Introduction
Pancreatic cancer is a common digestive malignant
tumor with low resection rate, high mortality rate and poor
prognosis, as the characteristics of this tumor are masked.
Pancreatic cancer patients who cannot undergo surgery are subjected
to chemotherapy as a fundamental treatment modality; this modality
is also a key component of systemic therapy (1). In pancreatic cancer chemotherapy,
gemcitabine (GEM) was initially recommended as a first-line drug by
the Food and Drug Administration (USA) in 1997. Since then,
research on combination chemotherapies, such as cytotoxic drugs
[5-fluorouracil (2), cisplatin
(3) and capecitabine (4)] and biological agents [erlotinib
(5), cetuximab (6) and bevacizumab (7)], as second-line modes of chemotherapy
has been extensively conducted. Although GEM is currently the
preferred drug for single chemotherapeutic applications in
pancreatic cancer, the inherent and acquired resistance of cancer
cells to GEM prevents the efficient improvement of the clinical
benefit and survival of patients. Furthermore, the efficiency of
this treatment is very low (12%) (8); as such, this drawback should be
resolved in clinical applications. However, related studies have
shown that the prognosis of pancreatic cancer in the past 10 years
has remained unchanged.
The resistance to GEM is induced by several factors.
Although numerous mechanisms have been presented, the main
mechanism remains unclear. This resistance is affected by several
key molecular factors, including deficiencies in drug uptake,
activation of DNA repair pathways, resistance to apoptosis,
enhancement of tumor microenvironments, overexpression of signaling
proteins, mutations in kinase domains, activation of alternative
pathways, mutations of genes and conversion to an
epithelial-mesenchymal transition-like phenotype. Hence,
GEM-resistance mechanisms involved in pancreatic cancer should be
investigated; furthermore, a highly efficient multi-target drug
with low toxicity should be developed to synergize current
chemotherapy drugs or reverse drug resistance for pancreatic cancer
treatment. The present study was conducted to establish a human
pancreatic cancer GEM-resistant cell line and determine its
biological characteristics for future studies.
Materials and methods
Cell culture and animal feeding
Human pancreatic cancer cell line PANC-1 was
purchased from the Shanghai Institute of Cell Biology, China. These
cells were incubated with RPMI-1640 + 10% fetal bovine serum at
37°C in a cell incubator with 5% CO2 and then digested
with 0.25% trypsinogen + 2% ethylene diaminetetra acetic acid for
passage at a ratio of 1:2–4 once at an interval of 2–3 days. Male
nude mice were obtained from the Animal Center of the Peking Union
Medical College, China. The mice were fed in a specific pathogen
free-grade animal room at the Fujian Medical University Animal
Center in strict accordance with aseptic principles. GEM (Hengda
Pharmaceutical Co., Ltd., Shanxi, China) was dissolved in normal
saline to obtain a final concentration of 100 mmol/l and stored at
−20°C.
Establishment of human pancreatic cancer
GEM-resistant cell line
To develop GEM-resistant PANC-1 cell line, we
exposed the cells to increasing concentrations of GEM (from 50
nmol/l to 2 μmol/l) with repeated subcultures until the cells
became fully resistant to GEM. Subsequently, the cells in the
logarithmic phase (1/well × 50 μl) were seeded in 96-well culture
plates containing 50 μl of supernatant liquid. This liquid had been
used to incubate fresh mouse spleen cells for 4 days and then
incubated the pancreatic cancer cells for 2 weeks to prepare the
cloning culture. Single cell colonies were selected by GEM. After
the cultures were cloned thrice, a stable cell clone termed
PANC-1RG7 with a uniformly resistant mechanism was obtained.
Morphology and ultrastructure
Cell size and the contours of PANC-1 and PANC-1RG7
cells were observed under an optical microscope. To observe
ultramicrostructure characteristics, we harvested 2×106
cells and washed them thrice with phosphate-buffered saline (PBS).
Subsequently, the cells were fixed in ice-cold 4% glutaraldehyde
for 2 h. The samples were subsequently fixed in 1% osmic acid for 2
h, gradually dehydrated with acetone and embedded in epoxy resin.
The cells were then observed under a transmission electron
microscope.
Cell growth curve
PANC-1 and PANC-1RG7 cells in the logarithmic phase
(5,000/well × 1 ml) were seeded in 24-well culture plates. After 24
h of attachment, the cells were harvested and counted under an
inverted microscope with 0.2% trypan blue dye. Three-wells of each
cell line were monitored daily for 12 days. The cell growth curves
of the 2 cell lines were drawn, and doubling time was calculated
using the following equation: Td = tx24 h
× [lg2/(lgNt-lgNo)], where No is the number of cells
when the logarithmic growth phase began, Nt is the number of cells
before cell death occurred, and t is the time between the 2 phases.
Each experiment was repeated thrice.
Cell cycle analysis by flow
cytometry
PANC-1 and PANC-1RG7 cells (1×106) were
harvested, washed thrice with PBS and fixed in ice-cold 75% alcohol
for >12 h. After fixation was completed, samples were stained
with 0.005% propidium iodide for 30 min in the dark at room
temperature, and then analyzed to determine the DNA content by
FACSCalibur (Becton-Dickinson, Mountain View, CA, USA). Each
experiment was repeated thrice.
Sulforhodamine B (SRB) assays
PANC-1 (1,000/well × 100 μl) and PANC-1RG7
(1,500/well × 100 μl) cells in the logarithmic phase were seeded in
96-well culture plates and incubated for 24 h until adherence
occurred. Then, cells were treated with different concentrations of
GEM, adriamycin (ADM), mitomycin C (MMC), paclitaxel (PTX),
methotrexate (MTX), vincristine (VCR), gefitinib (GEF), cisplatin
(DDP) and 5-fluorouracil (5-FU). Control cells were supplemented
with 100 μl of RPMI-1640 culture medium. The treated cells were
incubated with drugs for 96 h before SRB assays described
previously (9). The dose-effect
curve was plotted to calculate 50% inhibitory concentration
(IC50) and resistance index (RI). Each experiment was
repeated thrice.
Establishment of animal models and drug
intervention
After permission of Fujian Medical University
Laboratory Animal Welfare & Ethics Committee, PANC-1 and
PANC-1RG7 cells (5×106 cells suspended in 200 μl of
RPMI-1640) were injected percutaneously using a 29-gauge syringe
with a hypodermic needle on the right shoulder back of the mice. A
total of 40 integrated mice (20 in each cell line) with tumors
grown to a final size of ~0.4 cm in diameter were divided into 4
groups: 10 mice with PANC-1 and 10 mice with PANC-1RG7 were
included in the negative control group (injected intraperitoneally
with 10 ml/kg normal saline at 15, 18, 21, 24, 27 and 30 days); 10
mice with PANC-1 and 10 mice with PANC-1RG7 were included in the
GEM intervention group (injected intraperitoneally with 50 mg/kg
GEM at 15, 18, 21, 24, 27 and 30 days). We observed the general
conditions of the mice and tumors after they were sacrificed at 33
days.
Quantitative real-time polymerase chain
reaction analysis (qPCR)
PANC-1 and PANC-1RG7 cells (3×106) were
harvested. Total RNA was extracted and subjected to first-strand
complementary DNA as previously described (9). qPCR was performed using the ABI prism
7500 HT sequence detection system (Applied Biosystems, Foster City,
CA, USA) to detect the mRNA expression of deoxycytidine kinase
(dCK), 5′-nucleotidase (NT5), cytidine deaminase (CDA),
equilibrative nucleoside transporter 1 (ENT1), ENT2, ribonucleotide
reductase 1 (RRM1), RRM2, DNA polymerase A (POLA), multidrug
resistance protein 1 (MDR1), multidrug resistance-related protein
(MRP) and breast cancer resistance protein (BCRP). The forward and
reverse primers we designed are shown in Table I. Relative expression was calculated
using the ΔΔCt method and our result passed the validation
experiment. The results of control and treated cells are expressed
as an average of the triplicate samples of at least 3 independent
experiments.
 | Table ISequences of polymerase chain reaction
primers and sequence-specific probes of target genes and
β-actin. |
Table I
Sequences of polymerase chain reaction
primers and sequence-specific probes of target genes and
β-actin.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | Product length
(bp) |
|---|
| dCK |
ATCCAGCTTCCTTCTGTCATTCC |
CAACGAAGTGAGAGGCACCAG | 80 |
| NT5 |
TAATGGTATAAACACAGGATACCATCCT |
CATTATCTACTACAGCTTGCTACCTGACT | 85 |
| CDA |
GAAGCGTCCTGCCTGCA |
CTGGACCGTCATGACAATATACG | 382 |
| ENT1 |
TCTTCATGGCTGCCTTTGC |
GGCTTCACTTTCTTGGGCC | 79 |
| ENT2 |
CAAGACCTCATGGAAAGGGTG |
CCACTCTGAACCCTCTGGTCA | 124 |
| RRM1 |
GGCACCCCGTATATGCTCTA |
CCAGGGAAGCCAAATTACAA | 148 |
| RRM2 |
GGCTCAAGAAACGAGGACTG |
TCAGGCAAGCAAAATCACAG | 93 |
| POLA |
GGCTCGGATCTGTGAACCAA |
GGGCTCCATATCTGTTCCCG | 256 |
| MDR1 |
AGGTTCCAGGATTGGCGTCTT |
CCAGTCATTGCTGCGGTTTCA | 156 |
| MRP |
GCGAGTGTCTCCCTCAAACG |
TCCTCACGGTGATGCTGTTC | 118 |
| BCRP |
GATATGGATTTACGGCTTTGC |
CGATGCCCTGCTTTACCAA | 135 |
| β-actin |
AGTGTGACGTGGACATCCGCAAAG |
ATCCACATCTGCTGGAAGGTGGAC | 220 |
Western blotting
PANC-1 and PANC-1RG7 (9×106) cells were
harvested. Total protein fractions were extracted, separated on
SDS-PAGE and then exposed to specific antibodies using western
blotting described earlier (9). The
specific primary antibodies we used were mouse monoclonal
antibodies anti-human β-actin (sc-47778), ENT1 (sc-377283), ENT2
(sc-373871), NT5 (sc-32299), POLA (sc-137021), p-gp (sc-55510) (all
from Santa Cruz, USA), and MRP (no. ab32574; Abcam, USA); rabbit
polyclonal antibodies anti-human DCK (no. ab151966; Abcam, USA),
CDA (sc-134754), BCRP (sc-130933) (both from Santa Cruz), Akt (no.
BS1810) and mTOR (no. BS3611) (both from BioWorld, USA); rabbit
monoclonal antibodies anti-human PI3K (no. 4249; Cell Signaling
Technology, USA); and goat polyclonal antibodies anti-human RRM1
(sc-11733) and RRM2 (sc-10846) (both from Santa Cruz). Images were
analyzed using Quantity One 4.62. Each experiment was repeated
>3 times.
Statistical analysis
Experimental data are presented as the means ±
standard deviation (SD) and analyzed by SPSS 19.0. Comparisons were
performed using Student’s t-test between 2 groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Morphological and ultrastructure
characteristics
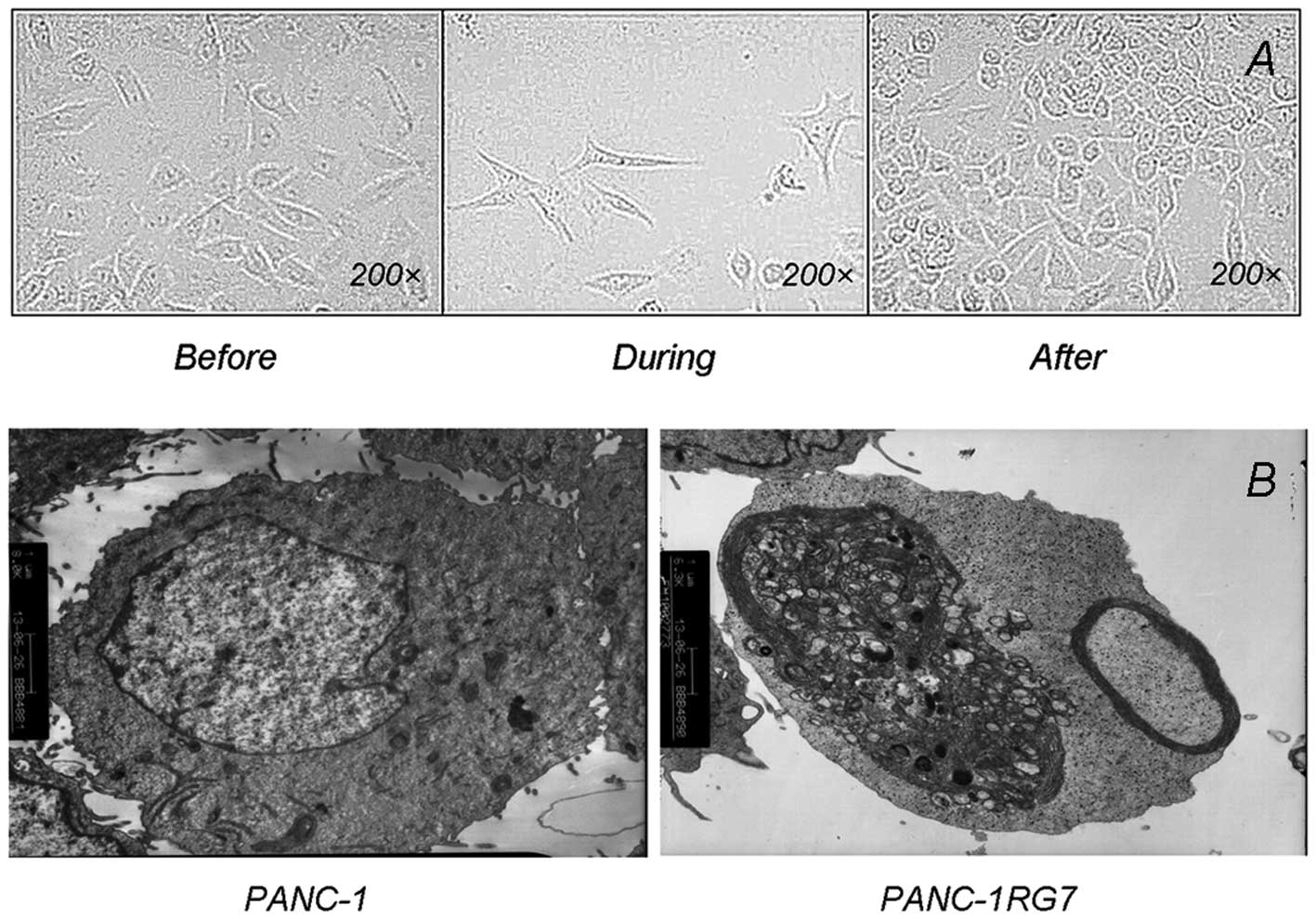
PANC-1 cells appeared fusiform under an optical
microscope at a magnification of ×200. During GEM intervention, the
cells appeared polygonal with elongated pseudopodia and growth
retardation; the size significantly increased and numerous vacuoles
were formed in the cytoplasm. Cell growth was gradually restored
after GEM was removed; a fusiform was formed but remained smaller
and grew more slowly than parental cells (Fig. 1A).
PANC-1 cells contained intact a cell membrane and
nucleus, numerous microvilli on the membrane, abundant organelles
and a satisfactory state under a transmission electron microscope.
However, PANC-1RG7 exhibited different ultrastructural
characteristics. In particular, small vacuoles and lipid droplets
were formed in the cytoplasm. The number of glycogen granules and
lysosomes increased significantly, the mitochondrial cristae was
broken as vacuolization occurred, and the rough endoplasmic
reticulum became swollen (Fig.
1B).
Cell growth curve
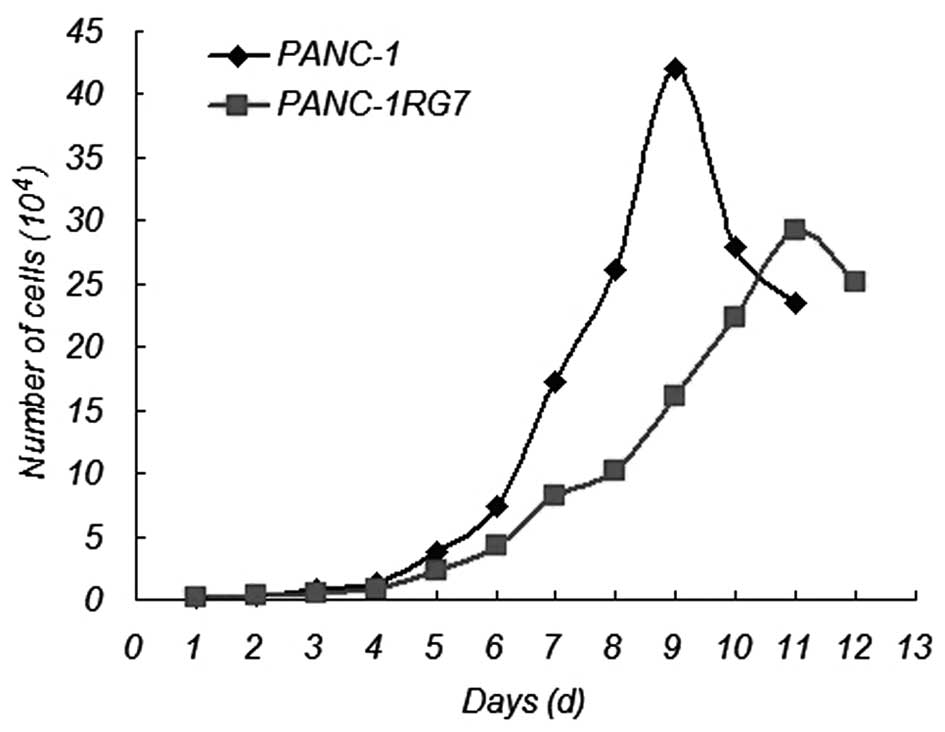
Compared with the parental cell PANC-1,
GEM-resistant pancreatic cancer PANC-1RG7 cells slowly grew at a
significant rate (Fig. 2). The
doubling times of PANC-1 and PANC-1RG7 cells were 25.83±2.03 and
33.83±2.15 h, respectively. The doubling time of PANC-1RG7 cells
was significantly increased (p<0.05).
Cell cycle analysis by flow
cytometry
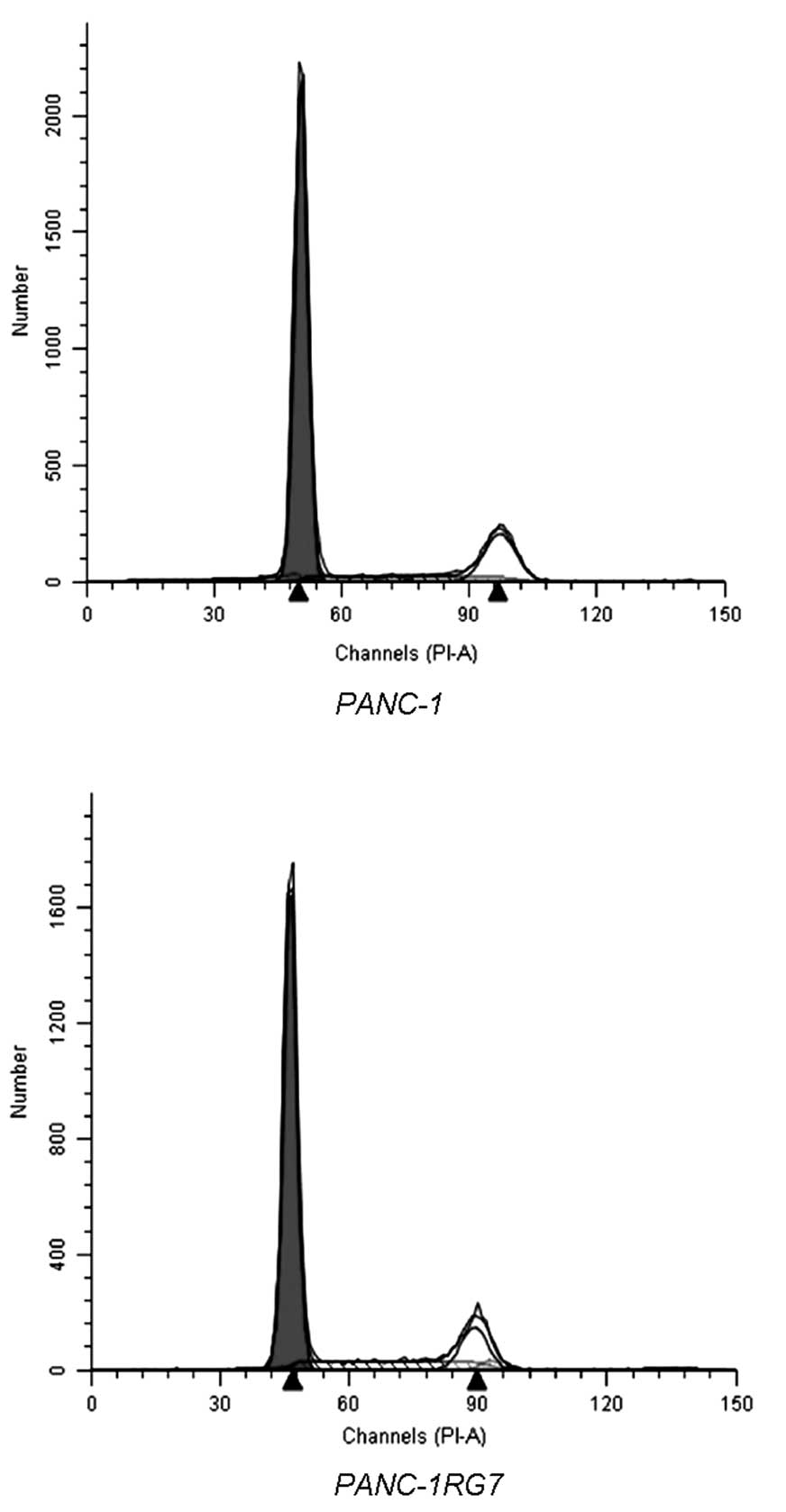
In PANC-1, 68.98±2.32 and 18.02±0.63% of the cells
were detected in the G0/G1 and S phase, respectively. In PANC-1RG7,
69.23±3.03 and 17.77±0.89% of the cells were detected in the G0/G1
and S phase, respectively. No significant difference was determined
(p>0.05; Fig. 3).
SRB assays
We detected 9 common chemotherapeutics, including
GEM, ADM, MMC, PTX, MTX, VCR, GEF, DDP and 5-FU. The
IC50 values of GEM, MTX, GEF, DDP and 5-FU were
statistically different between PANC-1 and PANC-1RG7; by contrast,
the IC50 values of the other drugs were not different
(Table II). The RIs of GEM, MTX,
GEF, DDP and 5-FU were 39.9, 2.24, 1.42, 2.35 and 7.00,
respectively. This result indicated that the PANC-1RG7 cells
established in this study expressed resistance to GEM and
cross-resistance to MTX, GEF, DDP and 5-FU.
 | Table IISRB assay results of PANC-1 and
PANC-1RG7 human pancreatic cancer cells treated with various
concentrations of GEM, ADM, MMC, PTX, MTX, VCR, GEF, DDP and 5-FU
for 96 h. |
Table II
SRB assay results of PANC-1 and
PANC-1RG7 human pancreatic cancer cells treated with various
concentrations of GEM, ADM, MMC, PTX, MTX, VCR, GEF, DDP and 5-FU
for 96 h.
| IC50 | PANC-1 | PANC-1RG7 | P-value |
|---|
| GEM (μmol/l) | 0.0081±0.0014 | 0.3233±0.0933 | 0.0023a |
| ADM (nmol/l) | 10.2380±2.4875 | 9.1433±2.4533 | 0.7011 |
| MMC (μmol/l) | 0.5689±0.5180 | 0.2545±0.1543 | 0.4972 |
| PTX (nmol/l) | 2.2618±0.2262 | 2.4840±0.1500 | 0.2682 |
| MTX (nmol/l) | 0.9346±0.1649 | 2.0948±0.1672 | 0.0199b |
| VCR (nmol/l) | 7.0513±2.3578 | 5.4185±1.7090 | 0.4265 |
| GEF (μmol/l) | 7.2575±0.5216 | 10.281±0.2890 | 0.0189b |
| DDP (μmol/l) | 0.6317±0.2159 | 1.4853±0.4649 | 0.0108b |
| 5-FU (μmol/l) | 1.9298±0.4420 | 13.509±2.7563 | 0.0001a |
Establishment of animal models and drug
intervention
We successfully established nude mouse subcutaneous
tumor models. The mice were sacrificed at 33 days. Subcutaneous
tumors were completely peeled off and weighed (Table III). PANC-1 tumors in the negative
control group were significantly smaller than PANC-1RG7 tumors
(p<0.05). This result indicated that PANC-1RG7 cells grew faster
in vivo than PANC-1 cells. A significant difference was
observed in tumor weights before and after GEM intervention was
administered in PANC-1 (p<0.05), but not in PANC-1RG7
(p<0.05). The inhibition rates of GEM in PANC-1 and PANC-1GR7
were 82.03 and 33.40%, respectively. This finding indicated that
the inhibition of GEM decreases in vivo.
 | Table IIISubcutaneous tumor weight of each
group of mice with pancreatic cancer (n=10, mean ± SD). |
Table III
Subcutaneous tumor weight of each
group of mice with pancreatic cancer (n=10, mean ± SD).
| Tumor weight
(g) | Control | GEM (50 mg/kg) |
|---|
| PANC-1 | 0.2118±0.0521 |
0.0381±0.0215a |
| PANC-1RG7 |
0.3247±0.1292b | 0.2163±0.0833 |
qPCR
We examined the expression levels of dCK, NT5, CDA,
ENT1, ENT2, RRM1, RRM2, POLA, MDR1, MRP and BCRP at mRNA levels by
qPCR. However, only CDA, MRP and BCRP expressions changed at mRNA
levels. The expression levels of these 3 genes in PANC-1RG7 were
lower than those in PANC-1 with a significant difference (Table IV).
 | Table IVmRNA expression of dCK, NT5, CDA,
ENT1, ENT2, RRM1, RRM2, POLA, MDR1, MRP and BCRP by qPCR. |
Table IV
mRNA expression of dCK, NT5, CDA,
ENT1, ENT2, RRM1, RRM2, POLA, MDR1, MRP and BCRP by qPCR.
| PANC-1RG7 gene | ΔΔCt |
2−ΔΔCt |
|---|
| dCK | 0.06±0.52 | 1.00±0.33 |
| NT5 | 0.62±1.33 | 0.86±0.77 |
| CDA | 5.88±0.69 | 0.02±0.01a |
| ENT1 | 0.70±0.22 | 0.62±0.09 |
| ENT2 | 0.38±0.68 | 0.83±0.36 |
| RRM1 | 0.04±0.43 | 1.00±0.29 |
| RRM2 | −0.04±0.40 | 1.05±0.30 |
| POLA | 0.45±1.55 | 0.98±0.67 |
| MDR1 | 1.27±1.44 | 0.55±0.46 |
| MRP | 1.48±0.28 | 0.36±0.07a |
| BCRP | 2.42±0.34 | 0.19±0.05a |
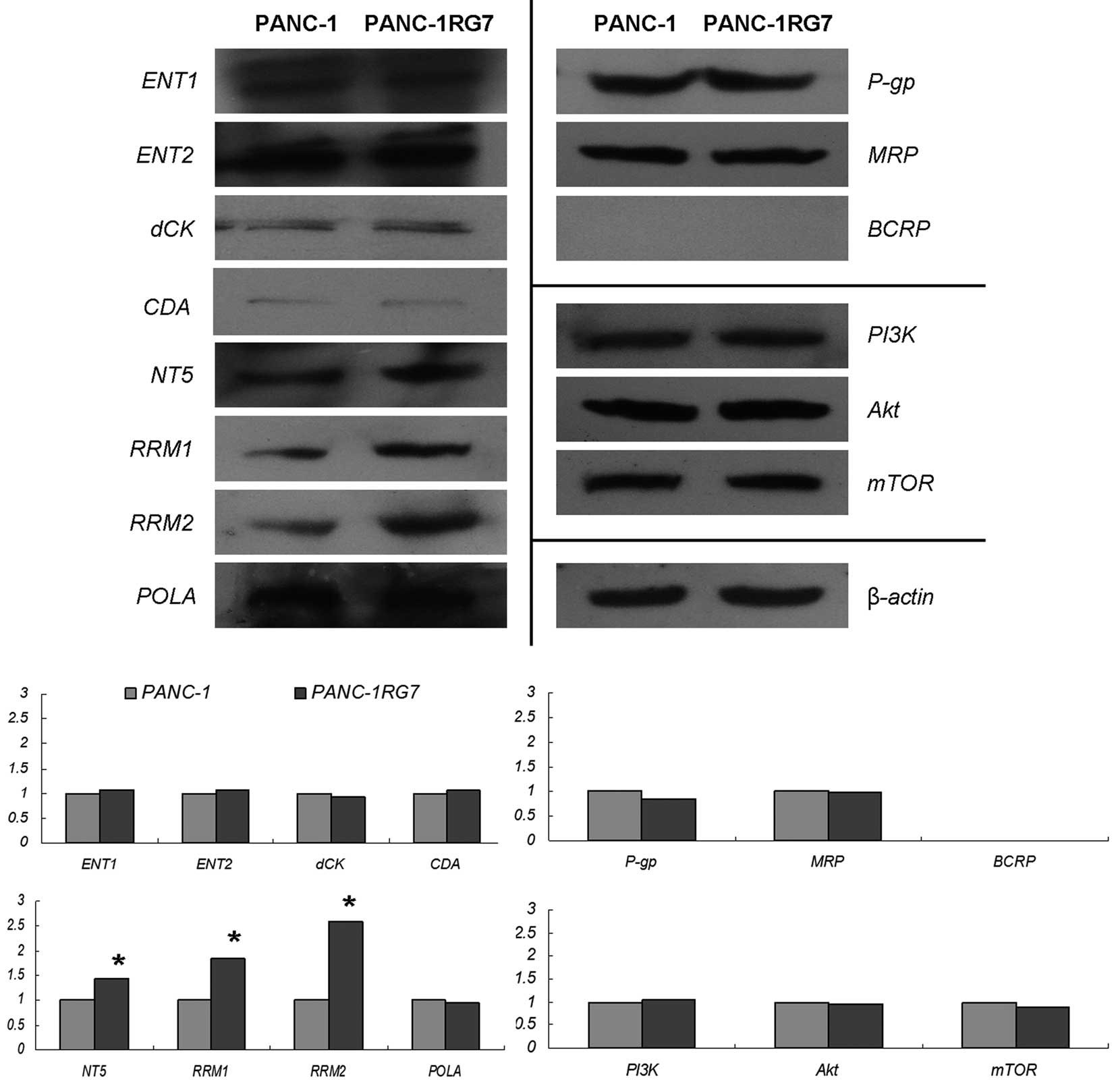
Western blotting
We further examined the expression levels of dCK,
NT5, CDA, ENT1, ENT2, RRM1, RRM2, POLA, MDR1, MRP and BCRP at
protein levels by western blotting. The key components of the
PI3K/Akt/mTOR signaling pathway were also determined. The examined
proteins, except BCRP, were expressed in PANC-1 and PANC-1RG7.
Compared with those in parental PANC-1, NT5, RRM1 and RRM2
expression levels were significantly increased in PANC-1RG7
(p<0.05). No changes in other proteins at a protein level were
noted (Fig. 4).
Discussion
Chemotherapy is the fundamental treatment modality
for pancreatic cancer patients who are unable to undergo surgery;
this modality is also a key component of systemic therapy (1). GEM is currently the preferred drug for
the treatment of pancreatic cancer by single chemotherapeutic
applications. However, the inherent and acquired resistance of
cancer cells to GEM limits its efficiency. Thus far, no effective
drug has improved the clinical benefits of GEM. Hence, resistance
to GEM remains a vital problem. Numerous mechanisms have been
presented, however, the main one remains unclear. In the present
study, a stable human pancreatic cancer GEM-resistant cell line was
established for use in further studies on GEM resistance.
Intermittent intervention in gradually increasing
concentration or pulse intervention in large concentrations can be
performed to establish drug-resistant cancer cell lines. The former
method can be used to simulate the intermittent administration of
drugs in clinical applications with a high achievement ratio and
increased stability. Although this method requires time-consuming
procedures, we performed this method in the present study. In
China, human pancreatic cancer cell line SW1990, which is derived
from pancreatic cancer accompanied by metastatic spleen, is
commonly used to establish a pancreatic cancer GEM-resistant cell
line (10–12), while MIA PaCa-2 derived from
pancreas tissues is used in other countries (13–15).
However, we chose human pancreatic cancer cell line PANC-1, which
is derived from pancreatic ductal carcinoma. Commonly used in
clinical practice, this cell line is highly sensitive to GEM owing
to its low differentiation. The stable cell clone termed PANC-1RG7
with a uniform resistant mechanism was obtained after GEM
intervention was conducted for 2 years and clone cultures were
prepared thrice. Our results showed that the RI of GEM was 39.9,
indicating low resistance.
Changes in the morphological characteristics of
resistant cells indicate acquired resistance. The established
GEM-resistant cells were smaller and grew more slowly than the
parental cells. Cell organs, such as lysosomes, mitochondria and
rough endoplasmic reticulum, significantly changed, as observed
under a transmission electron microscope. These changes may be
considered the basis of functional changes related to resistance
mechanisms. Multidrug resistance (16), characterized by cells that are
resistant not only to intervention drug but also to other
chemotherapeutics without structural or functional relationships
existed in PANC-1RG7 with cross-resistance to MTX, GEF, DDP and
5-FU, suggesting that a common mechanism could be implicated in
this resistance. As such, in vivo studies involving
xenografts are necessary to detect biological behavior and tumor
characterization. The established PANC-1RG7 cells indicated an
increased discernible invasion and growth compared with parental
PANC-1 cells; by contrast, in vitro studies showed a slow
growth. Further studies should be conducted to investigate the
possible mechanism implicated in the difference between in
vivo and in vitro processes.
ENTs facilitate the entry of GEM, a pyrimidine
analog, across the plasma membrane of cells. GEM is then
phosphorylated intracellularly by dCK via multiple steps to derive
diphosphate and triphosphate. The former is an active metabolite
inhibiting RRM, resulting in a decrease in intracellular dCTP;
thus, DNA synthesis is suppressed. The latter inhibits DNA
synthesis by interfering with the incorporation of endogenous dCTP
into DNA. Studies on GEM resistance mechanisms have shown that
factors involved in GEM metabolism and transport are related to
resistance. In the present study, the expression levels of the main
factors in PANC-1RG7 and parental PANC-1 were detected. CDA
expression at an mRNA level significantly decreased in PANC-1RG7,
yet it remained unchanged at a protein level. The expressions of
NT5, RRM1 and RRM2 proteins were significantly increased in
PANC-1RG7 compared with those in PANC-1RG7. By contrast, no change
was observed at the mRNA level. No linear relationship between mRNA
and protein expression was noted since mRNA undergoes a series of
regulatory processes, including microRNA regulation, translation,
post-translational modification (e.g., glycosylation and
phosphorylation), and protein transport, to express proteins. Thus,
the differences in the changes between mRNA and protein expression
could be acceptable. As active proteins, enzymes are involved in
activities more directly related to expressions at protein levels.
Hence, the overexpression of NT5, RRM1 and RRM2 was necessary to
induce the resistance of the established PANC-1RG7 to GEM.
Increased activities of RRM1 and RRM2 possibly
promote the conversion of nucleoside to deoxynucleoside and
accelerate DNA polymerization and repair, resulting in resistance
(17). In GEM metabolism and
transport, 4 factors, ENT1, dCK, RRM1 and RRM2, are implicated in
acquired resistance (18). As ENT1
expression decreases, GEM intake is reduced and cytotoxicity is
decreased in vivo (19). The
deficiency in dCK activities is one of the mechanisms by which
pancreatic cancer cells develop resistance to chemotherapeutic
drugs (20) since dCK is an
important factor in the intracellular conversion of GEM to an
active metabolite. However, ENT1 and dCK expression in PANC-1RG7
remained unchanged at the mRNA and protein levels. Enzyme activity
is not only affected by mRNA or protein expression; studies have
shown that dCK activity, protein and gene expression levels are
significantly correlated (21). No
dCK activity was detected directly due to limited experimental
conditions. However, dCK failed to induce PANC-1RG7 to develop
resistance to GEM. In a previous study, the overexpression of NT5
was observed in a human pancreatic cancer GEM-resistant cell line
(14); however, further studies
should be conducted to determine whether or not this overexpression
increases the removal of GEM.
P-gp, MRP and BCRP are 3 multidrug-resistant
proteins relevant to tumor stem cell. On the basis of the results
of expression detection, we found that P-gp and MRP proteins were
expressed in human pancreatic cancer PANC-1 cells; MDR1, MRP and
BCRP genes were also expressed in PANC-1 cells, indicating the
inherent resistance of PANC-1. The expressions did not increase
after GEM intervention was administered in the present study, and
the gene expression of MRP and BCRP decreased. However, studies
have yet to determine whether or not the resistance of cancer cells
to chemotherapeutics is attributed to the decrease in the gene
expression of MRP and BCRP. Further studies are required to
determine if these changes are correlated with cell resistance.
Nevertheless, the proteins and genes not implicated in the
resistance of PANC-1RG7 to GEM could be identified. Moreover,
changes in apoptotic signaling pathway are related to the
resistance of pancreatic cancer cells. Thus, apoptosis-regulatory
proteins are abnormally expressed in pancreatic cancer cells
(22). No changes in PI3K, Akt and
mTOR protein expression were observed in PANC-1RG7.
In summary, human pancreatic cancer GEM-resistant
cell line PANC-1RG7 was established in this study. These cells grew
slowly in vitro but rapidly in vivo. In vitro
and in vivo experimental results showed that PANC-1RG7
exhibited stable resistance to GEM and cross-resistance to other
chemotherapeutics, such as MTX, GEF, DDP and 5-FU. Of all the
factors related to GEM resistance, only RRM1 and RRM2 protein
expression increased in the resistant cells, thereby inducing
resistance to GEM. This result indicated that the overexpression of
RRM1 and RRM2 was necessary to induce the resistance of PANC-1RG7
to GEM.
Cancer-resistant cell lines established in
vitro remain the main tools used to study the mechanisms of
acquired tumor resistance. The established pancreatic cancer
GEM-resistant cell line PANC-1RG7 may also be used as an important
tool to investigate the acquired resistance of pancreatic cancer,
RRM, or new chemotherapy drugs that can reverse GEM resistance.
Acknowledgements
The present study was supported by the National
Science Foundation of China (no. 30772587), the Great Research
Project of Fujian Medical University (no. 09ZD012), and the Natural
Science Foundation of Fujian Province (nos. C0510012 and
2011J01188).
References
|
1
|
El Maalouf G, Le Tourneau C, Batty GN,
Faivre S and Raymond E: Markers involved in resistance to
cytotoxics and targeted therapeutics in pancreatic cancer. Cancer
Treat Rev. 35:167–174. 2009. View Article : Google Scholar
|
|
2
|
Berlin JD, Catalano P, Thomas JP, Kugler
JW, Haller DG and Benson AB III: Phase III study of gemcitabine in
combination with fluorouracil versus gemcitabine alone in patients
with advanced pancreatic carcinoma: Eastern Cooperative Oncology
Group Trial E2297. J Clin Oncol. 20:3270–3275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heinemann V, Quietzsch D, Gieseler F,
Gonnermann M, Schönekäs H, Rost A, Neuhaus H, Haag C, Clemens M,
Heinrich B, Vehling-Kaiser U, Fuchs M, Fleckenstein D, Gesierich W,
Uthgenannt D, Einsele H, Holstege A, Hinke A, Schalhorn A and
Wilkowski R: Randomized phase III trial of gemcitabine plus
cisplatin compared with gemcitabine alone in advanced pancreatic
cancer. J Clin Oncol. 24:3946–3952. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bernhard J, Dietrich D, Scheithauer W,
Gerber D, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schüller
J, Saletti P, Bauer J, Figer A, Pestalozzi BC, Köhne CH, Mingrone
W, Stemmer SM, Tàmas K, Kornek GV, Koeberle D and Herrmann R;
Central European Cooperative Oncology Group. Clinical benefit and
quality of life in patients with advanced pancreatic cancer
receiving gemcitabine plus capecitabine versus gemcitabine alone: a
randomized multicenter phase III clinical trial - SAKK
44/00-CECOG/PAN1.3.001. J Clin Oncol. 26:3695–3701. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos
D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M and
Parulekar W; National Cancer Institute of Canada Clinical Trials
Group. Erlotinib plus gemcitabine compared with gemcitabine alone
in patients with advanced pancreatic cancer: a phase III trial of
the National Cancer Institute of Canada Clinical Trials Group. J
Clin Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Philip PA, Benedetti J, Corless CL, Wong
R, O’Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC,
Rivkin SE, Khorana AA, Goldman B, Fenoglio-Preiser CM, Abbruzzese
JL and Blanke CD: Phase III study comparing gemcitabine plus
cetuximab versus gemcitabine in patients with advanced pancreatic
adenocarcinoma: Southwest Oncology Group-directed intergroup trial
S0205. J Clin Oncol. 28:3605–3610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kindler HL, Friberg G, Singh DA, Locker G,
Nattam S, Kozloff M, Taber DA, Karrison T, Dachman A, Stadler WM
and Vokes EE: Phase II trial of bevacizumab plus gemcitabine in
patients with advanced pancreatic cancer. J Clin Oncol.
23:8033–8040. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Storniolo AM, Enas NH, Brown CA, Voi M,
Rothenberg ML and Schilsky R: An investigational new drug treatment
program for patients with gemcitabine: results for over 3000
patients with pancreatic carcinoma. Cancer. 85:1261–1268. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang C, Yang A, Zhang B, Yin Q, Huang H,
Chen M and Xie J: PANC-1 pancreatic cancer cell growth inhibited by
cucurmosin alone and in combination with an epidermal growth factor
receptor-targeted drug. Pancreas. 43:291–297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niu BZ, Chen G, Li LJ, Wu YD and Zhao YP:
Drug resistance and activity changes of thioredoxin reductase in
pancreatic cancer cell strain SW1990 induced by gemcitabine.
Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 27:606–610. 2005.(In Chinese).
PubMed/NCBI
|
|
11
|
Yao J, Feng FY, Lin C, Zhang XY, Fu M,
Liang X and Yang Y: The mechanism of resistance to
2′,2′-difluorodeoxycytidine (gemcitabine) in a pancreatic cancer
cell line. Zhonghua Zhong Liu Za Zhi. 27:721–726. 2005.(In
Chinese).
|
|
12
|
An Y, Yao J, Wei JS, Lu ZP, Cai HH, Dai
CC, Qian ZY, Xu ZK and Miao Y: Establish a gemcitabine-resistant
pancreatic cancer cell line SW1990/GZ and research the relationship
between SW1990/GZ and pancreatic cancer stem cell. Zhonghua Wai Ke
Za Zhi. 48:999–1003. 2010.(In Chinese). PubMed/NCBI
|
|
13
|
Togawa A, Ito H, Kimura F, Shimizu H,
Ohtsuka M, Shimamura F, Yoshidome H, Katoh A and Miyazaki M:
Establishment of gemcitabine-resistant human pancreatic cancer
cells and effect of brefeldin-a on the resistant cell line.
Pancreas. 27:220–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kazuno H, Sakamoto K, Fujioka A, Fukushima
M, Matsuda A and Sasaki T: Possible antitumor activity of
1-(3-C-ethynyl-β-d-ribo-pentofuranosyl)cytosine (ECyd, TAS-106)
against an established gemcitabine (dFdCyd)-resistant human
pancreatic cancer cell line. Cancer Sci. 96:295–302. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kagawa S, Takano S, Yoshitomi H, Kimura F,
Satoh M, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Furukawa K,
Matsushita K, Nomura F and Miyazaki M: Akt/mTOR signaling pathway
is crucial for gemcitabine resistance induced by Annexin II in
pancreatic cancer cells. J Surg Res. 178:758–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uchiyama-Kokubu N and Watanabe T:
Establishment and characterization of adriamycin-resistant human
colorectal adenocarcinoma HCT-15 cell lines with multidrug
resistance. Anticancer Drugs. 12:769–779. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goan YG, Zhou B, Hu E, Mi S and Yen Y:
Overexpression of ribonucleotide reductase as a mechanism of
resistance to 2,2-difluorodeoxycytidine in the human KB cancer cell
line. Cancer Res. 59:4204–4207. 1999.PubMed/NCBI
|
|
18
|
Nakano Y, Tanno S, Koizumi K, Nishikawa T,
Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T and Kohgo
Y: Gemcitabine chemoresistance and molecular markers associated
with gemcitabine transport and metabolism in human pancreatic
cancer cells. Br J Cancer. 96:457–463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
García-Manteiga J, Molina-Arcas M, Casado
FJ, Mazo A and Pastor-Anglada M: Nucleoside transporter profiles in
human pancreatic cancer cells: role of hCNT1 in
2′,2′-difluorodeoxy-cytidine-induced cytotoxicity. Clin Cancer Res.
9:5000–5008. 2003.
|
|
20
|
Bergman AM, Pinedo HM and Peters GJ:
Determinants of resistance to 2′,2′-difluorodeoxycytidine
(gemcitabine). Drug Resist Updat. 5:19–33. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kroep JR, Loves WJ, van der Wilt CL,
Alvarez E, Talianidis I, Boven E, Braakhuis BJ, van Groeningen CJ,
Pinedo HM and Peters GJ: Pretreatment deoxycytidine kinase levels
predict in vivo gemcitabine sensitivity. Mol Cancer Ther.
1:371–376. 2002.PubMed/NCBI
|
|
22
|
Graber HU, Friess H, Zimmermann A, Korc M,
Adler G, Schmid R and Büchler MW: Bak expression and cell death
occur in peritumorous tissue but not in pancreatic cancer cells. J
Gastrointest Surg. 3:74–80. 1999. View Article : Google Scholar : PubMed/NCBI
|