Introduction
Ovarian cancer is one of the most common and lethal
gynecologic cancer in women worldwide, and the incidence of this
cancer has been increasing in many countries (1–3). More
than 200,000 new cases of ovarian cancer were diagnosed in 2011
worldwide (4). At present, most
ovarian cancer patients are diagnosed at an advanced stage due to
the lack of effective detection at early stages (5). Although advances in surgery and
chemotherapy have been developed, only ~30% of patients achieve a
5-year survival after diagnosis (6). To date, surgery is still of importance
for improving the effect of chemotherapy and the survival rate.
Chemotherapy is an important strategy and the combination of
platinum and taxane has been used as the reference standard for the
chemotherapy of ovarian cancer (7).
This standard combination shows effectiveness with a response rate
of ~80% in advanced ovarian cancer patients, while unfortunately
most of these patients relapse owing to drug resistance (8,9).
Therefore, identification of molecular biomarkers with
clinicopathologic and prognostic significance is critically
important for improving therapeutic methods and prolonging the
survival of ovarian cancer patients.
Insulin-like growth factor 2 (IGF2) is a mitogenic
peptide hormone, which is expressed in most tissues (10). Serum IGF2 is usually low in
newborns, increasing in childhood and remaining at a similar
concentration in adults, although it may decrease slightly in
healthy elders (11,12). Many studies have shown that IGF2
regulates cell growth, differentiation and metabolism (13). Particularly, it is highly expressed
during embryogenesis, and is important in promoting fetal growth
(14). Growing evidence has shown
that IGF2 can promote cancer development and progression (15). In hepatocellular carcinoma, the
expression of IGF2 is usually elevated in patients (16). In esophageal cancer, high IGF2
expression is associated with reduced disease-free survival
(17). The differential expression
of IGF2 between African-American and Caucasian patients is believed
to contribute to breast cancer survival disparities (18). However, the clinical significance of
IGF2 expression remains unclear in ovarian cancer.
In the present study, we also identified IGF2 as a
differentially expressed gene between tumor and non-tumor ovarian
tissues (19), and we confirmed its
expression in normal, corresponding non-cancerous and cancerous
ovarian tissues by reverse transcription-polymerase chain reaction
(RT-PCR), real-time quantitative PCR (RT-qPCR), western blotting
and IHC. Furthermore, the prognostic significance of IGF2
expression was analyzed in human ovarian cancers. Our study showed
that IGF2 expression is frequently higher in ovarian cancer
tissues, and the expression pattern may be an important prognostic
factor for ovarian cancer patients.
Materials and methods
Patient samples
A total of 72 tumor, 15 para-carcinoma and 10 normal
ovarian tissues were obtained from the Southwest Hospital in
Chongqing, China. The present study was approved by the ethics
Committee of the Southwest Hospital in Chongqing, China. Informed
consent was signed by all of the recruited patients.
Isolation of total RNA
Total RNAs were extracted from frozen tissues.
Approximately 2.0 µg of total RNAs was treated with DNase I
to eliminate the genomic DNA contamination, and then were
reverse-transcribed to generate cDNAs.
Analysis of IGF2 expression by RT-PCR and
RT-qPCR
IGF2 expression was determined by RT-PCR. A series
of PCRs with different cycles was performed. Based on the pilot
experiments, the appropriate cycles were chosen. Human β-actin was
amplified as an endogenous control. The primers for IGF2 and
β-actin were (5′-3′): IGF2-F, TAC TTC AGC AGG CCC GCA AG and
IGF2-R, GGT GAC GTT TGG CCT CCC TG; β-actin-F, TTC TAC AAT GAG CTG
CGT GTG and β-actin-R, GGG GTG TTG AAG GTC TCA AA. RT-qPCR was
performed using an iq5 Real-Time Detection system (Bio-Rad
Laboratories, Hercules, CA, USA) and GoTaq® qPCR Master
Mix (Promega). The relative gene expression was calculated by the
equation 2−ΔΔCT. The sequences of the primers were
(5′-3′): IGF2-F1, GAT GCT GGT GCT TCT CAC CT and IGF2-R1, CAG ACG
AAC TGG AGG GTG TC; β-actin-F2, TGA CGT GGA CAT CCG CAA AG and
β-actin-R2, CTG GAA GGT GGA CAG CGA GG. All RT-qPCRs were performed
in triplicate.
Tissue microarray generation
To construct the tissue microarray (TMA) slides, two
cores were obtained from each representative tumor and adjacent
non-cancerous tissue (within a distance of 20 mm). The
non-cancerous adjacent tissues were stained with hematoxylin and
eosin, and then reviewed histologically by two pathologists, and
compared with normal tissue. Duplicate cylinders from intratumoral
and peritumoral areas were obtained, and the TMA containing 72
tumor and 15 peritumoral ovarian tissues was constructed (20).
Western blot analysis
Western blotting (WB) was performed using the IGF2
antibody (Santa Cruz Biotechnology) as previously described
(21). Protein (100 µg) was
run on 8% SDS-PAGE and transferred to PVDF membranes (Millipore).
The membranes were blocked and incubated with the primary antibody
(IGF2, 1:1,000; Santa Cruz Biotechnology). Then, the membranes were
washed, incubated with the secondary antibody (1:3,000; Jackson
ImmunoResearch Laboratories), and developed with SuperSignal West
Pico chemiluminescent substrate (Pierce). The same membrane was
stripped and incubated with β-actin (Sigma) serving as an internal
control.
Immunohistochemical analysis
Immunohistochemistry was performed using the IGF2
antibody (1:50; Santa Cruz Biotechnology) as previously described
(22). Tumor cell staining was
evaluated and considered positive when immunoreactivity was ≥10%.
Positive staining was quantified and classified into 5 categories:
<10% positive cells as 0; 10 to 25% as 1; 26 to 50% as 2; 51 to
75% as 3 and ≥76% as 4. Staining intensity was graded as negative
(0), weak (1), moderate (2) or strong (3). All core biopsies were independently
reviewed by two pathologists, and the expression level was defined
by the sum of positive staining and intensity.
Statistical analysis
Statistical analyses were performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Results are expressed as
the mean ± standard deviation (SD). Measurement data were analyzed
by a Student’s t-test, and the Chi-square test was used to analyze
the differences between categorical variables. A Kaplan-Meier
survival database that contained the survival information of
ovarian cancer patients and gene expression data obtained by using
affymetrix microarrays was used (23). The probe set was 202409_at (there
are 3 IGF2 probe sets: 210881_s_at, 202410_x_at and 202409_at).
Although the probe sets are different, the result was similar. The
patients were grouped according to the median or auto selection of
the best cut-off value. P<0.05 was considered to be
statistically significant.
Results
IGF2 is frequently increased in human
ovarian cancer tissues
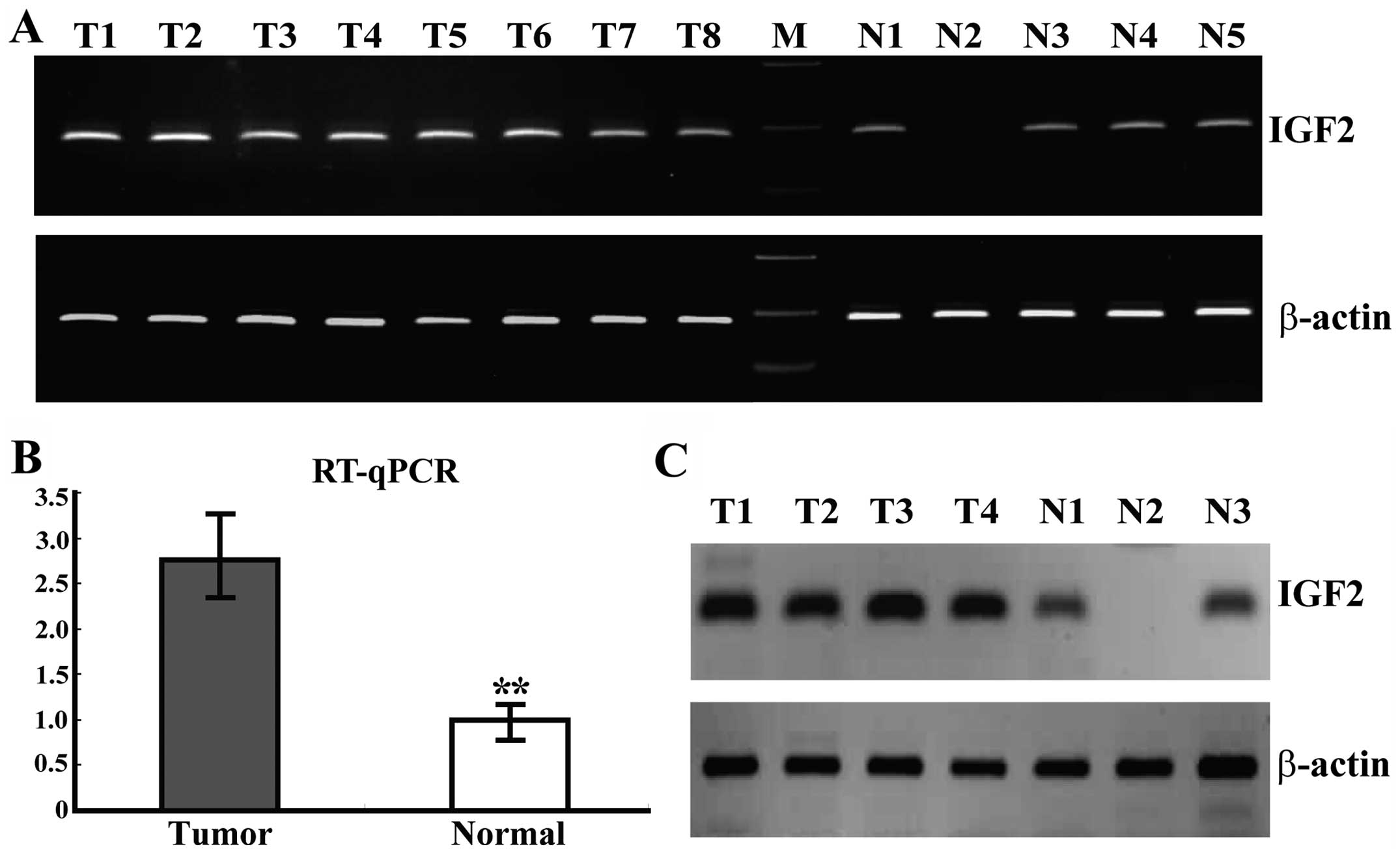
To investigate the level of IGF2 expression in human
ovarian cancers, RT-PCR and RT-qPCR assays were performed in 10
normal ovarian (10 different normal ovaries) and 35 ovarian cancer
tissues (35 different ovarian cancers). The expression of IGF2 in
most cases (30/35) of tumor tissues was upregulated compared to the
mean expression of the 10 normal ovarian tissues, and the mean
expression of IGF2 in cancer tissues (2.86±0.72) was markedly
higher than that in the normal ovarian tissues (1.00±0.18)
(P=0.000, Fig. 1A and B). The data
showed that IGF2 was frequently increased in the tumor tissues
compared to the frequency in the normal ones, which was further
confirmed by WB at the protein level (Fig. 1C).
IGF2 expression is correlated with
histological grade of ovarian cancer patients
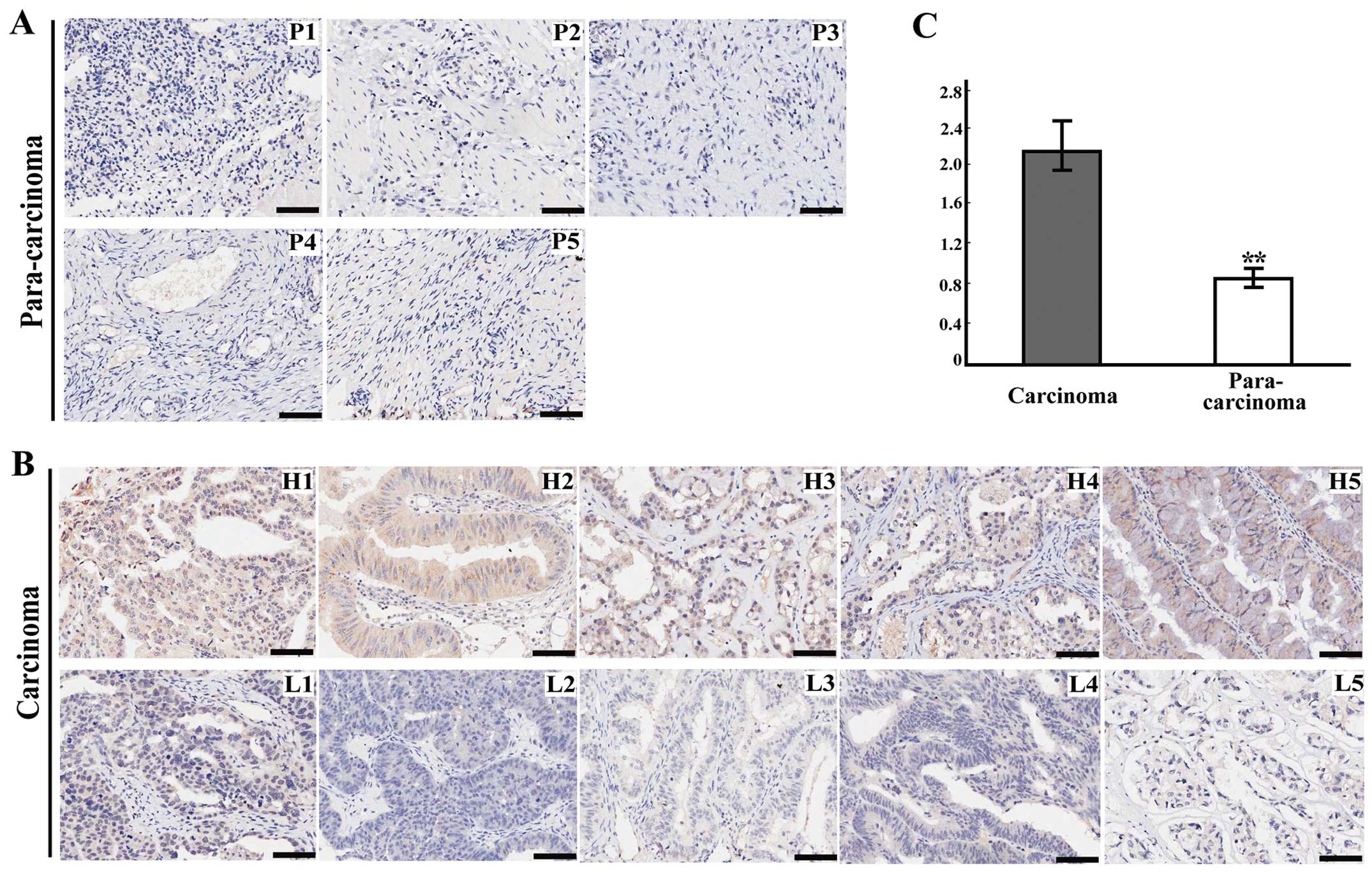
To confirm whether the IGF2 protein level is altered
in cancer, we also conducted IHC for IGF2 on a TMA containing 72
cancer and 15 para-carcinoma ovarian tissues. The IGF2 protein
level was increased in most (61/72) cancer tissues compared to the
level in the para-carcinoma ovarian tissues, and the mean
expression of IGF2 in cancer tissues (2.25±0.37) was evidently
higher than that in the para-carcinoma ovarian tissues (0.90±0.12)
(P=0.000, Fig. 2A–C). After
investigating the associations between IGF2 expression and
clinicopathologic features of the ovarian cancer patients, IGF2
expression was found to be significantly correlated with
histological grade (P=0.047). However, IGF2 expression was not
correlated with age, histological type, tumor size or location
(Table I).
 | Table ICorrelations of IGF2 expression with
clinicopathologic features of the ovarian cancer patients
(n=72). |
Table I
Correlations of IGF2 expression with
clinicopathologic features of the ovarian cancer patients
(n=72).
| Clinical feature | Total | IGF2 expression
| P-value |
|---|
| High (n=41) | Low (n=31) |
|---|
| Age (years) |
| <55 | 40 | 22 | 18 | 1.000 |
| ≥55 | 28 | 16 | 12 | |
| Histological
type |
| Serous | 39 | 22 | 17 | 1.000 |
| Other | 33 | 19 | 14 | |
| Histological
grade |
| 1+2 | 39 | 30 | 9 | 0.047 |
| 3 | 22 | 11 | 11 | |
| Tumor location |
| Left | 28 | 18 | 10 | 0.489 |
| Right | 26 | 13 | 13 | |
| Both | 18 | 10 | 8 | |
| Tumor size
(cm) |
| ≤10 | 29 | 17 | 12 | 1.000 |
| >10 | 32 | 19 | 13 | |
High expression of IGF2 is significantly
associated with poor overall survival (OS) of ovarian cancer
patients
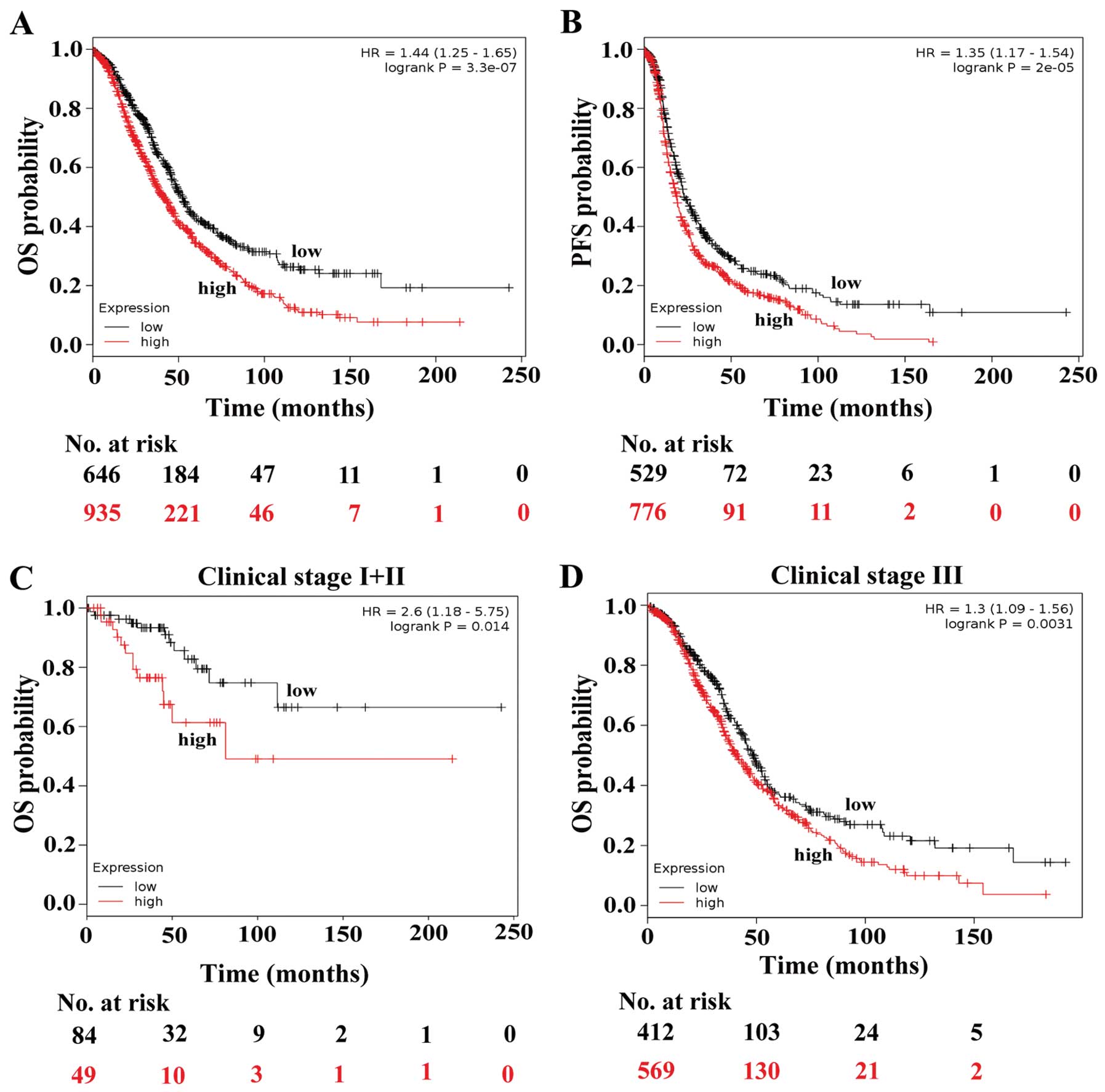
To investigate the correlation between IGF2
expression and survival of the tumor patients, we examined the
contribution of IGF2 expression to the OS of the ovarian cancer
patients in a clinical microarray database (23). This database collected gene
expression data obtained by using affymetrix microarrays and the OS
information of 1,648 ovarian cancer patients. The OS analysis
revealed that high expression of IGF2 predicts poorer survival of
ovarian cancer patients (HR=1.44, P=0.000, Fig. 3A). In addition, the patients with
high IGF2 expression also had a poorer progression-free survival
(PFS) compared with the low expression group (HR=1.35, P=0.000,
Fig. 3B).
High expression of IGF2 predicts poorer
survival of the patients at clinical stage I+II and III
To determine the association between IGF2 expression
and the OS of ovarian patients with different stages, we analyzed
the survival data of patients with different stages stratifying the
patients based on IGF2 expression. IGF2 expression was not
associated with OS time of ovarian patients at stage IV (P=0.301).
However, there was a statistically significant effect of high IGF2
expression on the poorer OS of the ovarian cancer patients at stage
I+II (HR=2.60, P=0.014) and III (HR=1.30, P=0.003, Fig. 3C and D). These findings suggest that
high expression of IGF2 is an unfavorable factor for the prognosis
of ovarian cancers at stage I+II and III.
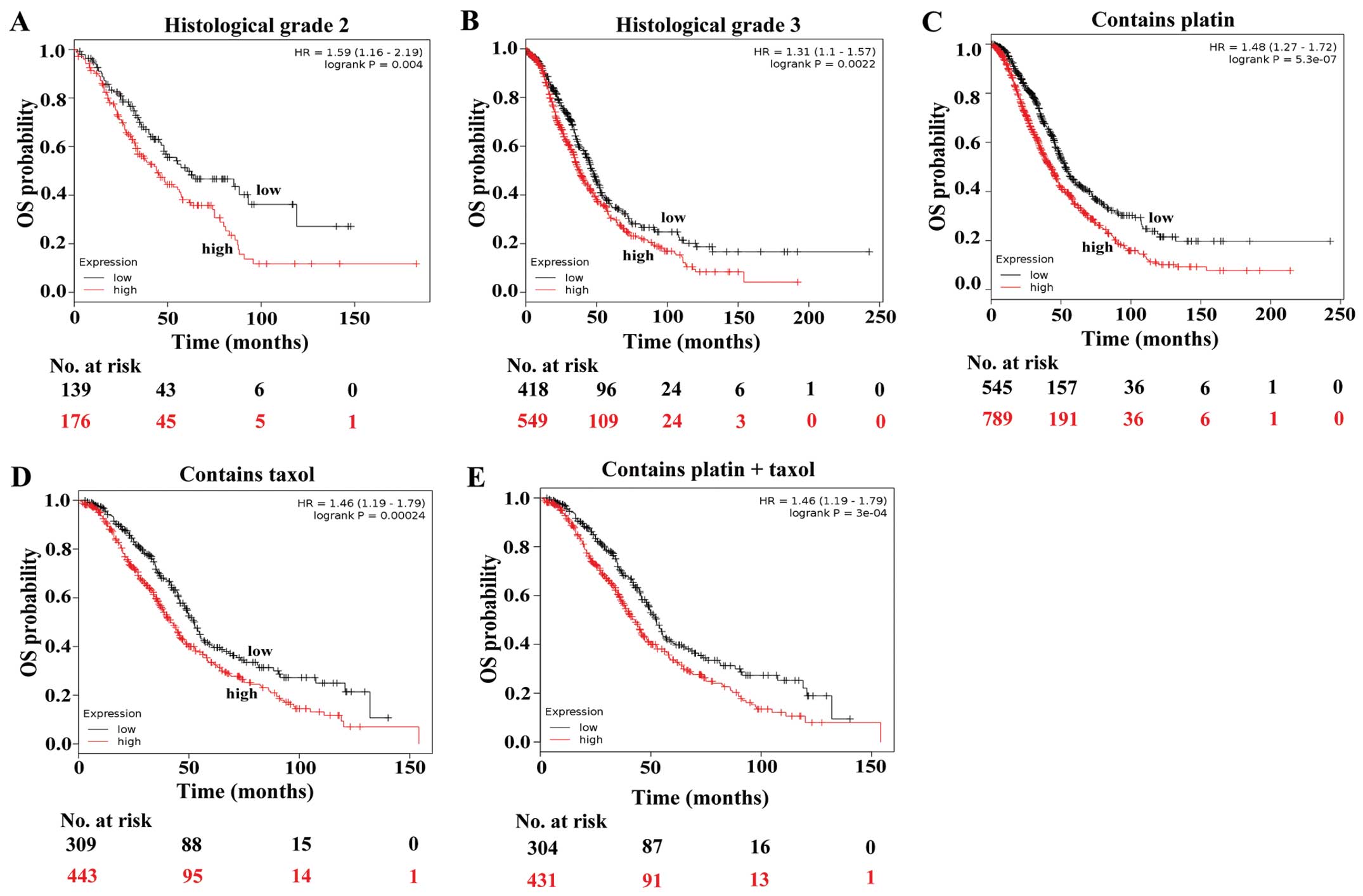
High expression of IGF2 predicts poorer
outcomes of the patients at grade 2 and 3
We then analyzed the survival data of patients at
different grades by stratifying the patients based on IGF2
expression. We observed a statistically significant effect of high
IGF2 expression on the poorer OS of the ovarian cancer patients at
grade 2 (HR=1.59, P=0.004) and 3 (HR=1.31, P=0.002, Fig. 4A and B), but no effect on that of
the patients at grade 1 (P=0.289). The results suggest that high
expression of IGF2 is an unfavorable factor for the prognosis of
ovarian cancers at grade 2 and 3.
High expression of IGF2 implies poorer
outcomes of the patients treated with chemotherapy containing
platin and taxol
We next aimed to ascertain whether high expression
of IGF2 also predicts poorer survival of the ovarian cancer
patients treated with different chemotherapeutic agents. We found a
statistically significant effect of high IGF2 expression on the
poorer OS of the ovarian cancer patients treated with chemotherapy
containing platin (HR=1.48, P=0.000) and chemotherapy containing
taxol (HR=1.46, P=0.00024, Fig. 4C and
D). To further validate the results, we analyzed the
association between IGF2 expression and OS times of the ovarian
patients treated with chemotherapy containing platin and taxol.
There was also a statistically significant effect of high IGF2
expression on the poorer OS of the patients treated with
chemotherapy containing platin and taxol (HR=1.46, P=0.000,
Fig. 4e). However, no effect of
high IGF2 expression was observed on the OS of the patients treated
with chemotherapy containing avastin, docetaxel, gemcitabine,
paclitaxel or topotecan.
Discussion
Accumulating data reveal the activation of IGF2 in
subsets of embryonic tumors, such as hepatoblastoma, neuroblastoma,
Wilms tumors and rhabdomyosarcoma (24,25).
Dysregulation of IGF 2 was also found in subsets of adult cancers,
and overexpression of IGF2 was detected in many tumors, for
example, in ~40% of colon carcinoma, 20% of hepatocarcinoma, 90% of
liposarcoma and adrenocortical carcinoma (10,26–28).
In the present study, we found that IGF2 expression was increased
in ~85.7% (30/35) of the ovarian cancer cases at the mRNA level and
84.7% (61/72) at the protein level, and the mean expression of IGF2
in the tumor tissues was obviously higher compared to that in the
non-tumor ovarian tissues (P=0.000).
Previous studies have shown that IGF2 mutations are
associated with risk for oral, colon and hepatocellular carcinoma
(29–31). Extensive evidence indicates that
increased IGF2 expression in tumors is associated with poorer
prognosis, for instance more rapid disease progression in chronic
myeloid leukemia, shorter time to disease recurrence in esophageal
cancer and higher mortality in breast cancer (17,18,32).
Moreover, transgenic mice overexpressing IGF2 have a high risk of
developing mammary gland adenocarcinoma and lung cancer, while the
animals with low IGF2 expression usually live longer, and have a
lower incidence of tumors (33–37).
In the present study, we found that the ovarian cancer patients
with high IGF2 expression had poorer OS and PFS compared with the
low expression group, suggesting that the expression of IGF2 could
act as a potential biomarker for prognostic evaluation of ovarian
cancers.
To further determine the association between IGF2
expression and OS of ovarian cancer patients with different
clinical stages, histological grades and chemotherapeutic
treatments, survival data were analyzed by stratifying the patients
based on the IGF2 levels. The results showed that high expression
of IGF2 may be an unfavorable factor for the prognosis of ovarian
cancer patients who were at clinical stage I+II and III,
histological grade 2 and 3 or treated with chemotherapy containing
platin and Taxol.
At present, the standard treatment for ovarian
cancer is surgery and systemic chemotherapy, usually with the
combination of taxol and platinum (6,7).
Unfortunately, the majority of these patients still succumb to
recurrent, progressive disease due to resistance to chemotherapy. A
recent study has shown that the silencing of IGF 2 can restore
taxol sensitivity in drugresistant ovarian cancer (38,39).
This study is consistent with our result that high expression of
IGF2 may be an unfavorable factor for the prognosis of ovarian
cancer patients.
Taken together, the present study revealed that IGF2
is upregulated in ovarian cancer tissues, and its expression may be
a potential marker for prognostic evaluation of ovarian cancers.
However, further investigation of the potential of IGF2 as a
therapeutic target is clearly warranted in ovarian cancer.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81172714).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen YL, Cheng WF, Chang MC, Lin HW, Huang
CT, Chien CL and Chen CA: Interferon-gamma in ascites could be a
predictive biomarker of outcome in ovarian carcinoma. Gynecol
Oncol. 131:63–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
White KL, Schildkraut JM, Palmieri RT,
Iversen ES Jr, Berchuck A, Vierkant RA, Rider DN, Charbonneau B,
Cicek MS, Sutphen R, et al Ovarian Cancer Association Consortium:
Ovarian cancer risk associated with inherited inflammation-related
variants. Cancer Res. 72:1064–1069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koyanagi T, Suzuki Y, Saga Y, Machida S,
Takei Y, Fujiwara H, Suzuki M and Sato Y: In vivo delivery of siRNA
targeting vasohibin-2 decreases tumor angiogenesis and suppresses
tumor growth in ovarian cancer. Cancer Sci. 104:1705–1710. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Rev Cancer. 11:719–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsofack SP, Meunier L, Sanchez L, Madore
J, Provencher D, Mes-Masson AM and Lebel M: Low expression of the
X-linked ribosomal protein S4 in human serous epithelial ovarian
cancer is associated with a poor prognosis. BMC Cancer. 13:3032013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marsh S: Pharmacogenomics of
taxane/platinum therapy in ovarian cancer. Int J Gynecol Cancer.
19(Suppl 2): S30–S34. 2009. View Article : Google Scholar
|
|
9
|
Kartalou M and Essigmann JM: Mechanisms of
resistance to cisplatin. Mutat Res. 478:23–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livingstone C: IGF2 and cancer. Endocr
Relat Cancer. 20:R321–R339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu H, Mistry J, Nicar MJ, Khosravi MJ,
Diamandis A, van Doorn J and Juul A: Insulin-like growth factors
(IGF-I, free IGF-I and IGF-II) and insulin-like growth factor
binding proteins (IGFBP-2, IGFBP-3, IGFBP-6, and ALS) in blood
circulation. J Clin Lab Anal. 13:166–172. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raynaud-Simon A: Levels of plasma
insulin-like growth factor I (IGF I), IGF II, IGF binding proteins,
type 1 IGF receptor and growth hormone binding protein in
community-dwelling elderly subjects with no malnutrition and no
inflammation. J Nutr Health Aging. 7:267–273. 2003.PubMed/NCBI
|
|
13
|
O’Dell SD and Day IN: Insulin-like growth
factor II (IGF-II). Int J Biochem Cell Biol. 30:767–771. 1998.
View Article : Google Scholar
|
|
14
|
Liu L, Greenberg S, Russell SM and Nicoll
CS: Effects of insulin-like growth factors I and II on growth and
differentiation of transplanted rat embryos and fetal tissues.
Endocrinology. 124:3077–3082. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu H and Rohan T: Role of the insulin-like
growth factor family in cancer development and progression. J Natl
Cancer Inst. 92:1472–1489. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
El Tayebi HM, Salah W, El Sayed IH, Salam
EM, Zekri AR, Zayed N, Salem ES, Esmat G and Abdelaziz AI:
Expression of insulin-like growth factor-II, matrix
metalloproteinases, and their tissue inhibitors as predictive
markers in the peripheral blood of HCC patients. Biomarkers.
16:346–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao R, DeCoteau JF, Geyer CR, Gao M, Cui
H and Casson AG: Loss of imprinting of the insulin-like growth
factor II (IGF2) gene in esophageal normal and adenocarcinoma
tissues. Carcinogenesis. 30:2117–2122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kalla Singh S, Tan QW, Brito C, De León M,
Garberoglio C and De León D: Differential insulin-like growth
factor II (IGF-II) expression: A potential role for breast cancer
survival disparity. Growth Horm IGF Res. 20:162–170. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng J, Dong Y, Li C, Zuo W, Meng G, Xu C
and Li J: Decreased expression of C10orf10 and its prognostic
significance in human breast cancer. PLoS One. 9:e997302014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY,
Xiao YS, Xu Y, Li YW and Tang ZY: Intratumoral balance of
regulatory and cytotoxic T cells is associated with prognosis of
hepatocellular carcinoma after resection. J Clin Oncol.
25:2586–2593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han F, Liu W, Jiang X, Shi X, Yin L, Ao L,
Cui Z, Li Y, Huang C, Cao J, et al: SOX30, a novel epigenetic
silenced tumor suppressor, promotes tumor cell apoptosis by
transcriptional activating p53 in lung cancer. Oncogene. Dec
1–2014.Epub ahead of print. View Article : Google Scholar
|
|
22
|
Han F, Dong Y, Liu W, Ma X, Shi R, Chen H,
Cui Z, Ao L, Zhang H, Cao J, et al: Epigenetic regulation of sox30
is associated with testis development in mice. PLoS One.
9:e972032014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gyorffy B, Lánczky A and Szállási Z:
Implementing an online tool for genome-wide validation of
survival-associated biomarkers in ovarian-cancer using microarray
data from 1287 patients. Endocr Relat Cancer. 19:197–208. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
El-Badry OM, Romanus JA, Helman LJ, Cooper
MJ, Rechler MM and Israel MA: Autonomous growth of a human
neuroblastoma cell line is mediated by insulin-like growth factor
II. J Clin Invest. 84:829–839. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhan S, Shapiro DN and Helman LJ:
Activation of an imprinted allele of the insulin-like growth factor
II gene implicated in rhabdomyosarcoma. J Clin Invest. 94:445–448.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tricoli JV, Rall LB, Karakousis CP,
Herrera L, Petrelli NJ, Bell GI and Shows TB: Enhanced levels of
insulin-like growth factor messenger RNA in human colon carcinomas
and liposarcomas. Cancer Res. 46:6169–6173. 1986.PubMed/NCBI
|
|
27
|
Cariani E, Lasserre C, Seurin D, Hamelin
B, Kemeny F, Franco D, Czech MP, Ullrich A and Brechot C:
Differential expression of insulin-like growth factor II mRNA in
human primary liver cancers, benign liver tumors, and liver
cirrhosis. Cancer Res. 48:6844–6849. 1988.PubMed/NCBI
|
|
28
|
Gicquel C, Bertagna X, Schneid H,
Francillard-Leblond M, Luton JP, Girard F and Le Bouc Y:
Rearrangements at the 11p15 locus and overexpression of
insulin-like growth factor-II gene in sporadic adrenocortical
tumors. J Clin Endocrinol Metab. 78:1444–1453. 1994.PubMed/NCBI
|
|
29
|
Yoon AJ, Zavras AI, Chen MK, Lin CW and
Yang SF: Association between Gly1619ARG polymorphism of IGF2R
domain 11 (rs629849) and advanced stage of oral cancer. Med Oncol.
29:682–685. 2012. View Article : Google Scholar
|
|
30
|
Hoyo C, Murphy SK, Schildkraut JM, Vidal
AC, Skaar D, Millikan RC, Galanko J, Sandler RS, Jirtle R and Keku
T: IGF2R genetic variants, circulating IGF2 concentrations and
colon cancer risk in African Americans and Whites. Dis Markers.
32:133–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Couvert P, Carrié A, Tezenas du Montcel S,
Vaysse J, Sutton A, Barget N, Trinchet JC, Beaugrand M, Ganne N,
Giral P, et al: Insulin-like growth factor 2 gene methylation in
peripheral blood mononuclear cells of patients with hepatitis C
related cirrhosis or hepatocellular carcinoma. Clin Res Hepatol
Gastroenterol. 36:345–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Randhawa GS, Cui H, Barletta JA,
Strichman-Almashanu LZ, Talpaz M, Kantarjian H, Deisseroth AB,
Champlin RC and Feinberg AP: Loss of imprinting in disease
progression in chronic myelogenous leukemia. Blood. 91:3144–3147.
1998.PubMed/NCBI
|
|
33
|
Bates P, Fisher R, Ward A, Richardson L,
Hill DJ and Graham CF: Mammary cancer in transgenic mice expressing
insulin-like growth factor II (IGF-II). Br J Cancer. 72:1189–1193.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moorehead RA, Sanchez OH, Baldwin RM and
Khokha R: Transgenic overexpression of IGF-II induces spontaneous
lung tumors: a model for human lung adenocarcinoma. Oncogene.
22:853–857. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rogler CE, Yang D, Rossetti L, Donohoe J,
Alt E, Chang CJ, Rosenfeld R, Neely K and Hintz R: Altered body
composition and increased frequency of diverse malignancies in
insulin-like growth factor-II transgenic mice. J Biol Chem.
269:13779–13784. 1994.PubMed/NCBI
|
|
36
|
Pravtcheva DD and Wise TL: Metastasizing
mammary carcinomas in H19 enhancers-Igf2 transgenic mice. J exp
Zool. 281:43–57. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bartke A, Chandrashekar V, Bailey B,
Zaczek D and Turyn D: Consequences of growth hormone (GH)
overexpression and GH resistance. Neuropeptides. 36:201–208. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang GS, Brouwer-visser J, Ramirez MJ,
Kim CH, Hebert TM, Lin J, Arias-Pulido H, Qualls CR, Prossnitz ER,
Goldberg GL, et al: Insulin-like growth factor 2 expression
modulates Taxol resistance and is a candidate biomarker for reduced
disease-free survival in ovarian cancer. Clin Cancer Res.
16:2999–3010. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brouwer-Visser J, Lee J, McCullagh K,
Cossio MJ, Wang Y and Huang GS: Insulin-like growth factor 2
silencing restores taxol sensitivity in drug resistant ovarian
cancer. PLoS One. 9:e1001652014. View Article : Google Scholar : PubMed/NCBI
|