Introduction
Lung cancer is the leading cause of cancer-related
mortality, with an extremely poor prognosis worldwide (1–3). It
has also been reported that ~30–40% of patients with lung cancer
eventually develop bone metastasis, not only impairing quality of
life but also increasing the incidence of cancer-related death
(3–5). Osteolytic metastasis develops in
certain types of cancer, such as lung cancer, and is featured as
upregulated osteoclast activity and downregulated osteoblast
capacity, resulting in bone destruction (6–8).
Therefore, novel therapeutic strategies for preventing and treating
bone metastasis in lung cancer should be developed.
As is well known, cancer cells secrete various
factors which enhance osteoclastic resorption, and the release of
certain factors from the skeletal matrix has been proven to enhance
bone metastasis (9,10). There are several factors
contributing to cancer cell migration to bone, such as
stromal-derived factor-1, monocyte chemotactic protein 1, and
receptor activator of nuclear factor κB ligand (RANKL). Other
factors enhancing cancer cell growth within bone, such as
transforming growth factor-β (TGF-β) and parathyroid hormone
(PTH)-related protein, have also been identified (11–13).
Takiguchi et al reported that CXCL14 promoted bone
metastasis through enhancement of cancer cell tropism to the bone
and/or recruitment of bone marrow cells around metastatic cancer
cells (14). Our recent study
illustrated that lung cancer-derived IL-8 increased
osteoclastogenesis via the phospholipase D signaling transduction
pathway (15).
Grapes are a rich source of polyphenols, including
stilbene and flavonol derivatives (16). Stilbenes, vine phytoalexins, are
associated with the beneficial effects of drinking wine.
Resveratrol, pterostilbene and piceatannol are naturally occurring
stilbene derivatives (17).
Previous studies have revealed that resveratrol and pterostilbene
possess antioxidant, anti-inflammatory and anticancer properties
(18–24). Moreover, even freeze-dried residue
from red wine significantly inhibits bone resorption (25). Red grapes are rich in flavonols, a
ubiquitous class of flavonoids, which along with other derivatives
of flavonoids such as myricetin, laricitrin and syringetin (SGN),
possess antioxidant effects (26–28).
SGN (3,5,7,4′-tetrahy-droxy-3′,5′-dimethoxyflavone), a flavonoid
derivative, exists in both grapes and wine (27–29).
The SGN content of red grapes is 3.22% (27). Syringetin-3-O-glycoside is
the major derivative present in red grapes, with
syringetin-3-glycosides coexisting with corresponding free
aglycones released by hydrolysis in wine (30). In a colorectal epithelial
adenocarcinoma cell model, SGN was shown to inhibit cellular
proliferation by decreasing cyclooxygenase-2 and cyclin D1
expression (31). Our previous
study indicated that SGN stimulated osteoblast differentiation via
the bone morphogenetic protein-2 (BMP2)/extracellular
signal-regulated kinase 1/2 (ERK1/2) signaling transduction pathway
(32). The present study
demonstrated that SGN inhibited human lung adenocarcinoma A549 and
CL1-5 cell-mediated osteoclastogenesis through the downregulation
of the AKT/mammalian target of rapamycin (mTOR) signaling pathway.
Moreover, SGN downregulated macrophage-colony stimulating factor
(M-CSF) and RANKL expression in A549-CM-stimulated osteoblasts and
inhibited osteoblast-mediated osteoclastogenesis.
Materials and methods
Chemicals
SGN and laricitrin were purchased from Extrasynthese
(Genay, France); resveratrol, pterostilbene, piceatannol and
myricetin were obtained from Sigma Chemical (St. Louis, MO, USA)
(Fig. 1). These were dissolved in
dimethylsulfoxide (DMSO) (Sigma Chemical) and stored at −20°C.
Control cultures received the carrier solvent (0.1% DMSO). All
other chemicals used were of the purest form available
commercially.
Cell culture
Human lung adenocarcinoma A549 cells were obtained
from the American Type Culture Collection (CCL-185) (Manassas, VA,
USA) and cultured in F-12K medium containing 10% fetal bovine serum
(FBS) (both from Gibco-BRL, Gaithersburg, MD, USA). CL1-5 human
lung adenocarcinoma cells were generously provided by Dr Pan-Chyr
Yang (Department of Internal Medicine, National Taiwan university
Hospital) (33,34), and cultured in RPMI-1640 medium
supplemented with 10% FBS and 1% penicillin-streptomycin (both from
Gibco-BRL). Human primary osteoblasts were cultured in osteoblast
growth medium (OBM) (both from Lonza, Walkersville, MD, USA). For
conditioned medium (CM) collection, the A549 and CL1-5 cells were
treated with vehicle (0.1% DMSO) or various concentrations of SGN
for 12 h. After washing, fresh culture medium was added and
cultured for another 24 h. The supernatants were collected,
filtered (0.22-mm) and identified as A549-CM, SGN-A549-CM, CL1-5-CM
and SGN-CL1-5-CM. Osteoblasts were cultured with A549-CM,
SGN-A549-CM, CL1-5-CM or SGN-CL1-5-CM (20%) for another 24 h, then
the supernatants were collected and filtered (0.22-mm). These
supernatants were grouped as osteoblast-CM (OB-CM), A549-OB-CM,
SGN-A549-OB-CM, CL1-5-OB-CM and SGN-CL1-5-OB-CM. All condition
media were frozen and stored at −80°C, with a single thawing for
study.
Osteoclast differentiation
Human peripheral blood, obtained from healthy adult
volunteers, was collected in syringes containing 1,000 U/ml of
preservative-free heparin. Peripheral blood mononuclear cells
(PBMCs) were isolated by density centrifugation using
Ficoll-Hypaque, and re-suspended in RPMI-1640 medium supplemented
with 10% heat-inactivated FBS. The PBMCs were then plated and
incubated overnight at 37°C. CD14+ monocytes were
isolated using CD14+ mAb-conjugated magnetic beads (MACS
MicroBeads; Miltenyi Biotec, Ltd., Bergisch Gladbach, Germany)
according to the manufacturer’s protocol. The purity of
CD14+ cells was 94–97%. Monocytes were grown in culture
medium containing vehicle or tested compounds preset at 200 ng/ml
human M-CSF and 100 ng/ml human RANKL (R&D Systems,
Minneapolis, MN, USA) for 14–21 days. The medium was replaced every
5 days. The Institutional Review Board of the Kaohsiung Medical
University Chung-Ho Memorial Hospital approved the study protocol,
and all participants provided written informed consent in
accordance with the Declaration of Helsinki. Osteoclast formation
was measured by quantifying cells positively stained by
tartrate-resistant acid phosphatase (TRAP) (Sigma-Aldrich, St.
Louis, MO, USA). Briefly, the cells were fixed with formaldehyde
for 30 min and then stained with naphthol AS-BI phosphate and a
tartrate solution for 1 h at 37°C, followed by counterstaining with
a hematoxylin solution. Osteoclasts were determined to be
TRAP-positive staining multi-nuclear (>3 nuclei) cells by light
microscopy. The total number of TRAP-positive cells and the number
of nuclei/TRAP-positive cell in each well were counted.
Bone resorption assay
CD14+ monocytes were plated into a
calcium phosphate apatite-coated 48-well plate for the bone
resorption assay (Cosmo Bio Co., Ltd., Tokyo, Japan) in the same
culture conditions as previously described. After a 14-day culture,
each well was washed with saline. A solution of 5% sodium
hypochlorite was left in the well for 5 min to detach the cells.
The pit area in each well was determined by AlphaEaseFC software
(Alpha Innotech Corporation, San Leandro, CA, USA).
Immunoblotting
CD14+ monocytes were pre-treated with
vehicle control (0.1% DMSO) or SGN (20 µM) for 1 h or
otherwise indicated times (time-dependent manner), and then M-CSF
(200 ng/ml)/RANKL (100 ng/ml) was added for 0.5, 1 and 6 h. The
expression of various proteins was assessed by immunoblotting. The
cells were lysed on ice for 15 min by M-PER lysis reagent (Thermo
Fisher Scientific, Waltham, MA, USA). The cell lysate was
centrifuged at 14,000 × g for 15 min, and the supernatant fraction
was collected for immunoblotting. Equivalent amounts of protein
were resolved by SDS-PAGE (6–8%) and transferred to polyvinylidene
difluoride membranes. After blocking for 1 h in 5% non-fat dry milk
in TBS, the membrane was incubated with the primary Ab for 1–16 h
(1 h for GAPDH; 16 h for phosphorylated AKT, mTOR, 4EBP1 and
p70S6K; and total protein of AKT, mTOR, 4EBP1 and p70S6K). The
membrane was then treated with the appropriate
peroxidase-conjugated secondary Ab, and the immunoreactive proteins
were detected using an ECL kit (Millipore, Billerica, MA, USA)
according to the manufacturer’s instructions. All antibodies were
obtained from Cell Signaling Technology (Beverly, MA, USA). To
quantify immunoblot images on unsaturated bands, densitometric
analysis was performed using AlphaEaseFC software.
Enzyme-linked immunosorbent assay
(ELISA)
M-CSF levels were assessed by the M-CSF ELISA kit
(R&D Systems). The RANKL and OPG levels in the osteoblasts were
quantified using the DuoSet ELISA (R&D Systems).
Statistical analysis
Data are expressed as means ± SD. Statistical
comparisons were carried out using analysis of variance.
Significant differences (p<0.05) between two test groups were
analyzed by the Student’s t-test.
Results
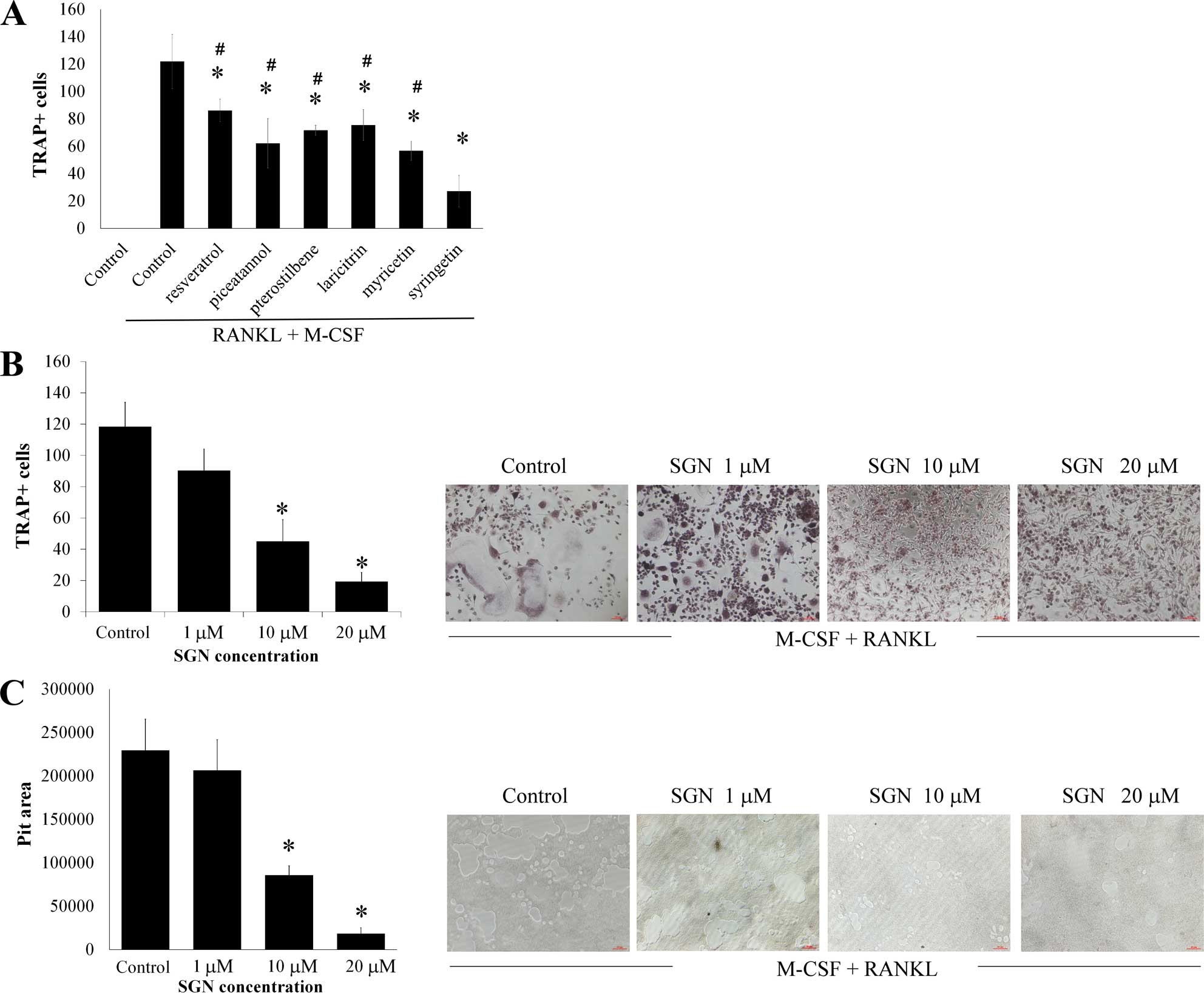
SGN exhibits a direct inhibitory effect
on osteoclast differentiation and bone resorption activity
To explore whether the novel agents inhibit
osteoclast development, the effects on osteoclast differentiation
by various polyphenols, including resveratrol, piceatannol,
pterostilbene, laricitrin, myricetin and SGN were assessed.
Treatment of the CD14+ monocytes with M-CSF and RANKL
caused formation of numerous TRAP-positive multi-nucleated
osteoclasts (Fig. 2A), whereas
osteoclast differentiation was significantly inhibited by
resveratrol, piceatannol, pterostilbene, laricitrin, myricetin and
SGN (20 µM). SGN showed the most significant inhibition of
osteoclast differentiation in comparison with resveratrol,
piceatannol, pterostilbene, laricitrin and myricetin. Consequently,
we selected SGN as the model for exploring the mechanism of lung
adenocarcinoma-related osteoclastogenesis. First, SGN treatment
inhibited osteoclast differentiation in a dose-dependent manner
(Fig. 2B). SGN treatment also
substantially reduced bone resorption in a dose-dependent manner
(Fig. 2C). These findings suggest
that SGN inhibits osteoclast differentiation and reduces bone
resorption activity in vitro.
SGN inhibits human lung
adenocarcinoma-mediated osteoclastogenesis
Next, we evaluated the effects of SGN on lung
adenocarcinoma-mediated osteoclastogenesis (Fig. 3A). The results showed that A549- and
CL-1-5-CMs markedly promoted osteoclastogenesis (Fig. 3B). However, the osteoclastic effect
induced by human lung adenocarcinoma A549- and CL1-5-CMs was
markedly inhibited by SGN. Furthermore, A549- and CL1-5-CMs
enhanced the bone resorption activity of the osteoclasts, yet this
effect was also reduced by SGN (Fig.
3C). Both results indicate that SGN suppresses human lung
adenocarcinoma A549 and CL1-5 cell-mediated osteoclast
differentiation and bone resorption activity.
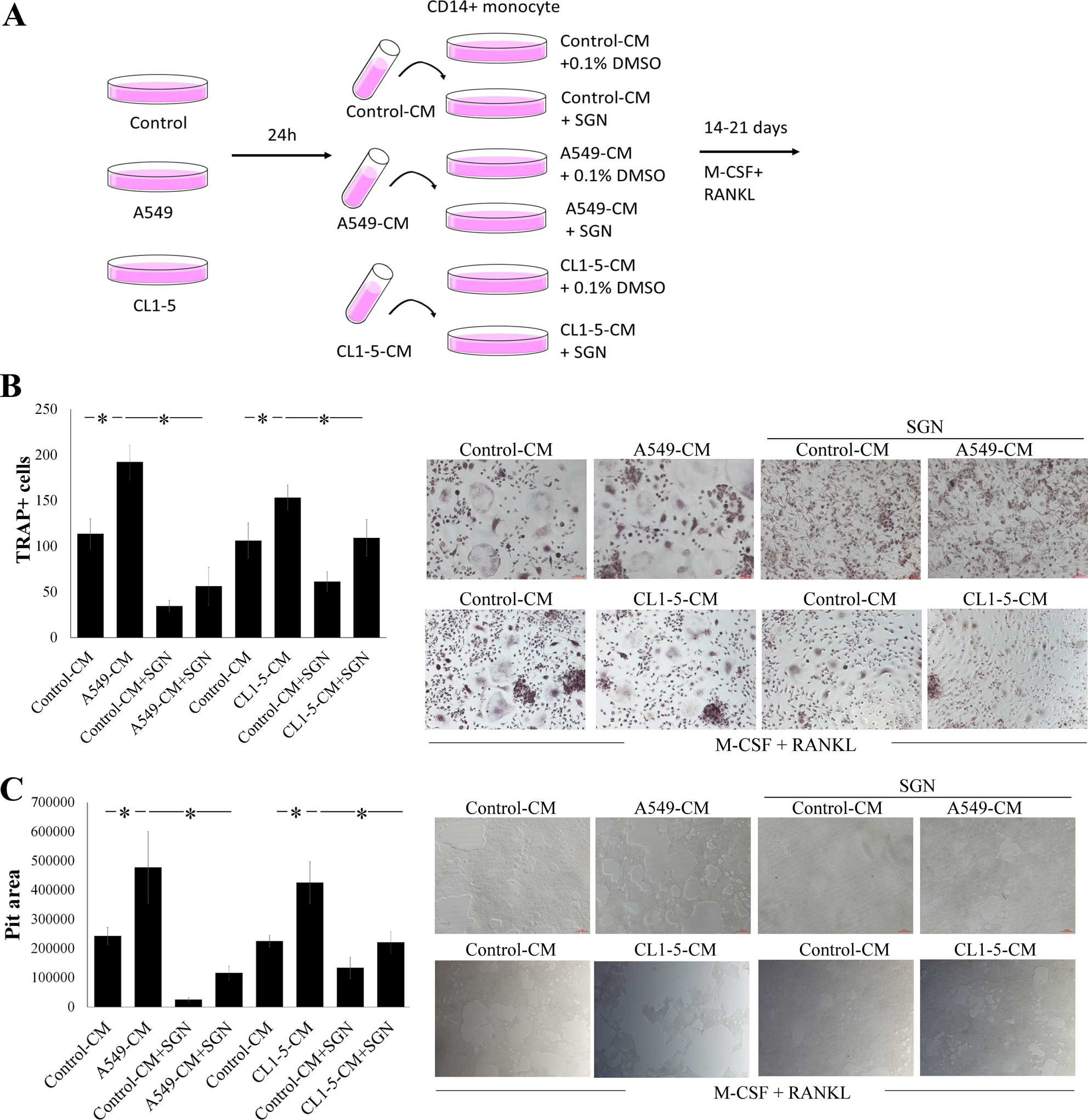 | Figure 3SGN inhibits human lung
adenocarcinoma cell-mediated osteoclastogenesis. (A) Flow chart of
the production of the control-, A549- and CL1-5-CM. SGN (20
µM) decreased (B) A549-mediated osteoclast differentiation
and (C) bone resorption. CD14+ monocytes were treated
with vehicle control (0.1% DMSO) or SGN (20 µM) in RPMI-1640
medium containing 20% of control-CM, A549-CM or CL1-5-CM for 21
(TRAP analysis) or 14 days (bone resorption assay). All media
contained RANKL (100 ng/ml) and M-CSF (200 ng/ml). The media were
replaced every 5 days. Osteoclast cells were stained for TRAP
activity, and bone resorption activity was determined by the bone
resorption assay kit. Each value is the mean ± SD of 3 independent
experiments. The pit areas were determined by AlphaEase FC
software. *p<0.05, significant difference with two
tested groups, as analyzed by the Student’s t-test. SGN,
syringetin; DMSO, dimethylsulfoxide; TRAP, tartrate-resistant acid
phosphatase; RANKL, receptor activator of nuclear factor κB ligand;
M-CSF, macrophage-colony stimulating factor. |
SGN suppresses osteoclastogenesis by
inhibiting AKT/mTOR
To clarify the molecular mechanism involved in SGN
osteoclastogenesis, signaling transduction pathways were
investigated. Treatment of M-CSF/RANKL increased the
phosphorylation of AKT and mTOR. The downstream targets of mTOR,
p70S6K and 4EBP1 were also activated by M-CSF/RANKL, suggesting
that the AKT/mTOR signaling transduction pathway was activated
during osteoclastogenesis. The osteoclastogenetic inducers (M-CSF
and RANKL) of AKT/mTOR expression were suppressed by SGN (Fig. 4A). Furthermore, both A549- and
CL1-5-CMs activated AKT/mTOR expression (Fig. 4B). Through treatment with both AKT
inhibitor IV and mTOR inhibitor (rapamycin) we further supported
the role of AKT/mTOR in osteoclast differentiation and bone
resorption (Fig. 4C and D). Both
AKT and mTOR inhibitors also suppressed A549 and CL1-5-mediated
osteoclasteogenesis (Fig. 4C and
D). In short, SGN not only inhibited M-CSF/RANKL-mediated
AKT/mTOR activation, yet also reduced the reinforcing effect of
lung adenocarcinoma on this signaling transduction pathway
(Fig. 4B). These results indicate
that SGN inhibits the AKT/mTOR signaling transduction pathway,
which is critical in osteoclastogenesis induced by both M-CSF/RANKL
and lung cancer cells.
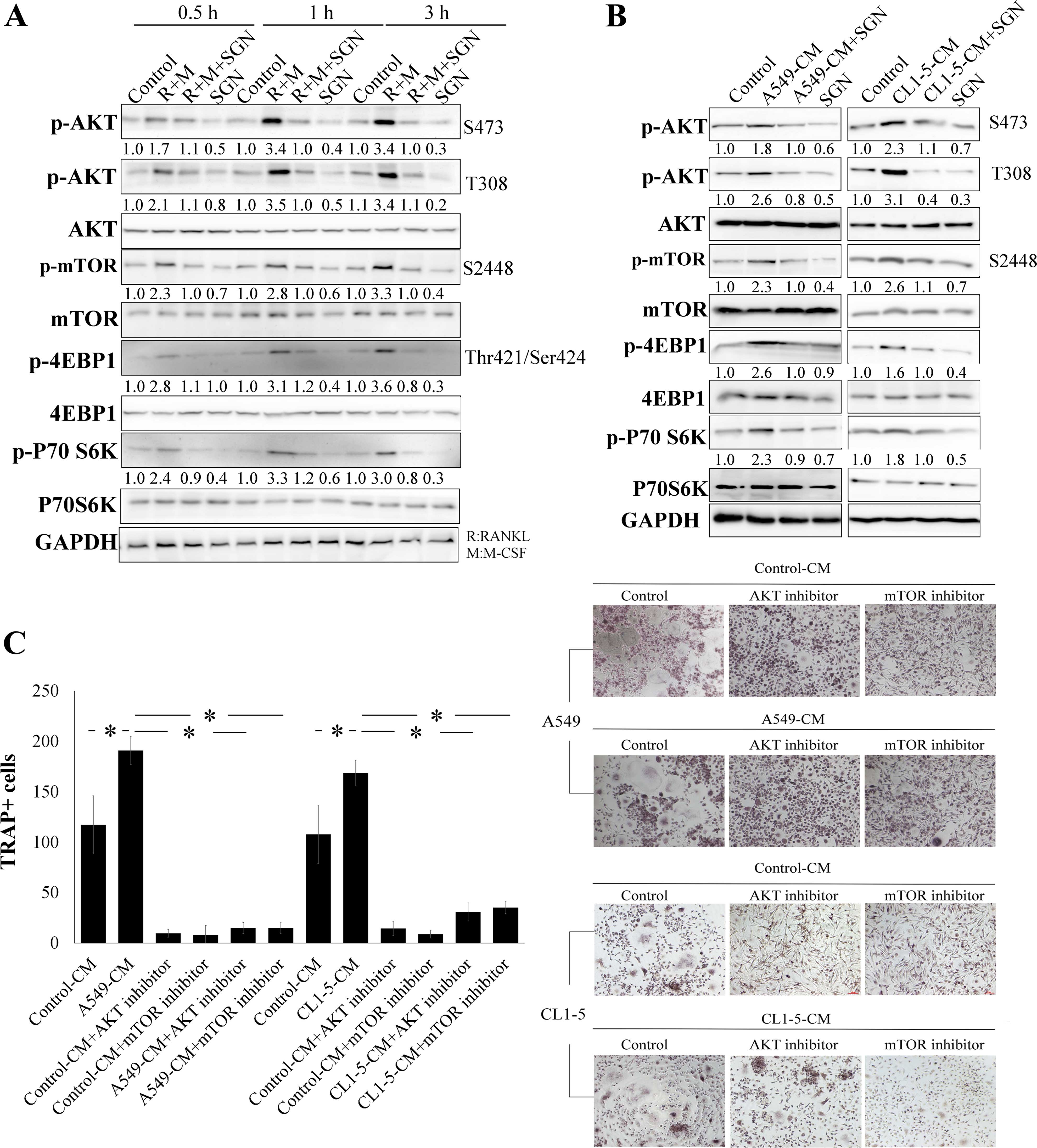 | Figure 4SGN suppresses osteoclastogenesis by
inhibiting AKT/mTOR signaling. (A) SGN (20 µM) reduced
M-CSF/RANKL-mediated AKT and mTOR signaling activation.
CD14+ monocytes were pre-treated with vehicle control
(0.1% DMSO) or SGN (20 µM) for 1 h and then M-CSF (200
ng/ml)/RANKL (100 ng/ml) were added for 0.5 h or the indicated
times (in a time-dependent manner). The expression of various
proteins was assessed by immunoblotting. (B) SGN decreased AKT/mTOR
activation induced by A549- and CL1-5-CM. CD14+
monocytes were pre-treated with vehicle control (0.1% DMSO) or SGN
(20 µM) for 1 h and then control-CM, A549-CM or CL1-5-CM
(20%) containing M-CSF (200 ng/ml)/RANKL (100 ng/ml) was added for
0.5 h or the indicated times (time-dependent manner). The
expression of various proteins was assessed by immunoblotting. (C)
AKT (AKT inhibitor IV) and mTOR (rapamycin) inhibitors decreased
osteoclast differentiation. CD14+ monocytes were treated
with vehicle control (0.1% DMSO), AKT (AKT inhibitor IV, 0.1
µM) and mTOR inhibitor (rapamycin, 0.5 µM) in
RPMI-1640 medium containing 20% of various control-CM, A549-CM and
CL1-5-CM for 21 days (TRAP analysis) or 14 days (bone resorption
assay). All media contained RANKL (100 ng/ml) and M-CSF (200
ng/ml). Each value is the mean ± SD of 3 independent experiments.
*p<0.05, significant difference between two tested
groups, as analyzed by the Student’s t-test. R, RANKL; M, M-CSF;
SGN, syringetin. (D) AKT (AKT inhibitor IV) and mTOR (rapamycin)
inhibitors decrease bone resorption. CD14+ monocytes
were treated with vehicle control (0.1% DMSO), AKT (AKT inhibitor
IV, 0.1 µM) and mTOR inhibitor (rapamycin, 0.5 µM) in
RPMI-1640 medium containing 20% of various control-CM, A549-CM and
CL1-5-CM for 21 days (TRAP analysis) or 14 days (bone resorption
assay). All media contained RANKL (100 ng/ml) and M-CSF (200
ng/ml). Each value is the mean ± SD of 3 independent experiments.
The pit areas were determined by Alphaease FC software.
*p<0.05, significant difference between two tested
groups, as analyzed by the Student’s t-test. RANKL, receptor
activator of nuclear factor κB ligand; M-CSF, macrophage-colony
stimulating factor; mTOR, mammalian target of rapamycin. |
SGN attenuates the stimulatory effect of
human lung adenocarcinoma on M-CSF and RANKL expression in
osteoblasts
Osteoblasts play a critical role in assisting bone
metastasis by increasing M-CSF and RANKL expression and decreasing
OPG expression (35). To verify
this, we examined the effect of SGN on secretions of M-CSF, RANKL
and OPG in osteoblasts by lung adenocarcinoma, A549- and CL1-5-CMs,
which markedly increased M-CSF and RANKL expression in the
osteoblasts (Fig. 5A and B). On the
other hand, A549- and CL1-5-CMs decreased OPG expression in the
osteoblasts (Fig. 5C). SGN
attenuated the stimulatory effect of lung adenocarcinoma on the
expression of M-CSF and RANKL, and reversed the effect of lung
adenocarcinoma on the suppression of OPG expression in the
osteoblasts (Fig. 5A–C).
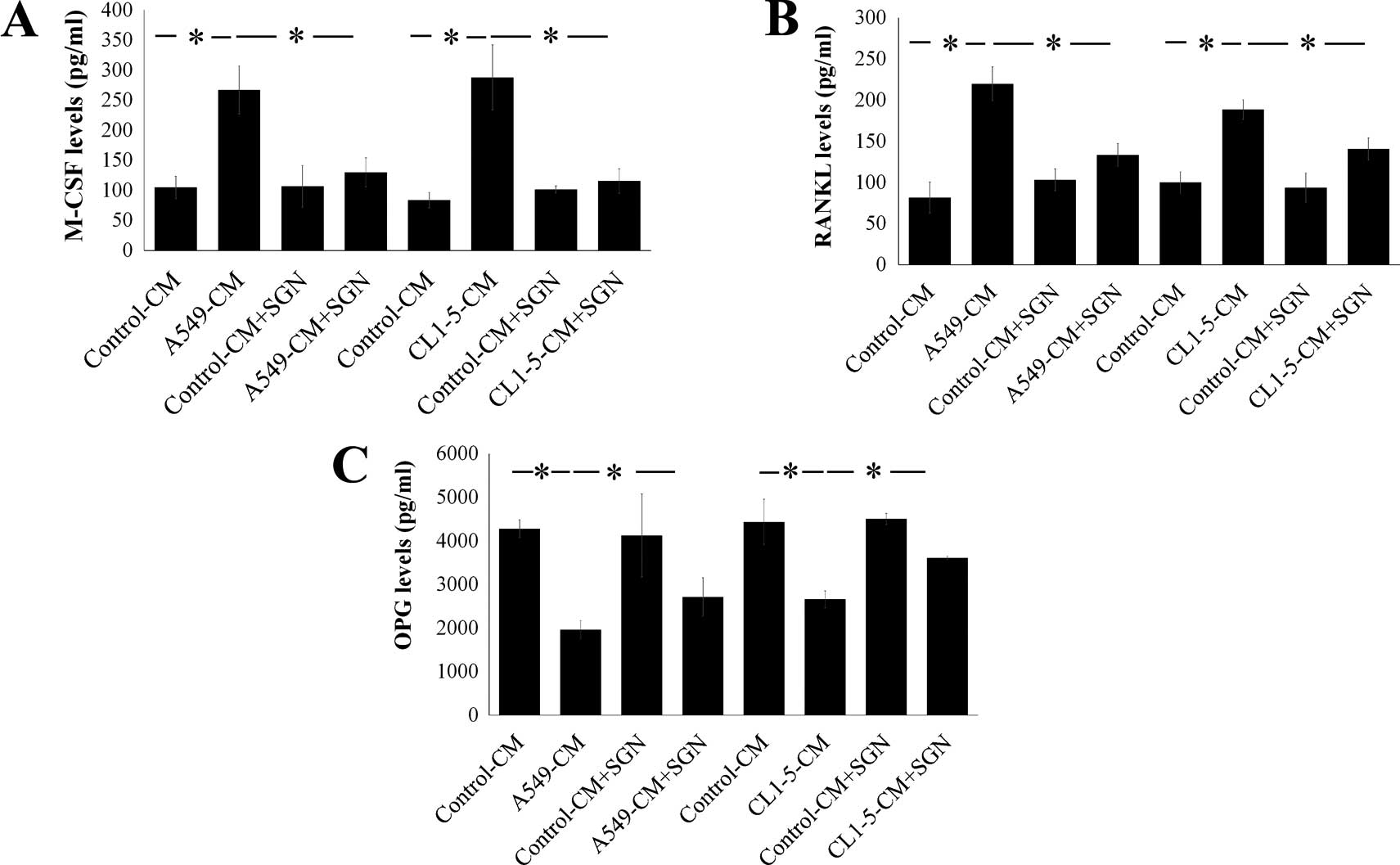 | Figure 5SGN attenuates the upregulation of
M-CSF and RANKL in osteoblasts after lung adenocarcinoma
stimulation. SGN (20 µM) decreased the stimulatory effect of
lung adenocarcinoma on the production of (A) M-CSF and (B) RANKL,
and reversed the suppressive effect of lung adenocarcinoma on the
production of (C) OPG in osteoblasts. A549 and CL1-5 cells were
treated with vehicle (0.1% DMSO) or SGN (20 µM) for 12 h.
After washing, fresh medium was added and cultured for another 24
h. The supernatants were collected, filtered (0.22 mm) and
identified as A549-CM, A549-CM+SGN, CL1-5-CM and CL1-5-CM+SGN.
Osteoblasts were cultured with A549-CM, A549-CM+SGN, CL1-5-CM and
CL1-5-CM+SGN (20%) for another 24 h, and the supernatants were then
collected. Levels of M-CSF, RANKL and OPG in the supernatants of
osteoblasts were assayed by M-CSF, RANKL and OPG ELISA kits,
respectively. Each value is the mean ± SD of 3 independent
experiments. *p<0.05 significant difference between
two tested groups, as analyzed by the Student’s t-test. SGN,
syringetin; RANKL, receptor activator of nuclear factor κB ligand;
M-CSF, macrophage-colony stimulating factor. |
SGN reduces the enhancing effect of human
lung adenocarcinoma on osteoblast-mediated osteoclastogenesis
The role of SGN in human lung
adenocarcinoma-mediated interaction between osteoblasts and
osteoclasts was further investigated (Fig. 6A). When compared with unstimulated
osteoblasts, human lung adenocarcinoma A549 and CL1-5 cell-treated
osteoblasts enhanced osteoclastogenesis. Although lung
adenocarcinoma A549 and CL1-5 cells promoted osteoblast-mediated
osteoclast differentiation and bone resorption, this effect was
altered by SGN (Fig. 6B and C).
These findings suggest that SGN inhibits the osteoblasts in lung
adenocarci-noma-mediated osteolytic bone metastasis.
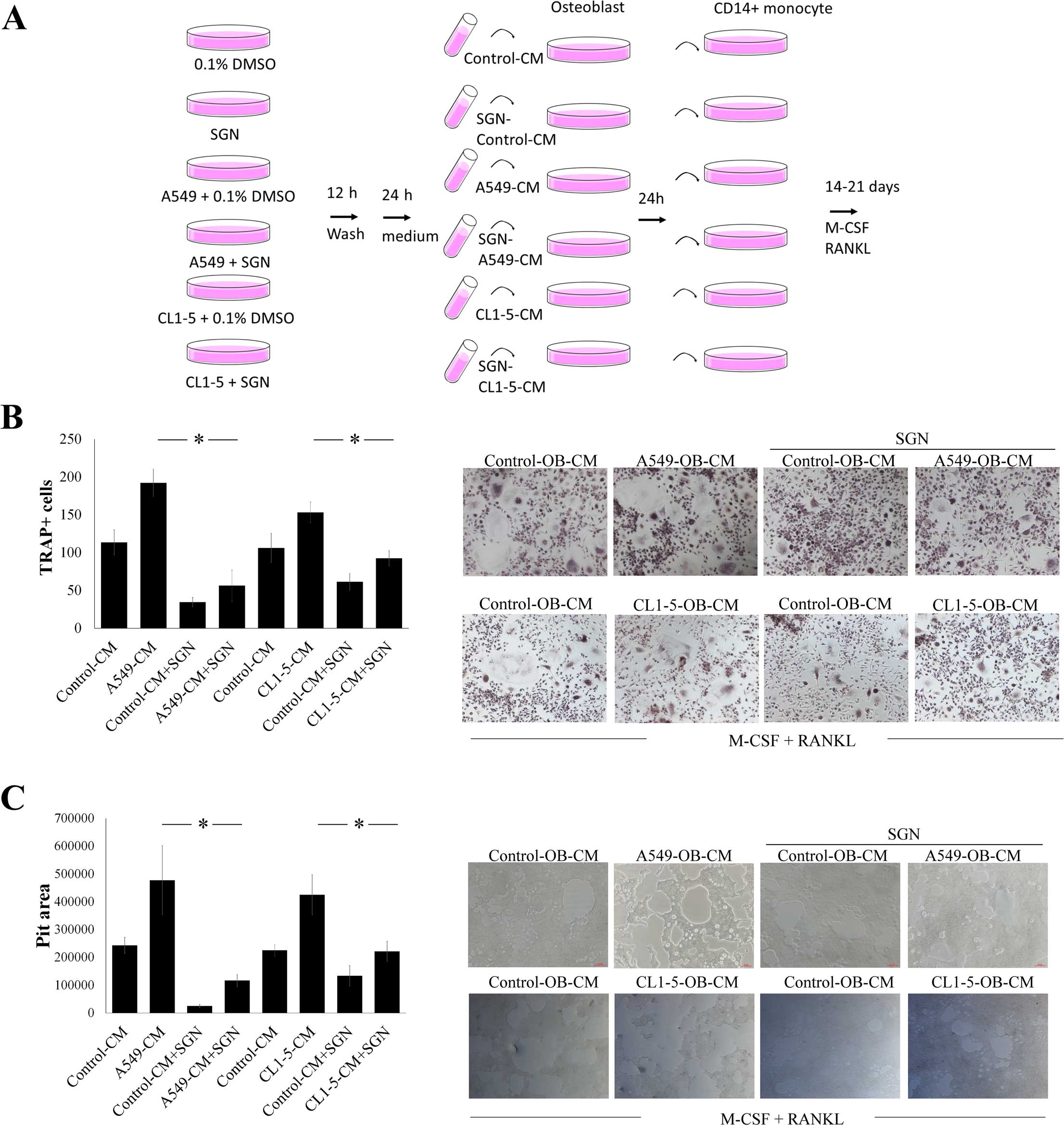 | Figure 6SGN reduces the stimulatory effect of
lung adenocarcinoma on osteoblast-mediated osteoclastogenesis. (A)
Flow chart of production of control-CM, A549-CM, SGN-A549-CM,
CL1-5-CM and SGN-CL1-5-CM, or various osteoblast conditioned media.
SGN (20 µM) reduced (B) osteoclast differentiation and (C)
bone resorption activity induced by lung adenocarcinoma-stimulated
osteoblasts. A549 and CL1-5 cells were treated with vehicle (0.1%
DMSO) or SGN (20 µM) for 12 h. After washing, fresh medium
was added and cultured for another 24 h. The supernatants were then
collected and filtered (0.22 mm). Osteoblasts were cultured with
control-CM, SGN-control-CM, A549-CM, SGN-A549-CM, CL1-5-CM and
SGN-CL1-5-CM (20%) for another 24 h, and the supernatants were
collected and filtered (0.22 mm). These supernatants were grouped
as osteoblast-CM (OB-CM), SGN-OB-CM, A549-OB-CM, SGN-A549-OB-CM,
CL1-5-OB-CM and SGN-CL1-5-OB-CM. CD14+ monocytes were
treated with various CMs of osteoblasts containing RANKL (100
ng/ml) and M-CSF (200 ng/ml) for 21 days (TRAP analysis) or 14 days
(bone resorption assay). All media contained RANKL (100 ng/ml) and
M-CSF (200 ng/ml). Osteoclast cells were stained for TRAP activity,
and bone resorption activity was determined by the bone resorption
assay kit. Each value is the mean ± SD of 3 independent
experiments. The pit areas were determined by AlphaEase FC
Software. *p<0.05, significant difference between two
tested groups, as analyzed by the Student’s t-test. SGN,
syringetin; RANKL, receptor activator of nuclear factor κB ligand;
M-CSF, macrophage-colony stimulating factor. |
Discussion
Bone metastasis in lung cancer patients is a
devastating event, since once it occurs, it markedly worsens
morbidity and mortality rates (36,37).
Sone and Yano demonstrated that compounds, bisphosphonates and
reveromycin A, which potentially suppress osteoclast activity, were
of benefit in treating lung cancer bone metastasis (38). Despite modern treatment strategies,
30–50% of patients still develop new bone metastases, skeletal
complications and disease progression, emphasizing the necessity
for novel therapies (39,40). Osteoclastic metastasis characterized
by lung cancer bone metastasis and osteoclast differentiation could
be a target for treatment strategy. Multi-modality therapy may be
required for lung cancer bone metastasis (36–38).
In the present study, SGN, a flavonoid derivative found in grapes
and wine, was first investigated to determine its suppressive
effects on osteoclast differentiation and bone resorption activity,
stimulated either by osteoclastogenic factors or lung cancer cells.
Furthermore, SGN was also found to inhibit the proosteoclastic
effect in osteoblasts stimulated by lung cancer cells. This
suggests that SGN possesses therapeutic potential in treating lung
adenocarcinoma bone metastasis (Fig.
7).
The balance between osteoblasts and osteoclasts can
be interrupted by metastasized cancer cells (6). Osteoblasts play vital roles in
regulating skeleton physiology, since they are not only precursors
of osteocytes, yet also influence osteoclast differentiation
(41,42). Osteoblasts promote osteoclast
differentiation from osteoclast precursors by producing M-CSF and
RANKL (43). In contrast,
osteoblasts inhibit osteoclastogenesis by secreting OPG, a decoy
receptor for RANKL, to block the binding of RANKL to its receptors
on pre-osteoclasts (44). Cancer
cells modify the pattern of M-CSF, RANKL and OPG expression in
osteoblasts, facilitating cancer cell-associated osteolytic bone
metastasis (45). In the present
study, human lung adenocarcinoma A549 and CL1-5 cells upregulated
M-CSF and RANKL expression yet downregulated OPG expression in
osteoblasts, thereby increasing osteoclast differentiation and bone
resorption activity. This upregulation of M-CSF and RANKL by human
lung adenocarcinoma cells was inhibited by SGN, resulting in lower
rates of lung cancer-associated osteoclastogenesis. The present
study revealed that SGN reversed the dysregulated interaction
between osteoblasts and osteoclasts in the skeletal
microenvironment of a lung adenocarcinoma model.
Furthermore, AKT is known as an important mediator
of proliferation, survival and differentiation in a variety of cell
types (46). It is involved in
regulating survival and differentiation of osteoclasts, and its
deficiency in osteoclasts leads to impaired bone resorption
(47,48). mTOR, a downstream molecule of AKT,
is also known to regulate osteoclast survival (49). Inhibition of the mTOR pathway by
rapamycin reduced the number of TRAP-positive multi-nucleated
osteoclasts in the chondro-osseous junction in rats (50). In the present study, osteoclast
differentiation induced by M-CSF/RANKL increased activation of the
AKT and mTOR signaling transduction pathway. Inhibitors of both AKT
and mTOR significantly inhibited osteoclast differentiation and
bone resorption activity, suggesting that the AKT/mTOR pathway
plays a crucial role in osteoclastogenesis. Moreover, human lung
adenocarcinoma A549 and CL1-5 cells increased the activation of
AKT/mTOR signaling, resulting in enhanced osteoclastogenesis. SGN
not only blocked M-CSF/RANKL-induced AKT/mTOR activation, yet also
prevented the reinforcing effect of lung adenocarcinoma on this
signaling transduction pathway. These data suggest that SGN is an
AKT/mTOR inhibitor, targeting both the induction of osteoclast
differentiation and the cancer-induced activation of AKT/mTOR.
In conclusion, SGN has protective potential against
human lung adenocarcinoma-mediated bone destruction by directly
decreasing cancer cell-mediated osteoclast differentiation and bone
resorption, and by restoring the balance of osteoblast-osteoclast
interaction. Taken together, SGN possesses dual ameliorating
effects on lung adenocarcinoma-associated osteolytic bone
metastasis.
Acknowledgments
The present study was supported by grants from the
National Science Council (NSC 101-2628-B-037-001-MY3 and NSC
101-2320-B-037-043-MY3), the Ministry of Science and Technology
(MOST 103-2320-B-037-006-MY3 and MOST 103-2314-B-037-052), the
Kaohsiung Medical University ‘Aim for the Top 500 Universities
Grant’ (grant no. KMU-DT103008), the Kaohsiung Medical University
‘Aim for the Top Universities Grant’ (grant nos. KMU-TP103A19 and
KMU-TP103A20) and the Kaohsiung Municipal Ta-Tung Hospital Research
Foundation (kmtth-102-032 and kmtth-103-019). The authors wish to
thank the Center for Resources, Research and Development of
Kaohsiung Medical University for its support with the
instrumentation.
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zükin M: Epidermal growth factor receptor
inhibitors in non-small cell lung cancer: Current status and future
perspectives. Rev Assoc Med Bras. 58:263–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuo PL, Liao SH, Hung JY, Huang MS and Hsu
YL: MicroRNA-33a functions as a bone metastasis suppressor in lung
cancer by targeting parathyroid hormone related protein. Biochim
Biophys Acta. 1830:3756–3766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al Husaini H, Wheatley-Price P, Clemons M
and Shepherd FA: Prevention and management of bone metastases in
lung cancer: A review. J Thorac Oncol. 4:251–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirsh V, Major PP, Lipton A, Cook RJ,
Langer CJ, Smith MR, Brown JE and Coleman RE: Zoledronic acid and
survival in patients with metastatic bone disease from lung cancer
and elevated markers of osteoclast activity. J Thorac Oncol.
3:228–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sterling JA, Edwards JR, Martin TJ and
Mundy GR: Advances in the biology of bone metastasis: How the
skeleton affects tumor behavior. Bone. 48:6–15. 2011. View Article : Google Scholar
|
|
7
|
Sims NA and Gooi JH: Bone remodeling:
Multiple cellular interactions required for coupling of bone
formation and resorption. Semin Cell Dev Biol. 19:444–451. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller RE, Jones JC, Tometsko M, Blake ML
and Dougall WC: RANKL inhibition blocks osteolytic lesions and
reduces skeletal tumor burden in models of non-small-cell lung
cancer bone metastases. J Thorac Oncol. 9:345–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pawelek JM and Chakraborty AK: The cancer
cell - leukocyte fusion theory of metastasis. Adv Cancer Res.
101:397–444. 2008. View Article : Google Scholar
|
|
10
|
Roato I: Interaction among cells of bone,
immune system, and solid tumors leads to bone metastases. Clin Dev
Immunol. 2013:3150242013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burger JA and Stewart DJ: CXCR4 chemokine
receptor antagonists: Perspectives in SCLC. Expert Opin Investig
Drugs. 18:481–490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsieh CJ, Kuo PL, Hou MF, Hung JY, Chang
FR, Hsu YC, Huang YF, Tsai EM and Hsu YL: Wedelolactone inhibits
breast cancer-induced osteoclastogenesis by decreasing Akt/mTOR
signaling. Int J Oncol. 46:555–562. 2015.
|
|
13
|
Cai Z, Chen Q, Chen J, Lu Y, Xiao G, Wu Z,
Zhou Q and Zhang J: Monocyte chemotactic protein 1 promotes lung
cancer-induced bone resorptive lesions in vivo. Neoplasia.
11:228–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takiguchi S, Korenaga N, Inoue K, Sugi E,
Kataoka Y, Matsusue K, Futagami K, Li YJ, Kukita T, Teramoto N, et
al: Involvement of CXCL14 in osteolytic bone metastasis from lung
cancer. Int J Oncol. 44:1316–1324. 2014.PubMed/NCBI
|
|
15
|
Hsu YL, Hung JY, Ko YC, Hung CH, Huang MS
and Kuo PL: Phospholipase D signaling pathway is involved in lung
cancer-derived IL-8 increased osteoclastogenesis. Carcinogenesis.
31:587–596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flamini R, Mattivi F, De Rosso M,
Arapitsas P and Bavaresco L: Advanced knowledge of three important
classes of grape phenolics: Anthocyanins, stilbenes and flavonols.
Int J Mol Sci. 14:19651–19669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rimando AM, Kalt W, Magee JB, Dewey J and
Ballington JR: Resveratrol, pterostilbene, and piceatannol in
vaccinium berries. J Agric Food Chem. 52:4713–4719. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan MH, Chang YH, Tsai ML, Lai CS, Ho SY,
Badmaev V and Ho CT: Pterostilbene suppressed
lipopolysaccharide-induced up-expression of iNOS and COX-2 in
murine macrophages. J Agric Food Chem. 56:7502–7509. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan MH, Chang YH, Badmaev V, Nagabhushanam
K and Ho CT: Pterostilbene induces apoptosis and cell cycle arrest
in human gastric carcinoma cells. J Agric Food Chem. 55:7777–7785.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan MH, Chiou YS, Chen WJ, Wang JM,
Badmaev V and Ho CT: Pterostilbene inhibited tumor invasion via
suppressing multiple signal transduction pathways in human
hepatocellular carcinoma cells. Carcinogenesis. 30:1234–1242. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan MH, Lin YT, Lin CL, Wei CS, Ho CT and
Chen WJ: Suppression of heregulin-β1/HER2-modulated invasive and
aggressive phenotype of breast carcinoma by pterostilbene via
inhibition of matrix metalloproteinase-9, p38 kinase cascade and
Akt activation. Evid Based Complement Alternat Med.
2011:5621872011. View Article : Google Scholar
|
|
22
|
Chiou YS, Tsai ML, Wang YJ, Cheng AC, Lai
WM, Badmaev V, Ho CT and Pan MH: Pterostilbene inhibits colorectal
aberrant crypt foci (ACF) and colon carcinogenesis via suppression
of multiple signal transduction pathways in azoxymethane-treated
mice. J Agric Food Chem. 58:8833–8841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiou YS, Tsai ML, Nagabhushanam K, Wang
YJ, Wu CH, Ho CT and Pan MH: Pterostilbene is more potent than
resveratrol in preventing azoxymethane (AOM)-induced colon
tumorigenesis via activation of the NF-E2-related factor 2
(Nrf2)-mediated antioxidant signaling pathway. J Agric Food Chem.
59:2725–2733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen RJ, Tsai SJ, Ho CT, Pan MH, Ho YS, Wu
CH and Wang YJ: Chemopreventive effects of pterostilbene on
urethane-induced lung carcinogenesis in mice via the inhibition of
EGFR-mediated pathways and the induction of apoptosis and
autophagy. J Agric Food Chem. 60:11533–11541. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mühlbauer RC, Lozano A, Reinli A and Wetli
H: Various selected vegetables, fruits, mushrooms and red wine
residue inhibit bone resorption in rats. J Nutr. 133:3592–3597.
2003.PubMed/NCBI
|
|
26
|
Singh H, Dixit S, Verma PC and Singh PK:
Evaluation of total phenolic compounds and insecticidal and
antioxidant activities of tomato hairy root extract. J Agric Food
Chem. 62:2588–2594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mattivi F, Guzzon R, Vrhovsek U, Stefanini
M and Velasco R: Metabolite profiling of grape: Flavonols and
anthocyanins. J Agric Food Chem. 54:7692–7702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Race EJ and Shrikhande AJ:
Anthocyanin transformation in Cabernet Sauvignon wine during aging.
J Agric Food Chem. 51:7989–7994. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Castillo-Muñoz N, Gómez-Alonso S,
García-Romero E and Hermosín-Gutiérrez I: Flavonol profiles of
Vitis vinifera red grapes and their single-cultivar wines. J Agric
Food Chem. 55:992–1002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Castillo-Muñoz N, Gómez-Alonso S,
García-Romero E, Gómez MV, Velders AH and Hermosín-Gutiérrez I:
Flavonol 3-O-glycosides series of Vitis vinifera Cv. Petit Verdot
red wine grapes. J Agric Food Chem. 57:209–219. 2009. View Article : Google Scholar
|
|
31
|
Gómez-Alonso S, Collins VJ, Vauzour D,
Rodríguez-Mateos A, Corona G and Spencer JPE: Inhibition of colon
adenocarcinoma cell proliferation by flavonols is linked to a G2/M
cell cycle block and reduction in cyclin D1 expression. Food Chem.
130:493–500. 2012. View Article : Google Scholar
|
|
32
|
Hsu YL, Liang HL, Hung CH and Kuo PL:
Syringetin, a flavonoid derivative in grape and wine, induces human
osteoblast differentiation through bone morphogenetic
protein-2/extracellular signal-regulated kinase 1/2 pathway. Mol
Nutr Food Res. 53:1452–1461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen HW, Lee JY, Huang JY, Wang CC, Chen
WJ, Su SF, Huang CW, Ho CC, Chen JJ, Tsai MF, et al: Curcumin
inhibits lung cancer cell invasion and metastasis through the tumor
suppressor HLJ1. Cancer Res. 68:7428–7438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chu YW, Yang PC, Yang SC, Shyu YC, Hendrix
MJ, Wu R and Wu CW: Selection of invasive and metastatic
subpopulations from a human lung adenocarcinoma cell line. Am J
Respir Cell Mol Biol. 17:353–360. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sambandam Y, Sundaram K, Liu A, Kirkwood
KL, Ries WL and Reddy SV: CXCL13 activation of c-Myc induces RANK
ligand expression in stromal/preosteoblast cells in the oral
squamous cell carcinoma tumor-bone microenvironment. Oncogene.
32:97–105. 2013. View Article : Google Scholar
|
|
36
|
Wu X, Liu T, Fang O, Leach LJ, Hu X and
Luo Z: miR-194 suppresses metastasis of non-small cell lung cancer
through regulating expression of BMP1 and p27kip1.
Oncogene. 33:1506–1514. 2014. View Article : Google Scholar
|
|
37
|
Hernández I, Moreno JL, Zandueta C,
Montuenga L and Lecanda F: Novel alternatively spliced ADAM8
isoforms contribute to the aggressive bone metastatic phenotype of
lung cancer. Oncogene. 29:3758–3769. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sone S and Yano S: Molecular pathogenesis
and its therapeutic modalities of lung cancer metastasis to bone.
Cancer Metastasis Rev. 26:685–689. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: A fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Coleman RE and McCloskey EV:
Bisphosphonates in oncology. Bone. 49:71–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Furugaki K, Moriya Y, Iwai T, Yorozu K,
Yanagisawa M, Kondoh K, Fujimoto-Ohuchi K and Mori K: Erlotinib
inhibits osteolytic bone invasion of human non-small-cell lung
cancer cell line NCI-H292. Clin Exp Metastasis. 28:649–659. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hsu YL, Huang MS, Yang CJ, Hung JY, Wu LY
and Kuo PL: Lung tumor-associated osteoblast-derived bone
morphogenetic protein-2 increased epithelial-to-mesenchymal
transition of cancer by Runx2/Snail signaling pathway. J Biol Chem.
286:37335–37346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takayanagi H: New immune connections in
osteoclast formation. Ann NY Acad Sci. 1192:117–123. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mountzios G, Dimopoulos MA, Bamias A,
Papadopoulos G, Kastritis E, Syrigos K, Pavlakis G and Terpos E:
Abnormal bone remodeling process is due to an imbalance in the
receptor activator of nuclear factor-kappaB ligand
(RANKL)/osteoprotegerin (OPG) axis in patients with solid tumors
metastatic to the skeleton. Acta Oncol. 46:221–229. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
McGrath EE: OPG/RANKL/RANK pathway as a
therapeutic target in cancer. J Thorac Oncol. 6:1468–1473. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Skeen JE, Bhaskar PT, Chen CC, Chen WS,
Peng XD, Nogueira V, Hahn-Windgassen A, Kiyokawa H and Hay N: Akt
deficiency impairs normal cell proliferation and suppresses
oncogenesis in a p53-independent and mTORC1-dependent manner.
Cancer Cell. 10:269–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moon JB, Kim JH, Kim K, Youn BU, Ko A, Lee
SY and Kim N: Akt induces osteoclast differentiation through
regulating the GSK3β/NFATc1 signaling cascade. J Immunol.
188:163–169. 2012. View Article : Google Scholar
|
|
48
|
Cao H, Zhu K, Qiu L, Li S, Niu H, Hao M,
Yang S, Zhao Z, Lai Y, Anderson JL, et al: Critical role of AKT
protein in myeloma-induced osteoclast formation and osteolysis. J
Biol Chem. 288:30399–30410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sugatani T and Hruska KA: Akt1/Akt2 and
mammalian target of rapamycin/Bim play critical roles in osteoclast
differentiation and survival, respectively, whereas Akt is
dispensable for cell survival in isolated osteoclast precursors. J
Biol Chem. 280:3583–3589. 2005. View Article : Google Scholar
|
|
50
|
Sanchez CP and He YZ: Bone growth during
rapamycin therapy in young rats. BMC Pediatr. 9:32009. View Article : Google Scholar : PubMed/NCBI
|