Introduction
Breast cancer (BC), the most frequent carcinoma in
women, is a multifactorial disease occurring as a result of
environmental and heritable factors. It is estimated that 5–10% of
all breast carcinomas are inherited, whereas the remaining 90–95%
are sporadic carcinomas. BRCA1 and BRCA2 genes are
responsible for 3–8% of all BCs and for 15–20% of familial cases
(1), and because these genes are
involved in maintaining genome integrity the complete loss of
function of encoded proteins leads to genomic instability (2,3).
Increased genetic susceptibility could be associated with genomic
instability, which could lead to chromosomal breakage. Sister
chromatid exchange (SCE) is an error-free recombination mechanism,
defined as a symmetrical exchange between portions of apparently
homologous sister chromatids (4).
Although little is known about its molecular basis, these exchanges
presumably involve DNA breakage and subsequent reunion of newly
synthesized DNA chains during replication (5,6). An
increase in SCE frequency is found in patients with various types
of cancers, such as uterine cervix (7) ovary (8), prostate (9), nasopharynx (10) and BC (11). Regarding BC, Roy et al
reported increased spontaneity of SCE frequency in hereditary BC
patients compared to their healthy relatives (12). Conversely, Cefle et al
(13) found no difference in SCE
frequency between patients with BC and their first-degree
relatives. Finally, in an observational study, Aristei et al
(14) showed an increase in SCE
frequency in early-stage BC patients compared to controls, with no
significant differences in SCE in patients with or without a family
history of cancer. In the present study, we evaluated the SCE
frequency in the peripheral blood lymphocytes of familial and
sporadic BC patients from the Apulian Caucasian population in order
to verify whether a difference exists among these groups. This is
the first study to investigate SCE frequency in this specific
population.
Materials and methods
Patient characteristics
SCE frequency was investigated in 22 familial and 59
sporadic female patients with a first diagnosis of primary BC, and
compared with 20 healthy control women with no history of any
cancer for the last three generations. The study was approved by
the Institutional Review Board of our Institute. Before undergoing
routine surgery, all patients signed an informed consent form
authorizing the Institute to utilize their blood sample for
research purposes according to ethical standards.
All the subjects came from the Apulian Caucasian
population, and none were cigarette smokers, or alcohol or drug
users. Furthermore, no subject had genetic disorders or chronic
diseases affecting the family. This information was controlled by
careful medical interviews. We stratified the 22 familial BC
patients in two group: ‘low-risk’ (n=11) and ‘high-risk’ (n=11)
patients for BRCA gene mutations. Eleven patients with a
familial history of BC and with a BRCAPro value ≥10% were eligible
for the genetic counseling program, and were screened for mutations
in the BRCA1 and BRCA2 genes as previously described
(1,15). Tumor characteristics including tumor
size, nodal status, tumor grade, estrogen receptor (ER),
progesterone receptor (PR), proliferative activity (MIB1) and human
epidermal growth factor receptor 2 (HER2) were provided by the
Pathology Department of our Institute (Table I).
 | Table IClinicopathological characteristics
and mean SCE in familial and sporadic breast cancer patients. |
Table I
Clinicopathological characteristics
and mean SCE in familial and sporadic breast cancer patients.
| Familial n=22 | Sporadic n=59 |
|---|
|
|
|
|---|
| Characteristics | No. of patients
(%) | Mean SCE | No. of patients
(%) | Mean SCE |
|---|
| Age (years) |
| ≤55 | 17 (77) | 5.5 | 27 (46) | 4.0 |
| >55 | 5 (23) | 4.8 | 32 (54) | 3.9 |
| Tumor size (cm) |
| ≤2 | 12 (55) | 5.2 | 27 (46) | 4.0 |
| >2 | 10 (45) | 5.4 | 32 (54) | 3.4 |
| Nodal status |
| Negative | 17 (81) | 5.4 | 32 (54) | 3.9 |
| Positive | 4 (19) | 5.3 | 27 (46) | 3.9 |
| Tumor grade |
| 1–2 | 15 (68) | 5.4 | 30 (51) | 3.9 |
| 3 | 7 (32) | 5.1 | 29 (49) | 3.9 |
| ER |
| Negative (≤10%) | 6 (27) | 5.1 | 15 (25) | 4.0 |
| Positive
(>10%) | 16 (73) | 5.4 | 44 (75) | 3.9 |
| PR |
| Negative (≤10%) | 12 (55) | 5.3 | 19 (32) | 4.0 |
| Positive
(>10%) | 10 (45) | 5.3 | 40 (68) | 3.9 |
| MIB1 |
| Negative (≤20%) | 16 (73) | 5.3 | 38 (64) | 3.9 |
| Positive
(>20%) | 6 (27) | 5.0 | 21 (36) | 4.1 |
| HER2a |
| Negative (0,
1+) | 14 (82) | 5.4 | 37 (67) | 3.9 |
| Positive (3+) | 3 (18) | 4.8 | 18 (33) | 3.9 |
The cut-off value for ER and PR was 10%. Tumors with
ER or PR expression were scored as positive when nuclear staining
was present in >10% of tumor cells and scored negative when ≤10%
of the tumor cells had nuclear staining. Cases with an MIB1 index
>20% were considered high proliferating tumors. The MIB1 cut-off
represents the median value of the scores relative to all BC
samples analyzed during the last five years at our Institute.
HER2 was scored as 0, 1+, 2+ or 3+ using a
monoclonal antibody (MoAb clone CB11; Novocastra Laboratories,
Ltd., Newcastle, UK), in accordance with the HercepTest scoring
system (Food and Drug Administration accepted): 0, no membranous
immunoreactivity or <10% of cells reactive; 1+, incomplete
membranous reactivity in >10% of cells; 2+, >10% of cells
with weak to moderate complete membranous reactivity; and 3+,
strong and complete membranous reactivity in >10% of cells.
Cytoplasmic immunoreactivity was ignored. Cases scoring 0 and 1+
were classified as negative. HER2 was considered to be positive if
immunostaining was 3+ or if a 2+ result showed gene amplification
by fluorescence in situ hybridization (FISH). In FISH
analyses, each copy of the HER2 gene and its centromere 17 (CEP17)
reference were counted. The interpretation followed the criteria of
the ASCO/CAP guidelines for HER2 immunohistochemistry
interpretation for BC (16):
positive if the HER2/CEP17 ratio was >2.2.
SCE assay
Venous blood from 81 BC patients and 20 healthy
controls was preserved in heparinized tubes. For SCE analyses,
blood cultures were prepared from blood samples in accordance with
the standard protocol on peripheral blood cultures.
5-Bromo-2′-deoxyuridine (BrdU; Sigma, St. Louis, MO, USA) was added
at a final concentration of 10 μg/ml at the 24th hour and incubated
for 72 h (4). The slides were
prepared in accordance with standard methods and were stained with
the fluorescence-plus-Giemsa technique (17). The slides were incubated for 15 min
in a bisbenzimide Hoechst 33258 solution (5 μg/ml) (bisbenzimide;
Histoline Laboratories S.R.L., Milan), and then washed with
distilled water. The slides were then incubated in fresh
phosphate-buffered water under a UV light source for 1 h. The
slides were washed again, incubated for 10 min in preheated 2×
saline-sodium citrate solution at 56°C in a water-bath, and, after
further rinsing, stained with 10× phosphate-buffered Giemsa
solution.
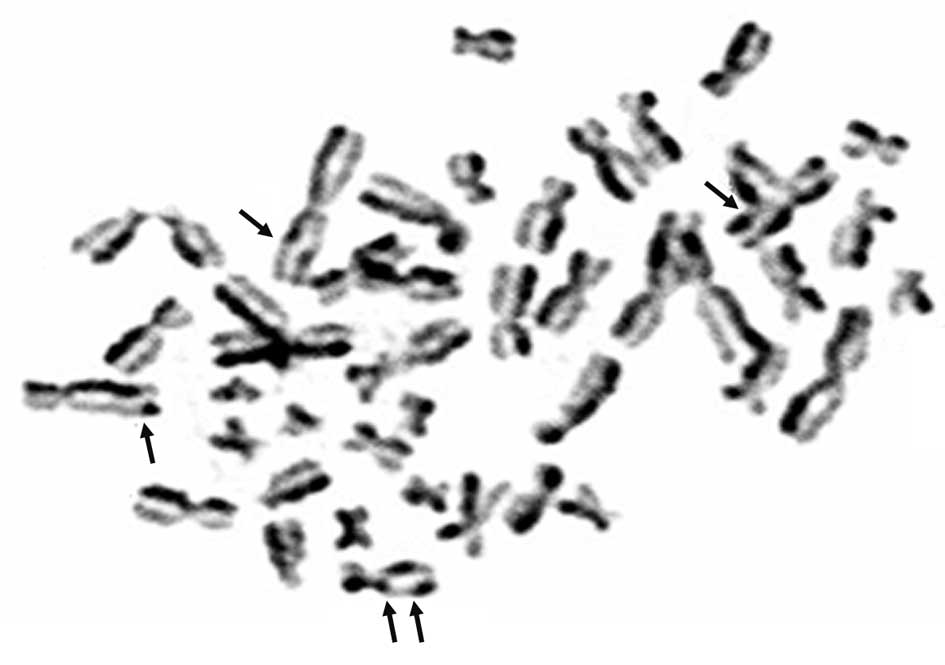
For each subject 50 metaphases were examined by two
observers, who had no knowledge of the patient information
(Fig. 1). Every metaphase was
scored for SCEs, and the individual mean value ± standard
deviation/cell was calculated. SCE baseline values of the familial
group were compared with those of the sporadic patients and with
those of the control group.
Statistical analysis
The statistical association of mean SCE frequency
with clinicopathologic characteristics was assessed using the
Fisher’s exact test and the Student’s t-test. The parametric
one-way analysis of variance and the post hoc Bonferroni’s multiple
comparison tests were carried out to compare the mean frequency of
SCEs per cell among the familial, sporadic and control groups. All
statistical differences were considered significant at the level of
P<0.05. Statistical analyses were performed using SPSS 14.0
statistical software (SPSS, Inc., Chicago IL, USA).
Results
The mean value of the SCE frequency in familial and
sporadic patients was 5.305±1.088 and 3.943±0.552, respectively,
whereas it was 3.197±0.649 in the control group. Statistical
analysis demonstrated that the SCE frequency in the familial
patients was significantly higher than that in both sporadic
patients (P<0.0001) and healthy controls (P<0.0001).
Moreover, SCE frequency in the sporadic patients was significantly
higher compared to that in the control group (P<0.0001)
(Fig. 2).
When comparing the mean SCE frequency value of
familial and sporadic patients with their clinicopathological
characteristics, we found that SCE frequency was always higher in
familial than in sporadic patients (Table I).
The mean ages of familial and sporadic patients and
the control group were 49, 57 and 37 years, respectively. No
significant association between the mean value of SCE frequencies
and the age of all subjects analyzed was found.
In accordance with the statistical analysis, no
significant difference in SCE frequency between low-risk and
high-risk patients was observed with respect to the BRCAPro value
(cut-off ≥10%). Furthermore, we correlated SCE frequency with the
BRCA mutational status of two patients (18%) with two
pathogenic mutations in the BRCA2 gene: 2029delCTTAT and
2049delTC. The SCE frequency was higher compared to patients
without BRCA2 mutations (data not shown).
Discussion
The aim of this preliminary study was to investigate
if a difference in SCE frequency existed between familial and
sporadic Caucasian BC patients from the Apulia Region. We used the
SCE assay, a classic cytomolecular technique which provides an easy
and accurate index to monitor DNA damage and DNA repair status. The
use of lymphocytes is based on the assumption of a hypothetical
association between the presence of chromosomal damage both in
lymphocytes and in tumor cells (18). Even though several studies have
investigated SCE frequency in BC patients (11–14),
this is the first report to analyze SCE in heredo-familial BC
patients as compared to sporadic patients in a Caucasian population
from Southern Italy. None of the patients or healthy controls were
cigarette smokers nor alcohol or drug users, as several authors
have reported an increase in the mean value of SCE due to lifestyle
factors (19,20).
In the present study, a significant increase in the
frequency of SCE was observed in familial compared to sporadic
patients, hypothesizing an increased genome susceptibility as
previously reported (13). However,
in our series of BC patients, we found an SCE frequency lower than
that of other studies (13,21). We believe that the frequency of SCE
of the BC patients from the Apulia Caucasian population is
different than that in other populations. Further studies are
needed to confirm our hypothesis. Furthermore, our results showed
that clinicopathological characteristics of familial and sporadic
tumors did not correlate with SCE frequency (14). SCE was always higher in the familial
than that in the sporadic tumors but the difference did not achieve
statistical significance. Lack of association could be due to the
small number of patients included in the analysis. This reflects
the difficulty in enrolling untreated non-smoking patients; this is
necessary to avoid confounding effects that could influence the
sensitive parameters being investigated in this study.
Considering that SCE showed a higher value in
familial patients, we suggest that SCE could be a biomarker
independent of the clinicopathological characteristics used in
clinical practice to characterize this type of BC phenotype.
Since age and genomic instability are associated
with failure of DNA repair and accumulation of DNA damage in the
genome, many authors have evaluated the association between SCE
frequency and age, but data in the literature are controversial
(4,14,19,22–24).
In accordance with other studies, we found no correlation between
age and SCE frequency, either in familial or in sporadic patients
(4,14).
It is known that BRCA1 and BRCA2 are
required for the maintenance of genomic integrity and that
BRCA gene products are necessary for the control of faithful
homologous recombination between sister chromatids in response to
DNA damage (2). Our statistical
analysis demonstrated no significant difference in SCE frequency
between ‘low-risk and high-risk’ patients. On the other hand, Trenz
et al (25) found no
significant difference between the SCE of patients carrying a
BRCA1 mutation and healthy donors without a family history
of cancer.
In the present study, we investigated the probable
relationship between BRCA mutations and the corresponding
values of SCE. We found an increase in SCE frequency in patients
with heterozygous germline mutations in BRCA2. This finding
is in agreement with a study by Kim et al (26), which suggests a role of the
BRCA2 mutation in the increase of SCE, as if the reduction
in BRCA2 gene dosage decreases the ability of cells to
maintain chromosome stability compared to wild-type level. In fact,
BRCA2 is involved in DNA repair, and the loss of function of
this protein leads to an increase in genomic instability.
In conclusion, in Caucasian patients from the Apulia
Region of Southern Italy with a family history of BC, we showed a
significant increase in SCE frequency, in particular in BRCA
mutation carriers. We suggest that SCE frequency may be used as a
biomarker to better characterize familial BC, and we hypothesize
that the BRCA gene could be involved in the molecular
mechanism of SCE. Further investigation of the correlation between
SCE and BRCA1/BRCA2 mutation carriers and subsequent
evaluation of SCE as a valuable marker for cancer prediction in
high-risk relatives are warranted.
Acknowledgements
We thank Patrizia Chiarappa and Vanessa Desantis for
management of the clinical samples. The authors also thank Caroline
Oakley for language revision (Scientific Direction).
References
|
1
|
Pilato B, Martinucci M, Danza K, Pinto R,
Petriella D, Lacalamita R, Bruno M, Lambo R, D’Amico C, Paradiso A
and Tommasi S: Mutations and polymorphic BRCA variants transmission
in breast cancer familial members. Breast Cancer Res Treat.
125:651–657. 2011. View Article : Google Scholar
|
|
2
|
Levin B, Lech D and Friedenson B: Evidence
that BRCA1- or BRCA2-associated cancers are not inevitable. Mol
Med. 18:1327–1337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O’Donovan PJ and Livingston DM: BRCA1 and
BRCA2: breast/ ovarian cancer susceptibility gene products and
participants in DNA double-strand break repair. Carcinogenesis.
31:961–967. 2010. View Article : Google Scholar
|
|
4
|
Tekcan A, Elbistan M and Ulusoy AN: Sister
chromatid exchanges in breast cancer patients who underwent
chemotherapy. J Toxicol Sci. 37:235–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Conrad S, Künzel J and Löbrich M: Sister
chromatid exchanges occur in G2-irradiated cells. Cell Cycle.
10:222–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
White JS, Choi S and Bakkenist CJ:
Transient ATM kinase inhibition disrupts DNA damage-induced sister
chromatid exchange. Sci Signal. 3:ra442010.PubMed/NCBI
|
|
7
|
Bozsakyova E, Wsolova L and Chalupa I:
Spontaneous and gamma-ray-induced sister chromatid exchanges in
patients with carcinoma of cervix uteri. Int J Radiat Biol.
81:177–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baltaci V, Kayikçioğlu F, Alpas I,
Zeyneloğlu H and Haberal A: Sister chromatid exchange rate and
alkaline comet assay scores in patients with ovarian cancer.
Gynecol Oncol. 84:62–66. 2002. View Article : Google Scholar
|
|
9
|
Dhillon VS and Dhillon IK: Chromosome
aberrations and sister chromatid exchange studies in patients with
prostate cancer: possible evidence of chromosome instability.
Cancer Genet Cytogenet. 100:143–147. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang LY, Lai MS, Huang SJ, Hsieh CY, Hsu
MM and Chen CJ: Increased sister chromatid exchange frequency in
peripheral lymphocytes of nasopharyngeal carcinoma and cervical
cancer patients. Anticancer Res. 14:105–107. 1994.PubMed/NCBI
|
|
11
|
Dhillon VS, Bhasker R, Kler RS and Husain
SA: Sister chromatid exchange (SCE) studies in breast cancer
patients: a follow-up study. Cancer Genet Cytogenet. 80:115–117.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roy SK, Trivedi AH, Bakshi SR, Patel RK,
Shukla PH, Patel SJ, Bhatavdekar JM, Patel DD and Shah PM:
Spontaneous chromosomal instability in breast cancer families.
Cancer Genet Cytogenet. 118:52–56. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cefle K, Ucur A, Guney N, Ozturk S,
Palanduz S, Tas F, Asoglu O, Bayrak A, Muslumanoglu M and Aydiner
A: Increased sister chromatid exchange frequency in young women
with breast cancer and in their first-degree relatives. Cancer
Genet Cytogenet. 171:65–67. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aristei C, Stracci F, Guerrieri P, Anselmo
P, Armellini R, Rulli A, Barberini F, Latini P and Menghini AR:
Frequency of sister chromatid exchanges and micronuclei monitored
over time in patients with early-stage breast cancer: results of an
observational study. Cancer Genet Cytogenet. 192:24–29. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mangia A, Chiriatti A, Tommasi S,
Menolascina F, Petroni S, Zito FA, Simone G, Schittulli F and
Paradiso A: BRCA1 expression and molecular alterations in familial
breast cancer. Histol Histopathol. 24:69–76. 2009.
|
|
16
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer
A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A,
Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler
TM and Hayes DF: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for human epidermal
growth factor receptor 2 testing in breast cancer. J Clin Oncol.
25:118–145. 2007. View Article : Google Scholar
|
|
17
|
Perry P and Wolff S: New Giemsa method for
the differential staining of sister chromatids. Nature.
251:156–158. 1974. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hagmar L, Bonassi S, Stromberg U, Brogger
A, Knudsen LE, Norppa H and Reuterwall C: Chromosomal aberrations
in lymphocytes predict human cancer: a report from the European
Study Group on Cytogenetic Biomarkers and Health (ESCH). Cancer
Res. 58:4117–4121. 1998.PubMed/NCBI
|
|
19
|
Barale R, Chelotti L, Davini T, Del Ry S,
Andreassi MG, Ballardin M, Bulleri M, He J, Baldacci S, Di Pede F,
Gemignani F and Landi S: Sister chromatid exchange and micronucleus
frequency in human lymphocytes of 1,650 subjects in an Italian
population: II. Contribution of sex, age, and lifestyle. Environ
Mol Mutagen. 31:228–242. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar JV, Saraswathi T, Ranganathan K,
Umadevi K, Joshua E and Rooban T: Sister chromatid exchanges in
smokers and smokers with alcohol habit. J Oral Maxillofac Pathol.
16:338–342. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Celik DA, Koşar PA, Ozçelik N and Eroğlu
E: Cytogenetic finding of breast cancer cases and in their
first-degree relatives. J Breast Cancer. 16:285–290. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ben Salah G, Kamoun H, Rebai A, Ben
Youssef A, Ayadi H, Belghith-Mahfoudh N, Fourati A, Ayadi H and
Fakhfakh F: Sister chromatid exchange (SCE) and high-frequency
cells (HFC) in peripheral blood lymphocytes of healthy Tunisian
smokers. Mutat Res. 719:1–6. 2011. View Article : Google Scholar
|
|
23
|
Bernardes de Jesus B and Blasco MA:
Telomerase at the intersection of cancer and aging. Trends Genet.
29:513–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jacobs PA, Maloney V, Cooke R, Crolla JA,
Ashworth A and Swerdlow AJ: Male breast cancer, age and sex
chromosome aneuploidy. Br J Cancer. 108:959–963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trenz K, Lugowski S, Jahrsdörfer U, Jainta
S, Vogel W and Speit G: Enhanced sensitivity of peripheral blood
lymphocytes from women carrying a BRCA1 mutation towards the
mutagenic effects of various cytostatics. Mutat Res. 544:279–288.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim MK, Zitzmann S, Westermann F, Arnold
K, Brouwers S, Schwab M and Savelyeva L: Increased rates of
spontaneous sister chromatid exchange in lymphocytes of
BRCA2+/− carriers of familial breast cancer clusters.
Cancer Lett. 210:85–94. 2004. View Article : Google Scholar : PubMed/NCBI
|