Introduction
Osteosarcoma is a malignant bone tumor that usually
develops in adolescents and young adults. The National Cancer
Institute estimates the number of new cases of osteosarcoma each
year to be 4.4 per one million among individuals aged 0–24 years
(1). Most treatment protocols for
osteosarcoma use an initial period of systemic chemotherapy prior
to definitive resection of the primary tumor. Notably, most of the
commonly used chemotherapeutic drugs kill normal as well as cancer
cells (2). Therefore, drugs
targeted specifically at cancer cells while leaving normal cells
intact, or even protecting normal cells, would be ideal for use as
part of a chemotherapy regimen.
Panax ginseng was discovered over 5,000 years
ago in the hills of Manchuria in China. Since then, the plant has
held its place as a highly venerated medicinal plant in traditional
Chinese medicine (3). Over recent
decades, the anticancer effects of ginsenosides have garnered
increasing attention because of their favorable safety and efficacy
profiles (4). Ginsenosides enhance
cytotoxic and humoral immune responses (5). Recent research has shown that
ginsenosides can inhibit the growth of several cancer cell lines
(6). The anticarcinogenic and
antimetastatic effects of ginsenoside Rg3 have been demonstrated
in vitro and in vivo (7). Ginsenoside Rg3 induces cell cycle
arrest and apoptosis in mammalian tumor cells (8). However, few published reports describe
the genotoxicity of ginsenoside Rg3. In contrast, the
cytoprotective effect of ginsenosides has been demonstrated in
other studies. Poon et al (9) showed that ginsenoside 20-Rg3 protected
against BaP-induced DNA damage in human dermal fibroblasts (HDFs).
Ginsenoside Rg3 was found to protect against
cyclophosphamide-induced DNA damage and cell apoptosis by reducing
oxidative stress (10). Ginseng
extract was also found to protect against γ-ray-induced DNA
double-strand breaks (11).
The results outlined above suggest that ginsenosides
could be used for the chemotherapeutic treatment of patients with
osteosarcomas. Theoretically, ginsenosides would destroy cancer
cells but leave normal cells unharmed. To prove our hypothesis, we
explored the effects of ginsenoside Rg3 on in vitro DNA
damage and apoptosis in human osteosarcoma cell lines. The presumed
cytoprotective effect of ginsenoside Rg3 was investigated in human
fibroblasts. Sensitive and quantitative detection assays (e.g., the
alkaline comet assay, measurements of γH2AX focus formation, flow
cytometry and DNA ladder assay) were used. The results confirmed
that ginsenoside Rg3 exhibited obvious genotoxicity against human
osteosarcoma cells and protected normal human cells against
MNNG-induced DNA damage and apoptosis.
Materials and methods
Chemicals and reagents
Ginsenoside Rg3 (purity, >96%) was purchased from
Tianping Pharmaceutical Co., Shanghai, China. The compound was
dissolved in dimethyl sulfoxide (DMSO).
N-methyl-N′-nitro-N-nitrosoguanidine (MNNG),
trypan blue, low-melting agarose (LMA), normal-melting agarose
(NMA), 2-amino-2-(hydroxymethyl)-1,3-propanediol (Tris), sodium
dodecyl sulfate (SDS), ethylenediaminetetraacetic acid (EDTA),
4′,6-diamidino-2-phenylindole (DAPI),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
Tween-20 and paraformaldehyde were obtained from Sigma Chemical Co.
(Silicon Valley, CA, USA). An apoptosis detection kit was obtained
from BD Pharmingen. Triton X-100, ethidium bromide (EtBr), fetal
bovine serum (FBS), xylene cyanol, and bromphenol blue were
obtained from Sangon Biotech Shanghai Co., Ltd. (Shanghai, China).
Propidium iodide (PI) and Dulbecco’s modified Eagle’s medium (DMEM)
were obtained from Gibco (Grand Island, NY, USA). Other common
chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd.
(Shanghai, China).
Cell culture
The human osteosarcoma MG-63, OS732, U-2OS and HOS
cell lines and human fibroblasts were purchased from the Cell Bank
of the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in DMEM supplemented
with 10% heat-inactivated FBS, penicillin (100 U/ml) and
streptomycin (100 U/ml). Cells were incubated at 37ºC in a 5%
CO2 incubator. The medium was exchanged once every 2
days. After treatment, the cells were harvested by
trypsinization.
Cell viability test
Cytotoxicity was measured using the MTT assay
(12). The trypan blue dye
exclusion assay was performed to confirm and verify cell viability.
The human osteosarcoma cells and fibroblasts were seeded at a
density of 1×104 cells/well in 100 μl of the cell
culture medium, and then placed into a 96-well plate. After 12 h of
incubation, the cells were treated with 0–300 μM ginsenoside Rg3
for 24 h. Then, MTT solution (5 mg/ml) was added to each well, and
the samples were incubated at 37ºC for 4 h. Thereafter, the
supernatant was removed and replaced with 100 μl of DMSO. Optical
density (OD) of the control and drug-treated wells was measured
using an automated microplate reader (Multiskan EX; Lab Systems,
Vantaa, Finland) at a test wavelength of 570 nm.
Alkaline comet assay
The alkaline comet assay was performed according to
the procedure described by Calderon-Segura et al (13), with slight modifications for the
evaluation of DNA single-strand breaks (SSBs). Cells were cultured
in each well of a 24-well plate, at a density of
1×105/ml. Then, MG-63 and U-2OS cells were treated with
0, 25, 50, 100 and 150 μM ginsenoside Rg3 for 24 h. The cells
treated by MNNG (20 μM) were used as the positive control and the
cells treated by 0 μM ginsenoside Rg3 were used as the negative
control in the genotoxicity study. Human fibroblasts were treated
with ginsenoside Rg3 (50 mM) and MNNG (20 mM) for 24 h. Then, cells
were collected, washed, and suspended in PBS (pH 7.4); 30-μl cell
samples (1×104 cells) were used and suspended in 110 μl
of 1% molten LMA at 37ºC. The mono-suspension was cast on a
microscopic slide covered with a layer of 0.8% NMA. The agarose was
gelled at 4ºC, after which the slides were immersed in a fresh
lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris-HCl, 1% Triton
X-100, 10% DMSO and pH 10.0) for 30 min at 4ºC. After lysis, the
slides were washed in distilled water three times and immersed in
fresh alkaline electrophoresis solution (300 mM NaOH, 1 mM EDTA, pH
13.0) for 10 min at 4ºC. An electric field was then applied at 20 V
(1 V/cm) and 300 mA for 10 min. The slides were neutralized to pH
7.5 in 0.4 mM Tris buffer and stained with 40 μl of 20 μg/ml EB.
The signal emitted was analyzed using an Olympus BX53 fluorescence
microscope (Olympus, Tokyo, Japan) with a 515- to 560-nm filter.
The extent of DNA migration was determined using an image analysis
system (CASP, www.casp.of.pl). Parameters such as tail
length (DNA migration from the nucleus), tail DNA (DNA content in
the tail), and the tail moment (migrated DNA in the tail multiplied
by the tail length) were recorded.
γH2AX focus staining
The phosphorylation of histone H2AX was used as a
marker of DNA double-strand breaks with slight modifications
(14). MG-63 and U-2OS cells
(1×105) were seeded onto 6-well culture plates and
treated with 0, 25, 50 and 100 μM of ginsenoside Rg3 and 20 μM MNNG
for 24 h. Human fibroblasts were treated with ginsenoside Rg3 (50
mM) and MNNG (20 mM) for 24 h. After treatment, the cells were
fixed in 4% paraformaldehyde for 15 min, washed with PBST (PBS
buffer pH 7.4 and 0.1% Tween-20), and permeabilized in 1% Triton
X-100 for 30 min. After blocking the cells with serum for 60 min,
the samples were incubated with a rabbit monoclonal anti-γH2AX
antibody (1:1,500; Cell Signaling Technology) overnight at 4ºC, and
then incubated with an Alexa594-conjugated anti-rabbit secondary
antibody (1:360; Cell Signaling Technology) for 60 min. To stain
the nuclei, cells were incubated in DAPI (1 mg/ml) for 15 min. The
cells were then mounted in Antifade media, and images were captured
using an Olympus BX53 fluorescence microscope (Olympus). The
objectives were set at wavelengths of 594 nm for γH2AX and 350 nm
for DAPI.
DNA extraction and detection of DNA
fragmentation
The DNA ladder assay was performed according to the
protocol published previously by our own laboratory. After the
cells were treated with ginsenoside Rg3 and MNNG at concentrations
of 50 and 20 μM, respectively, for 24 h, pellets containing
1×106 cells were lysed in lysis buffer (10 mM Tris-HCl,
pH 8.0, 25 mM EDTA, 0.5% SDS, 100 mM NaCl and 400 g/ml protease K)
for 120 min at 56ºC, then treated with 10 mg/ml RNase A for an
additional 50 min at 37ºC. The lysates were centrifuged (12,000 × g
for 30 min at 4ºC), and the supernatant was collected. The
fragmented DNA was extracted from the supernatant with a neutral
phenol:chloroform:isoamyl alcohol mixture (v/v/v, 25:24:1). The DNA
pellet was precipitated by adding isopropanol, then washed with 75%
ethanol, and dissolved in Tris-EDTA buffer (10 mM Tris-HCl, 1 mM
EDTA, pH 8.0). DNA fragmentation was detected by electrophoresis
through an agarose gel, and the bands were stained with ethidium
bromide for UV light visualization.
Detection of apoptotic incidence by flow
cytometry
Apoptotic incidence was measured using the Annexin
V-FITC Apoptosis Detection Kit I (BD Pharmingen) according to the
manufacturer’s instructions. Briefly, cells were treated with
ginsenoside Rg3 and MNNG at concentrations of 50 and 20 μM,
respectively, for 24 h. Then, cells were washed twice with cold PBS
and resuspended in 500 μl of binding buffer at a concentration of
1×106 cells/ml. Then, 5 μl of Annexin V-FITC solution
and 5 μl of propidium iodide (PI; 1 mg/ml) were added; cells were
incubated at 37ºC for 30 min. The cells were analyzed by flow
cytometry within 1 h. Apoptotic cells were counted and represented
as a percentage of the total cell count.
Statistical analysis
The data are expressed as the mean values (±SEM) of
3 independent experiments. The differences among the treated groups
and the negative control were compared by one-way analysis of
variance. The Newman-Keuls multiple comparisons test was applied;
the significance level was set at P<0.05. All statistical
analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, IL,
USA).
Results
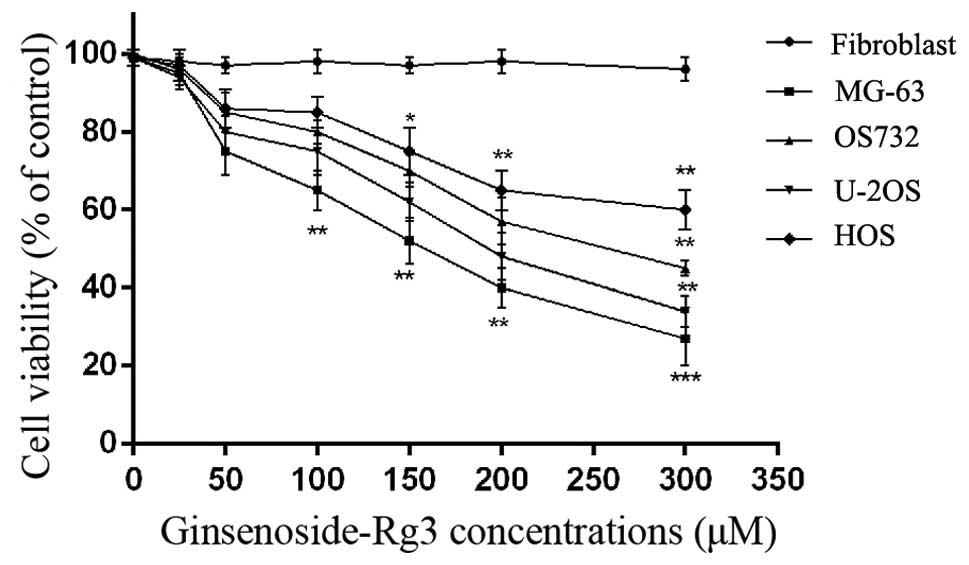
Cytotoxic effects of ginsenoside Rg3
To identify the cytotoxic effect of ginsenoside Rg3
on human osteosarcoma MG-63, OS732, U-2OS and HOS cell lines and
fibroblasts, we initially treated these cells with various
concentrations of ginsenoside Rg3 for 24 h. Cell viability was
estimated by MTT assay and trypan blue staining. Ginsenoside Rg3
triggered a concentration-dependent decrease in the viability of
MG-63 and U-2OS cells. According to our results, MG-63 and U-2OS
cells were the most sensitive to ginsenoside Rg3 and, thus, the two
cell lines were used in the subsequent experiments. Meanwhile,
ginsenoside Rg3 had no effect on the cell viability of the normal
human cells (human fibroblasts) (Fig.
1).
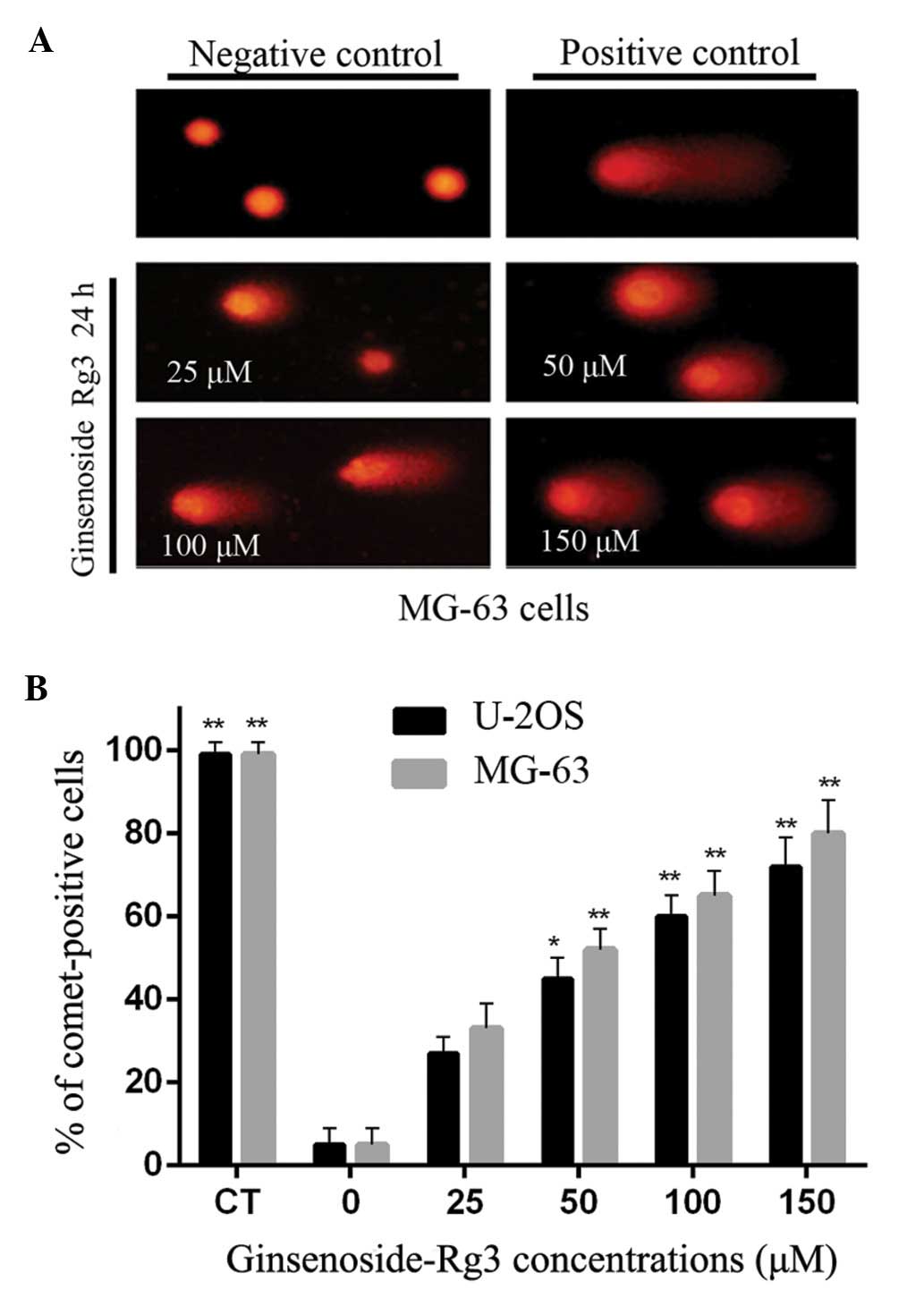
Alkaline comet assay for the
identification of DNA single-strand breaks
When subjected to the alkaline comet assay, DNA
fragments migrate to form a comet-like image. For the negative
control, comet heads contained high-density DNA and exhibited
smooth margins and intact nuclei. MG-63 and U-2OS cells accounted
for 6% of those in comet-like formations. In the groups treated
with ginsenoside Rg3, DNA comets exhibited broom-shaped tails. The
fluorescence intensity of their heads was weaker than that of the
negative control (Fig. 2A). After
treatment with ginsenoside Rg3, the percentages of comet-positive
MG-63 and U-2OS cells were significantly increased (P<0.01) at
50, 100 and 150 mM, when compared to the negative control (Fig. 2B).
The characteristics of MG-63 and U-2OS cells exposed
to ginsenoside Rg3, including mean (±SEM) tail length, tail DNA and
tail moment, are presented in Table
I. These results demonstrated that cells exposed to ginsenoside
Rg3 exhibited more severe DNA damage than the negative control
samples. Similar trends of DNA damage induced by ginsenoside Rg3
were noted in the MG-63 and U-2OS cells.
 | Table IParameters of DNA damage in the comet
assays for MG-63 and U-2OS cells that were exposed to the different
concentrations of ginsenoside Rg3. |
Table I
Parameters of DNA damage in the comet
assays for MG-63 and U-2OS cells that were exposed to the different
concentrations of ginsenoside Rg3.
| Comet assay
parameters |
|---|
|
|
|---|
| Treatment
group | Tail length
(μM) | Tail DNA (%) | Tail moment |
|---|
|
|
|
|
|---|
| Cell
lines/compounds | Concentrations | MG-63 | U-2OS | MG-63 | U-2OS | MG-63 | U-2OS |
|---|
| Negative
control | DMSO | 8.15±2.58 | 11.45±3.42 | 1.54±0.42 | 5.12±1.71 | 2.15±0.69 | 1.12±1.02 |
| Ginsenosides
Rg3 | 25 μM | 16.46±6.41 | 21.07±1.51 | 8.31±1.14a | 16.09±3.22 | 9.62±2.67 | 6.18±0.67 |
| 50 μM | 30.01±5.21 | 47.42±13.52b | 12.42±3.52b | 28.66±4.16b | 12.13±1.64 | 19.33±7.47b |
| 100 μM | 37.70±7.44b | 56.48±8.87b | 19.33±1.72b | 35.76±4.21b | 19.11±2.30b | 23.10±3.35b |
| 150 μM | 57.24±12.47b | 71.06±6.70b | 28.25±0.46b | 42.31±2.99b | 31.11±3.24b | 33.26±2.36b |
| Positive
control | MNNG 20 μM | 104.50±4.23b | 62.08±2.86b | 43.27±3.34b | 43.08±14.36b | 47.29±2.98b | 25.79±5.68b |
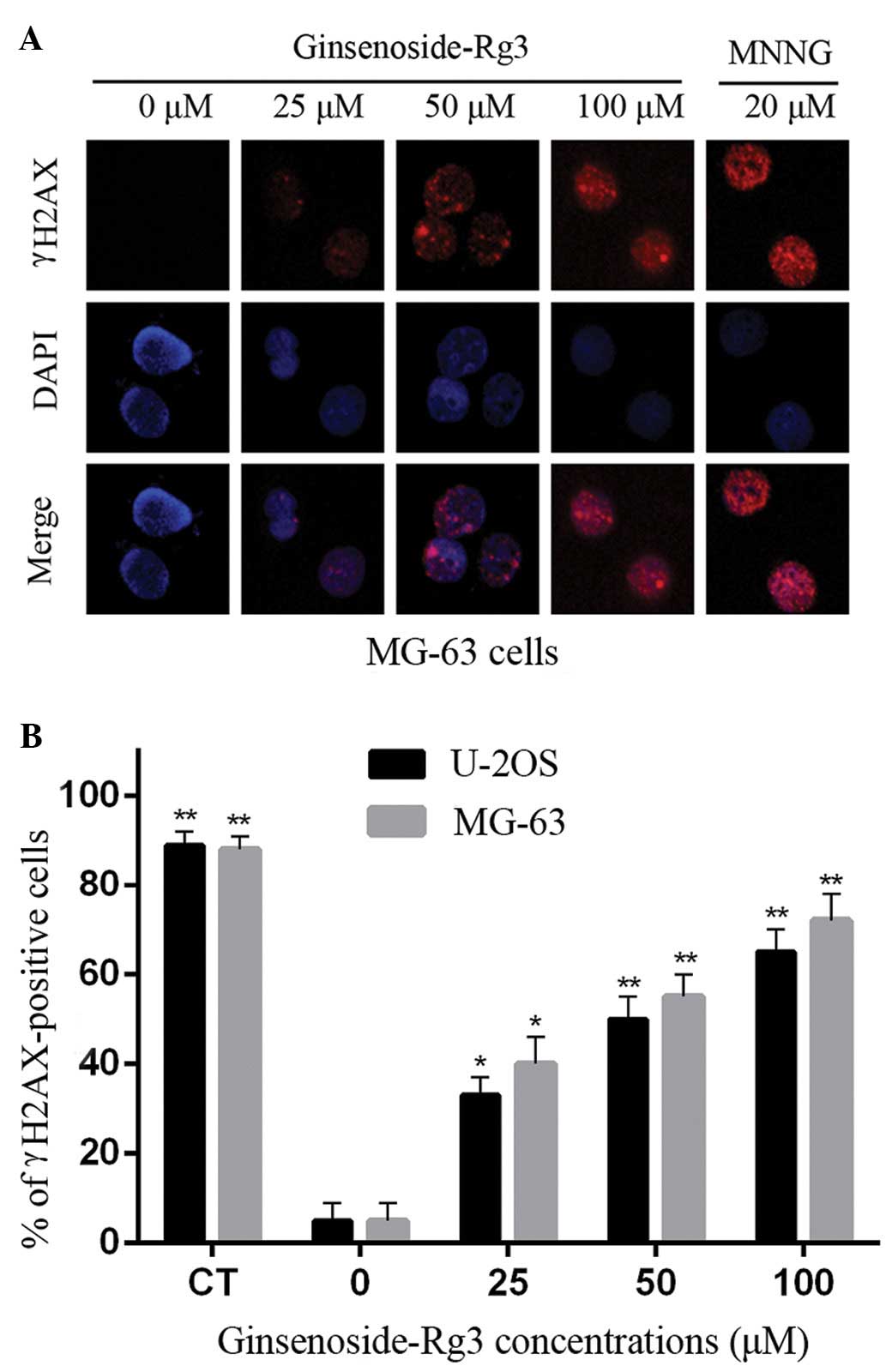
γH2AX focus staining for DNA
double-strand breaks
A threshold of ≥4 γH2AX foci per cell was determined
as optimal for quantifying the extent of DNA damage (15). Ginsenoside Rg3 caused a
concentration-dependent increase in the formation of γH2AX foci in
MG-63 and U-2OS cells. Representative immunofluorescent images of
the phosphorylation of histone H2AX in γH2AX-stained MG-63 cells
are shown in Fig. 3A.
Negative-control MG-63 and U-2OS cells had few γH2AX foci (~5% of
cells contained >4 foci). All of the treatments with ginsenoside
Rg3 and MNNG induced focus formation, thereby increasing the
percentage of γH2AX-positive cells. Ginsenoside Rg3 and MNNG both
exhibited distinct concentration-dependent effects (P<0.01) on
γH2AX focus formation in MG-63 and U-2OS cells (Fig. 3B).
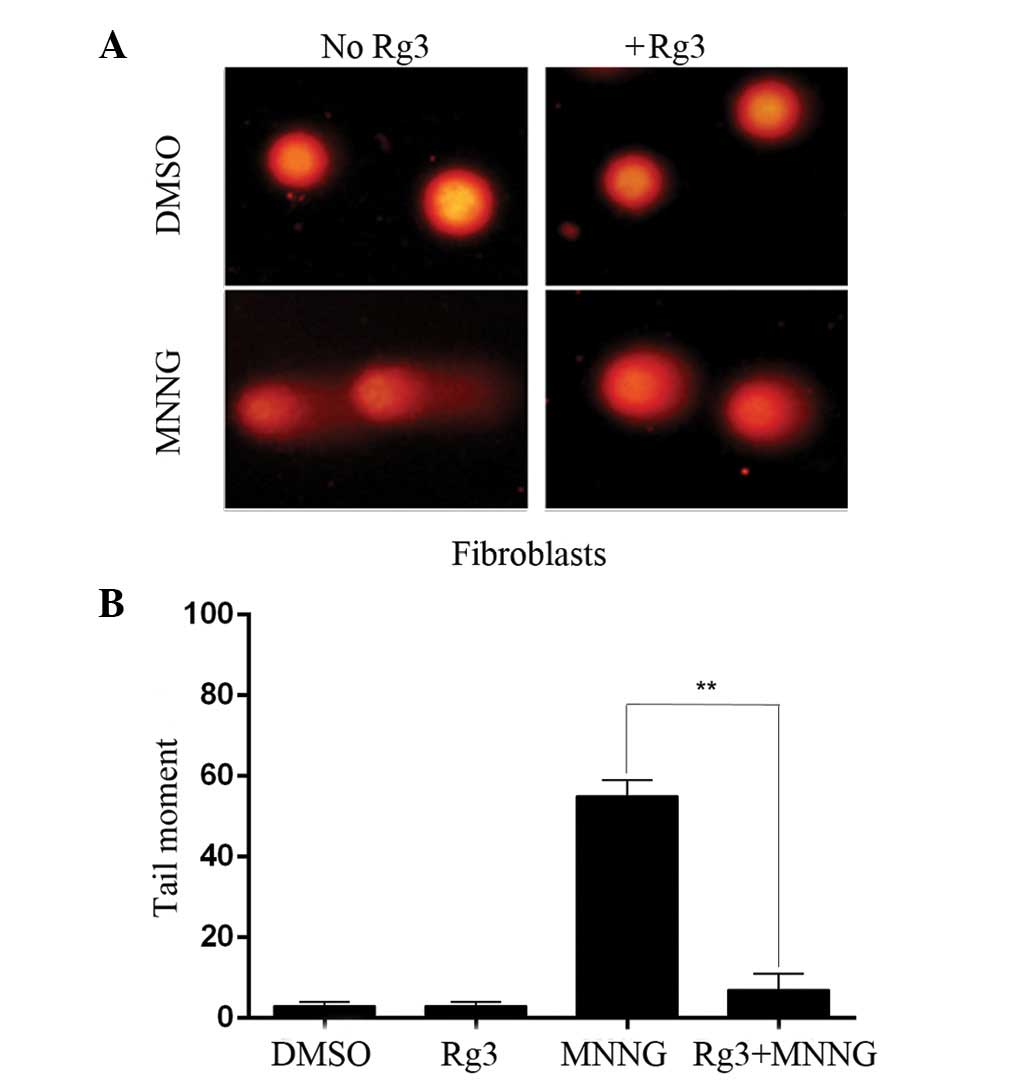
Effects of ginsenoside Rg3 on
MNNG-induced DNA damage in human fibroblasts
Ginsenoside Rg3 was reported to have a
cytoprotective effect (9). We,
therefore, sought to investigate the ability of ginsenoside Rg3 to
protect human fibroblasts against MNNG-induced DNA damage. The
number of MNNG-induced DNA single-strand breaks was measured using
the alkaline comet assay. The extent of DNA damage was
significantly greater in the MNNG treatment group as compared to
the DMSO treatment group. In the cells pretreated with 50 mM
ginsenoside Rg3 in combination with MNNG as opposed to MNNG alone,
the tail moments were significantly decreased. Treatment with
ginsenoside Rg3 alone did not induce any genotoxicity in the human
fibroblasts (Fig. 4). The
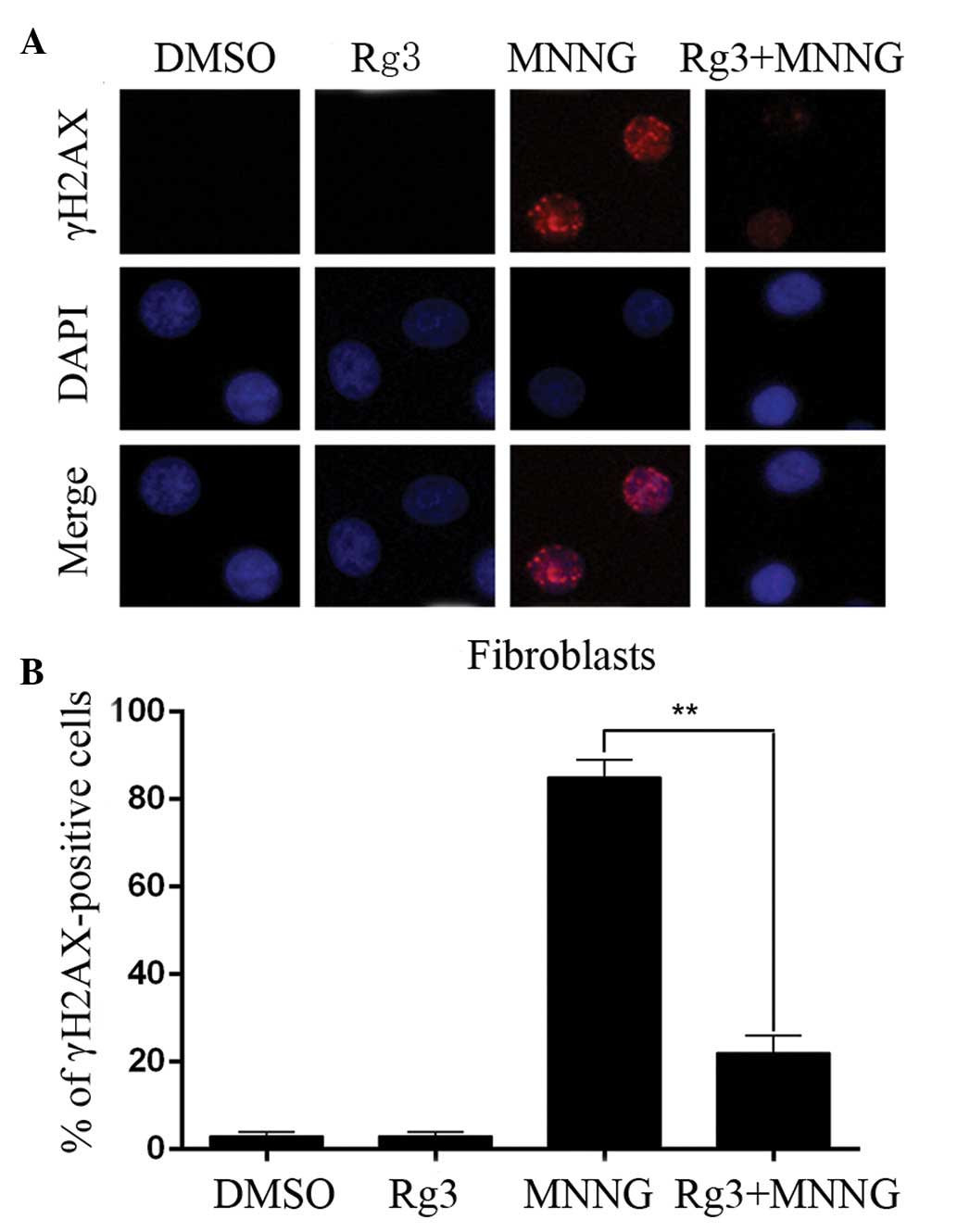
cytoprotective effect was further confirmed by staining γH2AX foci.
The percentage of γH2AX-positive cells in the DMSO and MNNG
treatment group was 5.3 and 86.9%, respectively. Ginsenoside Rg3
was effective in reducing the proportion of cells with MNNG-induced
DNA strand breakage from 86.9 to 28.3% (Fig. 5).
Effects of ginsenoside Rg3 on
MNNG-induced apoptosis in human fibroblasts
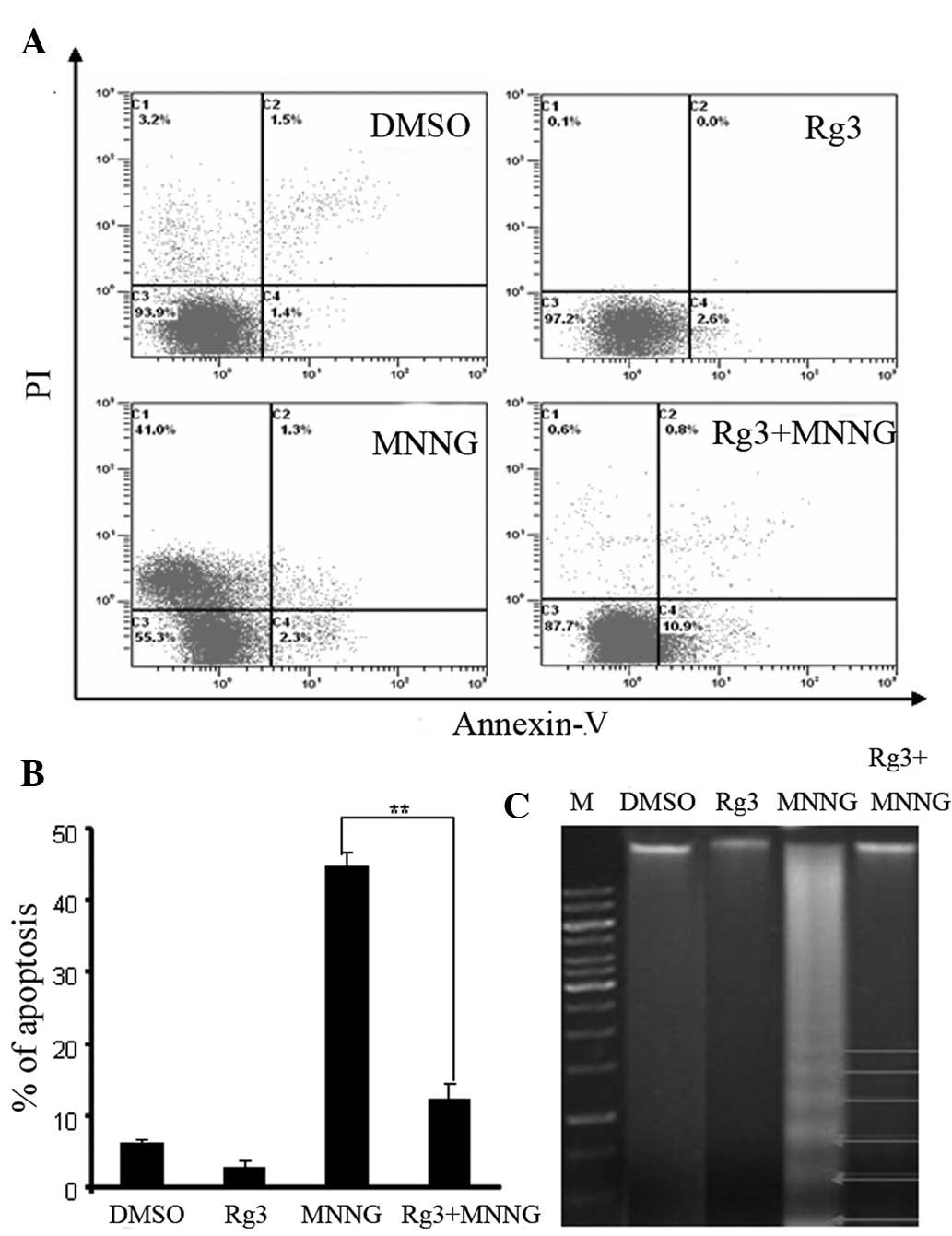
The rate of cell apoptosis was determined by flow
cytometry. Representative graphs of cells treated with ginsenoside
Rg3 and MNNG for 24 h after double staining with Annexin V-FITC and
PI are shown in Fig. 6A. The rate
of apoptosis in the MNNG-treated cells was 44.7±2.0%. When cells
were treated with ginsenoside Rg3 (50 mM) prior to MNNG
administration, the number of apoptotic human fibroblasts as
measured 24 h after pre-treatment was significantly decreased
(12.3±2.1%) (Fig. 6B). Cells in the
DMSO and ginsenoside Rg3 groups showed low rates of apoptosis. MNNG
induced a ladder-like pattern on agarose gel (Fig. 6C). Cotreatment with ginsenoside Rg3
(50 mM) induced minimal DNA fragmentation in human fibroblasts.
These results suggest that ginsenoside Rg3 alleviates MNNG-induced
cytotoxicity.
Discussion
Ginseng, an ancient and famous herbal drug in
traditional Chinese medicine, has been used in Chinese folklore for
more than 5,000 years. The most important pharmacological
components in ginseng are ginsenosides. Ginsenoside Rg3 is the
primary ginsenoside in ginseng. The compound can enhance immunity
(16), protect against the effects
of free radicals (17), suppress
the invasion and metastasis of various carcinoma cells (18) and inhibit tumor angiogenesis
(19).
The cytoprotective effect of ginsenosides has been
demonstrated in various studies. Ginsenoside Rg3 was found to
protect against DNA damage and cell apoptosis by reducing oxidative
stress (10), while ginsenoside
20(S)-protopanaxatriol protected endothelial cells against
oxidative stress through the regulation of intracellular redox
status (20).
Osteosarcoma is the most common type of bone cancer,
and the sixth most common type of cancer in children. Before any
major surgery to remove the tumor is undertaken, chemotherapy is
usually administered to shrink the tumor and facilitate surgery. It
may also kill any cancer cells that have spread to other areas of
the body. Conventional chemotherapeutic agents such as
cyclophosphamide are often toxic not only to tumor cells but also
to normal cells, limiting their therapeutic use in clinics. Natural
products are potentially valuable sources for the development of
new anticancer drugs due to their weak side-effects (21). However, no previous report has
examined whether ginsenoside Rg3 can influence human osteosarcoma
cell activity or induce DNA damage. Here, we confirmed that
ginsenoside Rg3 inhibits cell proliferation in human osteosarcoma
cells, particularly in MG-63 and U-2OS cells.
The alkaline comet assay is a form of single-cell
gel electrophoresis that can be used to detect DNA damage (22). γH2AX focus formation can be used in
this capacity as well. The alkaline comet assay is a sensitive
fluorescence microscope-based method that can be used to detect DNA
damage (22) caused primarily by
DNA strand breaks, DNA adduct formations, and DNA-DNA and
DNA-protein cross-links (23).
Moreover, the formation of DNA double-strand breaks could induce
the formation of γH2AX aggregations in nuclei. γH2AX focus
formation has been suggested as a sensitive way to detect DNA
double-strand breaks (24). The
phosphorylation of histone H2AX can be induced by replication
stress (25), ionizing radiation
(26), exogenous stress (27) and drugs that cause DNA damage
(28). A threshold of ≥4 γH2AX foci
per cell is optimal for determining the severity of DNA damage
(15). We, therefore, employed the
alkaline comet assay and measurements of the formation of γH2AX
foci to detect DNA damage induced by ginsenoside Rg3 in MG-63 and
U-2OS cells. Our results demonstrated a concentration-dependent
increase in the size of comet tails with a concomitant reduction in
head size. The images of representative comets clearly demonstrated
the amount of broken DNA liberated from the heads of the comets
during electrophoresis following treatment with increasing
ginsenoside Rg3 concentrations. A threshold of ≥4 γH2AX foci per
cell was optimal for measuring the extent of DNA damage. This
helped in efforts to identify the cellular DNA damage caused by
ginsenoside Rg3. Treatment with ginsenoside Rg3 demonstrated an
increase in the number of γH2AX-positive cells; approximately half
of these cells were positive following treatment at 25 mM, with
almost 76% positive cells noted following treatment with a 100 mM
solution (Fig. 3). In the present
study, we demonstrated that the presence of γH2AX foci may be
indicative of DNA strand breaks, which can be confirmed by comet
assay. These results are in concordance with those reported in
another study, showing that ginsenoside Rg3 mediates
antiproliferative and apoptotic activity in cancer cells (29).
Previous research has shown that MNNG can induce the
apoptosis of fibroblasts (14).
Flow cytometry and the DNA ladder assay were used to detect the
ability of ginsenoside Rg3 to protect against MNNG-induced
apoptosis in human fibroblasts. The ability of ginsenoside Rg3 to
protect against MNNG-induced DNA damage was also evaluated using
alkaline comet assay and γH2AX focus formation. The results
obtained showed that cotreatment with ginsenoside Rg3 significantly
decreased MNNG-induced DNA damage and apoptosis.
These results were consistent with those of other
studies. For example, red ginseng protected cells from
Helicobacter pylori-induced DNA damage and cytotoxicity
(30). Poon et al (9) showed that ginsenoside 20(S)-Rg3
can significantly decrease BaP-induced DNA damage using the TUNEL
and comet assays in human dermal fibroblasts. Ginsenoside
20(S)-Rg3 protected against cyclophosphamide-induced DNA
damage and cell apoptosis in mouse bone marrow cells and peripheral
lymphocyte cells (10). Ginseng was
also found to inhibit micronucleus formation and chromosomal
instability (31,32).
We found that ginsenoside Rg3 protected against the
effects of exposure to environmental contaminants. However, further
in vivo studies are required to elucidate the cytoprotective
effects of ginsenoside Rg3 in cells vulnerable to the genotoxic
effects of environmental contaminants. Zhou and Elledge (33) indicated that organisms respond to
DNA damage (DNA strand breaks) by activating a complex damage
response pathway. This pathway regulates known responses such as
cell cycle arrest and apoptosis. Previous reports also demonstrated
that ginsenoside Rg3 treatment increases the amount of time spent
by a cell in the G2/M phase, and therefore, the
likelihood that agents designed to damage DNA will in fact trigger
apoptosis (34).
There are some limitations to the present study. It
is not clear whether the induction of cell cycle arrest and
apoptosis were directly related to genotoxic effects. Further
studies will be necessary to elucidate the underlying
mechanism.
In conclusion, ginsenoside Rg3 is a strong genotoxic
agent that induces DNA damage in human osteosarcoma cells.
Moreover, ginsenoside Rg3 protected normal human fibroblasts
against the DNA damage and apoptosis induced by MNNG treatment
in vitro. Therefore, ginsenoside Rg3 may represent a
potential chemopreventive agent for the treatment of patients with
osteosarcomas.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81302340).
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bacci G, Balladelli A, Palmerini E, et al:
Neoadjuvant chemotherapy for osteosarcoma of the extremities in
preadolescent patients: the Rizzoli Institute experience. J Pediatr
Hematol Oncol. 30:908–912. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hemmerly TE: A ginseng farm in Lawrence
County Tennessee. Econ Bot. 31:160–162. 1977. View Article : Google Scholar
|
|
4
|
Toh DF, Patel DN, Chan EC, Teo A, Neo SY
and Koh HL: Anti-proliferative effects of raw and steamed extracts
of Panax notoginseng and its ginsenoside constituents on
human liver cancer cells. Chin Med. 6:4–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Molnar J, Szabo D, Pusztai R, et al:
Membrane associated antitumor effects of crocine-, ginsenoside- and
cannabinoid derivates. Anticancer Res. 20:861–867. 2000.PubMed/NCBI
|
|
6
|
Li B, Zhao J, Wang CZ, et al: Ginsenoside
Rh2 induces apoptosis and paraptosis-like cell death in colorectal
cancer cells through activation of p53. Cancer Lett. 301:185–192.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Peng H, Ou-Yang X and He X:
Research on the antitumor effect of ginsenoside Rg3 in B16 melanoma
cells. Melanoma Res. 18:322–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nag SA, Qin JJ, Wang W, Wang MH, Wang H
and Zhang R: Ginsenosides as anticancer agents: in vitro and
in vivo activities, structure-activity relationships, and
molecular mechanisms of action. Front Pharmacol.
3:252012.PubMed/NCBI
|
|
9
|
Poon PY, Kwok HH, Yue PY, et al:
Cytoprotective effect of 20S-Rg3 on
benzo[a]pyrene-induced DNA damage. Drug Metab Dispos.
40:120–129. 2012.
|
|
10
|
Zhang QH, Wu CF, Duan L and Yang JY:
Protective effects of ginsenoside Rg3 against
cyclophosphamide-induced DNA damage and cell apoptosis in mice.
Arch Toxicol. 82:117–123. 2008.
|
|
11
|
Kim TH, Lee YS, Cho CK, Park S, Choi SY
and Yool SY: Protective effect of ginseng on radiation-induced DNA
double strand breaks and repair in murine lymphocytes. Cancer
Biother Radiopharm. 11:267–272. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu JW, Yang F, Zhang Y and Li JY: Studies
on the cell immunosuppressive mechanism of Oridonin from Isodon
serra. Int Immunopharmacol. 7:945–954. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calderon-Segura ME, Gomez-Arroyo S,
Molina-Alvarez B, et al: Metabolic activation of herbicide products
by Vicia faba detected in human peripheral lymphocytes using
alkaline single cell gel electrophoresis. Toxicol In Vitro.
21:1143–1154. 2007.PubMed/NCBI
|
|
14
|
Izumchenko E, Saydi J and Brown KD:
Exonuclease 1 (Exo1) is required for activating response to
SN1 DNA methylating agents. DNA Repair. 11:951–964.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sokolov MV, Smilenov LB, Hall EJ, Panyutin
IG, Bonner WM and Sedelnikova OA: Ionizing radiation induces DNA
double-strand breaks in bystander primary human fibroblasts.
Oncogene. 24:7257–7265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bae EA, Han MJ, Shin YW and Kim DH:
Inhibitory effects of Korean red ginseng and its genuine
constituents ginsenosides Rg3, Rf, and Rh2 in mouse passive
cutaneous anaphylaxis reaction and contact dermatitis models. Biol
Pharm Bull. 29:1862–1867. 2006. View Article : Google Scholar
|
|
17
|
Tian J, Fu F, Geng M, et al:
Neuroprotective effect of 20(S)-ginsenoside Rg3 on cerebral
ischemia in rats. Neurosci Lett. 374:92–97. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iishi H, Tatsuta M, Baba M, et al:
Inhibition by ginsenoside Rg3 of bombesin-enhanced peritoneal
metastasis of intestinal adenocarcinomas induced by azoxymethane in
Wistar rats. Clin Exp Metastasis. 15:603–611. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shinkai K, Akedo H, Mukai M, et al:
Inhibition of in vitro tumor cell invasion by ginsenoside Rg3. Jpn
J Cancer Res. 87:357–362. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kwok HH, Ng WY, Yang MS, Mak NK, Wong RN
and Yue PY: The ginsenoside protopanaxatriol protects endothelial
cells from hydrogen peroxide-induced cell injury and cell death by
modulating intracellular redox status. Free Radic Biol Med.
48:437–445. 2010. View Article : Google Scholar
|
|
21
|
Mann J: Natural products in cancer
chemotherapy: past, present and future. Nat Rev Cancer. 2:143–148.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kassie F, Parzefall W and Knasmuller S:
Single cell gel electrophoresis assay: a new technique for human
biomonitoring studies. Mutat Res. 463:13–31. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cotelle S and Ferard JF: Comet assay in
genetic ecotoxicology: a review. Environ Mol Mutagen. 34:246–255.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu Y, Zhu W, Diao H, Zhou C, Chen FF and
Yang J: A comparative study of using comet assay and γH2AX foci
formation in the detection of
N-methyl-N′-nitro-N-nitrosoguanidine-induced
DNA damage. Toxicol In Vitro. 20:959–965. 2006.
|
|
25
|
Ward IM, Minn K and Chen J: UV-induced
ataxia-telangiectasia-mutated and Rad3-related (ATR) activation
requires replication stress. J Biol Chem. 279:9677–9680. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rogakou EP, Pilch DR, Orr AH, Ivanova VS
and Bonner WM: DNA double-stranded breaks induce histone H2AX
phosphorylation on serine 139. J Biol Chem. 273:5858–5868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanaka T, Huang X, Halicka HD, et al:
Cytometry of ATM activation and histone H2AX phosphorylation to
estimate extent of DNA damage induced by exogenous agents.
Cytometry A. 71:648–661. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Banáth JP and Olive PL: Expression of
phosphorylated histone H2AX as a surrogate of cell killing by drugs
that create DNA double-strand breaks. Cancer Res. 63:4347–4350.
2003.PubMed/NCBI
|
|
29
|
Wang CZ, Aung HH, Ni M, et al: Red
American ginseng: ginsenoside constituents and antiproliferative
activities of heat-processed Panax quinquefolius roots.
Planta Med. 73:669–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park S, Yeo M, Jin JH, et al: Rescue of
Helicobacter pylori-induced cytotoxicity by red ginseng. Dig
Dis Sci. 50:1218–1227. 2005.
|
|
31
|
Ivanova T, Han Y, Son HJ, Yun YS and Song
JY: Antimutagenic effect of polysaccharide ginsan extracted from
Panax ginseng. Food Chem Toxicol. 44:517–521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Panwar M, Samarth R, Kumar M, Yoon WJ and
Kumar A: Inhibition of benzo(a)pyrene induced lung adenoma by
Panax ginseng extract, EFLA400, in Swiss albino mice. Biol
Pharm Bull. 28:2063–2067. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou BB and Elledge SJ: The DNA damage
response: putting checkpoints in perspective. Nature. 408:433–439.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park HM, Kim SJ, Kim JS and Kang HS:
Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced
apoptosis in hepatoma cells through mitochondrial signaling
pathways. Food Chem Toxicol. 50:2736–2741. 2012. View Article : Google Scholar : PubMed/NCBI
|