Introduction
Malignant peripheral nerve sheath tumor (MPNST),
typically arising from Schwann cells of peripheral nerve sheaths
(1–3), is known as the most aggressive
peripheral nerve malignant tumors. MPNST is the main soft tissue
malignancy associated with neurofibromatosis type 1 (NF1) (4). It has been reported that ~80% of
MPNSTs are pathologically indicated as high-grade tumors (2). Furthermore, studies have shown that
20–50% of patients with MPNST also have NF1 and ~10% of patients
with NF1 will finally suffer from MPNST (1,4,5).
Traditional treatment methods including surgical resection,
chemotherapy and radiotherapy can not obtain an ideal curative
effect mainly due to the aggressive growth and metastasis of MPNST.
Therefore, it is urgent to clarify the underlying molecular
mechanism of MPNST for developing new molecular therapeutic
tools.
Eph receptors represent the largest family of
receptor tyrosine kinases (RTKs), which are capable of recognizing
signals from the cell environment and influencing cell-cell
interaction and cell migration (6–8).
Ephrins are the ligands to Eph receptors and they stimulate
bi-directional signaling of the Eph-ephrin axis. Ephrin-A3 (EFNA3)
is one of the ephrin ligands which could bind to EphA2, EphA3,
EphA5, EphA7, EphA8 and more poorly to EphA4. It is not only
expressed in skeletal muscle, spleen, thymus, prostate, testis,
ovary, small intestine and peripheral blood leukocytes, but is also
present in neuroblastomas, neural cancers and leukemias. The
dysregulated expression of EFNA3 has been observed in many types of
human cancer. The expression level of EFNA3 was found to be
upregulated 26-fold in squamous cell lung carcinoma, 3.8-fold in
liver cancer, 1.6-fold in colon cancer and downregulated 2.6-fold
in kidney carcinoma (9–12). Our previous study also showed that
mRNA expression levels of EFNA3 were significantly decreased in
MPNST cell lines (1). However, the
molecular mechanism by which EFNA3 mediates MPNST cells is still
unknown.
MicroRNAs (miRs), a kind of endogenous non-coding
RNAs, can serve as endogenous agents for RNA interference. Growing
evidence indicates that miRs deregulation is closely related to
certain pathological processes including tumorigenesis. miRs can
act as tumor suppressors or oncogenes depending on their targets
(13). Among these functional miRs,
miR-210 is frequently upregulated in various types of cancer, such
as glioblastoma (14), clear cell
renal cell carcinoma (15), lung
(16) and breast cancer (17,18).
miR-210 may play an oncogenic role in cancer initiation and
progression via regulating cellular growth, apoptosis, migration
and invasion (19,20). In MPNST, Presneau et al
identified 16 significantly differentially expressed miRs in MPNST
relatively to neurofibromas. Of these, miR-210 was identified with
increased expression (21). In
addition, our previous study (1)
indicated that miR-210 reduced the expression of its target gene
EFNA3 and stimulated growth and invasion of MPNST. Accordingly,
EFNA3 plays a role in MPNST progression and miR-210 acts as an
oncogene. However, the relationship between miR-210 and EFNA3 in
MPNST cells requires further investigation.
Overexpression and knockout of a specific gene are
the crucial strategies for gene function study. Transfection
strategies, which along with plasmid or lentiviral vectors, are
known as the powerful methods for overexpression of a specific
gene. Various technical tools have been developed to probe the
functions of genes, yet their application has been limited by low
efficacy and specificity (22).
Recently, transcription activator-like effector nucleases (TALENs)
emerged as a novel promising tool for gene function analysis.
TALENs are artificial restriction enzymes generated by fusing a TAL
effector DNA binding domain to a DNA cleavage domain. Transcription
activator-like effectors (TALEs) can be quickly engineered to bind
practically any desired DNA sequence (23). When these restriction enzymes are
introduced into cells, they can be used for gene knockout. In the
present study, TALENs and lentiviral transfection strategies were
applied to dissect the roles of EFNA3 in MPNST cells and to reveal
correlation between EFNA3 and miR-210. The present study may
facilitate better understanding of MPNST pathogenesis and the
development of potential therapeutic targets for MPNST.
Materials and methods
Cell culture
MPNST ST88-14 (NF1 wild-type) and sNF96.2 (NF1
mutant type) cell lines were purchased from the China Center for
Type Culture Collection (CCTCC; Wuhan, China). All the cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented
with 10% fetal bovine serum (FBS; Life Technologies, Carlsbad, CA,
USA), 100 IU/ml penicillin and 100 µg/ml streptomycin
sulfate at 37°C in a humidified incubator containing 5%
CO2.
Antibodies
Antibodies of EFNA3 and β-actin were obtained from
Santa Cruz Biotechnology (Dallas, TX, USA). Antibodies of focal
adhesion kinase (FAK), phosphorylation FAK (p-FAK), PI3K,
integrins, TNF-α and HIF-1α were purchased from ImmunoWay
Biotechnology (Newark, DE, USA). Antibodies of GTPase and VEGF were
purchased from Abzoom (Dallas, TX, USA).
Real-time RT-PCR
Total RNA was extracted from cells (MPNST cell line
ST88-14, T265p21, sNF96.2, YST-1 and MPNST-14 cells were purchased
from CCTCC or Schwann cells separated from human NF1 neurofibroma
tissues) with TRIzol reagent (Life Technologies) following the
manufacturer’s instructions. The expression of EFNA3 mRNA was
detected by real-time RT-PCR using the standard SYBR-Green RT-PCR
kit (Takara, Otsu, Japan) following the manufacturer’s
instructions. The specific primer pairs are as follows: EFNA3
sense, 5′-CTTGTGGCTCTGGTAATGTTTGG-3′ and anti-sense,
5′-GAGGAGGACGTGCTTATTGCTGT-3′; β-actin as an internal sense,
5′-AGGGGCCGGACTCGTCATACT-3′ and antisense,
5′-GGCGGCACCACCATGTACCCT-3′. The relative expression of the gene
mRNA was quantified using the GraphPad Prism 4.0 software (GraphPad
Software, San Diego, CA, USA) and the 2−ΔΔCt method
(24).
Design and construction of TALENs
TAL effector DNA binding domains were designed and
constructed based on TAL Effector Nucleotide Targeter 2.0 (25). The sites: gggaaaccggcatgcggt (left)
and ccccgactcactgctggt (right) were chosen. According to Sanjana
et al (26), the binding
pairs EFNA3-L and EFNA3-R were, respectively, assembled into the
pTALEN-v2-L and pTALEN-v2-R backbones, yielding pTALEN-EFNA3-L and
pTALEN-EFNA3-R.ST88-14 and sNF96.2 cells were transfected with a
mixture of pTALEN-EFNA3-L, pTALEN-EFNA3-R (untreated cells were
used as a control). Cells were trypsinized and resuspended after
transfection for 4 days. The transfected cells were expanded. To
confirm the disruption of EFNA3, western blotting and PCR were
performed. Whole cell extracts were analyzed by western blotting,
and the targeted exon was PCR-amplified from genomic DNA isolated
from individual clones. The following day, the medium was refreshed
and grown for an additional 24 h prior to harvesting for further
analysis.
Lentiviral transfection
The Lv-EFNA3 and Lv-NC lentiviral suspension was
purchased from GeneChem (Shanghai, China). The titer of the
lentiviral vectors was 2×1010 titer units (TU)/ml. The
ST88-14 and sNF96.2 cells were plated and cultured in 6-well plates
until cell fusion reached 60–70%. Then, 2.5×104 TU/well
Lv-EFNA3 or Lv-NC lentivirus was added to the cells under MOI
values of 50. To confirm the effect of the lentivirus on the
expression of EFNA3 gene, PCR and western blotting was performed to
determinate the mRNA and protein levels of EFNA3 in the ST88-14 and
sNF96.2 cells after infection with lentivirus for 6 days. The
transfected cells were expanded and harvested for further
analysis.
Cell viability assay
MPNST ST88-14 and sNF96.2 cells transfected with
pTALEN-EFNA3-L, pTALEN-EFNA3-R and pre-miR-210 or pre-miR-210 in
exponential growth were plated at a final concentration of
2×103 cells/well in 96-well plates. The viability of the
cells was evaluated by an MTT assay after 24, 48, 72 and 96 h of
seeding. The optical density at 570 nm (OD570) of each well was
measured with an ELISA reader (ELX-800 type; BioTek, Winooski, VT,
USA).
Cell invasion assay
The cell invasion assay was performed using a Cell
Invasion Assay kit (Chemicon International, Temecula, CA, USA)
according to the manufacturer’s guidelines. Briefly, ST88-14 and
sNF96.2 cells transfected with pTALEN-EFNA3-L and pTALEN-EFNA3-R or
pre-miR-210 or pTALEN-EFNA3-L, pTALEN-EFNA3-R and pre-miR-210 and
their corresponding negative control was placed in the upper
compartment of the chambers, and DMEM containing 10% FBS was added
in the lower chambers. After 24 h of incubation at 37°C, cells on
the upper face of the membrane were scraped using a cotton swab and
cells on the lower face were fixed, stained and observed under a
microscope. Then the dye on the membrane was dissolved with 10%
acetic acid, dispensed into 96-well plates (150 µl/well),
and the optical density at 570 nm (OD570) of each well was measured
with an ELISA reader (ELX-800 type).
Cell adhesion assay
Adhesion was assayed by plating cells in DMEM on
96-well plates pre-coated with bovine serum albumin (BSA) as a
control or 20 µg/ml fibronectin (FN) (both from Life
Technologies), respectively. Cells were pre-treated overnight with
MPNST cells and were allowed to adhere for 2 h. Wells were washed,
fixed with 4% paraformaldehyde and stained with crystal violet
(Life Technologies). Adhered cells were counted under a microscope
(AE31 type; Motic, HK, China) in five fields.
Western blotting
Cells were lysed in cell lysate, and then
centrifuged at 12,000 × g for 20 min at 4°C. The supernatant was
collected and denatured. Proteins were separated in 10% SDS-PAGE
and blotted onto polyvinylidene difluoride membrane (PVDF). The
PVDF membrane was treated with TBST containing 50 g/l skimmed milk
at room temperature for 4 h, followed by incubation with the
primary antibodies of EFNA3, FAK, p-FAK, PI3K, GTPase, integrins,
VEGF, TNF-α, HIF-1α and β-actin, respectively, at 37°C for 1 h.
Membranes were rinsed and incubated for 1 h with the correspondent
peroxidase-conjugated secondary antibodies. Chemiluminent detection
was performed with the ECL kit (Pierce Chemical Co., Rockford, IL,
USA).
Statistical analysis
Data are expressed as mean ± SD from at least three
separate experiments. Statistical analysis was carried out using
SPSS 15.0 software. The difference between the two groups was
analyzed by the Student’s t-test. A value of P<0.05 was
considered to indicate a statistically significant result.
Results
Knocked out or overexpressed EFNA3 in
ST88-14 and sNF96.2 cells
In the present study, ST88-14 and sNF96.2 cells were
selected to investigate the functions of EFNA3 in MPNST. TALENs are
emerging as a new powerful technique in the field of targeted
genome engineering. TALENs were applied for knockout of EFNA3 gene
in MPNST cells. After transfection with pTALEN-EFNA3-L and
pTALEN-EFNA3-R, the expression levels of EFNA3 mRNA and protein in
ST88-14 and sNF96.2 cells were analyzed by RT-PCR and western
blotting. The expression of EFNA3 mRNA and protein were detected
(Fig. 1A and B). These results
suggested that EFNA3 gene was effectively knocked out by
EFNA3-TALENs in ST88-14 and sNF96.2 cells.
EFNA3 was also overexpressed by lentiviral
transfection. After transfection with lentiviral recombinant
vectors, RT-PCR and western blotting were performed to analyze the
expression of EFNA3 mRNA and protein. As shown in Fig. 1C and D, the expression of EFNA3 mRNA
and protein increased significantly in transfected LV-EFNA3 cells
compared with transfected LV-NC vector and control cells. The above
indicated that EFNA3 gene was overexpressed effectively by the
LV-EFNA3 transfected in ST88-14 and sNF96.2 cells.
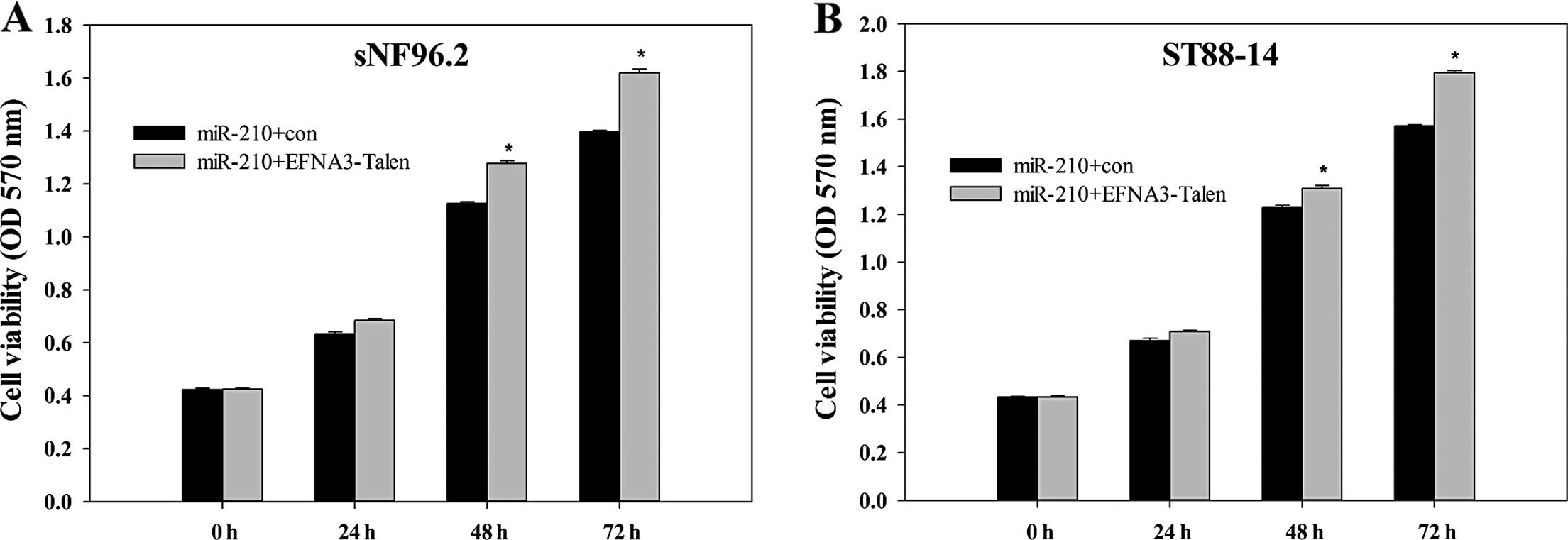
Effect of EFNA3 on the viability of MPNST
cells
MTT assay, generally applied to detect the viability
of the cellse, was employed to investigate the effect of EFNA3 on
the viability of ST88-14 and sNF96.2 cells. MPNST cells, with the
EFNA3 gene present or knocked out, was transfected with
pre-miR-210. Subsequently, the cell viabilities were evaluated
every 24 h for 3 days. As shown in Fig.
2A and B, although the MPNST cells were all transfected with
pre-miR-210, the viabilities still increased in ST88-14 and sNF96.2
cells when EFNA3 was knocked out. This suggested that the knockout
of EFNA3 promoted the viability of the MPNST cells.
Effect of EFNA3 on invasiveness of MPNST
cells
The rising invasiveness is an important feature of
malignant tumors. The effects of EFNA3 on invasiveness of MPNST
ST88-14 and sNF96.2 cells were examined by a Transwell assay. The
results showed that knockout of EFNA3 by introduction of
pTALEN-EFNA3-L and pTALEN-EFNA3-R, or overexpression of miR-210 by
introduction of pre-miR-210, both strongly increased the
invasiveness of ST88-14 and sNF96.2 cells (Fig. 3A and B). It was also noted that the
overexpression of miR-210 had more power in increasing the
invasiveness of the MPNST cells than that of the knockout of EFNA3.
In addition, the MPNST cells with both knockout EFNA3 and
overexpression of miR-210 increased the invasiveness of the cells
as well. As presented in Fig. 3,
the enhancement degree of invasiveness in MPNST cells with both the
knocked out EFNA3 and overexpressed miR-210 were greater than that
of EFNA3 knockout MPNST cells, but weaker than that of miR-210
overexpressed MPNST cells, suggesting that EFNA3 had a negative
effect on the invasiveness of the MPNST cells. Furthermore, EFNA3
may not be the only target gene of miR-210.
Effect of EFNA3 on adhesion of MPNST
The adhesion capability of cancer cells is closely
related with metastasis. To investigate the effect of EFNA3 on
adhesion of MPNST cells, EFNA3 in ST88-14 and sNF96.2 cells was
knocked out by TALENs that also overexpressed EFNA3 through
Lv-EFNA3 transfection. Our data showed that the adhesion capability
of MPNST cells was inhibited after knocking out EFNA3, while
overexpression of EFNA3 enhanced the adhesion capability of both
ST88-14 and sNF96.2 cells (Fig.
4A–D). These findings suggest that EFNA3 inhibits MPNST
metastasis by promoting the adhesion of MPNST cells.
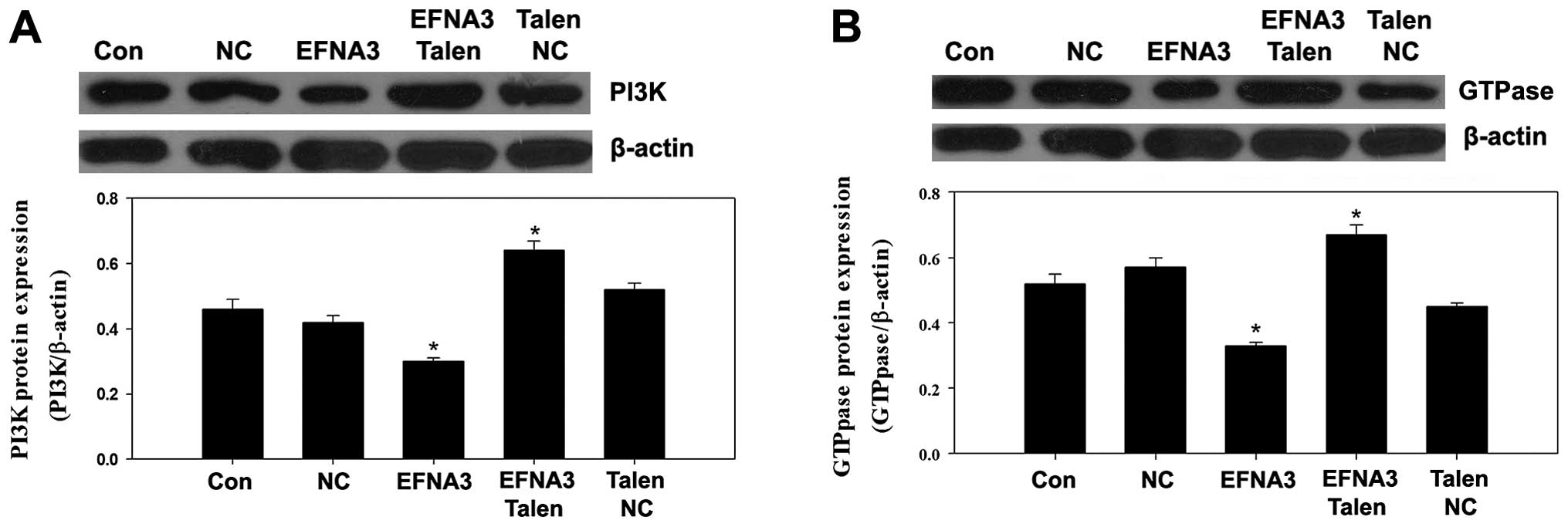
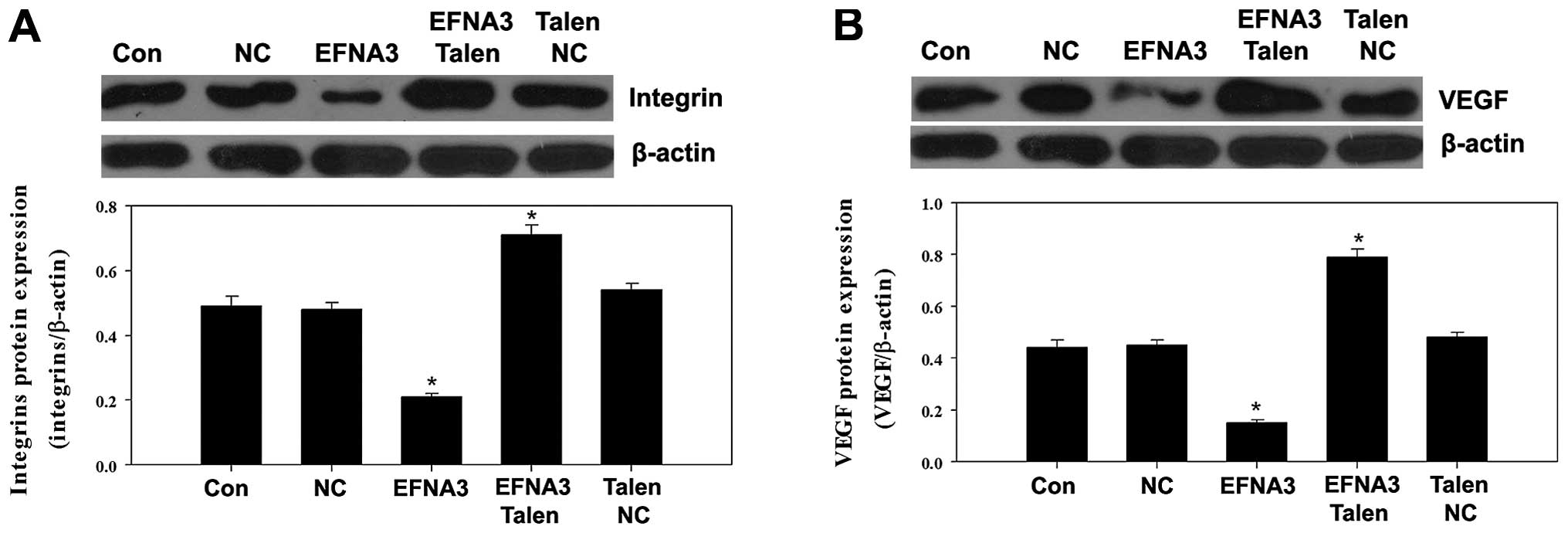
Molecular mechanism underlying the role
of EFNA3 in tumor angiogenesis
To explore the potential molecular mechanisms
underlying EFNA3-induced tumor angiogenesis, the expression of some
protein members of FAK signaling pathway including FAK, p-FAK,
phosphatidylinositol 3-kinase (PI3K), GTPase as well as integrins
and angiogenic factors including vascular endothelial growth factor
(VEGF), tumor necrosis factor α (TNF-α) and hypoxia-inducible
factor 1α (HIF-α) were determined by western blotting. We found
that knockout of EFNA3 notably decreased the protein expression of
p-FAK and TNF-α compared to the control groups (Fig. 5A and B), while the protein
expression levels of PI3K, GTPase, integrins, VEGF and HIF-α were
significantly increased (Figs.
6Figure 7–8). All these changes were beneficial to
the progression of the tumor. On the contrary, overexpression of
EFNA3 significantly upregulated the protein expression of p-FAK and
TNF-α compared to the control groups (Fig. 5A and B), yet the protein levels of
PI3K, GTPase, integrins, VEGF and HIF-α were notably reduced
(Figs. 6Figure 7–8). These data indicated that knockout and
overexpression of EFNA3 improved and inhibited progression of MPNST
cells, respectively. These data suggest that EFNA3 may function as
a tumor suppressor in MPNST.
Discussion
Ephrin ligands and their Eph receptors have been
proven to play a crucial role in mediating a wide range of
biological activities, such as angiogenesis, cell segregation, cell
adhesion, shape and motility. As several of these processes are
known to go awry during tumorigenesis and metastasis, Eph/ephrin
signaling has been identified to play a role in many human cancers,
such as lung, breast and prostate cancers, as well as melanoma and
leukemia (9). Ephrin-A3 (EFNA3) is
a GPI-anchored membrane protein and is widely expressed in human
organisms, such as skeletal muscle, spleen, thymus, prostate,
testis, and ovary (27). EFNA3 has
been proven to play an important role in the guidance of various
types of axons in the developing nervous system (28–30)
and in the control of dendritic spine morphology (31). EFNA3 has also been proposed to be
associated with some cancers. Iiizumi et al found that EFNA3
facilitated the growth of pancreatic cancer cells (32). Georgiou et al indicated that
EFNA3 served as an angiogenesis-specific gene and its expression
was upregulated in patients with breast cancer (33). Our previous study indicated that
EFNA3 may play a part in the process of miR-210 promotion of growth
and invasion of MPNST (1).
Subsequently, the functions of EFNA3 in MPNST were investigated in
this study.
In the present study, we investigated the function
of EFNA3 by gain- and loss-of-function strategies. EFNA3-TALENs and
Lv-EFNA3 were transfected into MPNST cells to knock out and
overexpress EFNA3, respectively. The EFNA3 mRNA and protein levels
were determined by RT-PCR and western blotting, and the results
indicated that EFNA3 gene in ST88-14 and sNF96.2 cells were
effectively knocked out by EFNA3-TALENs suggesting the promising
application of TALENs in cancer associated gene function study.
We further investigated the performance of ST88-14
and sNF96.2 cells with EFNA3 present or knockout, on cell
viability. The results suggested that knockout of EFNA3
significantly promoted the viability of MPNST cells even when
miR-210 was pre-upregulated. Notably, our previous study showed
that miR-210 promotes viability and proliferation of MPNST cells
through negative regulation of EFNA3 (1). In the present study, although the
MPNST cells were all transfected with pre-miR-210, the viabilities
still increased in ST88-14 and sNF96.2 cells when EFNA3 was further
knocked out, implying that EFNA3 interfered in the viability of
MPNST cells more directly than miR-210. Subsequently, the
invasiveness of ST88-14 and sNF96.2 cells transfected with
EFNA3-TALENs or pre-miR-210 or both recombinant vectors were
determined. Although the invasiveness of all transfected MPNST cell
lines was increased, the enhancement degree was different in each.
The enhancement degree of the invasiveness in the MPNST cells that
were transfected with both EFNA3-TALENs and pre-miR-210 was greater
than that of EFNA3-TALENs transfected cells but it was weaker than
that of pre-miR-210 transfected cells. These data suggest that
EFNA3 may not be the only target gene of miR-210. It was also
consistent with the findings in our previous study, in which the
ZNF462 gene was also indicated as a potential target of miR-210
(1). Besides, Fasanaro et al
also identified some other targets of miR-210, including E2F3, MNT,
APC, ACVR1B and CDK10, which were also demonstrated to be tumor
suppressors (34).
Angiogenesis is always closely associated with tumor
growth and metastasis. FAK signaling has been shown to promote
angiogenesis in embryonic development as well as various
physiological and disease processes in adult organism, including
tumor angiogenesis (35). In
addition, FAK signaling members and angiogenic factors have been
implicated in tumorigenesis with regards to the Eph/ephrin axis. A
study by Miao et al showed that EphA2 stimulation with
ephrin-A1 leads to the recruitment of the protein tyrosine
phosphatase SHP-2 to EphA2, followed by dephosphorylation of FAK
and paxillin (36). Brantley et
al validated the complementary expression of EphA2 in tumor
blood vessel endothelium and ephrin-A1 in tumor cells as the first
functional evidence of type-A Eph receptor regulation of pathogenic
angiogenesis in tumors (37).
Accordingly, the expression level of FAK signaling proteins and
angiogenic factors were determined by western blotting to further
clarify the potential molecular mechanisms underlying EFNA3-induced
tumor angiogenesis in the present study. The results indicated that
knockout of EFNA3 significantly decreased the expression of p-FAK.
However, the expression of PI3K, GTPase and intergins were
increased (Fig. 5). These suggested
that FAK signaling was negatively regulated by phosphorylation of
FAK. Generally, the FAK signaling is triggered by FAK
phosphorylation that led to actin reorganization through downstream
PI3K and GTPase activation (38).
In addition, FAK activation has been linked to integrin clustering
and is considered a critical step in the initiation of cell
migration (37). This process seems
to be positively regulated by FAK phosphorylation. However,
phosphorylation of FAK at its tyrosine phosphoacceptor site Tyr-407
has been reported to negatively regulate kinase activity and cell
migration (39). VEGF is a signal
protein produced by cells that stimulate vasculogenesis and
angiogenesis. It is part of the system that restores the oxygen
supply to tissues when blood circulation is inadequate. When VEGF
is overexpressed, it can contribute to cancer angiogenesis. HIF-α
is the protein which plays an essential role in cellular and
systemic responses to tumor mediated hypoxia. TNF-α is a member of
a group of cytokines which is able to induce fever, apoptotic cell
death, cachexia, inflammation and to inhibit tumorigenesis. VEGF,
HIF-α and TNF-α are known as angiogenic factors which are closely
related with tumorigenesis and angiogenesis. We also found that
knockout of EFNA3 significantly inhibited the expression of TNF-α
yet notably promoted the expression of VEGF and HIF-α. These
findings suggest that angiogenesis in MPNST cells was activated and
may promote tumor metastasis when EFNA3 is knocked out. On the
contrary, the expression of angiogenic factors was inversely
different when EFNA3 was upregulated. Collectively, our results
indicated that knockout of EFNA3 by TALENs may contribute to the
development and progression of MPNST and this effect may be
associated with increased viability and invasiveness, at least in
part, via promoting angiogenesis. Moreover, overexpression of EFNA3
was able to inhibit the progression of MPNST.
In summary, we demonstrated that EFNA3 serves as a
tumor suppressor in MPNST cells and it may play a critical role in
the FAK signaling and VEGF-associated tumor angiogenesis pathway.
These findings may not only facilitate better understanding of
MPNST pathogenesis, but also suggest EFNA3 as a promising target
for MPNST treatment.
References
|
1
|
Wang Z, Yin B, Wang B, Ma Z, Liu W and Lv
G: MicroRNA-210 promotes proliferation and invasion of peripheral
nerve sheath tumor cells targeting EFNA3. Oncol Res. 21:145–154.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Itani S, Kunisada T, Morimoto Y, Yoshida
A, Sasaki T, Ito S, Ouchida M, Sugihara S, Shimizu K and Ozaki T:
MicroRNA-21 correlates with tumorigenesis in malignant peripheral
nerve sheath tumor (MPNST) via programmed cell death protein 4
(PDCD4). J Cancer Res Clin Oncol. 138:1501–1509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Doorn PF, Molenaar WM, Buter J and
Hoekstra HJ: Malignant peripheral nerve sheath tumors in patients
with and without neurofibromatosis. Eur J Surg Oncol. 21:78–82.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ingham S, Huson SM, Moran A, Wylie J,
Leahy M and Evans DG: Malignant peripheral nerve sheath tumours in
NF1: improved survival in women and in recent years. Eur J Cancer.
47:2723–2728. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Evans DG, Baser ME, McGaughran J, Sharif
S, Howard E and Moran A: Malignant peripheral nerve sheath tumours
in neurofibromatosis 1. J Med Genet. 39:311–314. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Héroult M, Schaffner F and Augustin HG:
Eph receptor and ephrin ligand-mediated interactions during
angiogenesis and tumor progression. Exp Cell Res. 312:642–650.
2006. View Article : Google Scholar
|
|
7
|
Kuijper S, Turner CJ and Adams RH:
Regulation of angiogenesis by Eph-ephrin interactions. Trends
Cardiovasc Med. 17:145–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Irie F, Okuno M, Matsumoto K, Pasquale EB
and Yamaguchi Y: Heparan sulfate regulates ephrin-A3/EphA receptor
signaling. Proc Natl Acad Sci USA. 105:12307–12312. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Surawska H, Ma PC and Salgia R: The role
of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev.
15:419–433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Böhme B, Holtrich U, Wolf G, Luzius H,
Grzeschik KH, Strebhardt K and Rübsamen-Waigmann H: PCR mediated
detection of a new human receptor-tyrosine-kinase, HEK 2. Oncogene.
8:2857–2862. 1993.PubMed/NCBI
|
|
11
|
Fox BP and Kandpal RP: Invasiveness of
breast carcinoma cells and transcript profile: Eph receptors and
ephrin ligands as molecular markers of potential diagnostic and
prognostic application. Biochem Biophys Res Commun. 318:882–892.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walker-Daniels J, Coffman K, Azimi M, Rhim
JS, Bostwick DG, Snyder P, Kerns BJ, Waters DJ and Kinch MS:
Overexpression of the EphA2 tyrosine kinase in prostate cancer.
Prostate. 41:275–280. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu S, Lin S, Hu D, Feng Y, Tan Y and Peng
Y: Interactions of miR-323/miR-326/miR-329 and
miR-130a/miR-155/miR-210 as prognostic indicators for clinical
outcome of glioblastoma patients. J Transl Med. 11:102013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Redova M, Poprach A, Besse A, Iliev R,
Nekvindova J, Lakomy R, Radova L, Svoboda M, Dolezel J, Vyzula R,
et al: MiR-210 expression in tumor tissue and in vitro effects of
its silencing in renal cell carcinoma. Tumour Biol. 34:481–491.
2013. View Article : Google Scholar
|
|
16
|
Puisségur MP, Mazure NM, Bertero T,
Pradelli L, Grosso S, Robbe-Sermesant K, Maurin T, Lebrigand K,
Cardinaud B, Hofman V, et al: miR-210 is overexpressed in late
stages of lung cancer and mediates mitochondrial alterations
associated with modulation of HIF-1 activity. Cell Death Differ.
18:465–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hong L, Yang J, Han Y, Lu Q, Cao J and
Syed L: High expression of miR-210 predicts poor survival in
patients with breast cancer: a meta-analysis. Gene. 507:135–138.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rothé F, Ignatiadis M, Chaboteaux C,
Haibe-Kains B, Kheddoumi N, Majjaj S, Badran B, Fayyad-Kazan H,
Desmedt C, Harris AL, et al: Global microRNA expression profiling
identifies miR-210 associated with tumor proliferation, invasion
and poor clinical outcome in breast cancer. PLoS One. 6:e209802011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chan SY and Loscalzo J: MicroRNA-210: a
unique and pleiotropic hypoxamir. Cell Cycle. 9:1072–1083. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cui H, Grosso S, Schelter F, Mari B and
Krüger A: On the pro-metastatic stress response to cancer
therapies: evidence for a positive co-operation between TIMP-1,
HIF-1α, and miR-210. Front Pharmacol. 3:1342012. View Article : Google Scholar
|
|
21
|
Presneau N, Eskandarpour M, Shemais T,
Henderson S, Halai D, Tirabosco R and Flanagan AM: MicroRNA
profiling of peripheral nerve sheath tumours identifies miR-29c as
a tumour suppressor gene involved in tumour progression. Br J
Cancer. 108:964–972. 2013. View Article : Google Scholar :
|
|
22
|
Kim YK, Wee G, Park J, Kim J, Baek D, Kim
JS and Kim VN: TALEN-based knockout library for human microRNAs.
Nat Struct Mol Biol. 20:1458–1464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boch J: TALEs of genome targeting. Nat
Biotechnol. 29:135–136. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Doyle EL, Booher NJ, Standage DS, Voytas
DF, Brendel VP, Vandyk JK and Bogdanove AJ: TAL Effector-Nucleotide
Targeter (TALE-NT) 2.0: tools for TAL effector design and target
prediction. Nucleic Acids Res. 40:W117–W122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sanjana NE, Cong L, Zhou Y, Cunniff MM,
Feng G and Zhang F: A transcription activator-like effector toolbox
for genome engineering. Nat Protoc. 7:171–192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pulkkinen K, Malm T, Turunen M, Koistinaho
J and Ylä-Herttuala S: Hypoxia induces microRNA miR-210 in vitro
and in vivo ephrin-A3 and neuronal pentraxin 1 are potentially
regulated by miR-210. FEBS Lett. 582:2397–2401. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kullander K, Mather NK, Diella F, Dottori
M, Boyd AW and Klein R: Kinase-dependent and kinase-independent
functions of EphA4 receptors in major axon tract formation in vivo.
Neuron. 29:73–84. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cutforth T, Moring L, Mendelsohn M, Nemes
A, Shah NM, Kim MM, Frisén J and Axel R: Axonal ephrin-As and
odorant receptors: coordinate determination of the olfactory
sensory map. Cell. 114:311–322. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cang J, Kaneko M, Yamada J, Woods G,
Stryker MP and Feldheim DA: Ephrin-as guide the formation of
functional maps in the visual cortex. Neuron. 48:577–589. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murai KK, Nguyen LN, Irie F, Yamaguchi Y
and Pasquale EB: Control of hippocampal dendritic spine morphology
through ephrin-A3/EphA4 signaling. Nat Neurosci. 6:153–160. 2003.
View Article : Google Scholar
|
|
32
|
Iiizumi M, Hosokawa M, Takehara A, Chung
S, Nakamura T, Katagiri T, Eguchi H, Ohigashi H, Ishikawa O,
Nakamura Y, et al: EphA4 receptor, overexpressed in pancreatic
ductal adenocarcinoma, promotes cancer cell growth. Cancer Sci.
97:1211–1216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Georgiou GK, Igglezou M, Sainis I, Vareli
K, Batsis H, Briasoulis E and Fatouros M: Impact of breast cancer
surgery on angiogenesis circulating biomarkers: a prospective
longitudinal study. World J Surg Oncol. 11:2132013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fasanaro P, Greco S, Lorenzi M, Pescatori
M, Brioschi M, Kulshreshtha R, Banfi C, Stubbs A, Calin GA, Ivan M,
et al: An integrated approach for experimental target
identification of hypoxia-induced miR-210. J Biol Chem.
284:35134–35143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao J and Guan JL: Signal transduction by
focal adhesion kinase in cancer. Cancer Metastasis Rev. 28:35–49.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miao H, Burnett E, Kinch M, Simon E and
Wang B: Activation of EphA2 kinase suppresses integrin function and
causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol.
2:62–69. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brantley DM, Cheng N, Thompson EJ, Lin Q,
Brekken RA, Thorpe PE, Muraoka RS, Cerretti DP, Pozzi A, Jackson D,
et al: Soluble Eph A receptors inhibit tumor angiogenesis and
progression in vivo. Oncogene. 21:7011–7026. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kallergi G, Agelaki S, Markomanolaki H,
Georgoulias V and Stournaras C: Activation of FAK/PI3K/Rac1
signaling controls actin reorganization and inhibits cell motility
in human cancer cells. Cell Physiol Biochem. 20:977–986. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lim Y, Park H, Jeon J, Han I, Kim J, Jho
EH and Oh ES: Focal adhesion kinase is negatively regulated by
phosphorylation at tyrosine 407. J Biol Chem. 282:10398–10404.
2007. View Article : Google Scholar : PubMed/NCBI
|