Introduction
Breast cancer is a highly complex disease with large
inter-and intra-tumoral heterogeneity; the basal-like molecular
subtype of breast cancer has the poorest clinical outcome (1). Epidermal growth factor receptor (EGFR)
is frequently overexpressed in triple-negative breast cancer (TNBC)
and inflammatory breast cancer (IBC) in comparison with other
subtypes of breast cancer (2).
Highly-expressed EGFR is associated with a number of
characteristics including large tumor size, poor differentiation
and poor clinical outcome in a variety of cancer cells, including
breast and lung cancer cells (3).
In addition, elevated EGFR and CK5/14 expression was associated
with the status of CD44+/CD24− in basal-like
subtypes of breast cancer (4). A
population of CD44+/CD24−/low tumor cells has
also been associated with the aggressive basal-like breast cancer
(4). In a recent study, we also
reported that CD44 expression is regulated through
EGFR/ERK-dependent pathways in breast cancer cells (5).
Hyaluronan (HA) is a component of the extracellular
matrix and binds to its predominant cell-surface receptor CD44
(6,7). Aberrant expression of HA correlates
with poorly differentiated tumors, auxiliary lymph node status and
short overall survival time in breast cancer (8). HA/CD44 complex promotes multiple
signaling pathways that influence many tumor cell activities,
including abnormal growth, migration and invasion (9–11). The
standard form of CD44 is generally ubiquitously expressed on
epithelial cells and lymphocytes (7). The CD44 gene is composed of at least
20 exons that tissue-specific multiple variant isoforms
(CD44v1-v10) is produced by alternate mRNA splicing (12). Indeed, induction of CD44s alone
affected the growth characteristics of non-invasive luminal breast
cancer cells including the induction of cell proliferation, cell
migration and cell invasion in vitro (13). In addition, overexpression of CD44v3
isoforms promotes breast tumor cell migration and the attachment of
VEGF to the heparin sulfate sites on CD44v3 is responsible for the
onset of breast tumor-associated growth (14).
Zerumbone
[2,6,9,9-tetramethylcycloundeca-2,6,10-trien-1-one; (ZER)], a
monocyclic sesquiterpene derived from a Southeast Asian ginger, is
often used as an anti-inflammatory and antioxidant agent (15–17).
ZER has contributed to antitumor activities in a variety of
cancers, such as colon and gastric cancer (17). In addition, ZER triggers apoptosis
through modulation of the Bax/Bcl-2 ratio in HepG2 liver cancer
cells (17). Downregulation of
CXCR4 expression by ZER suppressed CXCL12-induced cell invasion
through the inhibition of NF-κB activity in breast and pancreatic
cancer cells (18). Despite these
studies, however, the regulatory mechanisms of ZER on EGFR
signaling pathways are not yet fully understood.
In the present study, we examined the role of ZER on
the regulatory mechanism of EGF-induced CD44 expression in breast
cancer cells. In particular, we found that ZER has an inhibitory
effect on the EGF/STAT3 signaling pathway in breast cancer cells.
EGF-induced CD44 expression also was regulated by the STAT3
dependent pathway.
Materials and methods
Reagents and cell culture
Dulbecco’s modified Eagle’s medium (DMEM), RPMI-1640
and the antibiotics were purchased from Life Technologies
(Rockville, MD, USA). Fetal bovine serum (FBS) was purchased from
HyClone (Logan, UT, USA). UO126 and LY294002 were purchased from
Tocris Bioscience (Ellisville, MO, USA). Mouse monoclonal anti-CD44
antibody was purchased from Cell Signaling Technology (Beverly, MA,
USA). The secondary HRP-conjugated antibodies, as well as the mouse
monoclonal anti-β-actin antibody, were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Rabbit monoclonal
phospho- and total-Akt, STAT3 and Erk1/2 antibodies were purchased
from Epitomics (Burlingame, CA, USA). ZER was a gift from Dr
Murakami (Kyoto University, Kyoto, Japan). EGF and TGF-α were
purchased from R&D Systems (Minneapolis, MN, USA). ALDEFluor™
kit was purchased from Stemcell Technologies (Durham, NC, USA).
SKBR3 and MDA-MB468 human breast cancer cells were
grown in a humidified atmosphere of 95% air and 5% CO2
at 37°C in RPMI-1640 supplemented with 10% FBS, 2 mM glutamine, 100
IU/ml penicillin and 100 μg/ml streptomycin.
ZER and specific inhibitor treatment
Cells were maintained in culture medium without FBS
for 24 h. Then, the culture media was replaced with fresh media
without FBS. Cells were pretreated with 5 or 10 μM concentrations
of ZER for 16 h prior to EGF treatment and were then treated with
50 ng/ml EGF for 24 h. In experiments involving specific
inhibitors, such as UO126, LY294002 and STAT3 inhibitor, each cell
was pretreated with specific inhibitors for 30 min prior to
treatment with EGF and they were then treated with EGF for 24
h.
Western blotting
The cell culture media (supernatants) and cell
lysates were used in the immunoblot analysis for CD44, EGFR, STAT3,
Erk, Akt and β-actin. Cells were lysed on ice for 30 min. The cell
lysate was collected into microtubes and samples were centrifuged
for 15 min at 12,000 rpm at 4°C. Supernatants were collected and
the protein concentrations were measured using the Bio-Rad Protein
Assay kit (Hercules, CA, USA). The proteins were boiled for 5 min
in Laemmli sample buffer and they were then electrophoresed in 10%
SDS-PAGE gels, respectively. The separated proteins were
transferred to PVDF membranes and the membranes were then blocked
with 10% skim milk in TBS with 0.01% Tween-20 for 15 min. The blots
were incubated with anti-CD44, EGFR, STAT3, ERK, AKT and β-actin
antibodies in 1% TBS/T buffer (0.01% Tween-20 in TBS) at 4°C
overnight. The blots were washed 3 times in TBS with 0.01% Tween-20
and they were subsequently incubated with anti-rabbit
peroxidase-conjugated antibody (1/2,000 dilution) in TBS/T buffer.
After 1 h incubation at room temperature (RT), the blots were
washed 3 times and ECL Prime reagents were used for
development.
Real-time PCR
Total RNA was extracted from the cells using the
TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the
manufacturer’s instructions. Isolated RNA samples were then used
for RT-PCR. Samples (1 μg of total RNA) were reverse-transcribed
into cDNA in 20 μl reaction volumes using a first-strand cDNA
synthesis kit for RT-PCR, according to the manufacturer’s
instructions (MBI Fermentas, Hanover, MD, USA).
The gene expression was quantified by real-time PCR
using a SensiMix SYBR kit (Bioline Ltd., London, UK) and 100 ng of
cDNA/reaction. The sequences of the primer sets used for this
analysis were: human CD44s (forward, 5′-CCA AGA TGA TCA GCC ATT CTG
G-3′ and reverse, 5′-AAG ACA TCT ACC CCA GCA AC-3′) and GAPDH as an
internal control (forward, 5′-ATT GTT GCC ATC AAT GAC CC-3′ and
reverse, 5′-AGT AGA GGC AGG GAT GAT GT-3′). An annealing
temperature of 60°C was used for all primers. PCRs were performed
in a standard 384-well plate format with an ABI 7900HT Real-Time
PCR detection system. For data analysis, the raw threshold cycle
(CT) value was first normalized to the housekeeping gene
for each sample to get the ΔCT. The normalized
ΔCT was then calibrated to the control cell samples to
get the ΔΔCT.
Cell viability
To confirm ZER toxicity, we used treatment with the
indicated concentrations of ZER and total cell numbers were
evaluated by Quick Cell Proliferation Assay kit II (BioVision,
Mountain View, CA, USA) according to the manufacturer’s protocol.
Briefly, CD44-positive cells, MDA-MB468 and SKBR3 human breast
cancer cells (5×104/well) were grown in a 96-well plate
in 100 μl/well of culture media in the absence or presence of ZER.
After incubating the cells for 24 h, 10 μl WST reagent was added to
each well. Viable cells were quantified photometrically at 480
nm.
ALDEFLUOR assay
The ALDEFLUOR kit (Stem Cell Technologies, Grenoble,
France) was used for the immuno-fluorescent detection of
intracellular ALDH enzyme activity, using a FACS-vantage
(Becton-Dickinson, San Diego, CA, USA), according to the
manufacturer’s instructions. Briefly, cells were incubated in
ALDEFLUOR assay buffer containing ALDH substrate (1 mM/l per 13,106
cells). In each experiment, a sample of cells was incubated, under
identical conditions, with 50 mM/l of diethylaminobenzaldehyde, a
specific ALDH inhibitor, as a negative control.
Statistical analysis
Statistical significance was determined using the
Student’s t-test. Data are presented as means ± SEM. All quoted
P-values are two-tailed and P-values <0.05 were considered to
indicate statistically significant differences. Microsoft Excel was
used for statistical analyses.
Results
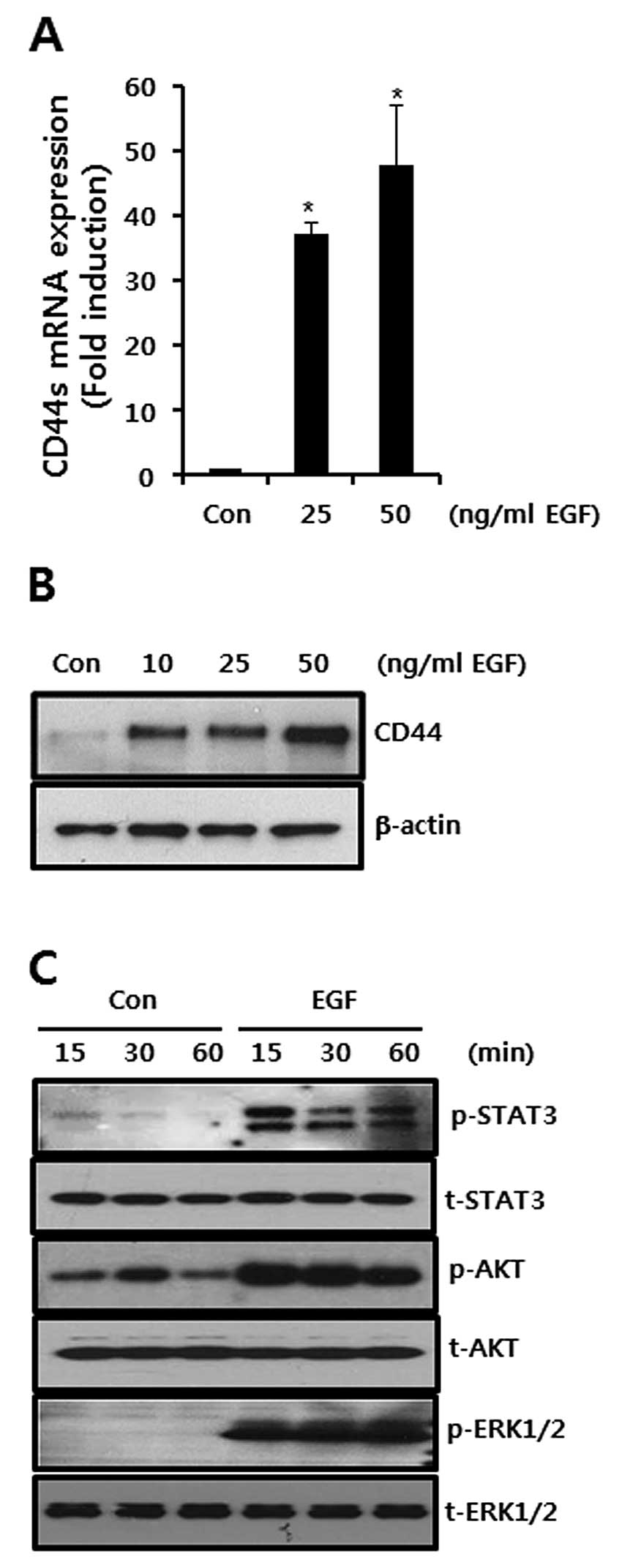
EGF augments the basal level of CD44 mRNA
and protein expression in the SKBR3 breast cancer cells
In the present study, we sought to verify the effect
of EGF on CD44 mRNA and protein expression. As a result, we treated
the CD44 with EGF for 24 h at the indicated concentrations. As
shown in Fig. 1A and B, the levels
of CD44 mRNA and protein expression were increased by EGF treatment
in a dose-dependent manner. After 50 ng/ml EGF treatment, the
levels of the CD44 mRNA expression were significantly increased to
48.1-fold that of the control level (Fig. 1A).
We also examined EGF-induced the phosphorylation of
STAT-3, ERK and AKT which are the downstream signaling molecules of
EGF. The levels of STAT-3, ERK and AKT phosphorylation were
significantly increased by 50 ng/ml EGF treatment in SKBR3 breast
cancer cells (Fig. 1C).
EGF ligand-induced CD44 mRNA and protein
expression levels are suppressed by MEK1/2 or STAT-3 inhibitors in
SKBR3 breast cancer cells
To verify the regulatory mechanism of EGF-induced
CD44 mRNA and protein expression, we pretreated cells with the
specific inhibitors, including UO, a MEK1/2 inhibitor; LY, a PI3K
inhibitor; and STAT3 IV, a STAT-3 inhibitor, respectively, for 30
min prior to 50 ng/ml EGF treatment. Our results showed that
EGF-induced CD44 protein and mRNA expression was decreased by UO or
STAT-3 IV inhibitors, respectively (Fig. 2A).
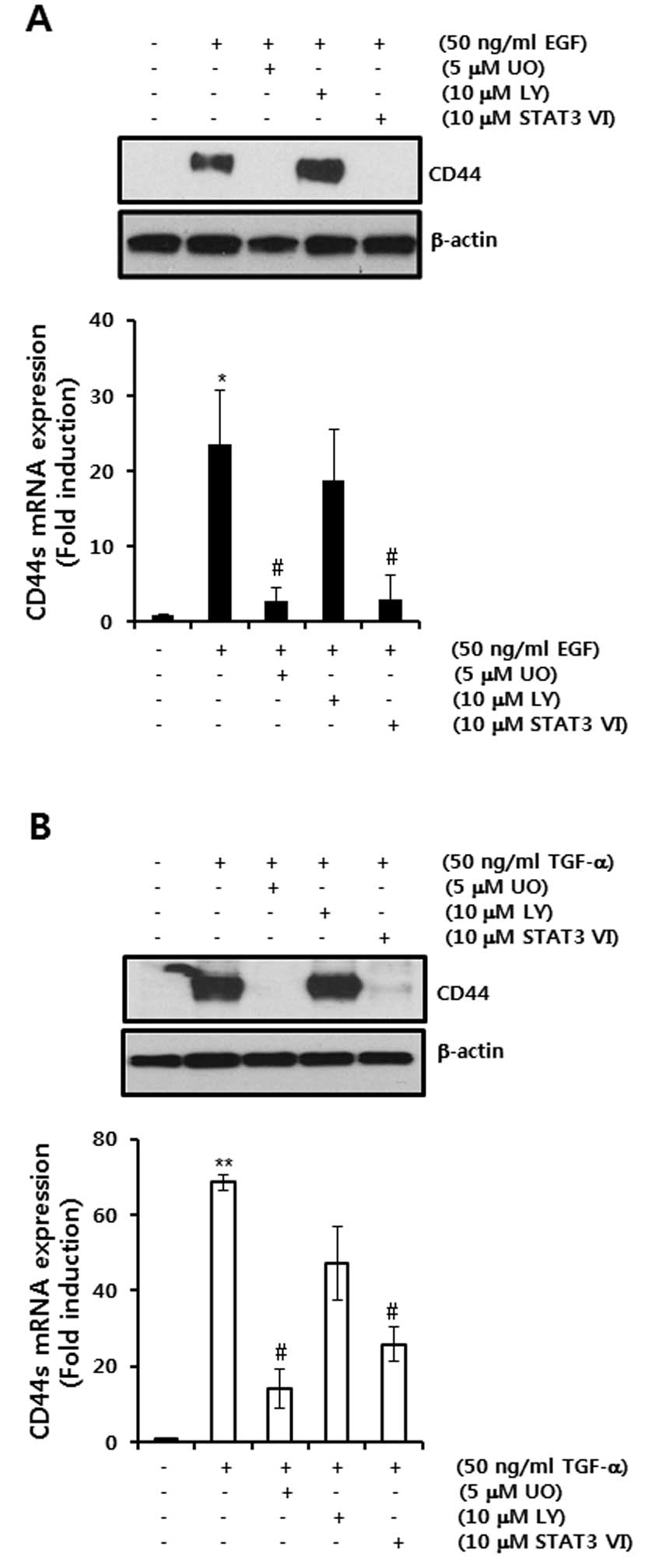 | Figure 2EGF ligand-induced CD44 mRNA and
protein expression levels are suppressed by a MEK1/2 inhibitor or
STAT3 inhibitor, respectively. (A and B) After serum-starvation for
24 h, SKBR3 cells were pretreated with 10 μM UO, LY and STAT3 VI,
respectively, for 30 min and were then treated with 50 ng/ml EGF
(A) or TGF-α (B) for 24 h, respectively. The levels of CD44 and
β-actin protein expression were analyzed by western blotting. The
levels of CD44s mRNA were analyzed by real-time PCR. The results
are representative of 3 independent experiments. The values shown
are the means ± SEM. *P<0.05, **P<0.01
vs. control. #P<0.05 vs. EGF-treated cells. Con,
control; UO, UO126; LY, LY294002; STAT3 VI, STAT3 inhibitor VI. |
The levels of CD44 mRNA expression were increased to
23.4-fold of the control level by 50 ng/ml EGF treatment (Fig. 2A). On the other hand, EGF-induced
CD44 mRNA and protein expression was significantly decreased by
3.8- and 4.2-fold of the control level by 10 μM UO and 10 μM STAT3
VI treatment, respectively (Fig.
2A). In addition, we confirmed the effect of another EGFR
ligand, TGF-α, on CD44 mRNA and protein. As expected, TGF-α-induced
CD44 protein and mRNA expression was also decreased by 10 μM UO and
10μM STAT3 VI treatment, respectively (Fig. 2B).
Next, we investigated the inhibitory effect of
specific inhibitors through the phosphorylation of signaling
molecules. Each inhibitor specifically decreased the
phosphorylation of STAT-3, AKT and ERK1/2 (Fig. 3). These results demonstrate that
EGF-induced CD44 expression is regulated through the MEK/ERK and
JAK/STAT3 dependent pathways in breast cancer cells.
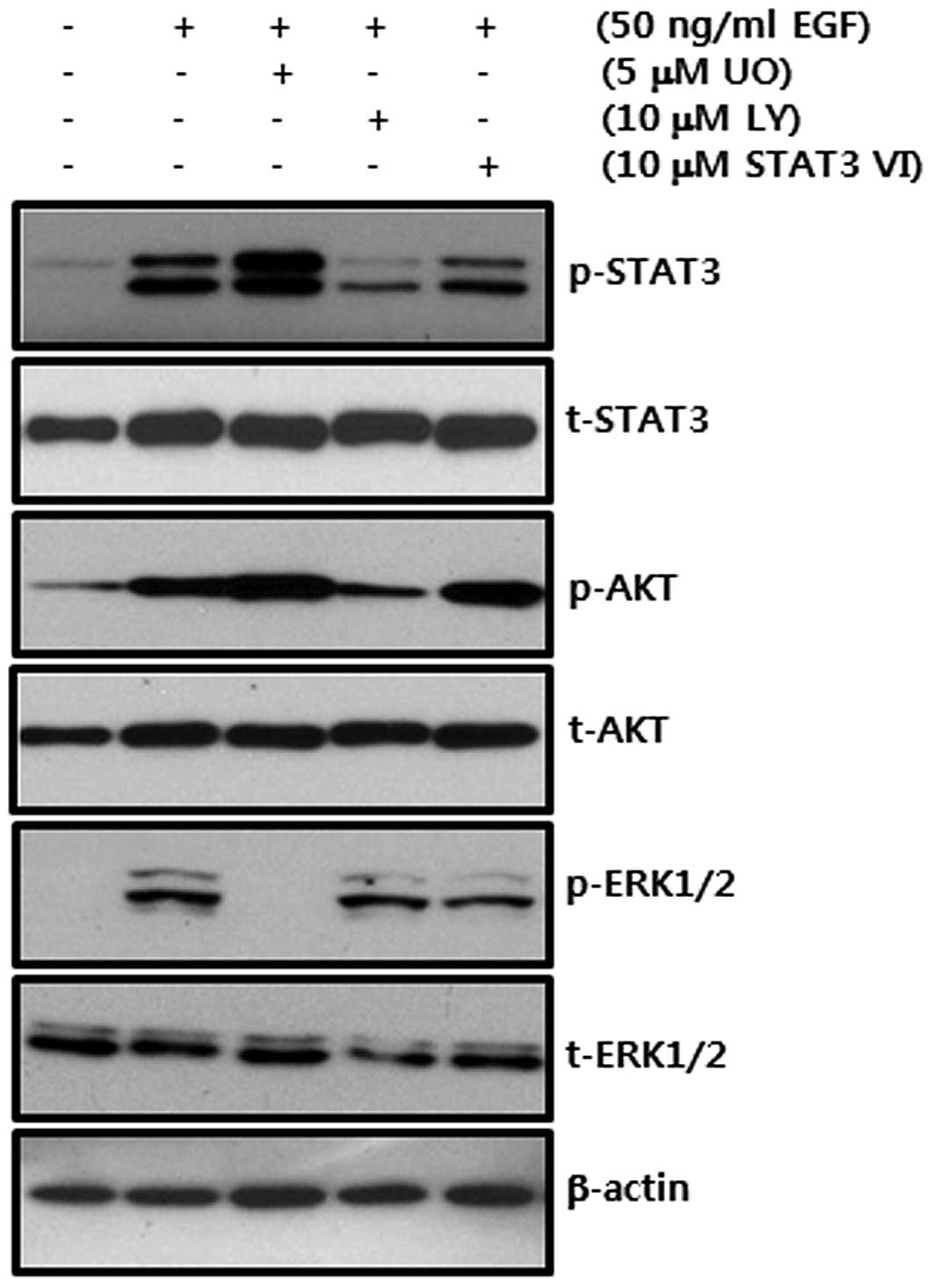 | Figure 3Effect of specific inhibitors on
EGF-induced phosphorylation of STAT3, AKT and ERK in SKBR3 breast
cancer cells. After serum-starvation for 24 h, SKBR3 cells were
pretreated with 10 μM UO, LY and STAT3 VI, respectively, for 30
min. They were then treated with 50 ng/ml EGF for 30 min. The
levels of STAT3, AKT and ERK phosphorylation by EGF were analyzed
by western blotting. The results are representative of 3
independent experiments. Con, control; UO, UO126; LY, LY294002;
STAT3 VI, STAT3 inhibitor VI. |
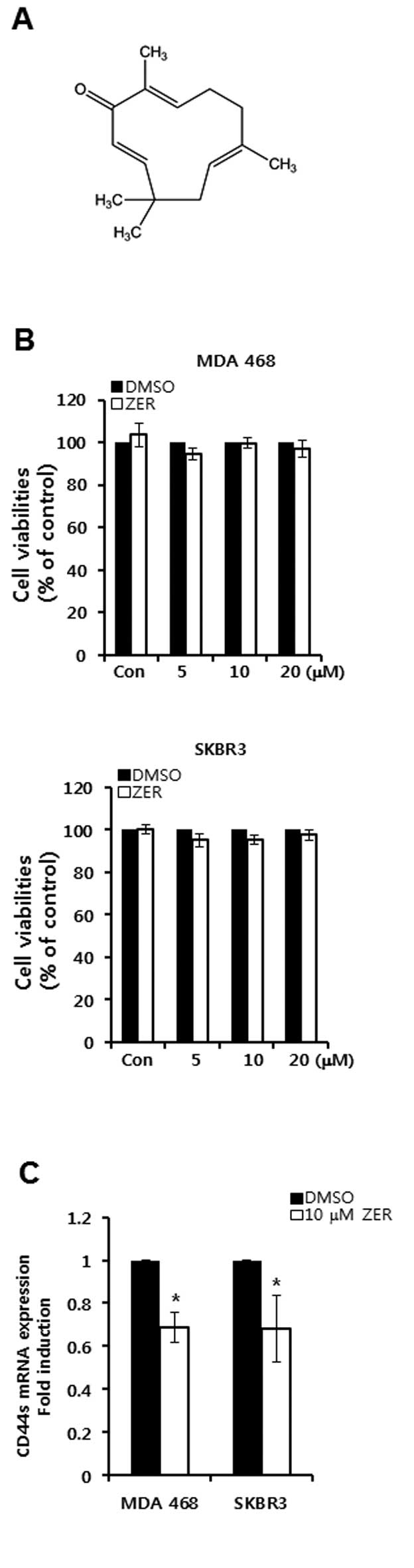
The basal level of CD44 is decreased by
ZER in CD44-positive breast cancer cells
In order to verify the effect of ZER on CD44
expression, we treated CD44-positive breast cancer cells with ZER.
The chemical structure of ZER is depicted in Fig. 4A. Cell viabilities were not altered
by ZER treatment in CD44-positive breast cancer cells (Fig. 4B). However, we found that the basal
level of CD44 mRNA expression was decreased by ZER treatment in
MDA-MB468 and SKBR3 breast cancer cells (Fig. 4C). The level of CD44 mRNA expression
was decreased by 0.69-fold (in MDA-MB468 cells) and 0.68-fold (in
SKBR3 cells) of the control level at 10 μM ZER, respectively
(Fig. 4C).
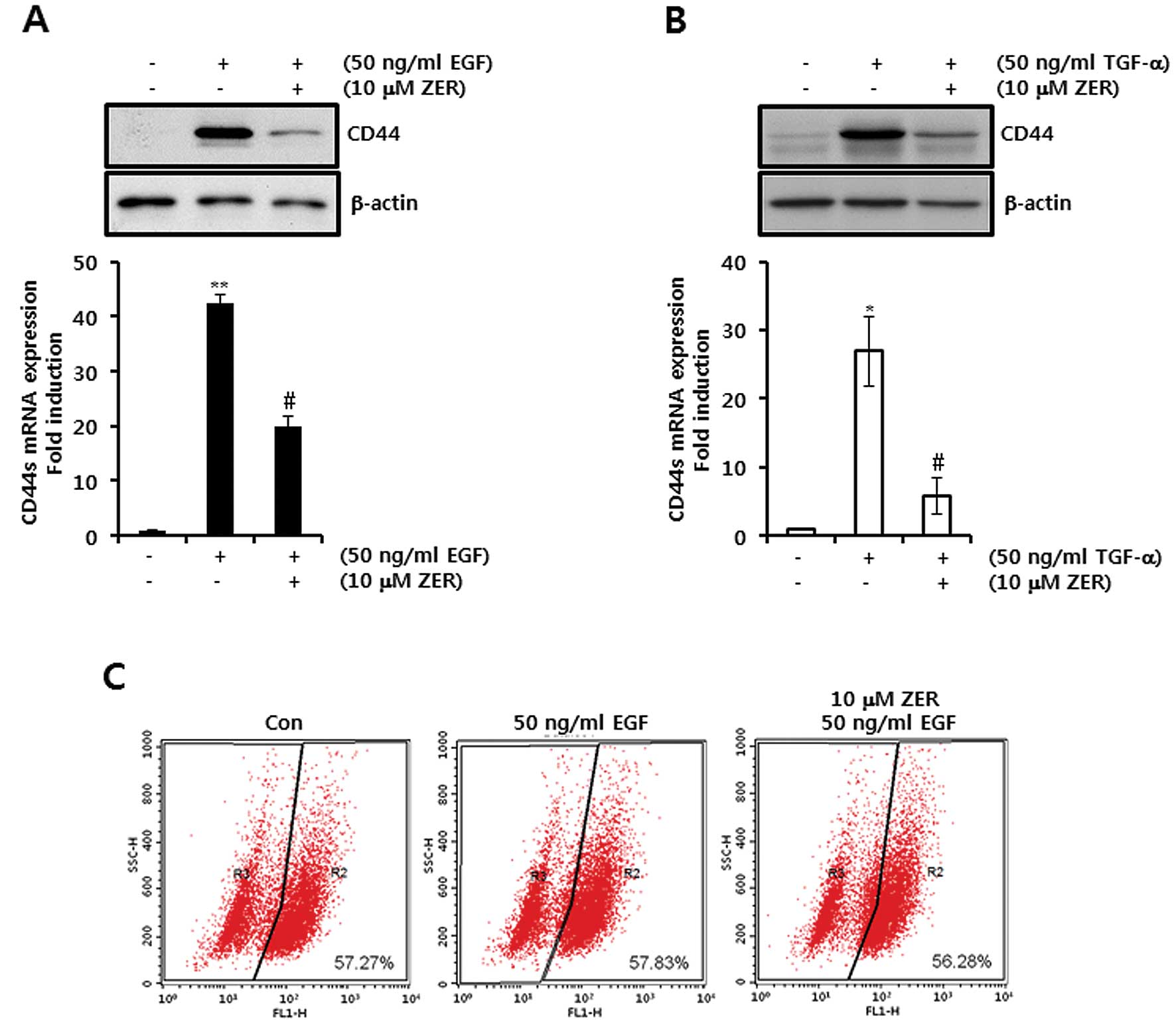
EGF ligand-induced CD44 mRNA and protein
expression levels are suppressed by ZER
To investigate the effect of ZER on EGF-induced CD44
expression, we pretreated cells with 10 μM ZER for 16 h prior to 50
ng/ml EGF treatment. Our results showed that the levels of CD44
protein and mRNA expression by EGF were significantly decreased
after ZER treatment (Fig. 5A). The
levels of EGF-induced CD44 mRNA expression were suppressed by
19.8-fold of control level by 10 μM ZER treatment (Fig. 5A). Under the same conditions, we
investigated the effect of ZER on TGF-α-induced CD44 protein and
mRNA expression. As expected, our results showed that the induction
of CD44 protein and mRNA expression in response to TGF-α was
decreased by ZER treatment (Fig.
5B). The levels of TGF-α-induced CD44 mRNA expression were
decreased by 5.9-fold of control level by 10 μM ZER treatment
(Fig. 5B).
Next, we examined the correlation between CD44
expression and ALDH enzyme activity. As shown in Fig. 5C, the ALDH+ population
was not changed; however, CD44 expression was altered by EGF and/or
ZER treatment. Therefore, we demonstrated that ZER may act as a
promising inhibitor of the EGF signaling pathway in breast cancer
cells.
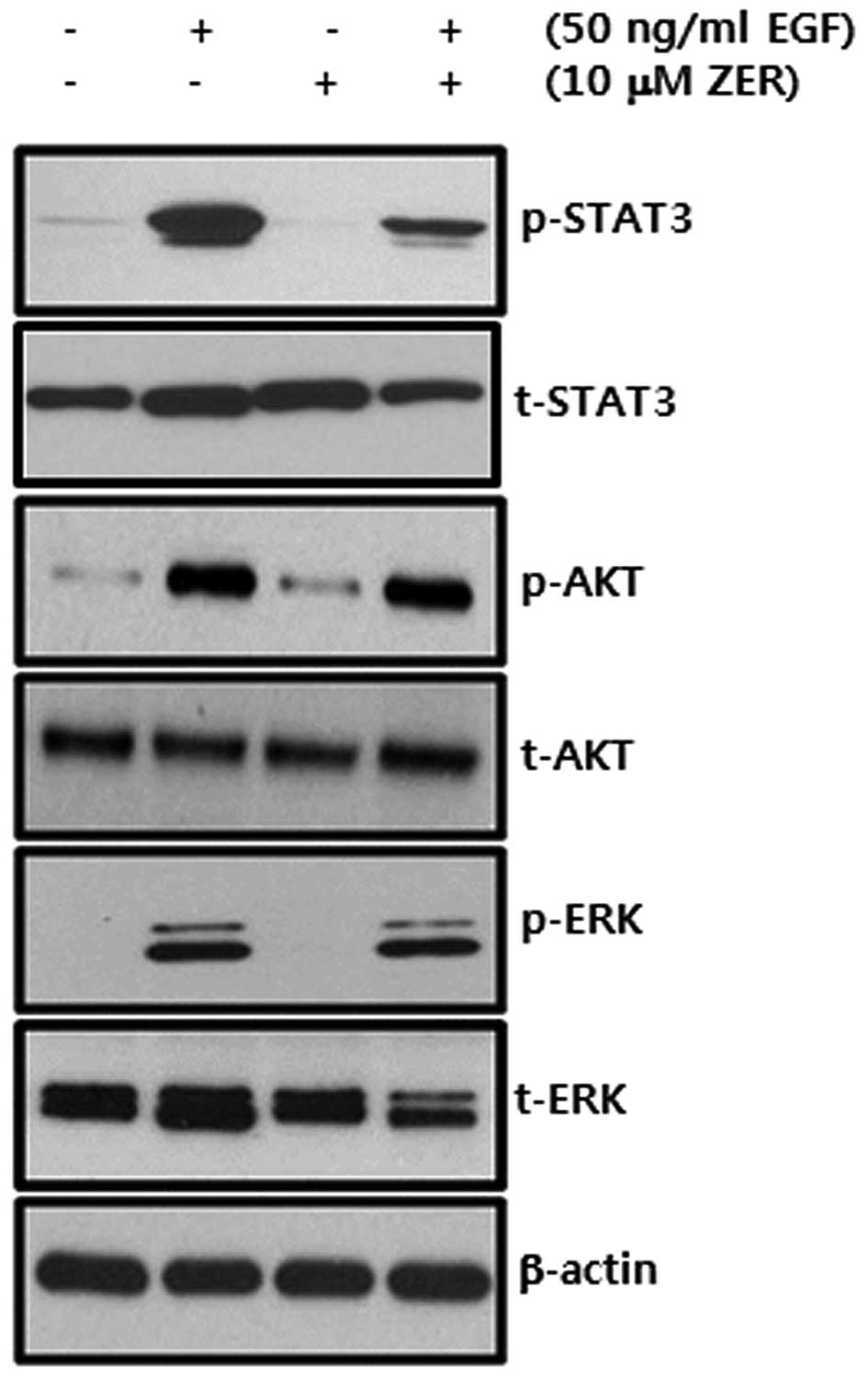
EGF-induced phosphorylation of STAT3 is
suppressed by ZER treatment in SKBR3 breast cancer cells
We investigated whether ZER regulates the
phosphorylation of EGF/EGFR downstream signaling molecules in
breast cancer cells. We pretreated cells with 10 μM ZER for 16 h
and then 50 ng/ml EGF for 30 min. We observed that EGF-induced
STAT-3 phosphorylation was markedly decreased by ZER but not ERK1/2
and AKT phosphorylation (Fig. 6).
Therefore, we demonstrated that ZER may suppress EGF-induced CD44
expression through the inhibition of the STAT-3 dependent pathway
in breast cancer cells.
Discussion
The EGFR and its ligands activate several mechanisms
underlying tumor progression, including cell proliferation,
survival and tumor invasion (19,20).
In particular, aberrant EGFR activation by frequent overexpression
or constitutive activation can promote tumor processes including
angiogenesis and metastasis; it is also associated with poor
prognosis in many human malignancies (21). The major signaling pathways
activated by EGFR are mediated by different intracellular signaling
cascades, including the phosphatidylinositol 3-kinase (PI3K)/Akt,
the Raf/MEK/ERK and the signal transducer and activator of
transcription (STAT) pathways (22,23).
In accordance with these previous studies, our results showed that
the phosphorylation of STAT3, Akt and ERK were significantly
increased by EGF treatment in breast cancer cells. Although we did
not observe data, MMP-9 expression, which is a drive gene of tumor
invasion and metastasis, also markedly increased under the same
conditions.
The EGF/EGFR signaling pathway stimulates cellular
interactions with ECM components, HA in particular, by upregulation
of CD44 expression in murine NR6 cells (24,25).
Overexpression of CD44 is involved in tumor progression, including
induction of tumor cell motility and enhancement of tumor growth
and metastasis (26). The promoter
region of CD44 contains AP-1 binding sites and EGF-induced AP-1
transcription activity mediates cell invasion through induction of
CD44 (27). We also reported that
the transcriptional activity of CD44 is regulated by an
EGFR/ERK-dependent pathway in breast cancer cells (5). Consistent with these reports, our
results showed that EGFR ligands, EGF and TGF-α augment the level
of CD44 expression in a dose-dependent manner. EGF-induced CD44
expression was also decreased by a MEK1/2 specific inhibitor,
UO126. In particular, EGFR ligand-induced CD44 expression was
markedly decreased by STAT-3 specific inhibitor, STAT3 VI.
Therefore, we demonstrated that the transcriptional activity of
STAT-3 plays a key role in the induction of CD44 in breast cancer
cells.
Zerumbone (ZER), a sesquiterpene, is an
anti-inflammatory agent (17); it
has apoptotic effects against a wide variety of tumor cells,
including colon cancer and leukemia cells (17,28).
In addition, ZER suppresses CXCL12-mediated invasion of breast and
pancreatic tumor cells through the downregulation of CXCR4
(18). The suppression of NF-κB
activity by ZER inhibits the secretion of angiogenic factors, such
as VEGF and IL-8, in pancreatic cancer cells (29). We also found that ZER abolishes the
basal levels of CD44 mRNA expression in CD44-positive breast cancer
cells. Furthermore, ZER significantly decreased EGFR ligand-induced
CD44 expression in SKBR3 breast cancer cells. In the present study,
we found for the first time that ZER significantly suppresses
EGF-induced phosphorylation of STAT3. Therefore, we demonstrated
that ZER inhibits EGFR ligand-induced CD44 expression through the
suppression of STAT3 activity.
To date, a variety of cancer stem/progenitor cells
from different tissues, including breast and colorectal cancer,
have illustrated highly expressed CD44 molecules (30,31).
In a recent study, Herishanu et al reported that anti-CD44
mAbs can also inhibit proliferation and induce apoptosis of
leukemia stem cells (32). However,
our results showed that the reduction of CD44 expression by ZER
treatment did not lead to the alteration of ALDH+
population. Therefore, we demonstrated that ZER is not directly
involved with the stemness of breast cancer cells.
In conclusion, we clearly demonstrated the
regulatory mechanism of ZER on EGF-induced CD44 expression in
breast cancer cells. Elevated STAT3 activity in response to EGF
ligands, including EGF and TGF-α, directly regulated the levels of
CD44 expression. On the other hand, EGF-induced CD44 expression was
suppressed by a STAT3 specific inhibitor, STAT3 VI. In particular,
EGF-induced STAT3 phosphorylation and CD44 expression were
significantly decreased by ZER treatment. Based on these findings,
we suggest that ZER may be a promising therapeutic drug for the
treatment of breast cancer through the blockage of the EGFR
signaling pathway.
Acknowledgements
This study was supported by a Korea Research
Foundation Grant funded by the Korean Government
(NRF-2012R1A1B4000493), and by a Samsung Biomedical Research
Institute grant (SMX1131701), and by a grant of the Korea Health
Technology R&D Project through the Korea Health Industry
Development Institute (KHIDI), funded by the Ministry of Health and
Welfare, Republic of Korea (HI09C1552).
References
|
1
|
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu
W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rakha EA, El-Sayed ME, Green AR, Lee AH,
Robertson JF and Ellis IO: Prognostic markers in triple-negative
breast cancer. Cancer. 109:25–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Normanno N, De Luca A, Bianco C, Strizzi
L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F and
Salomon DS: Epidermal growth factor receptor (EGFR) signaling in
cancer. Gene. 366:2–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Honeth G, Bendahl PO, Ringnér M, Saal LH,
Gruvberger-Saal SK, Lövgren K, Grabau D, Fernö M, Borg A and
Hegardt C: The CD44+/CD24− phenotype is
enriched in basal-like breast tumors. Breast Cancer Res.
10:R532008.
|
|
5
|
Kim S, Han J, Kim JS, Kim JH, Choe JH,
Yang JH, Nam SJ and Lee JE: Silibinin suppresses EGFR
ligand-induced CD44 expression through inhibition of EGFR activity
in breast cancer cells. Anticancer Res. 31:3767–3773.
2011.PubMed/NCBI
|
|
6
|
Gotte M and Yip GW: Heparanase,
hyaluronan, and CD44 in cancers: a breast carcinoma perspective.
Cancer Res. 66:10233–10237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naor D, Sionov RV and Ish-Shalom D: CD44:
structure, function, and association with the malignant process.
Adv Cancer Res. 71:241–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karihtala P, Soini Y, Auvinen P, Tammi R,
Tammi M and Kosma VM: Hyaluronan in breast cancer: correlations
with nitric oxide synthases and tyrosine nitrosylation. J Histochem
Cytochem. 55:1191–1198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naor D, Nedvetzki S, Golan I, Melnik L and
Faitelson Y: CD44 in cancer. Crit Rev Clin Lab Sci. 39:527–579.
2002. View Article : Google Scholar
|
|
10
|
Turley EA, Noble PW and Bourguignon LY:
Signaling properties of hyaluronan receptors. J Biol Chem.
277:4589–4592. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bourguignon LY: CD44-mediated oncogenic
signaling and cytoskeleton activation during mammary tumor
progression. J Mammary Gland Biol Neoplasia. 6:287–297. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tölg C, Hofmann M, Herrlich P and Ponta H:
Splicing choice from ten variant exons establishes CD44
variability. Nucleic Acids Res. 21:1225–1229. 1993.PubMed/NCBI
|
|
13
|
Ouhtit A, Abd Elmageed ZY, Abdraboh ME,
Lioe TF and Raj MH: In vivo evidence for the role of CD44s in
promoting breast cancer metastasis to the liver. Am J Pathol.
171:2033–2039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bourguignon LY, Gunja-Smith Z, Iida N, Zhu
HB, Young LJ, Muller WJ and Cardiff RD: CD44v3,8–10 is
involved in cytoskeleton-mediated tumor cell migration and matrix
metal-loproteinase (MMP-9) association in metastatic breast cancer
cells. J Cell Physiol. 176:206–215. 1998.
|
|
15
|
Takada Y, Murakami A and Aggarwal BB:
Zerumbone abolishes NF-κB and IκBα kinase activation leading to
suppression of antiapoptotic and metastatic gene expression,
upregulation of apoptosis, and downregulation of invasion.
Oncogene. 24:6957–6969. 2005.
|
|
16
|
Murakami A and Ohigashi H:
Cancer-preventive anti-oxidants that attenuate free radical
generation by inflammatory cells. Biol Chem. 387:387–392. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Murakami A, Takahashi D, Kinoshita T,
Koshimizu K, Kim HW, Yoshihiro A, Nakamura Y, Jiwajinda S, Terao J
and Ohigashi H: Zerumbone, a Southeast Asian ginger sesquiterpene,
markedly suppresses free radical generation, proinflammatory
protein production, and cancer cell proliferation accompanied by
apoptosis: the α,β-unsaturated carbonyl group is a prerequisite.
Carcinogenesis. 23:795–802. 2002.PubMed/NCBI
|
|
18
|
Sung B, Jhurani S, Ahn KS, Mastuo Y, Yi T,
Guha S, Liu M and Aggarwal BB: Zerumbone down-regulates chemokine
receptor CXCR4 expression leading to inhibition of CXCL12-induced
invasion of breast and pancreatic tumor cells. Cancer Res.
68:8938–8944. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Normanno N, Bianco C, Strizzi L, Mancino
M, Maiello MR, De Luca A, Caponigro F and Salomon DS: The ErbB
receptors and their ligands in cancer: an overview. Curr Drug
Targets. 6:243–257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Normanno N, Bianco C, De Luca A, Maiello
MR and Salomon DS: Target-based agents against ErbB receptors and
their ligands: a novel approach to cancer treatment. Endocr Relat
Cancer. 10:1–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lurje G and Lenz HJ: EGFR signaling and
drug discovery. Oncology. 77:400–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eccles SA: The epidermal growth factor
receptor/Erb-B/HER family in normal and malignant breast biology.
Int J Dev Biol. 55:685–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schulze WX, Deng L and Mann M:
Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol
Syst Biol. 1:2005.0008. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang M, Singh RK, Wang MH, Wells A and
Siegal GP: Epidermal growth factor modulates cell attachment to
hyal-uronic acid by the cell surface glycoprotein CD44. Clin Exp
Metastasis. 14:268–276. 1996.PubMed/NCBI
|
|
25
|
Zhang M, Wang MH, Singh RK, Wells A and
Siegal GP: Epidermal growth factor induces CD44 gene expression
through a novel regulatory element in mouse fibroblasts. J Biol
Chem. 272:14139–14146. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martin TA, Harrison G, Mansel RE and Jiang
WG: The role of the CD44/ezrin complex in cancer metastasis. Crit
Rev Oncol Hematol. 46:165–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lamb RF, Hennigan RF, Turnbull K,
Katsanakis KD, MacKenzie ED, Birnie GD and Ozanne BW: AP-1-mediated
invasion requires increased expression of the hyaluronan receptor
CD44. Mol Cell Biol. 17:963–976. 1997.PubMed/NCBI
|
|
28
|
Xian M, Ito K, Nakazato T, Shimizu T, Chen
CK, Yamato K, Murakami A, Ohigashi H, Ikeda Y and Kizaki M:
Zerumbone, a bioactive sesquiterpene, induces G2/M cell cycle
arrest and apoptosis in leukemia cells via a Fas- and
mitochondria-mediated pathway. Cancer Sci. 98:118–126. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shamoto T, Matsuo Y, Shibata T, Tsuboi K,
Nagasaki T, Takahashi H, Funahashi H, Okada Y and Takeyama H:
Zerumbone inhibits angiogenesis by blocking NF-κB activity in
pancreatic cancer. Pancreas. 43:396–404. 2014.PubMed/NCBI
|
|
30
|
Ali HR, Dawson SJ, Blows FM, Provenzano E,
Pharoah PD and Caldas C: Cancer stem cell markers in breast cancer:
pathological, clinical and prognostic significance. Breast Cancer
Res. 13:R1182011. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dalerba P, Dylla SJ, Park IK, Liu R, Wang
X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA,
Parmiani G, Castelli C and Clarke MF: Phenotypic characterization
of human colorectal cancer stem cells. Proc Natl Acad Sci USA.
104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Herishanu Y, Gibellini F, Njuguna N,
Hazan-Halevy I, Farooqui M, Bern S, Keyvanfar K, Lee E, Wilson W
and Wiestner A: Activation of CD44, a receptor for extracellular
matrix components, protects chronic lymphocytic leukemia cells from
spontaneous and drug induced apoptosis through MCL-1. Leuk
Lymphoma. 52:1758–1769. 2011. View Article : Google Scholar
|