Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cancer and the third most frequent cause of cancer-related
mortality globally, affecting >600,000 individuals annually
(1,2). Although there have been developments
in surgical strategies and percutaneous techniques such as ethanol
injection and radiofrequency ablation and transcatheter arterial
chemoembolization (TACE), the overall outcome for HCC patients
remains poor as HCC is commonly detected at a late stage when
therapeutic options are limited (3). Conventional cytotoxic therapies may
result in significant morbidity in patients with solid tumors
including HCC since these drugs affect rapidly dividing normal and
malignant cells, thereby limiting the survival of normal cells
(4). Therefore, it is necessary to
improve anticancer therapies that effectively and specifically
target liver tumor cells while minimizing the toxic side-effects
commonly associated with conventional cytotoxic therapies.
Sorafenib (SOR) (Nexavar, BAY 43-9006), an oral
multikinase small molecule inhibitor, has been shown to have
significant antitumor activity against various types of cancer
including HCC (5–7). SOR blocks tumor cell proliferation and
angiogenesis by targeting the Raf/mitogen-activated protein kinase
(MEK)/extracellular signal-regulated kinase (ERK) signaling pathway
and receptor tyrosine kinases (RTKs), such as vascular endothelial
cell growth factor receptor (VEGFR)-2, VEGFR-3, platelet-derived
growth factor receptor-β, fms-like tyrosine kinase receptor-3
(FLT3), RET and c-KIT (5,6). The results of phase III trials in
Europe and Asia showed that SOR increased the survival rate in
patients with advanced HCC (8,9).
Although results of SOR for patients with advanced HCC are
encouraging, treatment outcomes are poor due to unfavorable
pharmacokinetics, low tumor accumulation and other adverse effects
(10). Several studies have
demonstrated that combination therapies of SOR and TRAIL or other
chemotherapeutic agent are effective for HCC (11–18).
However, chemotherapeutic drug resistance often occurs and the
management of multi-drug resistant and recurrent or refractory
tumors pose a challenge for clinical oncologists.
A class of disintegrins and metalloproteinases,
known as ADAMs, has been shown to be involved in a variety of
signaling events that are aberrant in cancers as well as during
tumor progression (19). A
disintegrin and metalloproteinase 10 (ADAM10), a member of the ADAM
family, has been found to be upregulated in various types of cancer
and contributes to cancer progression and metastasis (20,21).
Consistent with these findings, our recent results showed that
ADAM10 is overexpressed in HCC tissues and there were significant
associations between the protein levels of ADAM10 and tumor grade,
amount of tumor differentiation, tumor size and the presence of
metastasis (22). In addition, the
RNA interference (RNAi)-mediated downregulation of endogenous
ADAM10 was found to decrease the cell migration and invasion of HCC
(23). Yang et al found that
ADAM10 plays an important role in modulating the chemosensitivity
of HCC cells to doxorubicin (24).
Findings of that study suggested that ADAM10 is involved in HCC
progression and metastasis and is important in modulating the
chemosensitivity of HCC. However, little is known regarding the
role of ADAM10 in the modulation of chemosensitivity of HCC cells
to SOR. Thus, in the present study, we investigated the effects of
modulating ADAM10 expression on the sensitivity of HCC cells to SOR
treatment and examined the molecular pathways involved. In
addition, tumor growth ability in nude mice was detected to define
SOR in combination with the siRNA-ADAM10 treatment effect on
tumorigenesis in vivo.
Materials and methods
Reagents
SOR (BAY 43-9006; Nexavar, LC Laboratories, Woburn,
MA, USA) was dissolved in sterile dimethyl sulfoxide (DMSO; Sigma,
St. Louis, MO, USA) for the in vitro experiments. DMSO was
added to cultures at 0.1% (v/v) final concentration as a vehicle
control. PI3K and phosphorylated PI3K (p-PI3K; Tyr458); Akt and
phosphorylated Akt (p-Akt; S473) primary antibodies were purchased
from Cell Signaling Technology (Beverly, MA, USA).
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ADAM10, MMP-2 and
MMP-9 were purchased from Sigma-Aldrich (St. Louis, MO, USA). The
HRP-conjugated goat anti-mouse IgG secondary antibody was obtained
from Amersham Biosciences (Uppsala, Sweden).
Cell culture
The human HCC cell lines, HepG2, was purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
HepG2 cells were cultured in Dulbecco’s modified Eagle’s medium
(DMEM) (Sigma-Aldrich) supplemented with 1% penicillin/streptomycin
(Gibco-BRL, Grand Island, NY, USA) and 10% heat-inactivated fetal
calf serum (FCS) (Invitrogen, Carlsbad, CA, USA).
Preparation of plasmid-based ADAM10 shRNA
vector and transfect in HepG2
The ADAM10 small-interfering RNA (siRNA)
(CAGTGTGCATTCAAGTCAA) and scrambled control (AATTCTCCGAACGTGTCACGT)
sequences, which do not target any gene product or have any
significant sequence similar to the human gene sequences, were
designed using siRNA Target Designer software (Promega, Madison,
WI, USA). The human ADAM10 and scrambled control short hairpin RNA
(shRNA) were synthesized (Shanghai GeneChem Co., Ltd., China) and
cloned into the pSUPER siRNA expression plasmid with the U6
promoter (Oligoengine, Seattle, WA, USA) as previously described
(25) and designated as psi-ADAM10
and psi-Scramble, respectively.
HepG2 cells were transduced with the plasmid
psi-ADAM10 and psi-Scramble using Lipofectamine™ 2000 transfection
reagent according to the manufacturer’s instructions. G418 (300
μg/ml; Sigma) was used to screen stably transfected clones. The
expression of ADAM10 was examined by quantitative RT-PCR (RT-qPCR)
and western blotting with an antibody against ADAM10 to validate
the silencing efficiency of the target gene after RNAi.
Quantitative RT-PCR
RT-qPCR for ADAM10 transcripts in HepG2 cells was
performed. First, total RNA was extracted from cultured cells using
TRIzol reagent (Invitrogen) according to the manufacturer’s
instructions. RNA was subsequently reverse-transcribed into cDNA by
a PrimeScript™ RT reagent kit according to the manufacturer’s
instructions (Takara, Dalian, China). RT-qPCR was conducted using
the SYBR-Green fluorescent dye method, and a Rotor Gene 3000
real-time PCR apparatus. ADAM10 gene-specific amplification was
confirmed by PCR with specific primers (sense,
5′-CTGCCCAGCATCTGACCCTAA-3′ and antisense,
5′-TTGCCATCAGAACTGGCACAC-3′) and subjected to melting curve
analysis. GAPDH was used as an internal control for
standardization. The primer sequences used were: β-actin, forward:
5′-GATCATTGCTCCTCCTGAGC-3′ and reverse: 5′-ACTCCTGCTTGCTGATCCAC-3′.
The PCR conditions were as follows: pre-denaturation at 95°C for 2
min, followed by 40 cycles of denaturation at 95°C for 10 sec, and
annealing/extension at 55°C for 20 sec. All the RT-qPCR tests were
performed in triplicate and after the third day of plasmid
transfections. The data were analyzed using the comparative Ct
method.
Western blotting
The cells were collected and homogenized in a lysis
buffer (Tris-HCl 50 mmol/l, EDTA 5 mmol/l, NaCl 150 mmol/l, sodium
deoxycholate 1%, Na3VO4 500 μmol/l, Triton
X-100 0.5%, AEBSF 10 μmol/l, NaF 10 mmol/l) on ice for 30 min. Cell
lysates were clarified by centrifugation (10,000 × g, 15 min), and
protein concentrations were determined using the Bradford reagent
(Sigma-Aldrich, Taufkirchen, Germany). Equal amounts of protein (15
μg/lane) from the cell lysates were separated on an 8–15%
SDS-polyacrylamide gel (SDS-PAGE) and transferred onto
nitrocellulose membranes (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA). The membrane was incubated for 2 h in
phosphate-buffered saline (PBS) plus 0.1% Tween-20 and 5% non-fat
skim milk to block non-specific binding. The membranes were then
incubated overnight at 4°C with primary antibodies. After washing,
the proteins were visualized using an ECL detection kit with the
appropriate HRP-conjugated secondary antibody (Amersham Pharmacia
Biotech, Piscataway, NJ, USA) for 2 h. All the assays were
performed after the third day of drug treatment.
Cell proliferation assay
The MTT assay was used to determine for cell
proliferation. Briefly, the cells were seeded in 8- of 96-well
plates at a density of 2×103 cells/well. After being
cultured for 24 h, the cells were treated with psi-ADAM10,
psi-Scramble, SOR or psi-ADAM10 in combination with SOR,
respectively. At 48 h after treatment, 20 μl of MTT (5 mg/ml;
Sigma) was added to each well followed by incubation at 37°C for 48
h. Centrifugation was then performed at 2,000 × g for 10 min. The
supernatant was removed, and 200 μl of DMSO was added to each well
followed by agitation for 10 min. The absorbance was measured using
a microplate reader at a wavelength of 490 nm. The experiment was
repeated three times. The inhibition rate was calculated according
to the formula: Inhibition rate (%) = [1-(average absorbance of
experimental group/average absorbance of blank control group)] ×
100%. The mean proliferation of cells without any treatment was
expressed as 100%.
Colony formation assay
HepG2 cells were seeded in 6-well culture plates at
1×104 cells/well, and were treated with specific drugs
when cells reached the logarithmic growth phase. After the cells
were incubated at 37°C for 10 days, and the medium was replaced
every 3 days. After washing three times with PBS, the colonies were
fixed with ice methanol for 30 min and stained with Giemsa for 10
min. The visible colonies were then counted.
TUNEL assay
To measure the effect of psi-AMAD10 in combination
with SOR on cell apoptosis, a TUNEL assay was carried out. Briefly,
after HepG2 cells were treated with psi-ADAM10, psi-Scramble, SOR
or psi-ADAM10 in combination with SOR for 24 h, cellular DNA
fragmentation was measured with the ApoTag Red In Situ Apoptosis
Detection kit (Chemicon International, Temecula, CA, USA) according
to the manufacturer’s instructions. To quantify the apoptotic
cells, the terminal deoxynucleotidyl transferase-mediated nick
end-labeling (TUNEL)-positive cells were counted using confocal
microscopy.
In addition, at the molecular level, we detected
apoptosis related to protein, survivin and Bcl-2 protein expression
by western blotting as an additional indicator of apoptosis.
Caspase-8 activity assay
HepG2 cells were collected and lysed in lysis buffer
(10 mM Tris-HCl, pH 7.5, 130 mM NaCl, 1% Triton X-100 and 10 mM
sodium pyrophosphate) following specific treatment. Cell lysates
were clarified by centrifugation at 14,000 × g for 5 min at 4°C and
clear lysates containing 50 μg of protein were incubated with 100
μM of enzyme-specific substrate (Ac-IEVD-pNA for caspase-8) in
assay buffer (50 mM Tris-HCl, pH 7.4, 1 mM EDTA and 10 mM EGTA) at
37°C for 1 h. Caspase-8 activity was determined by the cleavage of
colorimetric substrate monitored at 405 nm.
Wound-healing assay
To assess the effect of psi-ADAM10 in combination
with SOR psi-ADAM10 on cell migration, a wound-healing assay was
performed. Briefly, 1×105 HepG2 cells were plated in
12-well plates in DMEM containing 10% fetal bovine serum (FBS).
After 24 h, a scratch was made through the confluent cell
monolayer, and the cells were treated with psi-ADAM10,
psi-Scramble, SOR or psi-ADAM10 in combination with SOR,
respectively, in 3 ml of complete medium. After 48 h treatment, the
cells were stained with hematoxylin and eosin (H&E). Cells
invading the wound line were observed under an inverted
phase-contrast microscope using ×20, Leica DMR, Germany.
Experiments were performed in triplicate.
Transwell invasion assay
Cell invasion was determined using Transwell
chambers produced from polycarbonate membrane filters with a pore
size of 8-μm according to the manufacturer’s instructions (Costar,
USA) and the upper chambers were coated with Matrigel (BD
Biosciences, USA). The cells were transferred to the upper chamber
of each prepared Transwell chamber at a density of 4×105
cells/ml (100 μl) and treated with psi-ADAM10, psi-Scramble, SOR or
psi-ADAM10 in combination with SOR, respectively. The lower chamber
contained DMEM supplemented with 10% FBS. The cells were allowed to
migrate for 24 h at 37°C. Non-invading cells were removed from the
top surfaces with a cotton swab. The membranes were fixed in 95%
ethanol and stained with 0.1% crystal violet. The cells that had
penetrated to the bottom surface of each membrane were counted with
10 random fields on each microscope slide. In addition, cells were
quantified by measuring the absorbance of dye extracts at 570 nm in
100 ml of Sorenson’s solution (9 mg trisodium citrate, 305 ml
distilled water, 195 ml 0.1 N HCl, and 500 ml 90% ethanol).
Experiments were performed in triplicate.
Tumor xenograft assay
Fifty female BALB nude mice aged 4–6 weeks and
weighing 18–20 g, were purchased from the Institute of Laboratory
Animal Science, Jilin University (Changchun, Jilin, China), and
were maintained under specific pathogen-free (SPF) conditions and
provided with food and water ad libitum. The animal
experiments were carried out according to the standards of animal
care as outlined in the Guide for the Care and Use of Experimental
Animals of Jilin University. The study protocol was approved by the
Ethics Committee, The First Hospital of Jilin University.
Exponentially growing HepG2 cells were harvested and
a tumorigenic dose of 2×106 cells was injected
intraperitoneally into the BALB mice. Approximately 20 days after
the inoculation of HepG2 cells, the average tumor volume was at
120.28±8.23 mm3, and the mice were divided randomly into
5 groups (10 mice/group). The control group received 1% polysorbate
resuspended in deionized water. The remaining four groups were
treated with psi-Scramble (30 μg/50 μl/mouse), SOR (80 mg/kg body
weight), psi-ADAM10 (30 μg/50 μl/mouse) or SOR plus psi-ADAM10
(SOR, 40 mg/kg body weight; psi-ADAM10; 15 μg/50 μl/mouse)
intraperitoneally on alternative days for 3 weeks. The tumor size
was measured using calipers prior to administration of the
treatment injections and on the 7th, 14th and 21st days of
treatment. On the 21st day, the animals were sacrificed using
chloroform and their spleen tissue was collected and cultured for a
splenocyte surveillance study as previously described (26). Sections of each tumor tissue were
wax-embedded for H&E staining to study cell apoptosis in
vivo by TUNEL.
Statistical analysis
Data are presented as means ± SD. A statistical
comparison of more than two groups was performed using one-way
ANOVA followed by a Tukey’s post-hoc test. Statistical analyses
were undertaken using the GraphPad Prism version 5.01 (GraphPad
Software, San Diego, CA, USA) for Windows®. P<0.05
was considered to indicate a statistically significant result. The
images shown in the present study were obtained from at least three
independent experiments with similar results.
Results
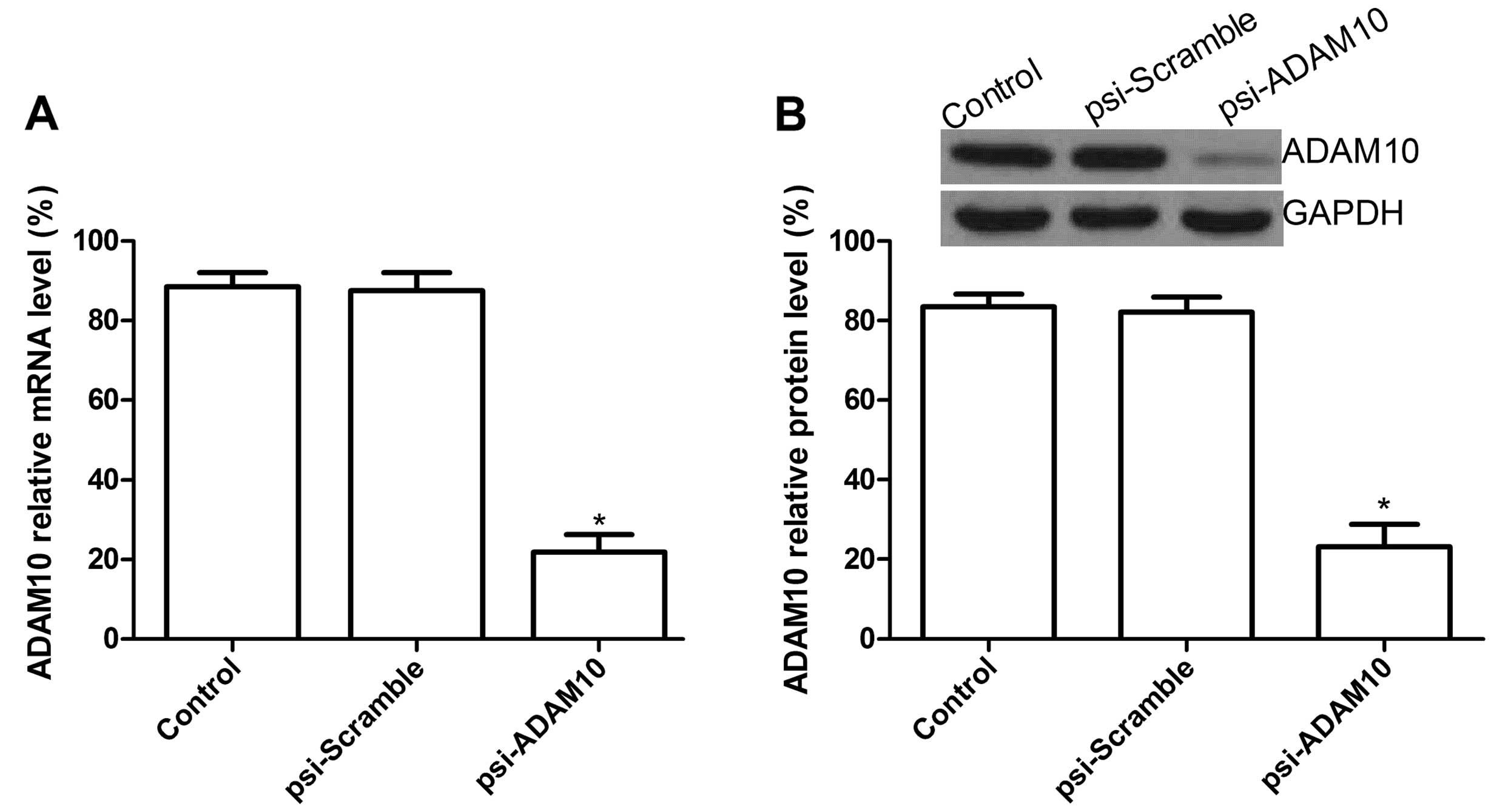
Downregulation of ADAM10 expression by
plasmid psi-ADAM10
To silence ADAM10 expression, we constructed a
recombinant plasmid-based shRNA against ADAM10, psi-Scramble.
Subsequently, we assessed the silencing ability of psi-ADAM10 in
the HCC cell line by RT-qPCR and western blotting following the
treatment of HepG2 cells with plasmid psi-ADAM10 for three days.
RT-qPCR results showed no significant inhibition in ADAM10 mRNA
expression in the control and psi-Scramble groups. Compared to the
psi-Scramble and control groups, mRNA expression in the psi-ADAM10
group was significantly decreased (Fig.
1A, P<0.01). On the protein level, no significant inhibition
in ADAM10 protein expression was found in the psi-Scramble and
control groups (P>0.05), while the band density markedly
decreased in the psi-ADAM10 group as compared with the psi-Scramble
and control groups (P<0.01) (Fig.
1B). These results demonstrated that silencing ADAM10
significantly decreased ADAM10 expression in the HCC cell line.
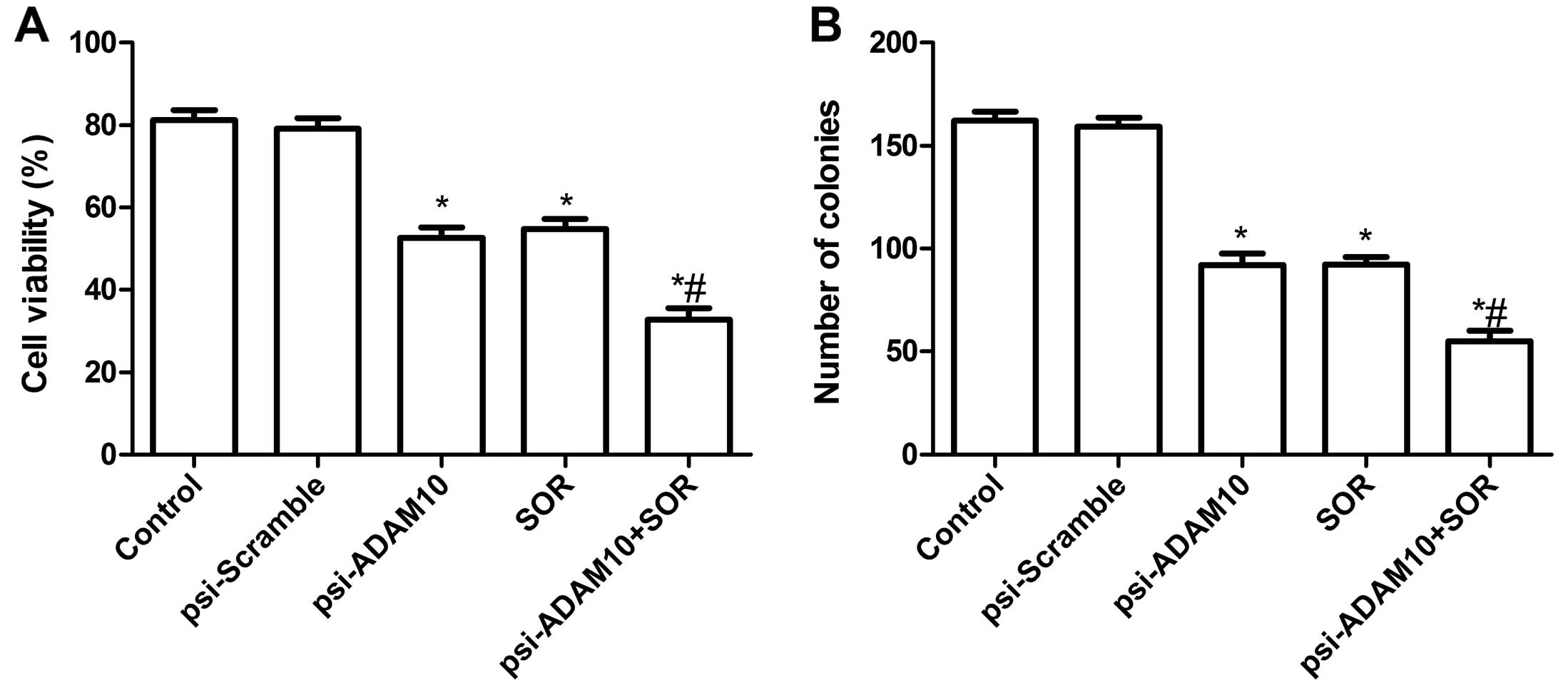
Effects of SOR and psi-ADAM10 alone or in
combination on HepG2 cell proliferation and cell colony
formation
To evaluate the effect of psi-ADAM10 and SOR alone
or their combination on the viability of HCC cells in vitro,
an MTT assay was performed for 48 h when HepG2 cells were treated
with psi-ADAM10 and SOR alone or both. It was found that the
inhibitory rates of psi-ADAM10 and SOR alone or the combination
treatment were higher than those of the control and psi-Scramble
groups (P<0.01, Fig. 2A). No
significant difference between the psi-Scramble and control groups
was observed (P>0.05, Fig. 2A).
In addition, the inhibitory rates of psi-ADAM10 in combination with
the SOR group were higher than the single treatment group
(P<0.05, Fig. 2A).
The effects of psi-ADAM10 and SOR alone or in
combination on the HepG2 cell colony formation were then analyzed.
Compared with the control and psi-Scramble groups, the number of
tumor cells per colony were significantly reduced in psi-ADAM10 and
SOR alone or the combination groups (P<0.01, Fig. 2B). The psi-Scramble in combination
with SOR resulted in an even greater percentage of reduction than
the higher doses of either drug alone (P<0.01, Fig. 2B).
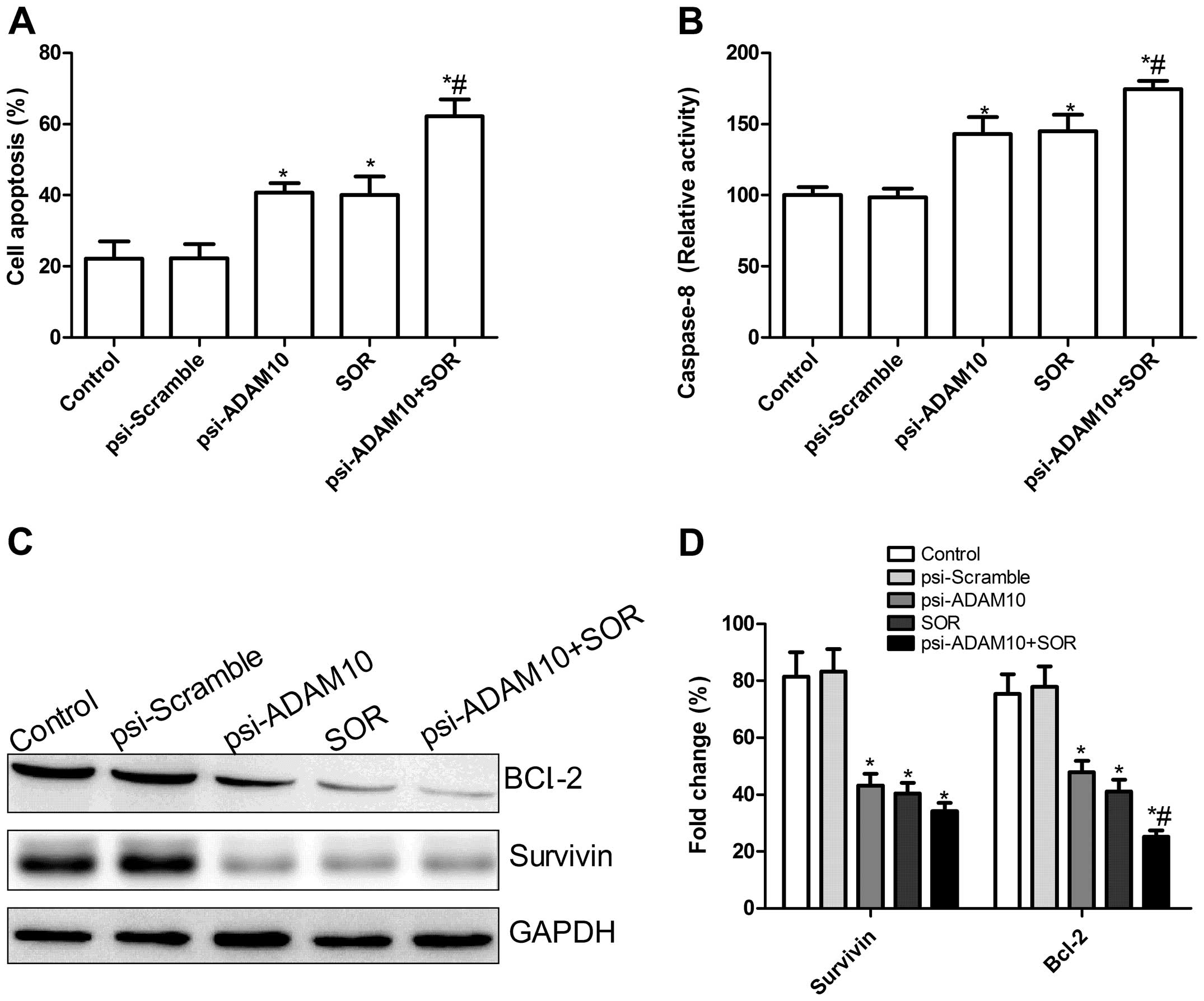
Effects of SOR and psi-ADAM10 alone or in
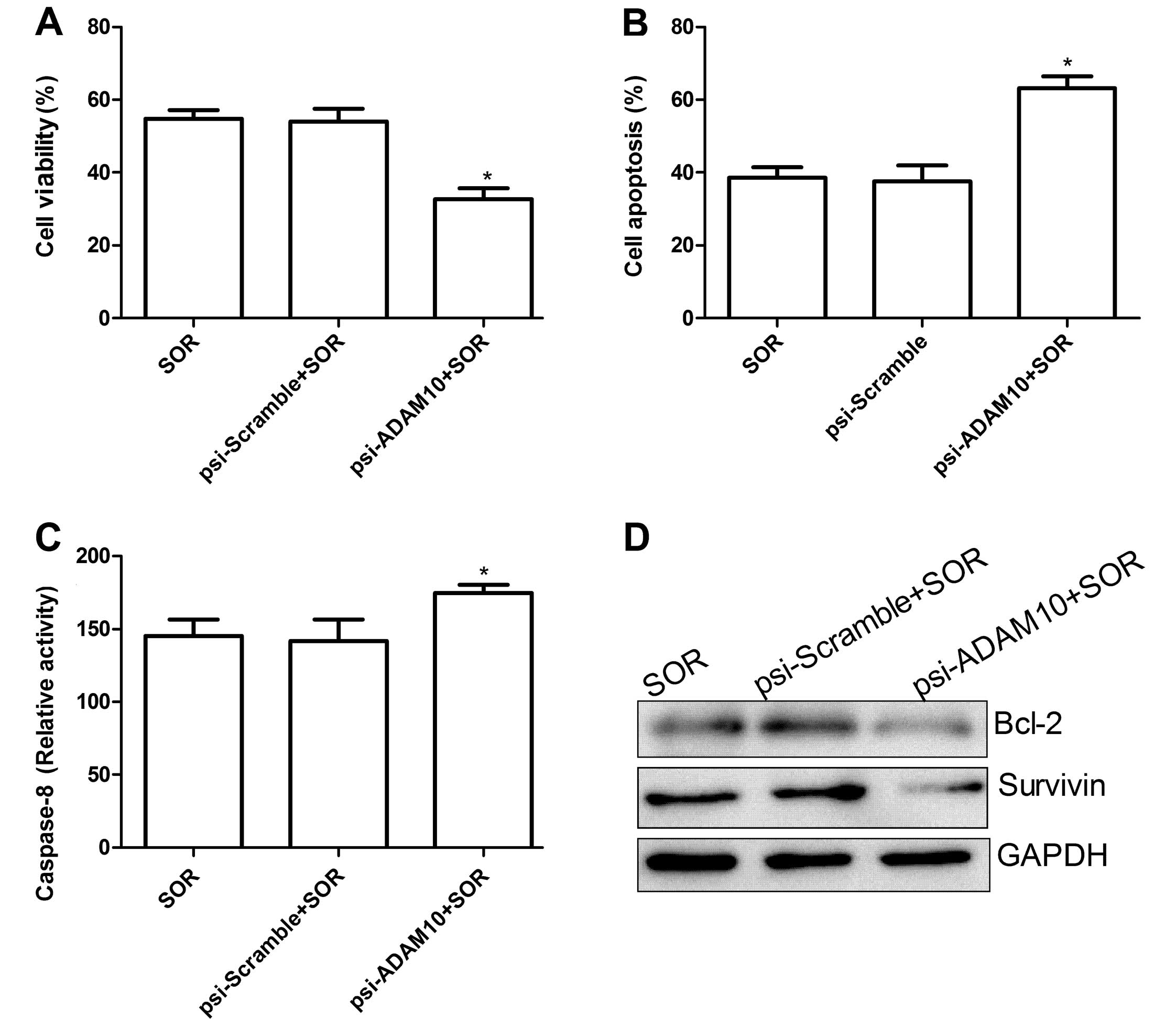
combination on HepG2 cell apoptosis
To investigate whether the psi-ADAM10 and SOR alone
or in combination induced apoptosis, the apoptosis was analyzed
after treatment with the specific drug. It was found that HepG2
cells treated with psi-ADAM10 and SOR alone or in combination
significantly induced cell apoptosis compared with the control and
psi-Scramble groups (Fig. 3A).
Treatment with the combination of psi-ADAM10 and SOR resulted in a
marked increase in apoptotic cells compare to the single drug
treatment group (P<0.01) (Fig.
3A).
To determine the possible mechanism of induction of
cell apoptosis of the combination with psi-ADAM10 and SOR,
caspase-8 activity was detected using ELISA. The results showed
that caspase-8 activity was significantly increased in psi-ADAM10
and SOR alone or in the combination treatment groups compared to
the control and psi-Scramble groups (P<0.05; Fig. 3B). Compared to the psi-ADAM10 and
SOR groups, the combination treatment significantly increased
caspase-8 activity (P<0.05; Fig.
3B). In addition, expression patterns of survivin and Bcl-2
were determined by western blotting. The results showed that the
combination with psi-ADAM10 and SOR significantly decreased the
expression of apoptosis inhibiting gene survivin and Bcl-2 in HepG2
cells compared to the single drug treatment, and control and
psi-Scramble groups (Fig. 3C and D,
P<0.05).
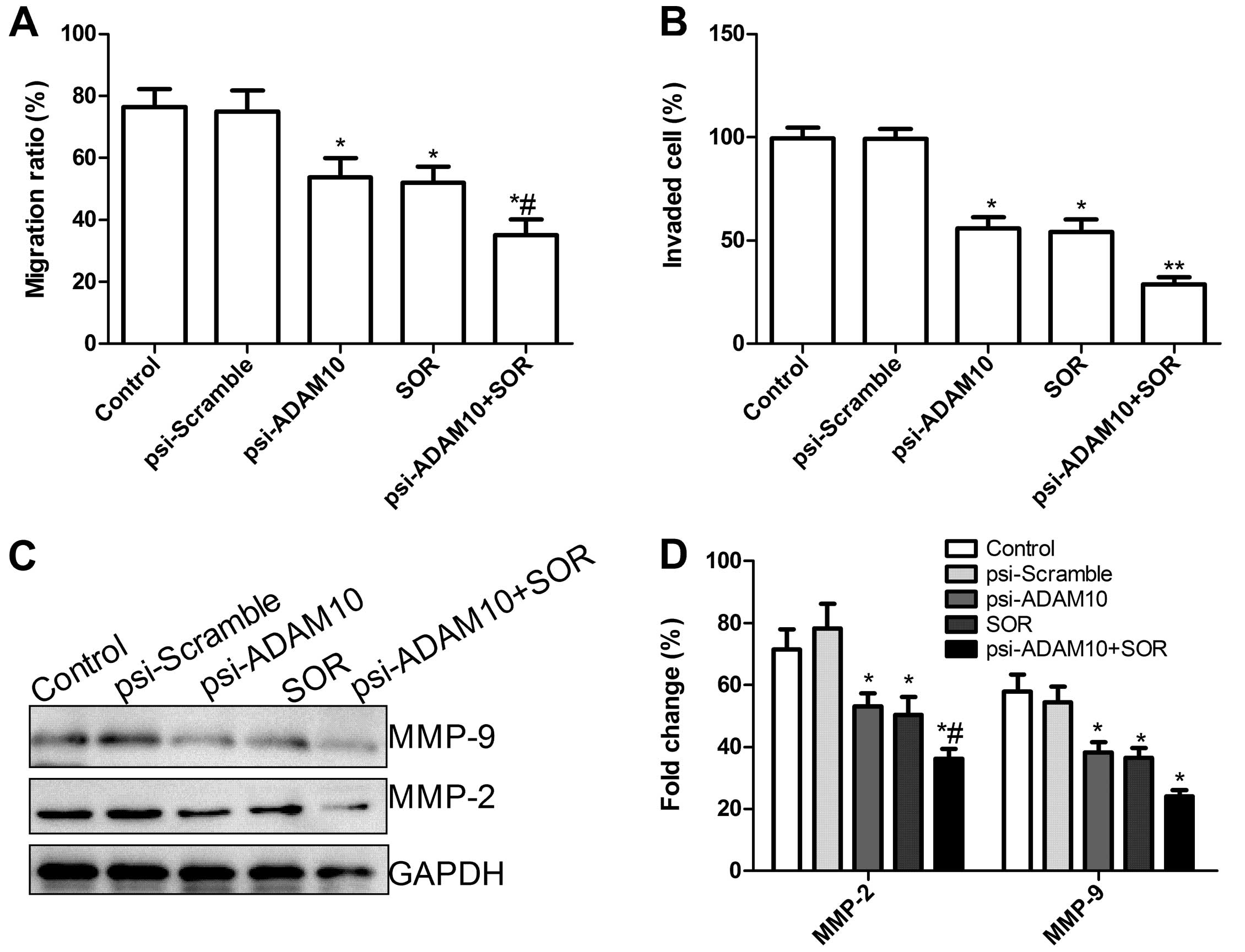
Effects of SOR and psi-ADAM10 alone or in
combination on HepG2 cell migration and invasion
To ascertain the inhibitory effect of psi-ADAM10 and
SOR alone or in combination on HCC cell motility in vitro, a
wound-healing assay was performed. As shown in Fig. 4A, cell migration in the psi-ADAM10
and SOR alone or the combination groups was significantly reduced
compared to the control and psi-Scramble groups when HepG2 cells
were treated with the indicated drug treatment for 48 h
(P<0.05). In addition, compared to the SOR and psi-ADAM10
groups, migration was significantly reduced in SOR in combination
with the psi-ADAM10 group.
To determine whether psi-ADAM10 and SOR alone or
their combination affected the invasiveness of human HCC HepG2
cells, the cells were treated with the indicated drug for 48 h. As
shown in Fig. 4B, the result of the
cell invasiveness assay showed that there was no significant
difference in the number of cells that had passed through the
simulated basement membrane between the control and psi-Scramble
groups. However, the number of cells that had passed through the
simulated basement membrane in the psi-ADAM10 and SOR alone or
combination group were significantly reduced when compared with the
control and psi-Scramble groups (all P<0.05). Compared to the
single treatment group, the cell invasion number was significantly
reduced (P<0.05, Fig. 4B).
To determine the potential mechanism of SOR in
combination with psi-ADAM10 inhibition of cell invasion in
vitro, the MMP-2 and MMP-9 protein expression was determined by
western blot analysis. Western blot analysis revealed a significant
decrease in MMP-2 and MMP-9 proteins in the psi-ADAM10 and SOR
alone or in the combination group compared to the control and
psi-Scramble groups alone (P<0.05, Fig. 4C and D). Compared to the single
treatment group, psi-ADAM10 in combination with the SOR group
decreased the protein expression of MMP-2 and MMP-9 (P<0.05,
Fig. 4C and D).
ADAM10 silencing sensitizes HCC cells to
SOR treatment
To determine whether the downregulation of ADAM10 by
siRNA affected the sensitivity of HCC cells to SOR, we stably
transfected plasmid psi-ADAM10 and psi-Scramble into HepG2 cells,
respectively, and then added SOR (20 μM) to HepG2 for further
treatment. After 48 h, cell proliferation and apoptosis were
determined. The results of the MTT assay showed that silencing
ADAM10 significantly inhibited cell proliferation in the presence
of SOR compared to the control and psi-Scramble groups in the
presence of SOR (Fig. 5A). In
addition, silencing of ADAM10 significantly induced cell apoptosis
in the presence of SOR (Fig. 5B).
Consistent with the increased apoptosis, the amount of cleaved
caspase-8 was profoundly elevated in ADAM10-silenced HCC cells in
the presence of SOR (Fig. 5C).
Moreover, ADAM10-deficient cells exhibited a reduced expression of
the anti-apoptotic factor survivin and Bcl-2 (Fig. 5D).
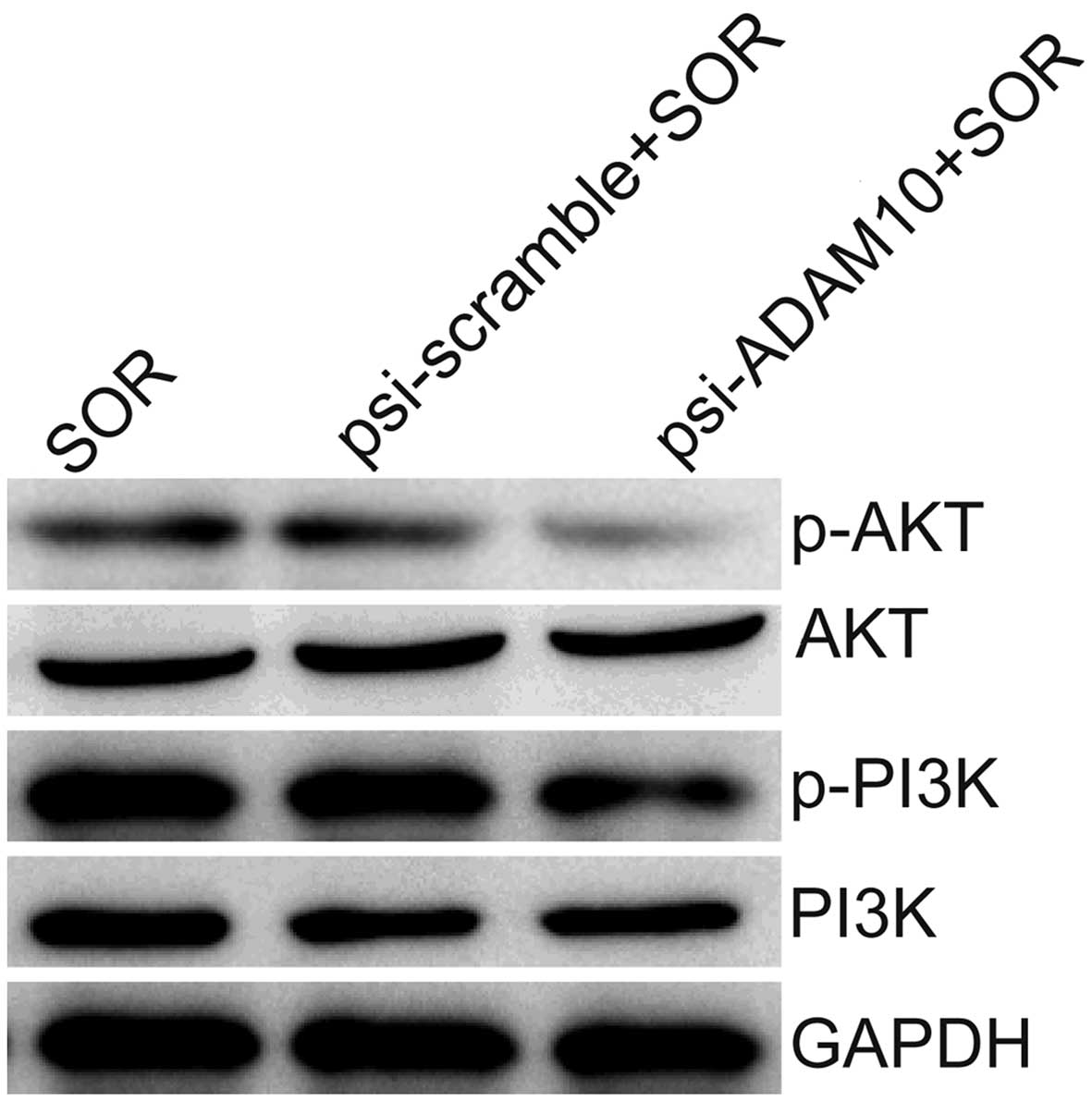
PI3K/Akt pathway is involved in
ADAM10-mediated chemoresistance to SOR
The PI3K/Akt pathway plays an important role in
regulating the chemoresistance of cancer cells (27–29).
We examined whether this signaling pathway mediated
ADAM10-dependent chemoresistance in HCC cells. Measurements of the
phosphorylation/activation pattern of PI3K and Akt was performed by
western blotting. Our results showed that the downregulation of
ADAM10 expression in HepG2 cells resulted in a marked reduction of
phosphorylated PI3K and Akt relative to mock cells, without
altering the total protein levels of PI3K or Akt in the presence of
SOR (Fig. 6).
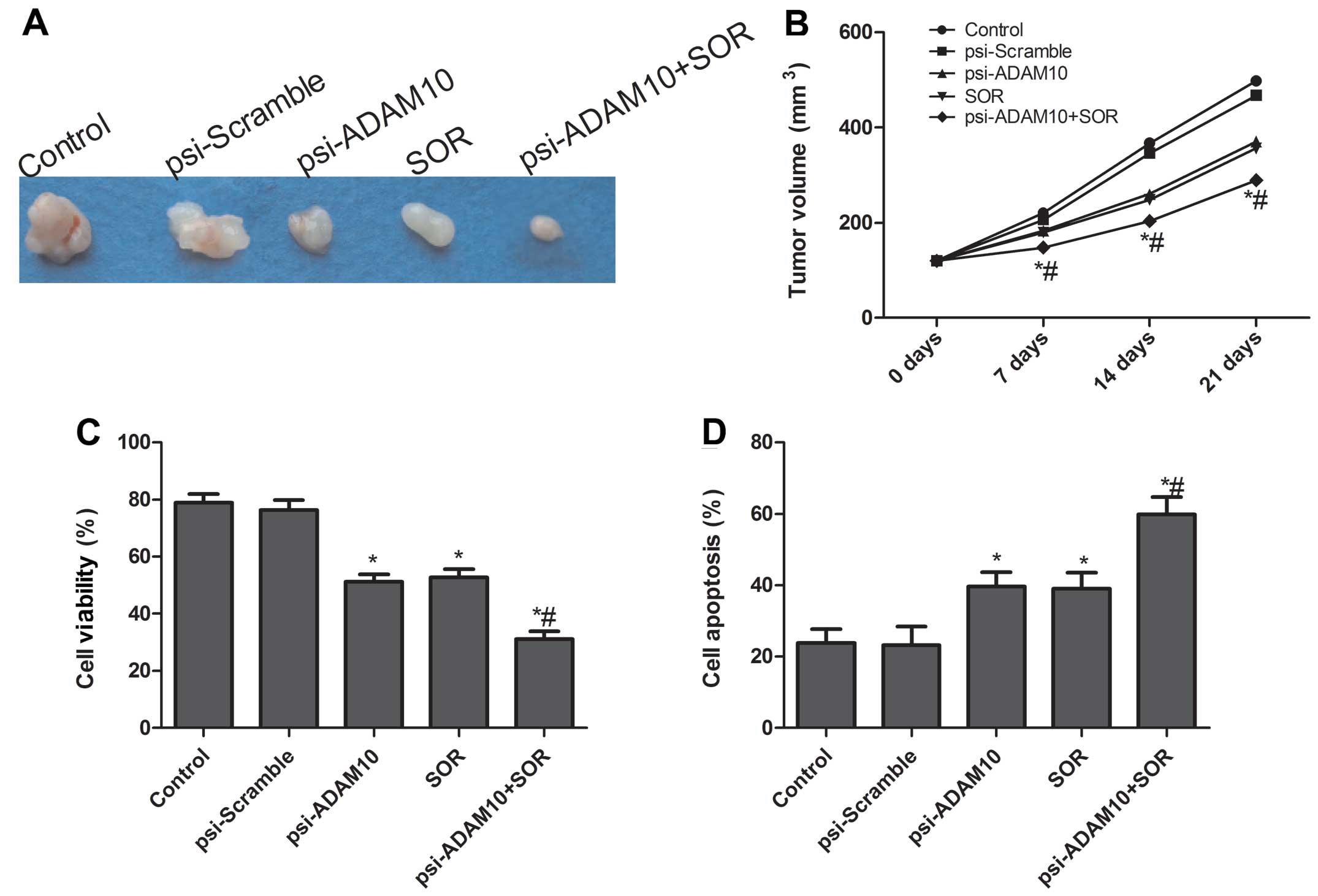
Effects of SOR and psi-ADAM10 alone or in
combination on tumor growth in a murine model
The in vivo therapeutic efficacy of
psi-ADAM10 and SOR alone or their combination on female BALB
mice-bearing HepG2 tumor cells was assessed. The results showed
that the tumor weight of psi-ADAM10 and SOR alone or the
combination group was lower than that of the control and
psi-Scramble groups (P<0.05, Fig.
7A). Compared with the results with either agent alone, the
combination of psi-ADAM10 and SOR greatly inhibited tumor growth
(Fig. 7A). In addition, we found
that tumor volume after treatment with psi-ADAM10 and SOR was
significantly reduced for the HepG2 tumor cells compared with the
control and psi-Scramble groups at different time points
(P<0.05, Fig. 7B). Treatment
with the combination of psi-ADAM10 and SOR resulted in marked
inhibition of the tumor volume compared to the psi-ADAM10 and SOR
groups alone (P<0.05, Fig.
7B).
To assess the efficacy of psi-ADAM10 and SOR alone
or combination in modulating splenocyte proliferation, MTT assay
were performed. As shown in Fig.
7C, the inhibitory rates of psi-ADAM10 and SOR alone or the
combination group were higher than those of the control and
psi-Scramble groups (P<0.05). Treatment with the combination of
psi-ADAM10 and SOR led to marked inhibition of cell proliferation
compared to the psi-ADAM10 and SOR groups alone. In addition, we
determined tumor tissue cell apoptosis in vivo by TUNEL. The
results showed that psi-ADAM10 and SOR alone or the combination
group significantly induced cell apoptosis compared to the control
and psi-Scramble groups (P<0.05, Fig. 7D). Compared with the results with
either agent alone, the combination of psi-ADAM10 and SOR greatly
induced tumor cell apoptosis in vivo. These results
demonstrated that the combination of psi-ADAM10 and SOR suppressed
the tumor growth of HCC in vivo.
Discussion
Sorafenib (SOR) is currently the standard of care
for hepatocellular carcinoma (HCC) patients with preserved liver
function who have metastatic or unresectable disease not amenable
to liver transplantation (30). In
two randomized controlled phase III trials, SOR significantly
improved overall survival (OS) and time to progression compared
with patients administered a placebo. However, the median OS in the
SOR arms of the two studies was moderately increased (8,9). As a
result, there is a need for new and effective HCC treatments to
improve patient outcome.
It has been reported that ADAM is involved in
various cell processes such as proliferation, differentiation,
migration and invasion (31,32). A
large number of studies suggest that ADAMs is important in cancer
cell survival (20–24,31,32).
ADAM10, a member of the ADAM family, has been shown to be
upregulated in various types of cancer including HCC (22). Accumulating evidence have
demonstrated that ADAM10 is involved in cancer cell progression and
metastasis (21–23,31–35).
For example, Klein et al (31) reported that ADAM10 can activate
Notch signaling by suppressing ectodomain shedding of δ-1, which
subsequently leads to a strong inhibitory effect on tumor cell
proliferation and apoptosis (33).
Endres et al showed that ADAM10 can cleave collagen type IV
in the basement membrane, which is relevant to tumor metastasis and
proliferation (34). In addition,
it has been shown that the combined therapy with shRNA combination
anticancer drug may achieve better antitumor activity (35). The study by Emdad et al
demonstrated that when melanoma differentiation-associated gene-7
(mda-7) through a replication-incompetent adenovirus (Ad.mda-7) and
gefitinib are used in combination, they may provide an effective
therapeutic approach for non-small cell lung cancer (NSCLC) since
these agents target various cell survival pathways and are equally
effective against NSCLC cells (36). Lu et al showed that
lentivirus-carrying COX-2 gene combination with tamoxifen (TAM) in
breast cancer cells significantly suppressed tumor growth in
vitro and in vivo (26).
Consistent with the abovementioned results, results of this study
show that plasmid psi-ADAM10 in combination with SOR treatment in
HCC cancer cells significantly suppressed proliferation, migration
and invasion, and induced tumor apoptosis in vitro and
suppressed tumor growth in vivo.
Resistance to chemotherapy poses a challenge for the
successful treatment of HCC patients. It has been reported that
ADAM10 decreases resistance to chemotherapeutic drugs (24,37).
Yang et al demonstrated that ADAM10 plays an important role
in modulating the chemosensitivity of HCC cells to doxorubicin
(24). Bai et al found that
the expression of microRNA-122, as a negative regulator of ADAM10,
sensitizes HCC cells to SOR against HCC (37). Consistent with their results,
results of the present study have shown that ADAM10 was involved in
the modulation of the chemosensitivity of HCC cells to SOR against
HCC and that the downregulation ADAM10 by siRNA sensitizes HCC
cells to SOR against HCC.
The mediation of drug resistance depends on cellular
changes, such as increased repair of DNA damage, alterations in the
cell cycle and/or reduction of apoptosis by the activation of
anti-apoptotic pathways (38). In
this regard, activation of the PI3K/AKT pathway is an important
requirement of cancer cells in order to escape cell death following
exposure to toxic stimuli. It has been shown that the
phosphorylation of AKT was significantly higher among patients who
received chemotherapy and this increase was associated with poor
prognosis in various types of cancer including HCC (39). Furthermore, accumulating evidence
has demonstrated that the activated PI3K/AKT signalling cascade
which promotes resistance against several chemotherapeutic drugs
was identified in various cell culture model systems including the
HCC cell line (24,40–42).
In the present study, the results have shown that ADAM10 is
important in modulating the chemosensitivity of HCC cells to SOR
against HCC, which, at least partially, involves the activation of
the PI3K/Akt pathway, a finding that is consistent with results of
a previous study (24).
In conclusion, our in vitro studies have
demonstrated that when plasmid psi-ADAM10 and SOR are used in
combination they may provide an effective therapeutic approach for
HCC since this combination may significantly suppress
proliferation, migration and invasion and induce tumor apoptosis
in vitro. Additionally, studies with in vivo murine
models confirmed that psi-ADAM10 combination with SOR may suppress
HCC tumor growth. Therefore, it is of note to consider their
combination as a novel therapeutic strategy for further evaluation
in clinical trials for the treatment of HCC.
Acknowledgements
This study was supported by the Science and
Technology Research and Innovation Team Fund of Jilin province
(JL2011038).
References
|
1
|
Venook AP, Papandreou C, Furuse J and de
Guevara LL: The incidence and epidemiology of hepatocellular
carcinoma: a global and regional perspective. Oncologist. 15(Suppl
4): 5–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Thorgeirsson SS and Grisham JW: Molecular
pathogenesis of human hepatocellular carcinoma. Nat Genet.
31:339–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar
|
|
6
|
Liu L, Cao Y, Chen C, et al: Sorafenib
blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and
induces tumor cell apoptosis in hepatocellular carcinoma model
PLC/PRF/5. Cancer Res. 66:11851–11858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gu FM, Li QL, Gao Q, et al: Sorafenib
inhibits growth and metastasis of hepatocellular carcinoma by
blocking STAT3. World J Gastroenterol. 17:3922–3932. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng AL, Kang YK, Chen Z, et al: Efficacy
and safety of sorafenib in patients in the Asia-Pacific region with
advanced hepatocellular carcinoma: a phase III randomised,
double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34.
2009. View Article : Google Scholar
|
|
10
|
Roy M, Luo YH, Ye M and Liu J: Nonsmall
cell lung cancer therapy: insight into multitargeted small-molecule
growth factor receptor inhibitors. Biomed Res Int.
2013:9647432013.PubMed/NCBI
|
|
11
|
Nojiri K, Sugimoto K, Shiraki K, et al:
Sorafenib and TRAIL have synergistic effect on hepatocellular
carcinoma. Int J Oncol. 42:101–108. 2013.PubMed/NCBI
|
|
12
|
Schmieder R, Puehler F, Neuhaus R, et al:
Allosteric MEK1/2 inhibitor refametinib (BAY 86-9766) in
combination with sorafenib exhibits antitumor activity in
preclinical murine and rat models of hepatocellular carcinoma.
Neoplasia. 15:1161–1171. 2013.
|
|
13
|
Hoffmann K, Glimm H, Radeleff B, et al:
Prospective, randomized, double-blind, multi-center, phase III
clinical study on transarterial chemoembolization (TACE) combined
with Sorafenib versus TACE plus placebo in patients with
hepatocellular cancer before liver transplantation - HeiLivCa
[ISRCTN24081794]. BMC Cancer. 8:3492008.PubMed/NCBI
|
|
14
|
Abou-Alfa GK, Johnson P, Knox JJ, et al:
Doxorubicin plus sorafenib vs doxorubicin alone in patients with
advanced hepatocellular carcinoma: a randomized trial. JAMA.
304:2154–2160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsu CH, Shen YC, Lin ZZ, et al: Phase II
study of combining sorafenib with metronomic tegafur/uracil for
advanced hepatocellular carcinoma. J Hepatol. 53:126–131. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pawlik TM, Reyes DK, Cosgrove D, Kamel IR,
Bhagat N and Geschwind JF: Phase II trial of sorafenib combined
with concurrent transarterial chemoembolization with drug-eluting
beads for hepatocellular carcinoma. J Clin Oncol. 29:3960–3967.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cabrera R, Pannu DS, Caridi J, et al: The
combination of sorafenib with transarterial chemoembolisation for
hepatocellular carcinoma. Aliment Pharmacol Ther. 34:205–213. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Petrini I, Lencioni M, Ricasoli M, et al:
Phase II trial of sorafenib in combination with 5-fluorouracil
infusion in advanced hepatocellular carcinoma. Cancer Chemother
Pharmacol. 69:773–780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mochizuki S and Okada Y: ADAMs in cancer
cell proliferation and progression. Cancer Sci. 98:621–628. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang YY, Ye ZY, Li L, Zhao ZS, Shao QS and
Tao HQ: ADAM 10 is associated with gastric cancer progression and
prognosis of patients. J Surg Oncol. 103:116–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SB, Schramme A, Doberstein K, et al:
ADAM10 is upregulated in melanoma metastasis compared with primary
melanoma. J Invest Dermatol. 130:763–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang W, Liu S, Liu K, et al: A
disintegrin and metalloprotease (ADAM)10 is highly expressed in
hepatocellular carcinoma and is associated with tumour progression.
J Int Med Res. 42:611–618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yue Y, Shao Y, Luo Q, Shi L and Wang Z:
Downregulation of ADAM10 expression inhibits metastasis and
invasiveness of human hepatocellular carcinoma HepG2 cells. Biomed
Res Int. 2013:4345612013.PubMed/NCBI
|
|
24
|
Yang CL, Jiang FQ, Xu F and Jiang GX:
ADAM10 overexpression confers resistance to doxorubicin-induced
apoptosis in hepatocellular carcinoma. Tumour Biol. 33:1535–1541.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brummelkamp TR, Bernards R and Agami R: A
system for stable expression of short interfering RNAs in mammalian
cells. Science. 296:550–553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang H, Li Z and Wang K: Combining
sorafenib with celecoxib synergistically inhibits tumor growth of
non-small cell lung cancer cells in vitro and in
vivo. Oncol Rep. 31:1954–1960. 2014.PubMed/NCBI
|
|
27
|
Gagnon V, Van Themsche C, Turner S,
Leblanc V and Asselin E: Akt and XIAP regulate the sensitivity of
human uterine cancer cells to cisplatin, doxorubicin and taxol.
Apoptosis. 13:259–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chekenya M, Krakstad C, Svendsen A, et al:
The progenitor cell marker NG2/MPG promotes chemoresistance by
activation of integrin-dependent PI3K/Akt signaling. Oncogene.
27:5182–5194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McDonald GT, Sullivan R, Paré GC and
Graham CH: Inhibition of phosphatidylinositol 3-kinase promotes
tumor cell resistance to chemotherapeutic agents via a mechanism
involving delay in cell cycle progression. Exp Cell Res.
316:3197–3206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Benson AB III, Abrams TA, Ben-Josef E, et
al: NCCN clinical practice guidelines in oncology: hepatobiliary
cancers. J Natl Compr Canc Netw. 7:350–391. 2009.PubMed/NCBI
|
|
31
|
Klein T and Bischoff R: Active
metalloproteases of the a disintegrin and metalloprotease (ADAM)
family: biological function and structure. J Proteome Res.
10:17–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seals DF and Courtneidge SA: The ADAMs
family of metalloproteases: multidomain proteins with multiple
functions. Genes Dev. 17:7–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao H, Zhu J, Cui K, et al:
Bioluminescence imaging reveals inhibition of tumor cell
proliferation by Alzheimer’s amyloid β protein. Cancer Cell Int.
9:152009.PubMed/NCBI
|
|
34
|
Endres K and Fahrenholz F: Upregulation of
the α-secretase ADAM10 - risk or reason for hope? FEBS J.
277:1585–1596. 2010.
|
|
35
|
Lin T, Gu J, Zhang L, et al: Enhancing
adenovirus-mediated gene transfer in vitro and in vivo by addition
of protamine and hydrocortisone. J Gene Med. 5:868–875. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Emdad L, Lebedeva IV, Su ZZ, et al:
Combinatorial treatment of non-small-cell lung cancers with
gefitinib and Ad. mda-7 enhances apoptosis-induction and
reverses resistance to a single therapy. J Cell Physiol.
210:549–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bai S, Nasser MW, Wang B, et al:
MicroRNA-122 inhibits tumorigenic properties of hepatocellular
carcinoma cells and sensitizes these cells to sorafenib. J Biol
Chem. 284:32015–32027. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McCubrey JA, Sokolosky ML, Lehmann BD, et
al: Alteration of Akt activity increases chemotherapeutic drug and
hormonal resistance in breast cancer yet confers an achilles heel
by sensitization to targeted therapy. Adv Enzyme Regul. 48:113–135.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kunter I, Erdal E, Nart D, et al: Active
form of AKT controls cell proliferation and response to apoptosis
in hepatocellular carcinoma. Oncol Rep. 31:573–580. 2014.PubMed/NCBI
|
|
40
|
Chiablaem K, Lirdprapamongkol K,
Keeratichamroen S, Surarit R and Svasti J: Curcumin suppresses
vasculogenic mimicry capacity of hepatocellular carcinoma cells
through STAT3 and PI3K/AKT inhibition. Anticancer Res.
34:1857–1864. 2014.PubMed/NCBI
|
|
41
|
Xiu P, Xu Z, Liu F, et al: Downregulating
sCLU enhances the sensitivity of hepatocellular carcinoma cells to
gemcitabine by activating the intrinsic apoptosis pathway. Dig Dis
Sci. Mar 27–2014.(Epub ahead of print).
|
|
42
|
Edling CE, Selvaggi F, Ghonaim R, Maffucci
T and Falasca M: Caffeine and the analog CGS 15943 inhibit cancer
cell growth by targeting the phosphoinositide 3-kinase/Akt pathway.
Cancer Biol Ther. 15:524–532. 2014. View Article : Google Scholar : PubMed/NCBI
|