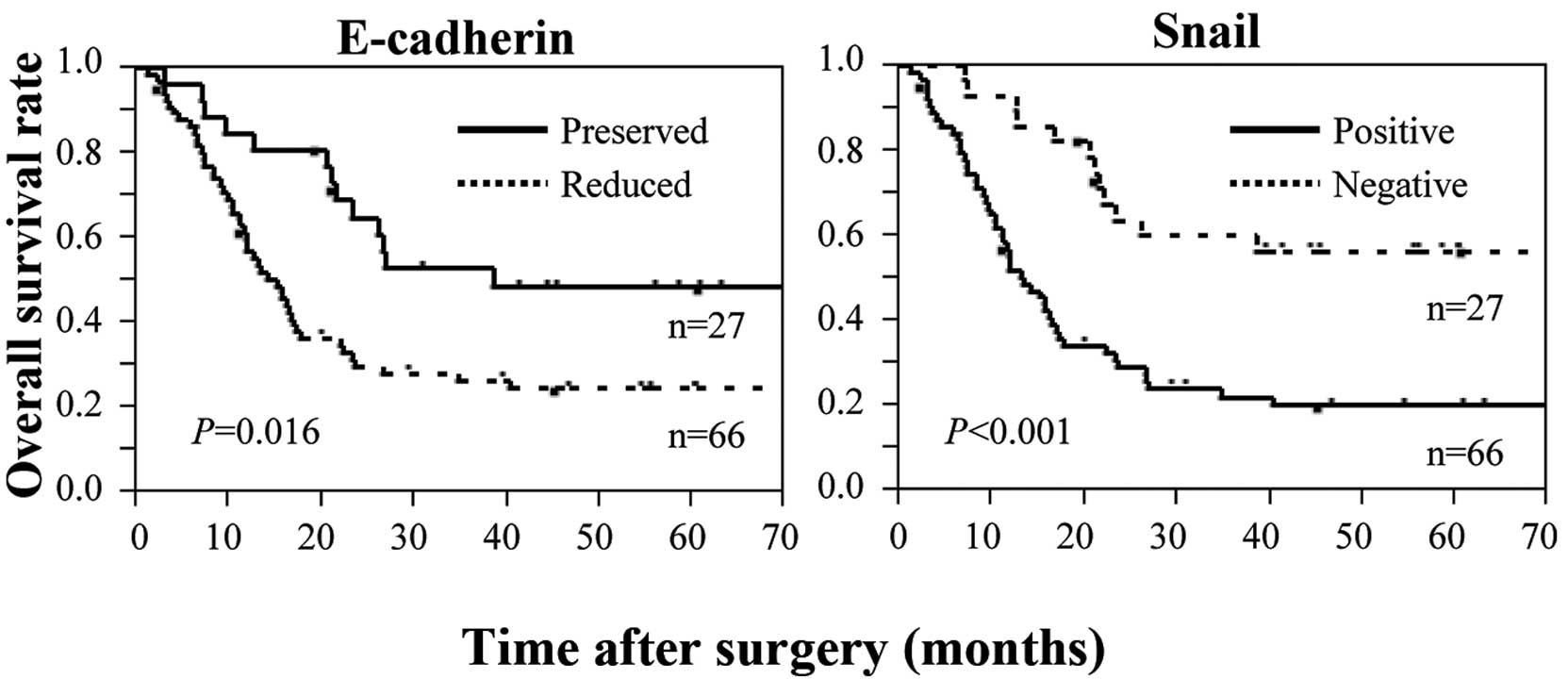
correlated significantly with short survival time (Fig. 2). In contrast, high expression of
Snail correlated significantly with short survival time (Fig. 2). Multivariate analysis identified
Snail expression as an independent prognostic factor, together with
tumor depth, in patients who received preoperative chemotherapy for
esophageal cancer (Table V).
Changes in E-cadherin and Snail
expression after chemotherapy and survival
In 65 of 93 patients with esophageal cancer who
underwent preoperative chemotherapy followed by surgery, we used
immunohistochemistry to compare biopsy samples obtained before
chemotherapy with the surgical specimens after chemotherapy. Among
these 65 patients, chemotherapy decreased the expression of
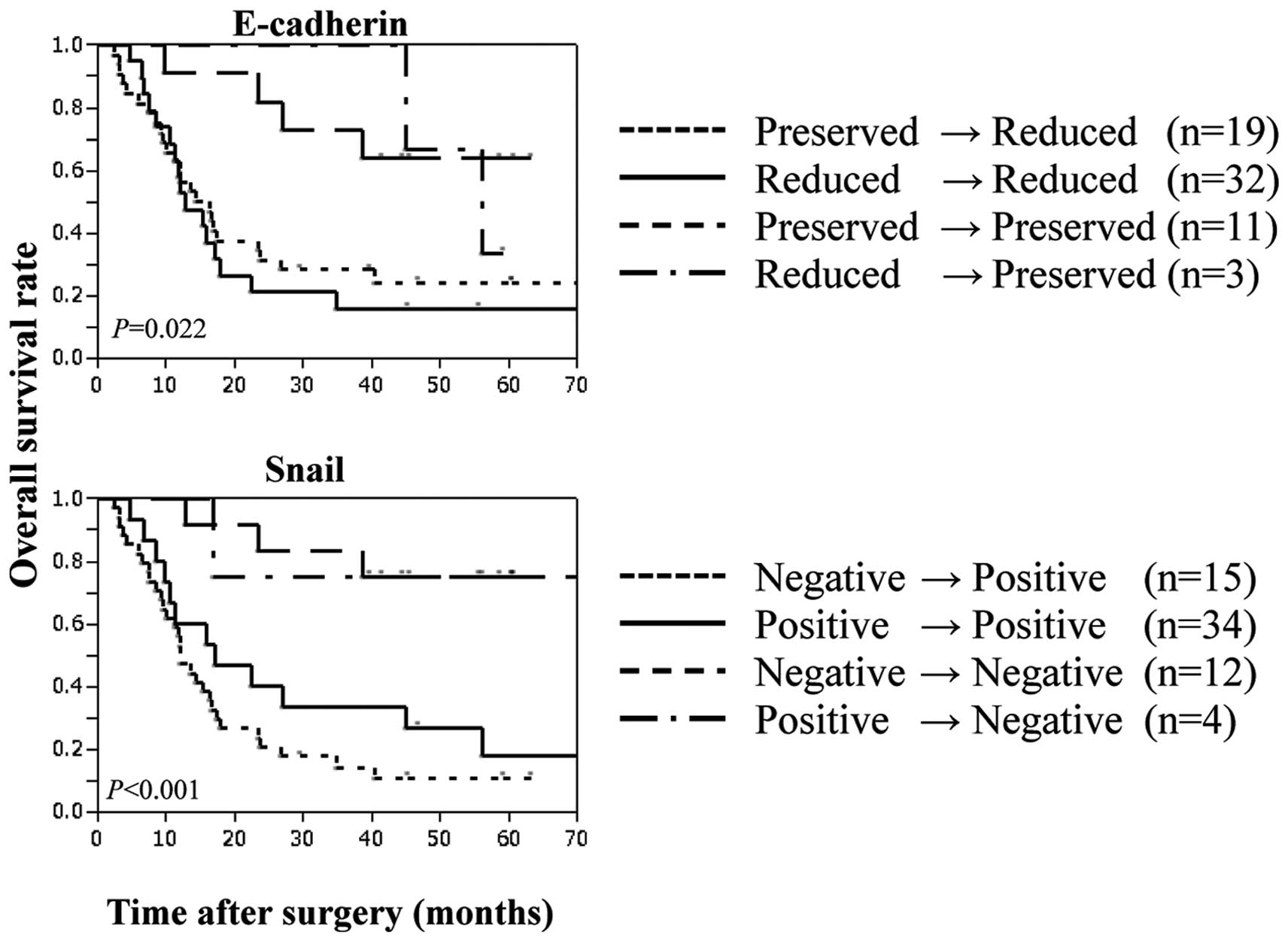
E-cadherin in 19 (preserved→reduced) (Table VI). The survival time was
significantly shorter in 51 patients with low E-cadherin expression
[including the above 19 patients and 32 patients who showed no
change in their low E-cadherin expression after chemotherapy
(reduced→reduced)], compared with 11 patients with preserved
expression of E-cadherin throughout chemotherapy (Fig. 2). With regard to Snail expression,
chemotherapy increased Snail expression in 15 of the 65 patients
(negative to positive). The survival time was significantly shorter
in 49 patients with positive Snail expression [including the above
15 patients and 34 patients who showed no change in positive Snail
expression after chemotherapy (negative to positive)], compared
with 12 patients with Snail-negative tumors throughout chemotherapy
(Fig. 3).
 | Table VIChanges in E-cadherin and Snail
expression after chemotherapy. |
Table VI
Changes in E-cadherin and Snail
expression after chemotherapy.
| Pre-CT biopsy | Residual | n | P-value |
|---|
| E-cadherin | Preserved | Reduced | 19 | <0.001 |
| Reduced | Reduced | 32 | |
| Preserved | Preserved | 11 | |
| Reduced | Preserved | 3 | |
| Snail | Negative | Positive | 15 | 0.019 |
| Positive | Positive | 34 | |
| Negative | Negative | 12 | |
| Positive | Negative | 4 | |
Discussion
Although recent evidence indicates that EMT does not
only cause increased metastasis but also contributes to
chemoresistance, there is no direct clinical evidence for a link
between mesenchymal phenotype and chemoresistance in human
malignancies. In this study, we examined the expression of
EMT-related markers in residual tumors after chemotherapy using
samples obtained from patients who underwent preoperative
chemotherapy for esophageal cancer. The results showed reduced
expression of E-cadherin (a marker of epithelial cells) and
increased expression of Snail, ZEB1 and N-cadherin (markers of
mesenchymal cells) in residual tumors after chemotherapy, compared
with chemo-naive tumors. Moreover, the reduced expression of
E-cadherin and increased expression of snail in residual tumors
were significantly associated with poor response to chemotherapy
and short survival time in patients who underwent preoperative
chemotherapy. These results suggest that residual esophageal tumors
after chemotherapy display mesenchymal features, resulting in
chemoresistance and poor prognosis.
Reduced expression of E-cadherin, which is a central
adhesion molecule located at cell-cell adhesion junctions, is one
of the characteristics findings during progression of EMT (26). Previous studies demonstrated that
the loss of E-cadherin is associated with tumor progression, tumor
metastasis and poor clinical outcome in various human carcinomas
(27–31). The association of E-cadherin
expression and drug sensitivity has been examined in several types
of human cancer. In colorectal cancer, E-cadherin was downregulated
in oxaliplatin-resistant colorectal cancer (CRC) cells (28). In gemcitabine-resistance pancreatic
cancer cells, E-cadherin expression was decreased and nuclear
localization of total β-catenin was increased (30). While the above studies showed
downregulation of E-cadherin in drug-resistant tumor cell lines,
there is little or no evidence for the clinical importance of
E-cadherin expression in drug-resistant human cancers. Using
samples from patients who underwent preoperative chemotherapy for
esophageal cancers, we demonstrated in this study the importance of
E-cadherin underexpression in chemoresistance in human esophageal
cancer.
Snail is recognized as a suppressor of E-cadherin
expression. Snail represses the transcription of E-cadherin by
binding to the E-box elements in the proximal E-cadherin promoter,
thereby triggering a complete EMT and resulting in enhanced tumor
invasiveness (30). Accumulating
evidence suggests the contribution of Snail expression to
therapeutic resistance in various cancers (28–30,33).
Paclitaxel-resistant ovarian cancer cells showed upregulation of
Snail expression, with marked enhancement of metastatic activity,
compared with control cells (30).
In head and neck cancer, Snail contributes to cisplatin resistance
by upregulating excision repair cross complementation group 1
(ERCC1), which plays a key role in nucleotide excision repair and
in platinum-induced DNA adducts (33). In the present study, upregulation of
Snail was observed in residual tumors after chemotherapy for
esophageal cancers and such high expression was significantly
associated with poor response to chemotherapy. These results
provide direct evidence for the important role of Snail expression
in chemoresistance in human esophageal cancer.
In the present study, we examined the relationship
between E-cadherin expression, as an epithelial marker, and the
expression of several mesenchymal markers (N-cadherin, vimentin,
Snail and ZEB1). In recent years, a switch from E-cadherin to
N-cadherin has been often used to monitor the progress of EMT
during embryonic development and cancer progression (34). In our study, although N-cadherin
expression was increased in residual tumors, compared with
chemo-naive tumors, we could not find significant inverse
relationship between E-cadherin and N-cadherin expression. Snail
and ZEB1 are well known transcription repressors of E-cadherin
(29,30,32,35),
and our results showed inverse correlation between E-cadherin and
Snail expression, although we could not find a significant
correlation between E-cadherin and ZEB1 expression.
Recent studies have indicated that cancer cells
undergoing EMT develop resistance to anticancer drugs. However, it
has been difficult to establish the role of EMT in chemoresistance
in human clinical samples. In the present study, we investigated
whether EMT confers resistance to chemotherapy by comparing the
expression of EMT-related markers in residual tumors after
chemotherapy with that in chemo-naive tumors. A few studies have
previously shown the presence of EMT in residual tumors after
conventional anti-cancer therapy. One such study demonstrated
recently mesenchymal features of tumor cells that had survived
conventional treatment, such as chemotherapy and endocrine therapy,
in human breast cancer (36). The
results of the present study demonstrating mesenchymal features of
tumor cells after chemotherapy in esophageal cancer provide further
support to the above previous studies.
One important problem in the present study is
whether tumor cells with initial mesenchymal phenotype survive the
chemotherapy or whether residual tumor cells acquire mesenchymal
features during chemotherapy. In this study, we compared the
expression of EMT-related markers such as E-cadherin and Snail
before and after chemotherapy in the same case, and found in
certain cases mesenchymal features in residual tumors after
chemotherapy compared with epithelial features before treatment.
This finding suggests that residual tumor cells seem to acquire
mesenchymal features during chemotherapy. However, the value of
immunohistochemistry in accurate assessment of gene expression in
biopsy samples is limited, because biopsy samples do not allow
accurate estimation of such events in the invasive front of tumors.
Recent studies have pointed to link between EMT phenotype and
development of cancer stem cells; cancer cells undergoing EMT
exhibit characteristic markers of cancer stem cells and properties
of cancer stem cells (17).
However, other studies have suggested that cancer stem cells from
solid tumors are not actually static entities but rather tumor
cells that transiently acquire stemness properties depending on the
tumor context (37), although the
traditional concept of cancer stem cells is a unidirectional
hierarchical model. These findings suggest that residual esophageal
cancer cells may transiently acquire mesenchymal features to
survive during chemotherapy. In support of this notion, one recent
study showed that cancer cell populations employ a dynamic strategy
in which individual cells transiently assume a reversibly
drug-tolerant state to protect the remaining population from
eradication by exposure to lethal anti-cancer drugs (38). Further studies are required to
ascertain whether esophageal cancer cells transiently acquire
mesenchymal features and stemness properties during chemotherapy in
human esophageal cancers.
In conclusion, the present study demonstrated
decreased expression of E-cadherin and increased expression of
Snail, ZEB1 and N-cadherin in residual tumors after chemotherapy in
human esophageal cancers, compared with chemo-naive tumors.
Moreover, in patients who underwent preoperative chemotherapy, the
reduced expression of E-cadherin and increased expression of Snail
in residual tumors correlated significantly with poor response to
chemotherapy and poor prognosis. These findings suggest that
residual tumors after chemotherapy for esophageal cancer switch to
mesenchymal phenotype, resulting in chemoresistance and poor
clinical outcome.
Acknowledgements
This study was supported by a Grant-in-Aid for Young
Scientists from the Ministry of Education, Culture, Sports, Science
and Technology of Japan.
References
|
1
|
Medical Research Council Oesophageal
Cancer Working Group. Surgical resection with or without
preoperative chemotherapy in oesophageal cancer: a randomised
controlled trial. Lancet. 359:1727–1733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kelsen DP, Ginsberg R, Pajak TF, Sheahan
DG, Gunderson L, Mortimer J, Estes N, Haller DG, Ajani J, Kocha W,
et al: Chemotherapy followed by surgery compared with surgery alone
for localized esophageal cancer. N Engl J Med. 339:1979–1984. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gebski V, Burmeister B, Smithers BM, Foo
K, Zalcberg J and Simes J: Australasian Gastro-Intestinal Trials
Group, Survival benefits from neoadjuvant chemoradiotherapy or
chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet
Oncol. 8:226–234. 2007. View Article : Google Scholar
|
|
4
|
Law S, Fok M, Chow S, Chu KM and Wong J:
Preoperative chemotherapy versus surgical therapy alone for
squamous cell carcinoma of the esophagus: a prospective randomized
trial. J Thorac Cardiovasc Surg. 114:210–217. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ando N, Iizuka T, Ide H, Ishida K, Shinoda
M, Nishimaki T, Takiyama W, Watanabe H, Isono K, Aoyama N, et al:
Japan Clinical Oncology Group, Surgery plus chemotherapy compared
with surgery alone for localized squamous cell carcinoma of the
thoracic esophagus: a Japan Clinical Oncology Group Study -
JCOG9204. J Clin Oncol. 21:4592–4596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yano M, Takachi K, Doki Y, Miyashiro I,
Kishi K, Noura S, Eguchi H, Yamada T, Ohue M, Ohigashi H, et al:
Preoperative chemotherapy for clinically node-positive patients
with squamous cell carcinoma of the esophagus. Dis Esophagus.
19:158–163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tepper J, Krasna MJ, Niedzwiecki D, Hollis
D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D and Mayer
R: Phase III trial of trimodality therapy with cisplatin,
fluorouracil, radiotherapy, and surgery compared with surgery alone
for esophageal cancer: CALGB 9781. J Clin Oncol. 26:1086–1092.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hilgenberg AD, Carey RW, Wilkins EW Jr,
Choi NC, Mathisen DJ and Grillo HC: Preoperative chemotherapy,
surgical resection, and selective postoperative therapy for
squamous cell carcinoma of the esophagus. Ann Thorac Surg.
45:357–363. 1998. View Article : Google Scholar
|
|
9
|
Ancona E, Ruol A, Santi S, Merigliano S,
Sileni VC, Koussis H, Zaninotto G, Bonavina L and Peracchia A: Only
pathologic complete response to neoadjuvant chemotherapy improves
significantly the long term survival of patients with resectable
esophageal squamous cell carcinoma: final report of a randomized,
controlled trial of preoperative chemotherapy versus surgery alone.
Cancer. 191:2165–2174. 2001.
|
|
10
|
Urschel JD, Vasan H and Blewett CJ: A
meta-analysis of randomized controlled trials that compared
neoadjuvant chemotherapy and surgery to surgery alone for
resectable esophageal cancer. Am J Surg. 183:274–279. 2002.
View Article : Google Scholar
|
|
11
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeisberg EM, Tarnavski O, Zeisberg M,
Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT,
Roberts AB, et al: Endothelial-to-mesenchymal transition
contributes to cardiac fibrosis. Nat Med. 13:952–961. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeisberg M, Yang C, Martino M, Duncan MB,
Rieder F, Tanjore H and Kalluri R: Fibroblasts derive from
hepatocytes in liver fibrosis via epithelial to mesenchymal
transition. J Biol Chem. 282:23337–23347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim KK, Kugler MC, Wolters PJ, Robillard
L, Galvez MG, Brumwell AN, Sheppard D and Chapman HA: Alveolar
epithelial cell mesenchymal transition develops in vivo during
pulmonary fibrosis and is regulated by the extracellular matrix.
Proc Natl Acad Sci USA. 103:13180–13185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: at the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Biddle A, Liang X, Gammon L, Fazil B,
Harper LJ, Emich H, Costea DE and Mackenzie IC: Cancer stem cells
in squamous cell carcinoma switch between two distinct phenotypes
that are preferentially migratory or proliferative. Cancer Res.
71:5317–5326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kurrey NK, Jalgaonkar SP, Joglekar AV,
Ghanate AD, Chaskar PD, Doiphode RY and Bapat SA: Snail and slug
mediate radioresistance and chemoresistance by antagonizing
p53-mediated apoptosis and acquiring a stem-like phenotype in
ovarian cancer cells. Stem Cells. 27:2059–2068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eyler CE and Rich JN: Survival of the
fittest: cancer stem cells in therapeutic resistance and
angiogenesis. J Clin Oncol. 26:2839–2845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baumann M, Krause M and Hill R: Exploring
the role of cancer stem cells in radioresistance. Nat Rev Cancer.
8:545–554. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyata H, Yoshioka A, Yamasaki M,
Nushijima Y, Takiguchi S, Fujiwara Y, Nishida T, Mano M, Mori M and
Doki Y: Tumor budding in tumor invasive front predicts prognosis
and survival of patients with esophageal squamous cell carcinomas
receiving neoadjuvant chemotherapy. Cancer. 115:3324–3334. 2009.
View Article : Google Scholar
|
|
23
|
Usami Y, Chiba H, Nakayama F, Ueda J,
Matsuda Y, Sawada N, Komori T, Ito A and Yokozaki H: Reduced
expression of claudin-7 correlates with invasion and metastasis in
squamous cell carcinoma of the esophagus. Hum Pathol. 37:569–577.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miller AB, Hoogstraten B, Staquet M and
Winkler A: Reporting results of cancer treatment. Cancer.
47:207–214. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Japan Esophageal Society. Japanese
classification of esophageal cancer, tenth edition: parts II and
III. Esophagus. 6:71–94. 2009. View Article : Google Scholar
|
|
26
|
Lombaerts M, van Wezel T, Philippo K,
Dierssen JW, Zimmerman RM, Oosting J, van Eijk R, Eilers PH, van de
Water B, Cornelisse CJ and Cleton-Jansen AM: E-cadherin
transcriptional downregulation by promoter methylation but not
mutation is related to epithelial-to-mesenchymal transition in
breast cancer cell lines. Br J Cancer. 94:661–671. 2006.PubMed/NCBI
|
|
27
|
Shiozaki H, Tahara H, Oka H, Miyata M,
Kobayashi K, Tamura S, Iihara K, Doki Y, Hirano S, Takeichi M and
Mori T: Expression of immunoreactive E-cadherin adhesion molecules
in human cancers. Am J Pathol. 139:17–23. 1999.
|
|
28
|
Yang AD, Fan F, Camp ER, van Buren G, Liu
W, Somcio R, Gray MJ, Cheng H, Hoff PM and Ellis LM: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kajiyama H, Shibata K, Terauchi M,
Yamashita M, Ino K, Nawa A and Kikkawa F: Chemoresistance to
paclitaxel induces epithelial-mesenchymal transition and enhances
metastatic potential for epithelial ovarian carcinoma cells. Int J
Oncol. 31:277–283. 2007.
|
|
30
|
Shah AN, Summy JM, Zhang J, Park SI,
Parikh NU and Gallick GE: Development and characterization of
gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol.
14:3629–3637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arumugam T, Ramachandran V, Fournier KF,
Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey
DJ and Choi W: Epithelial to mesenchymal transition contributes to
drug resistance in pancreatic cancer. Cancer Res. 69:5820–5828.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: an alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hsu DS, Lan HY, Huang CH, Tai SK, Chang
SY, Tsai TL, Chang CC, Tzeng CH, Wu KJ, Kao JY and Yang MH:
Regulation of excision repair cross-complementation group 1 by
Snail contributes to cisplatin resistance in head and neck cancer.
Clin Cancer Res. 16:4561–4571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hirohashi S and Kanai Y: Cell adhesion
system and human cancer morphogenesis. Cell Sci. 94:575–581.
2003.PubMed/NCBI
|
|
35
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Creighton CJ, Li X, Landis M, Dixon JM,
Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A,
Herschkowitz JI, et al: Residual breast cancers after conventional
therapy display mesenchymal as well as tumor-initiating features.
Proc Natl Acad Sci USA. 106:13820–13825. 2009. View Article : Google Scholar
|
|
37
|
Roesch A, Fukunaga-Kalabis M, Schmidt EC,
Zabierowski SE, Brafford PA, Vultur A, Basu D, Gimotty P, Vogt T
and Herlyn M: A temporarily distinct subpopulation of slow-cycling
melanoma cells is required for continuous tumor growth. Cell.
141:583–594. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sharma SV, Lee DY, Li B, Quinlan MP,
Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach
MA, et al: A chromatin-mediated reversible drug-tolerant state in
cancer cell subpopulations. Cell. 141:69–80. 2010. View Article : Google Scholar : PubMed/NCBI
|