Introduction
Osteosarcoma is the most common form of primary
malignant bone cancer in children and adolescents (1), and the 5-year survival rate of
osteosarcoma patients is 61.6% (2).
Although in the last few decades, multi-agent chemotherapy in
combination with surgery has resulted in an improvement in the
patient survival rate, which has increased to 70%, osteosarcoma is
still characterized by frequent relapse and metastatic disease
(3,4). Exploring novel therapeutic agents and
their molecular mechanisms are, therefore, necessary for improving
the outcome of osteosarcoma treatment.
In the present study, we screened 400 natural
compounds against human osteosarcoma U2OS, MG-63 and Saos-2 cells.
Several compounds were found cytotoxic, among which,
isoalantolactone showed strong antiproliferative effects against
osteosarcoma cells. Isoalantolactone has been reported to have a
variety of biological effects, including antifungal, antihelmintic,
and anticancer activities (5). A
previous study by Khan et al showed that isoalantolactone
did not exert any acute or chronic cytotoxicity in mouse liver and
kidney (6). Although there are a
few reports on the anticancer activities of isoalantolactone
(6–10), there is no report available on the
anti-osteosarcoma effect of isoalantolactone.
Apoptosis plays an important role in multiple steps
leading to cell death. Two major pathways are involved in
apoptosis: the extrinsic (death receptor) and the intrinsic
(mitochondrial) pathways (11). The
extrinsic pathways are involved in death receptor-induced
intracellular signaling and the cleavage and activation of
caspase-8 (12,13). Caspase-8 can then cleave effector
caspase-3 to induce apoptosis. The intrinsic cell death pathways
are mediated by modulation of Bcl-2 family proteins, mitochondrial
membrane potential dissipation and cytochrome c release
(14), which subsequently leads to
the activation of caspase-3, resulting in apoptosis. To date, no
report has addressed the role of isoalantolactone in the extrinsic
pathways. The present study was undertaken to investigate the
mechanisms of isoalantolactone-induced apoptosis in osteosarcoma.
Here, our results demonstrated that isoalantolactone inhibits cell
proliferation, triggers S phase and mainly G2/M phase arrest and
induces ROS-dependent apoptosis in U2OS cells via a novel mechanism
involving inhibition of NF-κBp65.
Materials and methods
Chemicals and antibodies
All of the chemicals were purchased from Sigma
unless otherwise stated. Isoalantolactone (purity >98%) was
purchased from Tauto Biotech Co., Ltd. (Shanghai, China). Fetal
bovine serum (FBS) was purchased from Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd. Nuclear protein extraction kit was
purchased from KeyGen (Nanjing, China), and the Annexin V-FITC
apoptosis detection kit, and Bcl-2, caspase-3 and -8 antibodies,
and the caspase inhibitor Z-VAD-FMK were purchased from Beyotime
Institute of Biotechnology (Shanghai, China). PARP, Bax, cyclin B1,
DR5, FADD, TRADD, RIP and NF-κBp65 antibodies were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). β-actin,
secondary anti-mouse and anti-rabbit antibodies were purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture and treatments
Human osteosacoma U2OS, MG-63 and Saos-2 cells were
maintained in DMEM supplemented with 10% FBS, 100 units/ml
penicillin and 100 μg/ml streptomycin at 37°C in a humidified
atmosphere with 5% CO2. The cells were treated with
various concentrations of drug dissolved in dimethyl sulfoxide
(DMSO) with a final concentration <1%. DMSO-treated cells were
used as the control.
Cell proliferation assay
The effects of isoalantolactone on cell viability
were evaluated by MTT assay as previously described (15). U2OS, MG-63 and Saos-2 cells were
treated with various concentrations (0–200 μM) of isoalantolactone
for 24 h. Following treatment, the MTT reagent was added (500
μg/ml), and the cells were further incubated at 37°C for 4 h.
Subsequently 150 μl DMSO was added to dissolve formazan crystals,
and the absorbance was measured at 570 nm in a microplate reader
(Thermo Scientific). IC50 values were calculated using
GraphPad Prism 5.
Flow cytometric analysis of
apoptosis
To determine apoptosis, U2OS cells were treated with
0, 20 or 40 μM isoalantolactone for 24 h. For the caspase inhibitor
and ROS inhibitor analyses, the cells were pretreated with 50 μM
Z-VAD-FMK and 3 mM NAC for 2 h and then incubated with 40 μM
isoalantolactone for 24 h. Following the treatment, the cells were
collected, washed with phosphate-buffered saline (PBS) and then
re-suspended in binding buffer containing Annexin V-FITC and
propidium iodide (PI), and incubated in the dark for 15 min at room
temperature. Then, samples were analyzed by flow cytometry (Beckman
Coulter EPICS XL) for the percentage of apoptotic cells.
Flow cytometric analysis of the cell
cycle
For cell cycle analysis, U2OS cells were treated
with 0, 20 or 40 μM isoalantolactone for 24 h. After the treatment,
the cells were collected, washed with PBS, and fixed with 70%
ethanol at 4°C overnight. After washing twice with PBS, the cells
were stained with a solution containing 50 μg/ml PI and 100 μg/ml
RNase A for 30 min in the dark at room temperature. Cell cycle
profiles were analyzed by flow cytometry (Beckman Coulter EPICS
XL).
Flow cytometric analysis of reactive
oxygen species (ROS) generation and mitochondrial membrane
potential (MMP) in U2OS cells
To determine the ROS generation and MMP, U2OS cells
were stained with 2′,7′-dichlorodihydrofluorescein diacetate
(DCFH-DA) and Rhodamine 123 (Rho-123) respectively, as previously
described (15). Briefly, U2OS
cells were incubated with 0, 20 or 40 μM isoalantolactone for 24 h.
After the treatment, the cells were harvested, washed with PBS, and
then incubated with DCFH-DA (10 μM) or Rho-123 (5 μg/ml) in the
dark for 30 min. After washing, the samples were analyzed for the
fluorescence of DCF or Rhodamine 123 by flow cytometry.
Immunoblotting and
immunoprecipitation
Following drug treatment, adherent and floating
cells were collected and centrifuged. Nuclear and cytosolic
proteins were extracted using a cytosolic and nuclear extraction
kit (KeyGen) according to the manufacturer’s instructions. Total
proteins were isolated as previously described (15). Briefly, U2OS cells were harvested,
and the cell pellets were re-suspended in lysis buffer and lysed on
ice for 30 min. After centrifugation for 15 min, the supernatants
were collected, and the protein concentration was measured by
NanoDrop 1,000 spectrophotometer (Thermo Scientific, USA). A total
of 40 μg protein was electrophoresed on 10–15% SDS-PAGE and
transferred to PVDF (Amersham Biosciences, Piscataway, NJ, USA)
membranes. After being blocked with 5% non-fat milk for 2 h and
washed with TBST, the membranes were incubated overnight at 4°C
with DR5 (1:1,000), FADD (1:1,000), TRADD (1:1,000), RIP (1:1,000),
NF-κBp65 (1:500), cyclin B1 (1:1,000), caspase-8 (1:500), caspase-3
(1:500), cleaved-PARP (1:1,000), Bcl-2 (1:1,000), Bax (1:1,000) and
β-actin (1:500) antibodies, respectively. After washing, the blots
were incubated with relevant secondary antibodies (1:5,000) for 1 h
at room temperature. Signals were detected using the ECL-Plus
chemiluminescence kit (Millipore Corporation) on X-ray film. All of
the bands were quantified by densitometry using ImageJ software.
For the immunoprecipitation (IP), the cells were incubated with
different concentrations of isoalantolactone (0, 20 and 40 μM), and
the lysates were incubated with anti-DR5 and protein G beads (YueKe
Institute of Biotechnology, Shanghai, China) at 4°C overnight.
Immunoblotting was carried out to analyze the association between
DR5 and FADD. The beads were washed three times with IP buffer [50
mM Tris-HCl (pH 7.4), 150 mM NaCl and 1% NP40], boiled in 2X SDS
loading buffer for 10 min, and then analyzed by immunoblotting.
Real-time PCR
The reverse transcription (RT) reaction was
performed using the PrimeScript™ RT Master Mix (Takara) in a final
volume of 10 μl containing 0.5 μg total RNA, 2 μl 5X PrimeScript
buffer, 0.5 μl Oligo(dT) primer, 0.5 μl PrimeScript RT enzyme and
RNase-free water. The RT reaction was performed at 37°C for 15 min
and then terminated by heating at 85°C for 5 sec. Real-time
quantitative PCR using SYBR®-Green (Roche) was performed
with LightCycler 480 Real-Time PCR System. Thermal protocol was as
follows: 95°C for 5 min plus 40 cycles at 95°C for 30 sec, 55°C for
30 sec and 72°C for 45 sec, with final elongation of 10 min at
72°C. The cyclin B1 primers were forward, 5′-GGC CAA AAT GCC TAT
GAA GA-3′ and reverse, 5′-AGA TGT TTC CAT TGG GCT TG-3′. The GAPDH
primers were forward, 5′-CGG AGT CA A CGG ATT TGG TCG TAT-3′ and
reverse, 5′-AGC CTT CTC CAT GGT GGT GAA GAC-3′. Human GAPDH was
measured as the internal control. Data analyses were performed
according to the 2−ΔΔCt method.
Statistical analysis of the data
The results are expressed as means ± standard error
of the mean (SEM) and were statistically compared with the control
group or within groups using one-way ANOVA followed by Tukey’s
multiple comparison test. The level of statistical significance was
regarded as P<0.05. All of the experiments were repeated at
least three times.
Results
Isoalantolactone inhibits proliferation
of U2OS cells
In the present study, we screened natural compounds
and their derivatives against human osteosarcoma U2OS, MG-63 and
Saos-2 cells to identify putative therapeutic compounds.
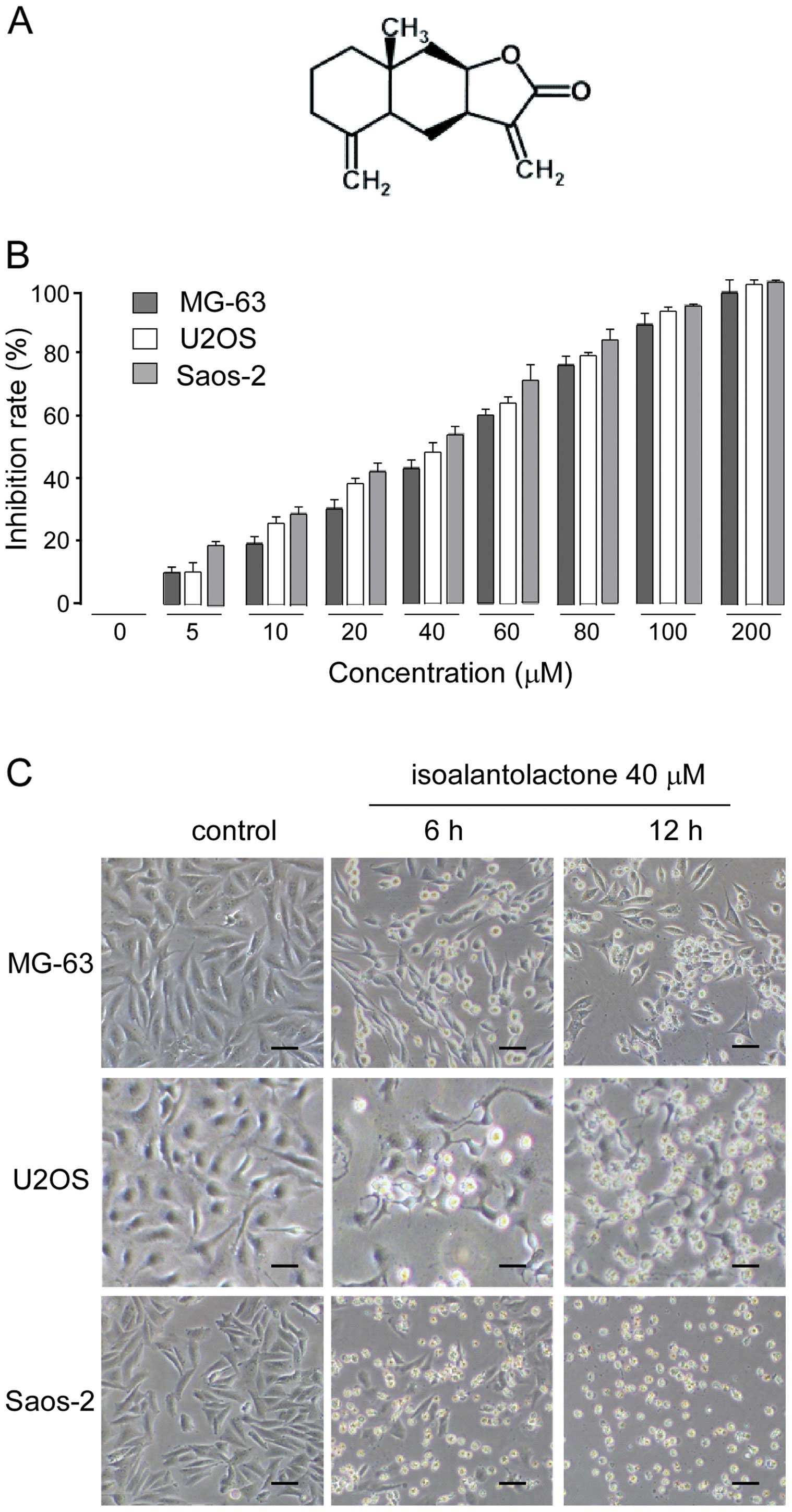
Isoalantolactone (chemical structure shown in Fig. 1A) exhibited a significant
antiproliferative effect on osteosarcoma cell lines in a
dose-dependent manner. The IC50 values of
isoalantolactone against U2OS, MG-63 and Saos-2 cells were 40, 50
and 40 μM, respectively (Fig. 1B).
The cytotoxic effect of isoalantolactone was also assessed by
observing morphological changes in the cells under phase-contrast
microscopy. As shown in Fig. 1C,
the control cells adhered to the well and displayed normal cell
morphology. After isoalantolactone treatment, the cells displayed
significant morphological changes, and acquired a round and
shrunken shape, an increase in floating cells and a reduction in
the total number of cells in a time-dependent manner. The data are
consistent with the results of the cell proliferation inhibition
studies, confirming that isoalantolactone inhibited the
proliferation of osteosarcoma cells.
Isoalantolactone induces S and mainly
G2/M phase cell cycle arrest in U2OS cells
Cell cycle regulation and apoptosis are two major
regulatory mechanisms for cell growth. When specific checkpoints
during the cell cycle are arrested, apoptotic cell death occurs
(16–20). To gain further insight into the
mechanisms underlying the cytotoxic effects of isoalantolactone on
U2OS cells, we next investigated the effect of this compound on the
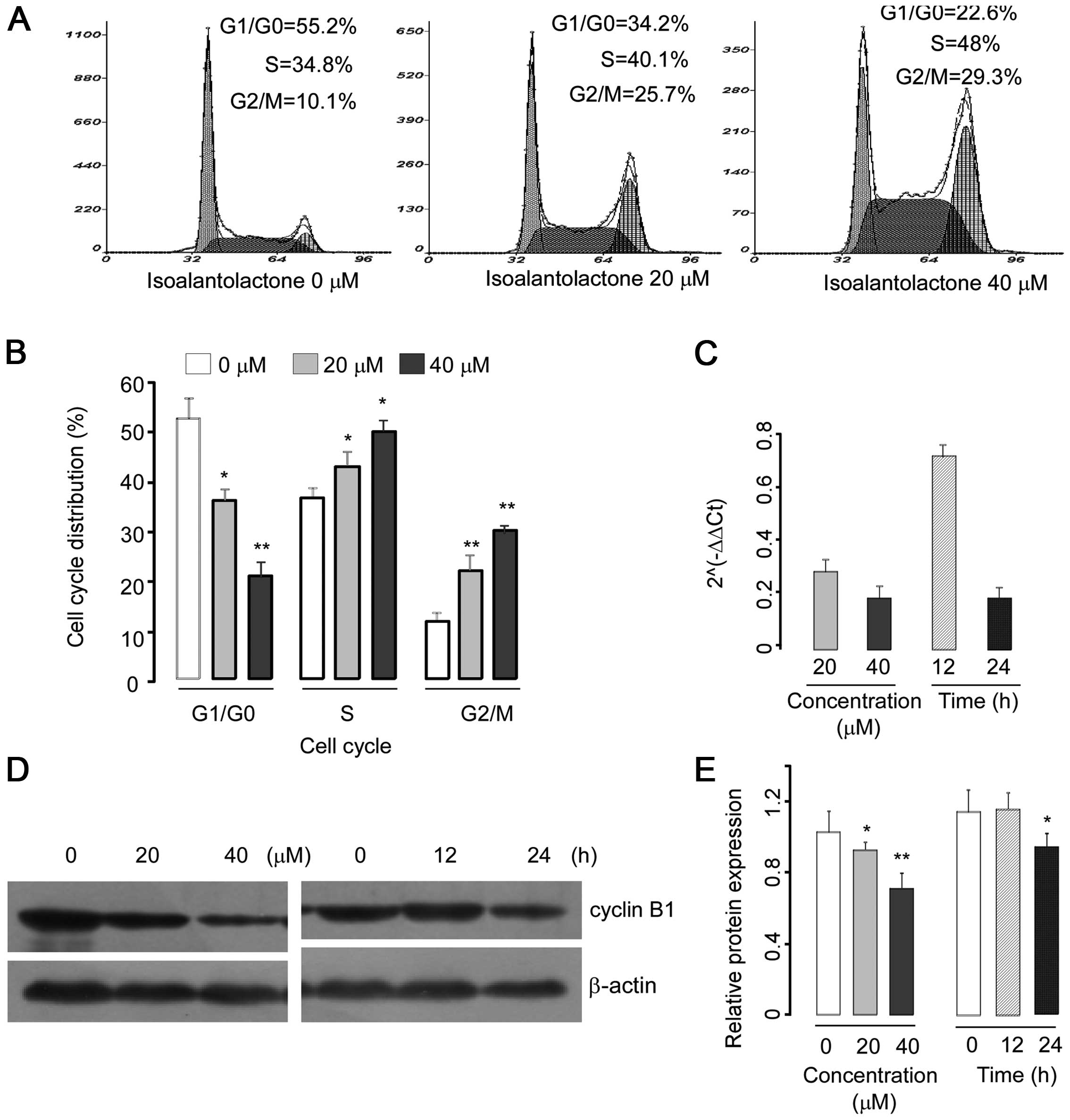
cell cycle phase profile. As shown in Fig. 2A, the percentages of cells that
accumulated in the S phase were 34.8, 40.1 and 48%; in the G2/M
phase, 10.1, 25.7 and 29.3%, following treatment with 0, 20 and 40
μM of isoalantolactone for 24 h, respectively. These results
indicated that isoalantolactone induced cell cycle arrest at the S
and mainly the G2/M phase.
To elucidate the molecular mechanism underlying G2/M
phase arrest, we investigated the expression of cyclin B1, a key
protein involved in the G2/M phase. U2OS cells were treated with
different concentrations of isoalantolactone (0, 20 or 40 μM) for
24 h or with 40 μM isoalantolactone for 0, 12 or 24 h,
respectively. The expression of cyclin B1 was assessed by western
blotting and real-time PCR. The results demonstrated that
isoalantolactone decreased the cyclin B1 expression at the mRNA and
protein levels in a dose- and time-dependent manner (Fig. 2C–E).
Isoalantolactone induces apoptosis in
U2OS cells
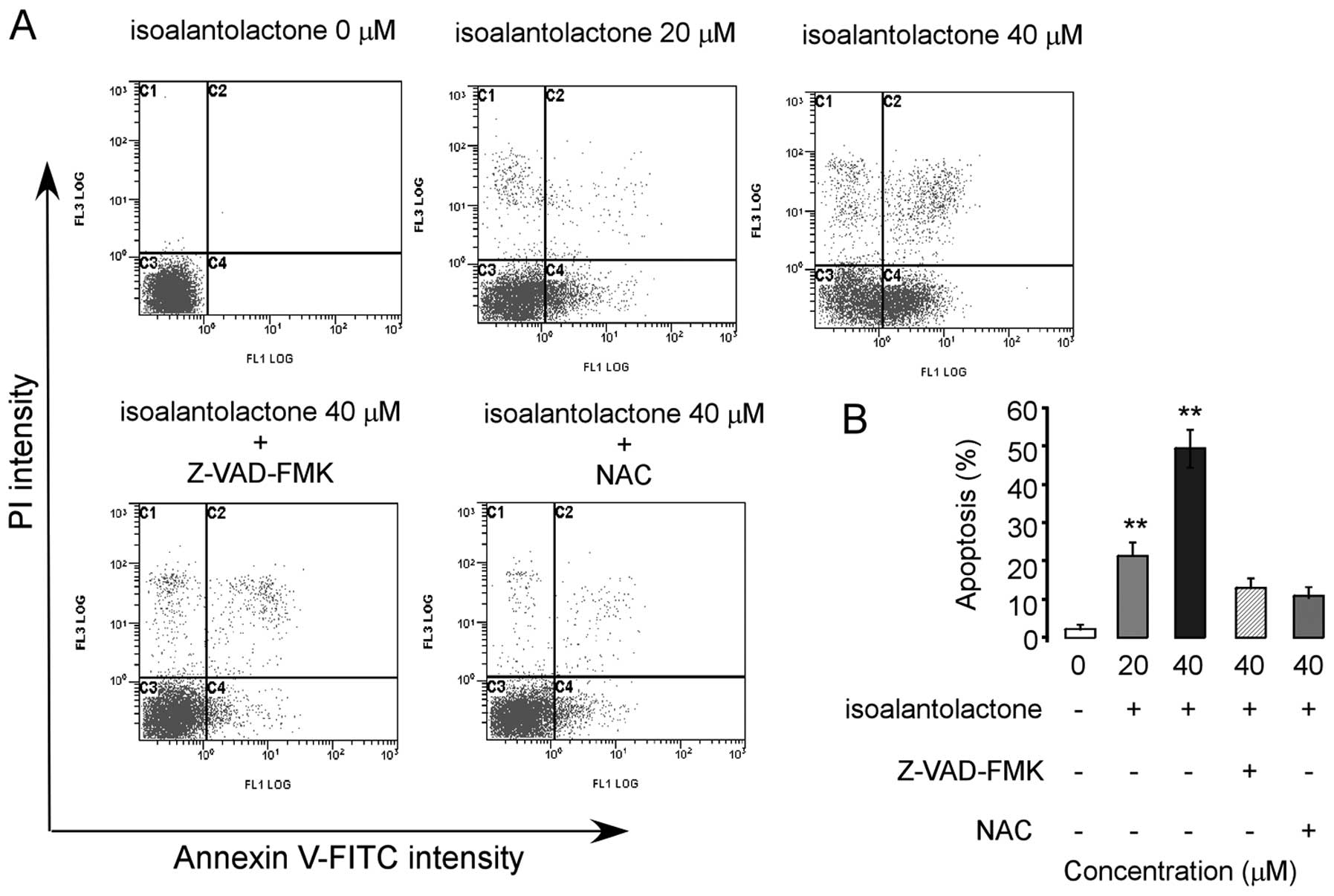
To confirm the nature of isoalantolactone-induced
cell death, the treated cells were stained with Annexin V-FITC and
PI, and the percentages of apoptotic and necrotic cells were
determined by flow cytometry. Our results demonstrated that
isoalantolactone triggered apoptosis in a dose-dependent manner
(Fig. 3). However,
isoalantolactone-induced apoptosis was markedly abrogated when the
cells were pretreated with N-acetylcysteine (NAC), a specific ROS
inhibitor or Z-VAD-FMK, a caspase inhibitor, suggesting that the
apoptosis-inducing effect of isoalantolactone in osteosarcoma cells
was mediated by reactive oxygen species and the caspases.
DR5/FADD/caspase-8 pathway is associated
with the isoalantolactone- induced apoptosis in U2OS cells
Death receptor 5 (DR5) signals apoptosis through
Fas-associating protein with death domain (FADD) and caspase-8
(21). To further explore the
mechanism underlying isoalantolactone-induced apoptosis, we
examined the effect of isoalantolactone on the death receptor
pathways by measuring levels of DR5 and FADD and caspase-8 using
western blotting. The data revealed that isoalantolactone increased
the expression of DR5, FADD and induced the cleavage of caspase-8
in a dose- and time-dependent manner (Fig. 4A–C). The results suggest that the
DR5/FADD/caspase-8 extrinsic pathways are associated with
isoalantolactone-induced apoptosis in osteosarcoma U2OS cells. We
next evaluated the physical interaction between DR5 and FADD in
U2OS cells using Co-IP assay. The results showed that in the
control group DR5 and FADD had almost no combination, while after
treatment with isoalantolactone the combination between DR5 and
FADD was significantly increased (Fig.
4D).
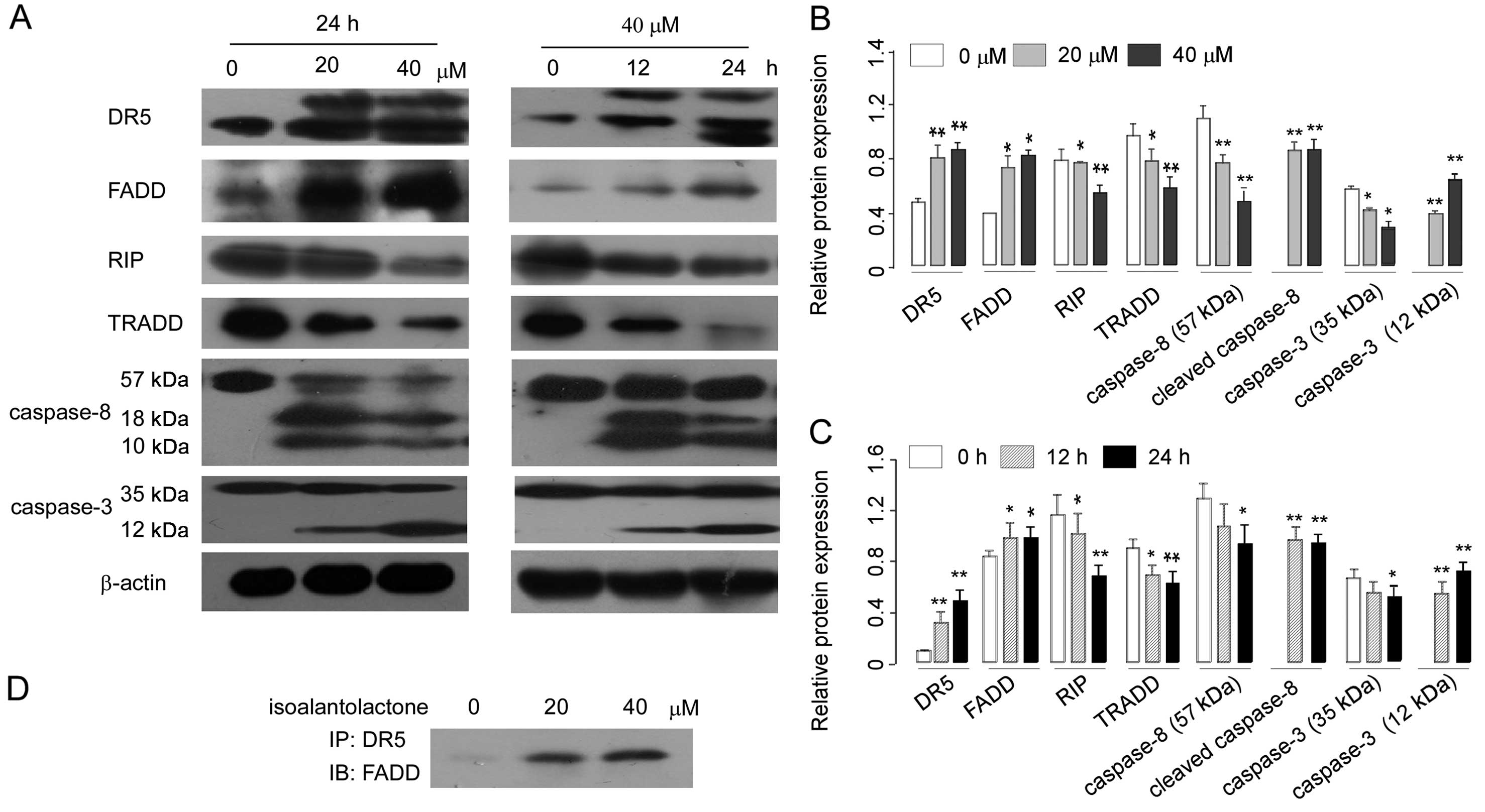 | Figure 4Effect of isoalantolactone on the
expression of death receptor apoptosis pathway proteins in U2OS
cells. (A) Representative images of DR5, FADD, RIP, TRADD, caspase
8 and 3 protein expression in a dose- and time-dependent manner as
detected by western blotting. β-actin was used as a control. (B and
C) The relative protein expression of DR5, FADD, RIP, TRADD,
pro-caspase 8, cleaved caspase 8, pro-caspase 3 and cleaved caspase
3 in a dose- and time-dependent manner. The data are represented as
the means ± SEM (n=3). *P<0.05 and
**P<0.01 compared to the control. (D) The cells were
incubated with different concentration of isoalantolactone (0, 20
and 40 μM), and the lysates were incubated with anti-DR5 and
protein G beads at 4°C overnight. Immunoblotting was carried out to
analyze the association between DR5 and FADD. IP,
immunoprecipitation; IB, immunoblotting. |
Many sesquiterpene lactone compounds have been
reported to induce apoptosis through inhibition of NF-κB activation
in various cancer cell lines (22,23),
and previous research suggests that DR5 can use a TRADD-dependent
pathway to activate NF-κB, which is independent of their ability to
induce apoptosis (24). Here, we
aimed to ascertain whether the apoptotic effect of isoalantolactone
in U2OS cells is associated with NF-κB. For this, levels of
TNF-receptor-associated death domain protein (TRADD),
receptor-interacting protein (RIP), and nuclear protein NF-κBp65
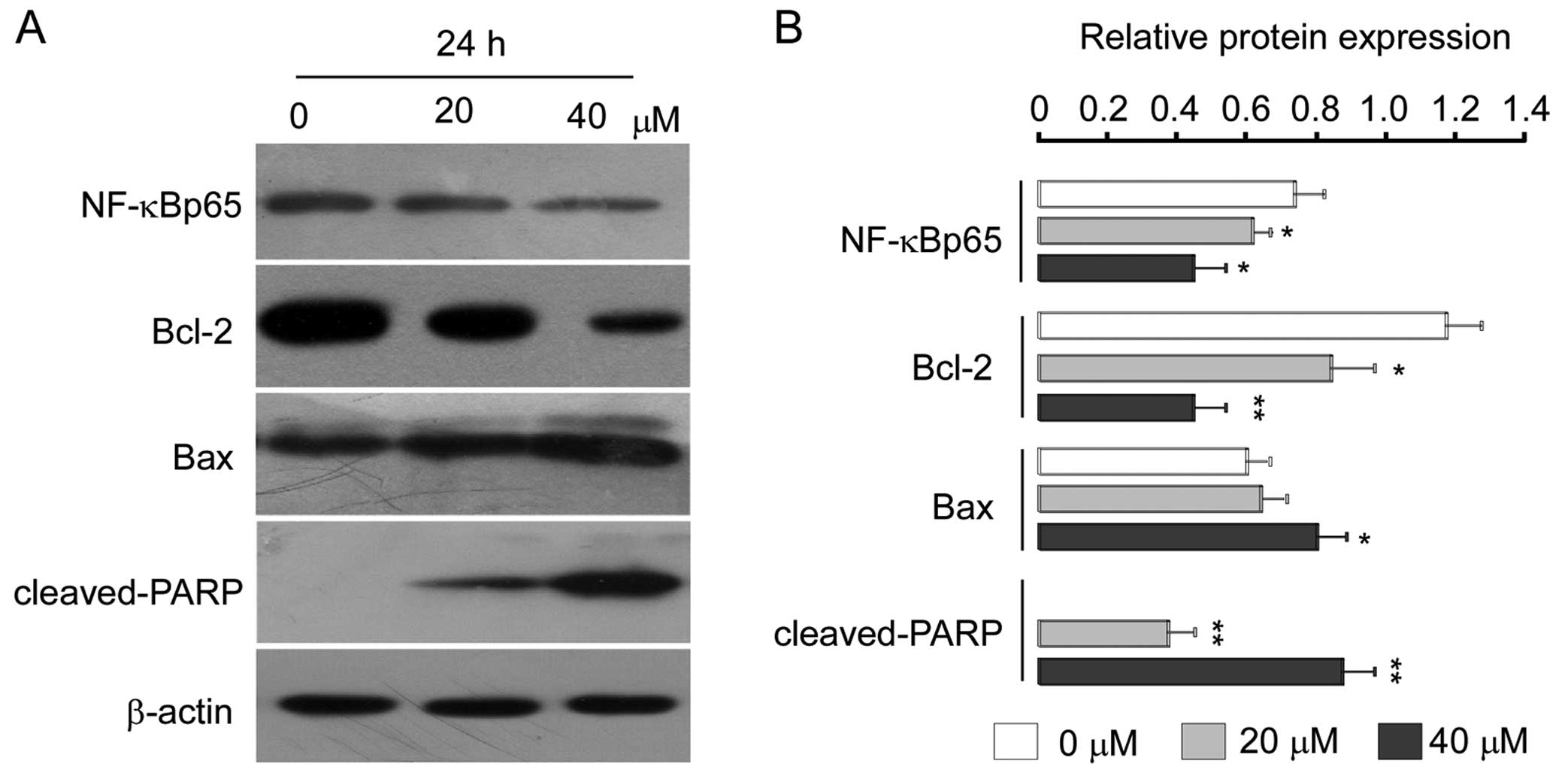
were assessed in the U2OS cells. Western blot analysis revealed
that treatment with isoalantolactone decreased the levels of TRADD
and RIP (Fig. 4A–C) and decreased
the levels of nuclear NF-κBp65 in a dose-dependent manner (Fig. 6). Further studies are required to
examine the association of these proteins after treatment of
isoalantolactone.
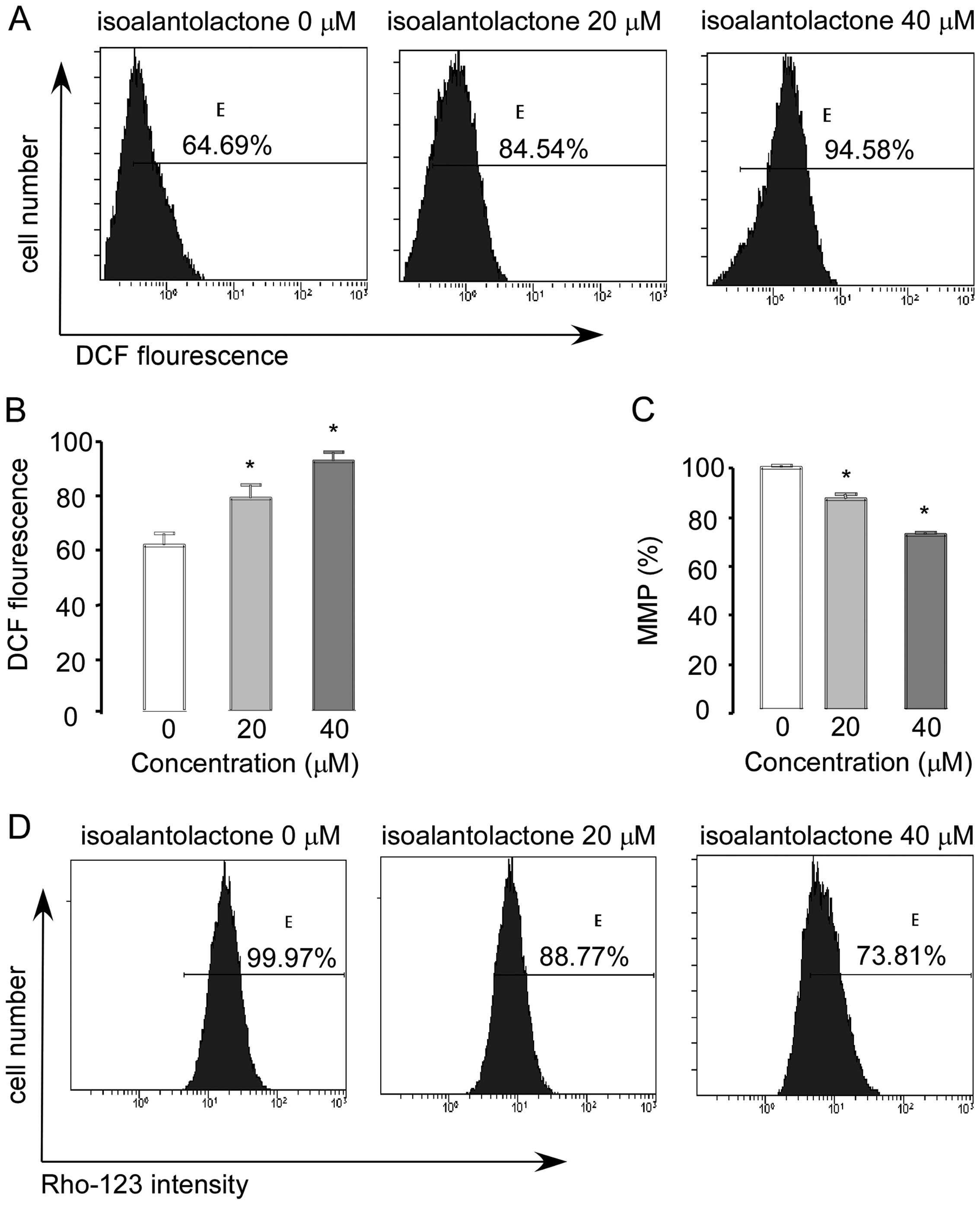
Effect of isoalantolactone on ROS
generation and mitochondrial membrane potential
Recent evidence suggests that ROS serve as
messengers for normal signal transductions at lower concentrations;
at higher concentrations, however, they become toxic and induce
cell death through various signaling pathways (25). As cancer cells contain a higher
level of ROS than normal cells, they can be easily poisoned by the
phytochemical targeting of ROS metabolism (25,26).
Many studies have reported that isoalantolactone-induced apoptosis
is linked with ROS generation (5,6). Here,
we hypothesized that the effect of isoalantolactone on U2OS cells
is associated with ROS. Therefore, we analyzed the level of ROS by
flow cytometry. The levels of ROS in the cells treated with 0, 20
and 40 μM isoalantolactone for 24 h were 64.69, 82.54 and 94.58%.
Our results demonstrated that isoalantolactone treatment
significantly increased ROS generation in the U2OS cells in a
dose-dependent manner (Fig. 5A and
B).
Next, we determined the effect of isoalantolactone
on mitochondrial membrane potential in U2OS cells. As shown in
Fig. 5C and D, the levels of MMP in
the cells treated with 0, 20 and 40 μM isoalantolactone for 24 h
were 99.97, 88.77 and 73.81%; Rho123 was significantly dissipated
in U2OS cells in a dose-dependent manner (Fig. 5C and D). These findings clearly
indicate that isoalantolactone-induced apoptosis is associated with
ROS generation and MMP disruption.
Isoalantolactone modulates Bcl-2 family
proteins
To further characterize this cell-specific apoptotic
effect of isoalantolactone in osteosarcoma U2OS cells, we analyzed
the levels of Bcl-2 family proteins after treatment with
isoalantolactone (0, 20 and 40 μM) in a dose-dependent manner. We
observed that expression of anti-apoptotic Bcl-2 was downregulated,
whereas the expression level of pro-apoptotic Bax was markedly
upregulated (Fig. 6). To examine
whether regulation of caspase-3 is necessary for
isoalantolactone-induced apoptosis, we investigated the expression
level of caspase-3 after treatment with 0, 20 and 40 μM
isoalantolactone for 24 h. As shown in Fig. 4A, isoalantolactone treatment was
found to result in a significant increase in cleaved caspase-3, and
its downstream target, PARP (Fig.
6) in a dose-dependent manner which is indicative of the
induction of apoptosis.
Discussion
Osteosarcoma is one of the most common primary
malignant tumors. Although neoadjuvant chemotherapy has improved
the overall survival of patients over the last several years, the
metastatic and toxic effects of chemotherapy still remain a major
drawback in the treatment of osteosarcoma patients. The goal of the
present study was to identify novel anti-osteosarcoma agents from
natural herbal compounds. Isoalantolactone did not exert any acute
or chronic cytotoxicity in liver and kidney, and sesquiterpene
lactones have drawn considerable attention in pharmacological
research due to their anti-neoplastic, anti-parasitic,
anti-inflammatory and antitumor activities (27–29).
However, its effects on osteosarcoma remained unexplored. In the
present study, we found that isoalantolactone strongly inhibited
the proliferation of osteosarcoma cells in a dose-dependent manner.
Therefore, we selected isoalantolactone compound to study the
molecular mechanisms of its antiproliferative effect against
osteosarcoma U2OS cells.
Isoalantolactone has been reported to induce cell
death in several human cancer cell lines, such as PANC-1 cells
(6), human prostate cancer cells
(8), and human gastric
adenocarcinoma SGC-7901 cells (9)
via cell cycle arrest and induction of apoptosis. In line with
previous reports, we found that isoalantolactone arrested the cell
cycle at the S and mainly the G2/M phase in U2OS cells. Cyclin B1,
the key protein in the regulation of G2/M transition, forms a
complex with cdc2 which is crucial in the transition from the G2 to
the M phase (30). Our results
demonstrated that isoalantolactone downregulated the expression of
cyclin B1 at the mRNA and protein levels in a dose- and
time-dependent manner. These data are in agreement with those of a
previous study (31), suggesting
that decreased expression of cyclin B1 may be a molecular mechanism
through which isoalantolactone induces S and mainly G2/M phase
arrest.
DR5 is a member of the tumor necrosis factor
receptor family, which plays an important role in the mediation of
extrinsic pathways. These receptors possess a cytoplasmic region
called the death domain (DD) (32,33).
Overexpression of death domain-containing receptors can induce
apoptosis in a ligand-independent manner (34,35).
Transient transfection of a full-length DR5 construct was found to
induce rapid apoptosis (24).
Bodmer et al (21) reported
that DR5 can recruit FADD through its death effector domain, and
FADD binds to caspase-8. Caspase-8 is the most proximal caspase in
the cascade of caspases, the activation of which eventually leads
to cell death (36). Overall, these
studies suggest that activation of the DR5/FADD/caspase-8 extrinsic
pathways may have therapeutic value for cancer patients. In the
present study, we found that isoalantolactone treatment increased
the expression of DR5 and FADD, and induced the binding of FADD to
DR5. It also decreased the expression of pro-caspase-8 and
increased the cleavage of caspase-8 in U2OS cells. The data clearly
demonstrated that isoalantolactone-induced apoptosis is associated
with the activation of DR5/FADD/caspase-8 extrinsic pathways. To
the best of our knowledge, this is the first report indicating that
isoalantolactone- induced apoptosis is associated with
DR5/FADD/caspase-8 extrinsic pathways.
Death domains are also found in TRADD and RIP, two
cytoplasmic adaptor proteins implicated in the mediation of
apoptosis by the death domain-containing receptors (37,38).
Overexpression studies have demonstrated that TRADD is required for
recruitment of several important molecules, including RIP, which
are indispensable for the activation of NF-κB and cell survival
(38,39). NF-κB is a transcription factor,
which can promote cell survival and proliferation. The constitutive
activation of NF-κB is associated with numerous types of cancers
(40–42). In the present study, we examined the
expression of nuclear protein NF-κBp65 and its regulatory proteins
TRADD and RIP. Our data indicated that isoalantolactone inhibits
NF-κB activation, and whether this is associated with TRADD and
RIP, more research needs to be performed to find the link between
these proteins after treatment of isoalantolactone.
Mitochondria play a critical role in anticancer
drug-induced apoptosis (43). The
Bcl-2 family proteins, which play a pivotal role in the intrinsic
apoptosis pathways, include both anti-apoptotic and pro-apoptotic
proteins such as Bcl-2 and Bax, respectively. The balance between
these two classes of proteins is critical for determining whether a
cell undergoes apoptosis or not (44–46).
ROS generation can inhibit the anti-apoptotic protein Bcl-2 and
activate the pro-apoptotic protein Bax to the outer mitochondrial
membrane (47,48). In the present study, we found that
isoalantolactone increased ROS generation, and the ROS inhibitor,
NAC, markedly reduced isoalantolactone-induced apoptosis in the
U2OS cells. Our results revealed that isoalantolactone-induced
apoptosis in U2OS cells was ROS-dependent. Moreover, flow
cytometric analysis of MMP showed that isoalantolactone led to
dissipation of mitochondrial membrane potential, and western blot
analysis of apoptosis-related proteins in U2OS cells revealed that
isoalantolactone downregulated the expression of Bcl-2 and
upregulated the expression of Bax, activated caspase-3 and its
downstream substrate PARP. Therefore, we conclude that
isoalantolactone promotes opening of the mitochondrial PTP by
increasing the Bax/Bcl-2 ratio and the isoalantolactone-induced
apoptosis is associated with intrinsic pathways.
In conclusion, our data provide evidence that
isoalantolactone inhibits the growth of human osteosarcoma cells by
inducing apoptosis. The present study is the first to describe the
role of ROS in the induction of apoptosis in osteosarcoma cells. In
addition, in the present study, we also found that isoalantolactone
upregulated DR5, FADD and cleaved caspase-8, increased the
interaction between DR5 and FADD, and inhibited the expression of
nuclear NF-κBp65, which, to our knowledge, is the first observation
that isoalantolactone upregulates expression of these proteins and
inhibits NF-κBp65 in osteosarcoma U2OS cells. The pathway that we
have described herein is novel and has not been previously
elucidated. Furthermore, isoalantolactone induced apoptosis in U2OS
cells by modulating mitochondrial Bcl-2 family proteins and
caspase-3. These findings support a prominent insight into how
isoalantolactone exerts its cytotoxic effect against osteosarcoma
cells. Thus, isoalantolactone may become a potential lead compound
for future development of anti-osteosarcoma therapy. Further
investigation is needed to validate the contribution of
isoalantolactone to tumor therapy in vivo.
References
|
1
|
Broadhead ML, Clark JC, Myers DE, Dass CR
and Choong PF: The molecular pathogenesis of osteosarcoma: a
review. Sarcoma. 2011:9592482011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kempf-Bielack B, Bielack SS, Jürgens H, et
al: Osteosarcoma relapse after combined modality therapy: an
analysis of unselected patients in the Cooperative Osteosarcoma
Study Group (COSS). J Clin Oncol. 23:559–568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu M, Zhang H, Hu J, et al:
Isoalantolactone inhibits UM-SCC-10A cell growth via cell cycle
arrest and apoptosis induction. PLoS One. 8:e760002013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan M, Ding C, Rasul A, et al:
Isoalantolactone induces reactive oxygen species mediated apoptosis
in pancreatic carcinoma PANC-1 cells. Int J Biol Sci. 8:533–547.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Konishi T, Shimada Y, Nagao T, Okabe H and
Konoshima T: Antiproliferative sesquiterpene lactones from the
roots of Inula helenium. Biol Pharm Bull. 25:1370–1372.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rasul A, Khan M, Yu B, et al:
Isoalantolactone, a sesquiterpene lactone, induces apoptosis in
SGC-7901 cells via mitochondrial and phosphatidylinositol
3-kinase/Akt signaling pathways. Arch Pharm Res. 36:1262–1269.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rasul A, Di J, Millimouno FM, et al:
Reactive oxygen species mediate isoalantolactone-induced apoptosis
in human prostate cancer cells. Molecules. 18:9382–9396. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seo JY, Park J, Kim HJ, et al:
Isoalantolactone from Inula helenium caused Nrf2-mediated
induction of detoxifying enzymes. J Med Food. 12:1038–1045.
2009.
|
|
11
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mühlethaler-Mottet A, Bourloud KB,
Auderset K, Joseph JM and Gross N: Drug-mediated sensitization to
TRAIL-induced apoptosis in caspase-8-complemented neuroblastoma
cells proceeds via activation of intrinsic and extrinsic pathways
and caspase-dependent cleavage of XIAP, Bcl-xL and RIP.
Oncogene. 23:5415–5425. 2004.
|
|
13
|
Kamachi M, Le TM, Kim SJ, Geiger ME,
Anderson P and Utz PJ: Human autoimmune sera as molecular probes
for the identification of an autoantigen kinase signaling pathway.
J Exp Med. 196:1213–1225. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang D, Lahti JM and Kidd VJ: Caspase-8
activation and bid cleavage contribute to MCF7 cellular execution
in a caspase-3-dependent manner during staurosporine-mediated
apoptosis. J Biol Chem. 275:9303–9307. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khan M, Yi F, Rasul A, et al:
Alantolactone induces apoptosis in glioblastoma cells via GSH
depletion, ROS generation, and mitochondrial dysfunction. IUBMB
Life. 64:783–794. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu YJ, Yang SH, Chien CM, et al: Induction
of G2/M phase arrest and apoptosis by a novel enediyne derivative,
THDB, in chronic myeloid leukemia (HL-60) cells. Toxicol In Vitro.
21:90–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Torres K and Horwitz SB: Mechanisms of
Taxol-induced cell death are concentration dependent. Cancer Res.
58:3620–3626. 1998.PubMed/NCBI
|
|
18
|
Gamet-Payrastre L, Li P, Lumeau S, et al:
Sulforaphane, a naturally occurring isothiocyanate, induces cell
cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer
Res. 60:1426–1433. 2000.PubMed/NCBI
|
|
19
|
Murray AW: Recycling the cell cycle:
cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Orren DK, Petersen LN and Bohr VA:
Persistent DNA damage inhibits S-phase and G2
progression, and results in apoptosis. Mol Biol Cell. 8:1129–1142.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bodmer JL, Holler N, Reynard S, et al:
TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat
Cell Biol. 2:241–243. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghantous A, Gali-Muhtasib H, Vuorela H,
Saliba NA and Darwiche N: What made sesquiterpene lactones reach
cancer clinical trials? Drug Discov Today. 15:668–678. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lyss G, Knorre A, Schmidt TJ, Pahl HL and
Merfort I: The anti-inflammatory sesquiterpene lactone helenalin
inhibits the transcription factor NF-κB by directly targeting p65.
J Biol Chem. 273:33508–33516. 1998.
|
|
24
|
Chaudhary PM, Eby M, Jasmin A, Bookwalter
A, Murray J and Hood L: Death receptor 5, a new member of the TNFR
family, and DR4 induce FADD-dependent apoptosis and activate the
NF-κB pathway. Immunity. 7:821–830. 1997.PubMed/NCBI
|
|
25
|
Juan ME, Wenzel U, Daniel H and Planas JM:
Resveratrol induces apoptosis through ROS-dependent mitochondria
pathway in HT-29 human colorectal carcinoma cells. J Agric Food
Chem. 56:4813–4818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schumacker PT: Reactive oxygen species in
cancer cells: live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu JQ, Gills JJ, Park EJ, et al:
Sesquiterpenoids from Tithonia diversifolia with potential
cancer chemopreventive activity. J Nat Prod. 65:532–536.
2002.PubMed/NCBI
|
|
28
|
Koch E, Klaas CA, Rüngeler P, et al:
Inhibition of inflammatory cytokine production and lymphocyte
proliferation by structurally different sesquiterpene lactones
correlates with their effect on activation of NF-κB. Biochem
Pharmacol. 62:795–801. 2001.PubMed/NCBI
|
|
29
|
Nam NH: Naturally occurring NF-κB
inhibitors. Mini Rev Med Chem. 6:945–951. 2006.
|
|
30
|
Lee SM, Kwon JI, Choi YH, Eom HS and Chi
GY: Induction of G2/M arrest and apoptosis by water extract of
Strychni Semen in human gastric carcinoma AGS cells.
Phytother Res. 22:752–758. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rasul A, Yu B, Zhong L, Khan M, Yang H and
Ma T: Cytotoxic effect of evodiamine in SGC-7901 human gastric
adenocarcinoma cells via simultaneous induction of apoptosis and
autophagy. Oncol Rep. 27:1481–1487. 2012.PubMed/NCBI
|
|
32
|
Scott FL, Stec B, Pop C, et al: The
Fas-FADD death domain complex structure unravels signalling by
receptor clustering. Nature. 457:1019–1022. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guicciardi ME and Gores GJ: Life and death
by death receptors. FASEB J. 23:1625–1637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gaeta ML, Johnson DR, Kluger MS and Pober
JS: The death domain of tumor necrosis factor receptor 1 is
necessary but not sufficient for Golgi retention of the receptor
and mediates receptor desensitization. Lab Invest. 80:1185–1194.
2000. View Article : Google Scholar
|
|
35
|
Sheikh MS and Fornace AJ Jr: Death and
decoy receptors and p53-mediated apoptosis. Leukemia. 14:1509–1513.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ramaswamy M, Efimova EV, Martinez O,
Mulherkar NU, Singh SP and Prabhakar BS: IG20 (MADD splice
variant-5), a proapoptotic protein, interacts with DR4/DR5 and
enhances TRAIL-induced apoptosis by increasing recruitment of FADD
and caspase-8 to the DISC. Oncogene. 23:6083–6094. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
MacEwan DJ: TNF ligands and receptors - a
matter of life and death. Br J Pharmacol. 135:855–875. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stanger BZ, Leder P, Lee TH, Kim E and
Seed B: RIP: a novel protein containing a death domain that
interacts with Fas/APO-1 (CD95) in yeast and causes cell death.
Cell. 81:513–523. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hsu H, Shu HB, Pan MG and Goeddel DV:
TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF
receptor 1 signal transduction pathways. Cell. 84:299–308. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bharti AC, Donato N, Singh S and Aggarwal
BB: Curcumin (diferuloylmethane) down-regulates the constitutive
activation of nuclear factor-κB and IκBα kinase in human multiple
myeloma cells, leading to suppression of proliferation and
induction of apoptosis. Blood. 101:1053–1062. 2003.PubMed/NCBI
|
|
41
|
Li Q, Yu YY, Zhu ZG, et al: Effect of
NF-κB constitutive activation on proliferation and apoptosis of
gastric cancer cell lines. European surgical research. Eur Surg
Res. 37:105–110. 2005.
|
|
42
|
Shen HM and Tergaonkar V: NFκB signaling
in carcinogenesis and as a potential molecular target for cancer
therapy. Apoptosis. 14:348–363. 2009.
|
|
43
|
Jeong SY and Seol DW: The role of
mitochondria in apoptosis. BMB Rep. 41:11–22. 2008. View Article : Google Scholar
|
|
44
|
Danial NN: BCL-2 family proteins: critical
checkpoints of apoptotic cell death. Clin Cancer Res. 13:7254–7263.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Frenzel A, Grespi F, Chmelewskij W and
Villunger A: Bcl2 family proteins in carcinogenesis and the
treatment of cancer. Apoptosis. 14:584–596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wei W, Huang H, Zhao S, et al:
Alantolactone induces apoptosis in chronic myelogenous leukemia
sensitive or resistant to imatinib through NF-κB inhibition and
Bcr/Abl protein deletion. Apoptosis. 18:1060–1070. 2013.
|
|
47
|
Felty Q, Xiong WC, Sun D, et al:
Estrogen-induced mitochondrial reactive oxygen species as
signal-transducing messengers. Biochemistry. 44:6900–6909. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jabs T: Reactive oxygen intermediates as
mediators of programmed cell death in plants and animals. Biochem
Pharmacol. 57:231–245. 1999. View Article : Google Scholar : PubMed/NCBI
|