Introduction
Chemotherapy is one of the principal modes of
treatment for cancer, but the effectiveness of chemotherapy is
limited by multidrug resistance (MDR) (1), which generally results from the
expression of P-glycoprotein (P-gp), the ATP-dependent efflux pump.
MDR reversal agents typically act by inhibiting the drug efflux
activity of P-gp, thereby increasing intracellular drug levels.
P-gp belongs to a family of ATP-binding cassette (ABC) transporters
that have been grouped into seven subfamilies, designated ABCA-ABCG
(2,3). ABCB1 encodes P-gp or MDR1, a 170-kDa
glycoprotein widely expressed in plasma cell membranes of healthy
human tissues and multidrug-resistant tumors (2,3). It
has been demonstrated that there is a minimum of four drug binding
sites on P-gp. These sites can be divided into two categories:
transport sites, at which translocation of drug across the cellular
membrane can occur, and regulatory sites, which modify P-gp
function. In addition, some agents can inhibit P-gp activity by
decreasing intracellular ATP supply and inhibiting P-gp ATPase
activity (4,5). To overcome the clinical problem of
P-gp-mediated MDR, several generations pharmacological inhibitors
of P-gp have been developed (6).
The first-generation inhibitors such as verapamil (the calcium
channel blockers) and the immunosuppressant cyclosporin, reverse
MDR by functioning as competitive substrates of P-gp (7), but they display low binding affinity
to P-gp, thus precluding further investigation after phase I
clinical trials (8). The
second-generation agents are analogues of the first generation
drugs (e.g. dexverapamil, valspodar and cinchonine) with supposedly
higher selectivity and activity; however, there are several factors
that limit their clinical use (9),
including that they are often inhibitors of other ABC transporters
as well (10–12). Another factor is that they may be
substrates of cytochrome P-450 (CYP), resulting in pharmacokinetic
interactions with increased host toxicity from cytotoxic drug
overexposure (13–16). The third-generation agents are
potentially able to overcome the limitations of previous generation
compounds (8,10,17–19);
they are not substrates of P-gp, they act through noncompetitive
inhibition of the pump, and bind with high affinity to it (20,21).
Several P-gp inhibitors have been tested in controlled clinical
trials; however, due to the cytotoxicity, no satisfactory results
have thus far been obtained (3,22).
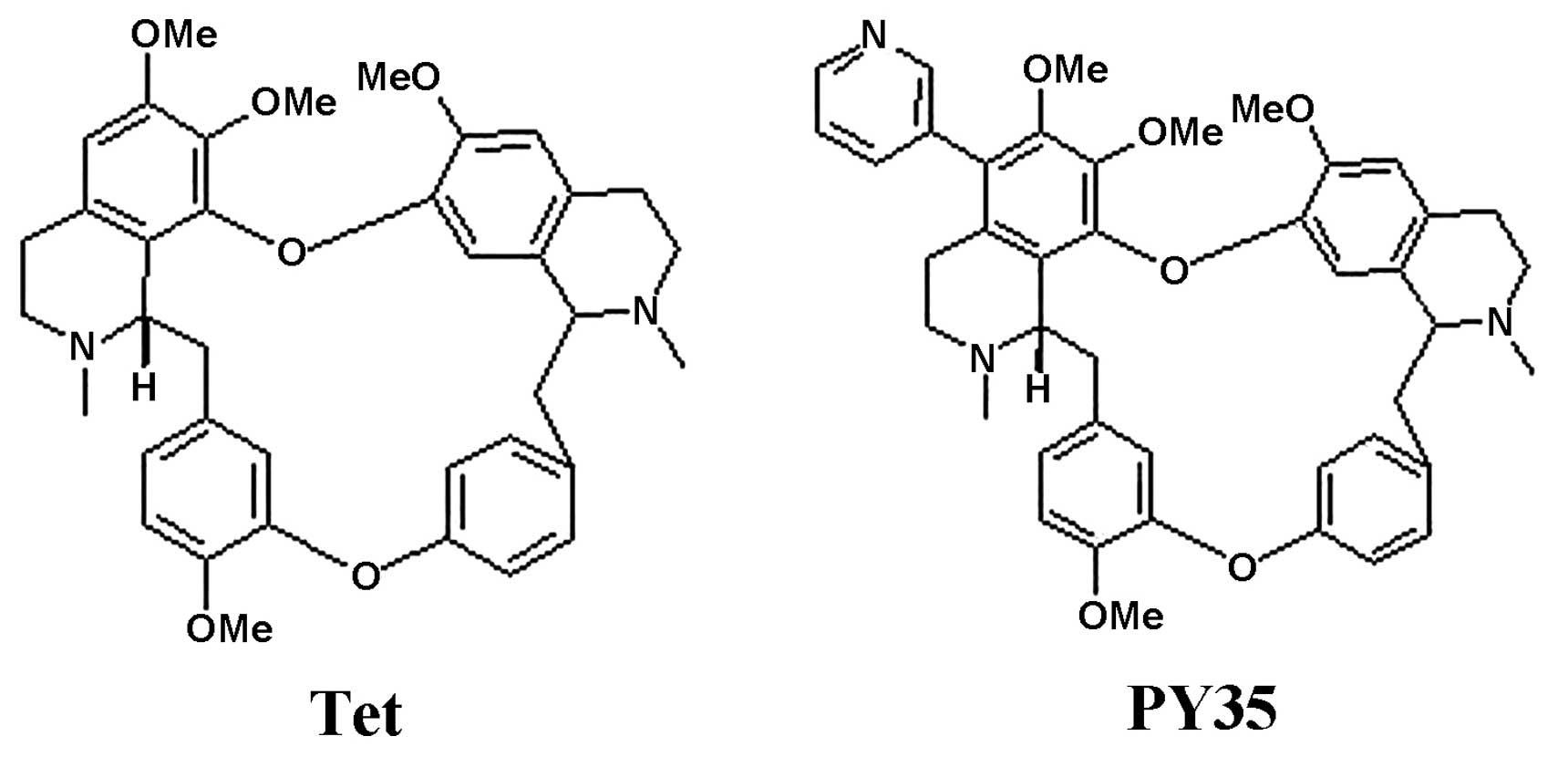
Many studies have focused on the Chinese traditional
medicine Tetrandrine (Tet), a bis-benzylisoquinoline alkaloid
derived from the Chinese medicinal herb Stephania tetrandra
(23). It is a voltage-activated,
L-type Ca2+ channel with its binding site at the
benzothiazepine receptor on the α1-subunit and it also blocks the
voltage-dependent T-type Ca2+ channel (24). Tet has been used in the treatment of
hypertension, cardiac arrhythmia and angina pectoris in China since
the 1950s (24). A previous study
showed that Tet can reverse P-gp-mediated MDR effectively in
vitro and in vivo (25).
A series of new bisbenzylisoquinoline alkaloids is partially
synthesized from Tet, and evaluated for their ability to reverse
P-gp-mediated MDR in cancer. PY35 is a novel 5-substituted Tet
derivative, synthesized by us. In the current study, the reversal
activity of PY35 on MDR was examined, and it showed that PY35
reverses the P-gp-mediated MDR in cancer cells, increases the
accumulation of cytotoxic agents in multidrug-resistant MCF-7/ADM
and K562/ADM cells, but it has no effect on P-gp expression.
Therefore, the combination of PY35 with a cytotoxic agent may help
overcome MDR in the clinic.
Materials and methods
Chemicals and reagents
Adriamycin (ADM), trypsin-EDTA solution, fetal
bovine serum (FBS), MTT (tetrazolium), Rhodamine-123 (Rho-123),
DAPI, P-gp and GAPDH mouse anti-human monoclonal antibody and
HRP-linked secondary antibody were obtained from Sigma Chemical
(St. Louis, MO, USA). RPMI-1640 was purchased from Gibco-BRL.
Tetrandrine (Tet) was a gift from Dr Jiekai Cheng, and PY35 with a
purity of >98% was synthesized by our lab. Both were evaluated
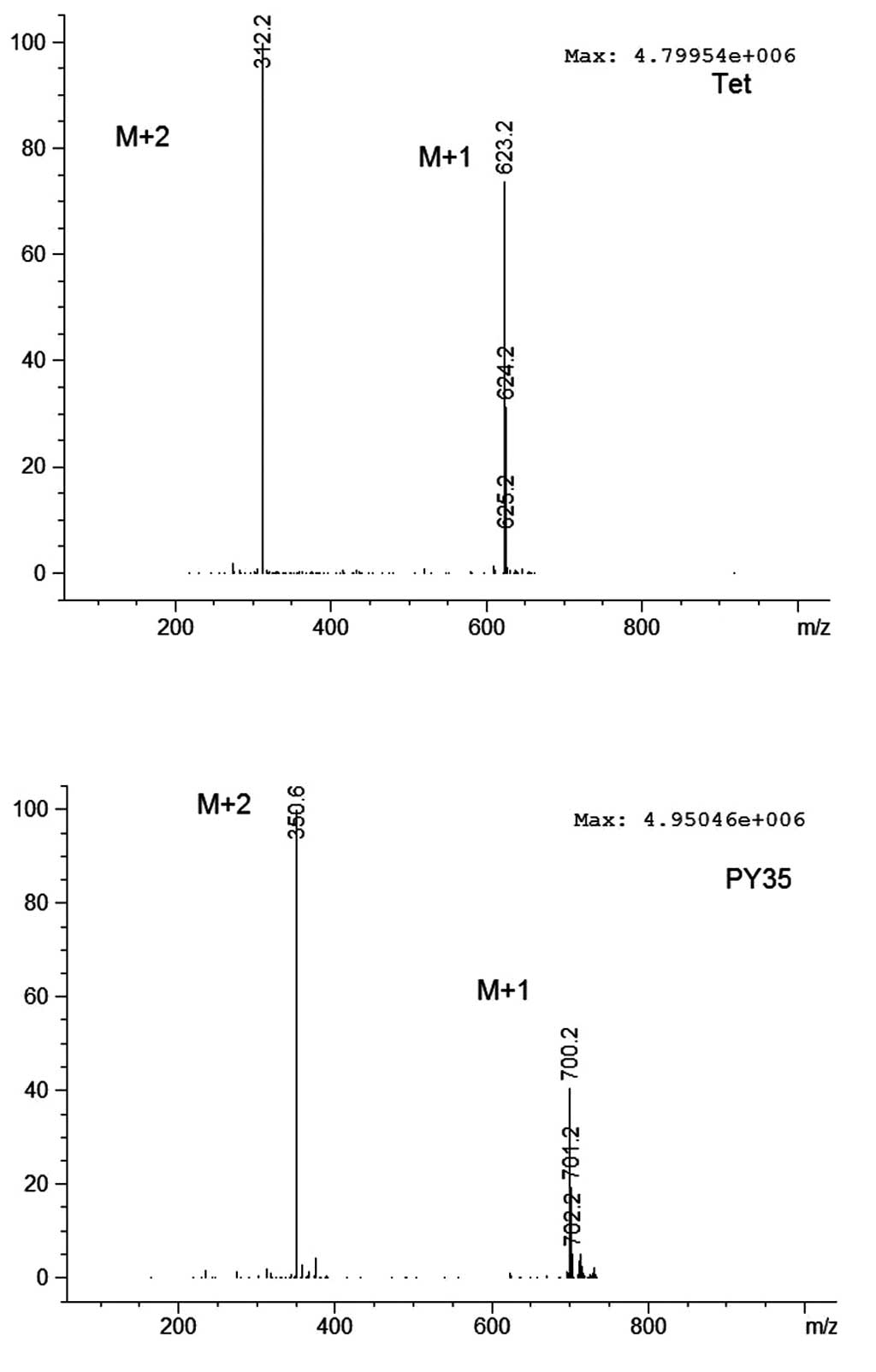
by chromatography, HRMS-ESI (Figs.
1 and 2, Table I) and NMR.
 | Table IThe HRMS data of Tet and PY35. |
Table I
The HRMS data of Tet and PY35.
| Compound name | Formula | Expected m/z | Found at m/z | Isotope diff.
(%) | Error (ppm) |
|---|
| Tet | C38H42N2O6 | 623.3116 | 623.3113 | 4.4 | −0.4 |
| PY35 | C43H45N3O6 | 700.3381 | 700.3383 | 1.5 | 0.2 |
Cell culture
Drug sensitive human breast carcinoma and MCF-7 and
human leukemia cell line K562 and their MDR sublines MCF-7/ADM and
K562/ADM were cultured in RPMI-1640 medium with 10% newborn bovine
serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a
humidified atmosphere of 5% CO2. To maintain MDR
phenotype, MCF-7/ADM and K562/ADM cells were cultured in the medium
containing 1 μg/ml ADM at least 1 week before the experiments.
MTT cytotoxicity assay
The cytotoxicity of PY35 was measured by the MTT
assay. Tumor cells were seeded in 96-well plates in 100 μl medium
per well and attached overnight. A series of concentrations of PY35
were added to each well and incubated for 2 days at 37°C, then the
100 μl of MTT were added to each well after removing the culture
medium and then incubated for an additional 4 h. The formed
formazan was dissolved in 150 μl DMSO after removing the culture
medium. The plates were shaken for 5 min and the optical density
was measured by the microplate reader (Tecan M1000) at a wavelength
of 570 nm. The IC50 values were calculated by Graphpad
Prism 6.0 software. According to the results of the cytotoxicity
assay, cytotoxic concentrations of PY35 were selected to determine
the reverse MDR effect. The reversal activity of PY35 on drug
resistance is expressed as the fold reversal calculated according
to the following equation: RVF=IC50 anticancer drug
alone/IC50 anticancer drug + inhibitor. Tet was used as
a control.
Rho-123 uptake assays
The assay was performed according to a previous
method (26). For the Rho-123
uptake assay, cells were incubated with 5 μM Rho-123, in the
presence or absence of PY35, at 37°C for 1 h. The cells were
observed under a laser scanning confocal microscope (LSCM, Zeiss
LSM 710, Germany) and analyzed with flow cytometry (FCM) (FACSAria;
BD Biosciences, Franklin Lakes, NJ, USA).
Intracellular accumulation of ADM
Intracellular accumulation of ADM was detected by
FCM. The K562/ADM and MCF-7/ADM cells were treated as previously
described. After being cultured for 48 h, cells were washed three
times with phosphate-buffered saline (PBS) and then suspended with
500 μl of PBS. Thereafter, each sample was measured by FCM at
excitation/emission wavelengths of 488/575 nm. The mean
fluorescence intensity (MFI) of ADM was calculated as MFI treated
group/MFI control group.
Western blotting
Following drug treatment, cells were washed three
times with ice-cold PBS, and total cells were lysed, total protein
was collected and was separated by SDS-PAGE and transferred to
polyvinylidine difluoride membranes. After blocking in 5% non-fat
milk in Tris-buffered saline with 0.1% Tween-20 (pH 7.6), mouse
anti-human P-gp monoclonal antibody and mouse anti-human GAPDH
monoclonal antibody were used as primary antibodies and HRP-linked
secondary antibody were used to detect P-gp protein.
Statistical analysis
Data are expressed as the means ± SD. Statistical
analysis of the data was performed using the Student’s t-test.
P<0.05 was considered statistically significant.
Results
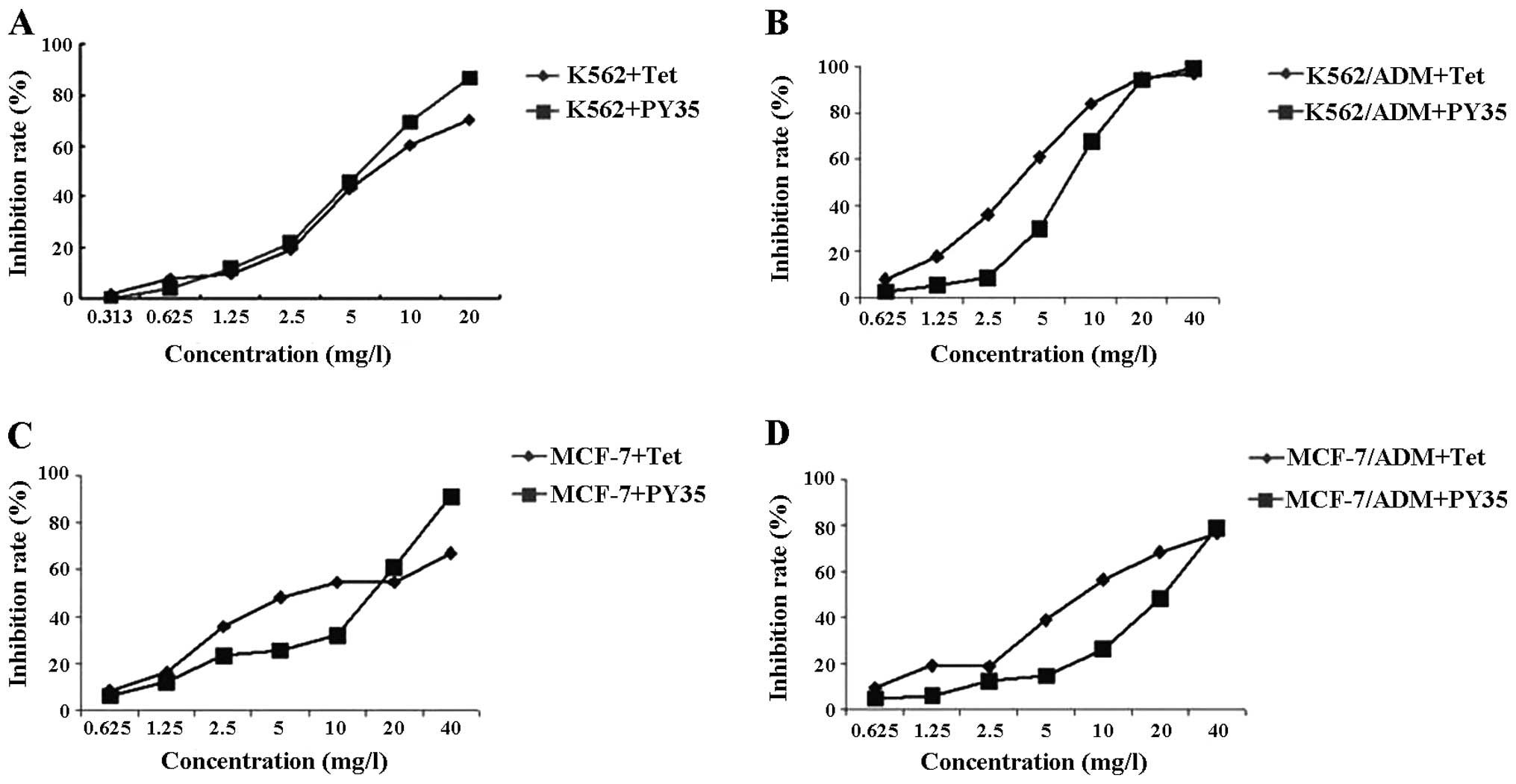
Evaluation of the cytotoxic effect of
PY35
As shown in Fig. 3,
>90% cells survived at the concentrations of 0.625 μg/ml PY35 on
these cell lines. Based on the results of the cytotoxicity assay
above, we used 0.625 μg/ml PY35 to evaluate the reversal
activity.
PY35 reverses MDR
The IC50 of ADM for K562, K562/ADM, MCF-7
and MCF-7/ADM cells were 0.45, 68.12, 4.50 and 112.72 μg/ml
respectively. The multiple of drug resistance was 149.95 and 25.05.
After the K562/ADM cells and MCF-7/ADM cells were treated with PY35
alone or in combination, IC50 was 2.34 and 2.98 μg/ml,
respectively. The reversal multiples were 29.14 and 37.83,
respectively. Between the single agent treatment groups and the
combined treatment groups, the differences were statistically
significant (P<0.05) (Table
II).
 | Table IIEffect of PY35 on the cytotoxicity of
ADM (expressed as IC50 values). |
Table II
Effect of PY35 on the cytotoxicity of
ADM (expressed as IC50 values).
| Cell lines |
|---|
|
|
|---|
| K562/ADM | MCF-7/ADM |
|---|
|
|
|
|---|
| Groups | IC50
(μg/ml) | RVF | IC50
(μg/ml) | RVF |
|---|
| ADM | 68.12±0.62 | | 112.72±1.81 | |
| ADM+Tet | 13.00±0.86a | 5.24 | 4.92±0.45c | 22.91 |
| ADM+PY35 | 2.34±0.53b | 29.14 | 2.98±0.47c | 37.83 |
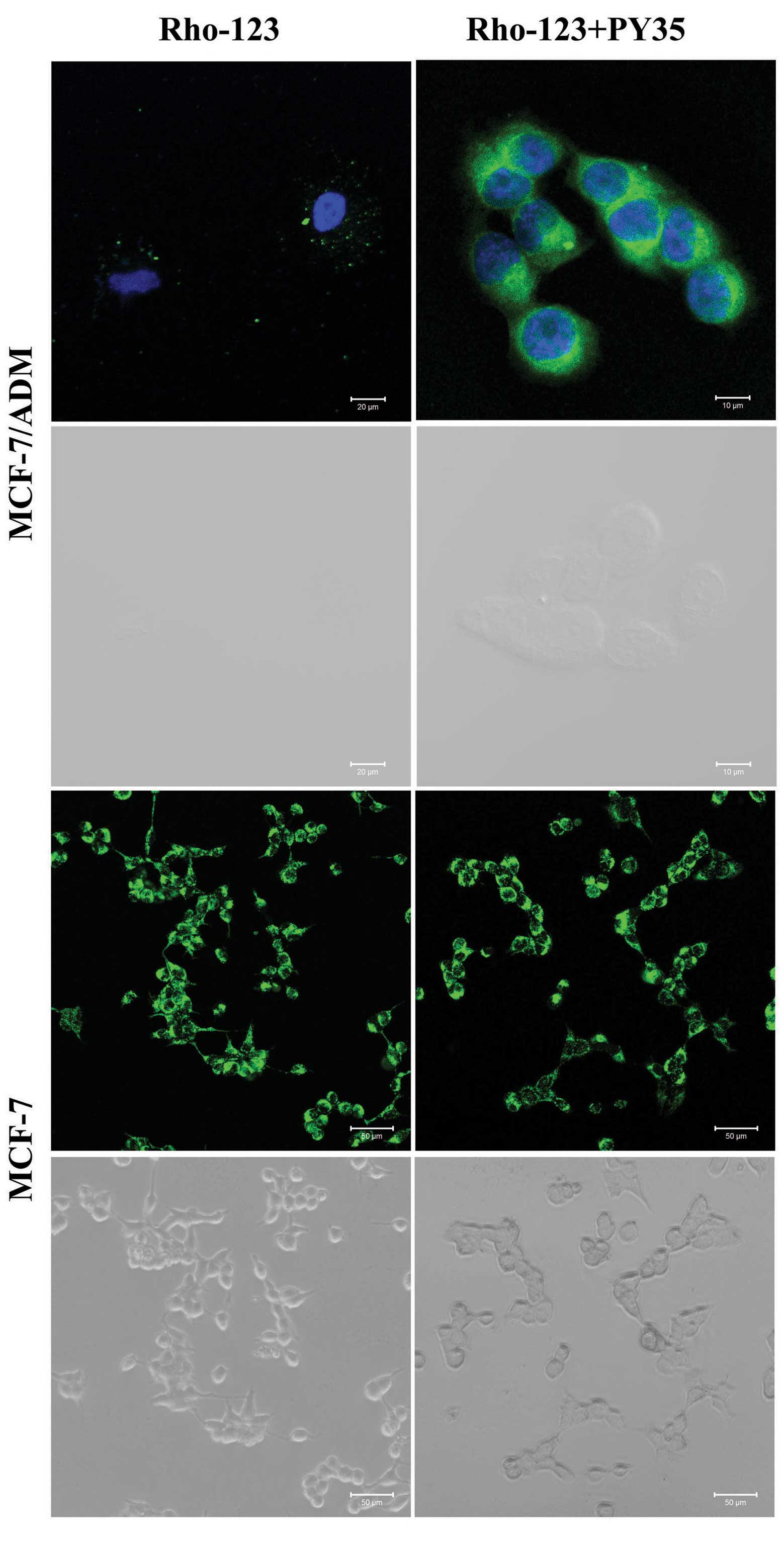
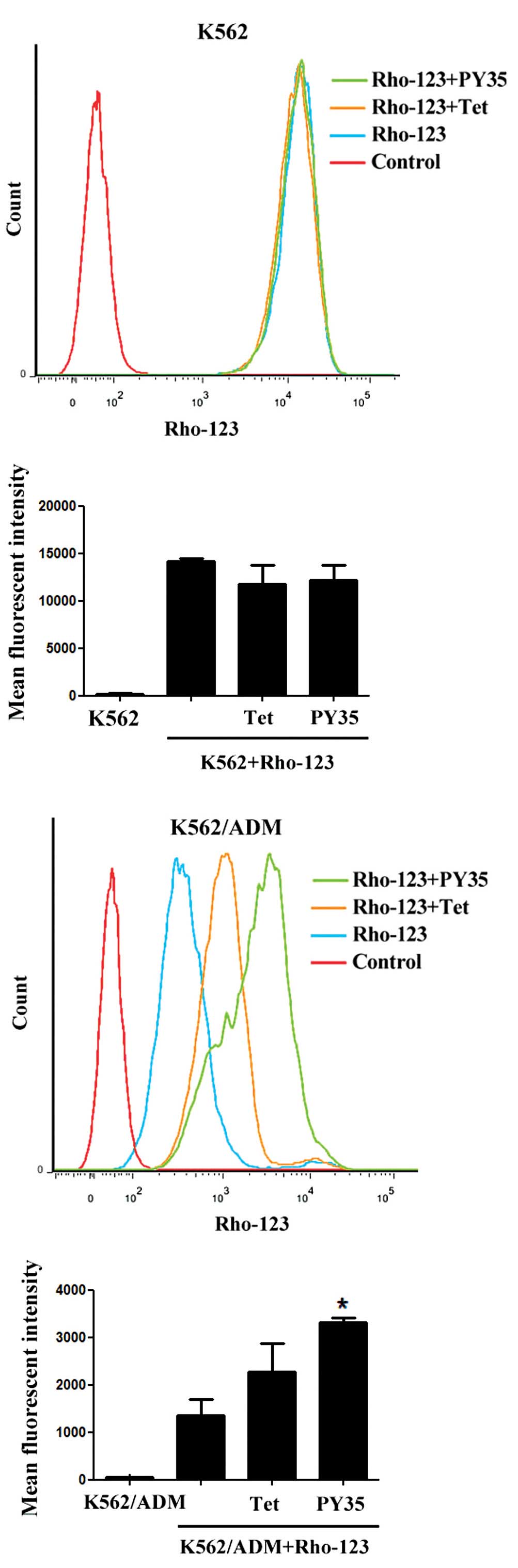
Rho-123 uptake assays
The intracellular uptake of the fluorescent dye
Rho-123 was evaluated in two MDR cell lines and their sensitive
counterparts by using FCM and LSCM. After incubation with Rho-123
for 1 h, the addition of PY35 led to significantly increased
intracellular Rho-123 levels in the resistant but not sensitive
cells. The results indicated that the amount of Rho-123
accumulation in cells treated with PY35 plus Rho-123 was
significantly higher than that in cells treated with Rho-123 alone
and control cells (Figs. 4–7).
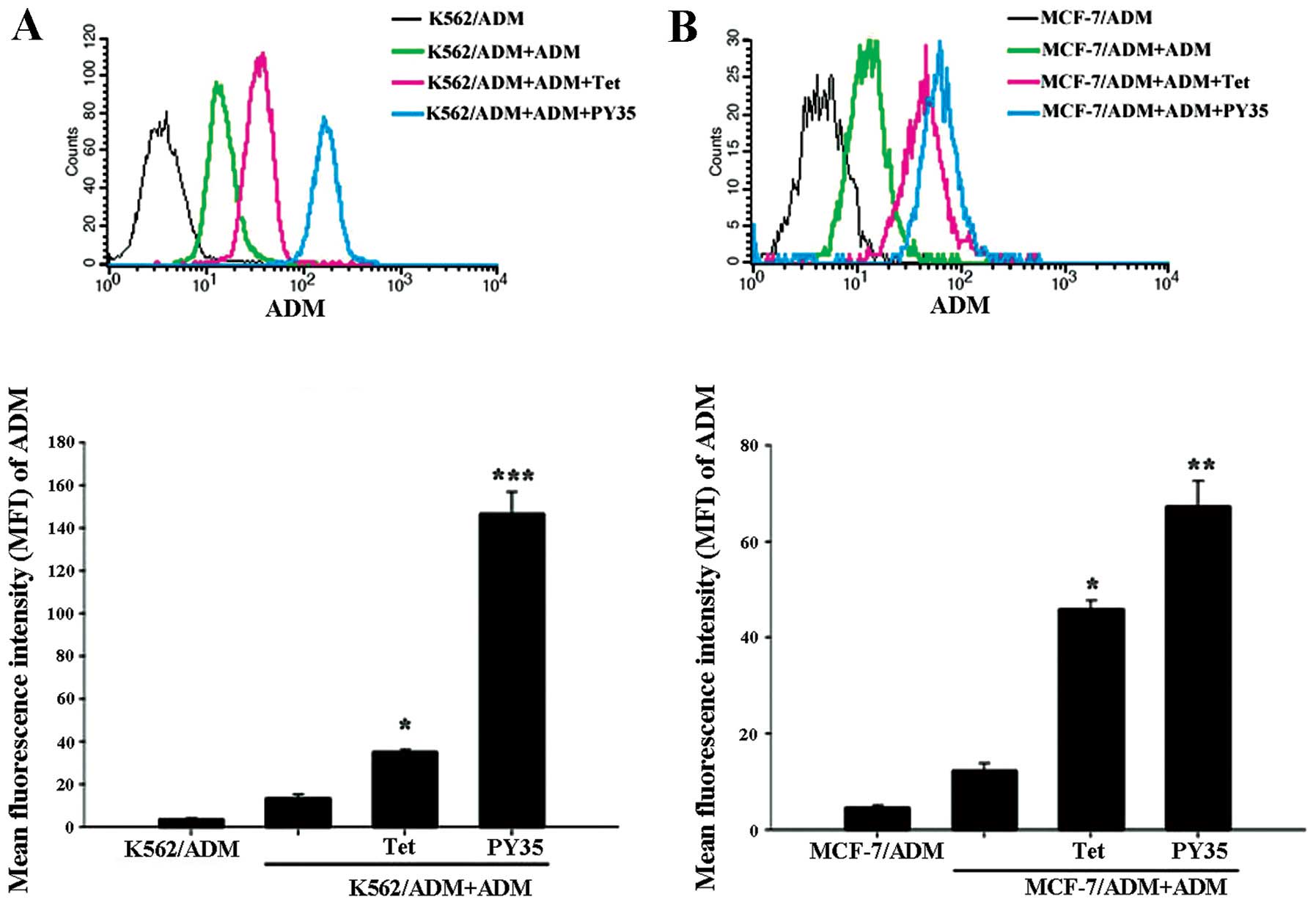
Effect of PY35 on cellular accumulation
of ADM
The MFI of ADM in K562/ADM cells and MCF-7/ADM cells
incubated with ADM, ADM+PY35 respectively were 13.86±1.21,
146.78±10.25, 12.35±1.58, and 67.34±5.34, respectively. Compared
with the ADM group, the ADM+PY35 group significantly enhanced the
intracellular ADM accumulation (Fig.
8).
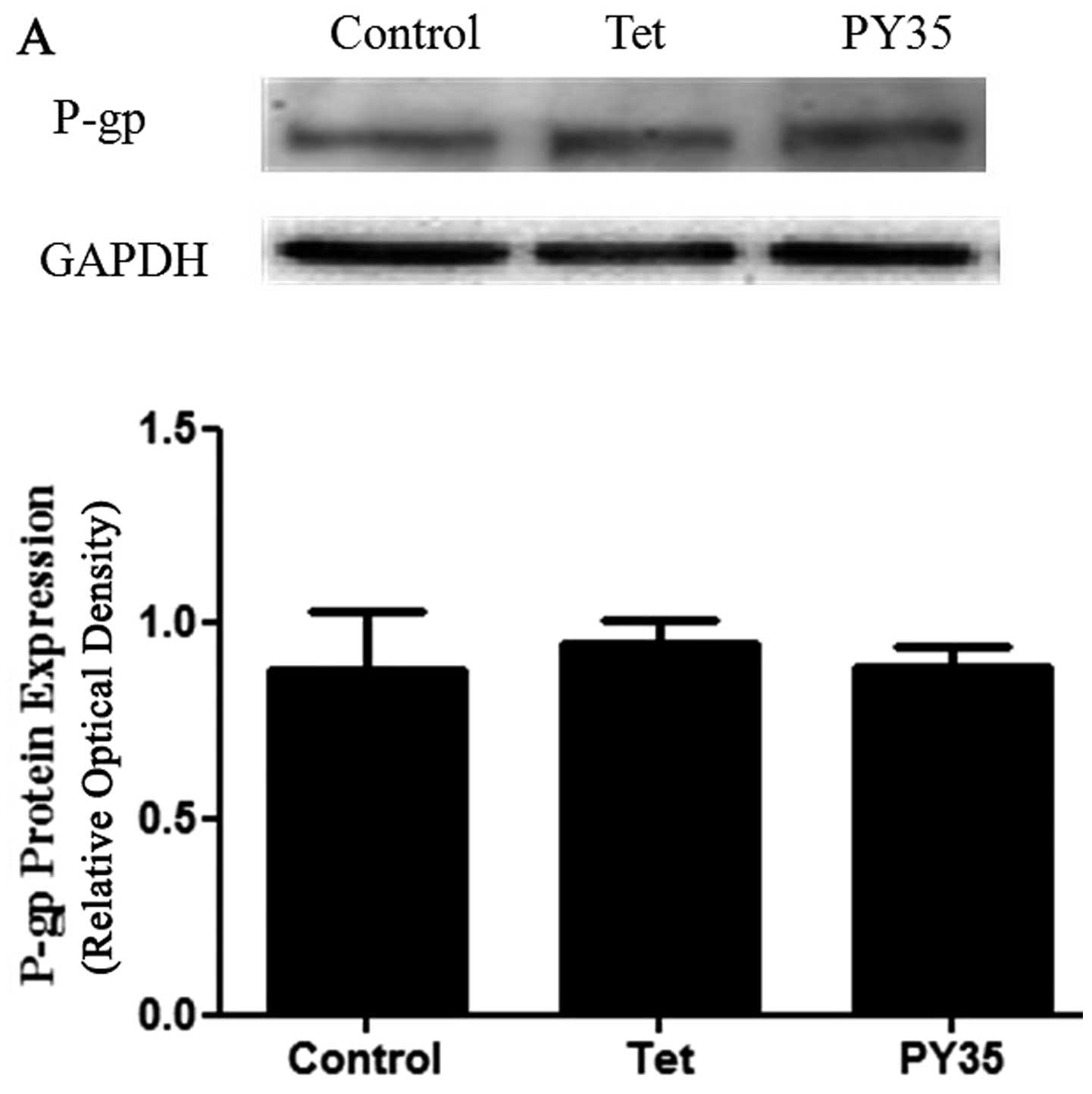
PY35 does not affect P-gp expression
To further investigate the MDR reversal mechanism of
PY35, we analyzed P-gp expression in K562/ADM and MCF-7/ADM cells
after they were treated with PY35 by western blotting; the
expression levels of P-gp were not affected by PY35 (Fig. 9A and B).
Discussion
Multidrug resistance (MDR) remains a major
impediment to successful chemotherapy. To overcome MDR, a wide
series of compounds have been studied as P-gp inhibitors and some
are undergoing clinical trials, although most have been stopped due
to toxicity.
Tetrandrine (Tet) is a benzylisoquinoline alkaloid
and studies have shown that it has a reversal effect on
P-gp-mediated MDR (2,3). It is an extremely potent MDR inhibitor
both in vitro and in vivo, without apparently
enhancing the toxicity of the coadministered drugs (27). PY35 is a novel 5-substituted Tet
derivative and this study showed that its reversal activity was
higher than the Tet; the reversal multiples of PY35 were 29.14 in
K562/ADM and 37.83 in MCF-7/ADM, while the reversal multiples of
Tet were 5.24 in K562/ADM and 22.91 in MCF-7/ADM respectively.
The P-gp functional activity was investigated with
Rho-123. Rho-123 is a fluorescence substrate that is applied to
investigate P-gp functional activity and is a sensitive measure of
MDR (28). When P-gp functional
activity decreased, the accumulation of the Rho-123 substrate
within cells increased and vice versa. The intracellular uptake of
the fluorescent dye Rho-123 was evaluated in two MDR cell lines and
their sensitive counterparts by using FCM and LSCM. After
incubation with Rho-123 for 1 h, the presence of PY35 led to
significantly increased intracellular Rho-123 levels in the
resistant but not sensitive cells. This demonstrates that PY35 can
reverse P-gp-mediated MDR by increasing the intracellular
concentration of Rho-123.
In the present study, the ADM accumulation assay was
used to evaluate the inhibitory effect of PY35 on P-gp in K562/ADM
and MCF-7/ADM cell lines. The results showed that the accumulation
in the cells treated with ADM and PY35 was significantly higher
than in the cells treated with ADM alone. The ADM accumulation
treated with PY35 was more compared to Tet, confirming that PY35
could effectively inhibit the drug efflux compared to Tet (Fig. 8).
P-gp expression and activity of P-gp were generally
viewed as the functional difference between resistant cells and
their sensitive parent cell lines. Western blot analysis showed
that the expression levels of P-gp in resistant cells treated with
Tet and PY35 had no statistical significance in terms of relative
gray-scale value (Figs. 9). The
expression levels of P-gp were not affected by the treatment with
PY35.
In conclusion, in vitro findings indicated
that the MDR reversal activity of PY35 was very high; it could
reverse P-gp-mediated MDR and the coadministration of PY35 and a
cytotoxic agent could increase the intracellular accumulation of
drugs. These findings confirm PY35 as a potential inhibitor to
overcome MDR, exhibiting clinical benefits in cancer treatment.
Our current hypothesis is that PY35 overcomes drug
resistance by blocking the calcium channel, leading to the
concentration of Ca2+ decreases in cells, then the ATP
production declines, thus the ATP-dependent P-gp efflux less
chemotherapeutic drugs. Further studies are required to confirm the
relationship between these pharmaceutical actions, which merits
further attention.
References
|
1
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: an evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gottesman MM: Mechanisms of cancer drug
resistance. Annu Rev Med. 53:615–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Szakacs G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Batrakova EV, Li S, Elmquist WF, Miller
DW, Alakhov VY and Kabanov AV: Mechanism of sensitization of MDR
cancer cells by Pluronic block copolymers: selective energy
depletion. Br J Cancer. 85:1987–1997. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Batrakova EV, Li S, Vinogradov SV, Alakhov
VY, Miller DW and Kabanov AV: Mechanism of pluronic effect on
P-glycoprotein efflux system in blood-brain barrier: contributions
of energy depletion and membrane fluidization. J Pharmacol Exp
Ther. 299:483–493. 2001.PubMed/NCBI
|
|
6
|
Woodhouse JR and Ferry DR: The genetic
basis of resistance to cancer chemotherapy. Ann Med. 27:157–167.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wigler PW: Cellular drug efflux and
reversal therapy of cancer. J Bioenerg Biomembr. 28:279–284. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Robert J and Jarry C: Multidrug resistance
reversal agents. J Med Chem. 46:4805–4817. 2003. View Article : Google Scholar
|
|
9
|
Nobili S, Landini I, Mazzei T and Mini E:
Overcoming tumor multidrug resistance using drugs able to evade
P-glycoprotein or to exploit its expression. Med Res Rev.
32:1220–1262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krishna R and Mayer LD: Multidrug
resistance (MDR) in cancer. Mechanisms, reversal using modulators
of MDR and the role of MDR modulators in influencing the
pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 11:265–283.
2000.PubMed/NCBI
|
|
11
|
Böhme M, Büchler M, Müller M and Keppler
D: Differential inhibition by cyclosporins of primary-active
ATP-dependent transporters in the hepatocyte canalicular membrane.
FEBS Lett. 333:193–196. 1993.PubMed/NCBI
|
|
12
|
Song S, Suzuki H, Kawai R, Tanaka C,
Akasaka I and Sugiyama Y: Dose-dependent effects of PSC 833 on its
tissue distribution and on the biliary excretion of endogenous
substrates in rats. Drug Metab Dispos. 26:1128–1133.
1998.PubMed/NCBI
|
|
13
|
Wandel C, Kim RB, Kajiji S, Guengerich P,
Wilkinson GR and Wood AJ: P-glycoprotein and cytochrome P-450 3A
inhibition: dissociation of inhibitory potencies. Cancer Res.
59:3944–3948. 1999.PubMed/NCBI
|
|
14
|
Bates S, Kang M, Meadows B, et al: A Phase
I study of infusional vinblastine in combination with the
P-glycoprotein antagonist PSC 833 (valspodar). Cancer.
92:1577–1590. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fischer V, Rodriguez-Gascon A, Heitz F, et
al: The multidrug resistance modulator valspodar (PSC 833) is
metabolized by human cytochrome P450 3A. Implications for drug-drug
interactions and pharmacological activity of the main metabolite.
Drug Metab Dispos. 26:802–811. 1998.PubMed/NCBI
|
|
16
|
Kang MH, Figg WD, Ando Y, et al: The
P-glycoprotein antagonist PSC 833 increases the plasma
concentrations of 6alpha-hydroxypaclitaxel, a major metabolite of
paclitaxel. Clin Cancer Res. 7:1610–1617. 2001.PubMed/NCBI
|
|
17
|
Mayur YC, Peters GJ, Prasad VV, Lemo C and
Sathish NK: Design of new drug molecules to be used in reversing
multidrug resistance in cancer cells. Curr Cancer Drug Targets.
9:298–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martelli C, Alderighi D, Coronnello M, et
al: N,N-bis(cyclohexanol)amine aryl esters: a new class of highly
potent transporter-dependent multidrug resistance inhibitors. J Med
Chem. 52:807–817. 2009. View Article : Google Scholar
|
|
19
|
Teodori E, Dei S, Martelli C and Scapecchi
S: N,N-bis(cyclohexanol)amine aryl esters: the discovery of a new
class of highly potent inhibitors of transporter-dependent
multidrug resistance (MDR). Curr Top Med Chem. 10:1715–1731. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang K, Wu J and Li X: Recent advances in
the research of P-glycoprotein inhibitors. Biosci Trends.
2:137–146. 2008.PubMed/NCBI
|
|
21
|
Thomas H and Coley HM: Overcoming
multidrug resistance in cancer: an update on the clinical strategy
of inhibiting P-glycoprotein. Cancer Control. 10:159–165.
2003.PubMed/NCBI
|
|
22
|
Fletcher JI, Haber M, Henderson MJ and
Norris MD: ABC transporters in cancer: more than just drug efflux
pumps. Nat Rev Cancer. 10:147–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
King VF, Garcia ML, Himmel D, et al:
Interaction of tetrandrine with slowly inactivating calcium
channels. Characterization of calcium channel modulation by an
alkaloid of Chinese medicinal herb origin. J Biol Chem.
263:2238–2244. 1988.PubMed/NCBI
|
|
24
|
Wang G and Lemos JR: Tetrandrine: a new
ligand to block voltage-dependent Ca(2+) and Ca(+)-activated K(+)
channels. Life Sci. 56:295–306. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen YJ: Potential role of tetrandrine in
cancer therapy. Acta Pharmacol Sin. 23:1102–1106. 2002.PubMed/NCBI
|
|
26
|
Ludescher C, Gattringer, Drach J, Hofmann
J and Grunicke H: Rapid functional assay for the detection of
multidrug-resistant cells using the fluorescent dye rhodamine 123.
Blood. 78:1385–1387. 1991.PubMed/NCBI
|
|
27
|
Fu LW, Zhang YM, Liang YJ, Yang XP and Pan
QC: The multidrug resistance of tumour cells was reversed by
tetrandrine in vitro and in xenografts derived from human breast
adenocarcinoma MCF-7/adr cells. Eur J Cancer. 38:418–426. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|