1. Introduction
Worldwide, breast cancer (BC) is the leading cause
of death in women due to malignant neoplasms. In Mexico, the
incidence of this disease reported in 2008 was 13,939 new cases per
100,000 inhabitants (1). In two
decades (1990–2010), the annual risk increased from 2 to 5%, being
more frequent in the north and central part of the country
(2). In 2010, the National
Institute of Statistics, Geography and Informatics of Mexico
(INEGI) reported that 19 of every 100 women enrolled into a medical
service, presented with a mammary neoplasm, and 47% of BC deaths
were in women with ages ranging from 45 to 64 (3). There are many risk factors for the
development of breast cancer. For the Mexican population, the
principal risk factors include reproductive factors (e.g. age at
menarche and menopause, nulliparity or late pregnancy and
non-breastfeeding women) and genetics (BRCA1 and BRCA2 genes)
(3). In contrast, a diet rich in
fruits and vegetables, low alcohol consumption and no smoking
habit, significantly reduce the probability to develop this disease
(2).
The treatment for BC in the primary stages (I and
II) generally consists of surgical procedures, followed by
chemotherapy (mainly anthracycline-type drugs) and/or radiotherapy
(depending on the stage of the cancer), offering successful and
even curative results in the majority of cases (4). Notwithstanding, the cardiotoxicity of
anthracyclines is well documented; the secondary effects are mainly
cardiomyopathy and cardiac syncope (5). Epidemiologic studies indicate that 50%
of patients exposed to anthracyclines develop cardiac abnormalities
after 10 to 20 years of chemotherapy; 40% of patients present with
arrhythmia and 5% with heart failure (6). In addition, patients in advanced
stages or with metastasis present a survival rate of 22% after 10
years of treatment (7).
In search for new alternatives to the conventional
treatment and/or for the improvement of known therapies, aspirin
has been one of the most attractive prevention strategy proposals.
There is a long literature record, of over 20 years, concerning the
study of aspirin and its effect on BC risk. An example of this is
the meta-analysis performed by Khuder and Mutgi (2001), where
evidence gathered from 1980 to 2000 was analyzed (8). In this work, the results highlighted
that women with estrogen receptor (ER)-positive (ER+)
tumors and who regularly consumed non-steroidal anti-inflammatory
drugs (NSAIDs), presented a 22% reduction in BC risk [relative risk
(RR) of 0.82; 95% confidence interval (CI), 0.75, 0.89]. In the
case of cohort studies, they reported an RR of 0.78 (95% CI,
0.62–0.99), and for case-control studies an RR of 0.87 (95% CI,
0.84–0.91).
More recently, Agrawal and Fentiman (2008) reported
that the use of NSAIDs decreased the risk of developing BC by 20%
(9). The authors proposed that
NSAIDs could be employed as coadjuvant or palliative treatment,
together with hormonal therapies, in women diagnosed with BC.
Nevertheless, a consensus regarding the ideal drug, the dosage, and
administration time has not been reached.
Due to the controversial use of NSAIDs, particularly
aspirin, and the risk of developing BC, in this review we carried
out a descriptive revision of the free access epidemiologic studies
from 2000 to 2012 listed in PubMed, using the following search key
words: ‘aspirin or acetylsalicylic acid and breast cancer’,
‘non-steroidal anti-inflammatory drugs’, ‘clinical studies’ and
‘epidemiological reports’. The main objective of this article was
to analyze whether aspirin reduces the risk of BC in women, and the
optimal conditions required to benefit from the use of this drug.
In order to organize the article, it was divided into three main
sections: i) epidemiologic studies, ii) aspirin as an inhibitor of
metastasis and iii)aspirin, mechanism of action. Table I summarizes the articles consulted
in this review.
 | Table IComparative table of studies
associating aspirin or NSAID use and the risk of breast cancer. |
Table I
Comparative table of studies
associating aspirin or NSAID use and the risk of breast cancer.
| Study | No. of
patients | RR/OR/SIR | 95% CI | Outcome | Authors, year of
publication (ref) |
|---|
| Case-control | 12,174
patients
34,934 control | RR=0.98 | 0.89–1.07 | Effect of NSAIDs on
9 different cancers (gastrointestinal and not intestinal) Reduction
in gastric cancers, 36 months of use previous to diagnosis of the
disease | Langman et
al, 2000 (29) |
| Case-control | 3,133
patients
3,062 control | OR=0.76 | 0.66–0.88 | OR=0.68 (95% CI,
0.54,0.86) for users of NSAIDs longer than 8 years | Cotterchio et
al, 2001 (31) |
| Cohort-study | 27,616
postmenopausal women | RR=0.71 | 0.58–0.87 | Aspirin consumers
at least 6 times/week | Johnson et
al, 2002 (10) |
| RR=1.01 | 0.83–1.25 | NSAID consumers at
least 6 times/week | |
| Cohort-study | 29,470 participants
(both genders)
SIR calculated for both genders | | | 150 mg of aspirin
during 9 years | Friis et al,
2003 (18) |
| SIR=0.9 | 0.7–1.1 | Colon cancer |
| SIR=0.1 | 0.8–1.2 | Rectal cancer |
| SIR=0.9 | 0.8–1.1 | Breast cancer |
| Cohort-study | 80,741
postmenopausal women
1,392 confirmed cases of breast cancer | RR=0.79 | 0.60–1.04 | NSAID regular use
(2 or more tablets/week; 5–9 years) | Harris et
al, 2003 (11) |
| RR=0.72 | 0.56–0.91 | NSAID regular use
(10 or more years) |
| RR=0.51 | 0.28–0.96 | Ibuprofen
(long-term use) |
| RR=0.79 | 0.60–1.03 | Aspirin (long-term
use) |
| Cohort-study | 734,899 women
enrolled
3,708 patients diagnosed with breast cancer | OR=0.77 | 0.62–0.95 | Daily doses of 75
mg of aspirin and 2,000 mg of paracetamol more effective dosages,
compared to non-users
Aspirin (1 year or longer)
Ibuprofen (1 year or longer) | Garcia-Rodriguez
and Gonzalez, Perez, 2004 (32) |
| OR=0.76 | 0.65–0.88 |
| Case-control | 1,442
cases
1,429 control users of aspirin, ibuprofen and acetaminophen | OR=0.8 | 0.66–0.97 | Ever use of aspirin
or other NSAIDs, once/week, 6 months or longer, ever vs.
non-users | Terry et al,
2004 (16) |
| OR=0.72 | 0.58–0.90 | Frequent users
(>7 tablets/week) |
| OR=0.74 | 0.60–0.93 | Aspirin users with
hormone receptor-positive tumors |
| OR=0.97 | 0.67–1.40 | Aspirin users with
hormone receptor-negative tumors |
| Cohort-study | 22,834
individuals | RR=1.07 | 0.84–1.35 | Cancer mortality in
women (aspirin users) | Ratnasinghe et
al, 2004 (12) |
| RR=0.82 | 0.49–1.36 | Breast cancer
mortality (aspirin users) |
| RR=0.90 | 0.73–1.13 | Cancer mortality in
men (aspirin users) |
| RR=0.98 | 0.84–1.14 | Aspirin use and
mortality both genders |
| Case-control | 1,478 breast cancer
patients
3,838 control | OR=0.84 | 0.64–0.97 | Regular use (1
tablet/week) | Swede et al,
2005 (33) |
| OR=0.80 | 0.67–0.96 | Occasional use |
| Cohort-study | 19,934 patients
aspirin 100 mg
19,942 patients aspirin placebo | RR=1.01 | 0.94–1.08 | Total
cancer
Breast cancer (1,230 cases)
No effect of aspirin was observed
in reduction of risk, except lung cancer (n=205 patients;
RR=0.78, 95% CI, 0.59–1.03) | Cook et al,
2005 (20) |
| RR=0.98 | 0.87–1.09 |
| Case-control | 7,006 incidents of
breast cancer | OR=0.78 | 0.63–0.97 | Regular use of
NSAIDs
Regular use of aspirin OR=0.86 | Zhang et al,
2005 (17) |
| Cohort-study | 114,460
patients | RR=0.98 | 0.86–1.13 | Breast cancer risk
for daily vs. no regular use of aspirin | Marshall et
al, 2005 (13) |
| 2,391 diagnosed
with BC | RR=1.09 | 0.97–1.21 | Breast cancer risk
for daily vs. no regular use of NSADs |
| RR=0.80 | 0.62–1.03 | Long term (≥5
years) daily aspirin users for ER/PR-positive BC |
| RR=1.81 | 1.12–2.92 | Long term (≥5
years) daily aspirin users for ER/PR-negative BC |
| Cohort-study | 77,413 patients
3,008 cases of breast cancer | | | No incidence
association was found with the use of NSAIDs or aspirin in either
short- or long-time use (≥5 years) | Jacobs et
al, 2005 (21) |
| RRa=1.05 | 0.88–1.26 | Long-time regular
use of NSAIDs compared with no use (≥30 pills/month; ≥5 years) |
| RRa=0.88 | 0.69–1.12 | Long-time regular
use of aspirin compared with nonuse (≥30 pills/month; ≥5
years) |
| Cohort-study | 22,507
postmenopausal women
3,487 cases of cancer
3,581 deaths | RR=0.84 | 0.77–0.90 | Aspirin users
compared with non-user cancer incidence | Bardia et
al, 2007 (24) |
| RR=0.87 | 0.76–0.99 | Aspirin users
compared with non-user cancer mortality |
| Cohort-study | 98,920 women | | | Multi-ethnic cohort
study (African-American, Caucasian, Japanese-American, Latina and
native Hawaiian women) | Gill et al,
2007 (28) |
| HRR=1.05 | 0.88–1.25 | Current users of
aspirin breast cancer risk ≥6 years of use |
| HRR=1.04 | 0.84–1.27 | Past users of
aspirin breast cancer risk for ≥6 years of use compared with
non-users |
| HRR=0.70 | 0.51–0.95 | Current users of
other NSAIDs was protective for ≥6 years of use |
| HRR=0.90 | 0.62–1.30 | Past users of other
NSAIDs was not protective for ≥6 years of use |
| Cohort-study | 69,810
men
76,303 women at enrollment
10,931 men and
7,196 women developed cancer | RRa=0.84 | 0.76–0.93 | Overall cancer
incidence in men (≥5 years of use) | Jacobs et
al, 2007 (22) |
| RRa=0.86 | 0.73–1.03 | Overall cancer
incidence in women (≥5 years of use) |
| RRa=0.68 | 0.52–0.90 | Colorectal cancer
(both genders); long-term daily aspirin use, lower incidence |
| RRa=0.81 | 0.70–0.94 | Prostate cancer;
long-term daily aspirin use in men |
| RRa=0.83 | 0.63–1.10 | Breast cancer;
long-term daily aspirin use in women
Aspirin 325 mg/day |
| Case-control | 1,527 NHW
cases
1,601 NHW control
798 H/NA cases
924 H/NA control | OR=0.56 | 0.33–0.96 | Recent aspirin use
decreased risk of BC among postmenopause H/NA | Slattery et
al, 2007 (34) |
| Case-control | 3,125 BC cases | OR=0.80 | 0.66–0.88 | NSAIDs, reduced
risk of BC | Kirsh et al,
2007 (15) |
| 0.60–0.84 |
ER+/PR+ |
| OR=0.80 | 0.62–1.03 |
ER−/PR−
Acetylsalicylic acid and nonacetylsalicylic acid use associated
with reduced risks |
| Cohort-study | 28,695
women
847 breast cancer cases | RRa=1.27 | 1.10–1.45 | NSAID users vs.
non-users, increase aspirin-only users, higher BC incidence than
non-aspirin NSAIDs
7.5 years follow-up | Friis et al,
2008 (19) |
| RRa=1.38 | 1.12–1.69 |
| RRa=1.25 | 1.04–1.49 |
| Cohort-study | 35,323
postmenopausal women | HR=0.65 | 0.43–0.97 | Low-dose aspirin,
≥4 days/week, at least 10 years.
Decreased risk of BC vs. no use | Ready et al,
2008 (27) |
| HR=0.78 | 0.61–0.98 | Moderate use of
NSIADs, 10 years, ≥ 3 days/week |
4,164
women
341 BC deaths | | | Aspirin associated
with a decreased risk of BC death | Holmes et
al, 2010 (43) |
| RR=1.07 | 0.70–1.63 | 1 day/week |
| RR=0.29 | 0.16–0.52 | 2–5 days/week |
| RR=0.36 | 0.24–0.54 | 6–7
days/week
Aspirin associated with distant recurrence: |
| RR=0.91 | 0.62–1.33 | 1 day/week |
| RR=0.40 | 0.24–0.65 | 2–5 days/week |
| RR=0.57 | 0.39–0.82 | 6–7 days/week |
2. Epidemiologic studies
In this section, 20 studies are reviewed, 12 of them
are cohort studies and the rest correspond to case-control studies.
Both categories comprise studies in which BC was assessed
exclusively and cancer studies where BC information was included.
These works are presented in a chronological fashion, from 2000 to
2012.
Cohort studies
Johnson and colleagues (2002) (10) carried out a cohort study in the US
in order to evaluate the effect of aspirin (and/or drugs with
aspirin in their formulation), NSAIDs, or drugs used in the
treatment of arthritis, in a population of 27,616 postmenopausal
women. Less than 4% of the women were diagnosed with BC (RR=0.80)
after 6 years of monitoring. In the aspirin group, the
multivariate-adjusted RR for BC was 0.71 (95% CI, 0.58–0.87),
considering those women that consumed aspirin at least 6 times a
week and independent of the BC stage. In comparison, for the rest
of the NSAIDs under the same conditions, the adjusted RR value was
1.01 (95% CI, 0.83–1.25). One year afterwards, the Women Health
Initiative (WHI) completed a study including 80,741 postmenopausal
women (50–79 years of age) during 10 years (11). During this period, less than 2% of
the participants were diagnosed with BC. Their results showed that
the regular use of NSAIDs (for more than 5 years but less than 10
years), taking two or more tablets per week (doses over 100 mg, no
further specification was given) reduced the incidence of BC by 21%
(RR=0.79; 95% CI, 0.60–1.04). An increment of 7 percentage units
was found in those women that had consumed NSAIDs for over 10 years
(RR=0.72; 95% CI, 0.56–0.91). Aspirin reduced by 21% the risk of BC
at doses higher than 100 mg/day; in comparison, ibuprofen was more
effective diminishing the risk by 49%. Importantly, they found that
NSAIDs had a major impact in women with characteristics such as
high mass index, lack of exercise, late pregnancy, family history
of BC and/or hormonal therapy. The authors did not mention whether
the cancer cases were diagnosed in situ or whether they were
invasive.
In another study carried out in the US, researchers
found a protective effect of aspirin against cancer, decreasing
mortality rates in both genders (12). The databases consulted were from the
First and Second National Health and Nutrition Examination Study
(NHANES I and II; 1971–1975 and 1976–1980, respectively) (12). However, the level of protection
depended on the type of neoplasia, gender and patient age. The
results showed a reduction in the mortality rate in men and women
that were aspirin users (RR=0.88, 95% CI, 0.85–0.99). For women
with breast and ovarian cancer, they found reduced mortality in
those patients that used aspirin (RR=0.82, 95% CI, 0.49–1.36). In
men, the use of aspirin reduced mortality by lung cancer (RR=0.69,
95% CI, 0.49–0.96). Nevertheless, they observed that when aspirin
was ingested daily for a period longer than 5 years, the risk to
develop bladder and central nervous system cancer was
increased.
In California, US, an evaluation of BC risk and the
use of NSAIDs was carried out in a population of 114,640 educators
(13). Data were obtained from the
California Teachers Study Cohort at baseline from 1995 to 1996, in
a region with a high prevalence of BC (13). At the beginning of the poll, the
participants were free of cancer. Six years later, 2,391 women were
diagnosed with BC and were classified into two different groups: i)
localized BC and ii) non-localized BC, including hormonal
receptor-positive and -negative cancer. The tested drugs were
aspirin, ibuprofen and acetaminophen. The last drug was included
for comparative purposes, even though it is prescribed for pain
relief. As a result of this cohort study, the researchers found no
association in BC risk between daily and no regular use of aspirin
and ibuprofen in combination with aspirin (RR=1.09, 95%
CI=0.97–1.21 for NSAIDs; and RR=0.98, 95% CI=0.86–1.13 for
aspirin); similar results to those previously reported for aspirin
in 1996 (14). Concerning the
hormone receptor groups, no statistically significant difference
was observed for those patients with ER+/progesterone
receptor (PR)+ breast cancer that were long-term aspirin
users (RR=0.80, 95% CI, 0.62–1.03). Meanwhile, in the
ER−/PR− BC group, the daily use of aspirin
for longer than 5 years increased the risk of BC (RR=1.81, 95%
CI=1.12–2.92). The same result was presented for patients with
long-term daily use of ibuprofen; it was associated with an
increased risk of BC (RR=1.51, 95% CI=1.17–1.95). Nevertheless, the
authors could not validate their results, arguing that it could be
a casualty.
Regarding the hormone receptor status, Kirsh and
colleagues evaluated the effect of NSAIDs and aspirin, considering
breast cancer risk according to ER+ or ER−
status, PR+ or PR− status, smoking habit and
arthritis, in women with incident BC (controls were randomly
selected women) (15). The
principal inclusion criteria for all participants were the daily
use of drugs (for at least 2 months), as well as a dose of 325 mg
for the majority of aspirin users (78% of users). Users of NSAIDs
presented a reduced risk of BC (OR, 0.76; 95% CI, 0.66–0.88);
similar for ER+/PR+ (OR, 0.71; 95% CI,
0.60–0.84) and for ER−/PR− (OR, 0.80; 95% CI,
0.62–1.03). The authors concluded that NSAIDs are more effective
for diminishing the BC risk when they are consumed for more than 7
years, regardless of whether the drug contains acetylsalicylic acid
or not.
Terry and colleagues (2004) evaluated patients with
hormone receptor-positive or -negative BC tumors who used NSAIDs
and the risk of cancer vs. a control population free of disease; no
age restriction was considered in this study (16). The research group found that
consuming aspirin once a week for at least 6 months corresponded to
an inverse association with BC risk (OR=0.80, 95% CI, 0.66–0.97)
with respect to never-users. On the other hand, for ibuprofen users
under the same conditions, no association with BC risk vs.
never-users was found (OR=0.91, 95% CI, 0.71–1.16). Not
surprisingly, acetaminophen was not associated with BC risk
(OR=1.02, 95% CI 0.80–1.31; users vs. never-users). However, a
reduction in risk was observed for users of aspirin with hormone
receptor-positive BC tumors (OR=0.74, 95% CI, 0.60–0.93), vs.
patients with hormone receptor-negative tumors (OR=0.97, 95% CI,
0.67–1.40). Aspirin decreased the BC risk, particularly in
postmenopausal women for each experimental subgroup studied
(ER+/PR+, ER+/PR−,
ER−/PR+), except in patients with tumors
negative for ER and PR, thus suggesting that it could have a
chemopreventive effect in specific BC subtypes. One year
afterwards, the same research group published an update of the
aforementioned investigation, including 444 new cases, for a total
of 7,006 patients with BC (17).
The authors continued their investigation regarding the association
between the regular use of NSAIDs and the risk of BC depending on
hormone receptor status of the tumor. Their findings showed that
the long-term use of NSAIDs diminished the risk of BC, independent
of the drug and the hormone receptor status of the tumor (OR=0.78,
95% CI, 0.63–0.97 for regular use of NSAIDs; OR=0.86 for aspirin
and OR=0.85 for ibuprofen).
A cohort study in Denmark with 29,470 individuals,
compared cancer incidence after exposure to aspirin at three
concentrations (75, 100 and 150 mg), against the expected cancer
rates obtained from the country (18). During the 9 years of the study,
2,381 individuals developed cancer vs. the 2,187 expected
[standardized incidence ratio (SIR)=1.09, 95% CI, 1.05–1.13].
Regarding BC, only 148 cases were registered in women and one in
men (SIR=0.9, 95% CI, 0.8–1.1; SIR=0.6, 95% CI, 0.0–3.2,
respectively) (18). Even though
their work had several strengths, there was not enough evidence to
support the use of low-dose aspirin as a chemopreventive agent in
cancer. Harris and colleagues reported similar findings in the same
year for aspirin at a dose of 150 mg; they did not differ in
regards to the observed risk (11).
In 2008, a Danish research group published another
work in which participants were selected from the database of the
Danish Diet, Cancer and Health Cohort (1993–1997) (19). A group of 28,695 Danish women
between 50 and 64 years of age completed a questionnaire. In this
study, other aspects related to risk were evaluated, such as
frequency and duration of use, in addition to the presence of
hormonal receptors. At the end of the survey (7.5 years), only 847
women were diagnosed with BC. Nevertheless, their work disclosed
that the use of NSAIDs, including aspirin, did not diminish the
risk of BC under any of the conditions mentioned before. On the
contrary, an increase in breast cancer incidence was found in NSAID
users compared to non-users (RR=1.27, 95% CI, 1.10–1.45). In the
case of aspirin users, an increment of BC incidence was noted
(RR=1.38, 95% CI, 1.12–1.69) compared to NSAID users (not aspirin
containing) (RR=1.25, 95% CI, 1.04–1.49).
Cook et al published similar results in 2005
(20). In this cohort study 39,876
healthy women participated and were randomly assigned to two
groups: aspirin and aspirin placebo. Aspirin treatment consisted of
a low concentration (100 mg), administered every other day for 10
years. For any cancer type (except non-melanoma skin cancer), the
dose was not effective and no reduction in cancer risk was observed
(n=2,865, RR=1.01, 95% CI, 0.94–1.08, P=0.87); in particular for
BC, the values for a sample of 1,230 patients were RR=0.98 (95% CI,
0.87–1.09, P=0.68). Such a result could be related to the aspirin
concentration used, since it has been observed that low doses of
aspirin do not inhibit COX-2. In order to have a positive effect,
aspirin must be ingested for longer periods. Likewise, the placebo
group exhibited a similar behavior as the aspirin group.
In the same year, 2005, another research group found
similar outcomes with almost twice as many patients (77,413 women)
who were followed up for close to 10 years (21). Participants completed questionnaires
from the Cancer Prevention Study II Nutrition Cohort (enrollments
in 1992 or 1993; updates in 1997 and 1999). As in previous cohort
study results, there was no direct association between current use
of NSAIDs, with an average intake of 60 pills or more per month and
BC incidence (NSAID users: RR=1.07, 95% CI, 0.96–1.21 as compared
to NSAID non-users; aspirin users: RR=1.01, 95% CI, 0.84–1.20 as
compared to non-users). Moreover, for those patients that reported
a consumption of ≥30 pills per month for at least 5 years, the
incidence of BC was not significant (RR=1.05, 95% CI, 0.88–1.26 for
total NSAIDs; RR=0.88, 95% CI, 0.69–1.12 for aspirin).
Two years later, in 2007, with information obtained
from the Cancer Prevention Study II Nutrition Cohort in the US,
aspirin was evaluated in regards to long-term daily use and the
cancer incidence at a dose of 325 mg/day in 10 different cancer
types, including BC (22).
Participants were diagnosed with cancer at the beginning of the
enrollment in 1992–1993 and during follow-up until 2003. By 2003,
10,931 men and 7,196 women were diagnosed with a neoplasm. The
incidence of cancer risk for men was associated with the use of
aspirin for 5 years or longer. For women, on the contrary, the
statistical difference was not significant, neither in general
cancer nor in BC risk (BC: RR=0.83, 95% CI=0.63–1.10). In this
study, it was demonstrated that high concentrations of aspirin were
not associated with cancer risk, while previous research reported
low doses and short-term aspirin administration (18,23).
In another cohort study carried out in 2007, which
also evaluated aspirin and BC risk, the researchers focused on
women smokers (22,507 patients) between 55 and 69 years of age
(24). Notably, they found that the
group of active smokers and aspirin consumers exhibited a reduction
in cancer risk, differing with non-smoker or ex-smoker patients.
This result could be attributed to the fact that smoking reduces
the expression of COX-2 (25,26).
However, in a subsequent study, no relationship between BC risk and
smoking status was noted (26).
Finally, Bardia and colleagues (24) postulated that the use of aspirin
could prevent the incidence of cancer by 4.7%, and mortality by 3.5
and 7.6% in coronary diseases. Even though the results were
promising, the research had various weaknesses such as the lack of
dose data, duration of treatment, and the criteria used for
population sample selection (the research was carried out in
postmenopausal and Caucasian women).
Another prospective study evaluated 35,323 women,
who were enrolled in the Vitamins and Lifestyle study (VITAL,
2008), where information regarding the use of NSAIDs, lifestyle and
BC risk factors was required (27).
Participants that used a low dose of aspirin 4 times/week or more,
for an average of 10 years, showed a decrease in BC risk (HR=0.65,
95% CI, 0.43–0.97) vs. non-users. Interestingly, frequent use and a
high dose of aspirin was associated with an increase in BC risk
(HR=1.26, 95% CI, 0.96–1.65). This study found a protective effect
of NSAIDs for long-term users of low doses or moderate frequency of
high doses, contrary to frequent users of high dose that showed an
increased risk.
Gill and colleagues (28) published a cohort study in 2007
integrating three relevant facts associated with BC risk in women:
hormone receptor status of BC, ethnicity and use of NSAIDs
(aspirin, acetaminophen, ibuprofen, naproxen, and indomethacin).
Mainly African-Americans, Caucasians, Japanese-Americans, Latinas
and native Hawaiians, residents of Hawaii and California formed the
multiethnic cohort even though other ethnicities were also
documented. Data were collected between 1993 and 1996 by
self-administered mail questionnaire; 98,920 women participated in
this study. The results showed that aspirin was not associated with
BC risk either for current or past users (HRR hazard rate ratio
=1.05, 95% CI, 0.88–1.25; HHR=1.04, 95% CI, 0.84–1.27 for ≥6 years
of use; respectively, compared with non-users); while, NSAIDs
(other than aspirin) presented a protective action against the risk
of BC in current users (HRR=0.70, 95% CI, 0.51–0.95, ≥6 years of
use). Regarding ethnicity and hormone receptor status, the
protective effect of NSAIDs (long-term use of NSAIDs other than
aspirin) was found in Caucasian and African-American women, and in
women with at least one positive hormone receptor. In conclusion,
the results suggested a protective effect of NSAIDs against BC risk
associated with time, ethnic group and hormone receptor status. The
use of NSAIDs for longer than 6 years decreased the risk by
30%.
In summary, according to the aforementioned studies,
there is not enough evidence yet to support aspirin as a possible
drug to lower BC risk. On one hand, some of the studies suggest
that aspirin could be a preventive treatment over long periods of
ingestion (longer than 3 years). However, the main problem with
long periods of aspirin consumption is its adverse reactions, such
as peptic ulcers and gastrointestinal hemorrhage. These secondary
effects are important to consider by physicians before prescribing
to women with the possibility to develop BC.
Case-control studies
In 2000, a study was published in the UK with data
collected from 1993 to 1995 (29).
In this study 12,174 recruited patients were divided into two large
groups: i) gastrointestinal cancer (esophagus, stomach, pancreas,
colon and rectum) and ii) no gastrointestinal cancer (bladder,
breast, lung and prostate); negative controls consisted of healthy
patients (34,934 individuals) (29). The effect of aspirin and other
NSAIDs was evaluated after 36 months of regular use. No important
statistical difference was observed for either group (OR=0.98, 95%
CI, 0.89–1.07, for patients that received at least 7 prescriptions
in the 13–36 months before cancer diagnosis). Similar results were
obtained by a research group at Duke University, in North Carolina,
US, who carried out a case-control study between 1996 and 2000.
Their results showed an inverse relationship between invasive BC
risk and the use of NSAIDs (OR=0.4, 95% CI, 0.3–0.6) (30). Contrary to the other two reports,
the findings of a study carried out in 2001 in Canada (3,133
patients and 3,062 controls), showed a benefit to women who had
ingested NSAIDs (31). A 24%
reduction in BC risk was observed (OR=0.76, 95% CI, 0.66–0.88),
when NSAID intake was daily and for at least 2 months; for those
patients with longer periods of intake (more than 8 years) they
exhibited improvement (OR=0.68, 95% CI=0.54–0.86).
In the effort to determine an optimal dose
administration of aspirin, García-Rodríguez et al studied
3,708 patients who were divided into three experimental groups
according to aspirin concentration: 75, 150 or 300 mg (32). In this case-control study, the lower
dose was more effective (the relative risk decreased 33%) in
comparison to the higher doses, where no significant differences
were obtained (aspirin RR=0.77; NSAIDs (non-aspirin) RR=1.00;
acetaminophen RR=0.76). Moreover, they reported that there was no
correlation between time of intake and benefit (32).
The majority of the epidemiologic studies performed
with aspirin are focused on dose-effect; few studies have consider
time as a key factor in the chemopreventive effect of this
medicine. However, Swede and colleagues reinvestigated this issue,
testing aspirin with 1,478 patients diagnosed with BC and 3,383
free of disease. The authors found that time was an important
variable in BC risk. Patients that consumed aspirin once a week for
1 year reported an RR of 0.84; and for those women consuming
aspirin daily for 10 years or more the RR value was 0.74 (33).
A more specific study was performed evaluating
genetic variation in the interleukin-6 (IL-6) gene, aspirin and BC
risk in the southeast region of the US (34). Four experimental groups were
included depending on their ethnic origin (non-Hispanic Caucasian
women, native Hispanic-American women with BC, and the respective
negative controls for each group). In the group of
Hispanic-American women, factors including postmenopausal status,
free of hormone use before the trial, and recent use of aspirin,
significantly decreased the BC risk (OR=0.56, 95% CI, 0.33–0.96).
The BC risk was not significant in the group of non-Hispanic
Caucasian women. Furthermore, the genotype and the haplotype of the
IL-6 gene modified the association between aspirin and BC
significantly. Women that were not exposed to hormones presented a
major benefit (P=0.06 for non-Hispanic Caucasian women, and 0.04
for native Hispanic-American women). According to their findings,
IL-6 may interfere either in inflammation or estrogen pathways, due
to its interactions with aspirin and the different associations of
exposure to hormones of postmenopausal women. Furthermore, their
data suggest that when estrogens are not present, the alleles
associated with low IL-6 production levels and inflammation have a
protective function. In addition, estrogen itself can modify levels
of IL-6; thus the effect can only be observed in the absence of
estrogens. Therefore, it can be concluded that IL-6 could amend the
relationship among estrogen, aspirin and BC risk.
3. Aspirin as an inhibitor of
metastasis
Aspirin has not only been studied as a
chemopreventive drug, but also as an inhibitor of metastasis. It is
known that patients with BC have a 50% likelihood to develop
metastasis after treatment (chemotherapy, radiation and/or
surgery). The maspin protein (mammary serpin) and its mRNA are
highly produced in normal breast epithelial cells (35), whereas their expression levels
diminish in the presence of BC (36). Maspin protein is a tumor-suppressor
serine protease, which is expressed in several tissues including
mammary epithelia, prostate, epidermis, lung, and in the stromal
cells of the cornea (35,36,61,62).
This protein is of special interest as it is able to inhibit
cellular invasion and metastasis and to act as a tumor suppressor
(37,60).
Girish and colleagues proposed that aspirin corrects
the production levels of maspin in human breast cells in
vitro and in patients, regulating maspin to normal levels
through the stimulation of nitric oxide (NO) synthesis independent
of the insulin receptor (37). An
epidemiologic example of this previously mentioned concept, is a
study carried out in India, where the effect of aspirin and the
incidence of metastasis in BC patients was determined (38). In this work, 35 women participated,
and the age range was from 41 to 64 years. All of the women were
diagnosed with BC and had been previously treated (chemotherapy,
radiation and/or surgery). The patients consumed a dose of aspirin
of 75 mg/70 kg body weight daily for 3 years. During this period of
time, plasmatic levels of NO and maspin were measured, and a
monthly surveillance of metastasis was performed by an oncologist
and occasionally by biopsy. The control group consisted of 35
healthy volunteers whose plasma maspin levels were measured. The
results showed that maspin levels were higher after 24 h of aspirin
administration; such levels were maintained during the 3 years of
treatment (initially maspin levels were 0.95±0.04 nM increasing to
4.63±0.05 nM at the end of treatment). Six patients developed
metastasis, and the rest of the patients were, apparently, free of
metastasis after 3 years. Researchers suggest that daily intake of
aspirin in patients with previously treated BC may reduce the
incidence of metastasis independent of the disease stage.
Recently, a large study performed in the UK with
17,285 patients was carried out in order to elucidate the
preventive effect of aspirin on distant metastasis comparing
adenocarcinomas vs. other cancer types (39). Patients were divided into four
groups according to the metastasis target: i) metastasis to distant
tissues (secondary tumors, particularly to the liver, lung, bone,
brain or other tissues distant to the primary tumor); ii)
metastasis to any specific region; iii) local invasion; and iv)
patients presenting rapid progression of disease and not enough
clinical information recovered. After data analysis, the authors
concluded that aspirin may help reduce the risk of distant
metastasis in certain types of cancers from 30 to 40%, and in 50%
of metastatic adenocarcinoma, using a low concentration (≤5 mg
daily) and long delivery formulation. Additionally, it was observed
that aspirin had a major impact on individuals with
adenocarcinomas, particularly in those cases where curative surgery
was performed (as in BC). The authors proposed that the
anti-metastatic activity of aspirin is mediated by platelets, based
on the knowledge that they participate in cancer development and
metastasis (40), protecting
tumoral cells during their travel through the bloodstream.
Moreover, platelets activate a coagulation system allowing
microthrombosis formation, thus facilitating the hosting of tumoral
cells in target tissues (41). In
addition, it is known that thrombocytosis is a common disease in
many cancer types and it is used as a poor prognostic indicator
(42).
Regarding aspirin and breast cancer metastasis, a
research group at Brigham and Women’s Hospital together with the
Harvard Medical School, performed a prospective observation study
in order to evaluate whether aspirin could decrease the risk of
death from BC (43). The study
consisted of 4,164 women (enrolled between 1976 and 2002; followed
up until death or June 2006). According to their results, the use
of aspirin reduced the risk of metastasis and BC death. The
adjusted relative risks (stage of cancer, menopausal status, BMI,
hormone receptor status) for distant recurrence were: for 1
day/week, RR=0.91 (95% CI, 0.62–1.33); 2–5 days/week, RR=0.40 (95%
CI, 0.24–0.65); and for 6–7 days/week, RR=0.57 (95% CI,
0.39–0.82).
Evidence to date regarding the use of aspirin as an
inhibitor of metastasis in patients diagnosed with cancer, suggests
that aspirin delays the invasion of cancer cells to other tissues
and in some cases prevents metastasis formation. The information
indicates that regular consumption of aspirin and long periods of
administration are required for a positive result.
4. Aspirin, mechanisms of action
NSAIDs can be classified depending on their
mechanisms of action on the COX enzyme: classical NSAIDs (including
aspirin) and COX-2 inhibitors (celecoxib and rofecoxib are mainly
used). The molecular mechanism by which aspirin and other classical
NSAIDs are capable of inhibiting COX-1 and COX-2, is by competing
with arachidonic acid for binding to the active site of
cyclooxygenase; this is a rapid and an irreversible union, followed
by the covalent acetylation of serine 530 in COX-1, hampering the
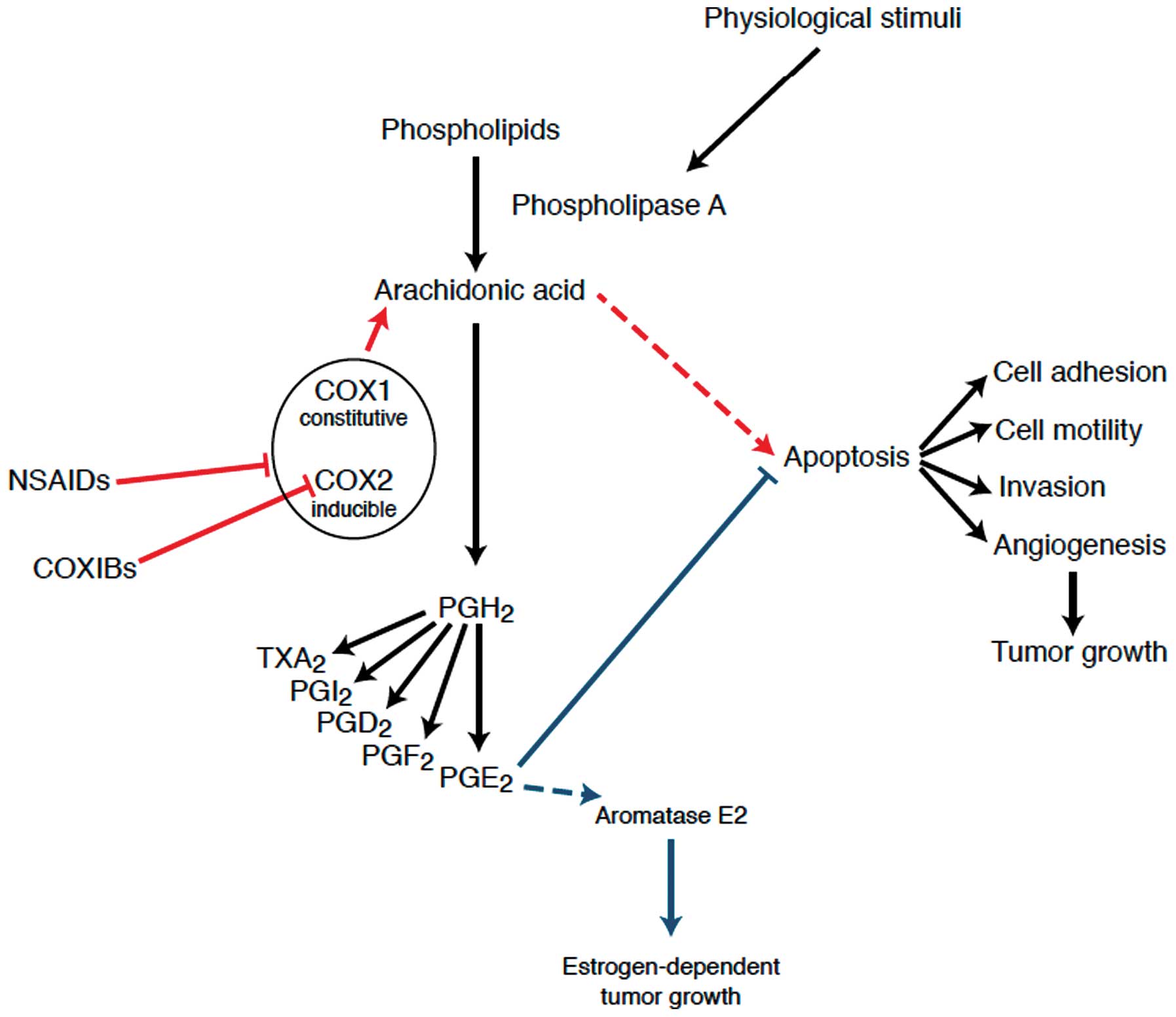
oxidation of arachidonic acid (Fig.
1) (44). After the same
modification, COX-2 can still convert arachidonic acid into
15R-hydroxyeicosatertraenoic acid (HETE), instead of PGG2 (45). On the other hand, it is believed
that specific inhibitors of COX-2 bind to valine 523, whereas in
COX-1 access to this binding site is blocked by the isoleucine
residue at the same position (46).
This difference explains the different degrees of inhibition
towards COX-1 and COX-2 that aspirin possesses.
As known, there are two major isoforms of the COX
enzyme, COX-1 that is expressed constitutively, and COX-2, which is
induced locally as part of an inflammatory cascade and during early
tumor development (46,47). COX-1 performs positive homeostatic
functions. It is antithrombotic when released to vascular
endothelium and is cytoprotective when produced by the gastric
mucous. Moreover, it provokes platelet aggregation and prevents
inappropriate bleeding when it is thromboxane A2-dependent
(48). On the other hand, COX-2, in
addition to its action in tissular damage response, could be
constitutively expressed in the brain, kidney, cells of the
pancreatic islet, ovary, gestational uterus and intestine (49,50).
COX-2 is present in several cancer cell lines and is implicated in
carcinogenesis, tumor growth, apoptosis and angiogenesis (51,52).
As these isoforms are encoded in independent genes with different
chromosomal localizations, their expression and regulation patterns
differ. More recently, a third isoform, named COX-3, was identified
as a COX-1 splice variant that may play an important role in fever
and pain processes (53).
These enzymes are responsible for catalyzing the
conversion of arachidonic acid to prostaglandin endoperoxide
(prostaglandin H2) and thromboxanes; both COX-1 and COX-2
participate in maintaining physiological regulation in the stomach,
platelets, kidneys and intestine (46). The principal side effects of aspirin
and NSAIDs such as gastropathy, renal insufficiency and impaired
vascular homeostasis among others, are due to a reduction in the
appropriate prostaglandins in these organs (46). Prostaglandins are short-lived
compounds acting as local mediators of continuous importance in
normal cellular reactions, but they appear to be increased in
several pathological conditions, particularly inflammation
(46), and it has also been shown
that the level of prostaglandins in various tumors is greater than
that of normal tissues (54).
Reports from the past 15 years, have shown that
COX-1 is localized in stromal cells adjacent to the tumor but not
in tumor cells. In contrast, high levels of COX-2 are localized
primarily in tumor cells but also appear in stromal cells (54). Such is the case of certain malignant
human breast tumors that produce more prostaglandin-like material
than do benign tumors or normal breast tissues (54).
Additional experimental data suggest that COX-2 can
be induced by a mutation of the tumor-suppressor gene APC,
subsequently increasing the expression of the nuclear transcription
factor PPAR-δ (55). Induction of
COX-2 expression in tumors can also occur by lipopolysaccharide
through the mitogen-activated protein kinase (MAPK) and protein
kinase C-(PKC) pathways (56).
Ceramide-stimulated activation of MAPK can also activate c-Jun
N-terminal kinase (JNK), which in turn can lead to increased COX-2
gene expression in human mammary epithelial cells (57). In breast tissue, induction of COX-2
expression can trigger prostaglandin production, which indirectly
stimulates cellular proliferation increasing the local biosynthesis
of estrogen and increasing the expression of the aromatase gene
(8,58). In humans, high levels of
prostaglandins have been related with metastatic potential and
reduced survival of patients (22).
Such a process could be reversible when COX-2 is inhibited using
NSAIDs with the purpose of reducing prostaglandin synthesis. For
those women who present with tumors with positive hormone
receptors, the use of aspirin could contribute to the suppression
of aromatase activity, reducing the intra-mammary prostaglandin
production, and thus, estrogen production (59). In particular, it has been shown that
E2 prostaglandin (PGE2) stimulates the transcription of
aromatase, increasing the estrogen levels and, moreover,
contributing to the progression of estrogen-dependent BC (60).
The induction and overexpression of COX-2 and its
main product PGE2 in human mammary tissue, have also
been associated with aromatase-catalyzed estrogen biosynthesis
(61). In addition, some
epidemiologic studies have reported that hormone-receptor-positive
breast tumors are more responsive to aspirin (62). The ability of aspirin and other
NSAIDs to protect against breast cancer might vary according to
hormone receptor status. For instance, in 2004, a case-control
study, reported a reduction in risk only in women with hormone
receptor-positive tumors, but not in women with hormone
receptor-negative tumors (9).
Moreover, COX-2 is related to the activation of carcinogens,
mutagenesis, angiogenesis, inhibition of apoptosis and metastasis
(53,63,64).
In contrast, COX-2 inhibition may reverse these processes; aspirin
and salicylates may also suppress NF-κB-related survival signaling
by inhibiting IKKα activation, leading to apoptosis (45).
Molecular studies have also revealed a strong
correlation between overexpression of COX-2 and other oncogenes,
such as HER-2/Neu, in malignant breast tumors (65,66). A
linkage between the expression of COX-2 and MDR1/Pgp 170 was shown
by immunohistochemical analyses in human breast tumor specimens,
and it is well known that overexpression of P-glycoprotein
contributes to primary chemotherapy resistance (66,67).
Regarding the possible anti-metastatic effect of
aspirin, it was demonstrated that the daily ingestion of aspirin in
patients previously treated with standard therapies indeed reduces
metastasis, assigning this effect to the maspin protein (38). Maspin is a serine protease inhibitor
of 42 kDa synthesized abundantly in normal mammary epithelial
cells, and it has been shown that the expression of this protein is
reduced in patients with breast cancer. Thus, the lack of
expression of maspin in breast cancer has been suggested to
indicate the presence of an aggressive and metastatic tumor
(36). Studies have revealed that
ingestion of aspirin increases the levels of serum nitric oxide
(NO) and maspin, both of which inhibited the growth of breast
cancer cells in vitro, as well as invasion and metastatic
processes in an animal model (9,38).
This finding suggests that aspirin participates in the restoration
of maspin synthesis at any stage of the disease, and also that
maspin presents beneficial effects at any stage of tumoral
progression. It has been observed that maspin is reduced in BC, or
even absent in invasive cancer; hence, there is a correlation
between the protein synthesis and its reduction according to cancer
type and stage (67). There is also
evidence of maspin as an inhibitor of angiogenesis (68).
On the other hand, in 2008, Burnett and
collaborators investigated the relationship between inflammation
and angiogenesis and how it is regulated by aspirin. They proposed
that aspirin alters macrophage regulation by inducing expression of
IL-10 in MCF-7 cells, and suggested aspirin as a therapeutic
strategy for modification of tumor-associated macrophages (TAMs),
which can initiate both angiogenesis and invasion (69).
Finally, regarding the mechanisms of aspirin as an
antitumor drug (70), Spitz et
al discuss the use of acetylsalicylic acid (ASA) and salicylic
acid (SA) in tumor cell viability and glucose metabolism. Both
drugs modulate an important glycolysis-regulatory intracellular
enzyme (6-phosphofructo-1-kinase, PFK) in a dose-dependent manner,
and the inhibition occurs due to the modulation of the enzyme
quaternary structure. The authors demonstrated that ASA, as well as
its precursor SA, decreased the viability of the human breast tumor
cell line MCF-7, diminishing its glucose consumption and PFK
activity.
5. Conclusions
One of the most significant discoveries along the
quest for novel cancer treatments and preventive therapies has been
the COX-2 inhibitors. These drugs have been demonstrated to
interfere directly with the carcinogenesis process, restoring
apoptosis. The most representative medicine of the last 100 years
is probably aspirin, a drug that is still currently under
investigation. The COX-2 gene is overexpressed in breast cancer;
furthermore, the presence of the COX-2 enzyme in tumoral tissue is
directly proportional to the density of cancer cells. Moreover,
some studies have revealed that high levels of prostaglandins are
linked to unfavorable patient characteristics, such as the risk to
develop metastasis and the reduction in survival rate, being good
indicators for the correct diagnosis of disease stage.
In respect to the epidemiologic studies reported
here, it is of great importance to homologate criteria in this type
of research, for example, the time of administration of the drug,
in order to truly assess the chemopreventive and anti-metastatic
effect of aspirin. This issue should be considered because of the
great variability existing in studies.
Evidence of the chemopreventive effect of aspirin
cited in this review, suggests that it is likely to lower BC risk
when administrated at doses greater than 100 mg/day for 3 or more
years. Such information suggests that aspirin may start being
favorable after a long period of regular use. However, this dosage
poses adverse reactions, such as, an increase in gastric ulcer risk
and gastrointestinal bleeding. Cancer is a multifactorial disease,
and its behavior is constantly being investigated toward a greater
understand and, at the same time, to propose less aggressive and
more effective therapies or, when possible, chemopreventive
alternatives. Aspirin could yield beneficial results for a specific
group of risk patients, but who should be monitored carefully if
there are any contraindications to the medicine. In conclusion,
aspirin is a suitable alternative in this non-stop search for
cancer prevention and treatment.
Acknowledgements
We express our gratitude to Dr Alberto Huberman from
INCMNSZ for improving this manuscript. Elizabeth Camacho Zavala
thanks Posgrado en Ciencias Biológicas, UNAM and to CONACyT for
scholarship support (no. 412740).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chávarri-Guerra Y, Villarreal-Garza C,
Liedke PE, et al: Breast cancer in Mexico: a growing challenge to
health and the health system. Lancet Oncol. 13:e335–e343.
2012.PubMed/NCBI
|
|
3
|
Rodríguez-Cuevas SA and Capurso-García M:
Epidemiology of breast cancer. Ginecol Obstet Mex. 74:585–593.
2006.(In Spanish).
|
|
4
|
Zentella-Dehesa A, Frías S,
Galicia-Vázquez G, et al: Cáncer de glándula mamaria y metástasis:
un creciente problema de salud pública en México. Mensaje
Bioquímico. Oria Hernández J, Rendón Huerta E, Reyes Vivas H,
Romero Álvarez I and Velázquez López I: Mexico: UNAM; XXXI. pp.
172–195. 2007
|
|
5
|
Spallarossa P, Altieri P, Aloi C, et al:
Doxorubicin induces senescence or apoptosis in rat neonatal
cardiomyocytes by regulating the expression levels of the telomere
binding factors 1 and 2. Am J Physiol Heart Circ Physiol.
297:H2169–H2181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Elliott P: Pathogenesis of cardiotoxicity
induced by anthracyclines. Semin Oncol. 33:S2–S7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rice J: Metastasis: The rude awakening.
Nature. 485:S55–S57. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khuder SA and Mutgi AB: Breast cancer and
NSAID use: a meta-analysis. Br J Cancer. 84:1188–1192. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agrawal A and Fentiman IS: NSAIDs and
breast cancer: a possible prevention and treatment strategy. Int J
Clin Pract. 62:444–449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnson TW, Anderson KE, Lazovich D and
Folsom AR: Association of aspirin and nonsteroidal
anti-inflammatory drugs use with breast cancer. Cancer Epidemiol
Biomarkers Prev. 11:1586–1592. 2002.PubMed/NCBI
|
|
11
|
Harris RE, Chlebowski RT, Jackson RD, et
al: Breast cancer and nonsteroidal anti-inflammatory drugs:
prospective results from the Women’s Health Initiative. Cancer Res.
63:6096–6101. 2003.
|
|
12
|
Ratnasinghe LD, Graubard BI, Kahle L,
Tangrea JA, Taylor PR and Hawk E: Aspirin use and mortality from
cancer in a prospective cohort study. Anticancer Res. 24:3177–3184.
2004.PubMed/NCBI
|
|
13
|
Marshall SF, Bernstein L, Anton-Culver H,
et al: Nonsteroidal anti-inflammatory drugs use and breast cancer
risk by stage and hormone receptor status. J Natl Cancer Inst.
97:805–812. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Egan KM, Stampfer MJ, Giovannucci E,
Rosner BA and Colditz GA: Prospective study of regular aspirin use
and the risk of breast cancer. J Natl Cancer Inst. 88:988–993.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kirsh VA, Kreiger N, Cotterchio M, Sloan M
and Theis B: Nonsteroidal anti-inflammatory drug use and breast
cancer risk: subgroup findings. Am J Epidemiol. 166:709–716. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Terry MB, Gammon MD, Zhang FF, et al:
Association of frequency and duration of aspirin use and hormone
receptor status with breast cancer risk. JAMA. 291:2433–2440. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Coogan PF, Palmer JR, Strom BL
and Rosenberg L: Use of nonsteroidal anti-inflammatory drugs and
risk of breast cancer: the case-control surveillance study
revisited. Am J Epidemiol. 162:165–170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Friis S, Sørensen HT, McLaughlin JK,
Johnsen SP, Blot WJ and Olsen JH: A population-based cohort study
of the risk of colorectal and other cancers among users of low-dose
aspirin. Br J Cancer. 88:684–688. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Friis S, Thomassen L, Sørensen HT, et al:
Nonsteroidal anti-inflammatory drug use and breast cancer risk: a
Danish cohort study. Eur J Cancer Prev. 17:88–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cook NR, Lee IM, Gaziano JM, et al:
Low-dose aspirin in the primary prevention of cancer. The Women’s
Health Study: a randomized controlled trial. JAMA. 294:47–55.
2005.
|
|
21
|
Jacobs EJ, Thun MJ, Connell CJ, et al:
Aspirin and other nonsteroidal anti-inflammatory drugs and breast
cancer incidence in a large U.S. cohort. Cancer Epidem Biomarkers
Prev. 14:261–264. 2005.PubMed/NCBI
|
|
22
|
Jacobs EJ, Thun MJ, Bain EB, Rodriguez C,
Henley SJ and Calle EE: A large cohort study of long-term daily use
of adult strength aspirin and cancer incidence. J Nat Cancer Inst.
99:608–615. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schreinemachers DM and Everson RB: Aspirin
use and lung, colon, and breast cancer incidence in a prospective
study. Epidemiology. 5:138–146. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bardia A, Ebbert JO, Vierkant RB, et al:
Association of aspirin and non-aspirin non-steroidal
anti-inflammatory drugs with cancer incidence and mortality. J Natl
Cancer Inst. 99:881–889. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Badawi AF, Habib SL, Mohammed MA, Abadi AA
and Michael MS: Influence of cigarette smoking on prostaglandin
synthesis and cyclooxygenase-2 gene expression in human urinary
bladder cancer. Cancer Invest. 20:651–656. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martey CA, Pollock SJ, Turner CK, et al:
Cigarette smoke induces cyclooxygenase-2 and microsomal
prostaglandin E2 synthase in human lung fibroblast: implications
for lung inflammation and cancer. Am J Physiol Lung Cell Mol
Physiol. 287:L981–L991. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ready A, Velicer CM, McTiernan A and White
E: NSAID use and breast cancer risk in the VITAL cohort. Breast
Cancer Res Treat. 109:533–543. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gill JK, Maskarinec G, Wilkens LR, Pike
MC, Henderson BE and Kolonel LN: Nonsteroidal anti-inflammatory
drugs and breast cancer risk: the multiethnic cohort. Am J
Epidemol. 166:1150–1158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Langman MJS, Cheng KK, Gilman EA and
Lancashire RJ: Effect of anti-inflammatory drugs overall risk of
common cancer: case-control study in general practice research
database. BMJ. 320:1642–1646. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moorman PG, Grubber JM, Millikan RC and
Newman B: Association between nonsteroidal anti-inflammatory drugs
(NSAIDs) and invasive breast cancer and carcinoma in situ of the
breast. Cancer Causes Control. 14:915–922. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cotterchio M, Kreiger N, Sloan M and
Steingart A: Nonsteroidal anti-inflammatory drug used and breast
cancer risk. Cancer Epidemiol Biormarkers Prev. 10:1213–1217.
2001.PubMed/NCBI
|
|
32
|
García Rodríguez LA and González Pérez A:
Risk of breast cancer among users of aspirin and other
anti-inflammatory drugs. Br J Cancer. 91:525–529. 2004.PubMed/NCBI
|
|
33
|
Swede H, Mirand AL, Menezes RJ and Moysich
KB: Association of regular aspirin use and breast cancer risk.
Oncology. 68:40–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Slattery ML, Curtin K, Baumgartner R, et
al: IL6, aspirin, nonsteroidal anti-inflammatory drugs, and breast
cancer risk in women living in the southwestern United States.
Cancer Epidemiol Biomarkers Prev. 16:747–755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hojo T, Akiyama Y, Nagasaki K, et al:
Association of maspin expression with the malignancy grade and
tumor vascularization in breast cancer tissues. Cancer Lett.
171:103–110. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maass N, Hojo T, Rosel F, Ikeda T, Jonat W
and Nagasaki K: Down regulation of the tumor suppressor gene maspin
in breast carcinoma is associated with a higher risk of distant
metastasis. Clin Biochem. 34:303–307. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Girish GV, Sinha N, Chakraborty K,
Bhattacharya G, Kahn NN and Sinha AK: Restoration by aspirin of
impaired plasma maspin level in human breast cancer. Acta Oncol.
45:184–187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bhattacharyya M, Girish GV, Ghosh R,
Chakraborty S and Sinha AK: Acetylsalicylic acid (aspirin) improves
synthesis of maspin and lowers incidence of metastasis in breast
cancer patients. Cancer Sci. 101:2105–2109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rothwell PM, Wilson M, Price JF, Belch JF,
Meade TW and Mehta Z: Effect of daily aspirin on risk of cancer
metastasis: a study of incident cancers during randomised
controlled trials. Lancet. 379:1592–1601. 2012.PubMed/NCBI
|
|
40
|
Bambace NM and Holmes CE: The platelet
contribution to cancer progression. J Thromb Haemostat. 9:237–249.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nature Rev Cancer.
9:239–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gay LJ and Felding-Habermann B:
Contribution of platelets to tumour metastasis. Nature Rev Cancer.
11:123–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Holmes MD, Chen WY, Li L, Hetzmark E,
Spiegelman D and Hankinson SE: Aspirin intake and survival after
breast cancer. J Clin Oncol. 28:1467–1472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Smith WL, DeWitt DL and Garavito RM:
Cyclooxygenases: structural, cellular, and molecular biology. Annu
Rev Biochem. 69:145–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zha S, Yegnasubramanian V, Nelson WG,
Isaacs WB and De Marzo AM: Cyclooxygenases in cancer: progress and
perspective. Cancer Lett. 215:1–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Giercksky KE: COX-2 inhibition and
prevention of cancer. Best Pract Res Clin Gastroenterol.
15:821–833. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ulrich CM, Bigler J and Potter JD:
Non-steroidal anti-inflammatory drugs for cancer prevention:
promise, perils and pharmacogenetics. Nature Review Cancer.
6:130–140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Moran EM: Epidemiological and clinical
aspects of nonsteroidal anti-inflammatory drugs and cancer risk. J
Environ Pathol Toxicol Oncol. 21:193–202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vane JR and Botting RM: Mechanism of
action of aspirin-like drugs. Semin Arthritis Rheum. 26:2–10. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Buttar NS and Wang KK: The “aspirin” of
the new millenium: cyclooxigenase-2 inhibitors. Mayo Clin Proc.
75:1027–1038. 2000.
|
|
51
|
Elwood PC, Gallagher AM, Duthie GG, Mur LA
and Morgan G: Aspirin, salicylates, and cancer. Lancet.
373:1301–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Langley RE, Burdett S, Tierney JF,
Cafferty F, Parmar MK and Venning G: Aspirin and cancer: has
aspirin been overlooked as an adjuvant therapy? Br J Cancer.
105:1107–1113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Meric JB, Rottey S, Olaussen K, Soria JC,
Khayat D, Rixe O and Spano JP: Cyclooygenase-2 as a target for
anticancer drug development. Crit Rev Oncol Hematol. 59:51–64.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hwang D, Scollard D, Byrne J and Levine E:
Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast
cancer. J Natl Cancer Inst. 90:455–460. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
He TC, Chan TA, Volgestein B and Kinzlet
KW: PPAR delta is an APC-regulated target of nonsteroidal
anti-inflammatory drugs. Cell. 99:335–345. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Davies G, Martin LA, Sacks N and Dowsett
M: Cyclooxygenase-2 (COX-2), aromatase and breast cancer: a
possible role for COX-2 inhibitors in breast cancer
chemoprevention. Ann Oncol. 13:669–678. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Subbaramaiah K, Chung WJ and Dannenberg
AJ: Ceramide regulates the transcription of cyclooxygenase-2.
Evidence for involvement of extracellular signal-regulated
kinase/c-Jun N-terminal kinase and p38 mitogen-activated protein
kinase pathways. J Biol Chem. 273:32943–32949. 1998. View Article : Google Scholar
|
|
58
|
Brueggemeier RW, Quinn AL, Parrett ML,
Joarder FS, Harris RE and Robertson FM: Correlation of aromatase
and cyclooxygenase gene expression in human breast cancer
specimens. Cancer Lett. 140:27–35. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Goss PE, Ingle JN, Martino S, et al: A
randomized trial of letrozole in postmenopausal women after five
years of tamoxifen therapy for early breast cancer. N Engl J Med.
349:1793–1802. 2003.PubMed/NCBI
|
|
60
|
Mazhar E, Ang R and Waxman J: COX
inhibitors and breast cancer. Br J Cancer. 94:346–350. 2006.
View Article : Google Scholar
|
|
61
|
Bosetti C, Gallus S and La Vecchia C:
Aspirin and cancer risk: an updated quantitative review to 2005.
Cancer Causes Control. 17:871–888. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Crew KD and Neugut AI: Aspirin and NSAIDs:
effects in breast and ovarian cancers. Curr Opin Obstet Gynecol.
18:71–75. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fosslien E: Molecular pathology of
cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 30:3–21.
2000.PubMed/NCBI
|
|
64
|
Bosetti C, Gallus S and La Vecchia C:
Aspirin and cancer risk: an update to 2001. Eur J Cancer Prev.
11:535–542. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Howe LR, Subbaramaiah K, Brown AMC and
Dannenberg AJ: Cyclooxygenase-2: a target for the prevention and
treatment of breast cancer. Endocr Relat Cancer. 8:97–114. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ratnasinghe D, Daschner PJ, Anver MR, et
al: Cyclooxygenase-2 P-glycoprotein-170 and drug resistance; is
chemoprevention against multidrug resistance possible? Anticancer
Res. 21:2141–2147. 2001.PubMed/NCBI
|
|
67
|
Streuli CH: Maspin is a tumour suppressor
that inhibits breast cancer tumour metastasis in vivo. Breast
Cancer Res. 4:137–140. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
68
|
Solomon LA, Munkarah AR, Schimp VL, et al:
Maspin expression and localization impact on angiogenesis and
prognosis in ovarian cancer. Gynecol Oncol. 101:385–389. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Burnett GT, Weathersby DC, Taylor TE and
Bremner TA: Regulation of inflammation and angiogenesis-related
gene expression in breast cancer cells and co-cultured macrophages.
Anticancer Res. 28:2093–2099. 2008.PubMed/NCBI
|
|
70
|
Spitz GA, Furtado CM, Sola-Penna M and
Zancan P: Acetylsalicylic acid and salicylic acid decrease tumor
cell viability and glucose metabolism modulating
6-phosphofructo-1-kinase structure and activity. Biochem Pharmacol.
77:46–53. 2009. View Article : Google Scholar : PubMed/NCBI
|