Introduction
Metastasis is the leading cause of cancer-related
mortality in almost all types of cancers, including colorectal
cancer (CRC). Although surgery, chemotherapy and radiotherapy have
long been the standards of CRC treatment, the long-term survival
and prognosis of patients remain quite poor due to tumor recurrence
and metastasis (1,2). Over 50% of patients with CRC present
with liver metastases or develop liver metastases (3). Thus, CRC remains a major global public
health issue.
Metastasis is a complex, multistep and dynamic
biological process, with epithelial-mesenchymal transition (EMT)
being a critical step during the cascade (4). EMT refers to the morphological and
molecular changes that occur when epithelial cells lose their
characteristics, gain mesenchymal properties and become motile,
which makes EMT a key event in tumor invasion and metastasis
(5–7). EMT could thus be a potential target
for CRC therapy.
Some complementary and alternative medical systems,
such as traditional Chinese medicine (TCM), have various approaches
to cancer treatment. TCM has the advantage of reducing cancer
therapy-induced toxicity (8). The
modified classic formula has been shown to further minimize the
side effects of surgery, radiation and chemotherapy. This formula
also increases immunity and improves survival (9–11).
Therefore, novel therapeutic strategies are essential to improve
the clinical management of patients with CRC.
Pien Tze Huang (PZH) was first prescribed by a royal
physician in the Ming Dynasty. PZH is a well-known traditional
Chinese formulation that is popularly known in Southeast Asia. The
main ingredients of PZH include Moschus, Calculus Bovis, Snake Gall
and Radix Notoginseng. These products ensure that PZH has heat
clearing, detoxification, blood circulation promotion, blood stasis
reduction and swelling reduction effects (12). Modern pharmacological studies have
proposed that PZH not only exhibits therapeutic effects on
hepatocellular carcinoma and CRC in clinical trials (13,14),
but also inhibits the growth of human colon carcinoma cells by
activating mitochondrion-dependent apoptosis (15). Moreover, PZH reportedly suppressed
the cell proliferation and tumor angiogenesis of CRC carcinoma
cells in vitro and in vivo (16,17).
To elucidate further the antitumor mechanism of action of PZH, we
evaluated its efficacy against tumor invasiveness in vivo
and in vitro and investigated its underlying molecular
mechanisms.
Materials and methods
Materials and methods
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin, and trypsin-EDTA were
purchased from Invitrogen (Carlsbad, CA, USA). E-cadherin,
N-cadherin, TGF-β, Smad2/3, P-Smad2/3, Smad4 and horseradish
peroxidase (HRP)-conjugated secondary antibodies were obtained from
Cell Signaling Technology (Beverly, MA, USA). All the other
chemicals used, unless otherwise indicated, were obtained from
Sigma Chemicals (St. Louis, MO, USA).
Preparation of PZH
PZH was obtained from and authenticated by the sole
manufacturer Zhangzhou Pien Tze Huang Pharmaceutical Co., Ltd.
(Zhangzhou, China; Chinese FDA, approval no. Z35020242). PZH stock
solution was prepared by dissolving PZH powder in
phosphate-buffered saline (PBS) to a concentration of 20 and 234
mg/ml before use. The PZH working solutions were prepared by
diluting the stock solution in either culture medium or saline.
Cell culture
Murine colon carcinoma CT-26 cells were obtained
from the Shanghai Cell Bank of the Chinese Academy of Sciences. The
cells were grown in DMEM containing 10% (v/v) FBS, 100 U/ml of
penicillin and 100 μg/ml of streptomycin in a 37°C humidified
incubator under 5% CO2. The cells were subcultured at 80
to 90% confluency.
Cell viability evaluation
Cell viability was assessed through an MTT
colorimetric assay. CT-26 cells were seeded into 96-well plates at
a density of 1×104 cells/well in 0.1 ml of medium. The
cells were treated with various concentrations of PZH for different
periods. At the end of the treatment, 100 μl of MTT (0.5 mg/ml in
PBS) was added to each well. The samples were then incubated for an
additional 4 h at 37°C. The purple-blue MTT formazan precipitate
was dissolved in 100 μl of DMSO. Absorbance was measured at 570 nm
using an ELISA reader (Model ELx800; BioTek, Winooski, VT,
USA).
Migration assay
The migration assay was performed using Transwell
cell culture chambers. Conditioned medium (500 μl of media with 10%
FBS) was added to the lower compartment of the chamber. CT-26 cells
(5×105) in 1% FBS-containing media were added to the
upper compartment of the chamber. After 24 h of incubation, the top
side of the insert membrane was scrubbed with a cotton swab,
whereas the bottom side was fixed with ice-cold methanol, stained
with 0.01% crystal violet and scored under a fluorescence
microscope (Olympus IX51 with DP70; Olympus, Center Valley, PA,
USA).
Matrigel invasion assay
The invasion assay was performed using Transwell
cell culture chambers (Corning Costar No. 3422; Corning, Tewksbury,
MA, USA) in accordance with the manufacturer’s instructions but
with specific modifications. Briefly, the upper surface of
polyvinylpyrrolidone-free polycarbonate filters (8.0-mm pore size;
Nuclepore Corp., Pleasanton, CA, USA) was pre-coated with 15 μl of
ice-cold Matrigel (BD Biosciences, Bedford, MA, USA) for 60 min at
room temperature. Conditioned medium (500 μl of medium with 10%
FBS) was added to the lower compartment of the chamber. CT-26 cells
(5×105) in 1% FBS-containing media were added to the
upper compartment of the chamber. After 24 h of incubation, the top
side of the membrane insert was scrubbed free of cells using a
cotton swab, whereas the bottom side was fixed with ice-cold
methanol, stained with 0.01% crystal violet and quantified.
Animals
Male BALB/c athymic (nude) mice and BALB/c mice
(with an initial body weight of 20 to 22 g) were obtained from
Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and
housed under pathogen-free conditions with controlled temperature
(22°C), humidity and a 12-h light/dark cycle. Food and water were
given ad libitum throughout the experiment. All the animal
treatments were performed in strict accordance with international
ethical guidelines and the National Institutes of Health Guide
Concerning the Care and the Use of Laboratory Animals. The
experiments were approved by the Institutional Animal Care and Use
Committee of Fujian University of Traditional Chinese Medicine.
CRC liver metastasis animal model
CT-26 cells were grown in culture and then detached
through trypsinization. Thereafter, the cells were washed and
resuspended in serum-free DMEM. Then, 1.5×106 of cells
mixed with Matrigel (1:1) were subcutaneously injected into the
right flank area of athymic nude mice, the growth of which was
monitored regularly. After 16 days of xenograft implantation,
tumors were selected for implantation to other nude mice using a
20-inoculating needle at an average size of 1 cm3. The
tumor tissues of 4–6 generations were chosen for orthotopic
transplantation into the cecum surface. Mice were anesthetized with
an i.p. injection of Nembutal (pentobarbital; Abbott Laboratories,
Abbott Park, IL, USA) at a dose of 70 mg/kg. After shaving, a small
incision was made through their skin over the cecum under a
dissecting microscope and by using microsurgical techniques. The
cecum was exteriorized through a small midline laparotomy, and a
piece of tumor tissue that was derived from CT-26 cells was sutured
to the cecal surface with a single Maxon 11/0 suture, which left
the tumor tissue buried in a ‘pouch’ that consisted of a double
cecal wall on each side. After implantation, the abdominal wall was
closed in two layers with Dexon 5/0. Food and water were given
ad libitum. A few days after the cecal implantation of the
colon tumor, the groups of mice were randomly assigned to receive
one of two treatments (n=6/group). The groups of mice were
intragastrically administered with a 234 mg/kg/day dose of either
PZH or saline daily. The animals were sacrificed 14 days after
tumor implantation. The tumor tissues were removed and weighed. A
portion of each tumor was fixed in 10% buffered formalin. The
remaining tissues were snap-frozen in liquid nitrogen and stored at
−80°C. We recorded all the macroscopic tumor deposits and
abnormalities in the liver. The number of liver metastases was
calculated. All neoplasms were identified through H&E
staining.
Histological examination by H&E
staining
The metastasized liver tissues were fixed with 10%
buffered formalin for 24 h. Samples were then paraffin-embedded,
sectioned and stained with H&E. Histopathological changes were
observed under a light microscope.
Immunohistochemistry
Immunohistochemical staining for E-cadherin,
N-cadherin, TGF-β, Smad2/3, P-Smad2/3 and Smad4 was performed as
follows. Briefly, after being fixed with 10% formaldehyde for 12 h,
tumor samples were processed for paraffin-embedded tumor slides.
The slides were subjected to antigen retrieval, and the endogenous
peroxidase activity was quenched with hydrogen peroxide. After
blocking nonspecific proteins with normal serum in PBS (0.1%
Tween-20), the slides were incubated with rabbit polyclonal
antibodies against E-cadherin, N-cadherin, Smad2/3, P-Smad2/3,
Smad4, TGF-β, ZEB1 and ZEB2 (all in 1:200 dilution). After being
washed with PBS, the slides were incubated with a biotinylated
secondary antibody followed by conjugated HRP-labeled streptavidin
(Dako) and then washed with PBS. The slides were then incubated
with diaminobenzidine (DAB, Sigma Chemicals) as the chromogen,
followed by counterstaining with diluted Harris’ hematoxylin (Sigma
Chemicals). After staining, five 5-power fields (400x) were
randomly selected for each slide. The average proportion of the
positive cells in each field was quantified using the True Color
Multi-Functional Cell Image Analysis Management System (Image-Pro
Plus; Media Cybernetics, Rockville, MD, USA). To rule out any
non-specific staining, PBS was used to replace the primary antibody
as a negative control.
Statistical analysis
All data are the means of 3 determinations and were
analyzed using SPSS Package for Windows (Version 11.5). Statistical
data analysis was performed using Student’s t-test and ANOVA.
Differences with P<0.05 were considered statistically
significant.
Results
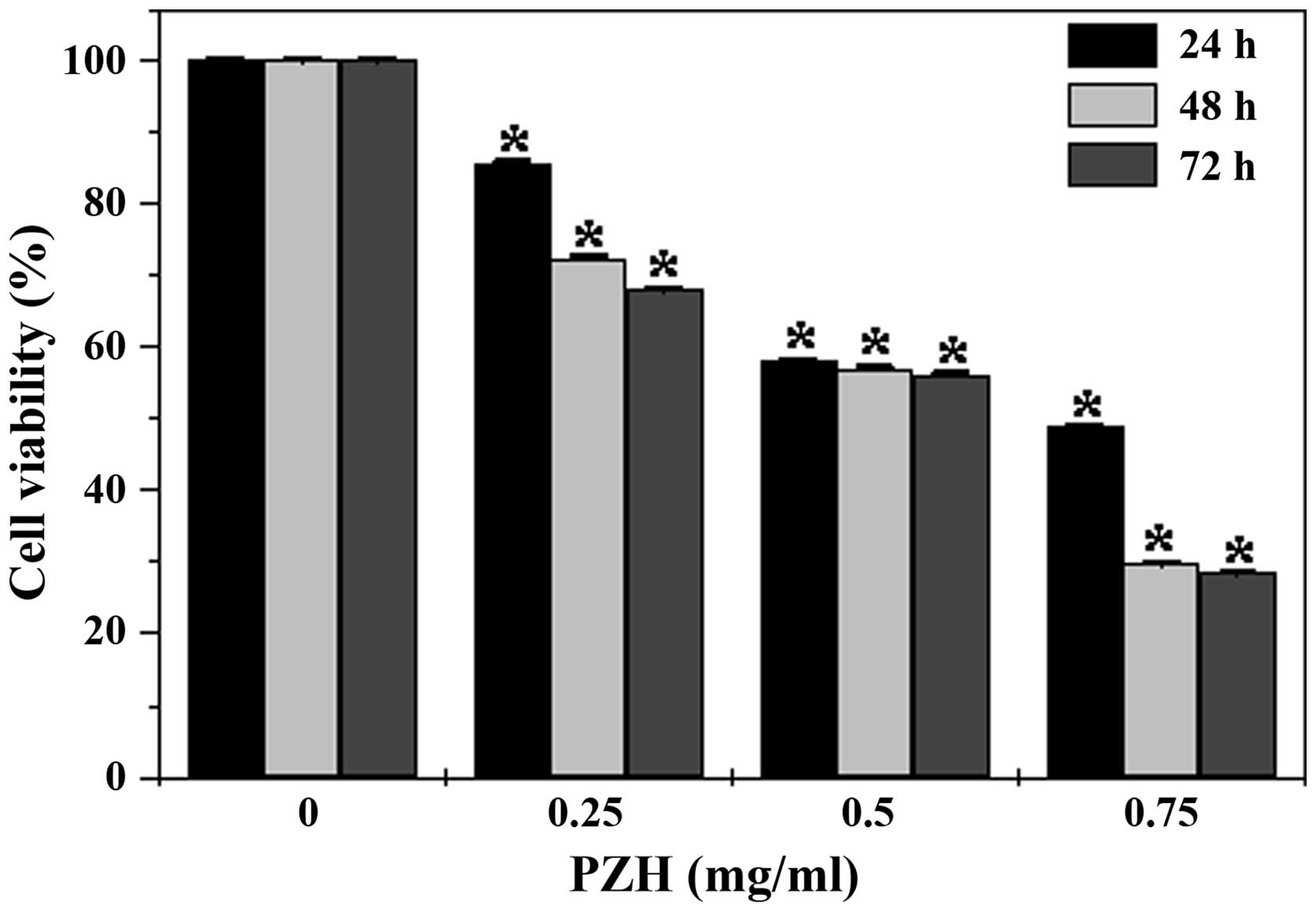
PZH inhibits the viability of CT-26
cells
The viability of the CT-26 cells was examined using
an MTT assay to compare the relative number of cells in the
PZH-treated monolayers with that in the untreated controls. As
shown in Fig. 1, treatment with
0.25 to 0.75 mg/ml of PZH for 24, 48 and 72 h, respectively reduced
cell viability by 14.3 to 51.1%, 27.4 to 60.3%, and 32.1 to 71.5%
compared with the untreated control cells (P<0.05). These data
indicate that PZH inhibited CT-26 cell growth and proliferation in
a dose- and time-dependent manner.
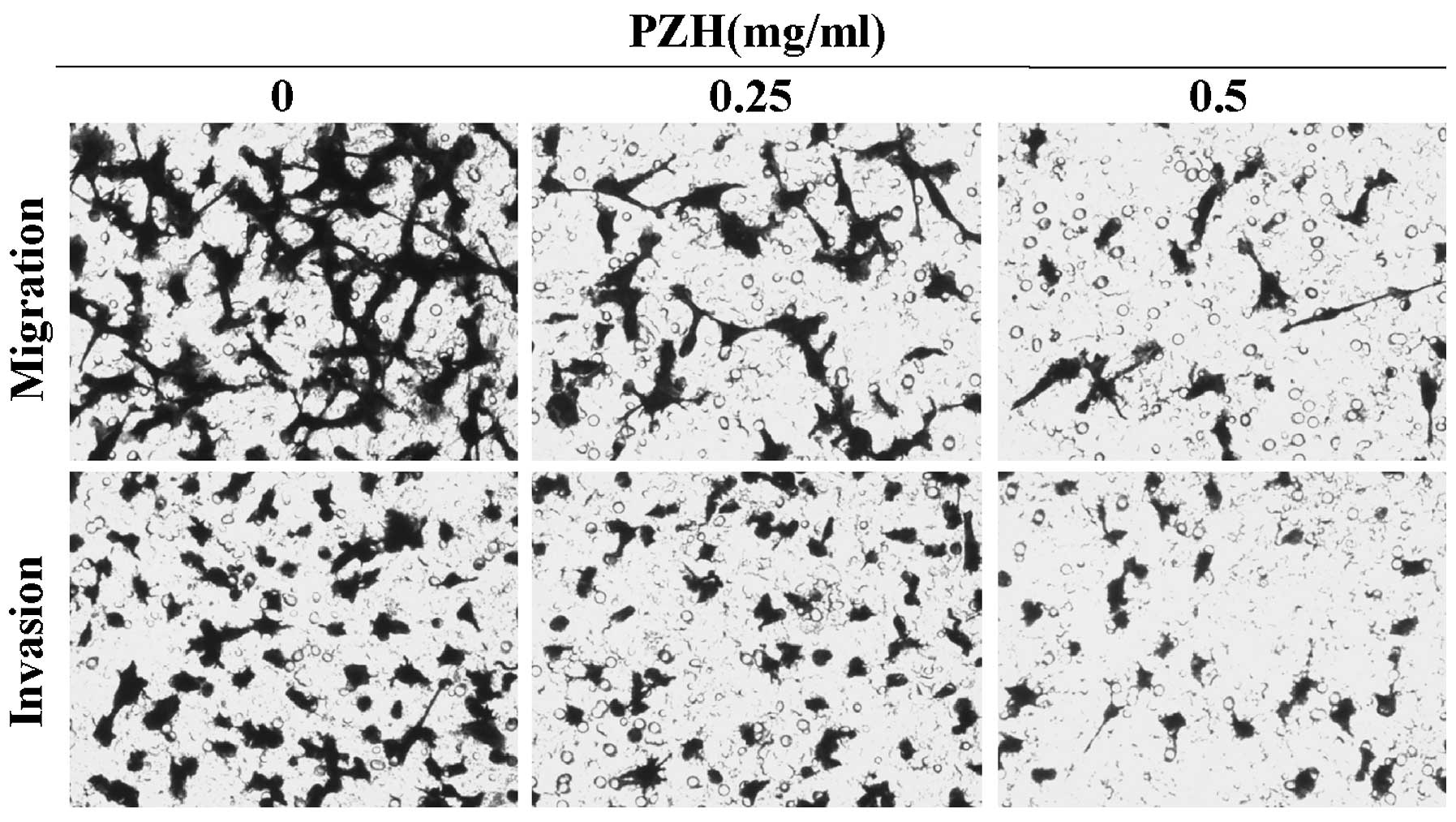
PZH inhibits migration and invasion of
CT-26 cells
To examine the effects of PZH on cell migration, we
performed Transwell migration assays with CT-26 cells. As
demonstrated in Fig. 2 (upper
panels), PZH effectively inhibited cell migration of CT-26 cells in
a dose-dependent manner. Furthermore, a Transwell invasion assay
was used to determine the invasive activity of the tumor cells
across the basement membrane (Fig.
2, lower panels). Overall, the results revealed that PZH
significantly decreased the invasive potential of CT-26 cells in a
dose-dependent manner.
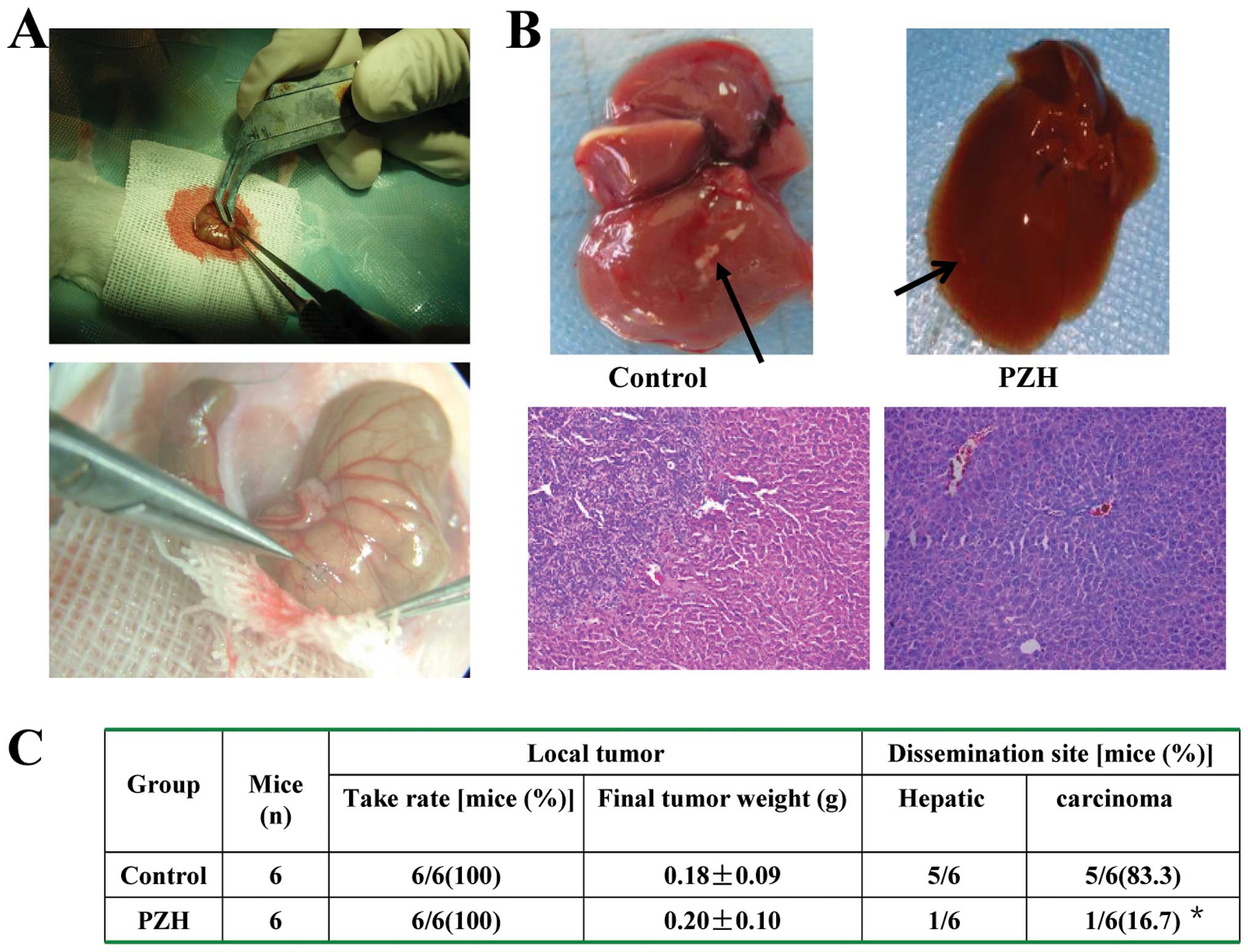
PZH inhibits tumor liver metastasis in
the orthotopic CRC mouse model
The antitumor effect on liver metastasis of PZH
in vivo was determined by examining the tumor weight and the
number of liver metastases in the orthotopic CRC mouse model. The
adverse effects were evaluated by measuring body weight changes. As
shown in Fig. 3A and B, PZH
treatment significantly reduced the number of tumor liver
metastases as compared with the control (P<0.01). After H&E
staining, histological changes in the liver samples of CRC mice
were observed under light microscopy. PZH treatment was observed to
have no effect on the changes in the tumor and body weights of the
mice (Fig. 3C). Thus, PZH
effectively suppressed CRC liver metastasis in vivo, but did
not significantly affect CRC growth and caused no apparent signs of
toxicity.
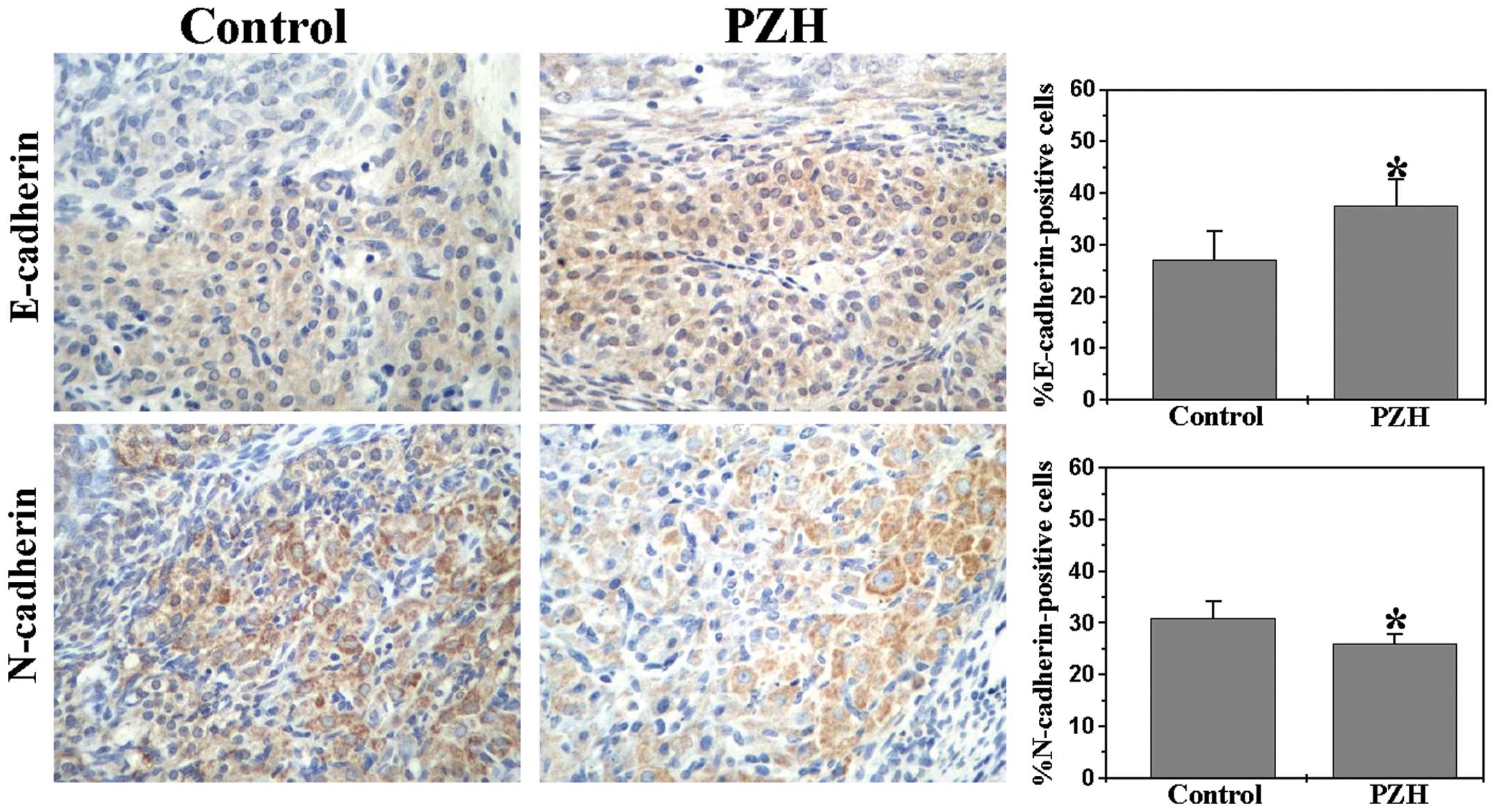
PZH inhibits EMT in the orthotopic CRC
mouse model
E-cadherin and N-cadherin, which are markers of EMT,
not only mediate cell migration and invasion properties, but also
contribute to metastasis (18,19).
Therefore, we further investigated E-cadherin, N-cadherin, and ZEB1
expression to explore further the anti-metastatic activity of PZH.
We performed immunohistochemical staining (IHS) analyses to examine
the protein expression of both E-cadherin and N-cadherin in the
orthotopic CRC mouse model. As shown in Fig. 4, the percentages of
E-cadherin-positive cells in the PZH-treated and control mice were
37.50±5.16 and 27.17±5.42% (P<0.01), respectively. By contrast,
the proportion of cells expressing N-cadherin was 26.01±1.78% in
the PZH-treated mice and 30.83±3.43% in the controls. These data
collectively suggest that the antitumor liver metastasis function
of PZH may be attributed to the inhibition of EMT of CRC cells.
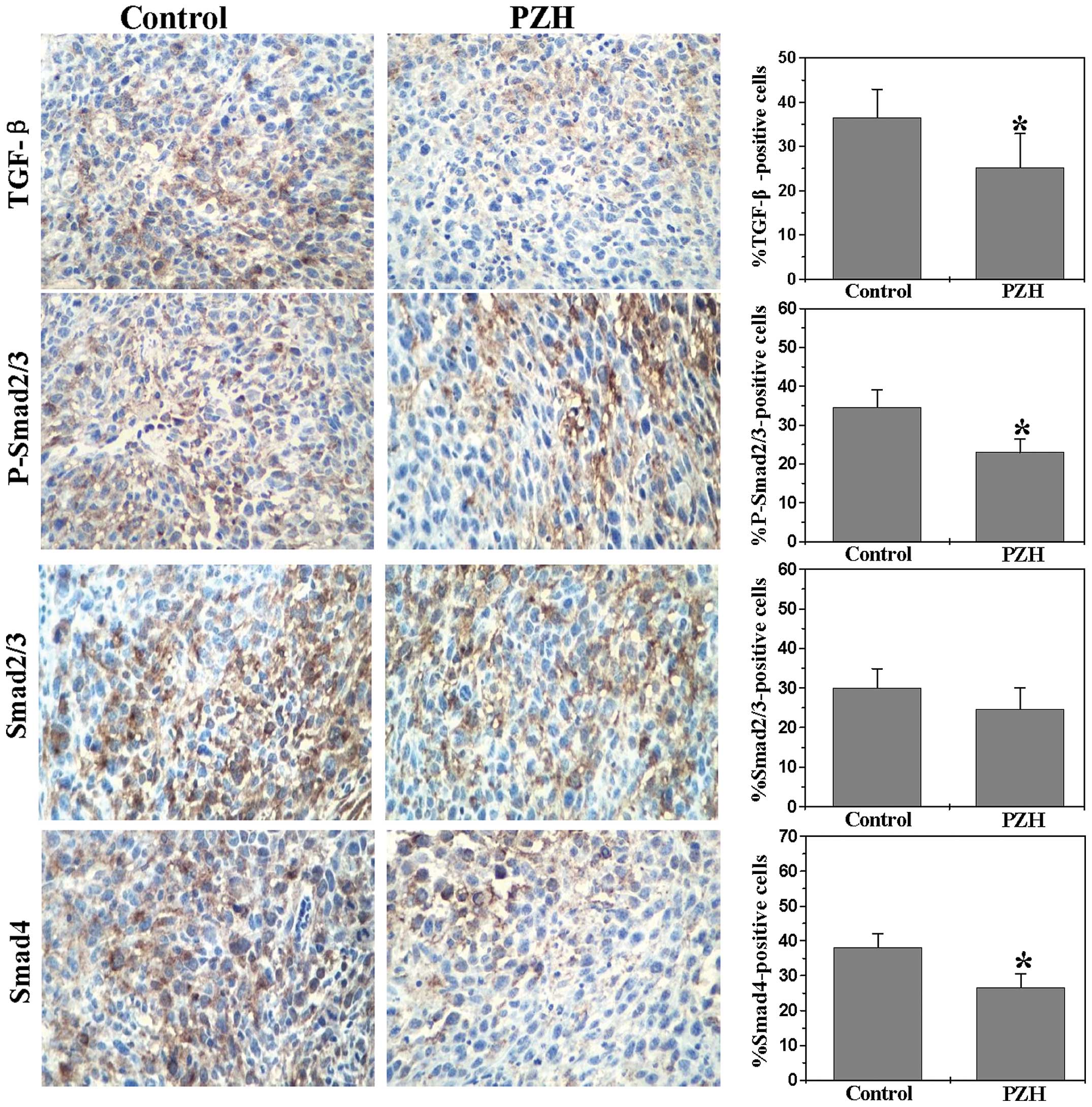
PZH inhibits activation of the TGF-β
pathway in the orthotopic CRC mouse model
Numerous pathways have been implicated in colorectal
carcinogenesis. Among these pathways is the TGF-β/Smad signaling
pathway, which is responsible for TGF-β-mediated cell growth
inhibition and apoptosis. TGF-β induces angiogenesis, inflammation
and EMT, thus providing a beneficial environment for tumor
progression and metastasis (20).
Therefore, we assessed the effect of PZH on the expression of key
mediators of the TGF-β/Smad pathway using IHS analyses. As shown in
Fig. 5, PZH treatment significantly
reduced the protein expression of TGF-β, P-Smad2/Smad3,
Smad2/Smad3, and Smad4 in the tumor tissues. The percentages of
TGF-β-, P-Smad2/Smad3-, Smad2/Smad3- and Smad4-positive cells in
the control group were 36.5±6.41, 34.5±4.55, 30.0±4.63 and
38.17±3.68%, respectively. By contrast, the percentages of TGF-β-,
P-Smad2/Smad3-, Smad2/Smad3- and Smad4-positive cells in the tumor
tissue of PZH-treated mice were 25.2±7.69, 23.0±3.40, 24.67±5.32
and 26.67±3.82%, respectively (P<0.01). These data suggest that
the in vitro inhibitory effect of PZH on tumor metastasis
could be mediated by the suppression of the TGF-β/Smad pathway.
Discussion
The occurrence of metastases due to tumor
progression causes the vast majority of cancer-related deaths.
Metastatic progression is a multi-step process that includes the
detachment of cancer cells from the primary tumor mass, migration
and invasion, thus enabling the re-establishment of malignant cells
at distant sites. TGF-β is a multifunctional cytokine and is a
potent inducer of EMT (21,22). Moreover, TGF-β can serve as a tumor
suppressor by inhibiting cell proliferation in the early stages of
tumor development while promoting metastasis in various cancer
models (23,24). CRC is associated with deregulated
levels of TGF-β, which predicts poor prognosis and limited
recurrence-free survival. A growing number of in vivo
studies have recently shown that the inhibition of TGF-β signaling
and transcription reduces the metastatic and/or invasive properties
of various experimental cancers, including CRC, presumably by
preventing the induction of migration and invasion in cancer cells
(25–27).
The function of the TGF-β signaling pathway depends
on the binding of ligands to cell membrane receptors, activating
cytoplasm mediators into the nucleus, and regulating expression of
their target gene. The Smad pathway is a major transducer of TGF-β
signaling (28). Smad2 and Smad3,
which are known as receptor-regulated Smads, are both
phosphorylated by the type 1 TGF-β receptor and form complexes with
Smad4 (29). These complexes
accumulate in the nucleus of the cell and regulate the
transcription of target genes, thus serving critical functions in
controlling cell proliferation, differentiation, apoptosis and cell
migration.
E-cadherin and N-cadherin are target genes of the
TGF-β signaling pathway. These genes are important for controlling
cell migration. The malignancy of carcinoma cells is characterized
by the loss of both cell-to-cell adhesion and cellular
differentiation, which have been repeatedly reported to correlate
with E-cadherin downregulation and N-cadherin upregulation
(30,31). Dysfunction or loss of E-cadherin and
increase in N-cadherin could be attributed to somatic mutations in
some tumor types, which include CRC and are associated with the
development of invasive carcinoma, metastatic dissemination and
poor clinical prognosis. Therefore, the deregulation of E-cadherin
and the upregulation of N-cadherin expression may contribute to
tumorigenesis.
In conclusion, the present study is the first to
demonstrate that PZH inhibits the invasiveness of CRC by
suppressing the TGF-β/Smad pathway, by promoting the expression of
E-cadherin and by suppressing the expression of N-cadherin. Our
findings suggest that PZH may be a potential novel therapeutic
agent for the treatment of cancers owing to its inhibition of CRC
metastasis.
Acknowledgements
We acknowledge- Projects 81373819 and 81202790
supported by the National Natural Science Foundation of China and
Project 2014J01359 by the Natural Science Foundation of Fujian.
Abbreviations:
|
CRC
|
colorectal cancer
|
|
PZH
|
Pien Tze Huang
|
|
EMT
|
epithelial-mesenchymal transition
|
|
TCM
|
traditional Chinese medicine
|
|
TGF-β
|
transforming growth factor-β
|
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gansler T, Ganz PA, Grant M, et al: Sixty
years of CA: a cancer journal for clinicians. CA Cancer J Clin.
60:345–350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Thomas A, Murray T and Thun M:
Cancer statistics. CA Cancer J Clin. 52:23–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klymkowsky MW and Savagner P:
Epithelial-mesenchymal transition: a cancer researcher’s conceptual
friend and foe. Am J Pathol. 174:1588–1593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moustakas A and Heldin CH: Signaling
networks guiding epithelial-mesenchymal transitions during
embryogenesis and cancer progression. Cancer Sci. 98:1512–1520.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shinto O, Yashiro M, Kawajiri H, et al:
Inhibitory effect of a TGFbeta receptor type-I inhibitor, Ki26894,
on invasiveness of scirrhous gastric cancer cells. Br J Cancer.
102:844–851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boose G and Stopper H: Genotoxicity of
several clinically used topoisomerase II inhibitors. Toxicol Lett.
116:7–16. 2000. View Article : Google Scholar
|
|
10
|
Newman D, Cragg G and Snader K: The
influence of natural products upon drug discovery. Nat Prod Rep.
17:215–234. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carmady B and Smith CA: Use of Chinese
medicine by cancer patients: a review of surveys. Chin Med.
9:222011. View Article : Google Scholar
|
|
12
|
Chinese Pharmacopoeia Commission, .
Pharmacopoeia of the People’s Republic of China. 1. Chinese Medical
Science and Technology Press; Beijing: pp. 573–575. 2010
|
|
13
|
Xu YY and Yu EX: Clinical analysis of the
effect of Pien Tze Huang in treatment of 42 patients with moderate
or advanced liver cancer. Shanghai J Tradit Chin Med. 12:4–5.
1994.
|
|
14
|
Gu ZX: Therapeutical observation of
advanced colon cancer. Chin Tradit Patent Med. 15:231993.
|
|
15
|
Lin JM, Wei LH, Chen YQ, Liu XX, Hong ZF,
Sferra TJ and Peng J: Pien Tze Huang induced apoptosis in human
colon cancer HT-29 cells is associated with regulation of the Bcl-2
family and activation of caspase 3. Chin J Integr Med. 17:685–690.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhuang QC, Hong F, Shen AL, et al: Pien
Tze Huang inhibits tumor cell proliferation and promotes apoptosis
via suppressing the STAT3 pathway in colorectal cancer mouse model.
Int J Oncol. 40:1569–1574. 2012.PubMed/NCBI
|
|
17
|
Shen AL, Hong F, Liu LY, Lin JM, Zhuang
QC, Hong ZF and Peng J: Effects of Pien Tze Huang on angiogenesis
in vivo and in vitro. Chin J Integr Med. 18:431–436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gumbiner BM: Regulation of
cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol.
6:622–634. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Elliott RL and Blobe GC: Role of
transforming growth factor Beta in human cancer. J Clin Oncol.
23:2078–2093. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Massagué J: TGFbeta signaling: receptors,
transducers, and Mad proteins. Cell. 85:947–950. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Caestecker MP, Piek E and Roberts AB:
Role of transforming growth factor-beta signaling in cancer. J Natl
Cancer Inst. 92:1388–1402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Massagué J, Blain SW and Lo RS: TGFbeta
signaling in growth control, cancer, and heritable disorders. Cell.
103:295–309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Sergina N, Ko TC, Gong J and
Brattain MG: Autocrine and exogenous transforming growth factor
beta control cell cycle inhibition through pathways with different
sensitivity. J Biol Chem. 279:40237–40244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oft M, Heider KH and Beug H: TGF-beta
signaling is necessary for carcinoma cell invasiveness and
metastasis. Curr Biol. 8:1243–1252. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Sun L, Myeroff L, et al:
Demonstration that mutation of the type II transforming growth
factor beta receptor inactivates its tumor suppressor activity in
replication error-positive colon carcinoma cells. J Biol Chem.
270:22044–22049. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Han W, Zborowska E, et al: Reduced
expression of transforming growth factor beta type I receptor
contributes to the malignancy of human colon carcinoma cells. J
Biol Chem. 271:17366–17371. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fukushima T, Mashiko M, Takita K, et al:
Mutational analysis of TGF-beta type II receptor, Smad2, Smad3,
Smad4, Smad6 and Smad7 genes in colorectal cancer. J Exp Clin
Cancer Res. 22:315–320. 2003.PubMed/NCBI
|
|
29
|
Miyaki M, Iijima T, Konishi M, et al:
Higher frequency of Smad4 gene mutation in human colorectal cancer
with distant metastasis. Oncogene. 18:3098–3103. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van Roy F and Berx G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miyoshi J and Takai Y: Structural and
functional associations of apical junctions with cytoskeleton.
Biochim Biophys Acta. 1778:670–691. 2008. View Article : Google Scholar : PubMed/NCBI
|