Introduction
Osteosarcoma is a rare highly malignant bone tumor
that occurs predominantly in adolescents and young adults. A
defining feature of osteosarcoma is the high rate of metastasis and
the most common site of metastasis is the lungs. In the era before
the use of chemotherapy, over 85% of post-surgery patients
developed metastases (1).
Chemotherapy routinely plays an important role in the treatment of
advanced osteosarcoma. At present, although the percentage of
patients cured has increased between 60 and 70% by neoadjuvant
chemotherapy coupled with limb-sparing surgery, the prognosis
remains poor for most patients with metastatic or recurrent
osteosarcoma (2). This poor
prognosis is mostly due to the development of drug resistance by
osteosarcoma cells after chemotherapy. Cisplatin or
cis-diamminedichloroplatinum (II) (CDDP) is one of the most
active anticancer agents, widely used against various tumors
including osteosarcoma. The mechanism of cisplatin anticancer
activity is generally believed to be its binding to DNA,
interfering with the cell repair mechanism, eventually leading to
cell death (3). However, the
success of chemotherapy depends on the sensitivity of the tumor to
CDDP, as osteosarcoma cells often acquire resistance to drugs and
even develop multiple drug resistance, which results in treatment
failure. In this regard, CDDP resistance has become one of the
major clinical issue to overcome. Alhough extensive research has
been carried out concerning the resistance to CDDP, there is no
mechanism to completely explain the clinical response to CDDP
therapy. For the purpose of fully understanding drug resistance,
establishment of cultured cell lines resistant to anticancer drugs
is primarily necessary. In the present study, we established a
human osteosarcoma subline resistant to CDDP and examined the
characteristics and mechanisms of CDDP resistance in this cell
line.
Materials and methods
Anticancer agents
CDDP was purchased from Hansoh Pharmaceutical Co.
(Lianyungang, China) and stored at a concentration of 1 mg/ml at
room temperature. Methotrexate (MTX) was purchased from Hualian
Pharmaceutical Co. (Shanghai, China) and stored at a concentration
of 1 mg/ml diluted in 0.9% NaCl at −20°C. Adriamycin (ADM) was
purchased from Hisun Pharmaceutical Co. (Taizhou, China) and stored
at a concentration of 2 mg/ml diluted in 0.9% NaCl at −20°C.
Paclitaxel (PTX) was purchased from Bristol-Myers Squibb (Shanghai,
China) and stored at a concentration of 6 mg/ml at −20°C.
Cells and cell culture
The human osteosarcoma cell line SOSP-9607 was
established in our laboratory as described previously (4). The cells were cultured in RPMI-1640
medium (HyClone, USA) supplemented with 10% fetal calf serum (FCS;
Sijiqing Co.), 100 U/ml of penicillin G and 100 μg/ml of
streptomycin, in a humidified atmosphere of 5% CO2 at
37°C. CDDP-resistant cells were established by stepwisely
increasing the concentrations of CDDP in the medium (5). CDDP was added to the cells at
concentrations from 0.1, 0.2, 0.5, 1 to 2 μg/ml when they grew to
60–70% confluency. After continuous exposure to CDDP for 2 days,
the medium was replaced with a fresh CDDP-free medium until the
surviving cells recovered favorably. When cells grew to the same
confluency, CDDP was added to the medium again. Each concentration
was repeated six times. After 12 months, cells that grew in the
medium with 2 μg/ml of CDDP were designated as SOSP-9607/CDDP cells
and stored for further investigation.
Growth curves and the doubling time
(Td)
The cells in an exponential growth phase
(1×105/5 ml) were seeded in a 60-mm plastic dish. The
numbers of cells were counted using a hemocytometer every 24 h for
7 days. The growth curve was plotted using the average number of
cells from triplicate plastic dishes. The doubling time
(Td) of each cell line was counted according to the
formula: Td = Δt × lg2/(lgNt −
lgN0); where N0 is the cell number at the
beginning and Nt is the cell number at the end, and Δt
is the time from N0 to Nt.
Cell cycle analysis by flow
cytometry
The SOSP-9607 and SOSP-9607/CDDP cells in the
logorithmic phase of growth were collected following trypsin
digestion. The cells were washed with PBS and fixed in 70% ethanol
at 4°C overnight. The fixed cells were washed with cold PBS and
stained with 50 mg/ml DNA-binding dye propidium iodide (PI;
Sigma-Aldrich, St. Louis, MO, USA) and 1.0 mg/ml RNase (Invitrogen,
Carlsbad, CA, USA) for 30 min at 37°C in the darkness and examined
with a fluorescence-activated cell sorting (FACS) flow cytometer
(BD Biosciences, San Jose, CA, USA). Each test was repeated in
triplicate.
Drug sensitivity assay
The 3-(4,5-dimethyl-2-thiazol) -2,5-diphenyl-2H
tetrazolium bromide (MTT) assay was applied to determine the
sensitivities of the cell lines SOSP-9607 and SOSP-9607/CDDP to the
drugs CDDP, MTX, PTX and ADM. Monodispersed cells in the
exponential growth phase following trypsinization were plated into
96-well plates at 5×103/well. After an overnight
incubation, the medium was replaced with a fresh one containing
various concentrations of the drugs mentioned above. Eight
different concentrations for each drug were used in triplicate
wells. Drug-free medium was added to the control and blank wells.
After the plates were incubated for 72 h, 20 μl of 5% MTT was added
to each well for 4 h at 37°C. Then the MTT solution was removed,
and the insoluble formazan crystals were dissolved in 150 μl of
dimethylsulfoxide (DMSO; Sigma). The absorbance was measured at
wavelengths of 490 and 630 nm using a Multiskan Ascent microplate
photometer (Thermo Labsystems, USA). Resistance indices (RIs) were
calculated by the ratio of the 50% inhibitory concentration
(IC50) values of the SOSP-9607/CDDP to SOSP-9607
cells.
Invasive capability of the cells
Transwell assay was used to examine the invasive
capability of the SOSP-9607/CDDP and SOSP-9607 cells. The 6.5-mm
Transwell insert (pore size, 8 μm; 24-well insert; Corning) was
pre-coated with 40 μl BD Matrigel (1:8 dilution, BD Biosciences).
The cells were inoculated on the filter at a density of
5×104 cells/filter and incubated with serum-free medium
for 48 h. The medium with 20% fetal bovine serum was added to the
lower chamber outside of the insert. The cells that invaded through
the Matrigel and reached the lower surface of the filter were fixed
with 95% ethanol for 30 min and then stained with 0.1% crystal
violet for 10 min. The numbers of cells in 10 random fields were
counted under a light microscope at ×200 magnification and the
average was calculated.
RT-PCR analysis
Total RNA was isolated from the cells in the
exponential growth phase with TRIzol (Invitrogen). Complementary
DNA (cDNA) was instructed by reverse transcription (RT) of 5 μg
each total RNA. Each cDNA sample was amplified with ExTaq (Takara,
Japan). PCR reactions for the indicated genes were carried out
using the following forward and reverse primers in Table I. The cycling conditions included:
denaturing at 94°C for 5 min, annealing at 55°C for 60 sec and
extension at 72°C for 60 sec, with total PCR cycles of 30, with a
final extension at 72°C for 10 min. Each of the amplified PCR
products was determined by electrophoresis on an 1.5% agarose gel.
The PCR results were observed under a Molecular Imager ChemiDoc XRS
System and analyzed with Quantity One Software (both from Bio-Rad,
Hercules, CA, USA). The ratio of the band density of the PCR for
the gene of interest and the housekeeping gene GAPDH was used as
the quantitative index of equal loading of cDNA for PCR samples
among the SOSP-9607 and SOSP-9607/CDDP cell lines.
 | Table IPrimers used for the RT-PCR
analysis. |
Table I
Primers used for the RT-PCR
analysis.
| Gene | Forward | Reverse |
|---|
| MDR1 |
5′-GAATCTGGAGGAAGACATGACC-3′ |
5′-TCCAATTTTGTCACCAATTCC-3′ |
| MRP1 |
5′-TCAGCCCTTCCTGACAAGCT-3′ |
5′-TCTCTGCTGCAGGAGGTCCG-3′ |
| MRP2 |
5′-TAGAGCTGGCCCTTGTACTC-3′ |
5′-TCAACTTCCCAGACATCCTC-3′ |
| LRP |
5′-CCCCCATACCACTATATCCATGTG-3′ |
5′-TCGAAAAGCCACTCATCTCCTG-3′ |
| ABCG2 |
5′-CAACCATTGCATCTTGGCTG-3′ |
5′-CAAGGCCACGTGATTCTTCC-3′ |
| GST-π |
5′-CATGCTGCTGGCAGATCAGG-3′ |
5′-CATTCATCATGTCCACCAGG-3′ |
| Bcl-2 |
5′-ATGTCCAGCCAGCTGCACCTGAC-3′ |
5′-GCAGAGTCTTCAGAGACAGCCAGG-3′ |
| Bax |
5′-GCTTCAGGGTTTCATCCAGG-3′ |
5′-AAAGTAGGAGAGGAGGCCGT-3′ |
| GAPDH |
5′-ACCACCATGGAGAAGGCTGG-3′ |
5′-CTCAGTGTAGCCCAGGATGC-3′ |
Statistical analysis
Data are expressed as mean ± standard deviation (SD)
and the experiments were repeated three times. Continuous variables
were analyzed using the Student’s t-test. P<0.05 was considered
to indicate a statistically significant result. All statistical
analyses were performed using SPSS 17.0 software (SPSS, Chicago,
IL, USA).
Results
Establishment of the cisplatin-resistant
cell line
We established SOSP-9607/CDDP cells by stepwise
exposure methods (from 0.1 μg/ml cisplatin) for 12 months. Under a
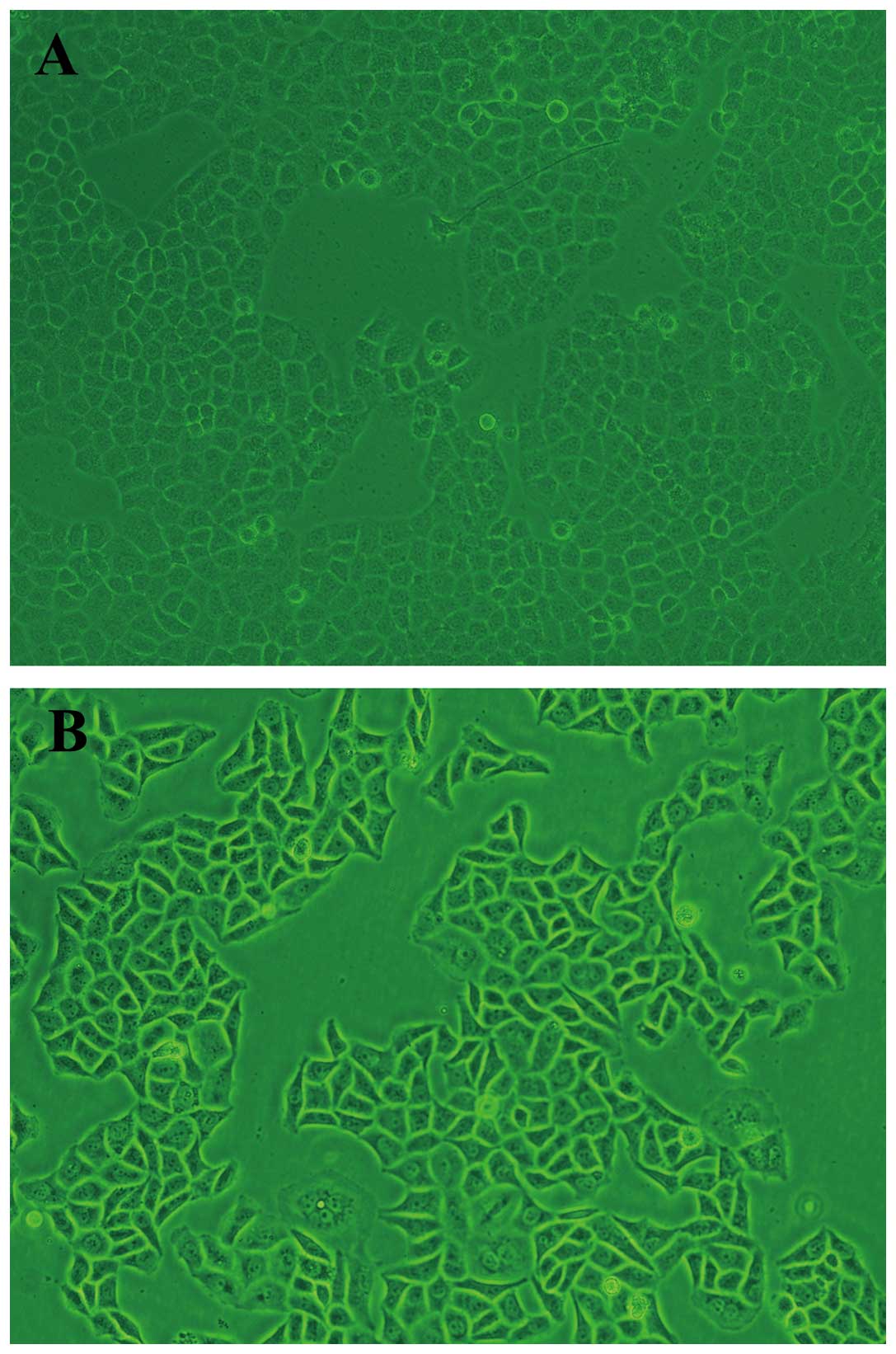
light microscope, both SOSP-9607 and SOSP-9607/CDDP cells grew
adhering to the bottom. SOSP-9607 cells were medium in volume, even
in size and oval in shape with little cytoplasm and a clear
nucleolus (Fig. 1A). SOSP-9607/CDDP
cells are larger than the SOSP-9607 cells in volume and trianglular
or irregular in shape with a markedly enlarged nucleus and
cytoplasm, and more multiple nucleoli (Fig. 1B).
Growth curves and doubling time
(Td)
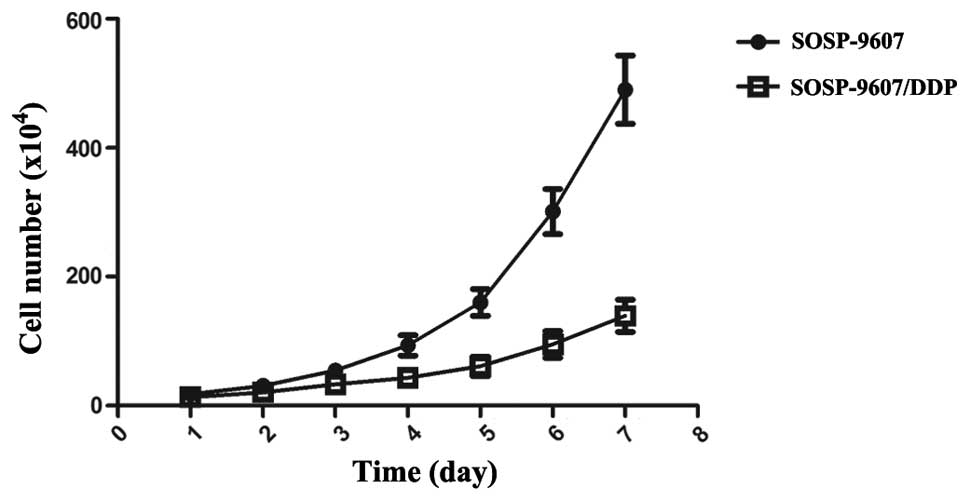
The cell growth curve was plotted with culture time
on the x-axis and the average cell counts/per day on the y-axis.
The SOSP-9607/CDDP cells grew more slowly than their SOSP-9607
parental cells, with a decrease in growth curves. The resistant
cell lines appeared to have a delay in the initiation of
logarithmic growth. According to the cell growth curves (Fig. 2), the Td of SOSP-9607 and
SOSP-9607/CDDP cells was 29.79±0.57 and 45.11±2.37 h, respectively,
which was significantly different (P<0.01).
Cell cycle analysis
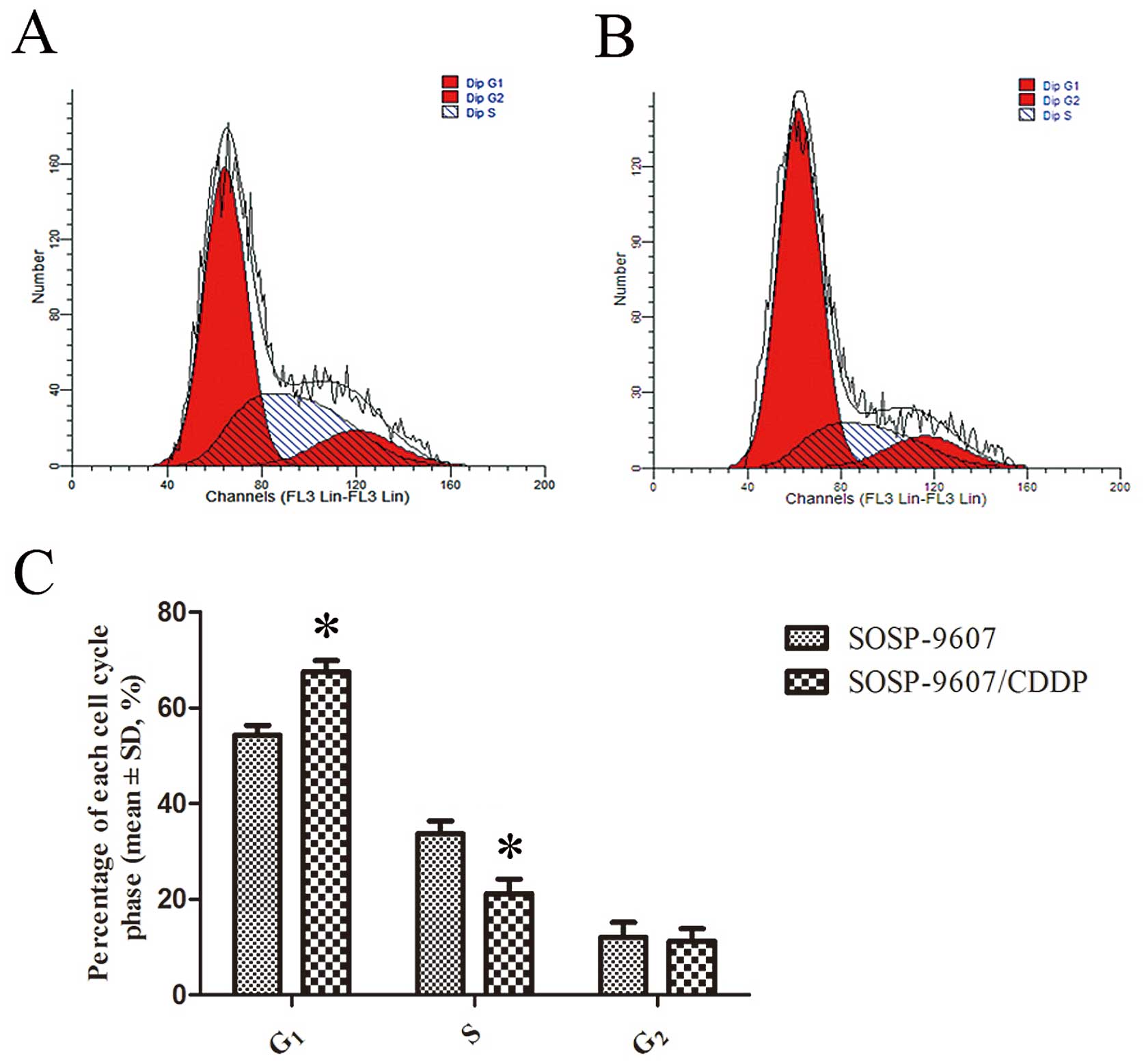
The cell cycle distribution was assessed with flow
cytometry. As shown in Fig. 3,
there were more SOSP-9607/CDDP cells in the
G0/G1 phase and less in the S phase as
compared to the SOSP-9607 cells (P<0.05). There was no
significant difference between the two cell lines in regards to the
G2/M phase.
Drug sensitivity assay
The results of the MTT assays showed that the
IC50 values for the SOSP-9607 and SOSP-9607/CDDP cells
to CDDP were 2.45±0.27 and 15.31±0.21 μg/ml, respectively. The RI
of the SOSP-9607/CDDP cells was 6.24 times, which means that this
cell line was highly resistant to CDDP. The SOSP-9607/CDDP cells
also had cross resistance to MTX and ADM, respectively, but showed
no resistance to PTX (Table
II).
 | Table IIDrug sensitivity of the SOSP-9607 and
the SOSP-9607/CDDP cells. |
Table II
Drug sensitivity of the SOSP-9607 and
the SOSP-9607/CDDP cells.
| IC50 (mean
± SD, μg/ml) | |
|---|
|
| |
|---|
| Agent | SOSP-9607 | SOSP-9607/DDP | RI |
|---|
| CDDP | 2.45±0.27 | 15.31±0.21 | 6.24a |
| ADM | 3.23±0.56 | 6.67±0.45 | 2.06a |
| MTX | 11.38±0.19 | 20.42±0.48 | 1.79a |
| PTX | 0.37±0.05 | 0.41±0.11 | 1.11 |
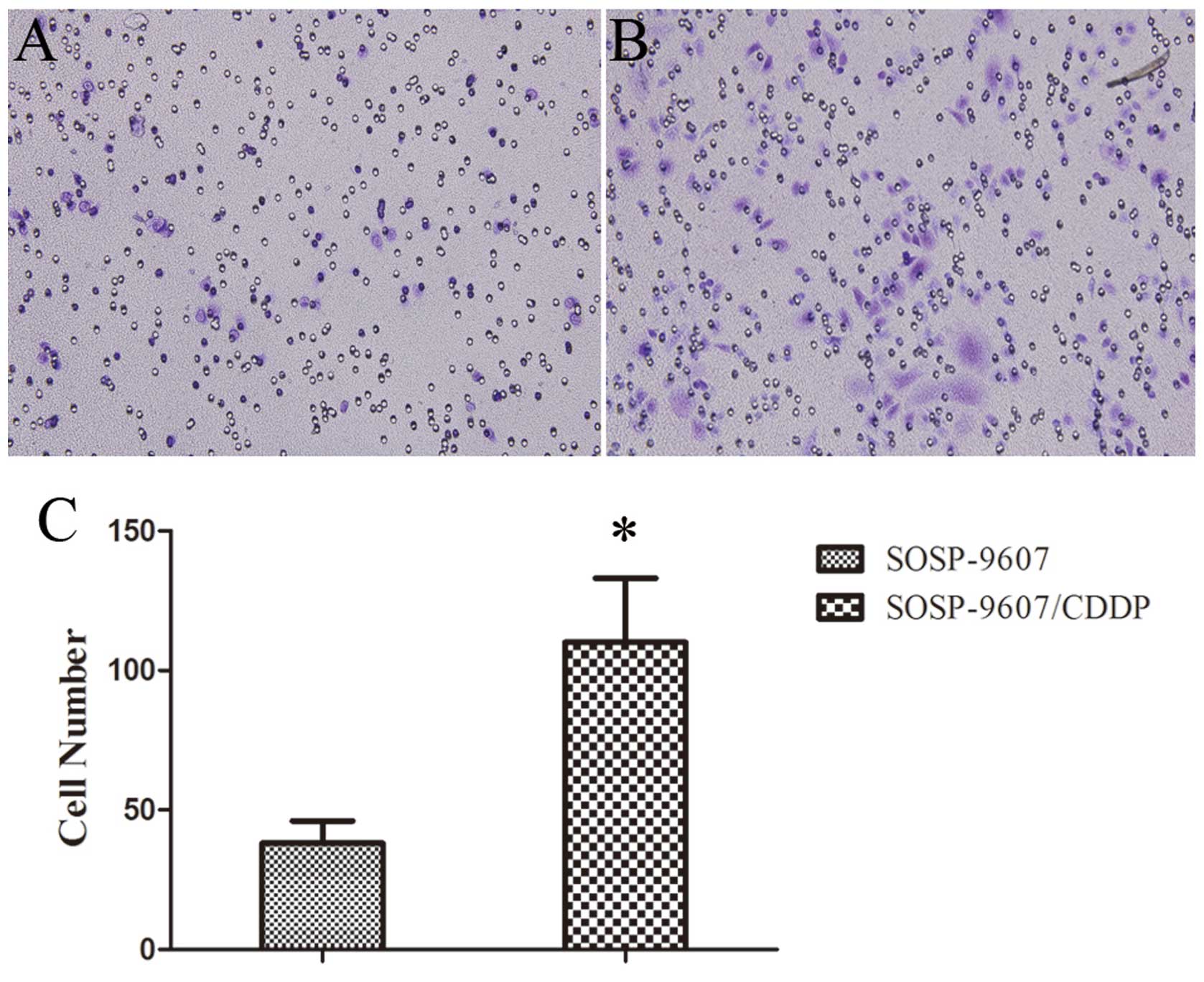
Invasive capability of the cells
Transwell assays with a layer of Matrigel on the top
inserts were performed to examine the invasive capability. After
incubation for 48 h, the number of cells that penetrated both the
Matrigel and membrane was counted. The results showed that the
invasive capability of the SOSP-9607/CDDP cells was increased
compared to the invasive capability of the parental SOSP-9607 cells
(P<0.01; Fig. 4).
Expression of drug resistance-related and
apoptosis-related genes
Gene expression analysis of MDR1, MRP1, MRP2, LRP,
ABCG2, GST-π, Bcl-2 and Bax is shown in Fig. 5A. After normalizing the mRNA
expression level of each gene relative to that of GAPDH, the levels
of MRP1, MRP2 and GST-π mRNA in the SOSP-9607/CDDP cells were more
highly expressed than those levels in the SOSP-9607 cells
(P<0.01); however, there were no differences in expression for
MDR1, LRP, ABCG2, Bcl-2 and Bax mRNA between the SOSP-9607 and
SOSP-9607/CDDP cells (Fig. 5B).
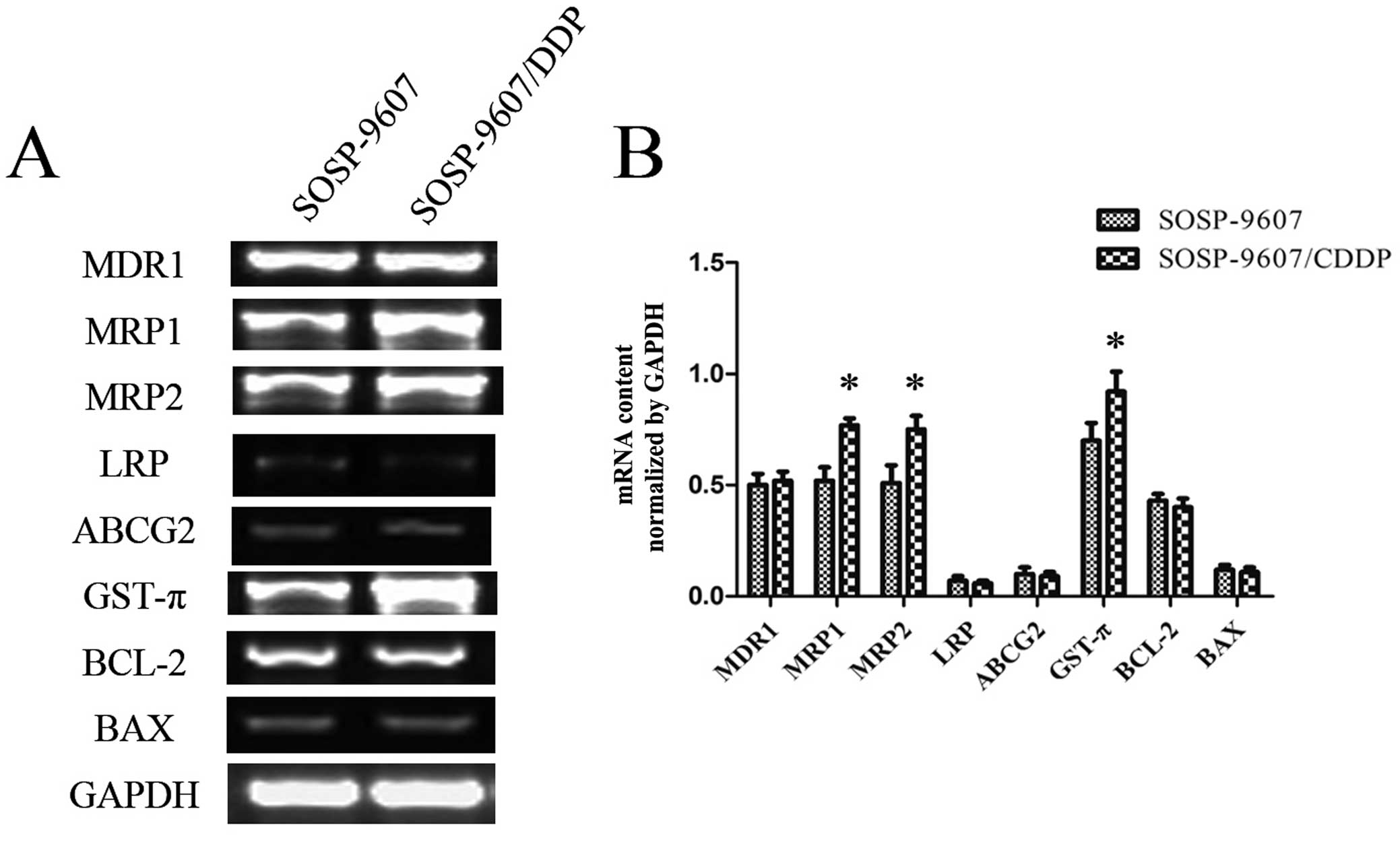 | Figure 5RT-PCR analysis of the SOSP-9607 and
SOSP-9607/CDDP cells. (A) Agarose gel electrophoresis image of
RT-PCR products for MDR1, MRP1, MRP2, LRP, ABCG2, GST-π, Bcl-2, Bax
and GAPDH. (B) mRNA expression levels of MDR1, MRP1, MRP2, LRP,
ABCG2, GST-π, Bcl-2, Bax after normalization relative to GAPDH.
*P<0.01. |
Discussion
Chemotherapy is one of the principal modes of
treatment for cancer, yet drug resistance is one of the major
impediments for effective chemotherapy in cancer patients. Cancer
cells may become cross-resistant to a broad spectrum of
chemotherapeutic agents after a single drug treatment, known as
multidrug resistance (MDR). MDR can be divided into two broad
categories: intrinsic or acquired (6). There are a number of mechanisms known
to be involved in cancer drug resistance, such as increased rates
of drug efflux, alterations in drug metabolism and mutation of drug
targets (7). However, there have
been few studies on osteosarcoma drug-resistant cell lines
(8–11), which has impeded research on the
mechanism of drug resistance of osteosarcoma. The establishment of
drug-resistant cancer cell lines is crucial for studying the
biological characteristics of resistant cells, the mechanisms of
drug resistance and the methods to overcome it. In the present
study, we established a CDDP-resistant osteosarcoma cell line in
vitro as a model for investigating chemotherapy resistance by
intermittent exposure of osteosarcoma parental cells to a high
concentration of CDDP with time-stepwise increments.
After an induction of 12 months, a CDDP-resistant
osteosarcoma cell line SOSP-9607/CDDP was established with a
typical MDR phenotype. As compared with the parental SOSP-9607
cells, SOSP-9607/CDDP cells showed 6.24-fold resistance to CDDP and
cross-resistance to other various drugs, including MTX and ADM. The
resistance of SOSP-9607/CDDP cells was not decreased after 3 months
of culturing in drug-free medium. This multidrug-resistant
characteristic of the SOSP-9607/CDDP cells might imply the failure
of chemotherapy combinations of CDDP with other anticancer drugs in
clinical practice.
The human osteosarcoma cell line SOSP-9607 was
established in our laboratory from a specimen of a 17-year-old
patient with pathologically diagnosed osteosarcoma (4). Morphologically, the SOSP-9607/CDDP
cells were larger than the SOSP-9607 cells in volume and showed
various differences in shape, nucleus and nucleoli. Our results are
consistent with those of another study. Wen et al (12) found that these ultrastructural
changes might facilitate the cisplatin-resistant cell survival
after CDDP therapy. Normally, resistant cells should grow faster
due to resistance. In this study, the resistant cell line grew
slower than the parental cell line and the doubling time of the
SOSP-9607/CDDP cells was higher than that of the SOSP-9607 cells
(45.11 vs. 29.79 h). Wang et al (13) found that this lower growth of
resistant cells might be caused by the existence of non-cycling
dormant cells. Our results suggest that after resistance was
established, the resistant cells changed their characteristics and
lost the majority of the proliferative ability. The growth delay of
the SOSP-9607/CDDP cell line warrants further study. Furthermore,
the percentages of SOSP-9607/CDDP cells in the
G0/G1 phases were significantly increased
when compared with these percentages in the SOSP-9607 cells in the
corresponding phases (Fig. 3).
There is a critical balance between cell cycle arrest (promoting
DNA repair and survival) and cell death following chemotherapy. In
response to DNA damage, some genes will be activated or inactivated
to induce cell cycle arrest in the G1 phase in order to
repair damaged DNA (14). A higher
proportion of SOSP-9607/CDDP cells was in the
G0/G1 phases, possibly indicating an enhanced
capacity in DNA damage repair, which could be considered as a
mechanism of drug resistance. The majority of cisplatin DNA damage
is removed by the NER system (15).
Excision repair cross-complementing rodent repair deficiency,
complementation group 1 (ERCC1) participates in the NER system.
Bellmunt et al found that ERCC1 expression was negatively
correlated with survival and/or responsiveness to cisplatin-based
regimens in several human neoplasms (16). The molecular mechanism of DNA damage
repair of the SOSP-9607/CDDP cell line requires further
investigation. The results of the invasion assays showed that the
invasive capability of the SOSP-9607/CDDP cells was increased
compared to that of the parental SOSP-9607 cells (P<0.01;
Fig. 4). The increased invasive
capability of the SOSP-9607/CDDP cells is involved in tumor
metastasis and progression.
The ATP-binding cassette (ABC) transporter family of
transmembrane proteins has been linked to resistance to commonly
used chemotherapeutics by promoting drug efflux. Several members of
this protein family have been studied extensively, such as
multidrug resistance protein 1 (MDR1, ABCB1), MDR-associated
protein 1 (MRP1, ABCC1) and breast cancer resistance protein (BCRP,
ABCG2) (17). Moreover, lung
resistance protein (LRP) could confer drug resistance by
redistributing drugs away from intracellular targets (18). Therefore, we used RT-PCR to detect
the expression levels of these genes in the SOSP-9607 and
SOSP-9607/CDDP cells. There were no differences in MDR1, LRP and
BCRP mRNA expression between the SOSP-9607 and SOSP-9607/CDDP
cells; however, MRP1 and MRP2 expression was significantly
increased in the SOSP-9607/CDDP cells when compared with that in
the SOSP-9607 cells. MRP overexpression is one of the major
mechanisms of cisplatin resistance. Borst et al suggested
that MRP1, MRP2, MRP3 and MRP5 also mediate some degree of
cisplatin resistance by increasing cisplatin export (19). In particular, other reports suggest
that MRP2 overexpression is responsible for an increased efflux of
cisplatin in resistant cells (20)
and MRP2 expression levels might predict the responsiveness of
tumors to platinum-based therapies (21). Our results also suggest that drug
resistance in the SOSP-9607/CDDP cells might be associated with a
mechanism mediated by MRP-induced changes in membrane transport
function. It is known that aquated cisplatin avidly binds to
cytoplasmic nucleophilic species, such as glutathione (GSH).
Elevated levels of GSH and glutathione S-transferase (GST) have
been observed in cisplatin-resistant cells, both in vitro
and ex vivo (22).
Furthermore, Lai et al found that overexpression of GST-π
increased GST activity in an adriamycin-resistant cell line.
Currently, it is generally accepted that increased GST activity is
mainly due to overexpression of GST-π (23). In the present study, GST-π
expression was significantly increased in the SOSP-9607/CDDP cells
when compared with that in the SOSP-9607 cells. These results are
consistent with those of another study in two cisplatin-resistant
endometrial cancer cell lines (24). Of note, glutathione S-conjugates are
readily extruded by cells via MRP1 or MRP2 (25), which possibly explain why MRP
expression levels are increased in cisplatin-resistant cells. In
short, these findings suggest that the mechanism of drug resistance
in SOSP-9607/CDDP cells may be associated with enhanced
inactivation of anticancer drugs induced by GST-π and increased
efflux of drug conjugates induced by MRP1 or MRP2. Recent studies
have demonstrated that anticancer drug resistance is induced by
mutation of the apoptosis mechanism of cancer cells. Proapoptotic
members (Bax, Bak, Bad and Bil) and antiapoptotic members (Bcl-2,
Bcl-XL and Mcl-1) of the BCL-2 protein family participate in these
lethal cascades, and the majority have been shown to modulate the
cellular response to cisplatin. Several studies have found that Bax
deficiency and overexpression of BCL-2 conferred resistance to CDDP
and to several other stressors in vitro (26,27).
In the present study, we compared the expression levels of BCL-2
and Bax in the SOSP-9607 and SOSP-9607/CDDP cells, yet no
difference was noted between the resistant cells and the parental
cells. However, the level of gene expression did not necessarily
parallel with the level of protein. Western blot and protein
function assays should be carried out to investigate whether the
BCL-2 protein family members participate in the mechanism of
resistance to CDDP in SOSP-9607/CDDP cells.
In conclusion, we established the stable
SOSP-9607/CDDP drug-resistant osteosarcoma cell line, which showed
cross resistance to other drugs. Thus, this cell line could serve
as a useful tool for further study concerning the molecular
mechanisms of osteosarcoma drug resistance and may lead to the
establishment of novel therapeutic strategies for osteosarcoma.
Acknowledgements
The authors would like to thank Yanhua Wen, Yunyan
Liu and Hong Zhao for their excellent technical assistance and
helpful discussions. This study was supported by grants from the
Postdoctoral Science Foundation of China (no. 2013M532203), Tangdu
Hospital Innovation Fund to X.Z. and the National Natural Science
Foundation of China (no. 31000660, no. 81372297 and no.
30973409).
References
|
1
|
Gorlick R and Khanna C: Osteosarcoma. J
Bone Miner Res. 25:683–691. 2010. View
Article : Google Scholar
|
|
2
|
Picci P: Osteosarcoma (osteogenic
sarcoma). Orphanet J Rare Dis. 2:62007. View Article : Google Scholar
|
|
3
|
Negoro K, Yamano Y, Fushimi K, Saito K,
Nakatani K, Shiiba M, Yokoe H, Bukawa H, Uzawa K, Wada T, Tanzawa H
and Fujita S: Establishment and characterization of a
cisplatin-resistant cell line, KB-R, derived from oral carcinoma
cell line, KB. Int J Oncol. 30:1325–1332. 2007.PubMed/NCBI
|
|
4
|
Chen X, Yang TT, Wang W, Sun HH, Ma BA, Li
CX, Ma Q, Yu Z and Fan QY: Establishment and characterization of
human osteosarcoma cell lines with different pulmonary metastatic
potentials. Cytotechnology. 61:37–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shibata K, Umezu T, Sakurai M, Kajiyama H,
Yamamoto E, Ino K, Nawa A and Kikkawa F: Establishment of
cisplatin-resistant ovarian yolk sac tumor cells and investigation
of the mechanism of cisplatin resistance using this cell line.
Gynecol Obstet Invest. 71:104–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gottesman MM: Mechanisms of cancer drug
resistance. Annu Rev Med. 53:615–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: an evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asada N, Tsuchiya H, Ueda Y and Tomita K:
Establishment and characterization of an acquired
cisplatin-resistant subline in a human osteosarcoma cell line.
Anticancer Res. 18:1765–1768. 1998.PubMed/NCBI
|
|
9
|
Maraldi NM, Zini N, Santi S, Scotlandi K,
Serra M and Baldini N: P-glycoprotein subcellular localization and
cell morphotype in MDR1 gene-transfected human osteosarcoma cells.
Biol Cell. 91:17–28. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oda Y, Matsumoto Y, Harimaya K, Iwamoto Y
and Tsuneyoshi M: Establishment of new multidrug-resistant human
osteosarcoma cell lines. Oncol Rep. 7:859–866. 2000.PubMed/NCBI
|
|
11
|
Niu BH, Wang JJ, Xi Y and Ji XY: The
establishment and characterization of adriamycin-resistant cell
lines derived from Saos-2. Med Sci Monit. 16:BR184–BR192.
2010.PubMed/NCBI
|
|
12
|
Wen J, Zheng B, Hu Y, Zhang X, Yang H, Luo
KJ, Zhang X, Li YF and Fu JH: Establishment and biological analysis
of the EC109/CDDP multidrug-resistant esophageal squamous cell
carcinoma cell line. Oncol Rep. 22:65–71. 2009.PubMed/NCBI
|
|
13
|
Wang C, Guo LB, Ma JY, Li YM and Liu HM:
Establishment and characterization of a paclitaxel-resistant human
esophageal carcinoma cell line. Int J Oncol. 43:1607–1617.
2013.PubMed/NCBI
|
|
14
|
Longley DB and Johnston PG: Molecular
mechanisms of drug resistance. J Pathol. 205:275–292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakai W, Swisher EM, Karlan BY, Agarwal
MK, Higgins J, Friedman C, Villegas E, Jacquemont C, Farrugia DJ,
Couch FJ, Urban N and Taniguchi T: Secondary mutations as a
mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature.
451:1116–1120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bellmunt J, Paz-Ares L, Cuello M, Cecere
FL, Albiol S, Guillem V, Gallardo E, Carles J, Mendez P, de la Cruz
JJ, Taron M, Rosell R and Baselga J; Spanish Oncology Genitourinary
Group. Gene expression of ERCC1 as a novel prognostic marker in
advanced bladder cancer patients receiving cisplatin-based
chemotherapy. Ann Oncol. 18:522–528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Li ZN, Du YJ, Li XQ, Bao QL and Chen
P: Expression of MRP1, BCRP, LRP, and ERCC1 in advanced
non-small-cell lung cancer: correlation with response to
chemotherapy and survival. Clin Lung Cancer. 10:414–421. 2009.
View Article : Google Scholar
|
|
19
|
Borst P, Evers R, Kool M and Wijnholds J:
A family of drug transporters: the multidrug resistance-associated
proteins. J Natl Cancer Inst. 92:1295–1302. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liedert B, Materna V, Schadendorf D,
Thomale J and Lage H: Overexpression of cMOAT (MRP2/ABCC2) is
associated with decreased formation of platinum-DNA adducts and
decreased G2-arrest in melanoma cells resistant to cisplatin. J
Invest Dermatol. 121:172–176. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamasaki M, Makino T, Masuzawa T, Kurokawa
Y, Miyata H, Takiguchi S, Nakajima K, Fujiwara Y, Matsuura N, Mori
M and Doki Y: Role of multidrug resistance protein 2 (MRP2) in
chemoresistance and clinical outcome in oesophageal squamous cell
carcinoma. Br J Cancer. 104:707–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen HH and Kuo MT: Role of glutathione in
the regulation of cisplatin resistance in cancer chemotherapy. Met
Based Drugs. 2010:4309392010. View Article : Google Scholar
|
|
23
|
Lai GM, Moscow JA, Alvarez MJ, Fojo AT and
Bates SE: Contribution of glutathione and glutathione-dependent
enzymes in the reversal of adriamycin resistance in colon carcinoma
cell lines. Int J Cancer. 49:688–695. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sagawa Y, Fujitoh A, Nishi H, Ito H,
Yudate T and Isaka K: Establishment of three cisplatin-resistant
endometrial cancer cell lines using two methods of cisplatin
exposure. Tumor Biol. 32:399–408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishikawa T: The ATP-dependent glutathione
S-conjugate export pump. Trends Biochem Sci. 17:463–468. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tajeddine N, Galluzzi L, Kepp O, Hangen E,
Morselli E, Senovilla L, Araujo N, Pinna G, Larochette N, Zamzami
N, Modjtahedi N, Harel-Bellan A and Kroemer G: Hierarchical
involvement of Bak, VDAC1 and Bax in cisplatin-induced cell death.
Oncogene. 27:4221–4232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jain HV and Meyer-Hermann M: The molecular
basis of synergism between carboplatin and ABT-737 therapy
targeting ovarian carcinomas. Cancer Res. 71:705–715. 2011.
View Article : Google Scholar : PubMed/NCBI
|