Introduction
EGR-1 belongs to the class of transcription factors
known as immediate-early genes and is rapidly induced by growth
factors to transduce the proliferative signal. Egr-1 induction by
external stimuli is generally transient; however, it seems to be
sustained in some prostate tumor cell lines and tumors, suggesting
that Egr-1 stimulates tumor cell growth, which may be important as
its expression level is enhanced with the degree of malignancy as
measured by the Gleason tumor grade (1). This increase seems to be specific to
prostate tumor cells as Egr-1 expression is low in mammary and lung
tumors, as well as most normal tissues (2–6). By
contrast, in breast, lung and brain tumor, Egr-1 expression is
often not present or decreased and when re-expressed, results in
growth inhibition (2,3,7,8). The
induction of Egr-1 by external stimuli is generally transient but
appears to be sustained in some prostate tumor cell lines and
tumors, suggesting that Egr-1 stimulates tumor cell growth
(9,10). Egr-1 is also involved in the
regulation of p53 in human melanoma cells leading to apoptosis
(11–13). The proapoptotic tumor-suppressor
gene PTEN is also directly regulated by EGR-1 (14). Previously, it was demonstrated that
the overexpression of Egr-1 in PC-3 and LNCaP prostate carcinoma
cell lines increases cell growth and independent anchorage
(15). Moreover, ablation of Egr-1
expression using small interfering RNA (siRNA) resulted in the
induction of cell apoptosis and cell death (15–18).
Another important factor associated with EGR-1
regulation is the transforming growth factor β1 (TGF-β1) (19). It has been previously shown that the
expression of EGR-1 in the HT1080 human fibrosarcoma cell line,
increases the secretion of transforming growth factor-β1 (TGF-β1)
in direct proportion to the amounts of EGR-1 expressed (20), and that EGR-1 protein specifically
binds to two GC-rich EGR-l-binding sites in the TGF-β1 gene
promoter and stimulates TGF-β1 promoter activity in HT1080 cells
(20). TGF-β1 is a potent
suppressive cytokine that inhibits immune system functions that
otherwise may be effective in generating antitumor immunity. TGF-β
is produced by many normal cells and is overexpressed in the tumor
cells that comprise the most common forms of human cancer,
including prostate carcinoma. TGF-β1 is induced by a variety of
signals including those of oncogenes and immediate-early genes,
whereas the expression of TGF-β2 and TGF-β3 is considerably more
developmentally and hormonally regulated (21). Thus, TGF-β1 influences various
pathologies including fibrosis, parasite induction, autoimmune
diseases and tumorigenesis (22–25).
Another protein that is involved in the regulation of EGR-1 is
p14ARF. This protein has emerged as an important tumor
suppressor that can trigger the growth suppression and apoptosis of
many cancer cells by a p53-dependent and -independent mechanism
(26,27), and loss of p14ARF is
likely to contribute to the failure of apoptosis in cancer.
p14ARF is encoded within the same INK4a locus that
encodes the p16 cyclin-dependent kinase inhibitor (27,28).
Unlike p16, which inhibits cell cycle progression,
p14ARF does not appear to have Cdk inhibitory activity.
Instead, p14ARF stabilized p53 function through a
complex formation with Mdm2, the principle mediator of p53
stability (29,30). A previous study demonstrated that
EGR-1 binds to p14ARF which is associated with the
tumor-suppressive effects of EGR-1 (31).
The results showed that TGF-β1 and p14ARF
activities in the presence of EGR-1 overexpression exist
independently of the presence of cells carrying a mutant p53 (PC-3
cells) or cells carrying a wild-type p53 (LNCaP cells). The effect
of EGR-1 on the growth of prostate cells may occur through multiple
mechanisms, but be independent of p53 expression control.
Materials and methods
Cell lines and culture
Human prostate carcinoma cell lines PC-3 and LNCaP
were a kind gift from Dr Dan Mercola (The SKCC; Sidney Kimmel
Cancer Center, La Jolla, CA, USA). The cells were cultured in
RPMI-1640 medium supplemented with 100 ml/l fetal bovine serum
(FBS), 8×105 U/l penicillin and 0.1 g/l streptomycin in
humidified incubator containing 50 ml/l CO2 at 37°C.
Tris-borate-EDTA and acrylamide:bisacrylamide (29:1)
were obtained from Bio-Rad Laboratories (Richmond, CA, USA). Egr-1
antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Lipofectamine was purchased from Life Technologies,
Inc. (Carlsbad, CA, USA) and TNF-α from Sigma Chemical Co. (St.
Louis, MO, USA). Complete Mini-EDTA-Free Protease Inhibitor
Cocktail Tablets and Annexin-V-Fluos were purchased from Roche
Diagnostics GmbH (Mannheim, Germany). Other reagents were purchased
from Sigma Chemical Co. (Taufkirchen, Germany). Phorbol
12-myristate 13-acetate (TPA), TNF-α and TGF-β1 were purchased from
Stratagene Inc. (La Jolla, CA, USA). Anti-Egr-1,
anti-p14ARF, anti-TGF-β1, and β-actin antibodies were
purchased from Santa Cruz Biotechnology.
siRNA preparation and transfection of
small interfering RNA
The sequences designed used to specifically target
human p14ARF and Egr-1 RNAs were:
for p14ARF, 5′-GAACAUGGU GCGCAGGUUCTT-3′;
and for Egr-1, 5′-AACGCAAGAGGC AUACCAAGA-3′. The scrambled
small interfering RNA (siRNA) oligonucleotides used as controls for
all RNA interference experiments were: 5′-AAAGGUGACGCUGACGAA GTT-3′
and 5′-CAAGAAAGGCCAGUCCAAGTT-3′. The cells were transfected with
siRNA oligonucleotide duplexes and cultured in medium without
antibiotics 24 h prior to transfection resulting in a confluence of
the cell monolayer by 60–80%. Egr-1-siRNA or non-silencing siRNA
(70 nmol) were mixed with Lipofectamine™ 2000 (Invitrogen-Life
Technologies) according to the manufacturer’s recommendation and
added to the cells. After 6 h at 37°C, the medium was replaced and
the cells were cultivated in RPMI-1640 supplemented with 10%
heat-inactivated FBS (9,16,17).
3-(4,5-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
PC-3 and LNCaP cells (5×105) were placed
in 96-well plates in RPMI-1640 containing 10% FBS at a final volume
of 0.1 ml. The following day, the cells were treated with the pTER
siRNA. MTT was added (20 ml/well of 5 g/l solution in PBS) after
culture for 24, 48 and 72 h. Following incubation at 37°C for 4 h,
the reaction was stopped by the addition of 100 ml DMSO. The
reaction product was quantified by measuring the absorbance at 490
nm using an ELISA reader (WALLAC 1420 VICTOR 2; Wallac, Turku,
Finland) and the Software HT-Soft (Perkin-Elmer). The samples were
assayed repeatedly in 6-wells.
Western immunoblot analysis
PC-3 and LNCaP prostate carcinoma cells lines
(5×105) were seeded in 6-well plates. Forty-eight hours
after transfection, the cells were collected and washed twice by
cold PBS, and each well was treated with 50 ml lysis buffer (2
mmol/l Tris-HCl pH 7.4, 50 mmol/l NaCl, 25 mmol/l EDTA, 50 mmol/l
NaF, 1.5 mmol/l Na3VO4, 1% Triton X-100, 0.1%
SDS, supplemented with protease inhibitors 1 mmol/l
phenylmethylsulfonylfluoride, 10 mg/l pepstatin, 10 mg/l aprotinin
and 5 mg/l leupeptin) (all from Sigma). Protein concentrations were
determined using the Bradford protein assay. Equal amounts of
protein (40 mg) were separated on a 15% SDS polyacrylamide gel and
transferred to a nitrocellulose membrane (Hybond C; Amersham,
Freiburg, Germany). The membranes were blocked in 5% non-fat dry
milk in TBS for 1 h at room temperature and probed with rabbit
anti-Egr-1 antibodies (1:500 dilution; Santa Cruz Biotechnology)
overnight at 4°C. After being washed 3 times with TBS containing
0.1% Tween-20, the membranes were incubated with anti-rabbit
IgG-horseradish-peroxidase (1:5,000; Santa Cruz Biotechnology), and
developed by luminol-mediated chemiluminescence (Appylgen
Technologies Inc., Beijing, China). To confirm equal protein
loading, membranes were reprobed with a 1:1,000 dilution of an
anti-actin antibody (Santa Cruz Biotechnology). Densitometric
analyses were performed using Scion Image software. Intestinal
alkaline phosphatase (IAP) activity was detected using an IAP kit
from Sigma-Aldrich.
DNA fragmentation assay
The cells were plated in 96-well plates 24 h prior
to treatment. Following treatment, DNA fragmentation was evaluated
by examination of cytoplasmic histone-associated DNA fragments
(mononucleosomes and oligonucleosomes) using a Cell Death Detection
ELISA kit (Roche Molecular Biochemicals, Indianapolis, IN, USA)
according to the manufacturer’s instructions.
Flow cytometry
PC-3 and LNCaP prostate carcinoma (5×105)
cell lines were seeded in triplicate in 6-well plates, and cultured
in RPMI-1640 supplemented with 100 ml/l FBS. After transfection for
48 h, the cells were collected and washed with ice-cold PBS, and
fixed in 70% ethanol overnight at 4°C. The fixed cells were
pelleted, washed in PBS, resuspended in PBS containing 0.1 mg/ml of
propidium iodide and analysed by flow cytometry.
Results
Expression of EGR-1, p14ARF
and TGF-β in human prostate carcinoma cell lines overexpressing
Egr-1
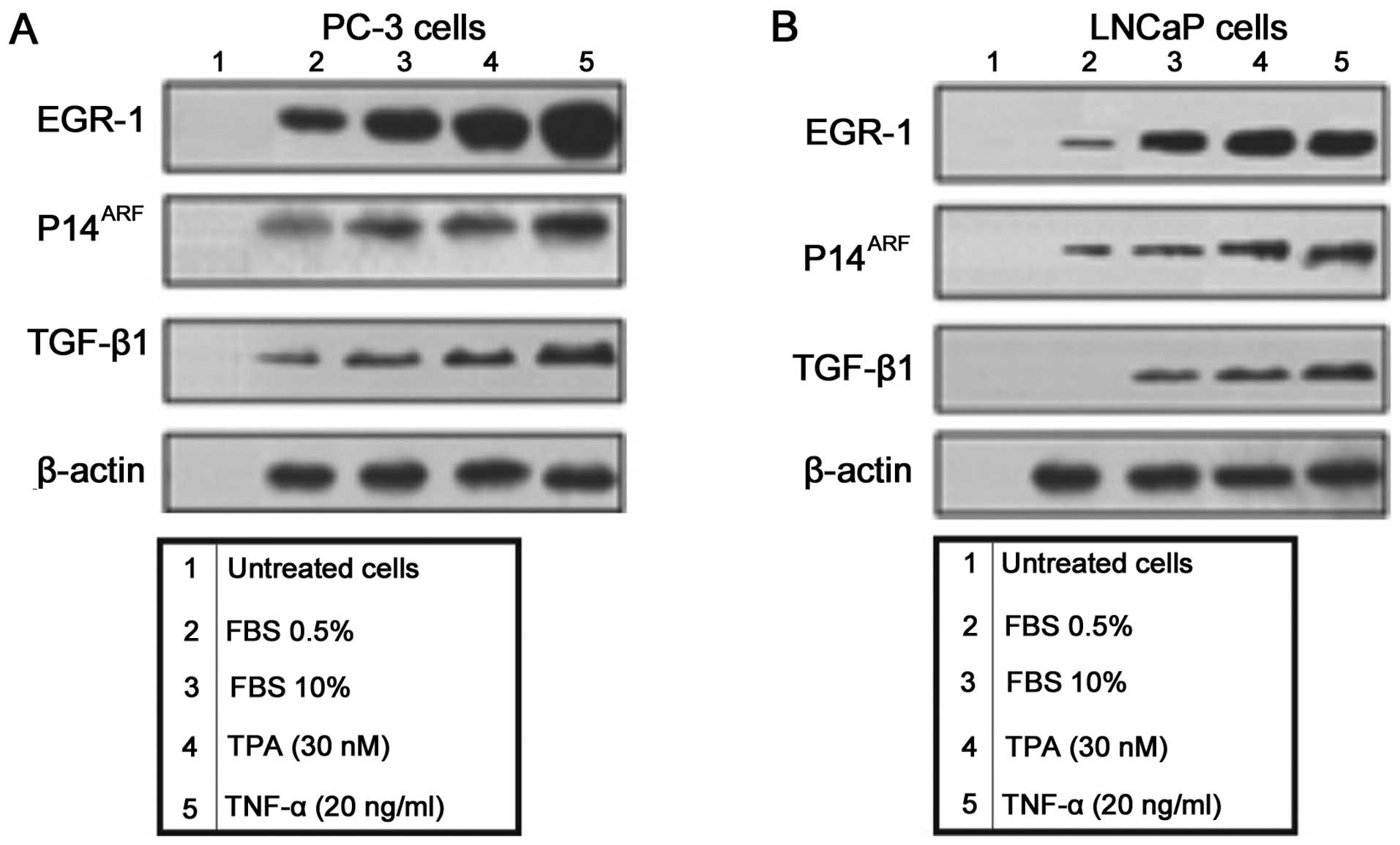
EGR-1 is an early response nuclear factor that is
important in the regulation of several genes (Fig. 1). To determine the effect of
overexpressing Egr-1 in the expression of EGR-1, p14ARF
and TGF-β1 in PC-3 (Fig. 1A) and
LNCaP (Fig. 1B), prostate carcinoma
cell lines the cells were treated with FCS 0.5%, FCS 10%, TPA (30
nM) and TNF-α (20 ng/ml). The protein expression of EGR-1,
p14ARF and TGF-β1 was assessed by western blot analysis
(Fig. 1).
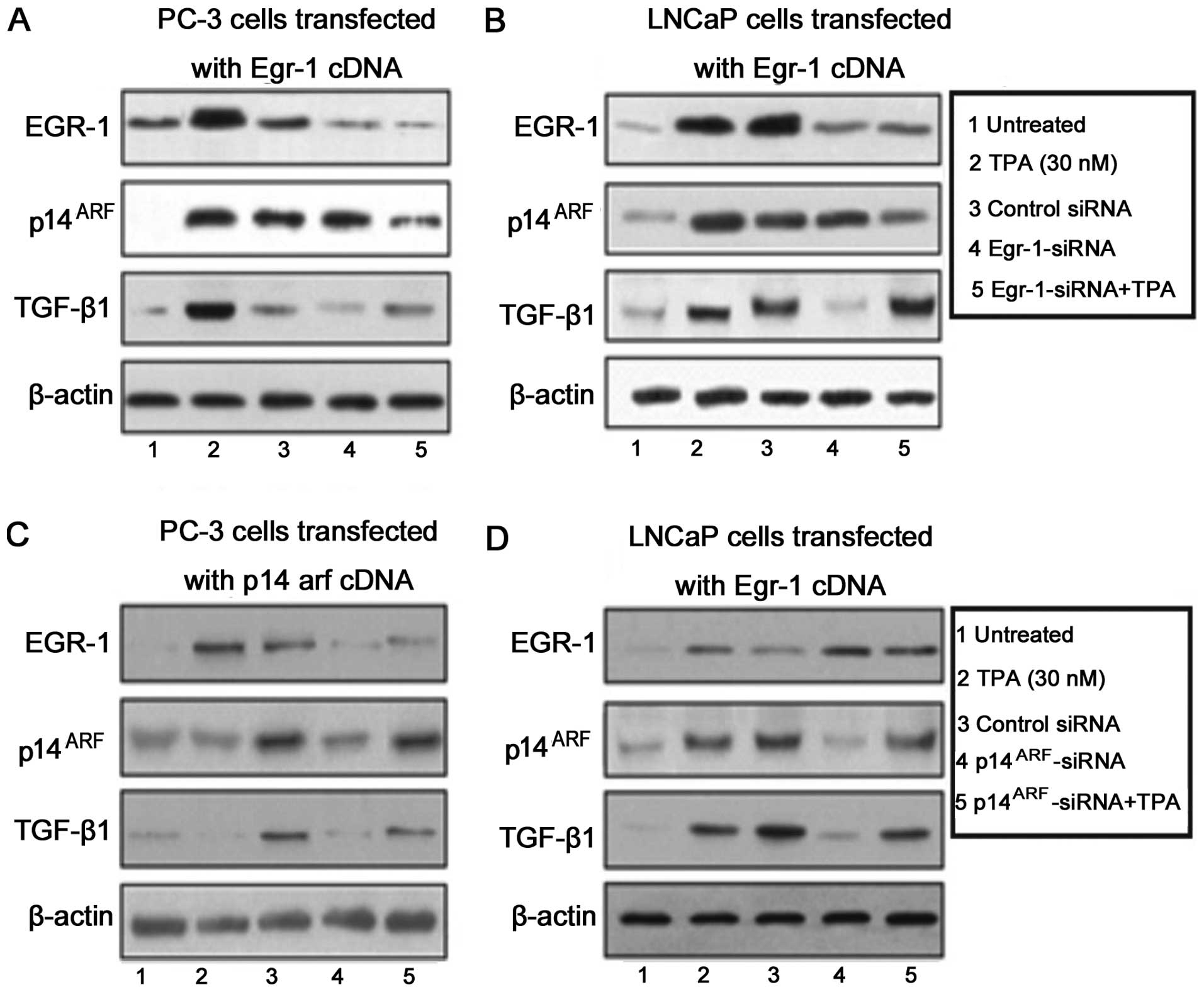
Knocking down Egr-1 expression by
Egr-1-siRNA, strongly decreased the activity of TGF-β 1 but only
moderately the expression of p14ARF and was able to
reverse the increasing effect of TPA (30 nM) only in PC-3
cells
PC-3 (Fig. 2A) and
LNCaP (Fig. 2B) prostate carcinoma
cells were transfected with a wild-type Egr-1-cDNA and the
expression of EGR-1, TGF-β1 and p14ARF proteins was
assessed by western blot analysis (Fig.
2A and B). We observed a strong induction of Egr-1 and
p14ARF after the treatment of cells with TPA and TNF-α
treatment (Fig. 2A and B, lanes 1
and 2). By contrast, TGF-β1 was significantly induced only after
TNF-α treatment in PC-3 cells (Fig.
2A, lane 3). The expression of TGF-β1 in LNCaP cells was
observed after treatment with TPA and TNF-α treatment (Fig. 2B, lanes 2, 3 and 5). A decrease in
the cell protein expression in the PC-3 (Fig. 2A) and LNCaP (Fig. 2B) prostate carcinoma cells was
observed following Egr-1 siRNA treatment (Fig. 2A and B, lane 4). Nevertheless,
Egr-1-siRNA was unable to reverse the effect of TPA in the
induction of TGF-β1 in LNCaP cells (Fig. 2B, lane 5).
To determine whether blocking the expression of
p14arf by a siRNA against p14arf affected the expression of EGR-1,
p14arf or TGF-β1, PC-3 (Fig. 2C)
and LNCaP (Fig. 2D) cells were
transfected with a vector carrying a p14arf cDNA. At 72 h after
transfection, the cells were treated with TPA (30 mM), TNF-α (20
ng/ml) and siRNA against p14arf and cultured for an additional 12
h. At the indicated time point, the cells were harvested and
analyzed for expression of EGR-1, p14ARF and TGF-β1. The
protein expression was assessed by western blot analysis. As shown
in Fig. 2C and D, p14arf-siRNA
strongly decreased EGR-1 and p14ARF expression but was
unable to reverse the effect of TPA.
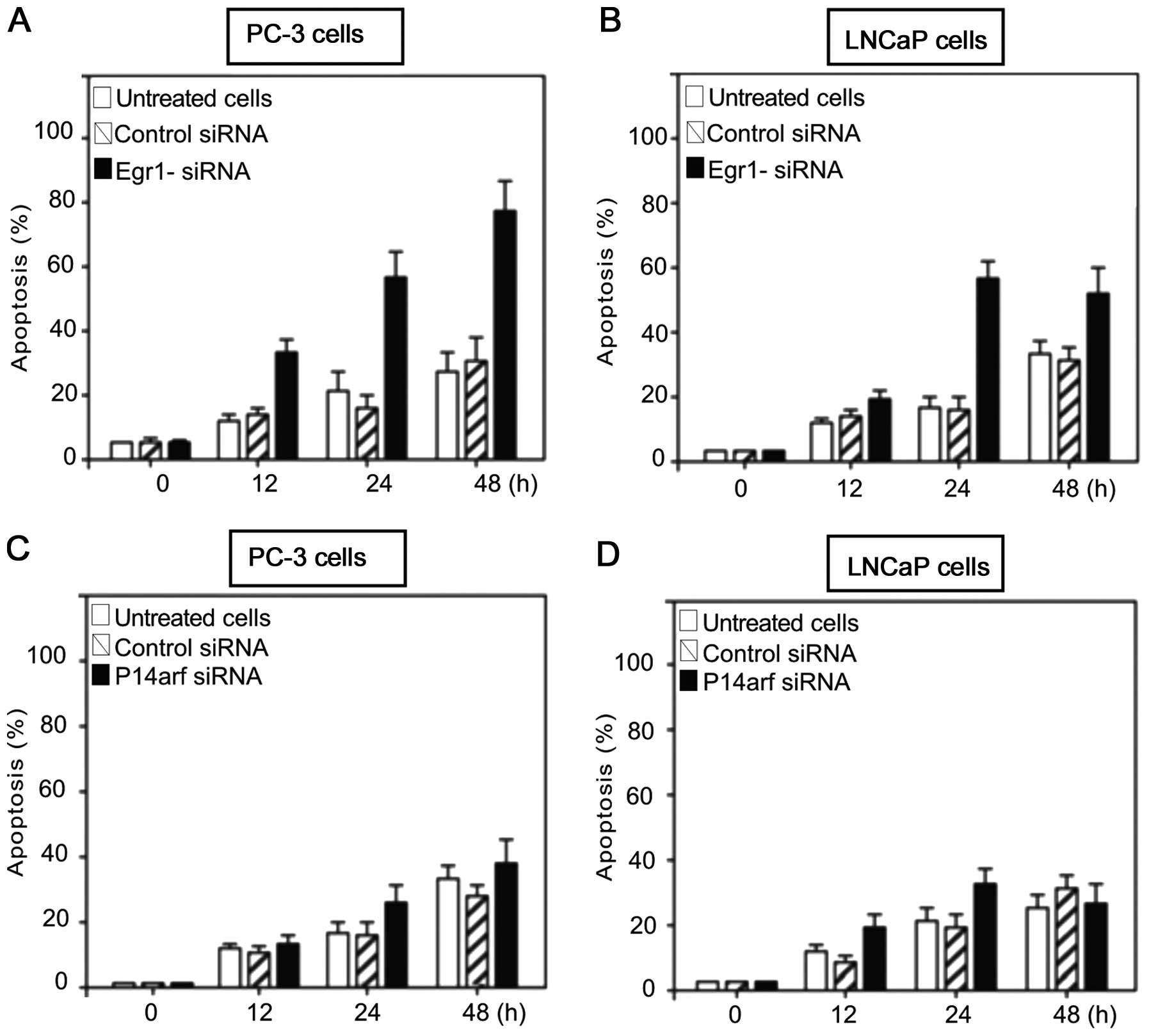
Inhibition of Egr-1 but not p14arf
increased apoptosis in PC-3 and LNCaP prostate carcinoma cell
lines
We determined whether blocking Egr-1 or p14arf
expression exerted an apoptotic effect on PC-3 or LNCaP cells
treated with siRNA against the abovementioned proteins. To this
effect, LNCaP cells were transfected with siRNA against Egr-1
(Fig. 3A) or against p14arf
(Fig. 3B) and with a non-specific
siRNA as the control. After culturing at the indicated times, the
cells were harvested and analysed for induction of apoptosis.
Results of the flow cytometric analysis showed that knocking down
Egr-1 using siRNA against Egr-1, significantly induced apoptosis in
PC-3 and LNCaP cells (Fig. 3A and
B). However, when PC-3 and LNCaP cells were treated with
p14arf-siRNA and analysed for induction of apoptosis, the effect
was only moderate (Fig. 3C and D)
and decreased after a 48-h transfection, suggesting a moderate role
for p14arf inhibition in controlling apoptosis. However, unlike
Egr-1-siRNA-treated PC-3 cells, the apoptotic activity decreased
after 48 h as compared to that observed in Fig. 3A and B. The results suggested that
the differential expression of EGR-1 and p53 was responsible for
the differences in apoptotic response demonstrated in PC-3 and
LNCaP cells (Fig. 3).
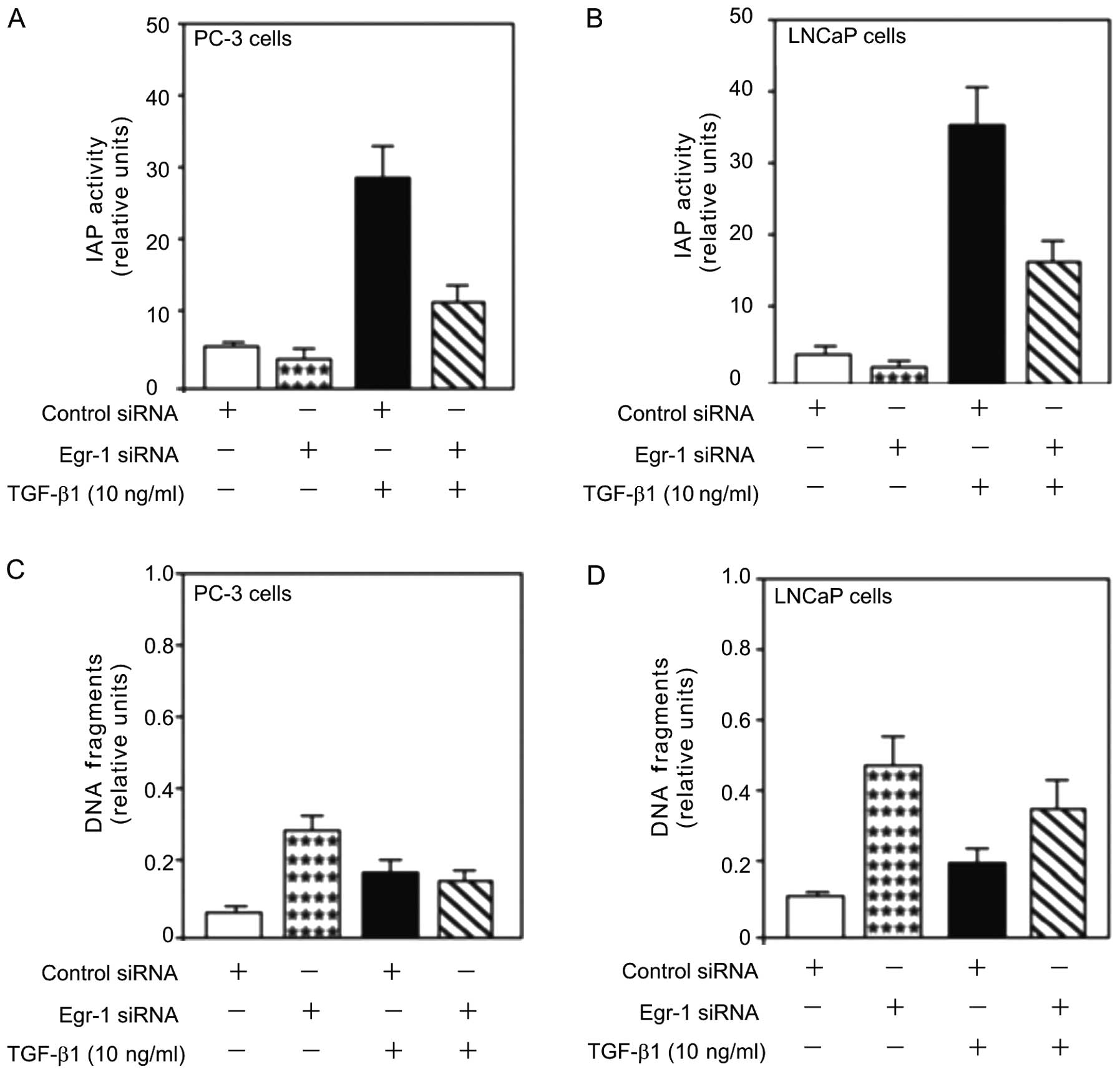
Egr-1 function in TGF-β1 induced
proliferation and cell survival
Since EGR-1 is a potential tumor inducer for
prostate cancer (1) and inhibition
of Egr-1 by siRNA-Egr-1 decreases TGF-β1-mediated proliferation
(6), we analysed the role of Egr-1
in TGF-β1-induced cell survival in PC-3 and LNCaP cells. Treatment
with TGF-β1 (10 ng/ml) increased IAP activity (Fig. 4A and B), an inhibitor of apoptosis
that plays a key role in preventing cell death by apoptosis.
However, this increase was attenuated by the transfection of siRNA
against Egr-1 (Egr-1-siRNA), suggesting a role for Egr-1 in
TGF-β1-mediated cell viability.
The aforementioned results showed that inhibition of
Egr-1 by siRNA enhanced TGF-β1-mediated cell viability in human
prostate cancer cells (Fig. 4A and
B). The effect of Egr-1 knockdown on TGF-β1-induced PC-3 and
LNCaP cell survival was assessed. TGF-β1 induced obvious cell
survival as shown by the decreased DNA fragmentation in the PC-3
and LNCaP cells (Fig. 4C and D) and
this decrease was partially attenuated by the knockdown of Egr-1
using Egr-1-siRNA transfection. Inhibition of Egr-1 expression
induced obvious cell death as shown by the increased DNA
fragmentation (Fig. 4C and D),
suggesting a survival role of Egr-1 in prostate cancer progression.
Collectively, the results suggested that Egr-1 is involved in
TGF-β-mediated prostate cell death, survival and
differentiation.
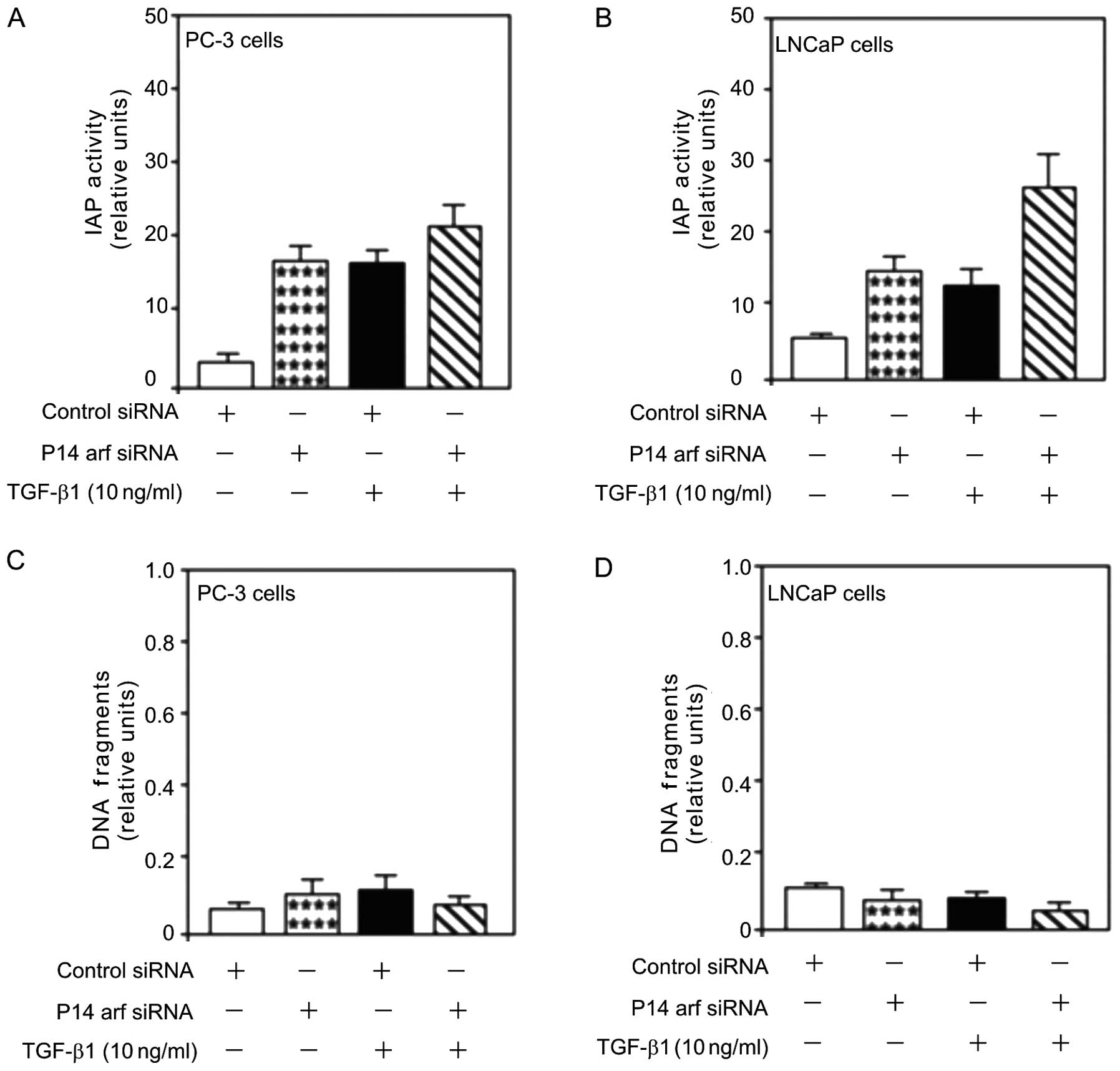
p14arf function in TGF-β1 induced
proliferation and cell survival
The role of p14ARF-induced cell death in
PC-3 and LNCaP cells was assessed. Treatment with TGF-β1 (10 ng/ml)
increased IAP activity (Fig. 5A and
B), an inhibitor of apoptosis. However, unlike Egr-1 (Fig. 4A and B), this increase was not
attenuated by the transfection of siRNA against p14ARF
(p14arf-siRNA) suggesting a key role of TGF-β1-mediated
proliferation in allowing cell viability in human prostate cancer
cells (Fig. 5A and B).
The effect of p14arf knockdown on TGF-β1-induced
PC-3 and LNCaP cell survival was examined. TGF-β1 induced obvious
cell survival as shown by the decreased DNA fragmentation in the
PC-3 and LNCaP cells (Fig. 5C and
D). This decrease was not attenuated by the knockdown of p14arf
using p14arf-siRNA transfection. Inhibition of p14arf expression
induced obvious cell survival as shown by the decreased DNA
fragmentation (Fig. 5C and D),
suggesting a cell death role for p14arf in prostate cancer.
Collectively, our results suggested that Egr-1 plays a role in
TGF-β1 and p14arf-mediated prostate cell death, survival and
differentiation.
Discussion
In previous studies, we demonstrated decreased
prostate carcinoma cell survival and independent anchorage by
Egr-1-siRNA (15,16) and defined a novel feedback
regulation of EGR-1 through the regulation of NF-κB and AP-1
(10). In the present study, we
delineated the signaling pathway involved in this regulation. The
results of the present study show that TGF-β1-treated cells
increased EGR-1 expression and decreased the p14ARF
suppressor effect in PC-3 and LNCaP prostate carcinoma cell lines.
This TGF-β1-mediated EGR-1 induction and further enhancement in the
increase of EGR-1 expression was independent of the presence or
absence of the tumor suppressor protein p14ARF.
Treatment of the PC-3 and LNCaP prostate carcinoma cell lines with
TPA (30 nM) or TNF-α (20 ng/ml) activated EGR-1, JNK-1 and JNK-3
(32) and attenuated cell apoptosis
(32). Findings of a recent study
have shown that the treatment of PC-3 and LNCaP cells with
Egr-1-siRNA induced cell survival, which was associated with the
activation p21Waf1/Cip1 protein and with a decreased JNK
expression (33). By contrast,
inhibition of JNK by a siRNA, did not attenuate EGR-1 expression
(18). Our observation that the
blockade of EGR-1 by a genetic mechanism (transfection of
Egr-1-siRNA) attenuated TGF-β1 induction, while it did not affect
p14ARF induction, suggests a functional role for EGR-1
activation in TGF-β1 induction in PC-3 and LNCaP prostate carcinoma
cell lines. The induction of proliferation by TGF-β1 via the
induction of EGR-1 may play a role in the autonomy of prostate
carcinoma cell growth and, thus, in the pathogenesis of prostate
cancer.
EGR-1 expression in certain cells, such as breast
and lung carcinoma cells, as well as most normal tissues, is low
(10). In addition, EGR-1
overexpression is correlated with loss of its co-repressor NAB2 in
primary prostate carcinoma (6–9). This
disruption of the balance between EGR-1 and NAB2 expression results
in high EGR-1 transcriptional activity in prostate carcinoma cells
(6).
Crosstalk between EGR-1 and TGF-β1 pathway has been
previously reported (34). The
levels of EGR-1 protein and mRNA were rapidly and transiently
upregulated by TGF-β1 in vitro, and EGR-1 promoter activity
was enhanced. Liu et al (25) observed that the EGR-1 gene product
directly controls TGF-β1 gene expression. Another study showed that
TGF-β1 treatment also activated the ERK1/2 signaling pathway,
causing an increase in EGR-1 (35).
However, the tumor-suppressor p14ARF protein showed
antagonist activity to the effect induced by EGR-1 in PC-3 and
LNCaP cells. Previously, Egr-1 and PTEN were identified as
mediators of p14ARF function (36). Egr-1-dependent expression of PTEN is
controlled by p14ARF through the ARF-mediated
sumoylation of Egr-1. This requires the phosphorylation of Egr-1 by
the protein kinase AKT, which promotes the association of Egr-1
with p14ARF fibroblasts or ARF-/-ARF (36). p14ARF has been identified
as a potent tumor suppressor in vitro and in vivo
(37,38). p14ARF has been shown to
protect against uncontrolled growth and tumorigenesis induced by
hyper-proliferative stimuli (39).
In agreement with these findings, results of this study have shown
that inhibition of p14ARF attenuated cell apoptosis,
leading to the enhancement of EGR-1 induction. p14ARF
was found to be primarily dependent on the presence of functional
p53. However, p14ARF is involved in p53-independent
mechanisms of cell cycle regulation and apoptosis induction,
respectively. Failure of apoptosis is central to the development of
cancer, and in many types of cancer, this failure results from loss
of p53, a key mediator of apoptosis (40). Consequently, the tumor-suppressor
p14ARF appears to be a possible target of the EGR-1
pathway.
In summary, the present study provides important
insights regarding the signaling mechanisms regulating EGR-1
expression and function in prostate carcinoma cell lines. The
importance of the antagonistic crosstalk between EGR-1 and
p14ARF in prostate carcinoma cell lines deserves further
investigation as a novel approach to cancer therapy.
Acknowledgements
We would like to thank Dr Peter T. Daniel,
Department of Hematology, Oncology and Tumor Immunology, University
Medical Center Charité, Campus Berlin-Buch, D-13125 Berlin-Buch,
Germany for providing the vector expressing the p14ARF cDNA. The
present study was supported by the Intramural Regular Research
Grant from the University of Tarapaca UTA-6710-14.
References
|
1
|
Eichelberger L, Koch OM, Eble J, Ulbright
T, Juliar B and Cheng L: Maximum tumor diameter is an independent
predictor of prostate-specific antigen recurrence in prostate
cancer. Mod Pathol. 18:886–890. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Virolle T, Krones-Herzig A, Baron V,
Gregorio GG, Adamson ED and Mercola D: Egr-1 promotes growth and
survival of prostate cancer cells: identification of novel Egr-1
target genes. J Biol Chem. 278:11802–11810. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eid MA, Kumar MV, Iczkowski KA, Bostwick
DG and Tindall DJ: Expression of early growth response genes in
human prostate cancer. Cancer Res. 58:2461–2468. 1998.PubMed/NCBI
|
|
4
|
Scharnhorst V, Menke AL and Attema J:
EGR-1 enhances tumor growth and modulates the effect of the Wilms’
tumor 1 gene products on tumorigenicity. Oncogene. 19:791–800.
2000.PubMed/NCBI
|
|
5
|
Pignatelli M, Luna-Medina R, Perez-Rendon
A, Santos A and Perez-Castillo A: The transcription factor early
growth response factor-1 (EGR-1) promotes apoptosis of
neuroblastoma cells. Biochem J. 373:739–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Houston P, Campbell CJ, Svaren J,
Milbrandt J and Braddock M: The transcriptional corepressor NAB2
blocks Egr-1-mediated growth factor activation and agiogenesis.
Biochem Biophys Res Commun. 283:480–486. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adamson ED and Mercola D: Egr-1
transcription factor, multiple roles in prostate tumor cell growth
and survival. Tumour Biol. 23:93–102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banks MF, Gerasimovskaya EV, Tucker D,
Frid MG, Carpenter TC and Stenmark KR: Egr-1 antisense
oligonucleotides inhibit hypoxia-induced proliferation of pulmonary
artery adventitial fibroblast. J Appl Physiol. 98:732–738. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parra E, Ferreira J and Ortega A:
Overexpression of EGR-1 modulates the activity of NF-κB and AP-1 in
prostate carcinoma PC-3 and LNCaP cell lines. Int J Oncol.
39:345–352. 2011.PubMed/NCBI
|
|
10
|
Yang SZ and Abdulkadir S: Early growth
response gene 1 modulates androgen receptor signaling in prostate
carcinoma cells. J Biol Chem. 278:39906–39911. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu C, Yao J, Mercola D and Adamson E: The
transcription factor EGR-1 directly transactivates the fibronectin
gene and enhances attachment of human glioblastoma cell line U251.
J Biol Chem. 275:20315–20323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krones-Herzig A, Mittal S, Yule K, Liang H
and English C: Early growth response 1 acts as a tumor suppressor
in vivo and in vitro via regulation of p53. Cancer Res.
65:5133–5141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sumathi P, Muthukkumar S, Han S, Sukhatme
V and Rangnekar V: Early growth response-1-dependent apoptosis is
mediated by p53. J Biol Chem. 272:20131–20138. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Virolle T, Adamson ED, Baron V, Birle D,
Mustelin T and de Belle I: The Egr-1 transcription factor directly
activates PTEN during irradiation-induced signalling. Nat
Cell Biol. 3:1124–1128. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parra E, Ortega A and Saenz L:
Downregulation of Egr-1 by siRNA inhibits growth of human prostate
carcinoma cell line PC-3. Oncol Rep. 22:1513–1518. 2009.PubMed/NCBI
|
|
16
|
Parra E, Ferreira J and Saenz L:
Inhibition of Egr-1 by siRNA in prostate carcinoma cell lines is
associated with decreased expression of AP-1 and NF-κB. Int J Mol
Med. 28:847–853. 2011.PubMed/NCBI
|
|
17
|
Parra E, Gutiérrez L and Ferreira J:
Increased expression of p21Waf1/Cip1 and JNK with
costimulation of prostate cancer cell activation by an siRNA Egr-1
inhibitor. Oncol Rep. 30:911–916. 2013.
|
|
18
|
Parra E, Ferreira J and Gutierrez L:
Decreased c-Abl activity in PC-3 and LNCaP prostate cancer cells
overexpressing the early growth response-1 protein. Oncol Rep.
31:422–427. 2014.PubMed/NCBI
|
|
19
|
Letterio JJ and Roberts AB: Regulation of
immune response by TGF-β. Annu Rev Immunol. 16:137–161. 1998.
|
|
20
|
Liu C, Yao J, de Belle I, Huang RP,
Adamson E and Mercola D: The transcription factor EGR-1 suppresses
transformation of human fibrosarcoma HT1080 cells by coordinated
induction of transforming growth factor-beta1, fibronectin, and
plasminogen activator inhibitor-1. J Biol Chem. 247:4400–4411.
1999. View Article : Google Scholar
|
|
21
|
Danielpour D, Dart LL, Flanders KC,
Roberts AB and Sporn MB: Immunodetection and quantitation of the
two forms of transforming growth factor-beta-1 and TGF-beta-2
secretion by cells in culture. J Cell Physiol. 138:79–86. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roberts AB and Sporn MB: Differential
expression of the TGF-β isoforms in embryogenesis suggests specific
roles in developing and adult tissue. Mol Reprod Dev. 32:91–98.
1992.
|
|
23
|
Millan FA, Denhez F, Kondaiah P and
Akhurst RJ: Embryonic gene expression patterns of TGF beta 1, beta
2 and beta 3 suggest different developmental functions in vivo.
Development. 111:131–143. 1991.PubMed/NCBI
|
|
24
|
Robert AB and Sporn MB: Physiological
actions and clinical applications of transforming growth factor-β
(TGF-β). Growth Factors. 8:1–9. 1993.
|
|
25
|
Liu Y, Liu H, Li J, Nadalin S,
Konigsrainer A, Weng H, Dooley S and Dijke P: Transforming growth
factor-β (TGF-β)-mediated connective tissue growth factor (CTGF)
expression in hepatic stellate cells requires Stat3 signaling
activation. J Biol Chem. 88:30708–30719. 2013.
|
|
26
|
Inoue K, Roussel MF and Sherr CJ:
Induction of ARF tumor suppressor gene expression and cell cycle
arrest by transcription factor DMP1. Proc Natl Acad Sci USA.
96:3993–3998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mao L, Merlo A, Bedi G, Shapiro GI,
Edwards CD, Rollins BJ and Sidransky D: A novel p16INK4a transcrpt.
Cancer Res. 55:2995–2997. 1995.PubMed/NCBI
|
|
28
|
Kamijo T, Weber JD, Zambetti G, Zindy F,
Roussel MF and Sherr C: Functional and physical interactions of the
ARF tumor suppressor with p53 and Mdm2. Proc Natl Acad Sci USA.
95:8292–8297. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haupt Y, Maya R, Kazaz A and Oren M: Mdm2
promotes the rapid degradation of p53. Nature. 387:296–299. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kubbutat MH, Jones SN and Vousden KH:
Regulation of stability by mdms. Nature. 387:299–303. 1997.
View Article : Google Scholar
|
|
31
|
Woods YL, Xirodimas DP, Prescott AR,
Sparks A, Lane DP and Saville MK: p14 Arf promotes small
ubiquitin-like modifier conjugation of Werners helicase. J Biol
Chem. 279:50157–50166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu J, Zhang SS, Saito K, Williams K,
Arimura Y, Ma Y, Ke Y, Baron V, Mercola D, Feng GS, Adamson E and
Mustelin T: PTEN regulation by Akt-Egr-1-ARF-PTEN axis. EMBO J.
28:21–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Parra E: Inhibition of JNK-1 by small
interference RNA induces apoptotic signalling in PC-3 prostate
cancer cells. Int J Mol Med. 30:923–930. 2012.PubMed/NCBI
|
|
34
|
Parra E and Ferreira J: Modulation of the
proliferative response of prostate cancer cells to cisplatin
treatment by JNK-1/JNK-2 siRNA. Oncol Rep. 30:1936–1942. 2013.
|
|
35
|
Friedrich B, Janessa A, Artunc F,
Karl-Aicher W, Muller GA, Alexander D and Risler T: DOCA and TGF-β
induce early growth response gene-1 (Egr-1) expression. Cell
Physiol Biochem. 22:465–474. 2008.
|
|
36
|
Rodriguez-Barbero A, Obreo J, Alvarez P,
Pandiella A, Bernabeu C and Lopez-Novoa JM: Endoglin modulation of
TGF-beta1-induced collagen synthesis is dependent on ERK1/2 MAPK
activation. Cell Physiol Biochem. 18:135–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gitenay D and Baron VT: Is Egr-1 a
potential target for prostate cancer therapy? Future Oncol.
5:993–1003. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kamijo T, Zindy F, Roussel MF, Quelle DE,
Downing JR, Ashmun RA, Grosveld G and Sherr CJ: Tumor suppression
at the mouse INK4a locus mediated by the alternative reading
frame product p19ARF. Cell. 91:649–659. 1997.
|
|
39
|
Sherr CJ and Weber JD: The ARF/p53
pathway. Curr Opin Genet Dev. 10:94–99. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Haupt S, Berger M, Goldb Z and Haupt Y:
Apoptosis - the p53 network. J Cell Sci. 116:4077–4085. 2003.
View Article : Google Scholar : PubMed/NCBI
|