Introduction
Breast cancer is the predominant type of cancer in
industrialized countries and the second leading cause of
cancer-related mortality in women (1). Metastasis, the development of
secondary tumors at a distant site, remains the major cause of
cancer mortality (2). Cancer
metastasis is a complex process which involves important steps of
cell migration and invasion (3).
Therefore, the control of metastasis and invasion represents an
important therapeutic target. Molecular mechanisms of cancer cell
invasion and metastasis involve a complex series of events. One
such early event involves proteolytic degradation of the
extracellular matrix (ECM) components, which provides biochemical
and mechanical barriers to cell movement in cancer cells (4,5). ECM
degradation requires extracellular proteinases, of which matrix
metalloproteinases (MMPs) play a critical role in breast
cancer.
MMPs are a family of zinc- and calcium-dependent
endopeptidases, consisting of four subclasses based on substrate
including collagenases, gelatinases, stromelysins and
membrane-associated MMPs according to substrate specificity and
domain homologies (6,7). Elevated MMP levels are functionally
linked to metastasis in many tumors including breast (2). Both MMP-2 and MMP-9 are the key
enzymes that control the rate of cell invasion and metastasis
(8). Although these two gelatinases
(MMP-2 and MMP-9) have similar properties, their gene expression is
differentially and specifically regulated by distinct regulatory
elements in their promoter regions (7). MMP-2 is commonly constitutively
present in tissues and it is maximally expressed in malignant
neoplasms as part of the host response to the presence of
neoplastic cells, rather than as part of an initial response to
invasion (9). In contrast,
synthesis and secretion of MMP-9 can be stimulated by a variety of
growth factors and inflammatory cytokines during pathological
processes and by agents such as phorbol 12-myristate 13-acetate
(PMA) and tumor necrosis factor-α (TNF-α) (9–11).
This difference can be explained by the presence of inducible
promoter elements, such as the nuclear factor-κB (NF-κB) and
activator protein-1 (AP-1) binding sites, which are involved in the
regulation of the MMP-9 gene transcription, but not in that of
MMP-2 (12,13). Therefore, it has been suggested that
the regulation of MMP-9 expression is a possible approach for the
development of anti-metastatic drugs (14,15).
Similar to other solid tumors, breast cancer is
difficult to treat. Some traditional methods, such as chemotherapy,
may cause strong side-effects and drug resistance in patients.
Therefore, there is a continuing need to find novel, efficient and
less toxic cancer therapeutic molecules. Natural products, such as
plant-derived drugs, play an increasingly important role in cancer
treatment due to their fewer side-effects and high efficacy. One of
these strategies is to determine the ability and mechanism of novel
anticancer agents to induce apoptosis in cancer cells. Apoptosis,
also known as programmed cell death, is a type of cell death
distinct from necrosis that plays an important role in the
regulation of various physiological and pathological conditions
(16). Apoptosis induction is
regarded as a major therapeutic target for cancer chemotherapy
(17–19). In mammalian cells, apoptosis is
regulated by the activation of two signaling pathways, an extrinsic
and an intrinsic one. The extrinsic pathway is regulated by death
receptors on the plasma membrane. In this pathway, ligand binding
to the receptors induces the activation of the caspase cascade. The
intrinsic pathway is regulated by mitochondrial proteins (20). Under most circumstances, activation
of either pathway eventually leads to proteolytic cleavage and thus
activation of caspases, a family of cysteine proteases that act as
common death effector molecules (21). Accordingly, caspases are responsible
for many biochemical and morphological hallmarks of apoptotic cell
death by cleaving a range of substrates in the cytoplasm or nucleus
(21). Several intracellular
anti-apoptotic molecules, such as members of the anti-apoptotic
B-cell lymphoma 2 (Bcl-2) and cellular inhibitor of apoptosis
protein (cIAP) families, as well as cellular FLICE-inhibitory
protein (FLIP), have been shown to inhibit the apoptotic signaling
cascade via inhibition of mitochondrial cytochrome c
release, apoptosome formation and recruitment of procaspase-8 to
the death receptor domain (22–25).
Receptor-mediated activation of caspase-8 is followed by activation
of caspase-3, which cleaves intracellular substrates leading to
cell death (26). Then, caspase-3
cleaves several target proteins, one of which is DNA repair enzyme,
PARP (27). Deregulation of
apoptosis programs can lead to resistance of cancers to current
treatment strategies, since the ability to activate cell death
programs in cancer cells critically determines the efficacy of
current cancer therapies (28).
Furthermore, apoptosis of circulating tumor cells can have an
impact on the metastatic process (29,30).
Targeting the apoptosis pathway in circulating tumor cells may
present a means to interfere with metastasis (28).
Celastrol, also known as tripterine, is one such
compound that was originally identified from the traditional
Chinese medicine Thunder God Vine or Tripterygium wilfordii
Hook F. almost three decades ago and is used for the treatment of
cancer and other inflammatory diseases (31). Celastrol is also known to inhibit
the proliferation of a variety of tumor cells, including leukemia,
glioma, prostate and breast cancer cells. The ability of celastrol
to modulate the expression of proinflammatory cytokines such as
interleukin-1 (IL-1), IL-6, IL-8 and TNF-α, to inhibit NF-κB
signaling, and to induce heat shock response and proteasome
activity has been reported (13,32,33).
Despite the diverse studies on the biological activities of
celastrol, the potential of celastrol against breast cancer
proliferation and invasion is poorly defined. In the present study,
we investigated the underlying pathways involved in inhibiting
TNF-α-induced anti-apoptotic gene expression and invasion in the
human breast cancer line MDA-MB-231 cells by celastrol. The results
showed that celastrol induced the apoptosis of breast cancer cells
and inhibited their invasion by downregulating MMP-9
expression.
Materials and methods
Cell culture and reagents
Two human breast cancer cell lines, MCF-7 and
MDA-MB-231, were grown in RPMI-1640 supplemented with penicillin
(100 U/ml)-streptomycin (100 U/ml) (Invitrogen, Carlsbad, CA, USA)
and 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan,
UT, USA). Cell cultures were grown to confluence and maintained in
a humidified atmosphere at 37°C and 5% CO2. All cells
were purchased from American Type Culture Collection (ATCC;
Manassas, VA, USA). TNF-α was obtained from R&D Systems
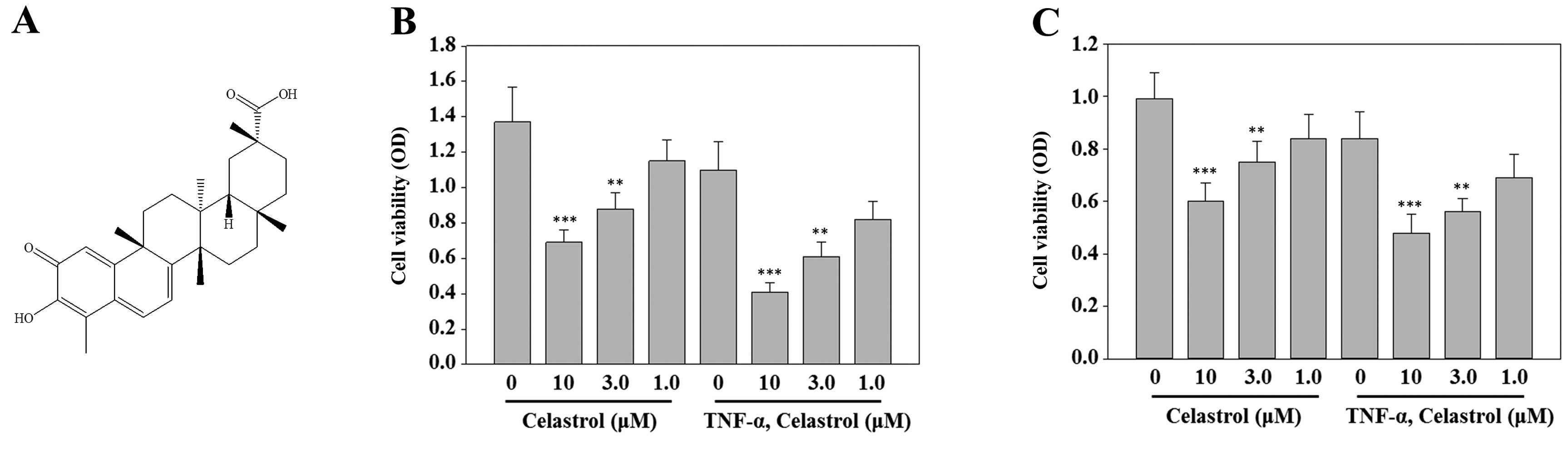
(Minneapolis, MN, USA). Celastrol was isolated from Tripterygium
wilfordii and its structure is shown in Fig. 1A. The purity of celastrol was
>98% in HPLC analysis.
Measurement of cell viability by MTT
assay
MCF-7 and MDA-MB-231 cells were seeded at
1×105 cells/ml in 96-well plates containing 100 μl of
RPMI-1640 with 10% FBS and incubated overnight. Celastrol was
dissolved in DMSO and DMSO was added to all plates to compensate
the same volume of DMSO. After 24 h, the cells were pretreated with
different concentrations of celastrol for 1 h, followed by
stimulation with or without TNF-α for 24 h. Subsequently, cells
were cultured with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
solution (5 mg/ml) (Sigma-Aldrich, St. Louis, MO, USA) for 3 h. The
viable cells converted MTT to formazan, which generated a
blue-purple color after dissolving in 150 μl of DMSO. The
absorbance at 570 nm was measured by an ELISA plate reader.
Apoptosis assays
Annexin V-staining was performed using Annexin
V-FITC apoptosis detection kit (BD Biosciences, San Diego, CA, USA)
following the manufacturer’s instructions. Briefly, after
incubation, cells were harvested, washed with phosphate-buffered
saline (pH 7.4), centrifuged, and stained with Annexin V-FITC and 2
mg/ml propidium iodide in binding buffer (10 mM HEPES, pH 7.4, 140
mM NaCl, 2.5 mM CaCl2) for 15 min at 37°C in the dark.
The samples were analyzed by flow cytometry using a FACScan flow
cytometer. The CellQuest software was used to analyze the data
(Becton-Dickinson).
Western blotting
Cell lysates and conditioned media were separated by
SDS-polyacrylamide gels and then transferred to a polyvinylidene
difluoride membrane (Millipore, Bedford, MA, USA). The membrane was
blocked with 5% skim milk and then incubated with the corresponding
antibody. Antibodies for cleaved PARP, cleaved caspase-3, cleaved
caspase-8, cIAP1 and cIAP2 were purchased from Cell Signaling
Technology (Beverly, MA, USA). Antibodies for MMP-1, MMP-2, MMP-9,
Bcl-2 and FLIP were obtained from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). Antibody for α-tubulin was from Sigma-Aldrich.
After binding an appropriate secondary antibody coupled to
horseradish peroxidase, proteins were visualized by enhanced
chemiluminescence according to the manufacturer’s instructions
(Amersham Pharmacia Biotech, Buckinghamshire, UK).
In vitro invasion assays
The ability of cells to invade through
Matrigel-coated filters (invasion) was determined using a modified
24-well Boyden chamber (Corning Costar, Cambridge, MA, USA; 8 μm
pore size) as was previously described (34). MDA-MB-231 cells were seeded at a
density of 5×104 cells in 100 μl RPMI-1640 containing
10% FBS in the upper compartment of Transwell. To determine the
effect of celastrol, various concentrations of celastrol and TNF-α
(20 ng/ml) were added to the lower or upper compartment of
Transwell. After incubation for 24 h at 37°C in 5% CO2,
the cells that did not penetrate the filter were completely wiped
out with a cotton swab, and the cells that had migrated to the
lower surface of the filter were fixed, stained, and counted in 5
randomly selected microscopic fields (x100) per filter.
Real-time PCR
Total RNA from MDA-MB-231 cells was obtained using
RNA Mini kit (Qiagen, Valencia, CA, USA). Total RNA (2 μg) was used
to perform reverse transcription-PCR (RT-PCR) using RT-PCR kit
(Invitrogen) according to the manufacturer’s protocol. The PCR
primers were: MMP-1, 5′-AGCTAGCTCAGGATGACATTGATG-3′ (sense) and
5′-GCCGATGGGCTGGACAG-3′ (antisense); MMP-2,
5′-TGAGCTCCCGGAAAAGATTG-3′ (sense) and 5′-TCAGCAGCCTAGCCAGTCG-3′
(antisense); MMP-9, 5′-CAACATCACCTATTGGATCC-3′ (sense) and
5′-CGGGTGTAGAGTCTCTCGCT-3′ (antisense); GAPDH,
5′-ACCACAGTCCATGCCATCAC-3′ (sense) and 5′-TCCACCACCCTGTTGCTGTA-3′
(antisense). The oligonucleotide sequences of the reaction products
were confirmed by sequencing.
Statistical analysis
All values are expressed as mean ± SD. A comparison
of the results was performed with one-way ANOVA and Tukey’s
multiple comparison tests (Graphpad Software, Inc., San Diego, CA,
USA). Statistically significant differences between groups were
defined as P-values <0.01.
Results
Celastrol is cytotoxic to breast cancer
cells
To evaluate the effect of celastrol on the viability
of breast cancer cells, MCF-7 and MDA-MB-231 cells were exposed to
increasing concentrations of celastrol (from 1 to 10 μM) in the
presence or absence of TNF-α (20 ng/ml) for 24 h, and the
percentage of viable cells was determined using the MTT assay.
Celastrol decreased cell viability in a dose-dependent manner in
MCF-7 and MDA-MB-231 breast cancer cells (Fig. 1B and C).
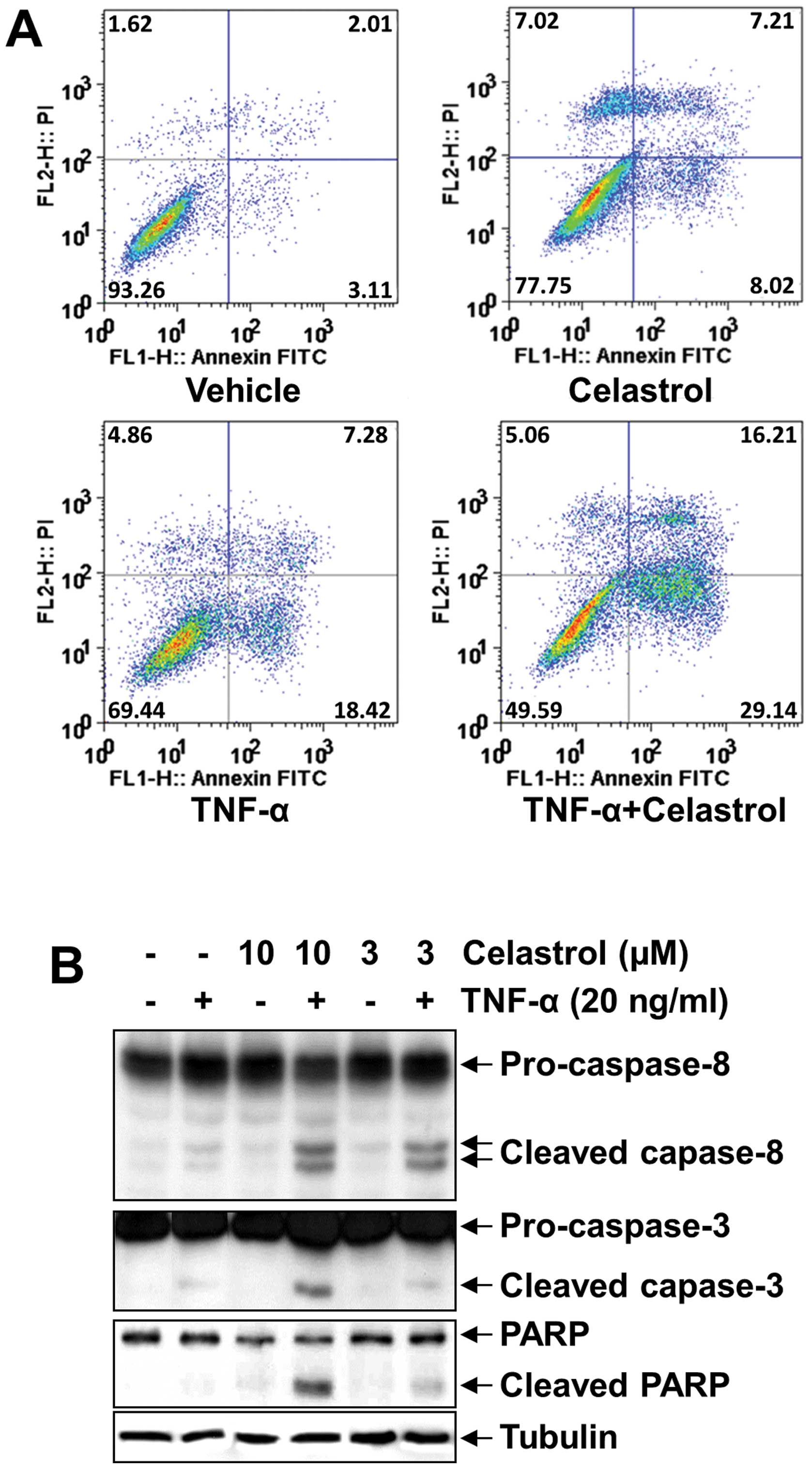
Celastrol potentiates TNF-α-induced
apoptosis
Next, we examined whether celastrol enhances
apoptosis by TNF-α. Celastrol potentiated TNF-α-induced apoptosis,
as assessed by Annexin V/PI double staining. As shown in Fig. 2A, combined treatment resulted in a
significant increase in the Annexin V-positive cell population
(45.35%), whereas no treatment (5.12%), treatment with TNF-α alone
(25.70%) or celastrol alone (15.23%) had only slight influence on
cell apoptosis. Since caspases are a group of cysteine proteases
critical for apoptosis of eukaryotic cells (35), we investigated whether celastrol
affects TNF-α-induced activation of caspase-8 and caspase-3. TNF-α
alone and celastrol alone did not affect the activation of
caspase-8 or caspase-3, whereas cotreatment with TNF-α and
celastrol potentiated their activation, as indicated by the
presence of cleaved caspases, and this activation was observed at a
concentration even as low as 3 μM (Fig.
2B, top two panels). We also used the PARP cleavage assay to
detect TNF-α-induced apoptosis. Similarly, celastrol
dose-dependently potentiated the effect of TNF-α-induced PARP
cleavage; however, TNF-α alone and celastrol alone did not induce
PARP cleavage (Fig. 2B, bottom
second panel). These results showed that celastrol enhances the
apoptotic effects of TNF-α.
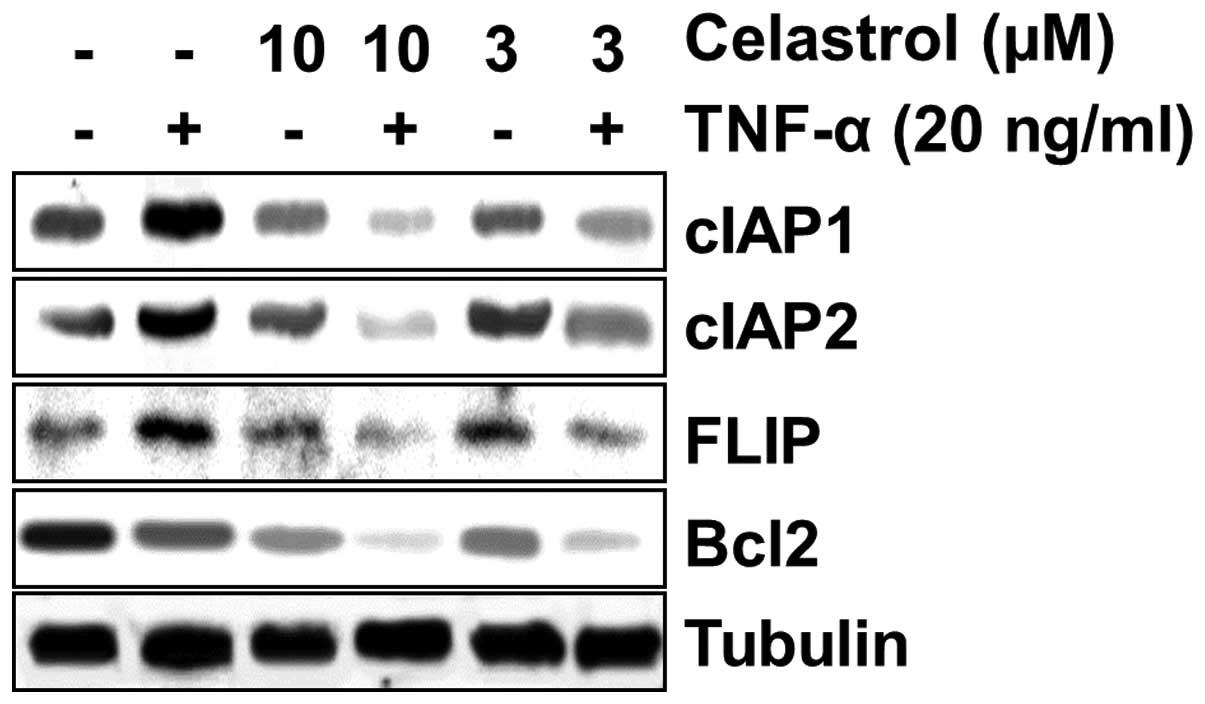
Celastrol inhibits TNF-α-induced
anti-apoptotic gene expression
Since TNF-α-induced apoptosis was potentiated by
celastrol, we investigated the effect of celastrol on TNF-α-induced
anti-apoptotic gene expression of cIAP1, cIAP2, FLIP, and Bcl-2.
MDA-MB-231 cells were preincubated with celastrol for 12 h and
subsequently stimulated with TNF-α for 12 h, and then the cIAP1,
cIAP2, FLIP and Bcl-2 expression levels were analyzed by western
blot analysis. TNF-α significantly induced the expression of
anti-apoptotic proteins, whereas celastrol markedly suppressed
TNF-α-induced expression of all the proteins in a dose-dependent
manner (Fig. 3).
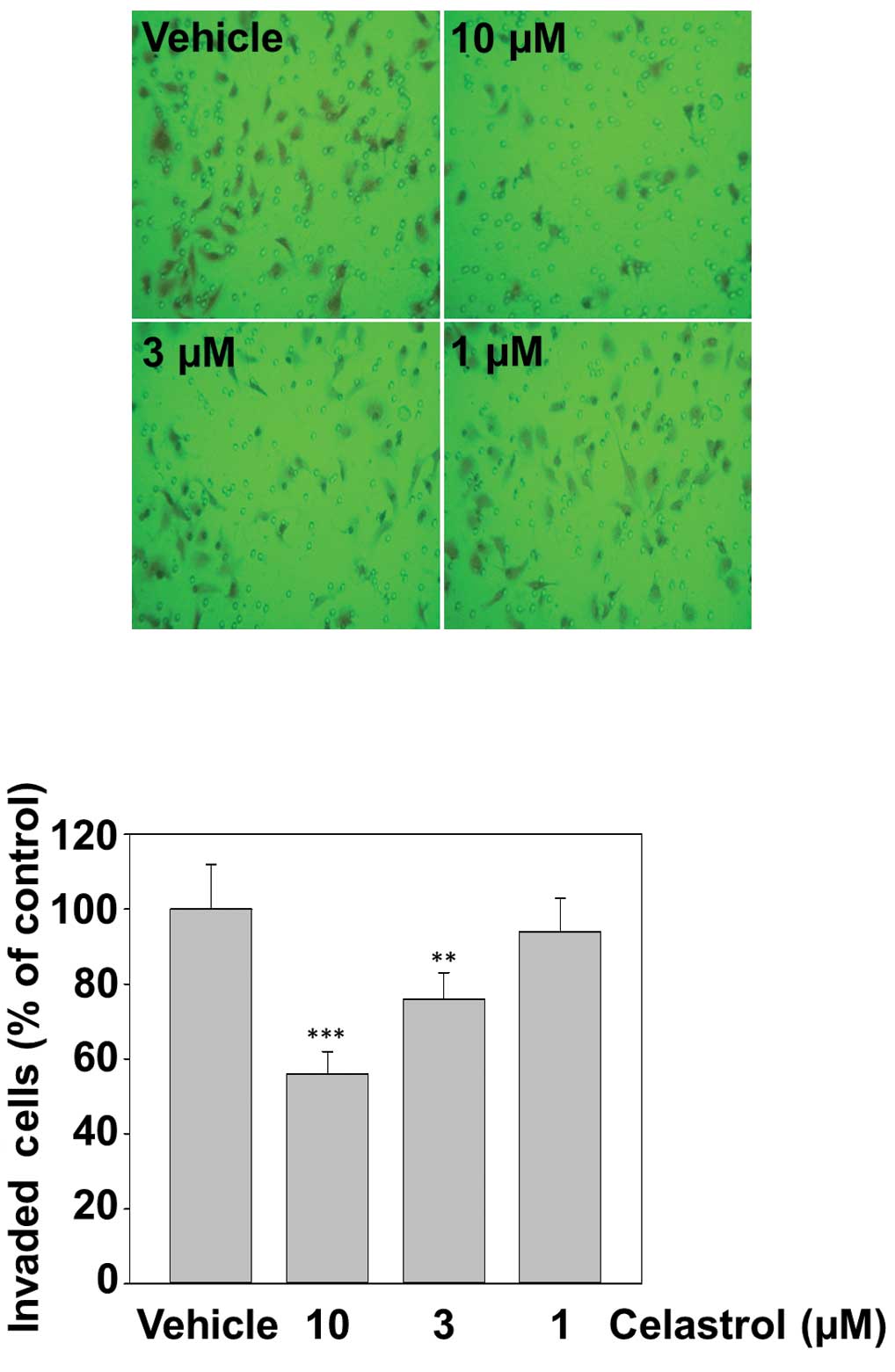
Celastrol inhibits TNF-α-induced invasion
of MDA-MB-231 cells
Cancer cell invasion is a critical step of tumor
metastasis (36). Whether celastrol
modulates invasion activity was examined in vitro with a
Matrigel invasion assay. MDA-MB-231 cells were seeded in the top
chamber of a Matrigel invasion chamber and incubated with various
concentrations of celastrol for 12 h and subsequently with TNF-α
(20 ng/ml) for 12 h. The cells that migrated to the lower chamber
were significantly decreased by celastrol in a dose-dependent
manner. This result could account for the anti-invasive activity of
celastrol (Fig. 4).
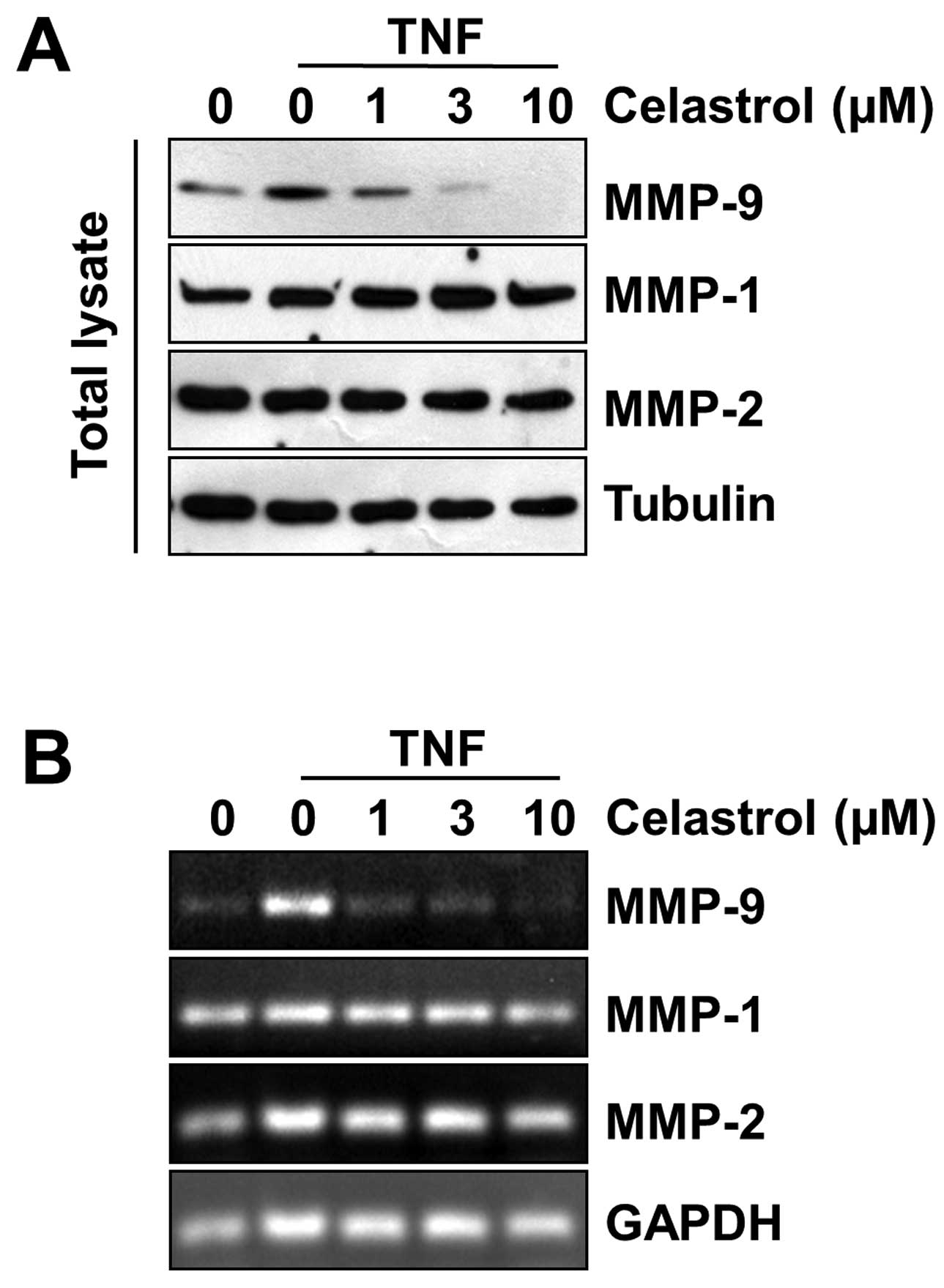
Celastrol inhibits MMP-9 gene
expression
In cancer cell metastasis, the degradation of ECM is
essential and is associated with the overexpression of proteolytic
enzymes including MMPs (3). MMP
(MMP-1, MMP-2, and MMP-9) activation has been found in the
metastasis of breast carcinoma cells. Therefore, the effects of
celastrol on TNF-α-induced MMP-1, MMP-2 and MMP-9 gene expression
were examined. Western blot analysis was performed to investigate
whether celastrol had inhibitory effects on TNF-α-induced
expression of MMPs. Although MMP-1 and MMP-2 protein expression
levels remained unaffected, TNF-α-induced expression of MMP-9 was
prevented by celastrol in a dose-dependent manner (Fig. 5A). To determine whether the
inhibition of MMP-9 gene expression by celastrol was due to a
decreased level of transcription, we performed RT-PCR analysis and
observed mRNA expression of MMP-9. In agreement with the above
findings, celastrol treatment of the cells was found to decrease
TNF-α-induced MMP-9 mRNA expression in MDA-MB-231 cells in a
dose-dependent manner, but it did not decrease MMP-1 and MMP-2 mRNA
expression (Fig. 5B).
Discussion
Celastrol is a remedial ingredient in the root
extracts of Thunder God Vine. Several screening studies on the
molecular libraries of Chinese herbs have identified celastrol as a
potent candidate with medicinal prospects for treating inflammatory
diseases and cancer (37). Although
several studies reported the possible involvement of celastrol in
tumor metastasis, no study has reported the effect on TNF-α-induced
metastasis by celastrol in human breast cancer cells. The aim of
the present study was to investigate the effect of celastrol on the
TNF-α signaling pathway that mediates apoptosis and metastasis in
human breast cancer cells.
The ability of anticancer drugs to induce the
cellular apoptosis of cancer cells is an important function of
cancer treatment (38,39). Our results showed that celastrol
inhibited the TNF-α-induced expression of anti-apoptotic proteins
such as cIAP1, cIAP2, FLIP and Bcl-2. Annexin V staining also
showed that TNF-α-induced apoptosis was enhanced by celastrol. In
addition, celastrol affected TNF-α-induced activation of caspase-8,
caspase-3 and PARP cleavage. In particular, celastrol had a
considerable effect on TNF-α-induced poly(ADP-ribose) polymerase
cleavage, indicating that the apoptotic effects of TNF-α are
enhanced by celastrol.
New anticancer agents have also been reported to
control various other processes involved in the malignant
transformation of cells, such as invasion and metastasis (40). Tumor metastasis is a multi-step and
complex process that includes cell division and proliferation,
proteolytic digestion of the ECM, cell migration through the
basement membranes to reach the circulation system, and the
remigration and growth of tumors at metastatic sites (11). MMPs play a key role in promoting
tumor metastasis and overexpression of MMP-9 has been shown to be
associated with the progression and invasion of tumors including
mammary tumors (11,41). Consequently, inhibiting MMP-9
expression may be critical in treating malignant tumors, including
breast carcinoma.
We observed an inhibitory effect of celastrol on
TNF-α-induced invasion in MDA-MB-231 breast cancer cells.
Accumulating evidence suggests that MMP-9 expression is strongly
implicated in breast cancer invasion. Effective anticancer agents
involved in anti-invasion have demonstrated the ability to
downregulate MMP-9 expression (42). Our data revealed that celastrol
inhibits TNF-α-induced MMP-9 expression at both the mRNA and
protein levels, but it does not influence MMP-1 and MMP-2
expression levels in MDA-MB-231 breast cancer cells. Notably, in
our study, the inhibition of TNF-α-induced invasion of MDA-MB-231
cells may correlate with that of the TNF-α-induced MMP-9
expression. These results suggest that the anti-invasion effect of
celastrol may be associated with the inhibition of MMP-9
expression.
In conclusion, the present study demonstrated that
the natural compound celastrol exhibits effective antitumor
properties. The observed antitumor activity inhibits the
proliferation of cancer cells and induces apoptosis. In addition,
our study provides evidence that celastrol can inhibit the invasion
of breast cancer cells through the downregulation of MMP-9
expression. As tumor metastasis is often associated with poor
prognosis and high mortality among breast cancer patients, there is
a growing need to discover and develop new therapeutic strategies
that target early tumor invasiveness or metastasis. In this regard,
celastrol is a promising agent against breast cancer invasion and
metastasis.
Acknowledgements
This study was partially supported by the National
Natural Science Foundation of China (nos. 81360496 and 81160250).
This study also received assistance from the Thousand Peoples Plan
by Foreign Expert Bureau, China.
Abbreviations:
|
ECM
|
extracellular matrix
|
|
MMPs
|
matrix metalloproteinases
|
|
TNF-α
|
tumor necrosis factor-α
|
|
PMA
|
phorbol 12-myristate 13-acetate
|
|
NF-κB
|
nuclear factor-κB
|
|
AP-1
|
activator protein-1
|
|
cIAP1
|
cellular inhibitor of apoptosis 1
|
|
FLIP
|
cellular FLICE-inhibitory protein
|
|
Bcl-2
|
B-cell lymphoma 2
|
References
|
1
|
Antonova L, Aronson K and Mueller CR:
Stress and breast cancer: from epidemiology to molecular biology.
Breast Cancer Res. 13:2082011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Decock J, Thirkettle S, Wagstaff L and
Edwards DR: Matrix metalloproteinases: protective roles in cancer.
J Cell Mol Med. 15:1254–1265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoon DS, Ferris R, Tanaka R, Chong KK,
Alix-Panabieres C and Pantel K: Molecular mechanisms of metastasis.
J Surg Oncol. 103:508–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Woessner JF Jr: Matrix metalloproteinases
and their inhibitors in connective tissue remodeling. FASEB J.
5:2145–2154. 1991.PubMed/NCBI
|
|
6
|
Kim JM, Noh EM, Kwon KB, et al: Curcumin
suppresses the TPA-induced invasion through inhibition of
PKCα-dependent MMP-expression in MCF-7 human breast cancer cells.
Phyto-medicine. 19:1085–1092. 2012.PubMed/NCBI
|
|
7
|
Yu HY, Kim KS, Moon HI, Kim KM, Lee YC and
Lee JH: JNP3, a new compound, suppresses PMA-induced tumor cell
invasion via NF-κB down regulation in MCF-7 breast cancer cells.
Biochem Biophys Res Commun. 421:190–196. 2012.PubMed/NCBI
|
|
8
|
Jin ML, Park SY, Kim YH, Park G and Lee
SJ: Halofuginone induces the apoptosis of breast cancer cells and
inhibits migration via downregulation of matrix
metalloproteinase-9. Int J Oncol. 44:309–318. 2014.PubMed/NCBI
|
|
9
|
Park SK, Hwang YS, Park KK, Park HJ, Seo
JY and Chung WY: Kalopanaxsaponin A inhibits PMA-induced invasion
by reducing matrix metalloproteinase-9 via PI3K/Akt- and
PKCdelta-mediated signaling in MCF-7 human breast cancer cells.
Carcinogenesis. 30:1225–1233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SO, Jeong YJ, Yu MH, et al: Wogonin
suppresses TNF-alpha-induced MMP-9 expression by blocking the
NF-kappaB activation via MAPK signaling pathways in human aortic
smooth muscle cells. Biochem Biophys Res Commun. 351:118–125. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chung TW, Moon SK, Chang YC, et al: Novel
and therapeutic effect of caffeic acid and caffeic acid phenyl
ester on hepato-carcinoma cells: complete regression of hepatoma
growth and metastasis by dual mechanism. FASEB J. 18:1670–1681.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan C and Boyd DD: Regulation of matrix
metalloproteinase gene expression. J Cell Physiol. 211:19–26. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim Y, Kang H, Jang SW and Ko J: Celastrol
inhibits breast cancer cell invasion via suppression of
NF-κB-mediated matrix metalloproteinase-9 expression. Cell Physiol
Biochem. 28:175–184. 2011.PubMed/NCBI
|
|
14
|
Ling H, Zhang Y, Ng KY and Chew EH:
Pachymic acid impairs breast cancer cell invasion by suppressing
nuclear factor-κB-dependent matrix metalloproteinase-9 expression.
Breast Cancer Res Treat. 126:609–620. 2011.PubMed/NCBI
|
|
15
|
Zhang S, Li Z, Wu X, Huang Q, Shen HM and
Ong CN: Methyl-3-indolylacetate inhibits cancer cell invasion by
targeting the MEK1/2-ERK1/2 signaling pathway. Mol Cancer Ther.
5:3285–3293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taylor RC, Cullen SP and Martin SJ:
Apoptosis: controlled demolition at the cellular level. Nat Rev Mol
Cell Biol. 9:231–241. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cotter TG, Lennon SV, Glynn JG and Martin
SJ: Cell death via apoptosis and its relationship to growth,
development and differentiation of both tumour and normal cells.
Anticancer Res. 10:1153–1159. 1990.PubMed/NCBI
|
|
18
|
Urbanska K, Trojanek J, Del Valle L, et
al: Inhibition of IGF-I receptor in anchorage-independence
attenuates GSK-3beta constitutive phosphorylation and compromises
growth and survival of medulloblastoma cell lines. Oncogene.
26:2308–2317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JH, Kwon HY, Sohn EJ, et al:
Inhibition of Wnt/β-catenin signaling mediates ursolic acid-induced
apoptosis in PC-3 prostate cancer cells. Pharmacol Rep.
65:1366–1374. 2013.
|
|
20
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Degterev A, Boyce M and Yuan J: A decade
of caspases. Oncogene. 22:8543–8567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Irmler M, Thome M, Hahne M, et al:
Inhibition of death receptor signals by cellular FLIP. Nature.
388:190–195. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chao DT and Korsmeyer SJ: BCL-2 family:
regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar
|
|
24
|
Wang CY, Mayo MW, Korneluk RG, Goeddel DV
and Baldwin AS Jr: NF-kappaB antiapoptosis: induction of TRAF1 and
TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation.
Science. 281:1680–1683. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yagita H, Takeda K, Hayakawa Y, Smyth MJ
and Okumura K: TRAIL and its receptors as targets for cancer
therapy. Cancer Sci. 95:777–783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Uchida M, Iwase M, Takaoka S, et al:
Enhanced susceptibility to tumor necrosis factor-related
apoptosis-inducing ligand-mediated apoptosis in oral squamous cell
carcinoma cells treated with phosphatidylinositol 3-kinase
inhibitors. Int J Oncol. 30:1163–1171. 2007.
|
|
27
|
Looi CY, Arya A, Cheah FK, et al:
Induction of apoptosis in human breast cancer cells via caspase
pathway by vernodalin isolated from Centratherum
anthelminticum (L.) seeds. PLoS One. 8:e566432013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fulda S: Targeting apoptosis signaling
pathways for anticancer therapy. Front Oncol. 1:232011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Larson CJ, Moreno JG, Pienta KJ, et al:
Apoptosis of circulating tumor cells in prostate cancer patients.
Cytometry A. 62:46–53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fehm T, Becker S, Becker-Pergola G, et al:
Presence of apoptotic and nonapoptotic disseminated tumor cells
reflects the response to neoadjuvant systemic therapy in breast
cancer. Breast Cancer Res. 8:R602006. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Calixto JB, Campos MM, Otuki MF and Santos
AR: Anti-inflammatory compounds of plant origin. Part II modulation
of pro-inflammatory cytokines, chemokines and adhesion molecules.
Planta Med. 70:93–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sethi G, Ahn KS, Pandey MK and Aggarwal
BB: Celastrol, a novel triterpene, potentiates TNF-induced
apoptosis and suppresses invasion of tumor cells by inhibiting
NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB
activation. Blood. 109:2727–2735. 2007.PubMed/NCBI
|
|
33
|
Yadav VR, Sung B, Prasad S, et al:
Celastrol suppresses invasion of colon and pancreatic cancer cells
through the downregulation of expression of CXCR4 chemokine
receptor. J Mol Med. 88:1243–1253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin HR, Jin SZ, Cai XF, et al:
Cryptopleurine targets NF-κB pathway, leading to inhibition of gene
products associated with cell survival, proliferation, invasion,
and angiogenesis. PLoS One. 7:e403552012.PubMed/NCBI
|
|
35
|
Wang J and Lenardo MJ: Roles of caspases
in apoptosis, development, and cytokine maturation revealed by
homozygous gene deficiencies. J Cell Sci. 113:753–757.
2000.PubMed/NCBI
|
|
36
|
Comen E, Norton L and Massague J: Clinical
implications of cancer self-seeding. Nat Rev Clin Oncol. 8:369–377.
2011.PubMed/NCBI
|
|
37
|
Salminen A, Lehtonen M, Paimela T and
Kaarniranta K: Celastrol: Molecular targets of Thunder God Vine.
Biochem Biophys Res Commun. 394:439–442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chandra-Kuntal K, Lee J and Singh SV:
Critical role for reactive oxygen species in apoptosis induction
and cell migration inhibition by diallyl trisulfide, a cancer
chemopreventive component of garlic. Breast Cancer Res Treat.
138:69–79. 2013. View Article : Google Scholar
|
|
39
|
Shi X, Zhao Y, Jiao Y, Shi T and Yang X:
ROS-dependent mitochondria molecular mechanisms underlying
antitumor activity of Pleurotus abalonus acidic
polysaccharides in human breast cancer MCF-7 cells. PLoS One.
8:e642662013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li F, Li C, Zhang H, et al: VI-14, a novel
flavonoid derivative, inhibits migration and invasion of human
breast cancer cells. Toxicol Appl Pharmacol. 261:217–226. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Scorilas A, Karameris A, Arnogiannaki N,
et al: Overexpression of matrix-metalloproteinase-9 in human breast
cancer: a potential favourable indicator in node-negative patients.
Br J Cancer. 84:1488–1496. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen HW, Chao CY, Lin LL, et al:
Inhibition of matrix metalloproteinase-9 expression by
docosahexaenoic acid mediated by heme oxygenase 1 in
12-O-tetradecanoylphorbol-13-acetateinduced MCF-7 human breast
cancer cells. Arch Toxicol. 87:857–869. 2013. View Article : Google Scholar : PubMed/NCBI
|