Introduction
Retinoblastoma (RB) is the most common primary
intraocular malignancy in children worldwide, with a prevalence
ranging from 1:15,000 to 1:20,000 in children under the age of 5
years and with an estimated 2,800 new cases in the US (1,2). Both
forms of this malignancy, hereditary and sporadic, are associated
with alteration or loss of the tumor-suppressor gene RB1. The RB1
protein (pRB) functions as a tumor suppressor by controlling the
cell cycle and differentiation via complex interaction with
multiple kinases and their inhibitors (3,4).
Evidence from many studies suggests that several genes and pathways
are dysregulated and misexpressed in RB, and the expression of RB1
can be modulated (5). Thus,
understanding the molecular mechanisms of RB progression more
thoroughly will undoubtedly benefit the development of new
therapeutic strategies.
MicroRNAs are small noncoding RNAs, 18–25
nucleotides in length, transcribed from non-protein-coding genes or
introns. They regulate gene expression by repressing translation
and cleaving their target mRNAs by binding to complementary sites
in their 3′-untranslated region (UTR). It has been demonstrated
that due to aberrant expression, miRNAs may function as tumor
suppressors or oncogenes according to the roles of their target
gene (6,7). Particularly, miRNAs can regulate
various cellular processes of tumor cells that involve the cell
cycle, differentiation, progression, apoptosis, proliferation,
migration and invasion (8–10). A number of miRNAs have been proven
to induce proliferation by targeting members of the pRB family
(11). Moreover, several miRNAs
have emerged as candidate components of oncogene or tumor
suppressor networks in RB, such as carcinogenic miR-17~92, and
tumor suppressor miR-365–2p, miR-34a and let-7 (12–15).
miR-101 as a tumor suppressor has recently attracted
much attention. It has been reported that the expression of miR-101
was downregulated during prostate cancer progression, and the
expression of EZH2 was upregulated concomitantly during this
process (16). Enhancer of zeste
homolog 2 (EZH2), a histone methyltransferase, is a catalytic
subunit of a Polycomb repressive complex 2 (PRC2) which methylates
histone H3 on lysine 27 (17).
Mounting evidence proves that EZH2 has properties consistent with
those of an oncogene, since overexpression of EZH2 promotes cell
proliferation, colony formation and enhanced cell migration and
invasion (18,19). The involvement of miR-101 in
carcinogenesis through downregulation of EZH2 has been subsequently
confirmed in bladder cancer, lung cancer, gastric cancer,
hepatocellular cancer and glioblastoma (20–26).
However, the function of miR-101 and EZH2 in RB has not yet been
thoroughly investigated.
In the present study, we investigated the expression
of miR-101 and EZH2 in RB patients by quantitative real-time
polymerase chain reaction (qRT-PCR). We reported that miR-101 was
significantly downregulated in RB patient tissues compared to
normal control retina, which was accompanied by the upregulation of
EZH2 in RB tumor tissues. Furthermore, we evaluated the association
between the expression of miR-101 and EZH2 with histopathological
and clinicopathological features. Finally, miR-101 was identified
to inhibit the expression of EZH2 directly and function as a tumor
suppressor by inhibiting cell growth and inducing cell cycle arrest
and cell apoptosis. To the best of our knowledge, this is the first
comprehensive and thorough study to exam the expression and
function of miR-101 and its target EZH2 in RB.
Materials and methods
Patients and tissue samples
This study was approved by the Research Ethics
Committee of Xi’an Jiaotong University. Written informed consent
was obtained from all of the patients. All specimens were handled
and made anonymous according to the ethical and legal standards. A
total of 87 RB tissues and 44 normal retinas were provided by the
Department of Ophthalmology, the First Affiliated Hospital, Xi’an
Jiaotong University. The ages of the subjects ranged from 1 month
to 14 years (median, 28 months). Among the patients, 52 (59.8%)
were affected unilaterally and 35 (40.2%) bilaterally. One tumor
from each patient with bilateral tumors was used in this study.
Histopathological analysis revealed that 66 (75.9%) of the tumors
were poorly differentiated (PD) and 21 (24.1%) were well
differentiated (WD). Invasion of the choroid, optic nerve or orbit
was detected in 52 (59.8%) tumors, while 35 (40.2%) tumors were
localized without invasion. Among the 52 invasive tumors, 39
exhibited choroidal invasion, 37 exhibited optic nerve invasion and
32 exhibited orbital invasion.
Quantitative reverse transcriptase PCR
(qRT-PCR) assay
The expression levels of miR-101 in RB and normal
retinal tissues were detected by qRT-PCR assay. Briefly, total RNA
was extracted from tissues using TRIzol reagent (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer’s protocol. miRNA
expression levels were then quantitated using TaqMan miRNA
real-time RT-PCR kit (Applied Biosystems) according to the
manufacturer’s protocol. Data were analyzed using 7500 software
v.2.0.1 (Applied Biosystems), with the automatic Ct setting for
adapting baseline and threshold for Ct determination. The universal
small nuclear RNA U6 (RNU6B) was used as an endogenous control for
miRNAs. Each sample was examined in triplicate, and the amounts of
PCR products produced were nonneoplasticized to RNU6B.
Cell culture
Human RB cell lines Y79 and WERI-RB1 were obtained
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China), where they were characterized by mycoplasma detection,
DNA-fingerprinting, isozyme detection and cell vitality detection.
They were cultured in DMEM (Invitrogen, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS)(HyClone, Logan, UT,
USA) and cultured in a humidified incubator at 37°C in 5%
CO2.
Oligonucleotide transfection
miR-101 mimics and inhibitors were chemically
synthesized by Shanghai GenePharma (Shanghai, China). Once the
cells were 80% confluent, miR-101 mimics or the inhibitor was
transfected into the RB cells with Lipofectamine 2000 (Invitrogen)
according to the manufacturer’s instructions. Cells were also
transfected with a scramble oligonucleotide as negative control
(NC). The expression level of miR-101 in the transfected RB cells
was determined by qRT-PCR.
Luciferase reporter assay
RB cells were seeded in a 96-well plate at 60%
confluency. After 24 h, cells were transfected with 120 ng of
miR-101 expression vector or negative control. Cells were
transfected with 30 ng of WT or MT 3′-UTR of EZH2 mRNA. Cells were
collected 48 h after transfection, and luciferase activity was
measured using a dual luciferase reporter assay system according to
the manufacturer’s protocol (Promega).
Cell viability assay
Cells were plated in 96-well plates
(0.5×104 cells/well) and transfected with NC, miR-101
mimics and inhibitors. Forty-eight hours later, 10 μl of MTT
reagent (5 mg/ml) was added to each well, and cells were incubated
at 37°C for another 4 h. The medium was removed, the cells were
solubilized in 150 μl of dimethylsulfoxide, and colorimetric
analysis was performed (wavelength, 490 nm). One plate was analyzed
immediately after the cells adhered (~4 h after plating), and the
remaining plates were assayed every day for the next 4 consecutive
days.
Soft agar colony formation assay
Cells seeded on a 6-well plate were covered with a
layer of 0.6% agar in DMEM supplemented with 10% FBS. After
transfection for 48 h, cells were trypsinized, gently mixed with
0.3% agar medium mixture containing selective antibiotics and
reseeded in triplicate onto a 6-well plate. After 4 weeks, the
resistant colonies were stained with 0.2% crystal violet and
counted under a microscope.
Flow cytometric analysis of cell cycle
distribution and cell apoptosis
The RB cells were transfected with NC, miR-101
mimics or miR-101 inhibitors. Forty eight hours post-transfection,
cells were trypsinized and analyzed for cell cycle distribution and
cell apoptosis. For cell cycle distribution, half of the cells of
each group was stained with propidium iodide (PI) and analyzed by
flow cytometry using FACSCalibur (BD Biosciences, San Diego, CA,
USA). For each group, 10,000 events were acquired. The percentages
of cells in the G1, S and G2 phases of the cell cycle were
calculated. The other half of cells of each group was used to
detect cell apoptosis using Annexin V-FITC and PI (BD Bioscience,
USA) following the manufacturer’s instructions. The apoptosis index
was calculated by adding the cells in the first and the cells in
the second group.
Statistical analysis
Statistical analysis was performed using IBM SPSS
statistical software (version 20.0). The differences in
characteristics between the 2 groups were examined by the
χ2 test or Fisher’s exact test. All P-values were
determined from 2-sided tests, and statistical significance was
based on a P-value of 0.05.
Results
Expression of miR-101 and EZH2 in
nontumor retina samples and retinoblastomas
Expression levels of miR-101 and EZH2 were analyzed
in 44 normal retina samples and 87 tumor samples by quantitative
real-time polymerase chain reaction (qRT-PCR) and normalized
against endogenous controls (U6 RNA or β-actin, respectively). As
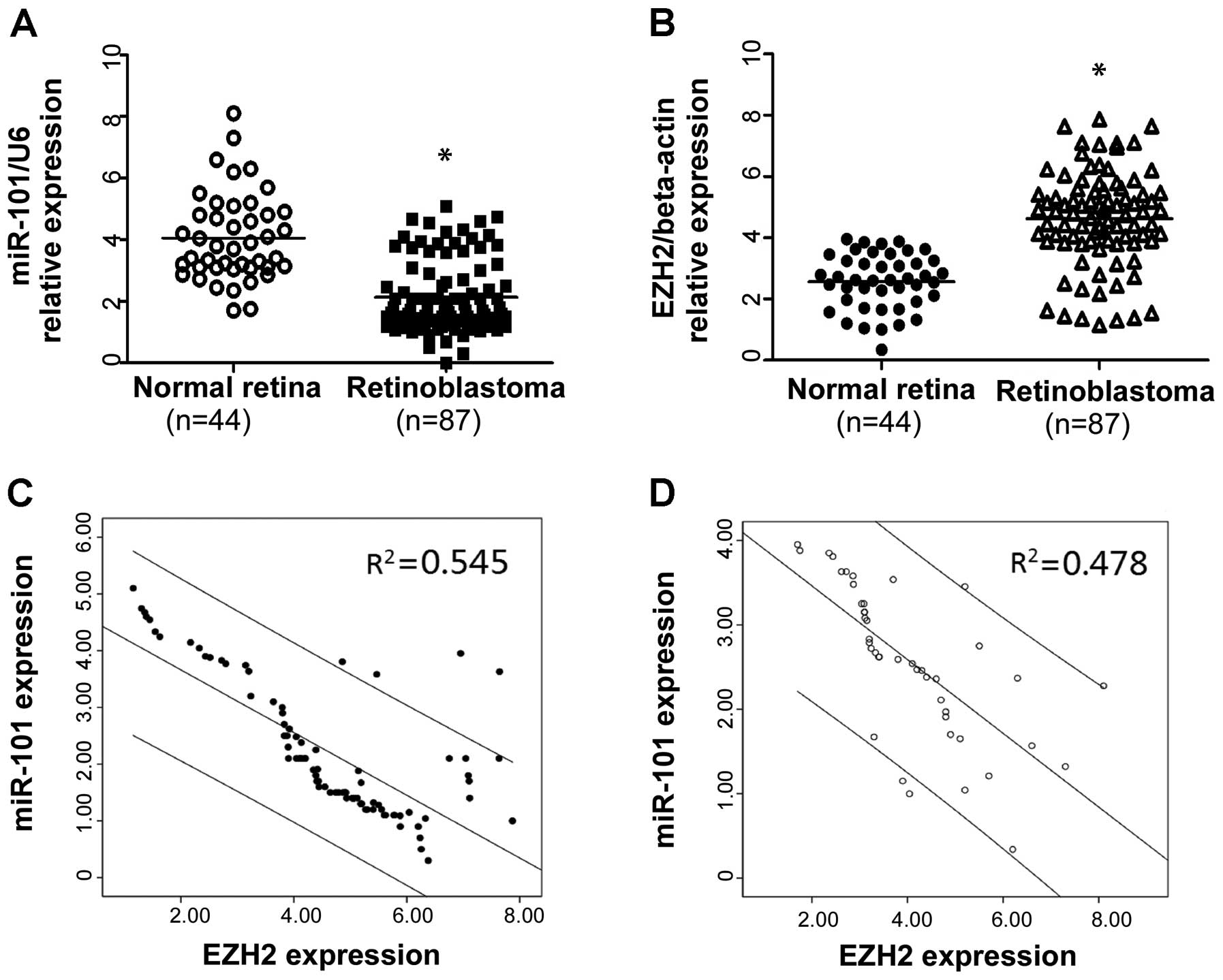
shown in Fig. 1A, the expression
level of miR-101 in RB tissues was found to be distinctly
downregulated compared to the nontumor retina tissues. In contrast,
weak or absent EZH2 expression was detected in the nontumor retina
tissues, but abundant expression of EZH2 was detected in the RB
tissues (Fig. 1B). Furthermore, we
detected an association among miR-101 expression and EZH2 in these
nontumor retina and RB cases. As shown in Fig. 1C and D, statistically significant
inverse correlations were revealed by Spearman’s correlation
analysis between mRNA levels of miR-101 and EZH2 in RB (r=−0.738;
P<0.001) and nontumor retina tissues (r=−0.691; P<0.001).
Taken together, our result suggests that miR-101 plays a
tumor-suppressor role and EZH2 plays an oncogenic role in RB. More
importantly, miR-101 was negatively correlated with the expression
of EZH2 in both the normal retina and RB.
Relationship between miR-101/EZH2 and
clinicopathological features of the retinoblastoma cases
To determine the clinical significance of miR-101
and its potential target EZH2 in RB, we analyzed the association of
miR-101 and EZH2 with various clinicopathological and
histopathological parameters of the RB cases. The median miR-101
and EZH2 expression levels in the 87 patients of RB were 2.74 and
4.72, respectively. The patients were divided into two groups
according to their expression levels of miR-101 and EZH2, using its
median as a cut off: high miR-101 expression group (n=34) and low
miR-101 expression group (n=53). Accordingly, a high EZH2
expression group (n=46) and a low EZH2 expression group (n=41) were
established. As shown in Table IA,
a significant association was found between low miR-101 expression
and the degree of invasion, subretinal and vitreous seeding
(P=0.045, P=0.001 and P=0.007, respectively). Whereas, EZH2 was
significantly upregulated in RB patients with invasion, subretinal
and vitreous seeding (P=0.018, P=0.004 and P=0.029, respectively).
No significant differences were observed in the miR-101 and EZH2
expression levels in regards to patient age, gender and
laterality.
 | Table IAssociation of miR-101 and EZH2
expression with clinicopathological and histopathological features
of the retinoblastoma cases. |
Table I
Association of miR-101 and EZH2
expression with clinicopathological and histopathological features
of the retinoblastoma cases.
| A,
Clinicopathological features |
|---|
|
|---|
| | miR-101
expression | | EZH2
expression | |
|---|
| |
| |
| |
|---|
| No. of cases | High
n | Low
n | P-value | High
n | Low
n | P-value |
|---|
| Age (years) | | | | 0.826 | | | 0.287 |
| <2 | 48 | 18 | 30 | | 28 | 20 | |
| ≥2 | 39 | 16 | 23 | | 18 | 21 | |
| Gender | | | | 0.829 | | | 0.394 |
| Male | 45 | 17 | 28 | | 26 | 19 | |
| Female | 42 | 17 | 25 | | 20 | 22 | |
| Laterality | | | | 0.655 | | | 0.284 |
| Unilateral | 52 | 19 | 33 | | 30 | 22 | |
| Bilateral | 35 | 15 | 20 | | 16 | 19 | |
| Invasion | | | | 0.045a | | | 0.018a |
| Noninvasive | 35 | 9 | 26 | | 13 | 22 | |
| Invasive | 52 | 25 | 27 | | 33 | 19 | |
| Subretinal
seeding | | | | 0.001a | | | 0.004a |
| Yes | 31 | 5 | 26 | | 23 | 8 | |
| No | 56 | 29 | 27 | | 23 | 33 | |
| Vitreous
seeding | | | | 0.007a | | | 0.029a |
| Yes | 37 | 8 | 29 | | 25 | 12 | |
| No | 50 | 26 | 24 | | 21 | 29 | |
|
| B,
Histopathological features |
|
|
Differentiation | | | | 0.004a | | | 0.013a |
| WD | 21 | 14 | 7 | | 6 | 15 | |
| PD | 66 | 20 | 46 | | 40 | 26 | |
| Necrosis | | | | 0.579 | | | 0.300 |
| None | 38 | 13 | 25 | | 23 | 15 | |
| Mild | 14 | 5 | 9 | | 8 | 6 | |
| Extensive | 35 | 16 | 19 | | 15 | 20 | |
| Optic nerve
invasion | | | | 0.026a | | | 0.082 |
| Yes | 37 | 9 | 28 | | 24 | 13 | |
| No | 50 | 25 | 25 | | 22 | 28 | |
| Choroidal
invasion | | | | 0.008a | | | 0.009a |
| Yes | 39 | 9 | 30 | | 27 | 12 | |
| No | 48 | 25 | 23 | | 19 | 29 | |
| Orbital
invasion | | | | 0.044a | | | 0.028a |
| Yes | 32 | 8 | 24 | | 22 | 10 | |
| No | 55 | 26 | 29 | | 24 | 31 | |
Relationship between miR-101/EZH2 and
histopathological features of retinoblastoma
The possible association between miR-101/EZH2 and
histopathologic features was then analyzed. As shown in Table IB, miR-101 was significantly
downregulated in RB cases with poor differentiation (PD) compared
to cases with well differentiation (WD) (P=0.004), whereas EZH2 was
significantly upregulated in RB cases with PD compared to WD
(P=0.013). Moreover, the expression of miR-101 was also
significantly associated with choroidal invasion (P=0.008),
invasion of the optic nerve (P=0.026) and orbital invasion
(P=0.044). EZH2 expression was significantly associated with
orbital invasion (P=0.028) and choroidal invasion (P=0.009). No
significant differences were observed between the other
histopathological features and the expression of miR-101 and
EZH2.
EZH2 is a direct target of miR-101 in
retinoblastoma cells
It has been proven that EZH2 is the direct target of
miR-101 in other cancer cells (26,27).
Considering the tissue-specific and developmental stage-specific
manner of microRNAs, we investigated the relationship of EZH2 and
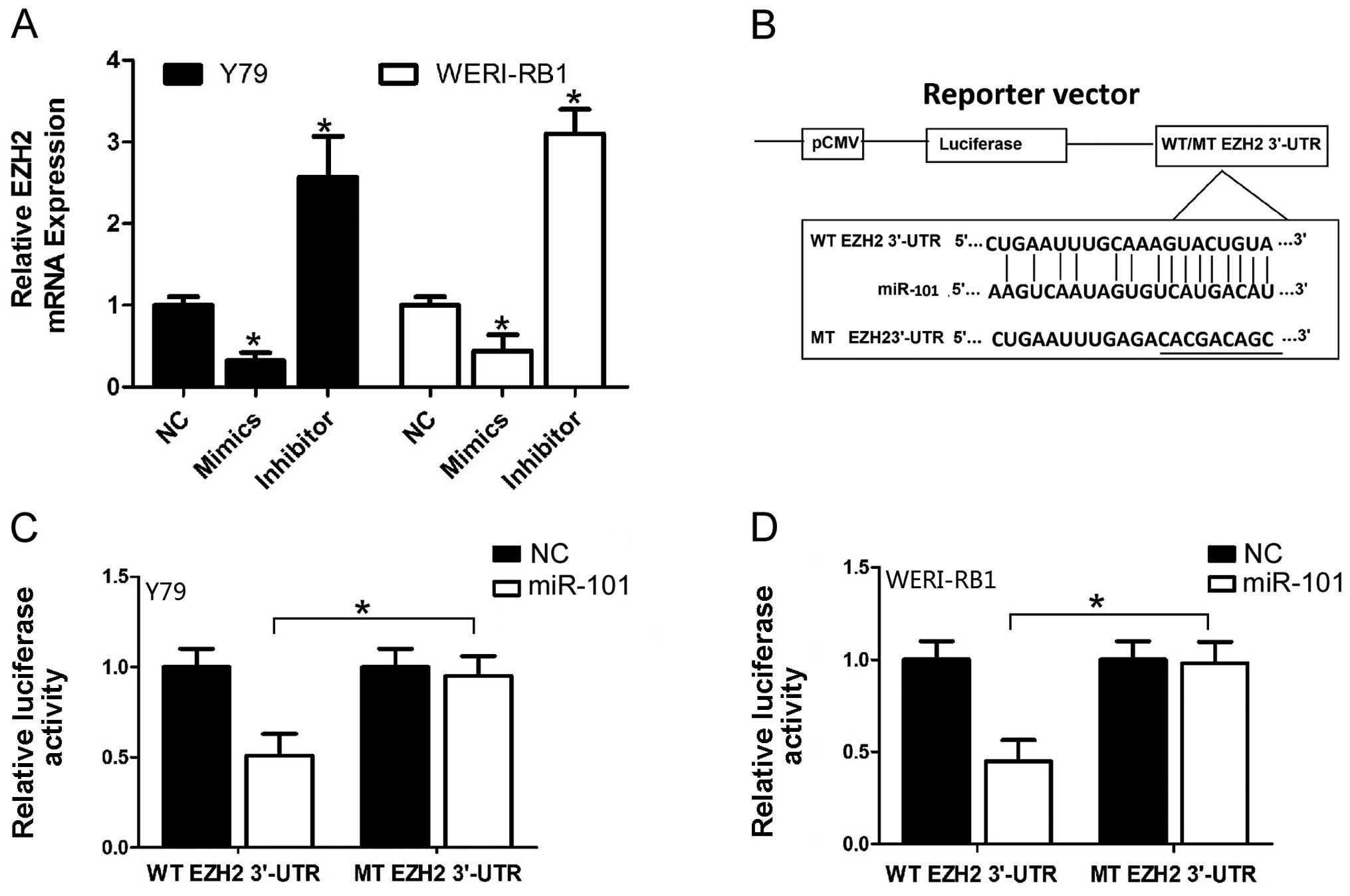
miR-101 in RB cell lines. In order to confirm whether EZH2 was a
target gene for miR-101 in RB cells, qRT-PCR was used to detect the
expression of EZH2 which was regulated by miR-101 in the RB cell
lines Y79 and WERI-RB1. The expression of EZH2 was significantly
downregulated after overexpression of miR-101 at the mRNA level in
RB cells (Fig. 2A). Furthermore, we
assessed the significance of miR-101 and EZH2 correlation in the RB
tissues. Previously, we determined the EZH2 mRNA and miR-101
expression in the same RB specimens by qRT-PCR. As shown in
Fig. 1C, a statistically
significant inverse correlation was revealed by Spearman’s
correlation analysis between the mRNA levels of miR-101 and EZH2 in
RB (r=−0.738; P<0.001). Taken together, our results suggest that
miR-101 negatively regulates the expression of its potential target
gene EZH2 in RB.
We further performed a luciferase reporter assay to
verify whether miR-101 directly targets the 3′-UTR of EZH2 in RB
cells. The target sequence of EZH2 3′-UTR (WT 3′-UTR) or the mutant
sequence (MT 3′-UTR) was cloned into a luciferase reporter vector
(Fig. 2B). Y79 and WERI-RB1 cells
were then transfected with the WT or MT 3′-UTR vector and miR-101
mimic. As shown in Fig. 2C, a
significant decrease in luciferase activity was demonstrated
between the EZH2 WT 3′-UTR group and the negative control group in
the Y79 cells (P<0.05). The suppressive effect was abrogated by
a point mutation in the core binding sites of the EZH2 3′-UTR. A
similar trend was also found in the WERI-RB1 cells (Fig. 2D). All these results indicate that
miR-101 exerts inhibitory effects on EZH2 expression via
interaction with 3′-UTR of EZH2 in RB cells.
Effect of miR-101 on cell viability and
proliferation of human retinoblastoma cell lines
It has been reported that EZH2 promotes cell
proliferation, colony formation and increased cell apoptosis in
many different cancer cells (16,19,28,29).
As the patients with adverse clinicopathological and
histopathological features were significantly associated with low
expression of miR-101 and EZH2 was found to be the direct target of
miR-101, we investigated whether miR-101 affects cell viability,
cell cycle distribution and cell apoptosis in RB cells. To confirm
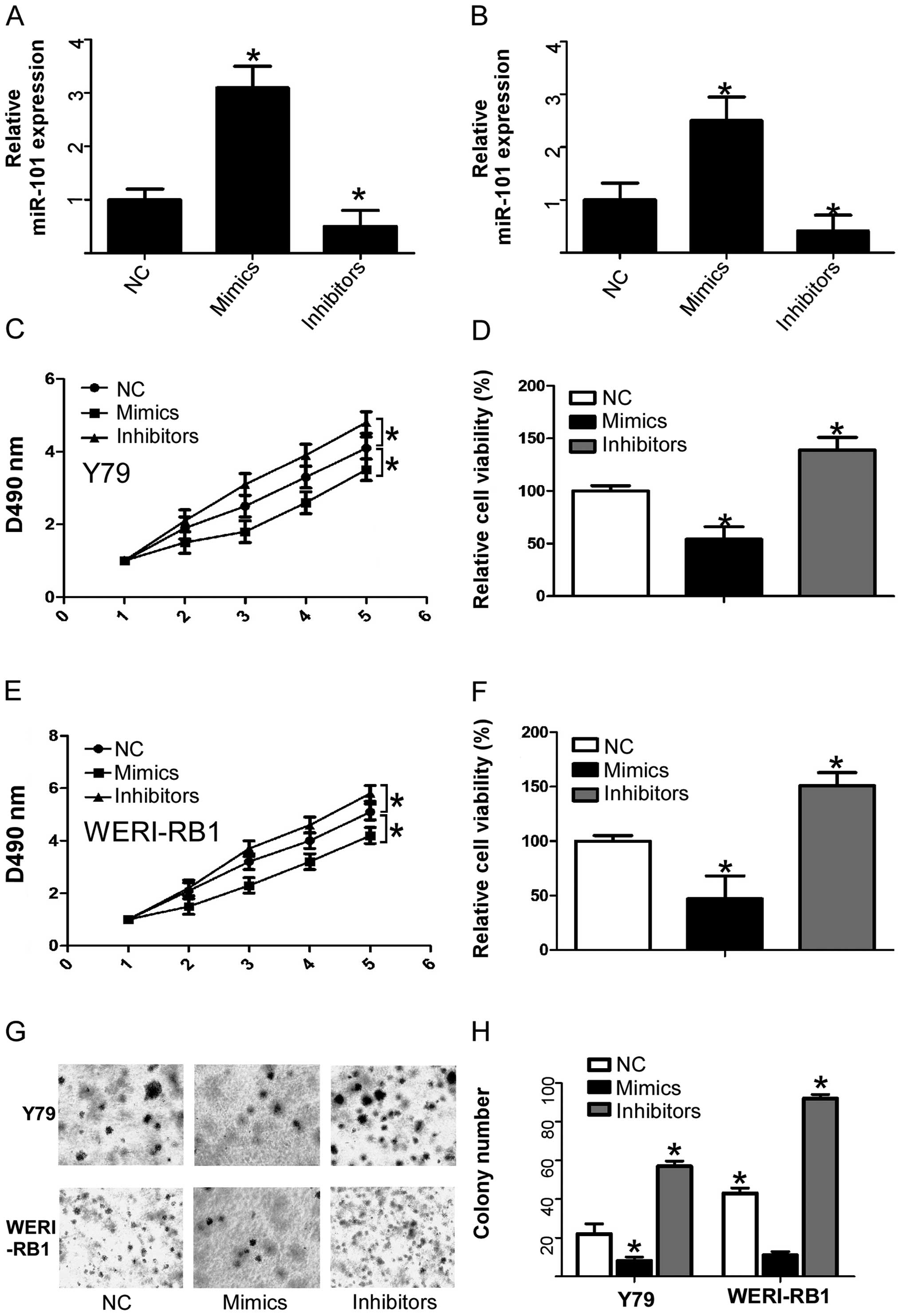
this possibility, we increased the miR-101 level in human RB cells
with miR-101 mimics or decreased the miR-101 level in human RB
cells with miR-101 inhibitors. Then human RB cell lines Y79 and
WERI-RB-1 were transiently transfected with miR-101 mimics or
inhibitors, respectively. As expected, transfection of miR-101
mimics or miR-101 inhibitors caused an increase or a decrease in
miR-101 expression, respectively, compared with the negative
control (Fig. 3A and B). After
confirming the efficiency of the miR-101 mimics and inhibitors, we
determined the effect of increased and decreased expression of
miR-101 on cell viability using an MTT assay. RB cells that were
transfected with miR-101 mimics showed a significant decrease in
cell viability when compared to the normal control. Whereas, RB
cells transfected with the miR-101 inhibitor showed a significant
increase in cell viability when compared to the normal control
(Fig. 3C–F). We then determined the
effect of increased and decreased expression of miR-101 on cell
proliferation using soft agar colony formation assays. RB cell
lines transfected with miR-101 mimics showed a significant decrease
in cell proliferation compared to the normal control, whereas, RB
cells transfected with miR-101 inhibitors showed a significant
increase in cell proliferation compared to the normal control
(Fig. 3G and H). These data suggest
that enforced expression of miR-101 can significantly suppress
human RB cell growth, and decreased expression of miR-101
significantly promotes human RB cell growth, which indicates a
tumor-suppressor role for miR-101 in human RB cells.
Effect of miR-101 on cell cycle and
apoptosis of human retinoblastoma cell lines
To further investigate the mechanism of
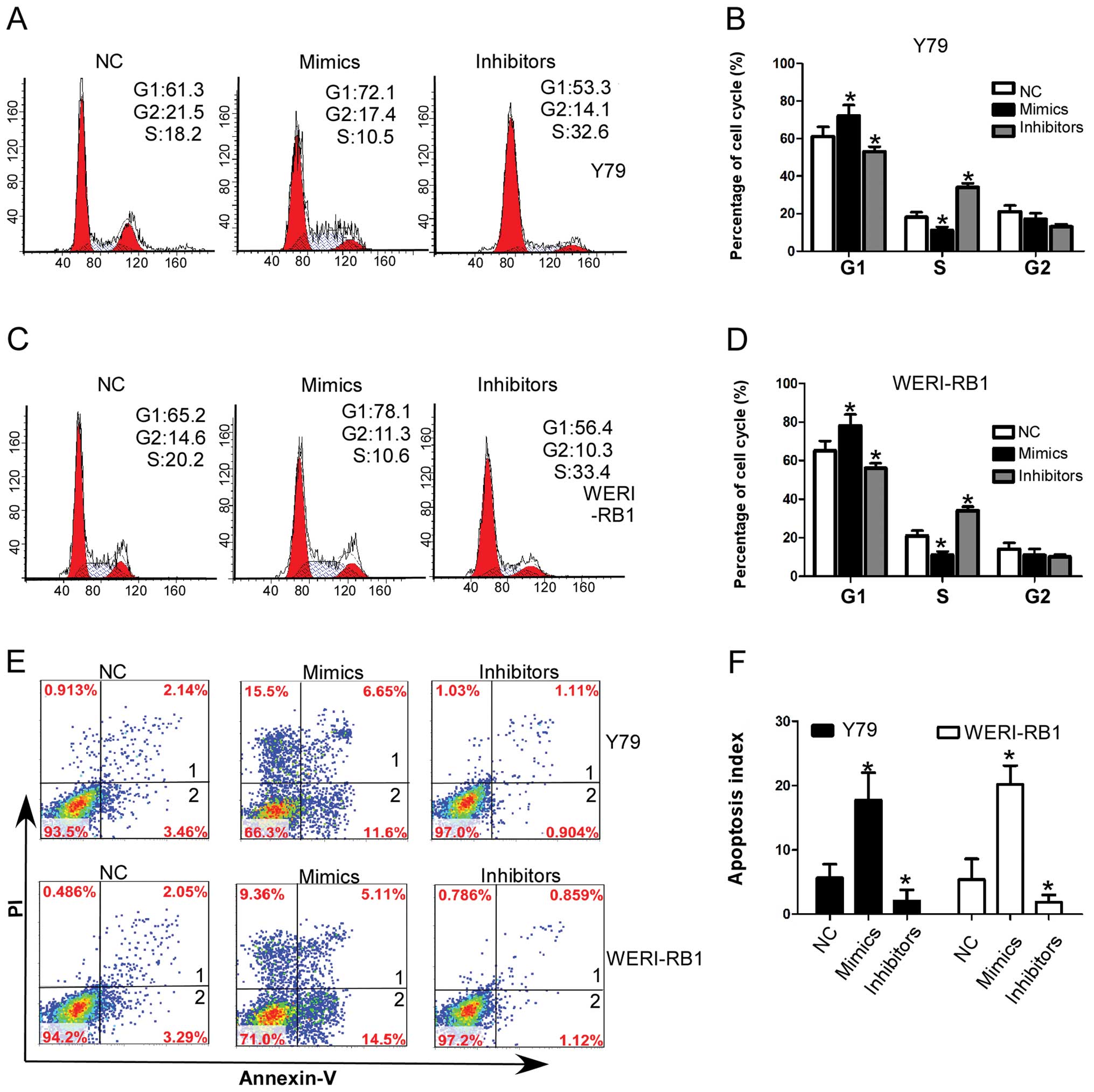
miR-101-induced cell growth inhibition, we assessed the change in
cell cycle distribution by flow cytometry. In RB cell lines, Y79
and WERI-RB1, miR-101 mimics significantly decreased the
percentages of cells in the S-phase, whereas the percentages of
cells were increased in the G1-phase compared to the normal control
transfected cells. whereas, miR-101 inhibitors significantly
increased the percentages of cells in the S-phase and decreased the
percentages of cells in the G1-phase compared to the normal control
transfected cells (Fig. 4A–D).
Previous studies confirmed that miR-101 is a p53-regulated
microRNA, and the activation of the p53 pathway results in
apoptosis in various cancer cells (30,31).
We hypothesized that the exogenous miR-101 administration may
result in activation of an apoptotic program in RB cells. Thus, we
evaluated the effect of miR-101 on cell apoptosis of RB cell lines.
As shown in Fig 4E and F, enhanced
expression of miR-101 significantly increased the percentage of
apoptotic cells, while decreased expression of miR-101
significantly decreased the apoptosis index of RB cells. Taken
together, these results indicate that miR-101 induces G1 arrest and
promotes cell apoptosis in RB cells.
Discussion
Several microRNAs have been identified as candidate
components of oncogene and tumor-suppressor networks in RB
(5), and the microRNA miR-101 has
been found to be a tumor suppressor and is a prognostic marker in
several types of cancer by targeting EZH2, MIF or SOX9 (20,21,32,33).
However, the function and clinical relevance of miR-101 in RB have
not yet been studied. In the present study, we found that miR-101
was weakly expressed in RB tissues compared to normal retina
tissues. At the same time, its potential target EZH2 had a higher
expression level in RB tissues than in normal retina. We also found
that miR-101 was inversely associated with the expression of EZH2
in normal retina and RB. Moreover, we demonstrated that decreased
expression of miR-101 and increased expression of EZH2 were
significantly associated with adverse clinicopathological and
histopathological features. Our results showed that miR-101 was
significantly associated with invasion, subretinal seeding,
vitreous seeding, choroidal invasion, orbital, optic nerve invasion
in RB patients. EZH2 was significantly associated with invasion,
subretinal seeding, vitreous seeding, orbital invasion and
choroidal invasion in the RB patients. These results provide
initial evidence to support that miR-101 functions as a tumor
suppressor in RB and that EZH2 is its potential target and has an
oncogenic role in RB.
To reveal the role of miR-101 in RB cells, we tested
the effect of miR-101 on cell growth and cell proliferation. Our
results showed that ectopic expression of miR-101 significantly
reduced cell growth and proliferation in RB cell lines Y79 and
WERI-RB1. These results indicate that miR-101 is a novel tumor
suppressor and plays important roles in the regulation of tumor
proliferation of RB. Next, we investigated the potential mechanism
of miR-101 in cell growth and proliferation and found that high
levels of miR-101 led to cell cycle arrest and increased cell
apoptosis. It has also been reported that miR-101 is downregulated
in non-small cell lung cancer and hepatocellular carcinoma cell
lines, resulting in the promotion of apoptosis and inhibition of
cell cycle progression (26,34,35).
Combining these studies and our results, we confirm that miR-101
functions as a tumor-suppressor gene by affecting the cell cycle
and cell apoptosis in these types of cancer.
The expression of miR-101 in cancers is still
controversial. Most studies indicate that miR-101 is significantly
downregulated in different types of cancers. However, one study
found that miR-101 was upregulated in prostate cancer tissues
(36). Our results make this
controversy even more complicated. Conkrite et al (37) found that miR-101 was upregulated in
human retinoblastoma compared to normal retina using microarray and
deep-sequencing analysis, which was opposite to our results. The
discrepancies between our study and their study concerning miR-101
expression in RB may be due to the differences in sample origin,
different analytical approaches or different technical platforms of
studies. In our study, we applied qRT-PCR as the main strategy to
detect the expression of miR-101. Consistent with most previous
studies, the expression of miR-101 was downregulated in RB compared
to normal retina tissues. In their study, they examined global
expression of microRNAs in human retinoblastoma using microarrays.
They found that miR-101 was upregulated in RB compared to normal
retina tissues, but they did not further identify their results
using qRT-PCR in a larger RB sample. However, it is also possible
that miR-101 has a dual role in the tumorigenicity of RB. To date,
several miRNAs with a dual role in cancer have been reported. For
example, miR-155 is upregulated in breast cancer, while the
expression of miR-155 is significantly decreased in pancreatic
tumors (38,39), which indicates the dual role of
miR-155 in cancer and the tissue-specific nature of microRNAs.
Thus, in order to further identify the tumor-suppressor role of
miR-101 in RB, we further investigated the effect of miR-101 on RB
cells.
As a tumor suppressive miRNA, miR-101 was reported
to be able to suppress the cell viability and colony formation
ability in various cancer cells (16,40,41).
In the present study, we hypothesized that miR-101 inhibits cell
growth and proliferation. As expected, ectopic expression of
miR-101 suppressed the cell viability and cell proliferation of RB
cell lines Y79 and WERI-RB1. Then we explored the potential
mechanism attributed to the proliferation inhibitory role of
miR-101 in RB cells. We found that ectopic expression of miR-101
resulted in cell cycle arrest in the G1 phase and promoted the cell
apoptosis of RB cells. Our study confirmed the tumor-suppressive
role of miR-101 in RB for the first time, and provides evidence for
the potential usefulness of miR-101 in miRNA-based cancer
therapy.
EZH2 is broadly overexpressed in aggressive solid
tumors and displays the properties of an oncogene, as
overexpression of EZH2 promotes cell proliferation, colony
formation, migration and invasion in vivo and in
vitro. EZH2 has been identified as a target of miR-101 by
luciferase reporter assay in many types of cancer, but not in RB.
In the present study, we examined the relationship of EZH2 and
miR-101 in RB tissues and investigated whether miR-101 directly
targets EZH2 in RB cells. We found that EZH2 was inversely
associated with miR-101 expression in RB tissues. The mRNA level of
EZH2 was also reduced in RB cells transfected with miR-101 mimics.
Using luciferase assay, we found that miR-101 directly targeted
EZH2 in RB cells. These result provide insight into the regulatory
mechanism of miR-101 in RB. Downregulation of miR-101 in RB cells
resulted in enhanced expression of EZH2, which consequently favored
tumor progression.
In summary, we showed that miR-101 was downregulated
and its target EZH2 was upregulated in RB tissues. Moreover,
downregulated miR-101 and upregulated EZH2 were significantly
associated with adverse clinicopathological and histopathological
features of RB. Finally, we found that ectopic expression of
miR-101 significantly reduced cell growth and proliferation in RB
cells through directly targeting EZH2, which was associated with
increased G1 phase arrest and cell apoptosis. These results suggest
that miR-101 plays a role in inhibiting the development and
progression of RB by targeting EZH2 and may potentially lead to a
novel strategy of therapy for RB.
Acknowledgements
The authors thank the local doctors and the patients
who participated in our study.
Abbreviations:
|
RB
|
retinoblastoma
|
|
miR-101
|
microRNA-101
|
|
EZH2
|
enhancer of zeste homolog 2
|
|
PRC2
|
polycomb repressive complex 2
|
|
WT
|
wild-type
|
|
MT
|
mutant-type
|
|
3′-UTR
|
3′-untranslated region
|
|
qRT-PCR
|
quantitative real-time polymerase
chain reaction
|
|
PD
|
poor differentiation
|
|
WD
|
well differentiation
|
References
|
1
|
Broaddus E, Topham A and Singh AD:
Incidence of retinoblastoma in the USA: 1975–2004. Br J Ophthalmol.
93:21–23. 2009.
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
3
|
Giacinti C and Giordano A: RB and cell
cycle progression. Oncogene. 25:5220–5227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lillington DM, Kingston JE, Coen PG, et
al: Comparative genomic hybridization of 49 primary retinoblastoma
tumors identifies chromosomal regions associated with
histopathology, progression, and patient outcome. Genes Chromosomes
Cancer. 36:121–128. 2003. View Article : Google Scholar
|
|
5
|
Reis AH, Vargas FR and Lemos B: More
epigenetic hits than meets the eye: microRNAs and genes associated
with the tumorigenesis of retinoblastoma. Front Genet.
3:2842012.PubMed/NCBI
|
|
6
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumar MS, Lu J, Mercer KL, Golub TR and
Jacks T: Impaired microRNA processing enhances cellular
transformation and tumorigenesis. Nat Genet. 39:673–677. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar
|
|
9
|
Jansson MD and Lund AH: MicroRNA and
cancer. Mol Oncol. 6:590–610. 2012. View Article : Google Scholar
|
|
10
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bueno MJ and Malumbres M: MicroRNAs and
the cell cycle. Biochim Biophys Acta. 1812:592–601. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kandalam MM, Beta M, Maheswari UK,
Swaminathan S and Krishnakumar S: Oncogenic microRNA 17–92 cluster
is regulated by epithelial cell adhesion molecule and could be a
potential therapeutic target in retinoblastoma. Mol Vis.
18:2279–2287. 2012.
|
|
13
|
Dalgard CL, Gonzalez M, deNiro JE and
O’Brien JM: Differential microRNA-34a expression and tumor
suppressor function in retinoblastoma cells. Invest Ophthalmol Vis
Sci. 50:4542–4551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Wang X, Wu G, Hou D and Hu Q:
MiR-365b-3p, down-regulated in retinoblastoma, regulates cell cycle
progression and apoptosis of human retinoblastoma cells by
targeting PAX6. FEBS Lett. 587:1779–1786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee YS and Dutta A: The tumor suppressor
microRNA let-7 represses the HMGA2 oncogene. Genes Dev.
21:1025–1030. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Varambally S, Cao Q, Mani RS, et al:
Genomic loss of microRNA-101 leads to overexpression of histone
methyltransferase EZH2 in cancer. Science. 322:1695–1699. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chase A and Cross NC: Aberrations of EZH2
in cancer. Clin Cancer Res. 17:2613–2618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Varambally S, Dhanasekaran SM, Zhou M, et
al: The polycomb group protein EZH2 is involved in progression of
prostate cancer. Nature. 419:624–629. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bracken AP, Pasini D, Capra M, Prosperini
E, Colli E and Helin K: EZH2 is downstream of the pRB-E2F pathway,
essential for proliferation and amplified in cancer. EMBO J.
22:5323–5335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Friedman JM, Liang G, Liu CC, et al: The
putative tumor suppressor microRNA-101 modulates the cancer
epigenome by repressing the polycomb group protein EZH2. Cancer
Res. 69:2623–2629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang HJ, Ruan HJ, He XJ, et al:
MicroRNA-101 is down-regulated in gastric cancer and involved in
cell migration and invasion. Eur J Cancer. 46:2295–2303. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smits M, Nilsson J, Mir SE, et al: miR-101
is down-regulated in glioblastoma resulting in EZH2-induced
proliferation, migration, and angiogenesis. Oncotarget. 1:710–720.
2010.PubMed/NCBI
|
|
23
|
Zhang JG, Guo JF, Liu DL, Liu Q and Wang
JJ: MicroRNA-101 exerts tumor-suppressive functions in non-small
cell lung cancer through directly targeting enhancer of zeste
homolog 2. J Thorac Oncol. 6:671–678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao P, Deng Z, Wan M, et al: MicroRNA-101
negatively regulates Ezh2 and its expression is modulated by
androgen receptor and HIF-1alpha/HIF-1beta. Mol Cancer. 9:1082010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Zhang X, Jia LT, et al:
c-Myc-mediated epigenetic silencing of microRNA-101 contributes to
dysregulation of multiple pathways in hepatocellular carcinoma.
Hepatology. 59:1850–1863. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu L, Beckebaum S, Iacob S, et al:
MicroRNA-101 inhibits human hepatocellular carcinoma progression
through EZH2 downregulation and increased cytostatic drug
sensitivity. J Hepatol. 60:590–598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo C, Merz PR, Chen Y, et al: MiR-101
inhibits melanoma cell invasion and proliferation by targeting MITF
and EZH2. Cancer Lett. 341:240–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakagawa S, Sakamoto Y, Okabe H, et al:
Epigenetic therapy with the histone methyltransferase EZH2
inhibitor 3-deazaneplanocin A inhibits the growth of
cholangiocarcinoma cells. Oncol Rep. 31:983–988. 2014.PubMed/NCBI
|
|
29
|
Yang YA and Yu J: EZH2, an epigenetic
driver of prostate cancer. Protein Cell. 4:331–341. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pushpavalli S, Ramaiah MJ, Srinivas CH, et
al: Effect of benzothiazole based conjugates in causing apoptosis
by regulating p53, PTEN and MAP kinase proteins affecting miR-195a
and miR-101–1. Cancer Cell Int. 11:362011.PubMed/NCBI
|
|
31
|
Ng SB, Yan J, Huang G, et al: Dysregulated
microRNAs affect pathways and targets of biologic relevance in
nasal-type natural killer/T-cell lymphoma. Blood. 118:4919–4929.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hiroki E, Akahira J, Suzuki F, et al:
Changes in microRNA expression levels correlate with
clinicopathological features and prognoses in endometrial serous
adenocarcinomas. Cancer Sci. 101:241–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Guo X, Xiong L, et al:
MicroRNA-101 suppresses SOX9-dependent tumorigenicity and promotes
favorable prognosis of human hepatocellular carcinoma. FEBS Lett.
586:4362–4370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yin J, Wang M, Jin C and Qi Q: miR-101
sensitizes A549 NSCLC cell line to CDDP by activating caspase
3-dependent apoptosis. Oncol Lett. 7:461–465. 2014.PubMed/NCBI
|
|
35
|
Xu Y, An Y, Wang Y, et al: miR-101
inhibits autophagy and enhances cisplatin-induced apoptosis in
hepatocellular carcinoma cells. Oncol Rep. 29:2019–2024.
2013.PubMed/NCBI
|
|
36
|
Lee KH, Chen YL, Yeh SD, et al:
MicroRNA-330 acts as tumor suppressor and induces apoptosis of
prostate cancer cells through E2F1-mediated suppression of Akt
phosphorylation. Oncogene. 28:3360–3370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Conkrite K, Sundby M, Mukai S, et al:
miR-17~92 cooperates with RB pathway mutations to promote
retinoblastoma. Genes Dev. 25:1734–1745. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kong W, Yang H, He L, et al: MicroRNA-155
is regulated by the transforming growth factor beta/Smad pathway
and contributes to epithelial cell plasticity by targeting RhoA.
Mol Cell Biol. 28:6773–6784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Volinia S, Calin GA, Liu CG, et al: A
microRNA expression signature of human solid tumors defines cancer
gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Su H, Yang JR, Xu T, et al: MicroRNA-101,
down-regulated in hepatocellular carcinoma, promotes apoptosis and
suppresses tumorigenicity. Cancer Res. 69:1135–1142. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li S, Fu H, Wang Y, et al: MicroRNA-101
regulates expression of the v-fos FBJ murine osteosarcoma viral
oncogene homolog (FOS) oncogene in human hepatocellular carcinoma.
Hepatology. 49:1194–1202. 2009. View Article : Google Scholar : PubMed/NCBI
|