Introduction
Glioma is the most common intracranial tumor
accounting for ~60% of all intracranial tumors (1). According to World Health Organization
(WHO) guidelines, glioma is divided into 4 grades. Among them,
grade IV tumor refers to glioblastoma multiforme (GBM) that is the
most aggressive form of glioma (2).
Despite the urgent demand for treatment solutions against this
malignant cancer, little is known about the causes or mechanisms of
its cancerous transformation and progression.
There is ample evidence that microRNAs (miRNAs), a
family of highly conserved small non-coding RNAs, have regulatory
functions in cancer progression (3). In previous studies, our group
demonstrated that miR-150 may present therapeutic strategies to
treat MLL-AF9-related leukemia by regulating multiple oncogenes
(4). Discovery of miRNAs provided a
new path to understand the molecular mechanism of glioma (5). Ciafrè et al (6) and Chan et al (5) observed that miR-221 and miR-21 were
significantly upregulated in GBMs using miRNA microarray analysis
with patient samples, respectively. In contrast, miR-181a/b/c were
downregulated in GBMs compared to the normal brain tissues.
Notably, a group of miRNAs including miR-16 and miR-195, which
belong to the miR-15/16 family involving miR-15a/b, miR-16,
miR-195, miR-424 and miR-497, are downregulated in human
glioblastoma cells, and their abnormal expression patterns are
associated with the survival rate of GBM patients compared to
non-tumorous cells (7–9). microRNA-503 (miR-503) is a member of
the miR-15/16 family and it was first reported as a highly elevated
miRNA in human retinoblastoma tissues using miRNA microarray
analysis (10,11). However, the relative expression of
miR-503 between GBM and normal brain as well as the function of
miR-503 on GBM is unclear.
In the present study, we first analyzed the
expression pattern of miR-503 in human GBM samples and cell lines
followed by functional investigation of miR-503 in human GBM cell
lines. Taken together, our results demonstrated that miR-503 is a
tumor suppressor in GBM with multiple aspects of antitumor effects
partially mediated by post-transcriptional downregulation of
insulin-like growth factor-1 (IGF-1R) expression, thereby
interfering with the PI3K/AKT pathway. These results elucidated a
novel molecular mechanism for the pathogenic mechanism in glioma
progression, and may thus provide novel support for the development
of targeted therapy.
Materials and methods
Human tissue samples
All human normal brain and glioma tissues from
patients were collected in the Department of Neurosurgery, Renmin
Hospital of Wuhan University from 2011 to 2013. Normal brain
tissues were obtained from patients with cerebral trauma.
Glioblastoma tissues were obtained according to the diagnosis of
clinical and pathological grading. Prior consent was obtained from
all patients and the study was approved by the institutional
research board.
Cell culture and miRNA transfection
Human glioma cell lines U251 and U87MG were from
ATCC (Manassas, VA, USA) and cultured according to methods
previously described (7). Cells at
50–70% confluence were transfected with miR-503 mimics or
non-specific mimics as negative control (NC) (RiboBio, Guangzhou,
China) using Lipofectamine® 2000 reagent (Invitrogen,
Carlsbad, CA, USA), respectively.
Bioinformatics and luciferase reporter
assay
Target genes of miR-503 were first predicted using
multiple target prediction algorithms: TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org/). The IGF-1R 3′ untranslated
region (3′UTR) was amplified from human genomic DNA using PCR and
cloned into pMIR-REPORT vector. The primers used were: forward,
5′-GGA CTA GTC TAG GAC TTC TTC ATG GGT CTT-3′ and reverse, 5′-ATG
AAG CTT GTG TCA CAA CCT AAG CAA AG-3′. Twenty-four hours before
transfection, U251 cells were seeded in a 24-well plate, the
pMIR-REPORT vector bearing miR-503 binding site (IGF-1R-3′UTR-wt)
or mutated binding site (IGF-1R-3′UTR-mut) constructs and pRL-TK
vector were transfected using Lipofectamine 2000 reagent.
Twenty-four hours after transfection, luciferase activities were
evaluated using the Dual Luciferase Reporter Assay System (Promega,
Madison, WI, USA) and the relative activity of Renilla
luciferase were normalized to that of firefly luciferase harbored
in the same reporter construct.
RNA extraction and quantification
assay
Total RNA from tissues and cell lines was extracted
with TRIzol reagent (Invitrogen) and reverse-transcribed with
RevertAid First Strand cDNA Synthesis kit (Fermentas, Vilnius,
Lithuania). The primer sequences for IGF-1R gene expression were:
IGF-1R forward, 5′-CCC AAG ACC CAG AAG GAA-3′ and reverse, 5′-ACT
CGT GCA GAG CAA AGG AT-3′; GAPDH primer forward, 5′-CTT CAA CGA CCA
CTT TGT-3′ and reverse, 5′-TGG TCC AGG GGT CTT ACT-3′. To analyze
miR-503 expression levels, the Bulge-Loop™ miRNA qRT-PCR primer
kits (RiboBio) were utilized according to the manufacturer’s
instructions. RNA input was normalized to the level of human U6
snRNA. Real-time PCR was performed using All-in-One™ qPCR mix
(GeneCopoeia, Guangzhou, China) with an iCycler thermal cycler
(Bio-Rad). The 2−ΔCt or 2−ΔΔCt methods were
used to calculate the relative expression level of miR-503 human
tissues or the expression levels of miR-503 and IGF-1R in cell
lines (n=3, means ± SEM).
Proliferation assays
U251 and U87MG cells were seeded in 96-well plates
with 6×103 cells/well 24 h prior to the transfection of
miR-503 mimics or NC and assayed 24, 48 and 72 h after
transfection. Twenty microliters of dimethyl thiazolyl diphenyl
tetrazolium bromide (MTT) (5 mg/ml) were added into each well and
the incubation continued for 4 h. Then, supernatant was removed and
100 μl dimethyl sulfoxide (DMSO) was added to each well to dissolve
the precipitate. Optical density (OD) value was measured at the
wavelength of 570 nm.
To further evaluate cell proliferation, cells
transfected with miR-503 mimics or NC were cultured for 20 h, and
then incubated for 4 h with DMEM containing 1 mCi/ml
[3H]thymidine (Amersham, Shanghai, China). The cells
were washed with ice-cold PBS, fixed with ice-cold 10%
trichloracetic acid, and solubilized by 0.3 M NaOH. The
incorporated radioactivity was quantified using a Beckman
scintillation counter.
Apoptosis assay
U251 and U87MG cells were transfected with miR-503
mimics or NC for 48 h. Cells were harvested in cold
phosphate-buffered saline (PBS), stained with Annexin V-FITC and
propidium iodide (PI) for 10 min and analyzed by flow cytometry
(EPICS Altra II; Beckman) and date SPSS version 17.0.
Cell cycle assay
Forty-eight hours after transfection, U251 and U87MG
cells were harvested in ice-cold PBS and then fixed with 70%
ice-cold ethanol for an additional 48 h at 4°C. The fixed cells
were incubated with PI for 30 min at 37°C in the dark. The DNA
content was analyzed by flow cytometry.
Wound healing assay
Transfected U251 cells were seeded in 6-well plates
at a density of 2×105 cells/well. At 80% confluence the
cells were scratched with a 10 μl plastic pipette tip to form a
straight wound and cultured for an additional 48 h. The wound
closure was measured under a microscope equipped with a camera.
Images of 3 random fields were captured at the time of 0, 24 and 48
h after wounding.
Cell migration and invasion assays
The details of cell migration and invasion assay
methods used are as previously described (7). Twenty-four-well Transwell plates (8-μm
pore size) (Corning, New York, USA) were used for these
experiments. Cells (3×104) were plated in the top
chamber for migration or invasion assay. The migration and invasion
rates were measured by photographing at 5 random fields.
Protein extraction and western
blotting
Forty-eight hours after transfection, cellular
proteins were extracted with lysis buffer supplemented with
protease inhibitors. The supernatant of the lysate was collected
and separated by SDS-PAGE, then transferred to PVDF membranes. The
proteins were respectively probed with primary antibodies,
incubated with HRP-conjugated secondary antibodies and then
visualized with an ECL detection system. Protein expression was
measured by ImageJ software.
Statistical analysis
Data are presented as mean values ± SEM, and were
analyzed by use of SPSS version 17.0 (SPSS, Chicago, IL, USA).
Significance between two group comparisons were analyzed with a
t-test, and relationships between more than two group comparisons
were analyzed with one-way ANOVA. p<0.05 was considered to
indicate a statistically significant difference. All experiments
were carried out in triplicate unless otherwise noted.
Results
miR-503 is downregulated in human GBM
tissue samples and cell lines compared to normal brain tissues
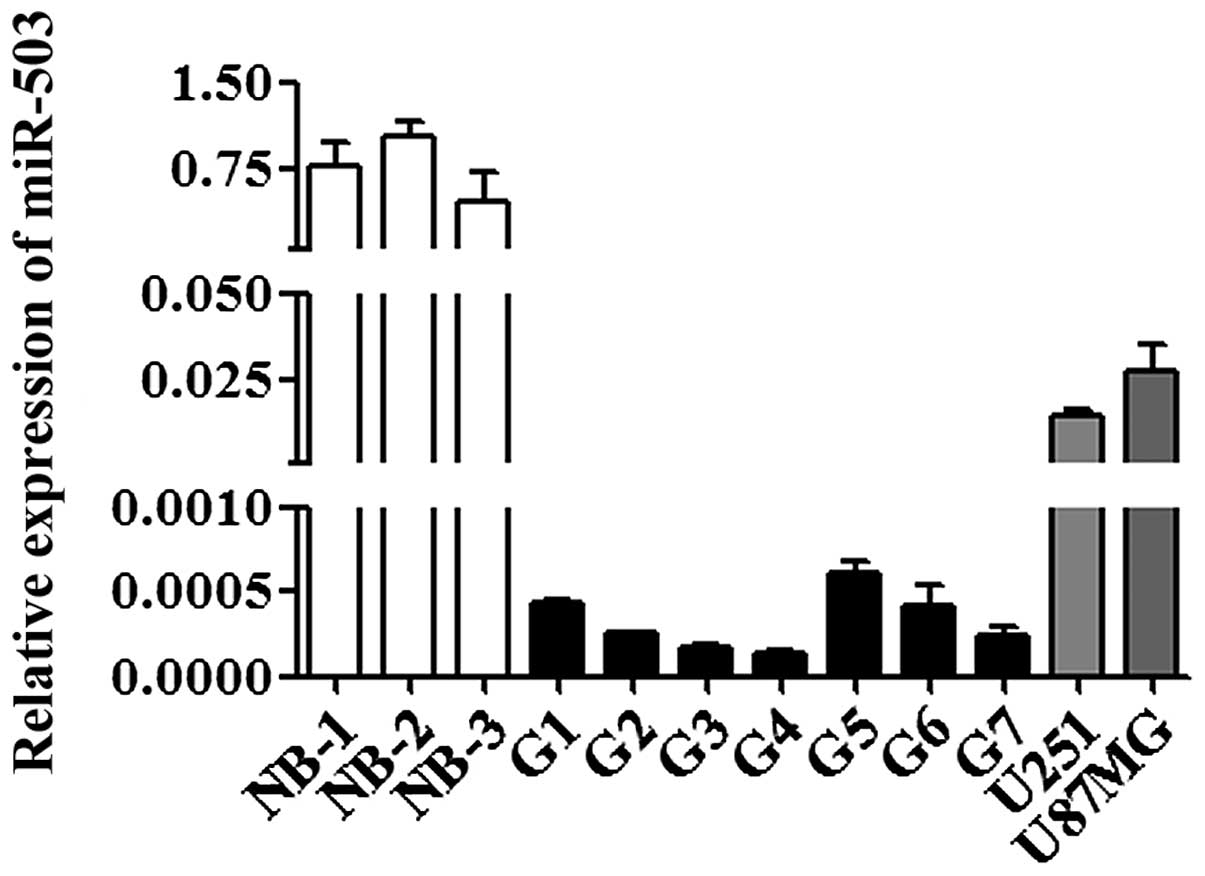
To evaluate the expression pattern of miR-503, we
performed quantitative RT-PCR analysis. The level of miR-503 was
significantly lower in human GBM tissue samples compared to normal
brain tissues (p<0.001; Fig. 1).
This is similar to the suppressed expression level of miR-503 in
human GBM cell lines (U251 and U87MG) (p<0.001; Fig. 1), suggesting a potential
tumor-suppressive function of miR-503 in GBM.
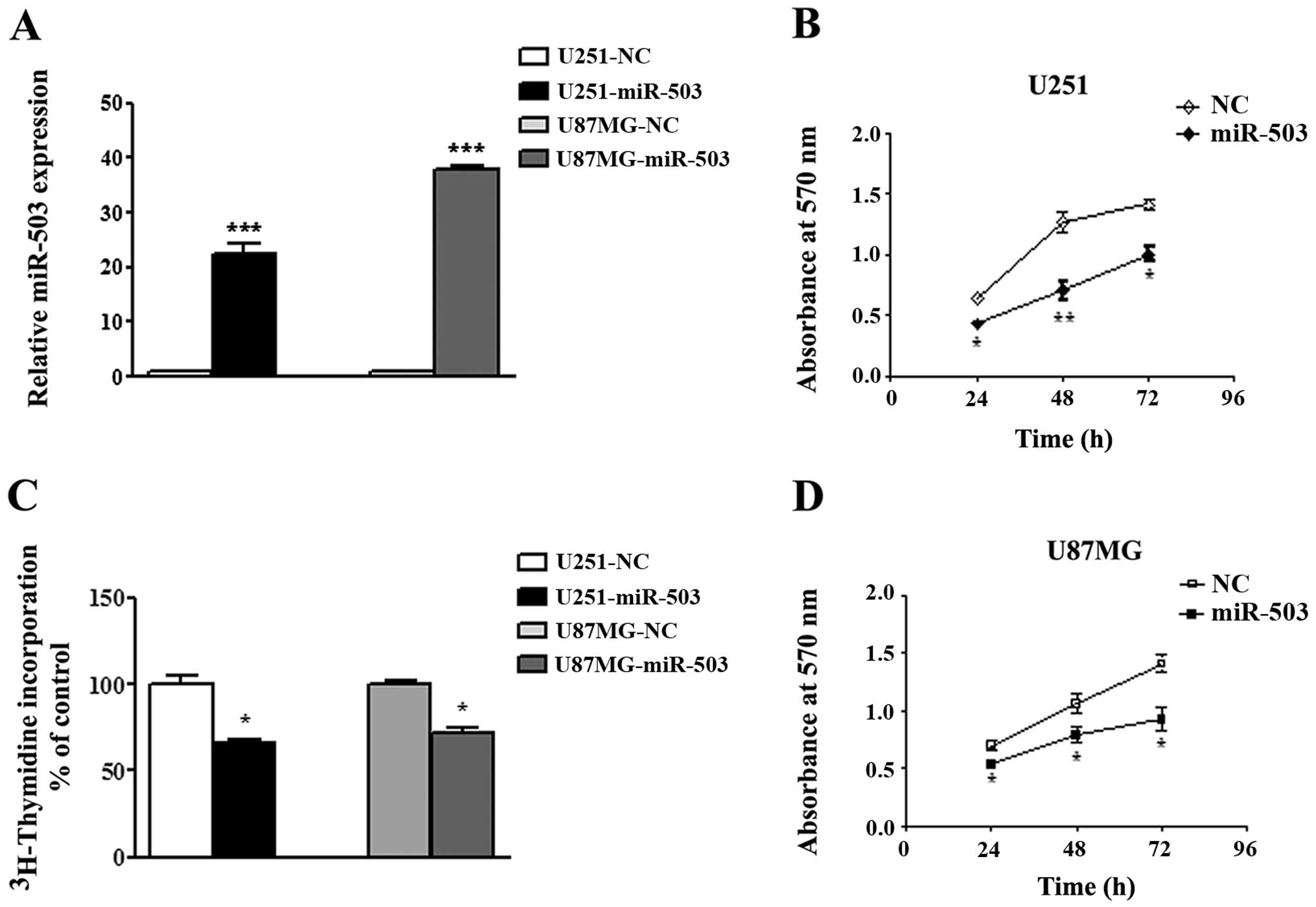
Overexpression of miR-503 inhibits the
propagation of GBM cell lines in vitro
To understand the function of miR-503 in GBM, we
utilized both gain of loss of function strategies in the cultured
cells. U251 and U87MG cells were transiently transfected with
miR-503 mimics or NC and efficiency of the transfection was
examined by real-time PCR assays at 48 h after transfection
(Fig. 2A). Next, the effect of
forced expression of miR-503 on cell proliferation was evaluated
using MTT assays at indicated time points. As expected,
transfection of both cell lines with miR-503 mimics
oligonucleotides resulted in a marked suppression of cell growth
activity compared to cells transfected with NC oligos after 24 h
(p<0.05; Fig. 2B and D), 48 h
(p<0.01 in U251 and p<0.05 in U87MG; Fig. 2B and D) and 72 h (p<0.05;
Fig. 2B and D). This inhibitory
effect of ectopic expression of miR-503 was further confirmed using
a [3H]thymidine incorporation assay (p<0.05; Fig. 2C). Taken together, these results
demonstrated an antiproliferative effect of miR-503 on GBM cell
lines.
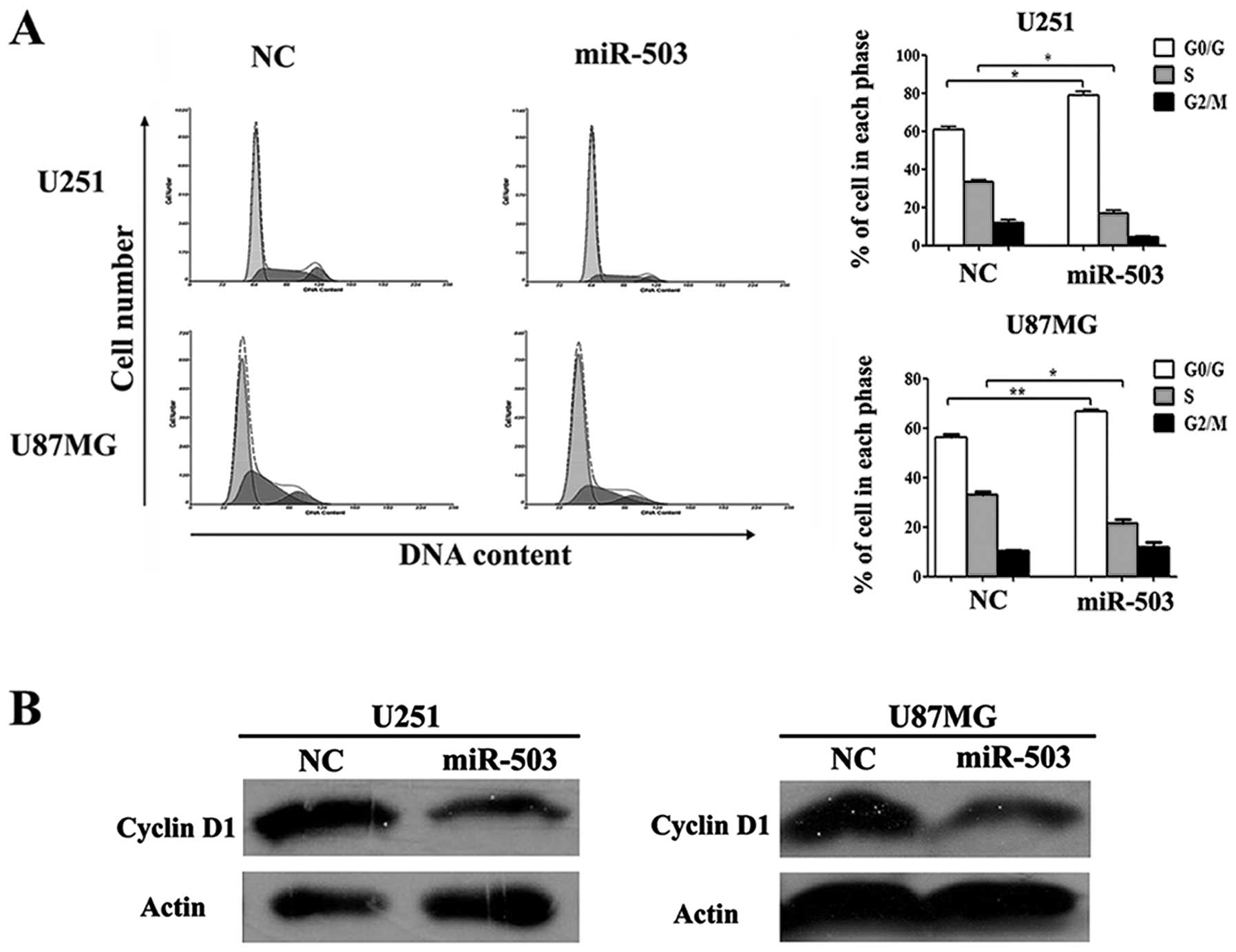
miR-503 induces G0/G1 phase arrest in
cell cycle distribution
To further define the antiproliferative ability of
miR-503 in glioblastoma cells, we analyzed cell cycle distribution
in GBM cells transfected with miR-503 mimics or NC control oligos
using flow cytometry analysis. As shown in Fig. 3A, the G0/G1 phase fraction of cells
with NC control was 60.97±1.16% (n=3, in U251 cells) and
56.43±1.04% (n=3, in U87MG cells), whereas cells transfected with
miR-503 mimics increased the percentage of cells in G0/G1 phase
79.07±1.91% (n=3, in U251 cells) and 66.77±0.88% (n=3, in U251
cells). Moreover, the average S phase fraction in cells with
miR-503 appeared significantly lower (mean, 16.40±1.82%, n=3, in
U251 cells and mean, 11.73±2.67%, n=3, in U87MG cells) compared to
NC-treated cells. Furthermore, the protein level of cyclin D1
(CCND1) which served as an active switch in the regulation of cell
cycle during G1/S transition displayed a significant reduction
observed by western blot analysis in both cell lines (Fig. 3B). These results suggested that
overexpression of miR-503 induced G0/G1 arrest, thereby delaying
the progression of cell cycle.
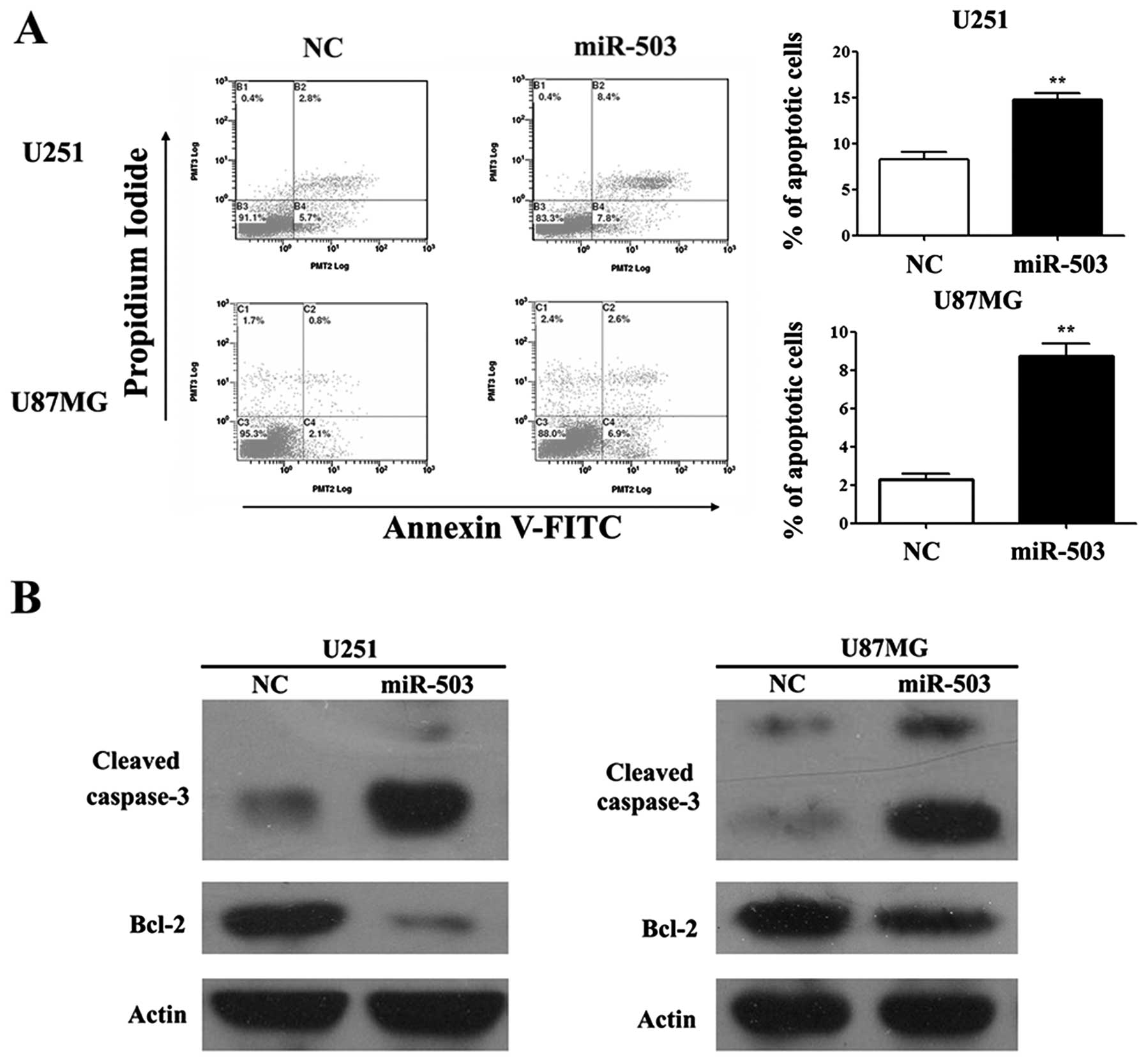
miR-503 enhances apoptosis of
glioblastoma
To evaluate the effects of miR-503 on GBM cancer
cell survival, we adopted cell apoptosis assays in both U251 and
U87MG cell lines. Forty-eight hours after transfection of miR-503
mimics or NC, cells were collected and analyzed for the binding of
Annexin V and PI penetration using flow cytometry. A markedly
induced cell apoptotic rate defined as the proportion of Annexin
V+PI+ population was observed in cells
transfected with miR-503 mimics compared to that of cells with NC
oligo transfection (p<0.01; Fig.
4A). Moreover, we also detected an increased level of cleaved
caspase-3 protein and Bcl-2 protein in cells with elevated miR-503
levels compared to NC (Fig.
4B).
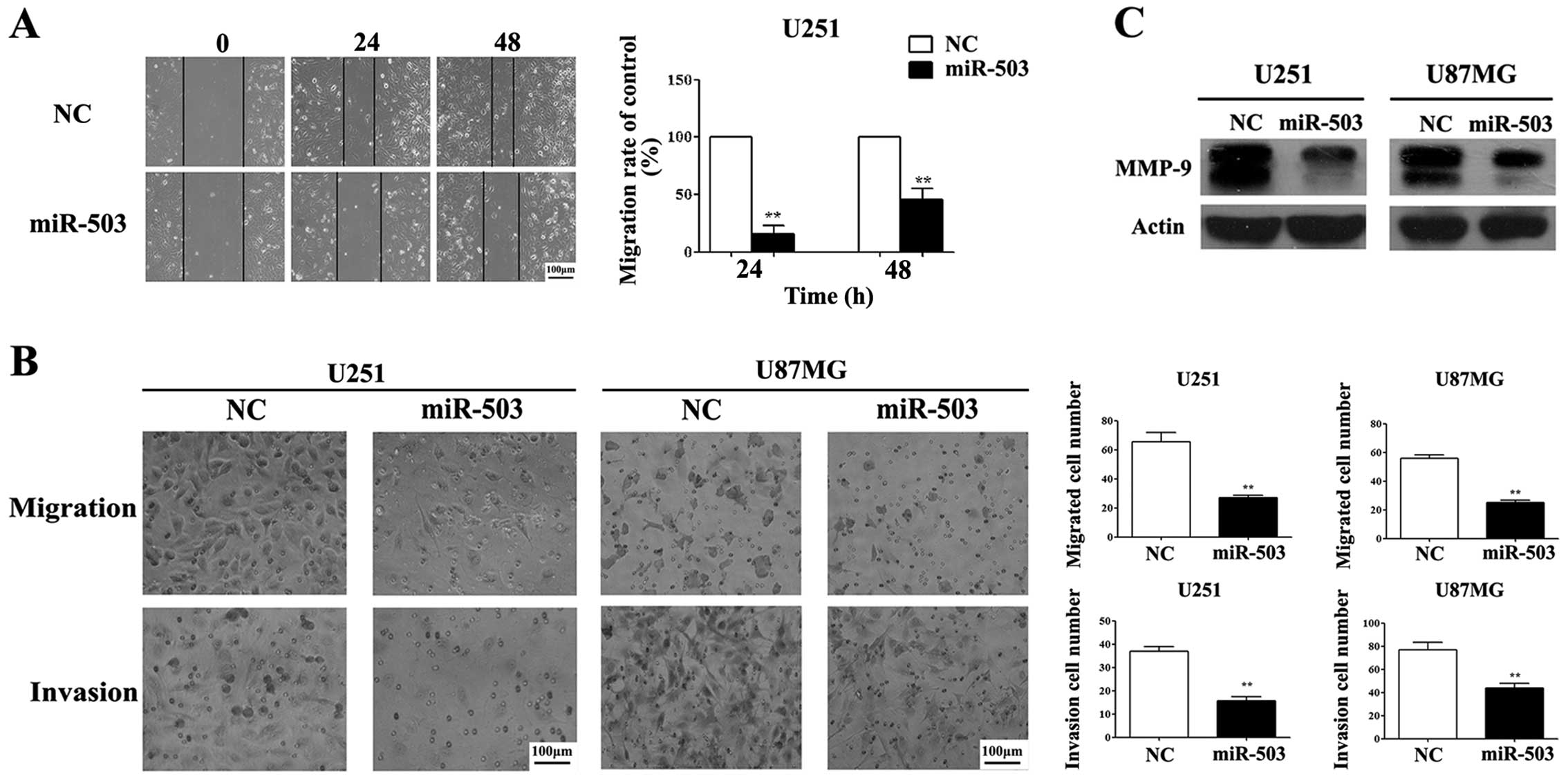
miR-503 inhibits cell migration and
invasion of glioblastoma cells
Based on our results of MTT and flow cytometry
(Figs. 2 and 4), we hypothesized that loss of miR-503 in
GBM cells may contribute to the invasion of GBM tumor. To test this
hypothesis, we performed a set of experiments to evaluate cell
migration and tumor invasion. First, we adopted cell migration
analysis using Transwell methods. U251 and U87MG cells transfected
with miR-503 mimics displayed a substantially suppressed migratory
and invasive capacity compared to their respective control groups
(Fig. 5B). As expected, the
inhibitory effect of miR-503 on cancer cell invasion was further
confirmed using the wound healing assays (Fig. 5A). Furthermore, we observed a
decrease in the MMP-9 protein level, a cell migration regulator in
gliomagenesis (12), in cells with
elevated miR-503 compared to control samples as shown by western
blot assays, suggesting a potential mechanism of miR-503 inhibiting
tumor invasion (Fig. 5C).
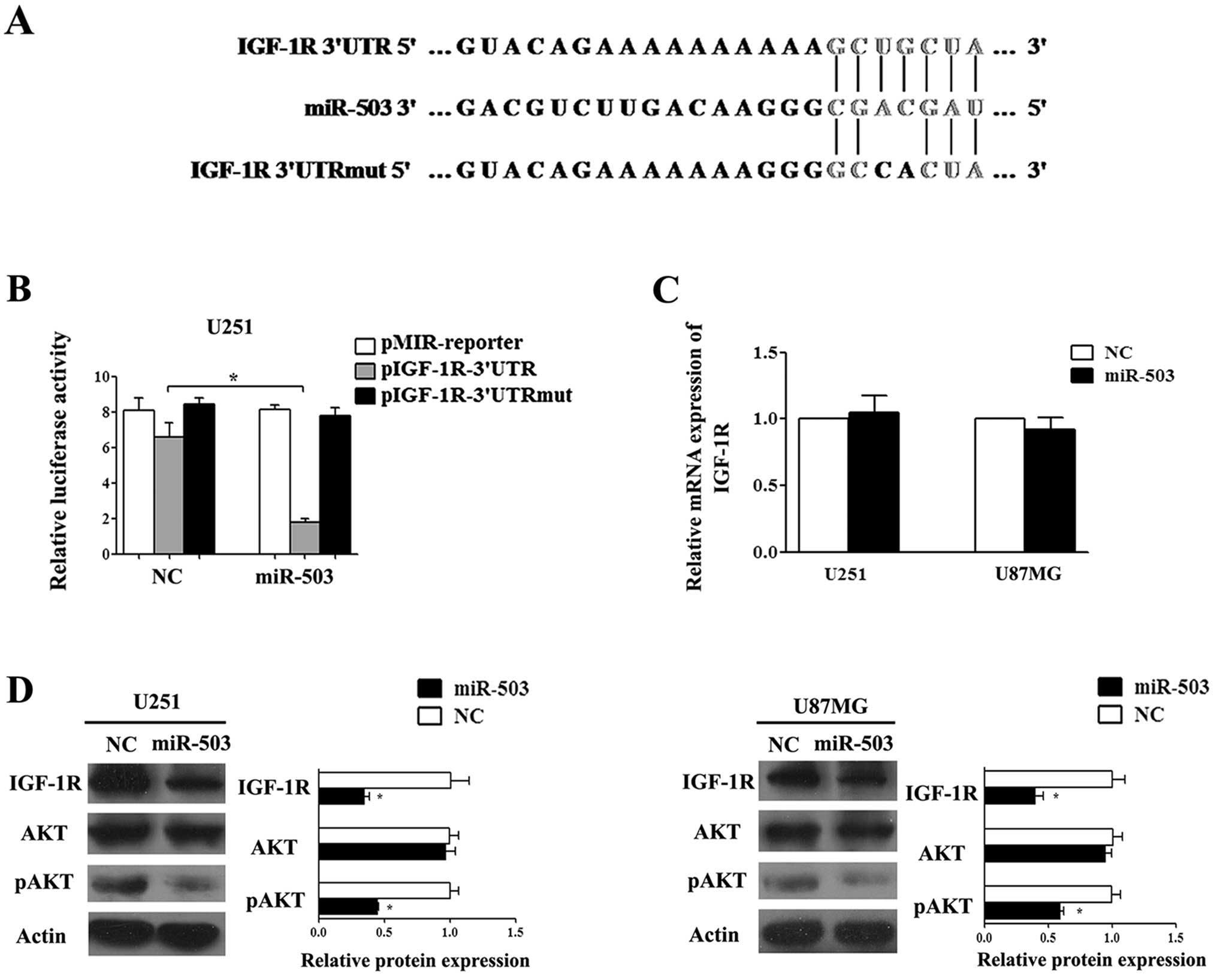
IGF-1R is a direct functional target of
miR-503 that partially mediates the effect of miR-503 through AKT
activation in glioblastoma cells
To investigate the responding molecular components
in miR-503 tumor-suppressive regulation, we sought to identify the
direct target gene in the context of glioblastoma. Surveying the
online algorithms miRanda and TargetScan, we found that IGF-1R, a
central receptor protein to facilitate tumor development mainly by
AKT and MAPK pathways, bears miR-503 target site in its 3′UTR
region, thus may serve as a potential target gene of miR-503
(Fig. 6A). To determine direct
regulatory binding of miR-503 on IGF-1R, we constructed a
luciferase reporter assay using pMIR-REPORT constructs containing a
segment of the 3′UTR with wild-type or mutated seed region.
Luciferase assays demonstrated that miR-503 suppressed activity of
luciferase in constructs carrying the wild-type target sites but
not the site with point mutations (Fig.
6B). Next, we examined the mRNA and protein levels of IGF-1R in
U251 and U87MG cells using real-time PCR and western blot analysis.
Our results showed that the level of IGF-1R protein was markedly
decreased but there was no apparent change in the IGF-1R mRNA
levels when miR-503 was overexpressed (Fig. 6C and D), suggesting miR-503 was
involved in the regulation of IGF-1R expression.
The activation of the AKT pathway is crucial for the
sensitivity of glioblastoma cells to IGF-1R inhibitors (13). Therefore, we examined both levels of
total AKT (AKT) and phosphorylated AKT (pAKT) proteins in U251 and
U87MG cells. Western blot results demonstrated that treatment with
miR-503 mimics reduced the expression of phosphorylated AKT,
whereas the effect of miR-503 on total AKT protein level was not
statistically significant (Fig.
6D). Collectively, these results suggest that miR-503 may
suppress GBM progression probably by inhibiting the IGF-1R/PI3K/AKT
pathway.
Discussion
In the present study, we found that miR-503 was
downregulated in GBM tissues and cell lines related to normal brain
tissues. Moreover, introduction of exogenous miR-503 inhibited cell
growth, migration and invasive ability, induced G0/G1 phase arrest
and enhanced apoptosis of U251 and U87MG human glioblastoma cells.
Further, we identified IGF-1R as a direct functional target of
miR-503 which exerts important effects on glioblastoma cells. These
results support an antineoplastic role for miR-503 in GBMs.
It is of note that the expression patterns of
miR-503 vary in different tumor tissues and cell lines and its
specific impact on the tumor biology needs to be further
investigated. miR-503 was overexpressed in human retinoblastoma
tissues as detected by miRNA microarray analysis. Corbetta et
al (11) also observed the
phenomenon in human parathyroid carcinomas and adenocortical
carcinomas (15,16). On the contrary, Zhou et al
reported that miR-503 was markedly downregulated in primary HCC
tissues compared to their adjacent non-cancerous liver tissues
(17) and a similar observation was
reported in endometrioid endometrial cancer and cisplatin-resistant
non-small cell lung cancer cells (18–20).
In our study, we found that miR-503 was significantly downregulated
in GBM tissues and two glioma cell lines compared to three normal
brain tissues. A putative reason for such tumor-specific expression
patterns of miR-503 may be due to the feedback adjustment, tumor
progression stages and diversity of internal environment between
different types of tumor.
miR-503 is a member of the miR-15/16 family and all
the family members shared a common characteristic of 5′-end AGCAGC
motifs in the mature miRNA (21).
As some group members of the miR-15/16 family have similar
sequences and consistent expression profiles in different human
tissues, they may have similar or synergistic effects in a certain
pathogeneses (22–24). For example, downregulation of
miR-195 and miR-497 may strongly affect cell cycle progression and
lead to an aberrant cell proliferation in hepatocellular carcinoma
cell lines (25). Given that
miR-16-1 and miR-195 played tumor-suppressor roles in human
glioblastoma cells, we hypothesized that miR-503 may have a similar
antitumor effect as other members of the miR-15/16 family in human
glioblastoma cells (7,8). Consistent with this hypothesis, we
found that elevated miR-503 level not only suppressed cellular
proliferation, inhibited cell migration and invasion, but also
enhanced apoptosis in U251 and U87MG cells.
miR-503 may also be a potent cell cycle regulator by
targeting multiple cell cycle-related proteins. Caporali et
al (26) and Sarkar et
al (27) identified CCNE1 and
CDC25A as direct targets of miR-503 in endothelial cells and
osteosarcoma cells respectively, leading to the block in G0/G1 and
G2/M phase transitions. Moreover, Jiang et al demonstrated
that miR-503 may reduce S phase cell populations by targeting CCND1
3′UTR and resulted in human head and neck carcinoma cell growth
inhibition (28). Our results
further confirmed that miR-503 may suppress the endogenous CCND1
protein level and induce G0/G1 phase arrest in GBM cell lines.
These results suggested that miR-503 may play a key role in cell
cycle regulation. The progression of glioma is correlated with
increased expression level of IGF-1R (29). IGF-1R is known to play a pivotal
role in regulating cell proliferation and survival in the process
of gliomagenesis (30–32). IGF-1R exerts its effect primarily
through activating MAPK kinases and the PI3K/AKT pathways, that may
further influence the invasion of glioma cells (33,34).
Moreover, recent studies demonstrated that a number of cell
cycle-related and invasion-associated genes are regulated by
IGF-1R, including CCND1 and MMP-2/MMP-9 in human glioma and head
and neck cancer, which function through MAPK and PI3K/AKT pathways
(35–41). In the present study, we found that
IGF-1R was a direct target of miR-503 and indicated the
IGF-1R/PI3K/AKT pathway may contribute to the biological effects of
miR-503.
To the best of our knowledge, this is the first
description of the tumor-suppressive role of miR-503 in GBMs and
its functions through regulating IGF-1R and its AKT activation,
leading to potent suppression in cancer cell proliferation,
survival, migration and invasion. In summary, we conceived a
specific mechanism of miR-503 regulatory impacts on a variety of
tumors, including the current discovery in glioblastoma (Fig. 7). In combination with previous and
current studies, we highlighted a potential regulatory mechanism of
miR-503 on cell cycle and apoptosis-related protein in GBM cells
(26,28,42).
Collectively, our study provided a possible therapeutic application
against glioma by a gene therapy approach with introduction of
exogenous miR-503.
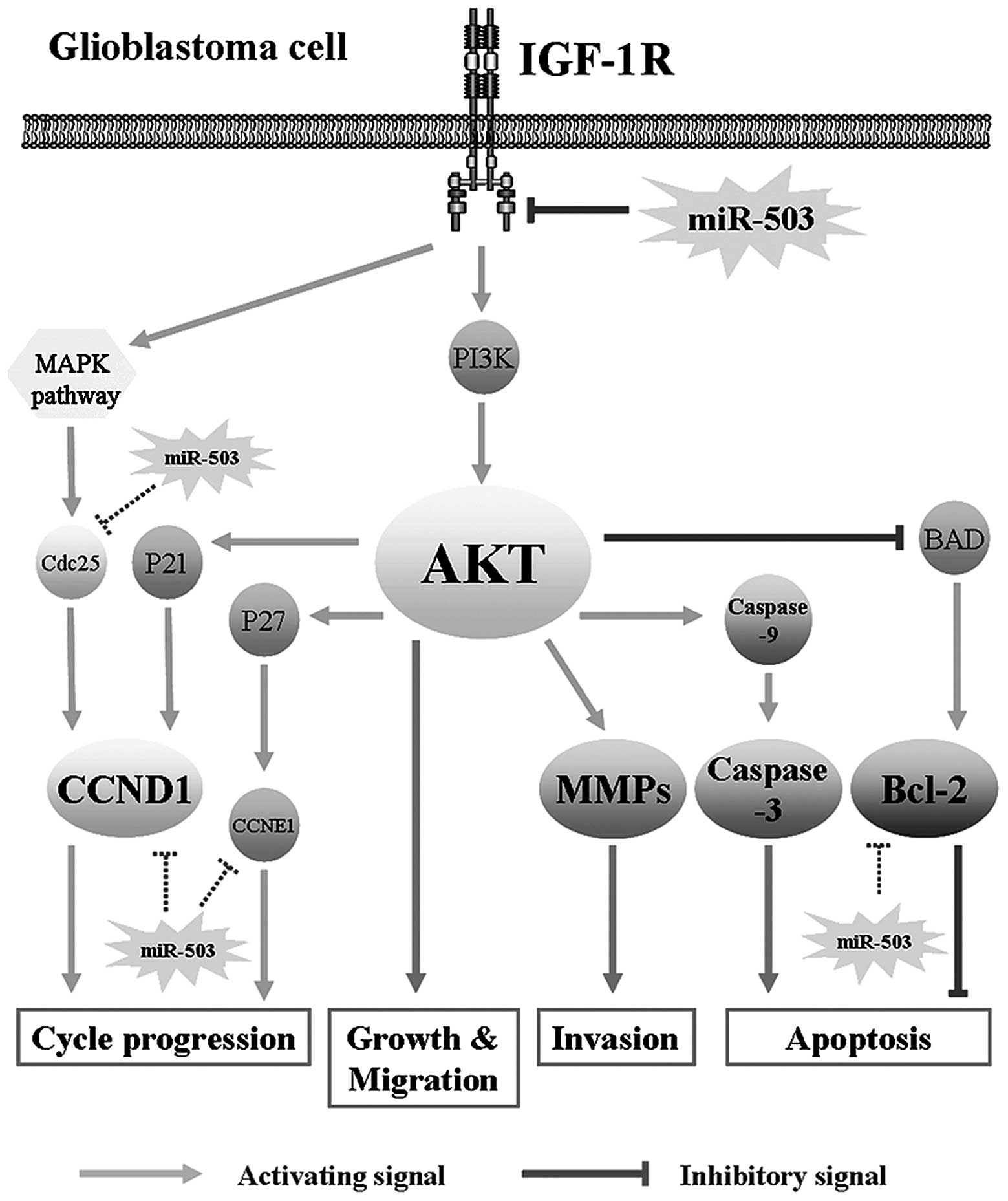 | Figure 7Model of miR-503 mediates
constitutive suppression of the IGF-1R pathway leading to a less
aggressive glioma phenotype. The IGF-1R/AKT pathway is a core
signaling pathway in glioblastoma oncogenesis. miR-503 influenced
AKT pathway by targeting IGF-1R, and consequently inhibits cell
growth, cell cycle progression, cell migration and invasion, and
induces cell apoptosis. Among them, AKT may regulate cell cycle
progression by P21/CCND1, influence cellular invasiveness by MMP-9,
affect apoptosis by caspase-3 and Bcl-2. In addition to directly
target IGF-1R, miR-503 may also influence GBM pathogenesis by
directly regulating Bcl-2, CDC25, CCND1 and CCNE1 according to
previous research. miR-503, microRNA-503; IGF-1R, insulin-like
growth factor-1; GBM, glioblastoma multiforme. |
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (nos. 81171577, 30901816, 8137179,
81371422 and 81171127).
References
|
1
|
von Deimling A, Louis DN and Wiestler OD:
Molecular pathways in the formation of gliomas. Glia. 15:328–338.
1995.PubMed/NCBI
|
|
2
|
Louis DN, Ohgaki H, Wiestler OD, et al:
The 2007 WHO classification of tumours of the central nervous
system. Acta Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 435:834–838.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bousquet M, Zhuang G, Meng C, et al:
miR-150 blocks MLL-AF9-associated leukemia through oncogene
repression. Mol Cancer Res. 11:912–922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ciafrè SA, Galardi S, Mangiola A, et al:
Extensive modulation of a set of microRNAs in primary glioblastoma.
Biochem Biophys Res Commun. 334:1351–1358. 2005.PubMed/NCBI
|
|
7
|
Zhang QQ, Xu H, Huang MB, et al:
MicroRNA-195 plays a tumor-suppressor role in human glioblastoma
cells by targeting signaling pathways involved in cellular
proliferation and invasion. Neuro Oncol. 14:278–287. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Ling N, Bai Y, et al: MiR-16-1 plays
a role in reducing migration and invasion of glioma cells. Anat
Rec. 296:427–432. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lakomy R, Sana J, Hankeova S, et al:
MiR-195, miR-196b, miR-181c, miR-21 expression levels and
O-6-methylguanine-DNA methyltransferase methylation status
are associated with clinical outcome in glioblastoma patients.
Cancer Sci. 102:2186–2190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caporali A and Emanueli C: MicroRNA-503
and the extended microRNA-16 family in angiogenesis. Trends
Cardiovasc Med. 21:162–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao JJ, Yang J, Lin J, et al:
Identification of miRNAs associated with tumorigenesis of
retinoblastoma by miRNA microarray analysis. Childs Nerv Syst.
25:13–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fisher JF and Mobashery S: Mechanism-based
profiling of MMPs. Methods Mol Biol. 622:471–487. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hägerstrand D, Lindh MB, Peña C, et al:
PI3K/PTEN/Akt pathway status affects the sensitivity of high-grade
glioma cell cultures to the insulin-like growth factor-1 receptor
inhibitor NVP-AEW541. Neuro Oncol. 12:967–975. 2010.PubMed/NCBI
|
|
14
|
Corbetta S, Vaira V, Guarnieri V, et al:
Differential expression of microRNAs in human parathyroid
carcinomas compared with normal parathyroid tissue. Endocr Relat
Cancer. 17:135–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tömböl Z, Szabó PM, Molnár V, et al:
Integrative molecular bioinformatics study of human adrenocortical
tumors: microRNA, tissue-specific target prediction, and pathway
analysis. Endocr Relat Cancer. 16:895–906. 2009.
|
|
16
|
Özata DM, Caramuta S, Velázquez-Fernández
D, et al: The role of microRNA deregulation in the pathogenesis of
adrenocortical carcinoma. Endocr Relat Cancer. 18:643–655.
2011.PubMed/NCBI
|
|
17
|
Zhou B, Ma R, Si W, et al: MicroRNA-503
targets FGF2 and VEGFA and inhibits tumor angiogenesis and growth.
Cancer Lett. 333:159–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao F, Zhang W, Chen L, et al:
MicroRNA-503 inhibits the G1/S transition by downregulating cyclin
D3 and E2F3 in hepatocellular carcinoma. J Transl Med. 11:1952013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu YY, Wu HJ, Ma HD, et al: MicroRNA-503
suppresses proliferation and cell-cycle progression of endometrioid
endometrial cancer by negatively regulating cyclin D1. FEBS J.
280:3768–3779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiu T, Zhou L, Wang T, et al: miR-503
regulates the resistance of non-small cell lung cancer cells to
cisplatin by targeting Bcl-2. Int J Mol Med. 32:593–598.
2013.PubMed/NCBI
|
|
21
|
Finnerty JR, Wang WX, Hébert SS, et al:
The miR-15/107 group of microRNA genes: evolutionary biology,
cellular functions, and roles in human diseases. J Mol Biol.
402:491–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Babak T, Zhang W, Morris Q, et al: Probing
microRNAs with microarrays: tissue specificity and functional
inference. RNA. 10:1813–1819. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baskerville S and Bartel DP: Microarray
profiling of microRNAs reveals frequent coexpression with
neighboring miRNAs and host genes. RNA. 11:241–247. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peltier HJ and Latham GJ: Normalization of
microRNA expression levels in quantitative RT-PCR assays:
identification of suitable reference RNA targets in normal and
cancerous human solid tissues. RNA. 14:844–852. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Furuta M, Kozaki K, Tanimoto K, et al: The
tumor-suppressive miR-497-195 cluster targets
multiple cell-cycle regulators in hepatocellular carcinoma. PLoS
One. 8:e601552013.PubMed/NCBI
|
|
26
|
Caporali A, Meloni M, Völlenkle C, et al:
Deregulation of microRNA-503 contributes to diabetes
mellitus-induced impairment of endothelial function and reparative
angiogenesis after limb ischemia. Circulation. 123:282–291. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sarkar S, Dey BK and Dutta A: MiR-322/424
and -503 are induced during muscle differentiation and promote cell
cycle quiescence and differentiation by down-regulation of Cdc25A.
Mol Biol Cell. 21:2138–2149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang Q, Feng MG and Mo YY: Systematic
validation of predicted microRNAs for cyclin D1. BMC Cancer.
9:1942009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang R, Mircean C, Shmulevich I, et al:
Pathway alterations during glioma progression revealed by reverse
phase protein lysate arrays. Proteomics. 6:2964–2971. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carapancea M, Alexandru O, Fetea AS, et
al: Growth factor receptors signaling in glioblastoma cells:
therapeutic implications. J Neurooncol. 92:137–147. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Friend KE, Khandwala HM, Flyvbjerg A, et
al: Growth hormone and insulin-like growth factor-I: effects on the
growth of glioma cell lines. Growth Horm IGF Res. 11:84–91. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Resnicoff M, Abraham D, Yutanawiboonchai
W, et al: The insulin-like growth factor I receptor protects tumor
cells from apoptosis in vivo. Cancer Res. 55:2463–2469.
1995.PubMed/NCBI
|
|
33
|
Bellacosa A, Kumar CC, Di Cristofano A and
Testa JR: Activation of AKT kinases in cancer: implications for
therapeutic targeting. Adv Cancer Res. 94:29–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakada M, Niska JA, Tran NL, et al:
EphB2/R-Ras signaling regulates glioma cell adhesion, growth, and
invasion. Am J Pathol. 167:565–576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jones RA, Campbell CI, Petrik JJ and
Moorehead RA: Characterization of a novel primary mammary tumor
cell line reveals that cyclin D1 is regulated by the type I
insulin-like growth factor receptor. Mol Cancer Res. 6:819–828.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wilsbacher JL, Zhang Q, Tucker LA, et al:
Insulin-like growth factor-1 receptor and ErbB kinase inhibitor
combinations block proliferation and induce apoptosis through
cyclin D1 reduction and Bax activation. J Biol Chem.
283:23721–23730. 2008. View Article : Google Scholar
|
|
37
|
Schlenska-Lange A, Knüpfer H, Lange TJ, et
al: Cell proliferation and migration in glioblastoma multiforme
cell lines are influenced by insulin-like growth factor I in vitro.
Anticancer Res. 28:1055–1060. 2008.PubMed/NCBI
|
|
38
|
Oh SH, Kang JH, Kyu Woo J, et al: A
multiplicity of anti-invasive effects of farnesyl transferase
inhibitor SCH66336 in human head and neck cancer. Int J Cancer.
131:537–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Saikali Z, Setya H, Singh G and Persad S:
Role of IGF-1/IGF-1R in regulation of invasion in DU145 prostate
cancer cells. Cancer Cell Int. 8:102008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kang DW and Min do S: Platelet derived
growth factor increases phospholipase D1 but not phospholipase D2
expression via NFκB signaling pathway and enhances invasion of
breast cancer cells. Cancer Lett. 294:125–133. 2010.PubMed/NCBI
|
|
41
|
Feng X, Aleem E, Lin Y, et al: Multiple
antitumor effects of picropodophyllin in colon carcinoma cell
lines: Clinical implications. Int J Oncol. 40:1251–1258.
2012.PubMed/NCBI
|
|
42
|
Min S, Liang X, Zhang M, et al: Multiple
tumor-associated microRNAs modulate the survival and longevity of
dendritic cells by targeting YWHAZ and Bcl2 signaling pathways. J
Immunol. 190:2437–2446. 2013. View Article : Google Scholar : PubMed/NCBI
|