Introduction
Pancreatic ductal adenocarcinoma (PDA) is almost
uniformly lethal with an estimated annual number of 45,220 new
cases resulting in ~38,460 annual mortalities and a 5-year survival
rate of <5% (1–3). Late initial diagnosis, aggressive
metastatic behavior and resistance to chemoradiotherapy render
pancreatic cancer one of the most difficult malignancies to treat.
Surgical resection is potentially curative in a minority of
patients. However, almost 80% of the patients are diagnosed with
locally advanced disease that precludes surgical intervention.
Systemic gemcitabine alone or in combination with 5-FU, irinotecan
and oxaliplatin (FOLFIRINOX) is the current standard of care for
advanced pancreatic cancer, providing short-term symptomatic
improvement with minor impact on survival (4–6). Thus,
the development of novel agents targeting novel biomarkers for the
treatment of pancreatic cancer is imperative.
Pristimerin (PM) is a quinonemethide triterpenoid
present in various plant species in the Celastraceae and
Hippocrateaceae families. PM has shown potent
antiproliferative and apoptosis-inducing activity in various types
of cancer cells including pancreatic cancer cells (7–11).
Induction of apoptosis by PM involved the generation of reactive
oxygen species (ROS), activation of caspases, mitochondrial
dysfunction and the inhibition of nuclear factor κB (NF-κB), Akt
and MAP kinases (12,13).
Telomeres are nucleoprotein structures present at
the end of chromosomes that play an essential role in chromosomal
stability and protection from end-to-end fusion (14). Telomeres shorten progressively
during each cell division due to the gradual loss of the telomeric
DNA sequence (15). When the
telomere length becomes critically short, it triggers replicative
senescence or apoptosis. Maintaining the telomere length by
incorporating hexameric DNA repeats (TTAGGG) to the 3′ flanking end
of DNA strands is the function of telomerase, a ribonucleoprotein
complex. Human telomerase comprises the RNA template (hTERC) and
RNA-dependent DNA polymerase human telomerase reverse transcriptase
(hTERT) (16,17). hTERC serves as a template for
hTERT-mediated telomere extension. In addition, hTERT associates
with several proteins including a six-protein complex known as
shelterin for proper functioning (18,19).
Deregulated telomerase activity is associated with the promotion of
tumorigenesis and neoplastic growth of cancer (20,21).
Approximately 90% of human cancer types exhibit activated
telomerase (22). hTERT expression
and telomerase activity are elevated in pancreatic cancers with PDA
showing significantly higher telomerase activity (23–25).
The high incidence of active telomerase in PDA suggests that
targeting telomerase is a promising strategy for the treatment of
this disease.
In the present study, we investigated the effect of
PM on the expression of hTERT and hTERT telomerase activity in the
MiaPaCa-2 and Panc-1 PDA cell lines. PM inhibited hTERT mRNA,
native and phospho-hTERT protein and telomerase activity. PM also
downregulated proteins that regulate hTERT transcriptionally and
post-translationally.
Materials and methods
Reagents
PM was purchased from Sigma Chemicals (St. Louis,
MO, USA). Antibodies against PARP-1, Akt, p-Akt
(Ser473), Sp1, c-Myc, NF-κB and β-actin were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Anti-hTERT and p-TERT antibodies were obtained from Abcam, Inc.
(Cambridge, MA, USA). The CellTiter 96® AQueous One
Solution Proliferation Assay System was purchased from Promega
(Madison, WI, USA). The Annexin V-FITC Apoptosis Detection Kit II
was obtained from BD Biosciences Pharmingen (San Diego, CA, USA)
and the TRAPeze Telomerase Detection kit was purchased from
Millipore (Temecula, CA, USA).
A stock solution (100 mM) of PM was prepared in
dimethyl-sulfoxide (DMSO) and test concentrations were prepared by
diluting the stock solution in tissue culture medium.
Cell lines
MiaPaCa-2 and Panc-1 PDA cell lines were obtained
from the American Type Culture Collection (ATCC; Rockville, MD,
USA). The two cell lines were grown in Dulbecco’s modified Eagle’s
medium (DMEM) tissue culture medium (Gibco-BRL, Rockville, MD, USA)
supplemented with 10% fetal bovine serum, 1%
penicillin/streptomycin and 25 mM HEPES buffer. The cells were
incubated at 37°C in a humidified atmosphere consisting of 5%
CO2, 95% air and maintained by splitting the cultures
twice a week.
MTS assay
Tumor cells (1×104) in 100 μl of
tissue culture medium were seeded in each well of 96-well plates.
After a 24-h incubation to allow cells to adhere, the cells were
treated with PM at concentrations ranging from 0 to 5 μM.
The cultures were incubated for an additional 72 h and cell
viability was then determined by the colorimetric
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazo
lium (MTS) assay using the CellTiter 96 AQueous One Solution
Proliferation Assay System. This assay measures the bioreduction of
the tetrazolium compound MTS in the presence of the
electron-coupling reagent phenazine methosulfate by intracellular
dehydrogenases. MTS and phenazine methosulfate were added to the
culture wells, and the cultures were incubated for 2 h at 37°C. The
absorbance, which is directly proportional to the number of viable
cells in the cultures, was measured at 490 nm using a microplate
reader.
Cell cycle analysis
The distribution of cells in various cell cycle
phases was analyzed by measuring cell DNA content. Untreated
(control) (2×106) or PM-treated cells were fixed in 70%
ethanol overnight at 4°C. The cells were washed twice and
resuspended in 0.8 ml of phosphate-buffered saline (PBS). In total,
100 μl of DNAse free RNAse (500 μg/ml) and 100
μl of propidium iodide (PI) (500 μg/ml) were added to
each tube and the tubes were incubated at room temperature in the
dark for 30 min. Cell DNA content was determined by flow cytometry
using an Accuri C6 flow cytometer (Accuri Cytometers, Inc., Ann
Arbor, MI, USA).
Annexin V-FITC binding
Induction of apoptosis was assessed by the binding
of Annexin V to phosphatidylserine, which was externalized to the
outer leaflet of the plasma membrane early during induction of
apoptosis. Briefly, MiaPaCa-2 and Panc-1 cells treated with PM (0–5
μM) for 24 h were resus-pended in the binding buffer
provided in the Annexin V-FITC Apoptosis Detection Kit II. The
cells were mixed with 5 μl of the Annexin V-FITC reagent, 5
μl of PI and incubated for 30 min at room temperature in the
dark. The stained cells were analyzed by flow cytometry.
Western blotting
Cell lysates were prepared by CHAPS detergent lysis
[1% Triton X-100 (v/v), 10 mM Tris-HCl (pH 7.5), 5 mM EDTA, 150 mM
NaCl, 10% glycerol, 2 mM sodium vanadate, 5 μg/ml leupeptin,
1 μg/ml aprotinin, 1 μg/ml pepstatin A and 10
μg/ml 4-2-aminoethyl-benzenesulfonyl fluoride]. The lysates
were clarified by centrifugation at 14,000 × g for 10 min at 4°C,
and protein concentrations were determined by the Bradford assay.
Samples (50 μg) were boiled in an equal volume of sample
buffer [20% glycerol, 4% SDS, 0.2% bromophenol blue, 125 mM
Tris-HCl (pH 7.5) and 640 mM 2-mercaptoethanol] and separated on
10% SDS-polyacrylamide gels. Proteins resolved on the gels were
transferred to nitrocellulose membranes. The membranes were blocked
with 5% milk in 10 mM Tris-HCl (pH 8.0), 150 mM NaCl with 0.05%
Tween-20 (PBS) and incubated with protein-specific antibodies
followed by an HRP-conjugated secondary antibody. Immune complexes
were visualized with an enhanced chemiluminescence detection system
from Amersham Corp. (Arlington Heights, IL, USA) and protein bands
were imaged.
Transfections
For hTERT overexpression, semi-confluent cell
cultures were transfected with 10 μg of empty or hTERT
expression plasmid (pCI-neo-hTERT) DNA using Lipofectamine Plus
reagent. After transfection for 48 h, the cells were analyzed for
the expression of hTERT by immuno-blotting.
For the silencing of hTERT, the cells were
transfected with double-stranded siRNA-hTERT or a non-targeting
siRNA sequence using a SignalSilence siRNA kit (Cell Signaling
Technology, Beverly, MA, USA). Briefly, 2×106 tumor
cells were plated in a 60-mm Petri dish for 24 h and treated with 3
ml of transfection medium containing 20 μg Lipofectamine and
100 nM siRNA for 48 h. The hTERT expression was analyzed by
immunoblotting.
Statistical analysis
Data are presented as means ± SD. The differences
between the control and treatment groups were analyzed using the
Student’s t-test. P<0.05 was considered to indicate a
statistically significant result.
Results
PM inhibits proliferation and cell-cycle
progression in PDA cells
The effect of PM on proliferation of PDA cells
(MiaPaCa-2 and Panc-1 cells) was examined using the MTS assay.
Briefly, the cells were treated with PM at concentrations of 0–5
μM for 72 h and the viability of the cultures was
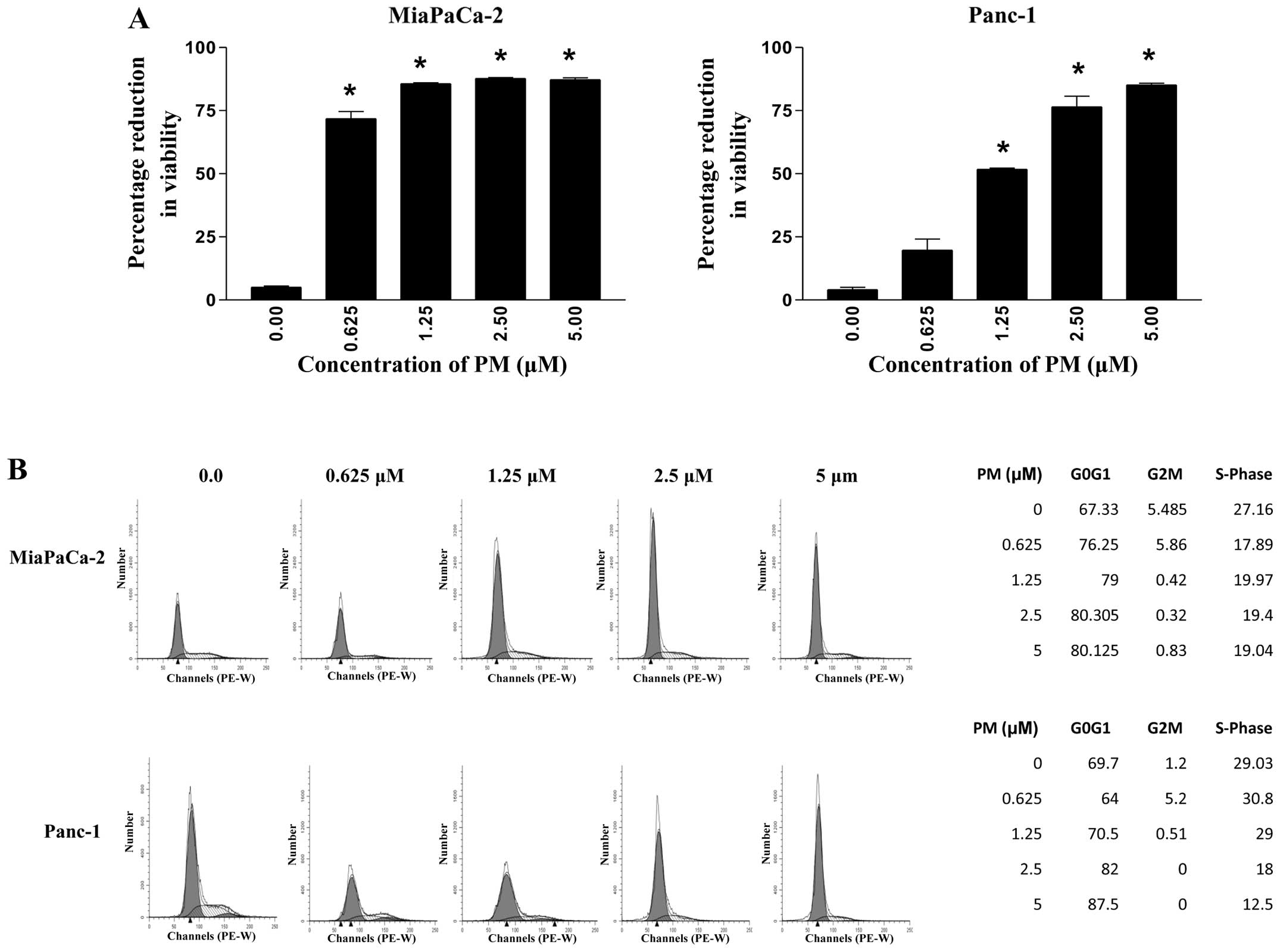
determined. As shown in Fig. 1A, a
significant (MiaPaCa-2, 71%) to measurable (Panc-1, 19%) reduction
in viability was observed at 0.625 μM PM. In the MiaPaCa-2
cells the viability reached a plateau (85–87%) at 1.25-5 μM
PM. On the other hand, reduction of viability in the Panc-1 cells
occurred in a dose-dependent manner (51, 76 and 85% at 1.25, 2.5
and 5 μM PM). Thus, PM significantly reduced the
proliferation of the two PDA cell lines at PM concentrations of
1.25–5 μM.
The effect of PM on cell cycle progression was then
analyzed. MiaPaCa-2 and Panc-1 cells were treated with PM (0–5
μM) for 24 h, stained with PI and cell DNA content of the
cells was measured by flow cytometry. As shown in Fig. 1B, treatment with PM resulted in cell
cycle arrest in G1-phase with a cell distribution of 67.3, 76.2,
79, 80.3 and 80.1% in MiaPaCa-2 cells and 69.7, 64, 70.5, 82 and
87.5% in Panc-1 cells at 0, 0.625, 1.25, 2.5 and 5 μM PM,
respectively.
PM induces apoptosis in PDA cells
Whether the cell cycle arrest leads to the induction
of apoptosis was investigated by measuring the binding of Annexin
V-FITC and cleavage of PARP-1 in tumor cells treated with PM. Thus,
MiaPaCa-2 and Panc-1 cells were treated with PM (0–5 μM) for
24 h and the binding of Annexin V-FITC was determined by flow
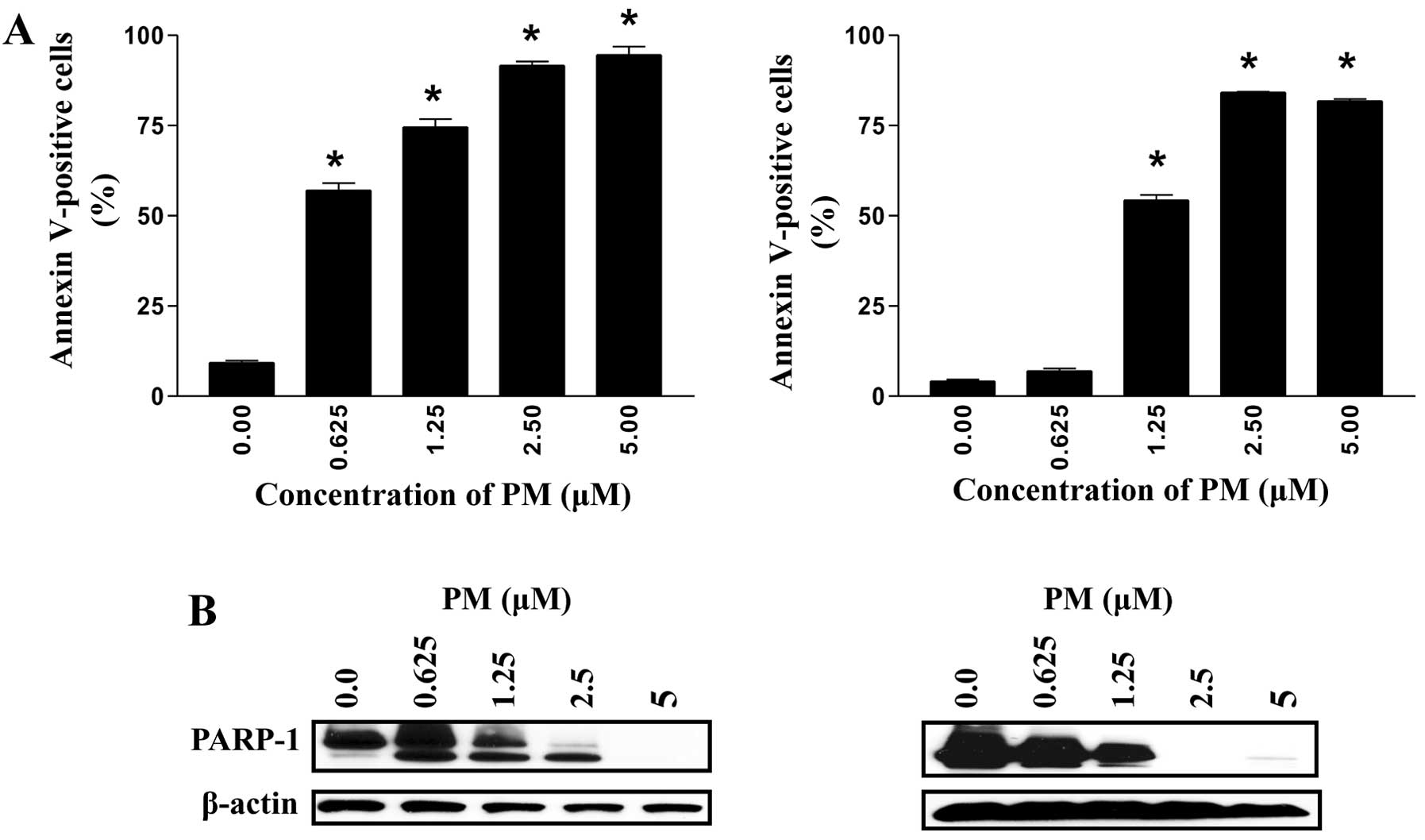
cytometry. As shown in Fig. 2, only
a small percentage of untreated MiaPaCa-2 and Panc-1 cells bound to
Annexin V-FITC (9–4%, respectively). By contrast, the percentage of
Annexin V-FITC binding cells in the two cell lines increased in a
dose-dependent manner following treatment with PM at 0.625–5
μM (MiaPaCa-2, 57–91%; Panc-1, 7–81%).
The induction of apoptosis was confirmed by the
cleavage of PARP-1. Thus, the tumor cells were treated with PM as
described above and cleavage of PARP-1 was determined by western
blotting. As shown in Fig. 2B,
treatment with PM induced the cleavage of native PARP-1 (110-kDa
fragment) as identified by the emergence of an 89-kDa cleaved
PARP-1 fragment in the two cell lines treated with PM. The increase
in Annexin V-FITC binding and the cleavage of PARP-1 demonstrated
induction of apoptosis in PDA cells by PM.
PM inhibits hTERT expression in PDA
cells
Reactivation of telomerase promotes the
proliferation of tumor cells. Therefore, we determined the effect
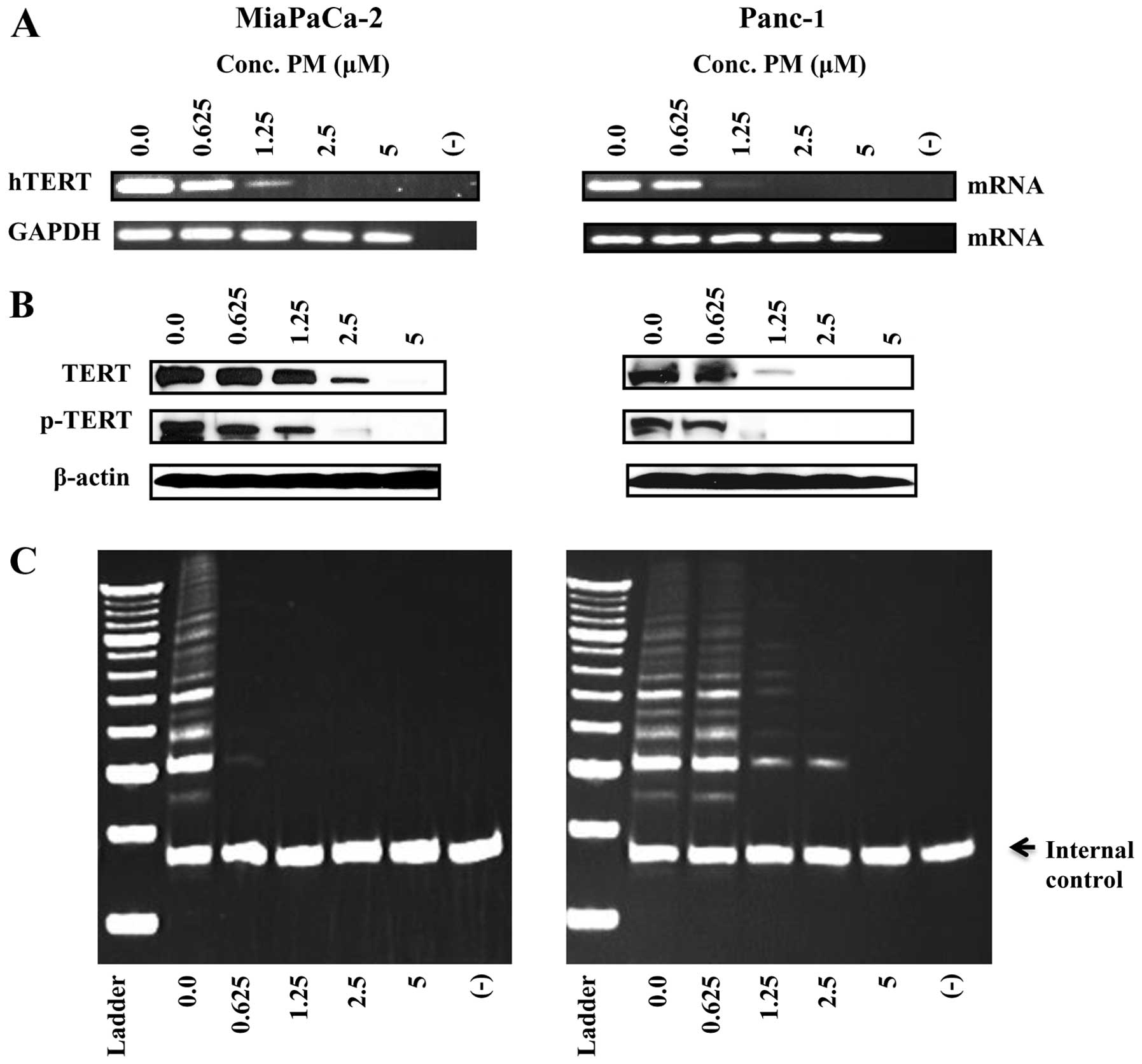
PM exerts on hTERT expression and its telomerase activity. Briefly,
hTERT mRNA was analyzed by RT-PCR and hTERT protein by western
blotting following treatment of the tumor cells with PM. Treatment
with PM at concentrations of 1.25-5 μM for 48 h completely
inhibited hTERT mRNA in the two cell lines (Fig. 3A). PM at these concentrations,
however, had no effect on the expression of the housekeeping gene
GAPDH.
Treatment with PM (1.25–5 μM) also resulted
in partial to complete reduction in the native and
phosphorylated-hTERT (Ser826) protein in the two cell
lines.
PM inhibits telomerase activity
To determine whether PM affects hTERT telomerase
activity, MiaPaCa-2 and Panc-1 cells were treated with PM (0–5
μM) for 48 h and the cells were extracted in CHAP lysis
buffer. The telomerase activity in the extracts was measured using
the PCR-based TRAP assay. As shown in Fig. 3C, the telomerase activity in the
MiaPaCa-2 cells was completely abolished even at the lowest
concentration of 0.625 μM PM, as determined from the
complete loss of DNA laddering. Telomerase activity was also
markedly reduced in Panc-1 cells at 1.25 and 2.5 μM PM and
completely abolished at 5 μM.
hTERT regulates the response to PM in PDA
cells
To identify the involvement of telomerase in
mediating the antiproliferative and apoptosis-inducing activity of
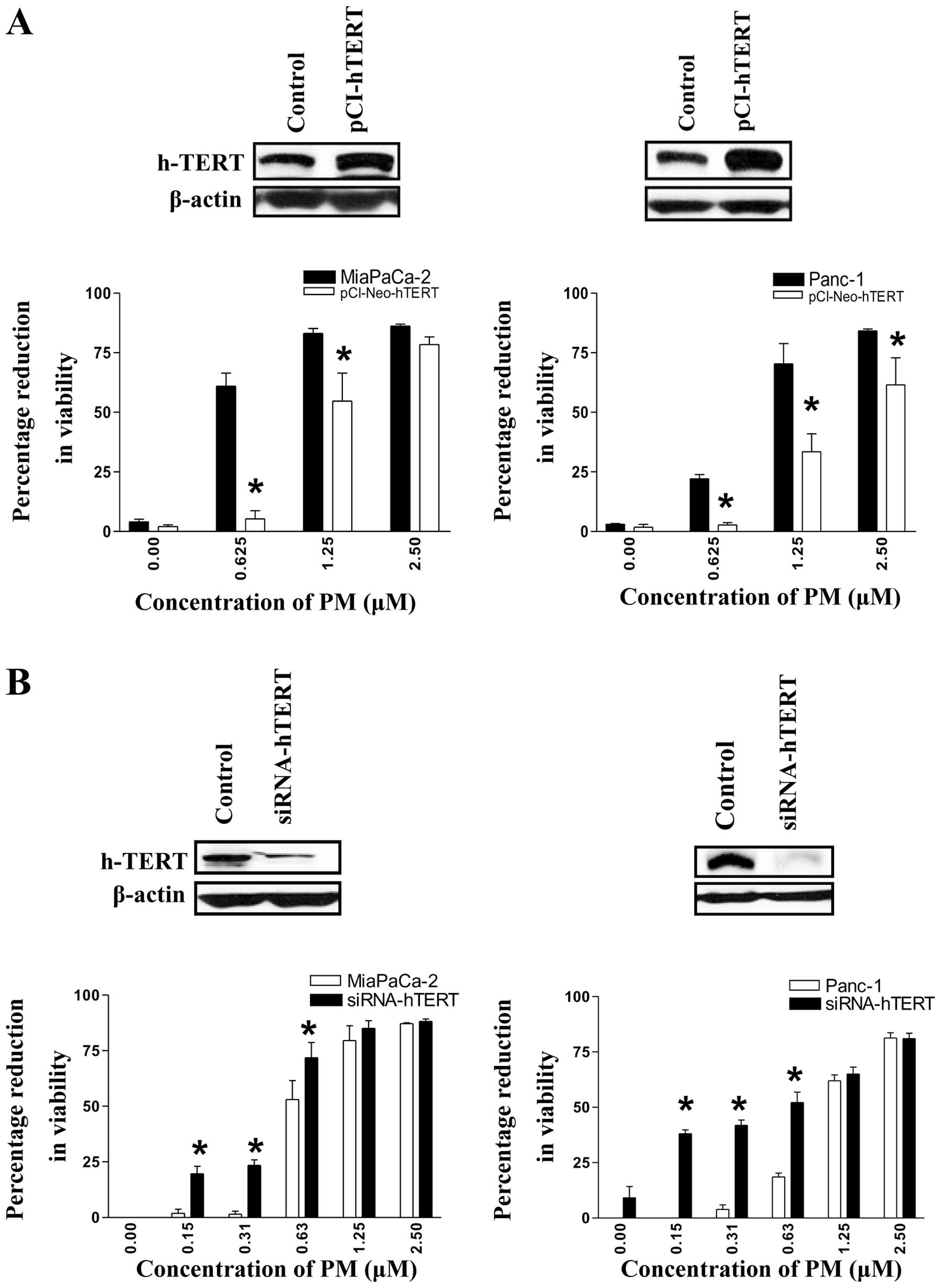
PM, we genetically altered the expression of hTERT in MiaPaCa-2 and
Panc-1 cells and measured their response to PM. To increase hTERT
expression, the tumor cells were transfected with pCI-neo-hTERT
expression vector for 48 h and the response to PM was measured in
the MTS assay. As expected, the transfected cells showed higher
levels of hTERT (Fig. 4A) and
demonstrated a significantly reduced sensitivity to PM,
particularly at lower concentrations of PM (0.625 and 1.25
μM) compared to the control cells (MiaPaCa-2, 5 vs. 61%, and
54 vs. 83%; Panc-1, 3 vs. 22%, and 33 vs. 70%). Similarly, to
investigate the effect of a reduced expression of hTERT on the
response to PM, tumor cells were transfected with hTERT siRNA for
48 h. As shown in Fig. 4B,
transfection with siRNA-hTERT significantly reduced the levels of
hTERT in the two cell lines (insets). A decrease in hTERT levels
significantly (p<0.05) increased the sensitivity of the two cell
lines to PM at concentrations that were otherwise inactive or only
slightly active (MiaPaCa-2, 19.6, 23 and 72% vs. 2, 1.4 and 53%;
Panc-1, 38, 42 and 52% vs. 0, 4 and 18% at 0.157, 0.312 and 0.625
μM PM). Transfection with empty plasmid or non-targeting
siRNA had no effect on response of cells to PM (data not shown).
These data demonstrated that hTERT regulates the response to PM in
pancreatic cancer cells.
PM inhibits hTERT regulatory
proteins
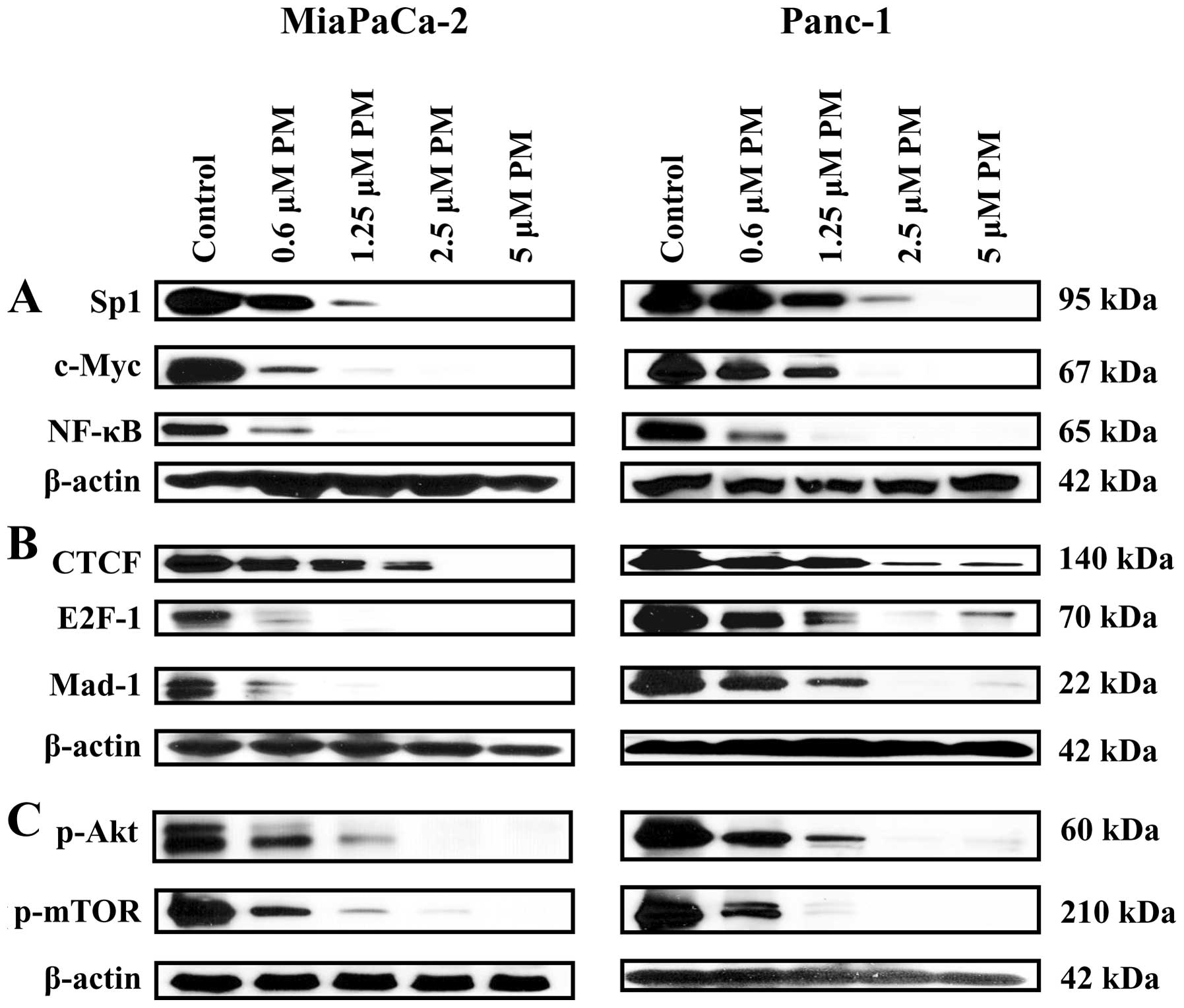
Transcription factors such as c-Myc, Sp1, NF-κB and
STAT-3 control the transcription of the hTERT gene (26,27)
and post-translationally, the phosphorylation of hTERT on
Ser227 and Ser826 residues by Akt is
necessary for the activation of telomerase activity and the nuclear
translocation of hTERT (28,29).
Therefore, we assessed the effect of PM on the levels of these
hTERT regulatory proteins. Treatment with PM (0.625–5 μM)
for 48 h partially to completely inhibited Sp1, c-Myc and NF-κB
(Fig. 5A). Of note, the inhibitory
factors that negatively regulate the transcription of the
hTERT gene (CTCF, E2F1 and Mad-1) were also reduced or
completely inhibited following treatment with PM (Fig. 5B). Furthermore, p-Akt and p-mTOR
that post-translationally regulate hTERT were also inhibited by PM
(Fig. 5C). These data demonstrated
that PM downregulates hTERT expression and activity through the
inhibition of transcription factors (Sp1, c-Myc and NF-κB) and
signaling molecules p-Akt and p-mTOR, respectively.
Discussion
Identification of novel agents and new targets for
treating pancreatic cancer is imperative. Several studies have
shown the antiproliferative and apoptosis-inducing activity of
pristimerin (PM) in various types of tumor cells, including
pancreatic cancer cells, through the inhibition of antiapoptotic or
prosurvival pathways (9–13), providing some insights into the mode
of action of PM. Telomerase, the enzyme that plays a crucial role
in maintaining telomere length and chromosomal stability is
activated in >90% of all human types of cancers including
pancreatic cancer (22). Activated
telomerase promotes tumorigenesis and tumor growth (20,21).
By contrast, the inhibition of telomerase induces telomere
shortening and apoptosis in cancer cells. Of all the pancreatic
cancers, PDA shows the highest increase in telomerase activity
(24). However, the significance of
telomerase in mediating the antitumor activity of PM in PDA cells
has not been investigated. Thus, the present study was undertaken
to examine the role of telomerase in mediating the proapoptotic
activity of PM in PDA cells. PM inhibited cell proliferation and
blocked cell-cycle progression in the G1-phase. The inhibition of
cell proliferation and cell cycle arrest in G1-phase by PM may have
led to induction of apoptosis in the two cell lines as demonstrated
by the increased binding of Annexin V and cleavage of PARP-1. These
results confirm the findings of another study that also reported
cell cycle arrest in G1-phase by PM in PDA cells (11).
Since cell proliferation and apoptosis are regulated
by telomerase, we investigated whether inhibition of cell
proliferation and induction of apoptosis correlate with the
expression and telomerase activity of hTERT in tumor cells treated
with PM. Specifically, analysis of hTERT mRNA by RT-PCR showed
attenuation of hTERT mRNA by PM. Western blot analysis also showed
a decrease in the levels of basal and phosphorylated hTERT. PM also
significantly inhibited hTERT telomerase activity as measured by
the TRAP assay. Thus, the inhibition of cell proliferation and
induction of apoptosis by PM correlated with the inhibition of
hTERT and its telomerase activity and suggested that inhibition of
telomerase is part of the mechanism by which PM inhibits
proliferation and induces apoptosis in PDA cells. However, further
study is required to determine whether inhibition of hTERT by PM
results in the shortening of telomeres and whether PM binds and
degrades the RNA template of telomerase.
The present study has also demonstrated the
relevance of hTERT in mediating the antitumor activity of PM in PDA
cells. We found that the expression level of hTERT influenced the
response of PDA cells to PM. An increase in the expression of hTERT
through gene knockin rendered PDA cells more resistant to PM. On
the other hand, knockdown of the expression of hTERT with siRNA
hTERT increased the sensitivity of PDA cells to concentrations of
PM that are otherwise inactive or only slightly active (0.156–0.625
μM). These data suggested that hTERT is a potential
molecular target of PM in PDA cells.
hTERT expression is driven by the binding of
transcription factors c-Myc, Sp1, NF-κB and STAT-3 to their
consensus sequences in hTERT promoter (26,27).
Inhibition of these transcription factors by PM impacted
transcription of the hTERT gene. We found that PM inhibited
Sp1, c-Myc and NF-κB in the two PDA cell lines, suggesting that
inhibition of these transcription factors is at least partially
responsible for the reduced hTERT gene transcription and
reduced hTERT protein in cells treated with PM. By contrast,
various repressors of gene transcription, such as CTCF, E2F-1 and
Mad-1 were also reduced or inhibited in cells treated with PM. This
result suggests that inhibition of the transcription factors Sp1,
c-Myc and NF-κB may be sufficient for the inhibition of hTERT
expression by PM.
Post-translationally, phosphorylation of hTERT on
Ser227 and Ser826 by protein kinase B/Akt is
required for nuclear import and activation of its telomerase
activity (28,29). The inhibition of telomerase activity
in PDA cells may result from the inhibition of kinase B/Akt by PM.
PM inhibited activated Akt (p-Akt). Thus, inhibition of hTERT
telomerase activity may be due to the lack of phosphorylation of
hTERT by Akt. hTERT is also regulated epigenetically through
chromatin remodeling and DNA methylation at the hTERT promoter
(30). However, whether PM impacts
these epigenetic events is currently being investigated.
Collectively, results of the present study identified telomerase as
a potential target that may be exploited for the treatment of PDA
with PM.
Acknowledgments
This study was supported by the NIH grant 1R01
CA130948 from the National Cancer Institute.
References
|
1
|
Pancreatic Cancer-National Cancer
Institute, U.S. National Institutes of Health: Cancer Gov.
http:www.cancer.gov/cancer-topics/types/pancreatic.
Retrieved 06-04-2010.
|
|
2
|
Maitra A and Hruban RH: Pancreatic cancer.
Annu Rev Pathol. 3:157–188. 2008. View Article : Google Scholar
|
|
3
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mulcahy MF, Wahl AO and Small W Jr: The
current status of combined radiotherapy and chemotherapy for
locally advanced or resected pancreas cancer. J Natl Compr Canc
Netw. 3:637–642. 2005.PubMed/NCBI
|
|
5
|
Pino SM, Xiong HQ, McConkey D and
Abbruzzese JL: Novel therapies for pancreatic adenocarcinoma. Curr
Oncol Rep. 6:199–206. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vaccaro V, Sperduti I and Milella M:
FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N
Engl J Med. 365:768–769; author reply 769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Costa PM1, Ferreira PM, Bolzani Vda S,
Furlan M, de Freitas Formenton Macedo Dos Santos VA, Corsino J, de
Moraes MO, Costa-Lotufo LV, Montenegro RC and Pessoa C:
Antiproliferative activity of pristimerin isolated from Maytenus
ilicifolia (Celastraceae) in human HL-60 cells. Toxicol In Vitro.
22:854–863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang H, Landis-Piwowar KR, Lu D, Yuan P,
Li L, Reddy GP, Yuan X and Dou QP: Pristimerin induces apoptosis by
targeting the proteasome in prostate cancer cells. J Cell Biochem.
103:234–244. 2008. View Article : Google Scholar
|
|
9
|
Yan YY, Bai JP, Xie Y, Yu JZ and Ma CG:
The triterpenoid pristimerin induces U87 glioma cell apoptosis
through reactive oxygen species-mediated mitochondrial dysfunction.
Oncol Lett. 5:242–248. 2013.
|
|
10
|
Wu CC, Chan ML, Chen WY, Tsai CY, Chang FR
and Wu YC: Pristimerin induces caspase-dependent apoptosis in
MDA-MB-231 cells via direct effects on mitochondria. Mol Cancer
Ther. 4:1277–1285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Zhou Y, Zhou H, Jia G, Liu J, Han
B, Cheng Z, Jiang H, Pan S and Sun B: Pristimerin causes G1 arrest,
induces apoptosis, and enhances the chemosensitivity to gemcitabine
in pancreatic cancer cells. PLoS One. 7:e438262012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu Z, Jin Y, Chen C, Li J, Cao Q and Pan
J: Pristimerin induces apoptosis in imatinib-resistant chronic
myelogenous leukemia cells harboring T315I mutation by blocking
NF-kappaB signaling and depleting Bcr-Abl. Mol Cancer. 9:1122010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deeb D, Gao X, Liu YB, Pindolia K and
Gautam SC: Pristimerin, a quinonemethide triterpenoid, induces
apoptosis in pancreatic cancer cells through the inhibition of
pro-survival Akt/NF-κB/mTOR signaling proteins and anti-apoptotic
Bcl-2. Int J Oncol. 44:1707–1715. 2014.PubMed/NCBI
|
|
14
|
Greider CW: Chromosome first aid. Cell.
67:645–647. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blackburn EH: Structure and function of
telomeres. Nature. 350:569–573. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kilian A, Bowtell DD, Abud HE, Hime GR,
Venter DJ, Keese PK, Duncan EL, Reddel RR and Jefferson RA:
Isolation of a candidate human telomerase catalytic subunit gene,
which reveals complex splicing patterns in different cell types.
Hum Mol Genet. 6:2011–2019. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng J, Funk WD, Wang SS, Weinrich SL,
Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, et al:
The RNA component of human telomerase. Science. 269:1236–1241.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Palm W and de Lange T: How shelterin
protects mammalian telomeres. Annu Rev Genet. 42:301–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Boeck G, Forsyth RG, Praet M and
Hogendoorn PC: Telomere-associated proteins: Cross-talk between
telomere maintenance and telomere-lengthening mechanisms. J Pathol.
217:327–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blasco MA and Hahn WC: Evolving views of
telomerase and cancer. Trends Cell Biol. 13:289–294. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Newbold RF: The significance of telomerase
activation and cellular immortalization in human cancer.
Mutagenesis. 17:539–550. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Janknecht R: On the road to immortality:
hTERT upregulation in cancer cells. FEBS Lett. 564:9–13. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohuchida K, Mizumoto K, Yamada D,
Yamaguchi H, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M and
Tanaka M: Quantitative analysis of human telomerase reverse
transcriptase in pancreatic cancer. Clin Cancer Res. 12:2066–2069.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grochola LF, Greither T, Taubert HW,
Möller P, Knippschild U, Udelnow A, Henne-Bruns D and Würl P:
Prognostic relevance of hTERT mRNA expression in ductal
adenocarcinoma of the pancreas. Neoplasia. 10:973–976. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hashimoto Y, Murakami Y, Uemura K,
Hayashidani Y, Sudo T, Ohge H, Fukuda E, Sueda T and Hiyama E:
Detection of human telomerase reverse transcriptase (hTERT)
expression in tissue and pancreatic juice from pancreatic cancer.
Surgery. 143:113–125. 2008. View Article : Google Scholar
|
|
26
|
Kyo S, Takakura M, Taira T, Kanaya T, Itoh
H, Yutsudo M, Ariga H and Inoue M: Sp1 cooperates with c-Myc to
activate transcription of the human telomerase reverse
transcriptase gene (hTERT). Nucleic Acids Res. 28:669–677. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Konnikova L, Simeone MC, Kruger MM,
Kotecki M and Cochran BH: Signal transducer and activator of
transcription 3 (STAT3) regulates human telomerase reverse
transcriptase (hTERT) expression in human cancer and primary cells.
Cancer Res. 65:6516–6520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang SS, Kwon T, Kwon DY and Do SI: Akt
protein kinase enhances human telomerase activity through
phosphorylation of telomerase reverse transcriptase subunit. J Biol
Chem. 274:13085–13090. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chung J, Khadka P and Chung IK: Nuclear
import of hTERT requires a bipartite nuclear localization signal
and Akt-mediated phosphorylation. J Cell Sci. 125:2684–2697. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meeran SM, Ahmed A and Tollefsbol TO:
Epigenetic targets of bioactive dietary components for cancer
prevention and therapy. Clin Epigenetics. 1:101–116. 2010.
View Article : Google Scholar
|