Introduction
Gliomas are the most common primary malignant brain
tumors that originate from neuroepithelial tissue (1–3). They
are associated with a late diagnosis, poor prognosis, and high
mortality rate. Despite aggressive multimodal therapy with maximal
resection followed by the latest available therapeutic
interventions, these tumors still have a dismal prognosis with a
median survival rate of only 2 years and a 3-year survival rate of
only 10% (4). There is currently no
effective treatment for glioma. Glioma is a highly complicated and
heterogeneous tumor condition that results from the aberrant
activation of numerous important signaling pathways (5). Understanding the molecular mechanisms
of glioma is of great importance in the search for a curative
therapy.
Integrin-linked kinase (ILK) is a highly conserved
serine-threonine protein kinase that interacts with the cytoplasmic
domains of integrin subunits. Since its initial discovery in 1996,
ILK has emerged as a key regulator of the phosphatidylinositol
3-kinase (PI3-K) signaling pathway, which activates protein kinase
B (PKB)/Akt activity and inhibits cyclin D1, thereby inhibiting
proliferation of different types of cancer cells (6). Overexpression of ILK has been shown to
promote cell migration and invasion (7–11). In
particular, inhibition of E-cadherin transcription is thought to be
a key mechanism by which ILK promotes cancer cell invasion and
migration.
In the present study, we investigated the role of
ILK in a human glioma cell line (U251). Our results demonstrated an
important and essential role of ILK in two key aspects of glioma,
including apoptosis and the migration and invasion process.
Materials and methods
Cell culture
The human glioma cell line U251 was obtained from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China)
and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco,
Grand Island, NY, USA) supplemented with 10% fetal bovine serum
(FBS; HyClone, Logan, UT, USA) and penicillin/streptomycin (100
U/ml and 100 mg/ml) at 37°C in a 5% CO2 humidified
atmosphere.
Plasmid construction and stable
transfection
To generate a ILK stable expressing cell line, U251
cells were seeded into 6-well plates at a density of
1×106 cells/well. When U251 cell confluency reached
75–80%, the U251 cells were transfected with different
PGFP-V-RS-shRNA constructs using Lipofectamine™ 2000 (Invitrogen,
Carlsbad, CA, USA), according to the manufacturer’s protocol. One
clone was transfected with ILK-PGFP-V-RS-shRNA and the other was
transfected with empty-PGFP-V-RS-shRNA. At 48 h post-transfection,
stable clones were selected with puromycin (8 µg/ml) for 3
weeks. Then the stable clones were screened for GFP expression by a
fluorescence microscope and assayed for ILK expression by
quantitative reverse transcriptase-PCR (qRT-PCR) and western blot
analysis. ILK-PGFP-V-RS-shRNA was named U251-si and
empty-PGFP-V-RS-shRNA was named U251-N. The control cell line named
U251 was not transfected.
Quantitative real-time PCR
Total RNA was extracted from cells using TRIzol
reagent (Life Technologies, Rockville, MD, USA) according to the
manufacturer’s instructions. First-strand complementary DNA (cDNA)
was synthesized from 1 µg of total RNA using the Prime
Script First Strand cDNA Synthesis kit (Takara, Dalian, China).
Quantitative real-time PCR reactions were carried out with cDNA (1
µl) and the SYBR-Green Master Mix (Takara) on a Real-Time
Quantitative Thermal Block (Biometra, Göttingen, Germany). The
sequences of primers are listed in Table I. β-actin served as an internal
control. The specificity of the PCR was confirmed by melting curve
analysis. Data were treated using the comparative threshold cycle
(CT) method (12).
 | Table ISequences of the primers. |
Table I
Sequences of the primers.
| Genes | Sequences |
|---|
| ILK | F:
5′-TGAAGACACAAACAGACG-3′ |
| R:
5′-TCAAGGATAGGCACAATC-3′ |
| E-cadherin | F:
5′-ATGCCGCCATCGCTTACAC-3′ |
| R:
5′-CGACGTTAGCCTCGTTCTCA-3′ |
| Cyclin D1 | F:
5′-ACCTGAGGAGCCCCAACAA-3′ |
| R:
5′-TCTGCTCCTGGCAGGCC-3′ |
| β-actin | F:
5′-GTTGCCCTGAGGCTCTTTTCC A-3′ |
| R:
5′-ACCACCAGACAGCACTGTGTTG-3′ |
Western blot analysis
Proteins were extracted with a buffer composed of 50
mM Tris-HCl, pH 7.5, 0.1% Triton X-100, ethylenediaminetetraacetic
acid (EDTA) 5 mM, and a cocktail of protease inhibitors. The
following primary antibodies were used: anti-E-cadherin, anti-ILK,
anti-Bcl-2, anti-cyclin D1, and anti-actin (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA).
Cell cycle analysis
Cells were cultured with or without drug treatment
for 24 h and subsequently analyzed. Control cells were treated with
the vehicle control PTE [PEG300/ethanol/Tween 80/citrate
(63:29:7.8:0.2, w/v/w/w)]. Cells were harvested, fixed with cold
70% ethanol, and stored overnight at −20°C, followed by staining
with propidium iodide staining buffer (1 mg/ml RNase A, 0.1% Triton
X-100, 50 µg/ml propidium iodide in PBS).
Stained samples were analyzed by flow cytometry with
a FACSCalibur (Becton-Dickinson, San Jose, CA, USA) or with WinMDI
2.9 freeware to determine cell cycle distribution. The percentage
of cells in each cell cycle phase was calculated relative to the
total cells in the G1-G0, S and G2-M phases after prior exclusion
of pre-G1-G0 events.
Wound healing assay
Cells were plated in 6-well plates at a density of
1×105 cells per well and grown to approximately 80%
confluency. The monolayer was scraped with a sterile 200-µl
pipette tip after removal of the culture medium. Subsequently, the
culture was washed twice with serum-free medium. After that, cells
were maintained in DMEM, the scratched areas were photographed at
0, 12 and 24 h after wounding using computer-assisted microscopy.
Cell migration was calculated as the percentage of cell coverage to
the initial cell-free zone.
Transwell invasion assay
Cell invasion was evaluated using a Transwell
chamber (Corning Costar, Cambridge, MA, USA) equipped with a
Matrigel-coated filter membrane (8-µm pores). Briefly, the
filters were pre-coated with 0.5 µg basement membrane
proteins (Matrigel; BD Biosciences, San Jose, CA, USA) and allowed
to dry overnight at room temperature. Cells in FBS-free medium were
seeded in the upper chambers, and the lower wells contained 10% FBS
medium. After incubation at 37°C for 24 h, non-migratory cells on
the upper side of the insert were removed with a cotton swab. Cells
that had passed through the filter were fixed in methanol and
stained with hematoxylin. For quantification, six randomly selected
fields on the lower side of the insert were photographed using
computer-assisted microscopy.
Statistical analysis
Statistical analyses were performed with SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as
means ± SD. Differences between groups were analyzed by one-way
ANOVA. A p-value <0.05 was considered statistically
significant.
Results
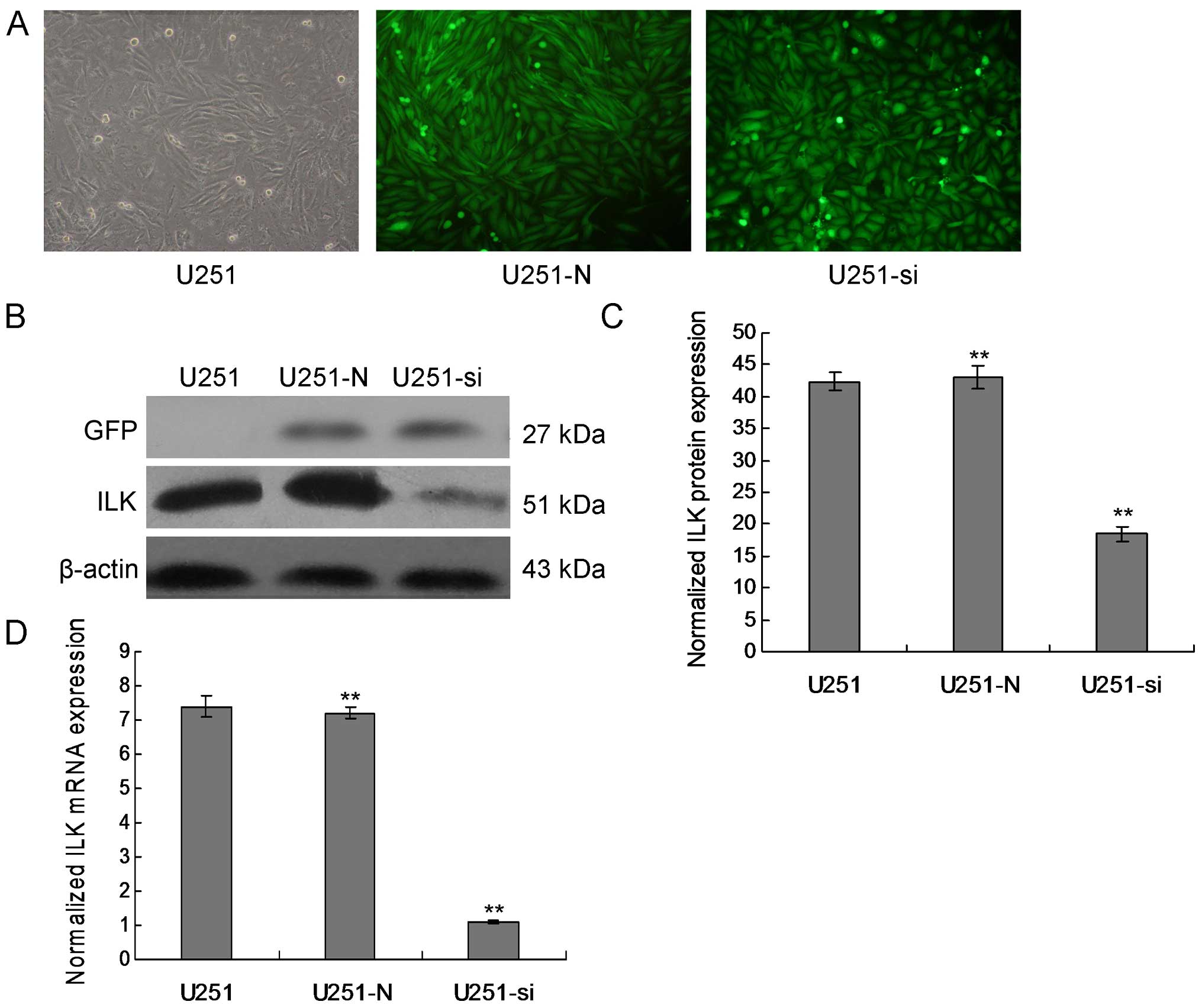
Stable downregulation of ILK in the U251
cells
One effective way to understand the physiological
role of ILK in glioma is to inhibit the expression of endogenous
ILK in glioma cell line models. The first efficient knockdown of
ILK expression was achieved by the successful delivery and
expression of short-hairpin (sh)RNA targeting ILK in the U251
cells. We detected expression of ILK in the U251 cells using
immunofluorescence staining and western blot analysis.
Immunofluorescence staining showed that ILK was mainly localized at
the cell membrane and that immune intensity was obviously decreased
in the the ILK-downregulated cells as compared with the control
cells. ILK demonstrated low expression at both the mRNA and protein
levels in the ILK-knockdown stable transfected clones compared with
the empty-vector transfected cells (Fig. 1, p<0.01).
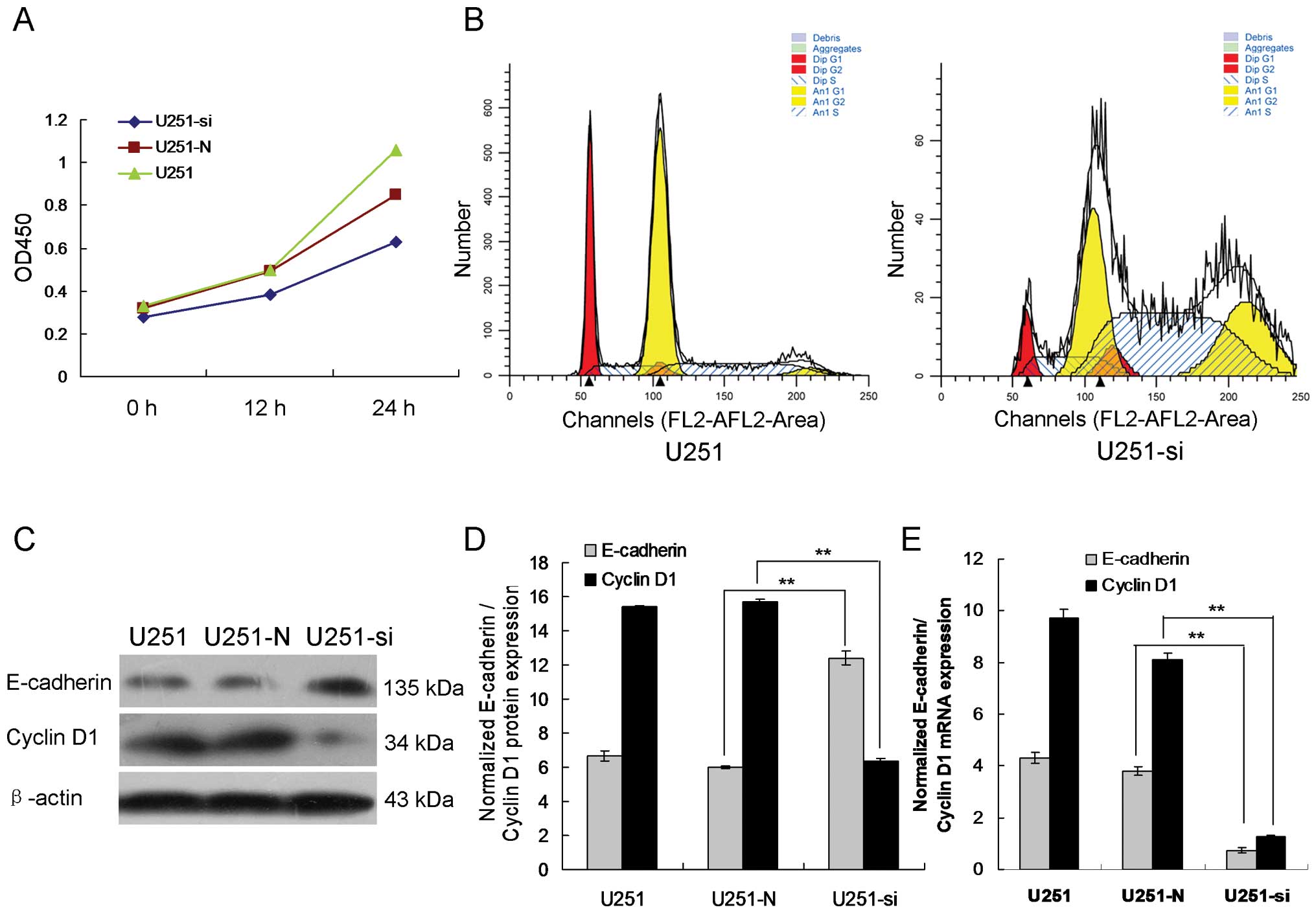
Knockdown of ILK suppresses glioma cell
growth
To determine the effect of ILK expression on glioma
cell growth, proliferation curves of the ILK-knockdown stable
clones and their corresponding non-targeted controls were compared.
As shown in Fig. 2A, similar
proliferation rates were observed for U251, U251-N, and U251-si
cells during the first 48 h. However, starting from 72 h, a
significant difference in cell growth was observed between the
ILK-knockdown clones and the non-targeted controls. U251-N was
found to have a higher proliferation rate when compared to the
U251-si cells. This trend persisted until the last day of the
proliferation assay. As shown in Fig.
2B, flow cytometry showed that the cell cycle was arrested in
the G2/M phase. These results indicate that ILK-knockdown
suppressed the cell proliferation of glioma cells.
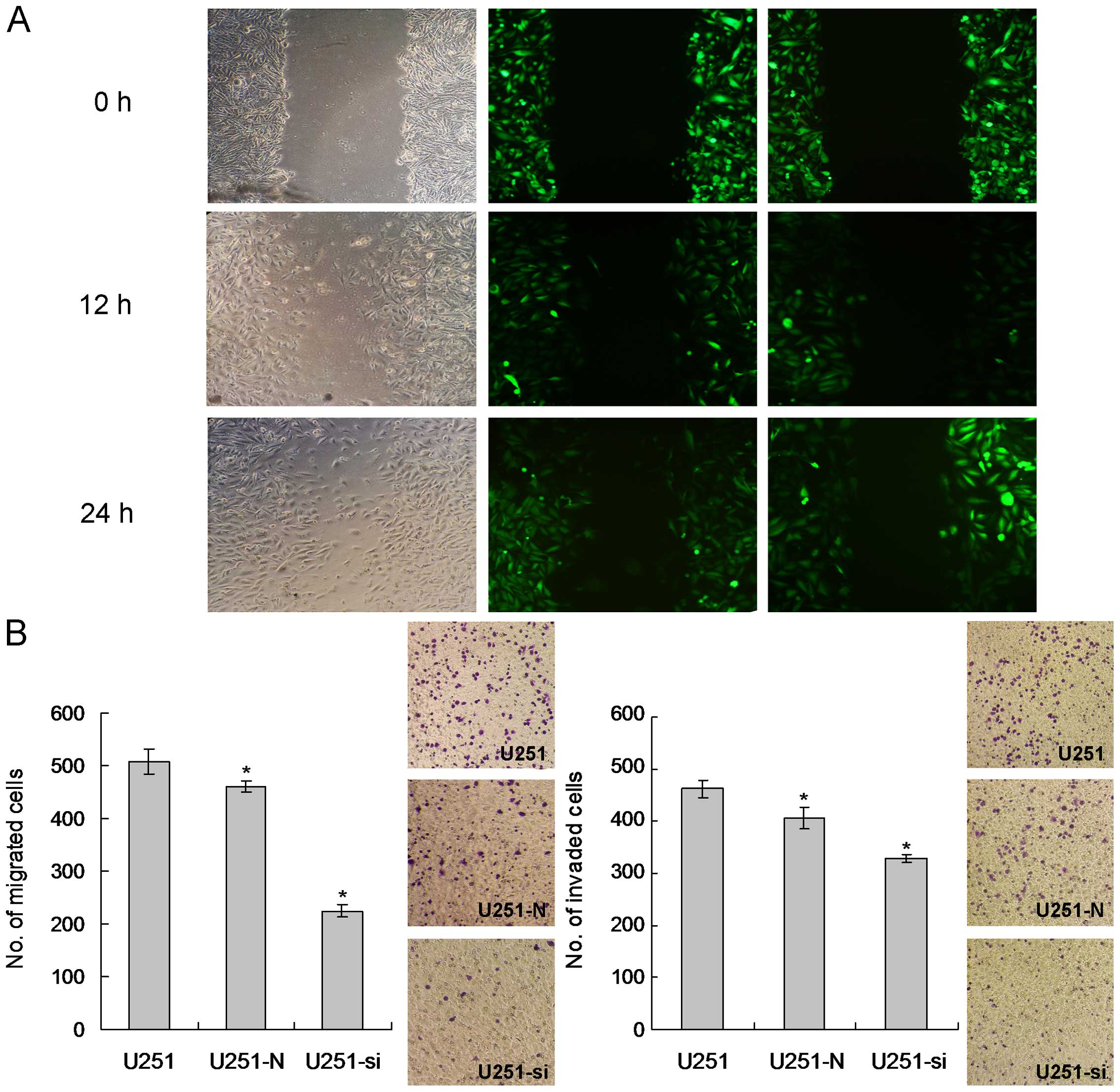
Knockdown of ILK reduces the migratory
and invasive potentials of glioma cells
To assess the migratory ability of the ILK-knockdown
stable clones, wound healing and a Transwell migration chamber were
employed. Differences in wound-closure ability were obvious in the
ILK-knockdown stable clones. Wounds in the U251-N control clones
were almost closed in 24 h, yet the wounds in the U251-si stable
clones were still clearly observed (Fig. 3A). The Transwell migration assay
revealed that the number of migrated cells in the U251-N control
clones was significantly higher when compared to the U251-si stable
clones (Fig. 3B). Cell invasiveness
of the ILK-knockdown stable clones was assessed by seeding the
cells onto a Matrigel-coated invasion chamber. More invaded cells
were observed in the U251-N control while significantly fewer cells
were able to invade through the Matrigel in the U251-si stable
clones. Collectively, these results indicated that knockdown of ILK
suppressed cell invasion.
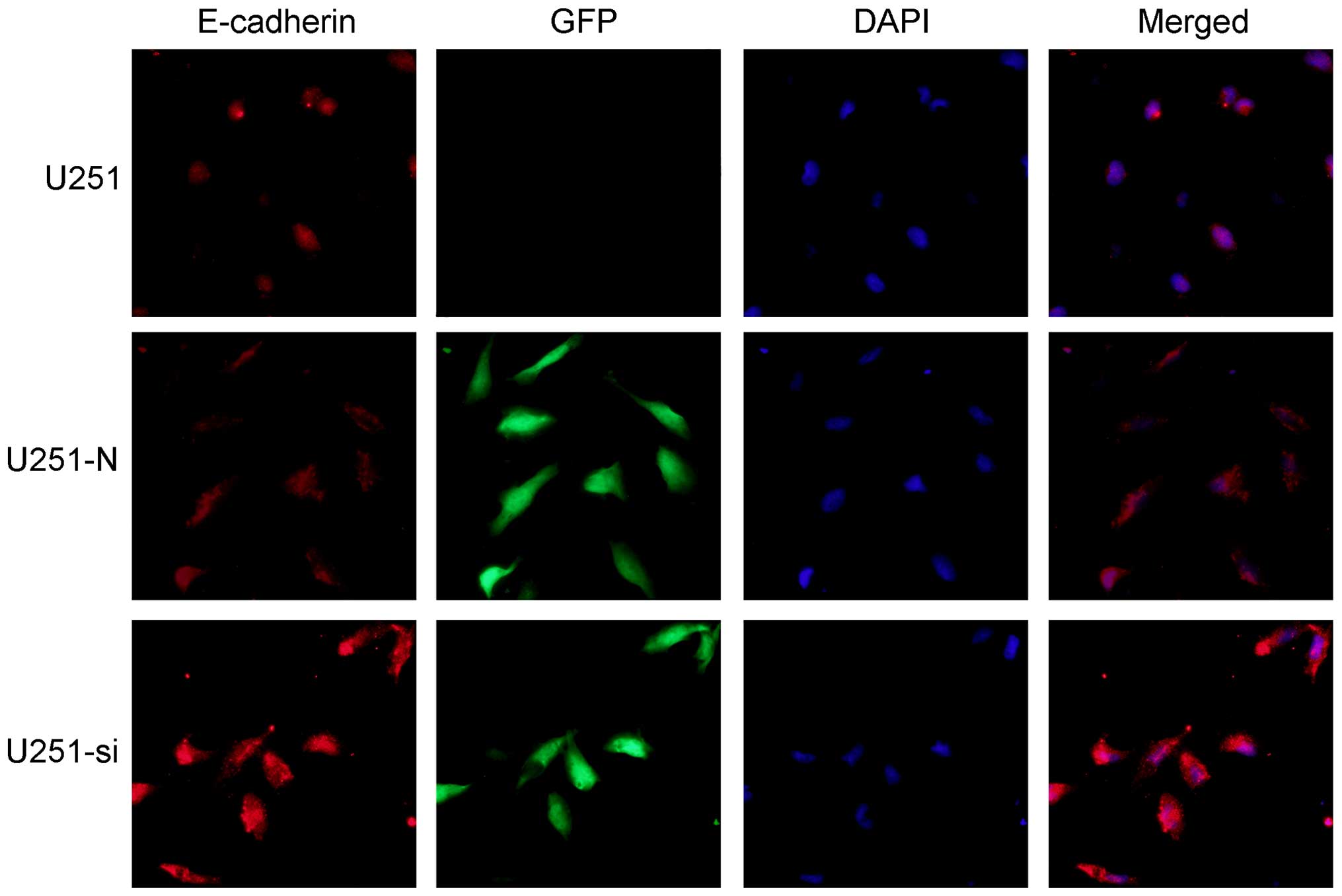
Inhibition of ILK expression upregulates
E-cadherin and downregulates cyclin D1 in the glioma cells
To investigate whether E-cadherin contributes to the
cell migration and invasion induced by knockdown of ILK, we
detected expression of E-cadherin in the U251 cells using
immunofluorescence staining, real-time PCR and western blot
analysis. Immunofluorescence staining showed that exogenous
E-cadherin was mainly localized at the cell-cell contacts and that
immune intensity was obviously increased in the U251-si cells as
compared with the U251-N cells (Fig.
4). Meanwhile, western blot analysis consistently showed that
the protein expression level of E-cadherin was significantly
increased in the U251-si cells as compared to the U251-N cells
(Fig. 2C and D, p<0.01). The
real-time PCR data were also in direct agreement with the results
of the western blot analysis (Fig.
2E, p<0.01). Our data indicated that downregulation of
E-cadherin was involved in the ILK-induced cell migration and
invasion.
As shown in Fig. 2C,
the protein level of cyclin D1 was significantly reduced in the
U251-si cells. Additionally, such treatment resulted in a marked
reduction in mRNA expression in the U251-si cells (Fig. 2E). Collectively, these results
suggest that upregulation of cyclin D1 is involved in ILK-induced
cell proliferation.
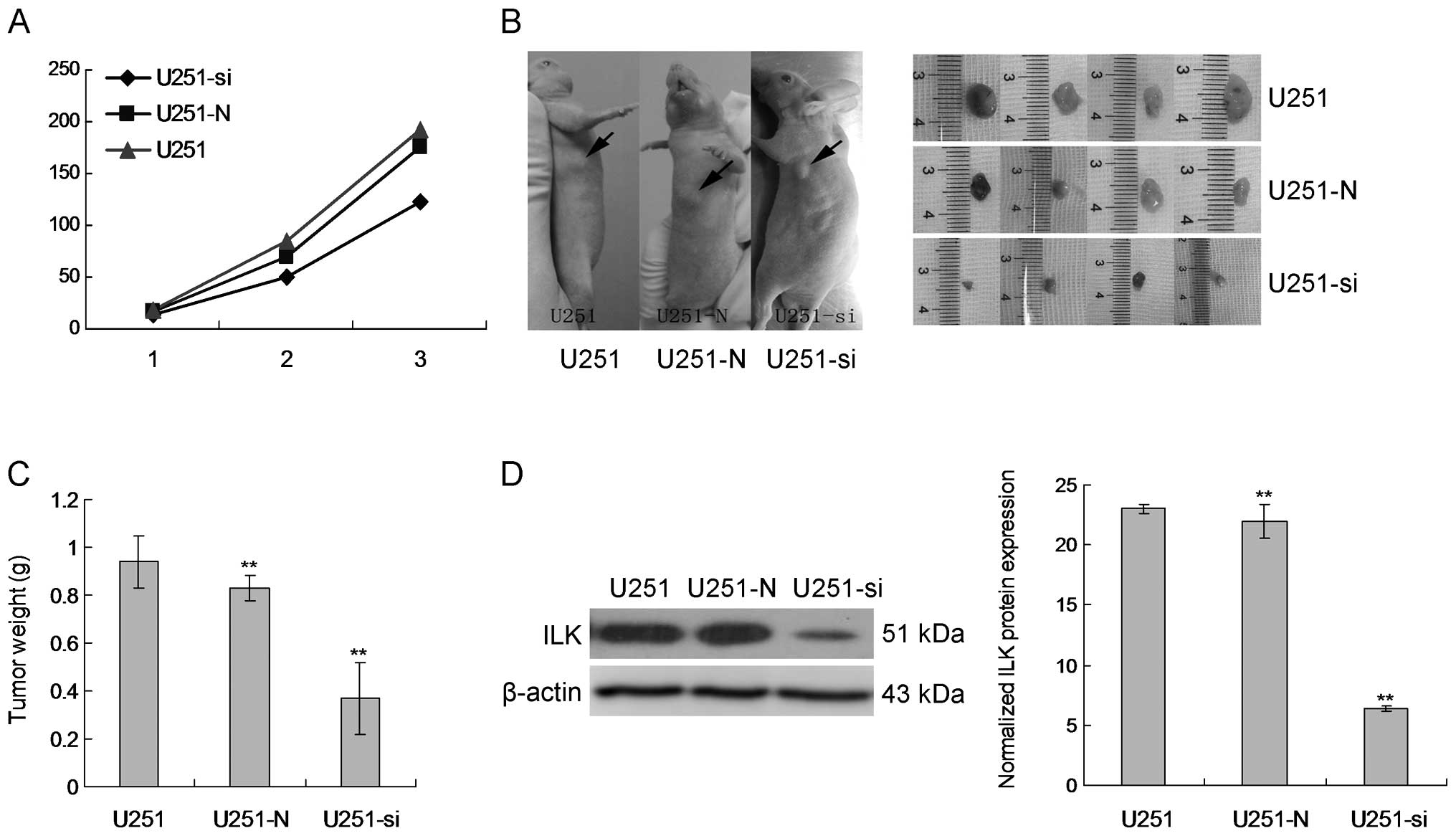
Knockdown of ILK impairs glioma cell in
vivo tumorigenicity
U251-si stable clones were subjected to nude mouse
injection. After subcutaneous injection of the cells into the nude
mice, tumor growth was monitored carefully and the size of the
tumors formed was measured weekly. Starting from week 3, larger
tumors were formed in the mice injected with the NT control clones
while smaller tumors were formed from the stable clones (Fig. 5A). At week 3, the animals injected
with U251-si were sacrificed due to oversized tumors (Fig. 5B). Tumors were then harvested from
the mice, photographed and weighed. Tumors formed from the U251-N
control clones were significantly larger than the tumors from the
U251-si stable clones. This trend was also reflected in the weight
of the tumors where the average tumor weight from the U251-N
control clones was significantly higher than that from the U251-si
clones (Fig. 5C). This result
demonstrated that suppression of ILK in U251 cells attenuated the
ability of U251 cells to form tumors in nude mice. In addition,
protein was extracted from the excised tumors and analyzed for ILK
expression by western blot analysis. ILK protein expression was
decreased in the tumor tissues of the mice injected with the
U251-si clones (Fig. 5D).
Discussion
ILK is widely expressed in several types of human
cancers including glioma and has been positively correlated with
tumor growth and closely related to tumor apoptosis, proliferation,
invasion and migration (13,14).
ILK is a ubiquitously expressed serine/threonine protein kinase
that binds directly to the cytoplasmic domains of β1 and β3
integrins and has been shown to have clinical/prognostic and
functional significance in various human cancers.
In spite of the potential significance of ILK in
glioma, the functional role of ILK and elucidation of its
associated pathways in glioma have not been clearly defined. To
understand the functions of ILK, endogenous ILK expression in
glioma cell lines was silenced by shRNA. ILK-knockdown stable
clones displayed suppressed cell proliferation and
anchorage-independent growth. Additionally, motility and
invasiveness of the cells were largely impeded upon ILK depletion.
Glioma cells with suppressed ILK expression displayed an inhibited
ability to form tumors in nude mice. All of these studies
corroborate our findings that ILK exerts an oncogenic effect on
glioma cell lines.
Recently, ILK has been shown to induce E-cadherin
shedding and to promote cell migration and invasion in lung cancer
cells (15). Thus, we sought to
study the effects of ILK on E-cadherin expression. E-cadherin is a
transmembrane glycoprotein that mediates calcium-dependent
intracellular adhesion in normal epithelial cells. Selective loss
of E-cadherin function or expression has been linked with cancer
progression and metastasis. In the present study, our results
showed that inhibition of ILK increased E-cadherin expression at
the mRNA and protein levels and decreased cell migratory and
invasive abilities in the glioma cells. Our results strongly
suggest that ILK suppresses E-cadherin expression to inhibit
invasion and migration. However, the mechanisms underlying tumor
metastasis by ILK remain to be further elucidated.
AKT is an important transcription factor that plays
a pivotal role in promoting and maintaining an invasive phenotype
by regulating multiple target genes, including Bcl-2,
metalloproteinase-9 (MMP-9) and cyclin D1 (16). Notably, previous research (17,18)
has also shown that AKT phosphorylates ILK and mediates its
nucleocytoplasmic shuttling and functions in the nucleus. Cyclin D1
is the target of AKT, which has also been implicated in glioma
(19–21). In the present study, we demonstrated
that cyclin D1 in the ILK-knockdown cells was markedly decreased
compared with the level in the control cells. We also demonstrated
that proliferation of human glioma cells in response to cyclin D1
were inhibited upon inhibition of ILK activity or expression in
vitro. Finally, we also showed that inhibition of ILK resulted
in a statistically significant suppression of tumor growth in a
mouse xenograft model of U251 tumor growth in nude mice. These data
suggest that ILK may be considered as an inhibitor for the
suppression of tumor growth.
In conclusion, the results of the present study
revealed that ILK knockdown reduced glioma cell migration and
invasion at least partly through upregulation of E-cadherin and
suppressed glioma cell growth through downregulation of cyclin D1.
ILK plays a pivotal role in apoptosis, proliferation, invasion and
migration. Our results suggest that ILK may serve as a promising
therapeutic target for glioma.
Acknowledgments
The present study was funded by the Natural Science
Foundation of Hebei Province (no. 2012201136) and the Medical
Science Special Foundation of Hebei University (no. 2012A2004).
References
|
1
|
Lu Y, Chopp M, Zheng X, Katakowski M,
Buller B and Jiang F: MiR-145 reduces ADAM17 expression and
inhibits in vitro migration and invasion of glioma cells. Oncol
Rep. 29:67–72. 2013.
|
|
2
|
DeAngelis LM: Brain tumors. N Engl J Med.
344:114–123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R: Malignant glioma: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20(Suppl 4): 126–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Che H, Song J, Guo S, Wang W and Gao G:
Inhibition of xenograft human glioma tumor growth by
lentivirus-mediated gene transfer of alphastatin. Oncol Rep.
29:1101–1107. 2013.
|
|
6
|
Delcommenne M, Tan C, Gray V, Rue L,
Woodgett J and Dedhar S: Phosphoinositide-3-OH kinase-dependent
regulation of glycogen synthase kinase 3 and protein kinase B/AKT
by the integrin-linked kinase. Proc Natl Acad Sci USA.
95:11211–11216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peroukides S, Bravou V, Varakis J,
Alexopoulos A, Kalofonos H and Papadaki H: ILK overexpression in
human hepatocellular carcinoma and liver cirrhosis correlates with
activation of Akt. Oncol Rep. 20:1337–1344. 2008.PubMed/NCBI
|
|
8
|
Yan Z, Yin H, Wang R, Wu D, Sun W, Liu B
and Su Q: Overexpression of integrin-linked kinase (ILK) promotes
migration and invasion of colorectal cancer cells by inducing
epithelial-mesenchymal transition via NF-κB signaling. Acta
Histochem. 116:527–533. 2014. View Article : Google Scholar
|
|
9
|
Zhao D, Tang XF, Yang K, Liu JY and Ma XR:
Overexpression of integrin-linked kinase correlates with aberrant
expression of Snail, E-cadherin and N-cadherin in oral squamous
cell carcinoma: Implications in tumor progression and metastasis.
Clin Exp Metastasis. 29:957–969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hannigan GE, McDonald PC, Walsh MP and
Dedhar S: Integrin-linked kinase: Not so ‘pseudo’ after all.
Oncogene. 30:4375–4385. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mao Q, Lin C, Gao J, Liang X, Gao W, Shen
L, Kang L and Xu B: Mesenchymal stem cells overexpressing
integrin-linked kinase attenuate left ventricular remodeling and
improve cardiac function after myocardial infarction. Mol Cell
Biochem. 397:203–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alberton CL, Kanitz AC, Pinto SS, Antunes
AH, Finatto P, Cadore EL and Kruel LF: Determining the anaerobic
threshold in water aerobic exercises: A comparison between the
heart rate deflection point and the ventilatory method. J Sports
Med Phys Fitness. 53:358–367. 2013.PubMed/NCBI
|
|
13
|
Serrano I, McDonald PC, Lock F, Muller WJ
and Dedhar S: Inactivation of the Hippo tumour suppressor pathway
by integrin-linked kinase. Nat Commun. 4:29762013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo L, Liu H, Dong Z, Sun L, Peng Y and
Liu F: Small interfering RNA targeting ILK inhibits EMT in human
peritoneal mesothelial cells through phosphorylation of GSK-3β. Mol
Med Rep. 10:137–144. 2014.PubMed/NCBI
|
|
15
|
Yu J, Shi R, Zhang D, Wang E and Qiu X:
Expression of integrin-linked kinase in lung squamous cell
carcinoma and adenocarcinoma: Correlation with E-cadherin
expression, tumor microvessel density and clinical outcome.
Virchows Arch. 458:99–107. 2011. View Article : Google Scholar
|
|
16
|
Min HS, Choe G, Kim SW, Park YJ, Park J,
Youn YK, Park SH, Cho BY and Park SY: S100A4 expression is
associated with lymph node metastasis in papillary microcarcinoma
of the thyroid. Mod Pathol. 21:748–755. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robertson BW and Chellaiah MA: Osteopontin
induces betacatenin signaling through activation of Akt in prostate
cancer cells. Exp Cell Res. 316:1–11. 2010. View Article : Google Scholar
|
|
18
|
Plante I, Charbonneau M and Cyr DG:
Activation of the integrin-linked kinase pathway downregulates
hepatic connexin32 via nuclear Akt. Carcinogenesis. 27:1923–1929.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rathod SS, Rani SB, Khan M, Muzumdar D and
Shiras A: Tumor suppressive miRNA-34a suppresses cell proliferation
and tumor growth of glioma stem cells by targeting Akt and Wnt
signaling pathways. FEBS Open Bio. 4:485–495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zong H, Cao L, Ma C, Zhao J, Ming X, Shang
M and Xu H: Association between the G870A polymorphism of cyclin D1
gene and glioma risk. Tumour Biol. 35:8095–8101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou CX and Gao Y: Aberrant expression of
beta-catenin, Pin1 and cyclin D1 in salivary adenoid cystic
carcinoma: Relation to tumor proliferation and metastasis. Oncol
Rep. 16:505–511. 2006.PubMed/NCBI
|